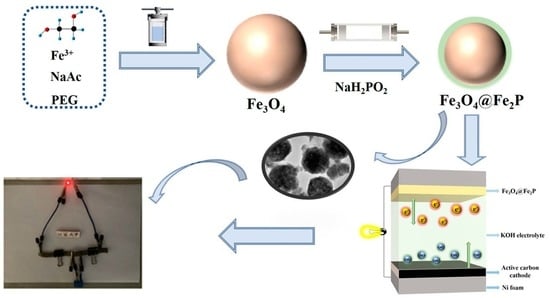
Construction of Fe3O4@Fe2P Heterostructures as Electrode Materials for Supercapacitors
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
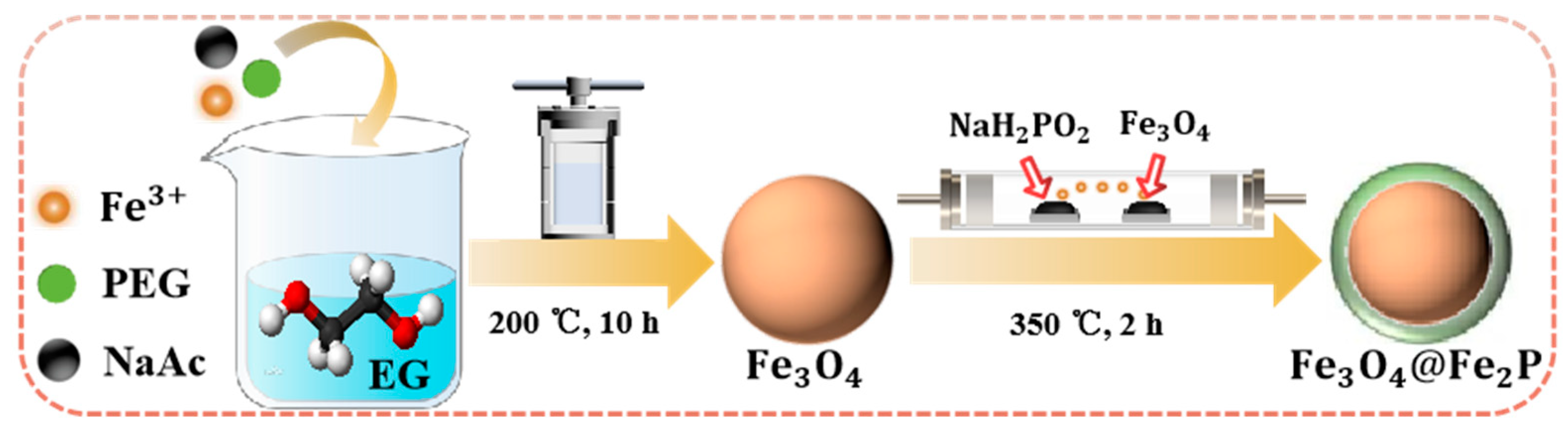
2.2. Synthesis of Fe3O4@Fe2P
2.2.1. Preparation of Fe3O4
2.2.2. Preparation of Fe3O4@Fe2P Heterostructure
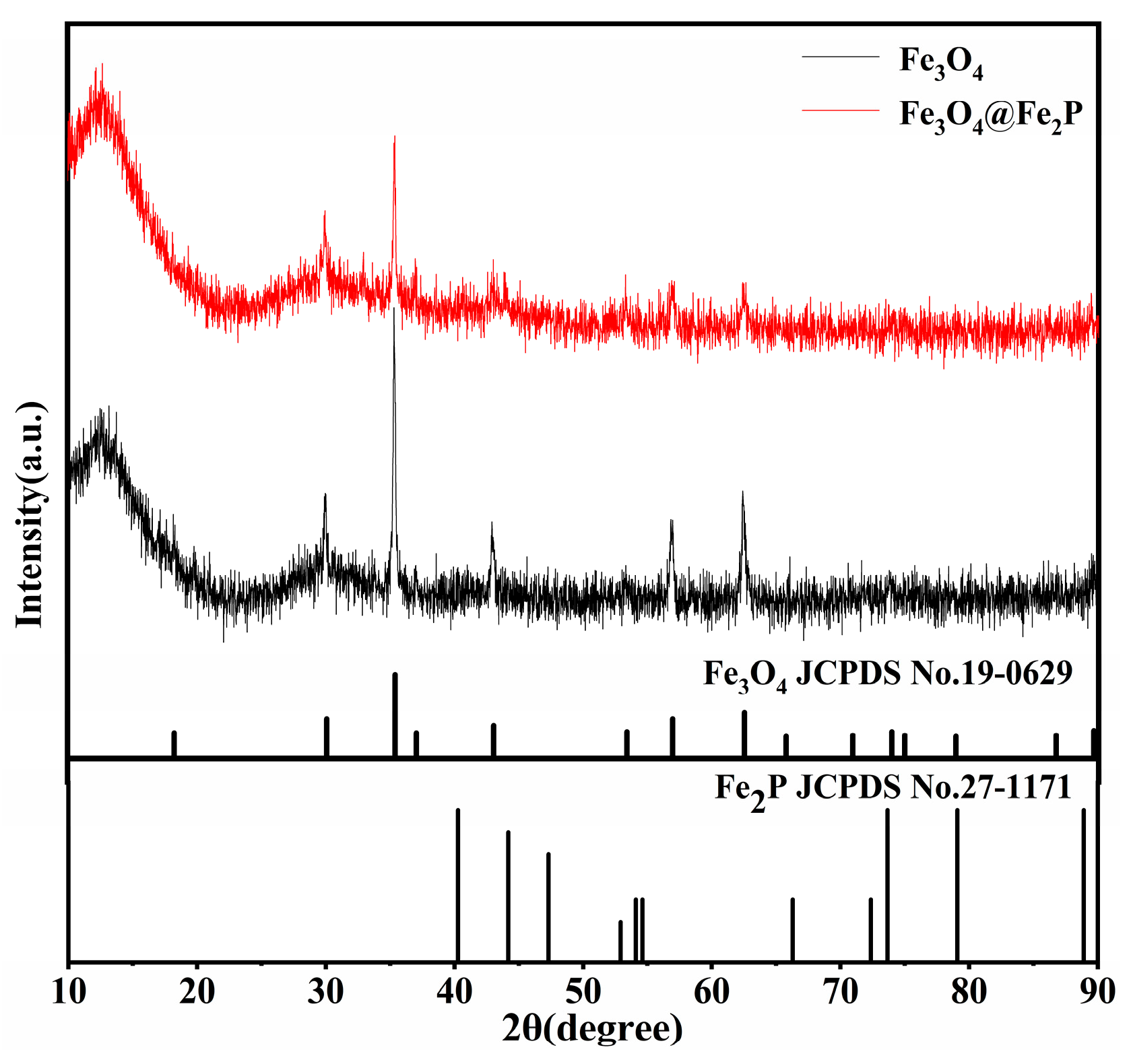
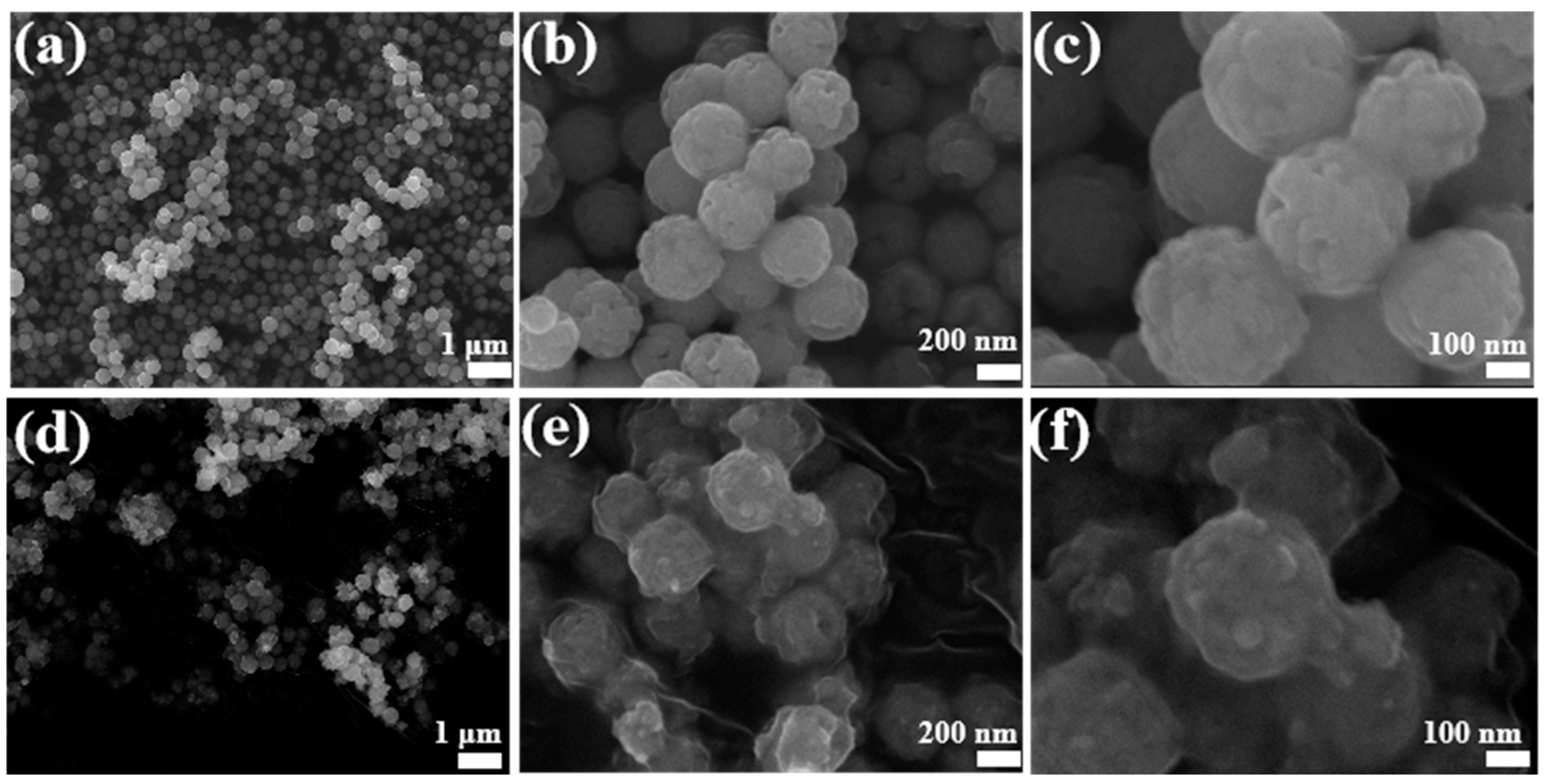
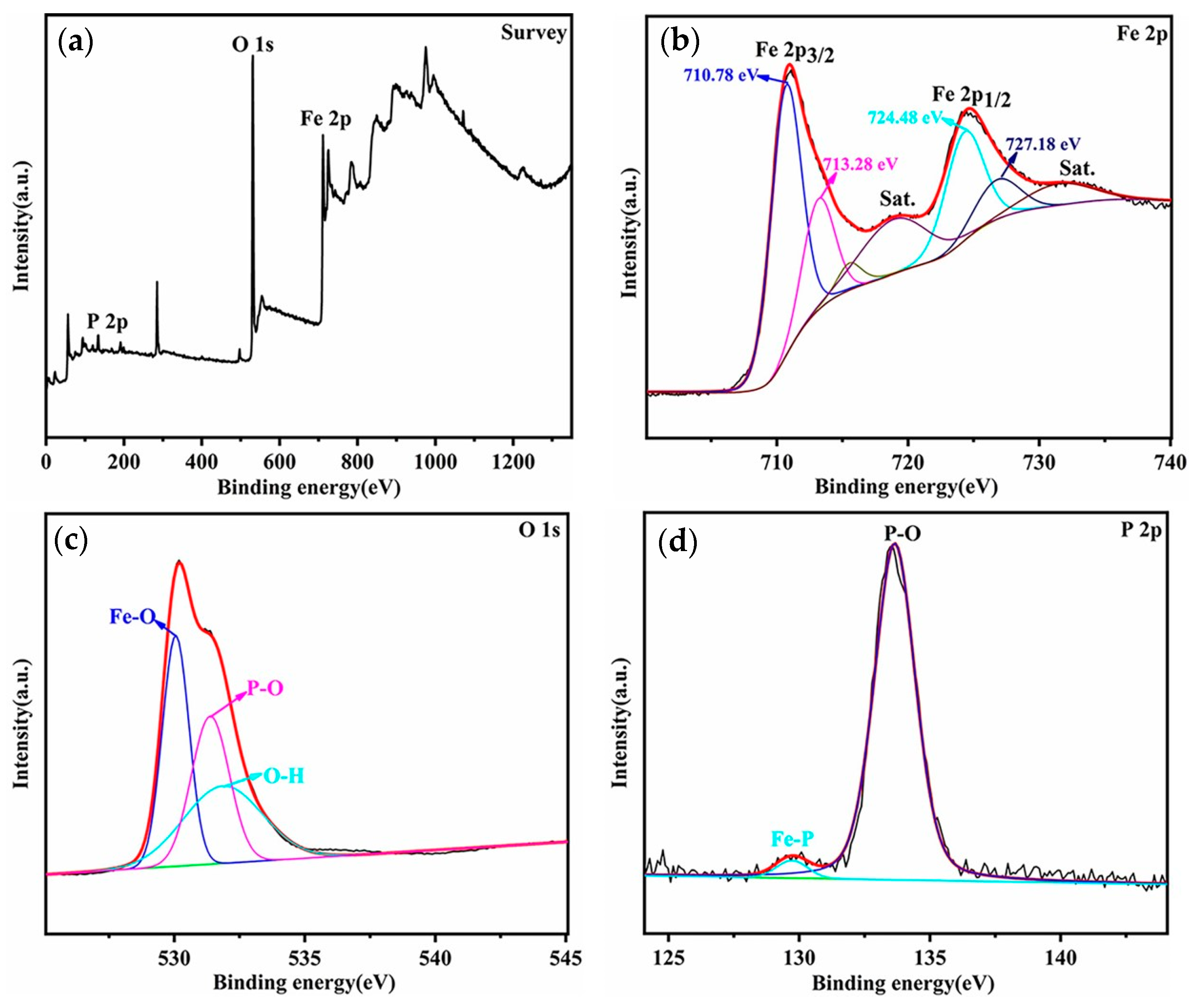
2.3. Characterization
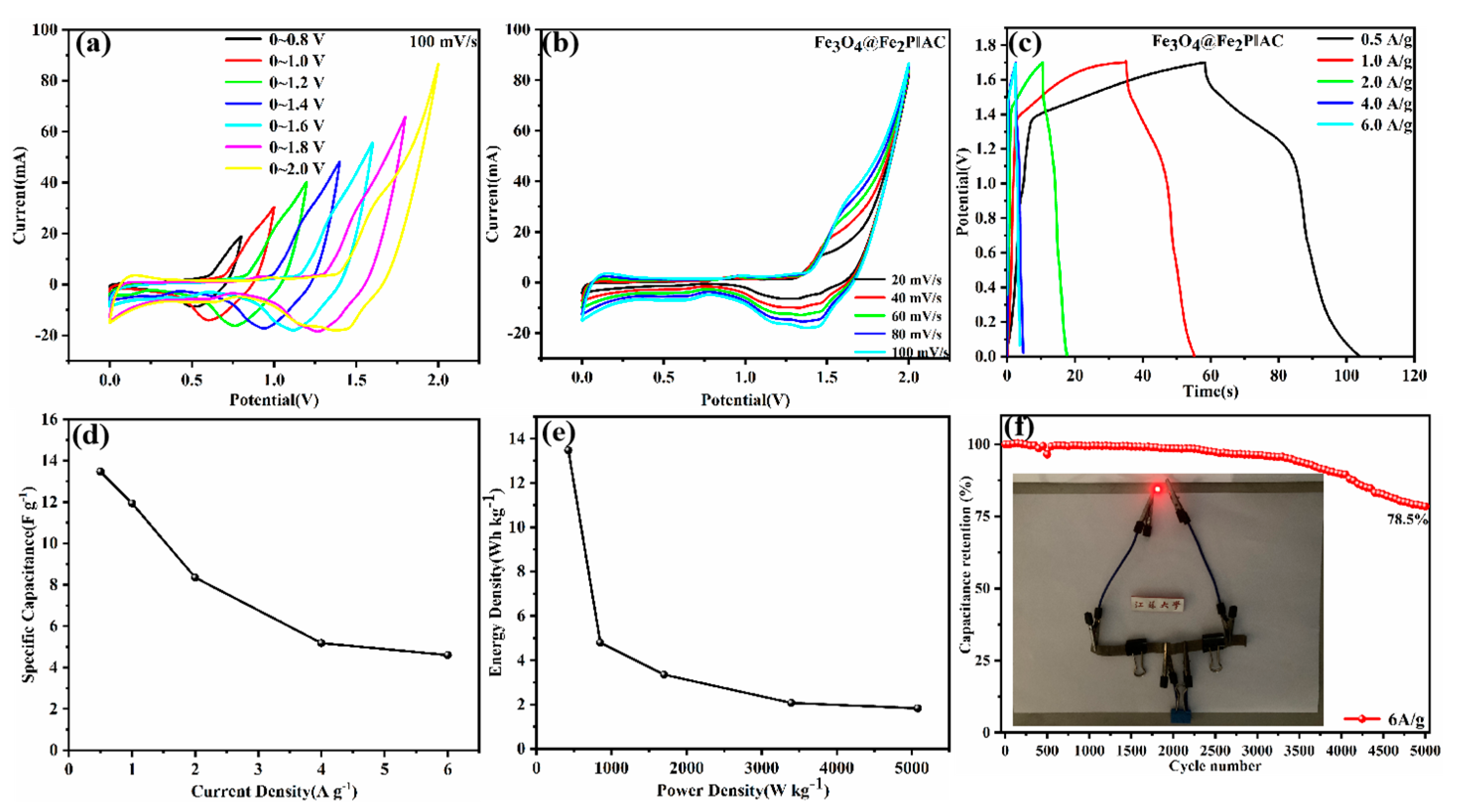
2.4. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dubal, D.P.; Ayyad, O.; Ruiz, V.; Gómez-Romero, P. Hybrid energy storage: The merging of battery and supercapacitor chemistries. Chem. Soc. Rev. 2015, 44, 1777–1790. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Yu, Z.; Duong, B.; Abbitt, D.; Thomas, J. Highly Ordered MnO2Nanopillars for Enhanced Supercapacitor Performance. Adv. Mater. 2013, 25, 3302–3306. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wang, T.; Jing, X.; Zhang, L. MnO/C/Sepiolite 3D-network aerogel as electrode material for supercapacitors. Mater. Chem. Phys. 2023, 303, 127744. [Google Scholar] [CrossRef]
