Trends in Automotive Battery Cell Design: A Statistical Analysis of Empirical Data
Abstract
1. Introduction
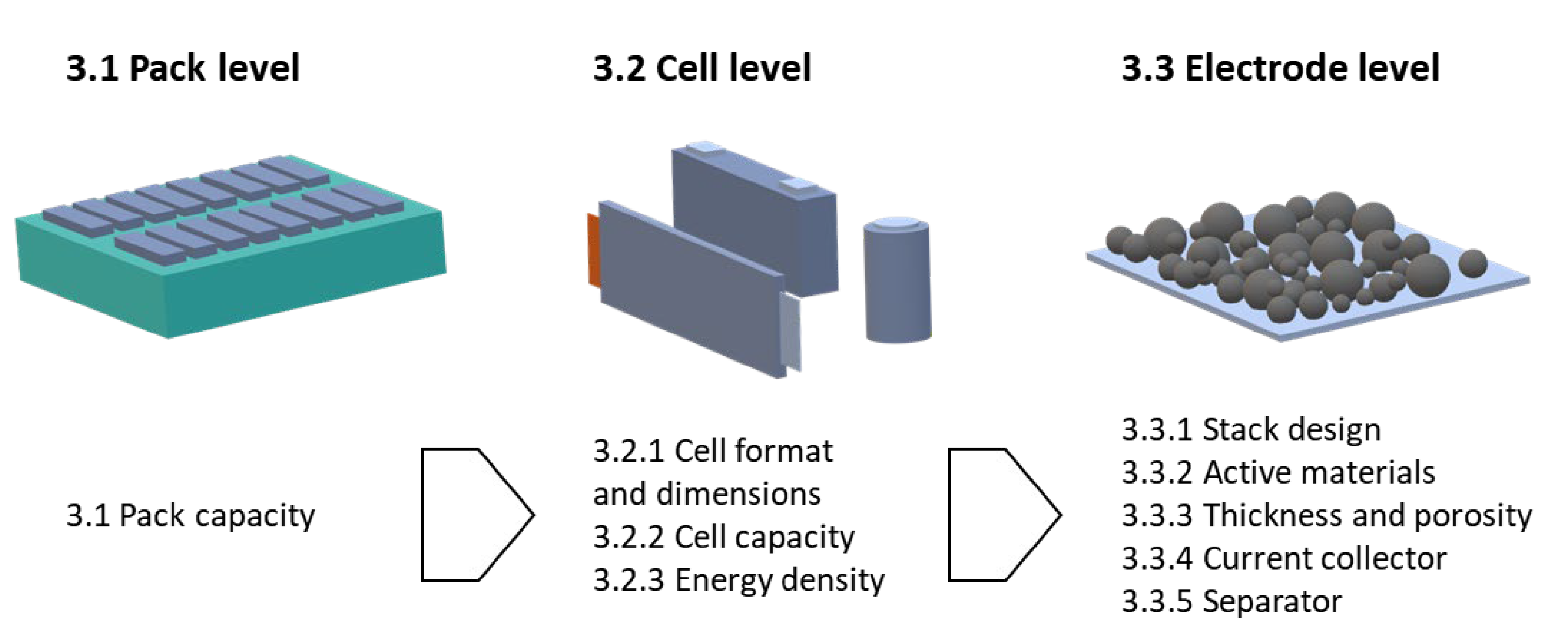
2. Data and Methods
3. Results
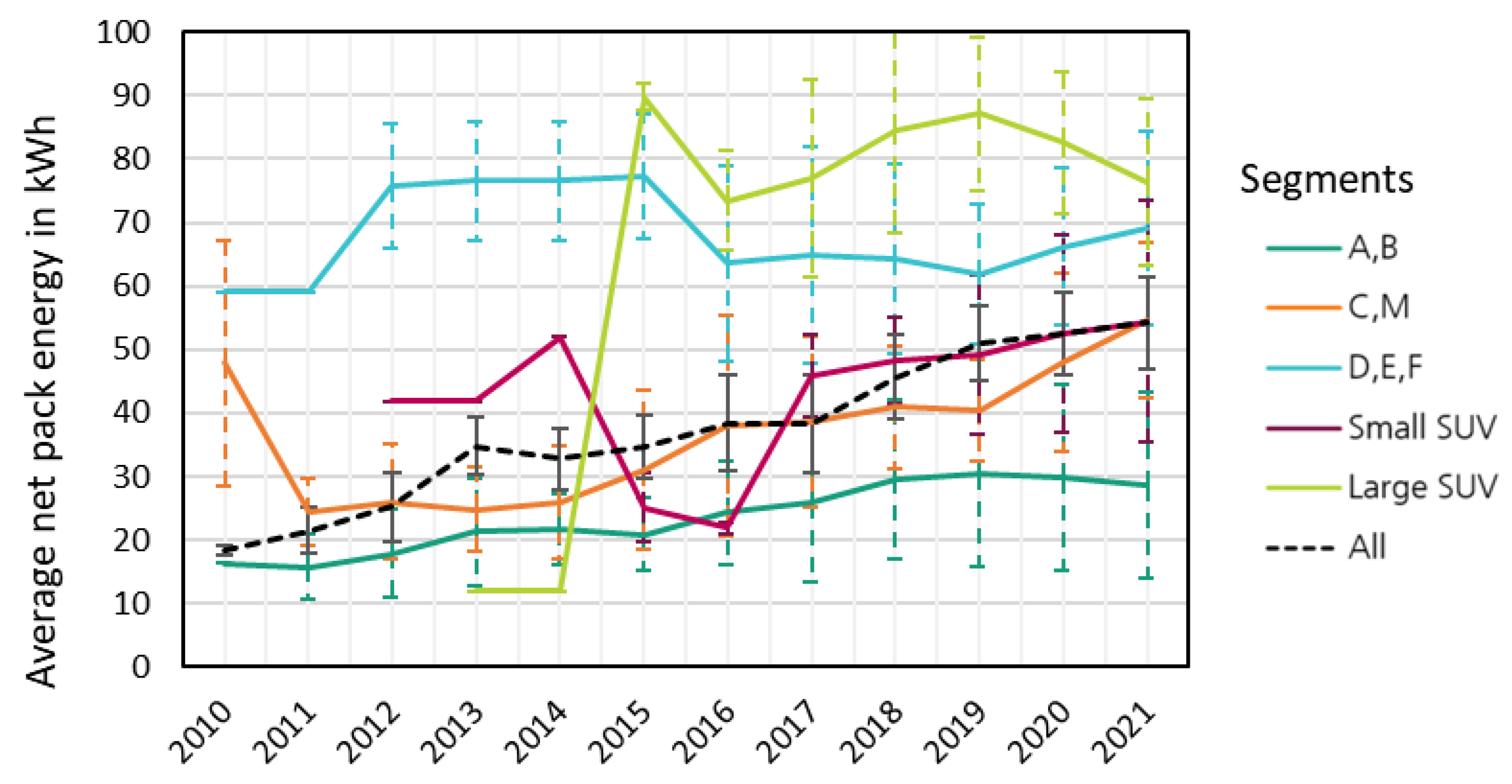
3.1. Pack capacity
3.2. Cell Properties
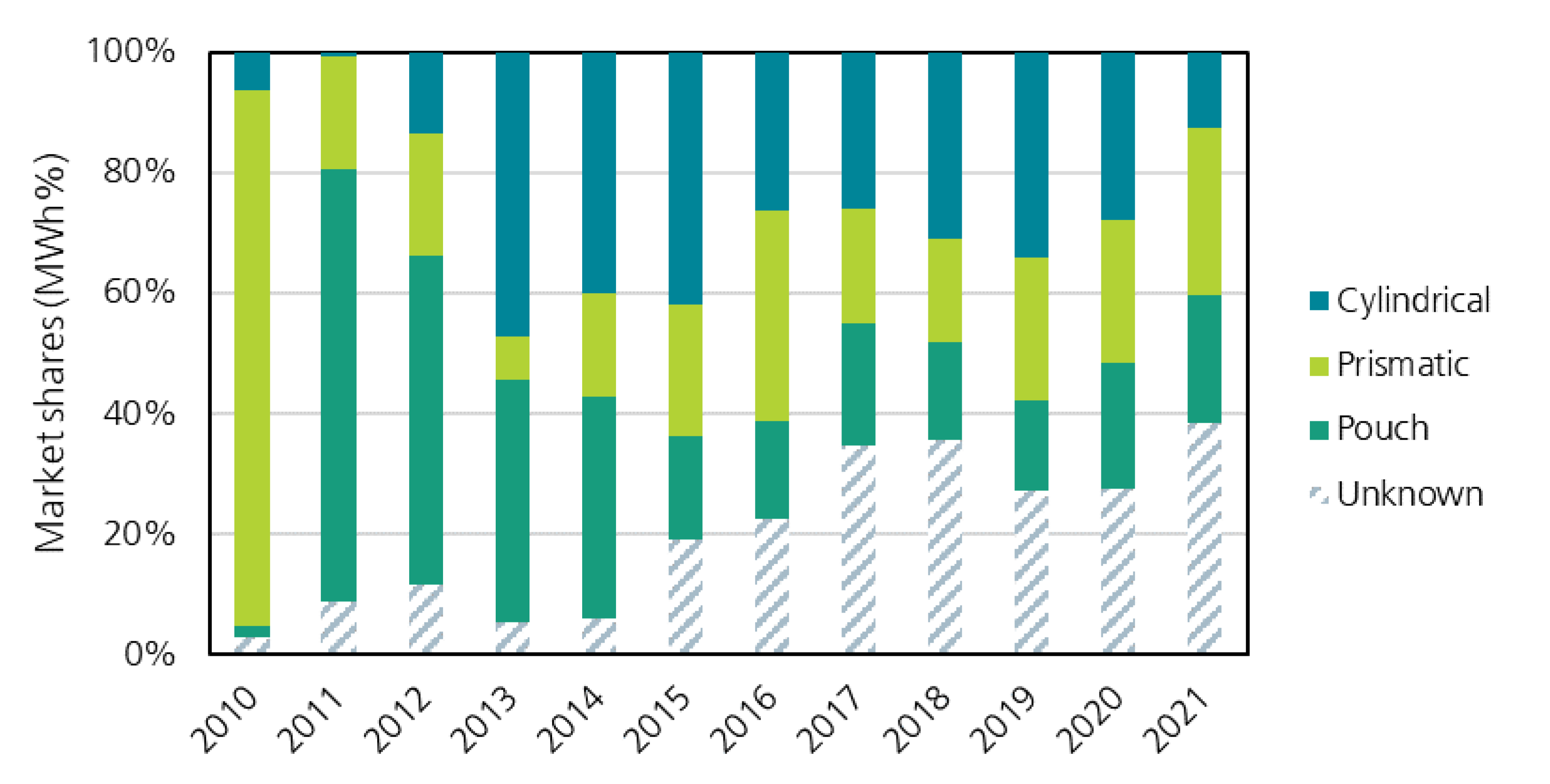
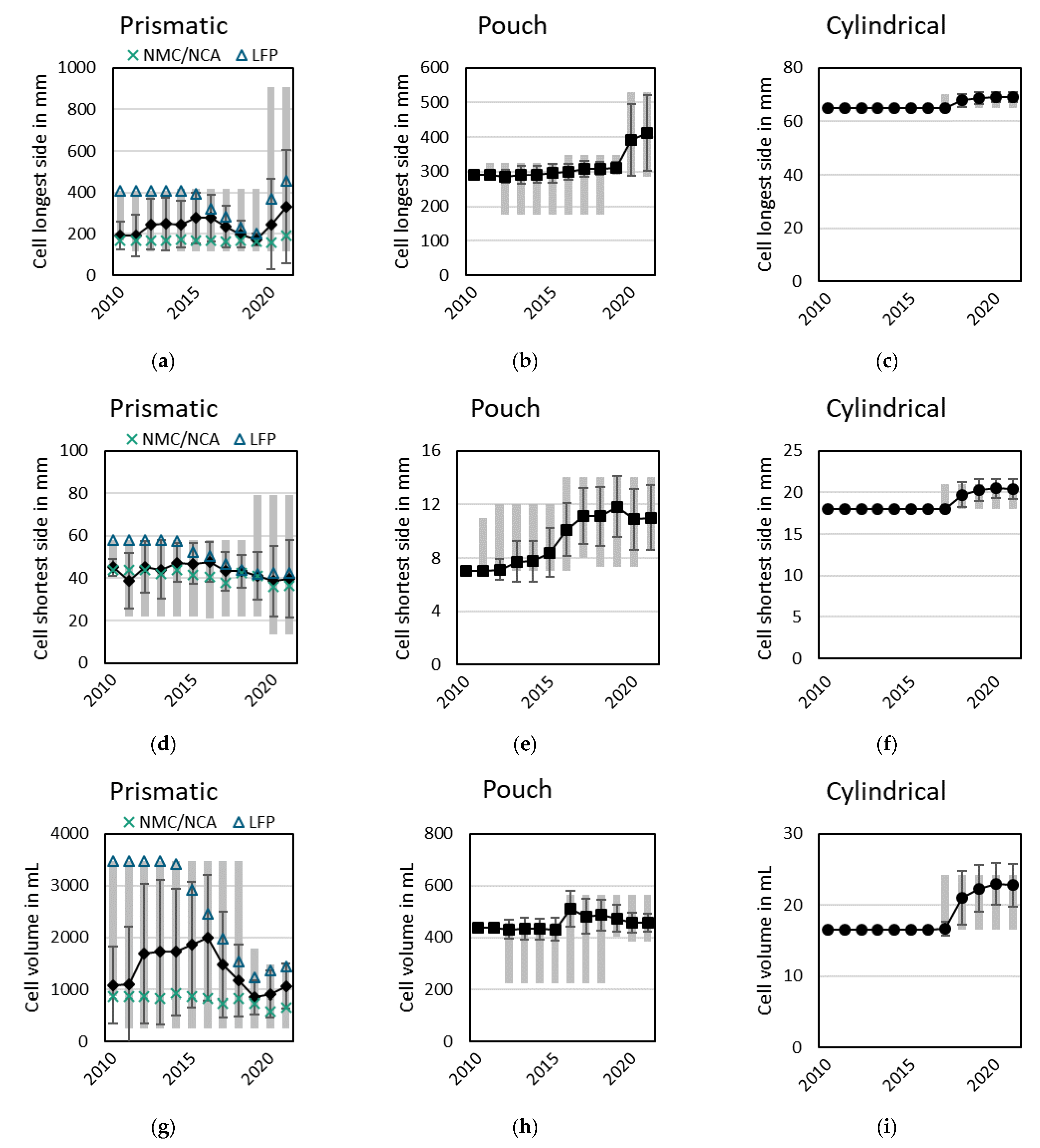
3.2.1. Cell Formats and Outer Cell Dimensions