- Tan, Y.B.; Lee, J.M. Graphene for supercapacitor applications. J. Mater. Chem. A 2013, 1, 14814–14843. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Wei, Q.; Lv, H.; Tian, Y.; Tong, Z.; Liu, X.; Hao, J.; Qu, H.; Zhao, J.; et al. 3D self-supported nanopine forest-like Co3O4@CoMoO4 core–shell architectures for high-energy solid state supercapacitors. Nano Energy 2015, 19, 222–233. [Google Scholar] [CrossRef]
- Shown, I.; Ganguly, A.; Chen, L.-C.; Chen, K.H. Conducting polymer-based flexible supercapacitor. Energy Sci. Eng. 2015, 3, 2–26. [Google Scholar] [CrossRef]
- Liu, X.; Pickup, P.G. Ru oxide/carbon fabric composites for supercapacitors. J. Solid State Electrochem. 2009, 14, 231–240. [Google Scholar] [CrossRef]
- Nithya, V.D.; Arul, N.S. Progress and development of Fe3O4 electrodes for supercapacitors. J. Mater. Chem. A 2016, 4, 10767–10778. [Google Scholar] [CrossRef]
- Li, G.; Li, R.; Zhou, W. A Wire-Shaped Supercapacitor in Micrometer Size Based on Fe3O4 Nanosheet Arrays on Fe Wire. Nano-Micro Lett. 2017, 9, 46. [Google Scholar] [CrossRef]
- Tang, X.; Jia, R.; Zhai, T.; Xia, H. Hierarchical Fe3O4@Fe2O3 core–shell nanorod arrays as high-performance anodes for asymmetric supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 27518–27525. [Google Scholar] [CrossRef] [PubMed]
- Panicker, N.J.; Dutta, J.C.; Sahu, P.P. Confined growth of NiCo2S4 on 2D/2D porous carbon self-repairing g-C3N4/rGO heterostructure for enhanced performance of asymmetric supercapacitors. Chem. Eng. J. 2023, 463, 142376. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Yang, T.-H.; Yue, S.; Zheng, H.-B.; Liu, X.-P.; Gao, P.-Z.; Qin, H.; Xiao, H.-N. Effects of Alternating Magnetic Fields on the OER of Heterogeneous Core–Shell Structured NiFe2O4@(Ni, Fe) S/P. ACS Appl. Mater. Interfaces 2023, 15, 11631–11641. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhu, F.; Ge, B.; Sun, L.; Liu, Y.; Shi, W. MOF derived ZnO/C@(Ni, Co) Se2 core–shell nanostructure on carbon cloth for high-performance supercapacitors. Chem. Eng. J. 2022, 427, 130788. [Google Scholar] [CrossRef]
- Wang, Y.; Adekoya, D.; Sun, J.; Tang, T.; Qiu, H.; Xu, L.; Zhang, S.; Hou, Y. Manipulation of edge-site Fe–N2 moiety on holey Fe, N codoped graphene to promote the cycle stability and rate capacity of Li–S batteries. Adv. Funct. Mater. 2019, 29, 1807485. [Google Scholar] [CrossRef]
- Oyama, S.T.; Clark, P.; Wang, X.; Shido, T.; Iwasawa, Y.; Hayashi, S.; Ramallo-López, J.M.; Requejo, F.G. Structural Characterization of Tungsten Phosphide (WP) Hydrotreating Catalysts by X-ray Absorption Spectroscopy and Nuclear Magnetic Resonance Spectroscopy. J. Phys. Chem. B 2002, 106, 1913–1920. [Google Scholar] [CrossRef]
- Wei, X.; Song, Y.; Song, L.; Liu, X.D.; Li, Y.; Yao, S.; Xiao, P.; Zhang, Y. Phosphorization Engineering on Metal–Organic Frameworks for Quasi-Solid-State Asymmetry Supercapacitors. Small 2021, 17, 2007062. [Google Scholar]