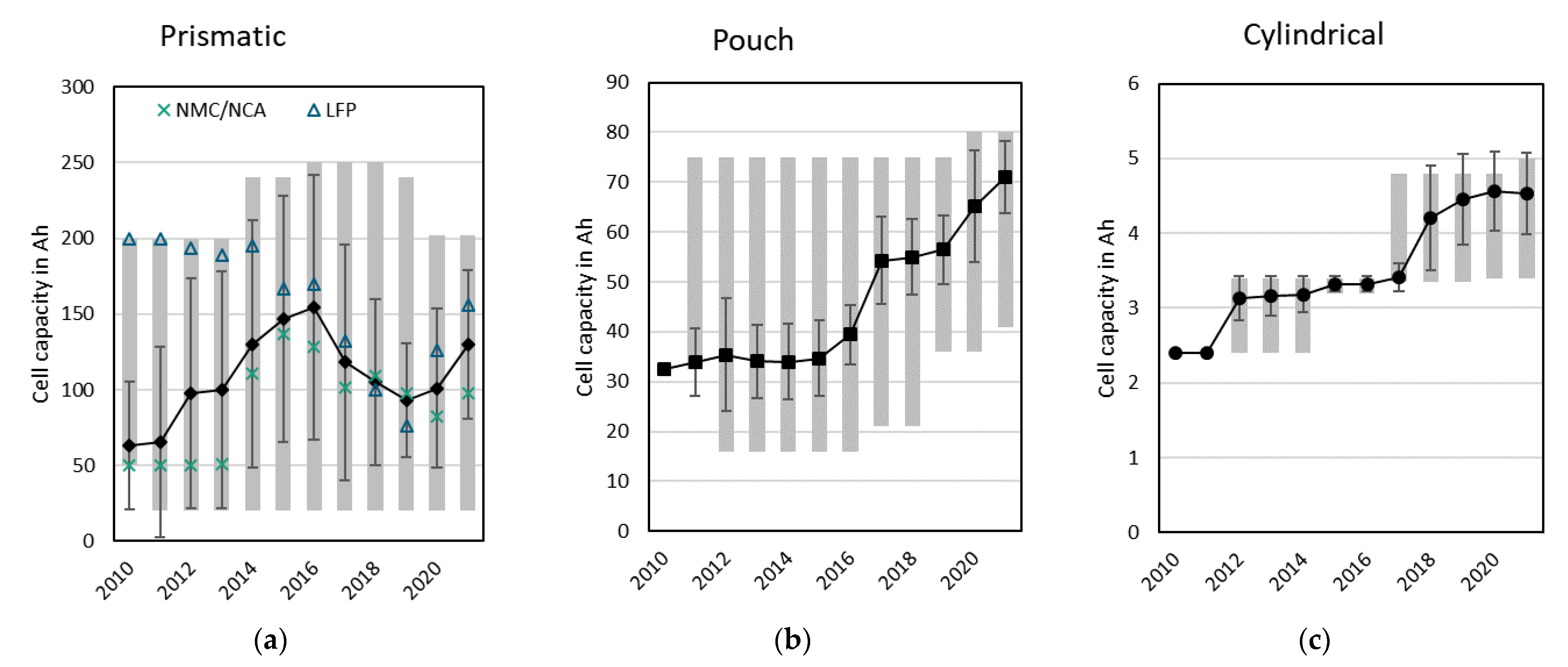
3.2.2. Cell Capacities
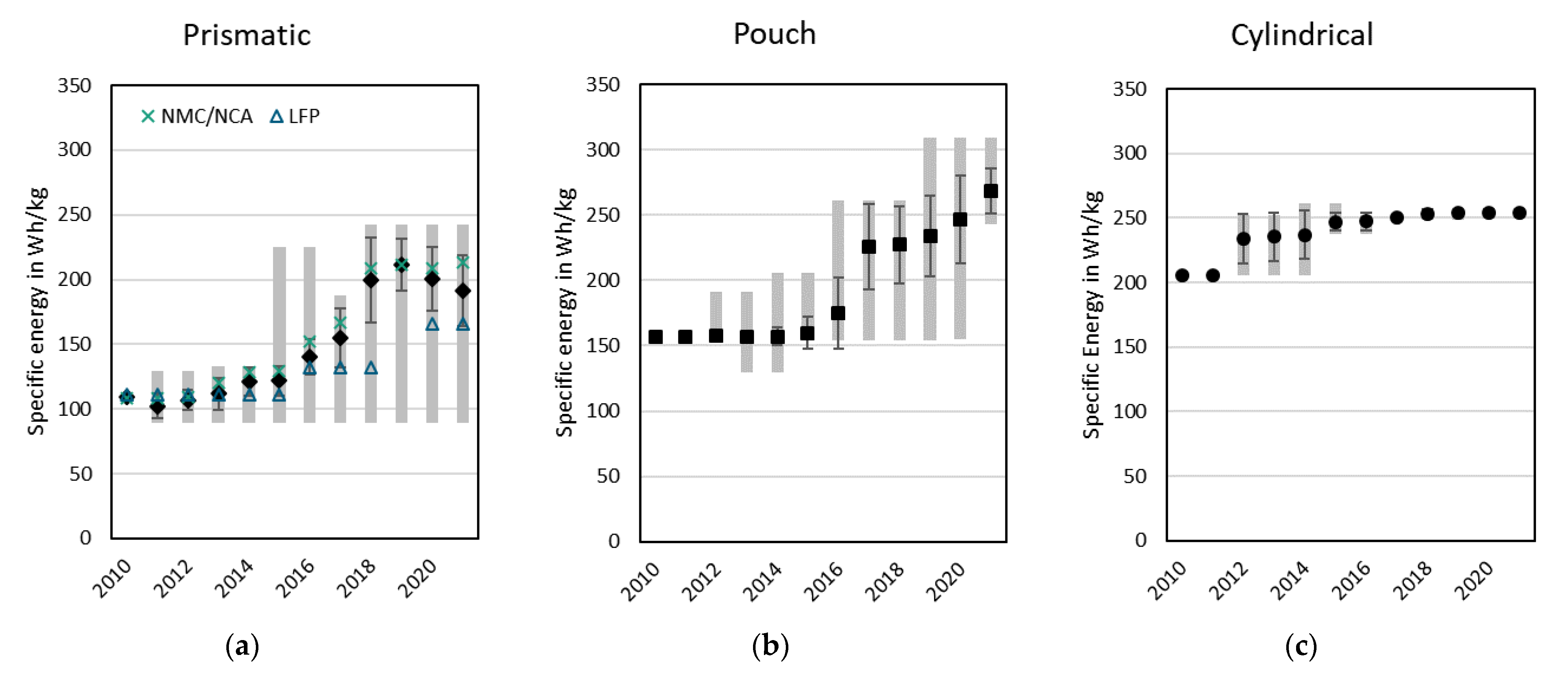
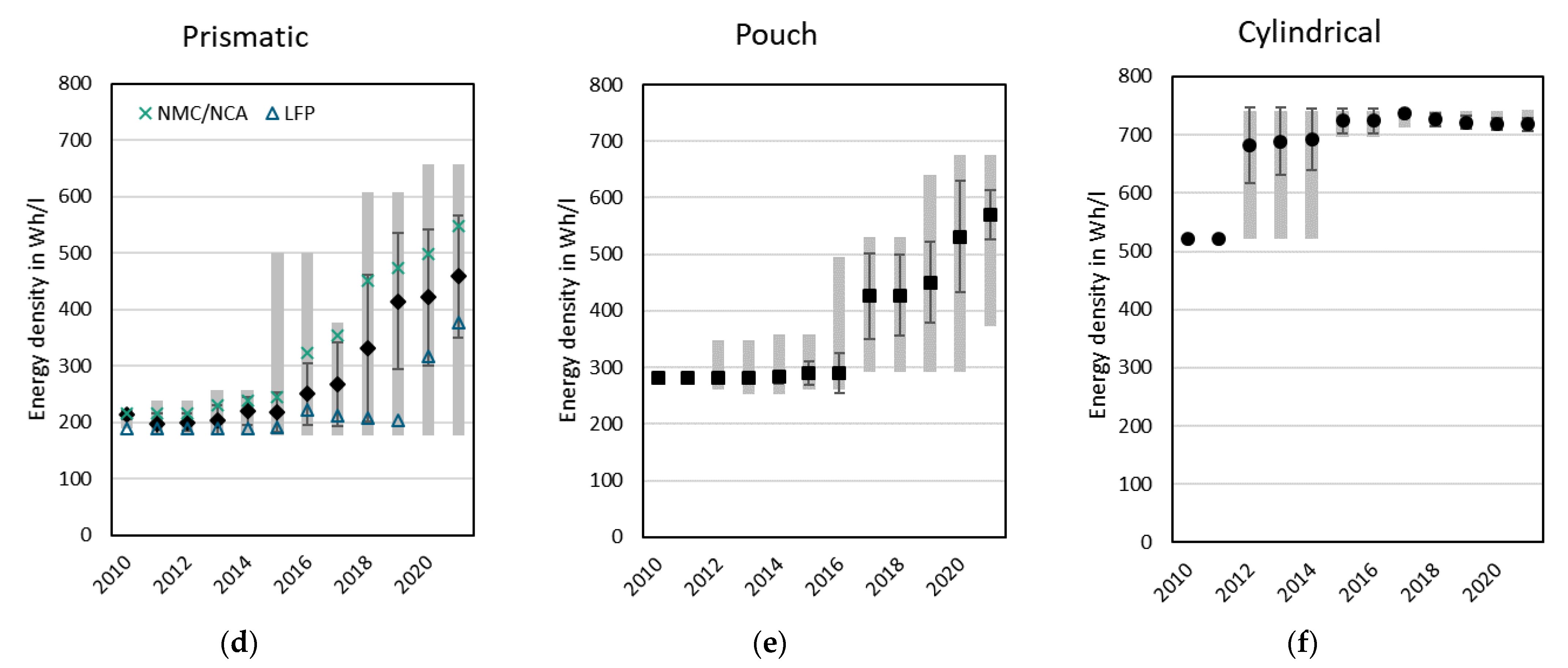
3.2.3. Specific Energy and Energy Density
3.3. Electrode Properties
3.3.1. Stack Design
3.3.2. Cathode and Anode Active Materials
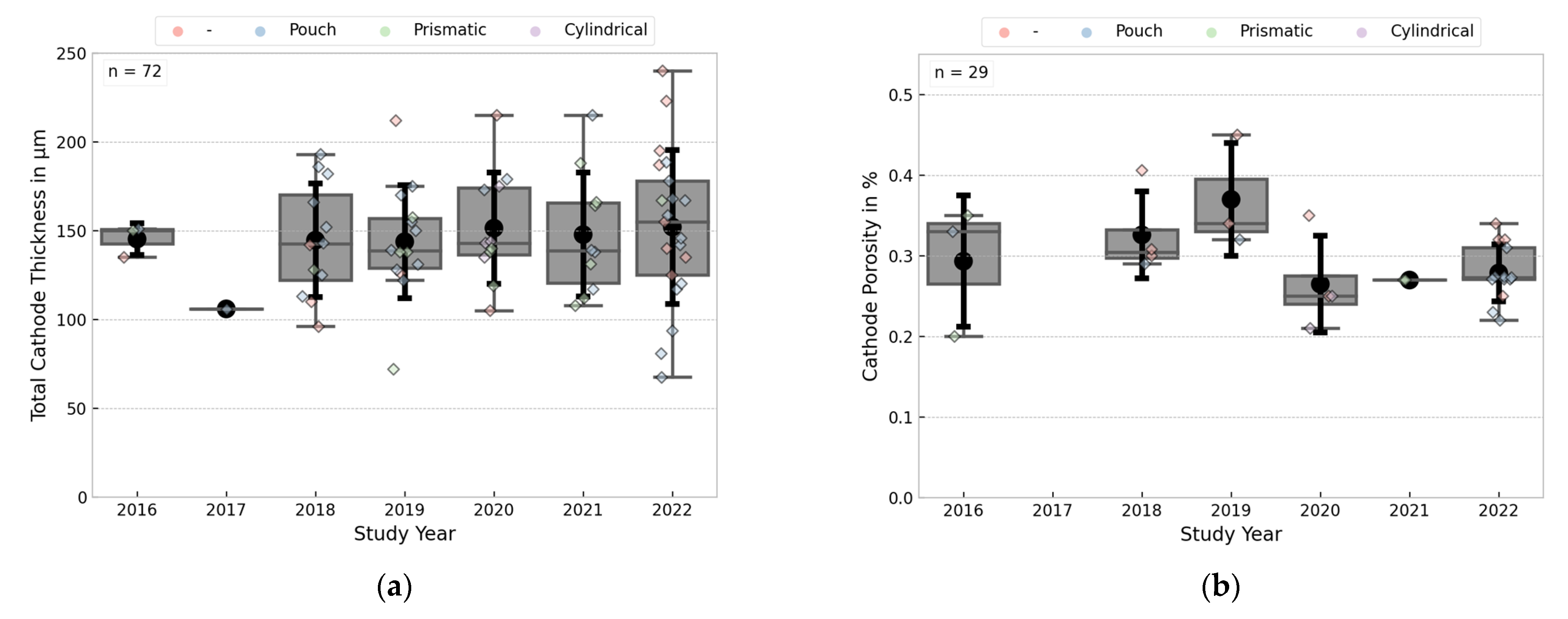
3.3.3. Electrode Thicknesses and Porosities