- Cuña, A.; da Silva, E.L.; Malfatti, C.F.; Gonçalves, G.R.; Schettino, M.A.; Freitas, J.C.C. Porous Carbon-Based Nanocomposites Containing Fe2P Nanoparticles as Promising Materials for Supercapacitor Electrodes. J. Electron. Mater. 2019, 49, 1059–1074. [Google Scholar] [CrossRef]
- Saha, S.; Samanta, P.; Murmu, N.C.; Kuila, T. A review on the heterostructure nanomaterials for supercapacitor application. J. Energy Storage 2018, 17, 181–202. [Google Scholar] [CrossRef]
- Mohamed SR, E.; Abdul-Aziz MR, R.; Saber, S.; Khabiri, G.; Khalil, A.S.G. Precise Engineering of Fe3O4/MWCNTs Heterostructures for High-Performance Supercapacitors. J. Alloys Compd. 2023, 957, 170281. [Google Scholar]
- Ciucci, F. Modeling electrochemical impedance spectroscopy. Curr. Opin. Electrochem. 2018, 13, 132–139. [Google Scholar] [CrossRef]
- Yang, M.; Ning, H.; Xiao, L.; Cui, F.; Zhang, F. Mn3O4/MnS heterostructure for electrode and asymmetric supercapacitor under high charge/discharge current. Electrochim. Acta 2022, 424, 140630. [Google Scholar] [CrossRef]
- Ran, F.; Yang, X.; Xu, X.; Li, S.; Liu, Y.; Shao, L. Green activation of sustainable resources to synthesize nitrogen-doped oxygen-riched porous carbon nanosheets towards high-performance supercapacitor. Chem. Eng. J. 2021, 412, 128673. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, L.; Huan, W.; Liang, X.; Liu, X.; Yang, Y. A study on synthesis and properties of Fe3O4 nanoparticles by solvothermal method. Glas. Phys. Chem. 2010, 36, 325–331. [Google Scholar] [CrossRef]
- Fan, H.; Niu, R.; Duan, J.; Liu, W.; Shen, W. Fe3O4@carbon nanosheets for all-solid-state supercapacitor electrodes. ACS Appl. Mater. Interfaces 2016, 8, 19475–19483. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, L.; Zhang, L.; Guo, Y.; Chen, X.; Holze, R.; Tang, T. Preparation of Fe3O4@polypyrrole composite materials for asymmetric supercapacitor applications. New J. Chem. 2021, 45, 16011–16018. [Google Scholar] [CrossRef]
- Qiu, Z.; Peng, Y.; He, D.; Wang, Y.; Chen, S. Ternary Fe3O4@C@PANi nanocomposites as high-performance supercapacitor electrode materials. J. Mater. Sci. 2018, 53, 12322–12333. [Google Scholar] [CrossRef]
- Li, Z.; Yao, Y.; Zheng, Y.; Gao, T.; Liu, Z.; Zhou, G. Fabrication of core-shell Fe3O4@C@MnO2 microspheres and their application in supercapacitors. J. Electrochem. Soc. 2018, 165, E58. [Google Scholar] [CrossRef]
- El-Gendy, D.M.; Ghany, N.A.A.; Allam, N.K. Green, single-pot synthesis of functionalized Na/N/P co-doped graphene nanosheets for high-performance supercapacitors. J. Electroanal. Chem. 2019, 837, 30–38. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, W.; Kou, Z.; Li, J.; Wang, T.; Guo, J. One-step microwave-assisted solvothermal nano-manufacturing of Ni2P nanosphere as high-performance supercapacitors. Ionics 2021, 27, 801–810. [Google Scholar] [CrossRef]