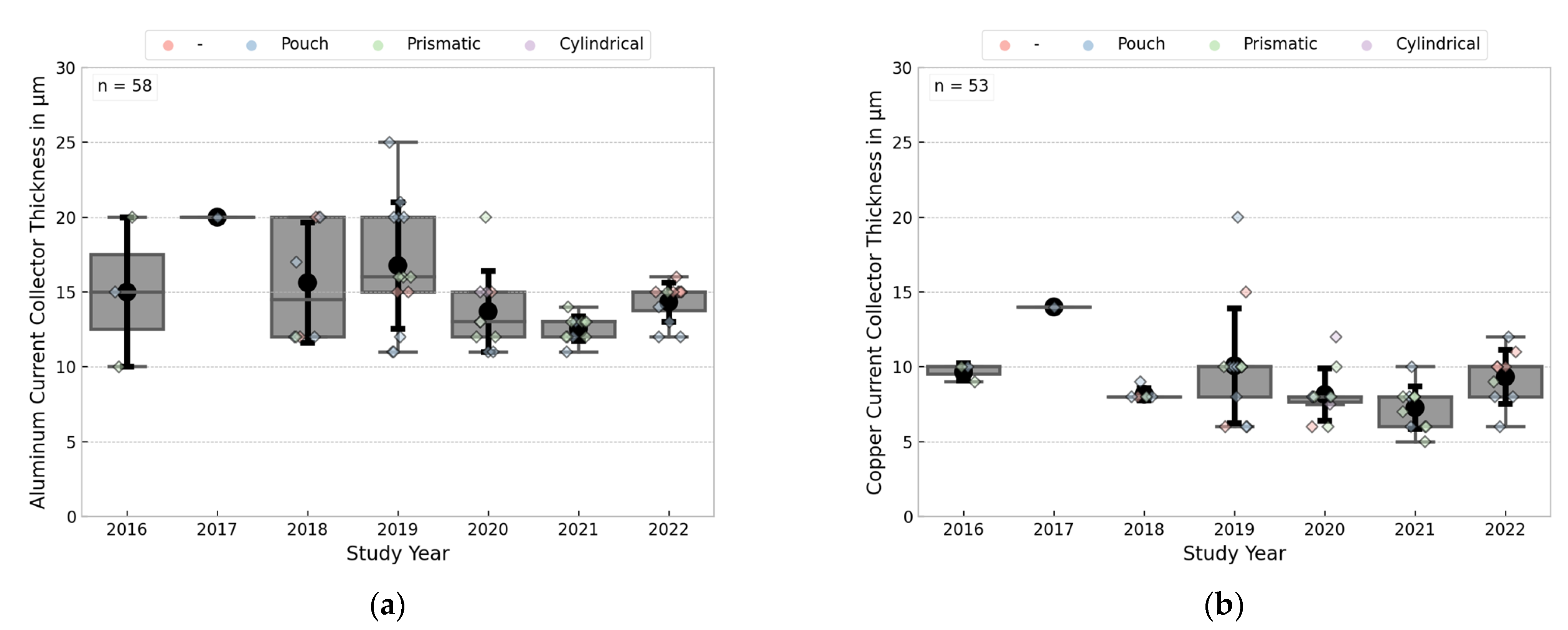
3.3.4. Current Collector Thicknesses
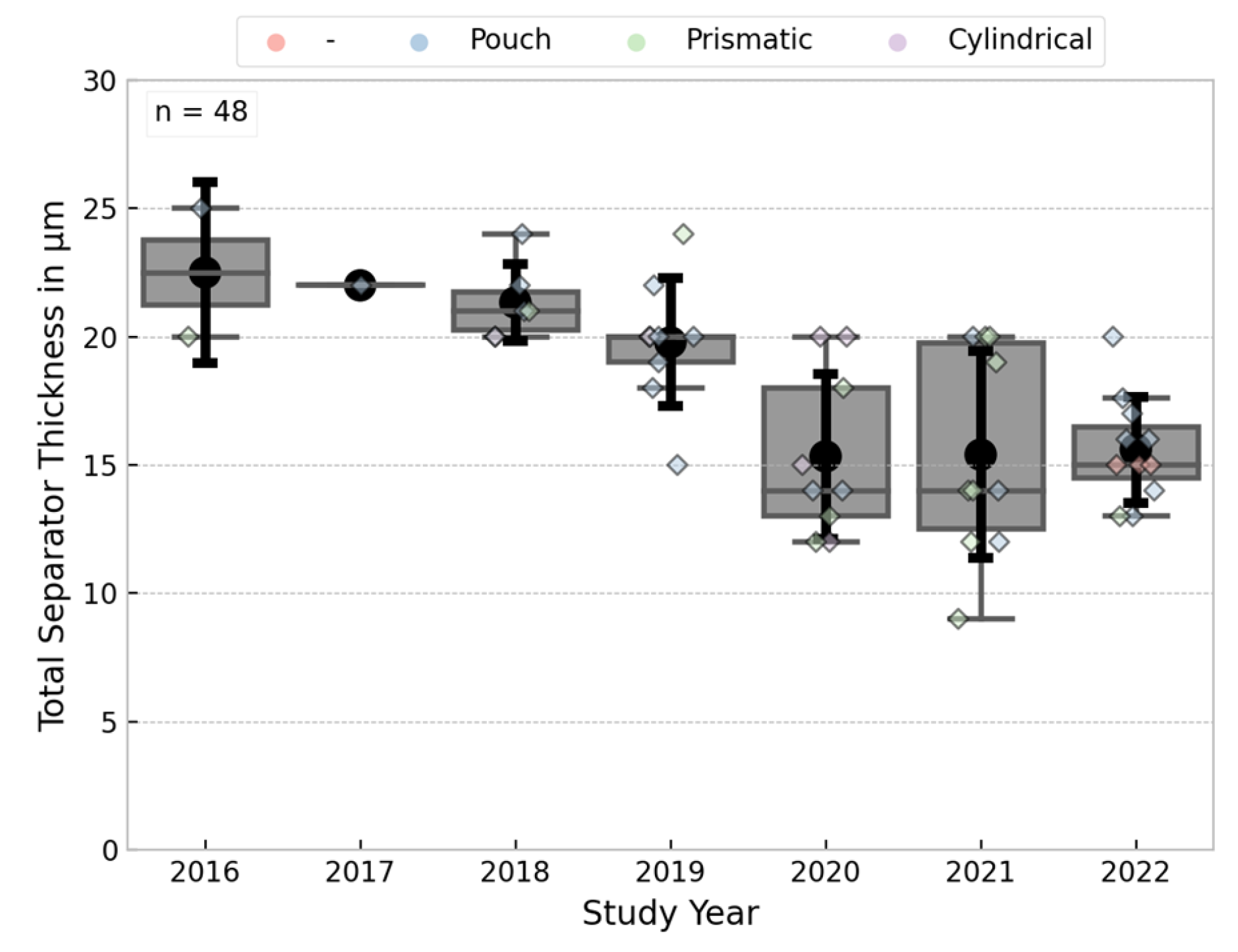
3.3.5. Separator Thickness
4. Discussion
4.1. Data Limitations
4.2. Discussion of Results
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BEV | Battery Electric Vehicle |
| C2P | Cell-to-Pack |
| EV | Electric Vehicle |
| HP | High Power |
| HE | High Energy |
| Li-ion | Lithium-ion |