- Liu, S.; Chen, Y.; Ren, J.; Wang, Y.; Wei, W. An effective interaction in polypyrrole/nickel phosphide (PPy/Ni2P) for high-performance supercapacitor. J. Solid State Electrochem. 2019, 23, 3409–3418. [Google Scholar] [CrossRef]
- Arun, T.; Prabakaran, K.; Udayabhaskar, R.; Mangalaraja, R.; Akbari-Fakhrabadi, A. Carbon decorated octahedral shaped Fe3O4 and α-Fe2O3 magnetic hybrid nanomaterials for next generation supercapacitor applications. Appl. Surf. Sci. 2019, 485, 147–157. [Google Scholar] [CrossRef]
- Song, L.; Han, Y.; Guo, F.; Jiao, Y.; Li, Y.; Liu, Y.; Gao, F. Mesoporous Nickel-Based Zeolite Capsule Complex with Fe3O4 as Electrode for Advanced Supercapacitor. J. Nanomater. 2018, 2018, 9813203. [Google Scholar] [CrossRef]
- Tu, C.; Li, X.; Lu, C.; Luo, Q.; Li, T.; Zhu, M. A sequential process to synthesize Fe3O4@MnO2 hollow nanospheres for high performance supercapacitors. Mater. Chem. Front. 2022, 6, 1938–1947. [Google Scholar] [CrossRef]
- Mondal, S.; Rana, U.; Malik, S. Reduced graphene Oxide/Fe3O4/polyaniline nanostructures as electrode materials for an all-solid-state hybrid supercapacitor. J. Phys. Chem. C 2017, 121, 7573–7583. [Google Scholar] [CrossRef]
- Lin, T.W.; Dai, C.S.; Hung, K.C. High energy density asymmetric supercapacitor based on NiOOH/Ni3S2/3D graphene and Fe3O4/graphene composite electrodes. Sci. Rep. 2014, 4, 7274. [Google Scholar] [CrossRef]
- Sun, J.; Zan, P.; Yang, X.; Ye, L.; Zhao, L. Room-temperature synthesis of Fe3O4/Fe-carbon nanocomposites with Fe-carbon double conductive network as supercapacitor. Electrochim. Acta 2016, 215, 483–491. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Zhou, C.; Wang, C.; Ba, X.; Li, Y.; Huang, X.; Liu, J. Carbon-Stabilized High-Capacity Ferroferric Oxide Nanorod Array for Flexible Solid-State Alkaline Battery-Supercapacitor Hybrid Device with High Environmental Suitability. Adv. Funct. Mater. 2015, 25, 5384–5394. [Google Scholar] [CrossRef]



| Electrode | Electrolyte | Current Density (A g−1) | Specific Capacitance (F g−1) | Ref. |
|---|---|---|---|---|
| Fe3O4@Fe2P | 1 M KOH | 0.5 | 651.25 | This work |
| Fe3O4/carbon | 6 M KOH | 0.5 | 586 | [25] |
| Fe3O4@polypyrrole | 1 M H2SO4 | 1 | 290.2 | [26] |
| Fe3O4@C@PANi | 1 M KOH | 0.5 | 420 | [27] |
| Fe3O4@C@MnO2 | 1 M Na2SO4 | 0.5 | 158 | [28] |
| Na/N/P-GNS | 5 M H2SO4 | 499 | [29] | |
| Ni2P | 6 M KOH | 1 | 404.2 | [30] |
| PPy/Ni2P | 1 M Na2SO4 | 1 | 476.5 | [31] |
| Fe3O4 and α-Fe2O3 | 6 M KOH | 0.5 | 274 | [32] |