| MY | Model Year |
| OEM | Original Equipment Manufacturer |
| xEV | Different electric vehicles, including hybrids, plug-in hybrids, BEVs, and fuel-cell vehicles |
References
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 2018, 3, 267–278. [Google Scholar] [CrossRef]
- Masias, A.; Marcicki, J.; Paxton, W.A. Opportunities and Challenges of Lithium Ion Batteries in Automotive Applications. ACS Energy Lett. 2021, 6, 621–630. [Google Scholar] [CrossRef]
- Ding, Y.; Cano, Z.P.; Yu, A.; Lu, J.; Chen, Z. Automotive Li-Ion Batteries: Current Status and Future Perspectives. Electrochem. Energy Rev. 2019, 2, 1–28. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Deng, J.; Bae, C.; Denlinger, A.; Miller, T. Electric Vehicles Batteries: Requirements and Challenges. Joule 2020, 4, 511–515. [Google Scholar] [CrossRef]
- Davis, S.J.; Lewis, N.S.; Shaner, M.; Aggarwal, S.; Arent, D.; Azevedo, I.L.; Benson, S.M.; Bradley, T.; Brouwer, J.; Chiang, Y.-M.; et al. Net-zero emissions energy systems. Science 2018, 360, eaas9793. [Google Scholar] [CrossRef]
- Xu, C.; Dai, Q.; Gaines, L.; Hu, M.; Tukker, A.; Steubing, B. Future material demand for automotive lithium-based batteries. Commun. Mater. 2020, 1, 99. [Google Scholar] [CrossRef]
- Marklines. Marklines Co L Automotive Industry Portal. Available online: http://www.marklines.com/ (accessed on 27 January 2023).
- IEA. Global EV Outlook 2022; IEA: Paris, France, 2022; Available online: https://www.iea.org/reports/global-ev-outlook-2022 (accessed on 24 January 2023).