| Electrode | Current Density (A g−1) | Number of Cycles | Retention Rate (%) | Ref. |
|---|---|---|---|---|
| Fe3O4@ Fe2P||AC | 6 | 5000 | 78.5 | This work |
| Fe3O4/carbon||CPY | 5 | 5000 | 70.8 | [25] |
| Fe3O4@Ni||AC | 1 | 1000 | 80.3 | [33] |
| Fe3O4@MnO2-HNS||AC | 2 | 5000 | 70.6 | [34] |
| rGO/Fe3O4/PANI | 1 | 5000 | 78 | [35] |
| NiOOH/Ni3S2/3D-G||Fe3O4/rGO | 1 | 2000 | 74 | [36] |
| Fe3O4/Fe-CNTs||AC | 5 | 3000 | 78.9 | [37] |
| CNTs(+)||Fe3O4-C(−) | 1 M Na2SO4 | 1000 | 67.7 | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, C.; Tu, C.; Yang, Y.; Ma, Y.; Zhu, M. Construction of Fe3O4@Fe2P Heterostructures as Electrode Materials for Supercapacitors. Batteries 2023, 9, 326. https://doi.org/10.3390/batteries9060326
Lu C, Tu C, Yang Y, Ma Y, Zhu M. Construction of Fe3O4@Fe2P Heterostructures as Electrode Materials for Supercapacitors. Batteries. 2023; 9(6):326. https://doi.org/10.3390/batteries9060326
Chicago/Turabian StyleLu, Congcong, Chengyu Tu, Yu Yang, Yunping Ma, and Maiyong Zhu. 2023. "Construction of Fe3O4@Fe2P Heterostructures as Electrode Materials for Supercapacitors" Batteries 9, no. 6: 326. https://doi.org/10.3390/batteries9060326
APA StyleLu, C., Tu, C., Yang, Y., Ma, Y., & Zhu, M. (2023). Construction of Fe3O4@Fe2P Heterostructures as Electrode Materials for Supercapacitors. Batteries, 9(6), 326. https://doi.org/10.3390/batteries9060326