- Cornet, A.; Heuss, R.; Tschiesner, A.; Hensley, R.; Hertzke, P.; Möller, T.; Schaufuss, P.; Conzade, J.; Schenk, S.; von Laufenberg, K. Why the Automotive Future is Electric: Mainstream EVs Will Transform the Automotive Industry and Help Decarbonize the Planet; IAA: Westchester, IL, USA, 2021. [Google Scholar]
- Volta Foundation. The Battery Report 2022; Volta Foundation: San Francisco, CA, USA, 2023; Available online: https://www.volta.foundation/annual-battery-report (accessed on 15 February 2023).
- Link, S.; Neef, C.; Wicke, T.; Hettesheimer, T.; Diehl, M.; Krätzig, O.; Degen, F.; Klein, F.; Fanz, P.; Burgard, M.; et al. Development Perspectives for Lithium-Ion Battery Cell Formats. 2022. Available online: https://www.isi.fraunhofer.de/content/dam/isi/dokumente/cct/2022/Development_perspectives_for_lithium-ion_battery_cell_formats_Fraunhofer_2022.pdf (accessed on 20 February 2023).
- Kwade, A.; Haselrieder, W.; Leithoff, R.; Modlinger, A.; Dietrich, F.; Droeder, K. Current status and challenges for automotive battery production technologies. Nat. Energy 2018, 3, 290–300. [Google Scholar] [CrossRef]
- Lain, M.J.; Brandon, J.; Kendrick, E. Design Strategies for High Power vs. High Energy Lithium Ion Cells. Batteries 2019, 5, 64. [Google Scholar] [CrossRef]
- EUCAR. Battery Requirements for Future Automotive Applications: EG BEV&FCEV; EUCAR: Brussels, Belgium, 2019. [Google Scholar]
- Rauscher, S. Einfluss von Material-und Beschichtungsparametern auf die Elektrodenmorphologie und die Leistungsparameter von Lithiumionen-Zellen. Ph.D. Thesis, Universität Ulm, Ulm, Germany, 2014. [Google Scholar] [CrossRef]
- Yang, X.-G.; Liu, T.; Wang, C.-Y. Thermally modulated lithium iron phosphate batteries for mass-market electric vehicles. Nat. Energy 2021, 6, 176–185. [Google Scholar] [CrossRef]
- Thompson, D.L.; Hartley, J.M.; Lambert, S.M.; Shiref, M.; Harper, G.D.J.; Kendrick, E.; Anderson, P.; Ryder, K.S.; Gaines, L.; Abbott, A.P. The importance of design in lithium ion battery recycling—A critical review. Green Chem. 2020, 22, 7585–7603. [Google Scholar] [CrossRef]
- Schmidt, A.; Smith, A.; Ehrenberg, H. Power capability and cyclic aging of commercial, high power lithium ion battery cells with respect to different cell designs. J. Power Sources 2019, 425, 27–38. [Google Scholar] [CrossRef]
- Wagner, R.; Preschitschek, N.; Passerini, S.; Leker, J.; Winter, M. Current research trends and prospects among the various materials and designs used in lithium-based batteries. J. Appl. Electrochem. 2013, 43, 481–496. [Google Scholar] [CrossRef]
- Al Hallaj, S.; Maleki, H.; Hong, J.; Selman, J. Thermal modeling and design considerations of lithium-ion batteries. J. Power Sources 1999, 83, 1–8. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, Y.; Huang, R.; Chen, J.; Chen, F.; Liu, Z.; Yu, X.; Roskilly, A.P. Study on the thermal interaction and heat dissipation of cylindrical Lithium-Ion Battery cells. Energy Procedia 2017, 142, 4029–4036. [Google Scholar] [CrossRef]
- Mao, C.; Ruther, R.E.; Li, J.; Du, Z.; Belharouak, I. Identifying the limiting electrode in lithium ion batteries for extreme fast charging. Electrochem. Commun. 2018, 97, 37–41. [Google Scholar] [CrossRef]
- Mussa, A.S.; Liivat, A.; Marzano, F.; Klett, M.; Philippe, B.; Tengstedt, C.; Lindbergh, G.; Edström, K.; Lindström, R.W.; Svens, P. Fast-charging effects on ageing for energy-optimized automotive LiNi1/3Mn1/3Co1/3O2/graphite prismatic lithium-ion cells. J. Power Sources 2019, 422, 175–184. [Google Scholar] [CrossRef]
- Preger, Y.; Barkholtz, H.M.; Fresquez, A.; Campbell, D.L.; Juba, B.W.; Romàn-Kustas, J.; Ferreira, S.R.; Chalamala, B.R. Degradation of Commercial Lithium-Ion Cells as a Function of Chemistry and Cycling Conditions. J. Electrochem. Soc. 2020, 167, 120532. [Google Scholar] [CrossRef]
- Han, X.; Lu, L.; Zheng, Y.; Feng, X.; Li, Z.; Li, J.; Ouyang, M. A review on the key issues of the lithium ion battery degradation among the whole life cycle. Etransportation 2019, 1, 100005. [Google Scholar] [CrossRef]
- Wassiliadis, N.; Steinsträter, M.; Schreiber, M.; Rosner, P.; Nicoletti, L.; Schmid, F.; Ank, M.; Teichert, O.; Wildfeuer, L.; Schneider, J.; et al. Quantifying the state of the art of electric powertrains in battery electric vehicles: Range, efficiency, and lifetime from component to system level of the Volkswagen ID.3. Etransportation 2022, 12, 100167. [Google Scholar] [CrossRef]
- Mauler, L.; Duffner, F.; Zeier, W.G.; Leker, J. Battery cost forecasting: A review of methods and results with an outlook to 2050. Energy Environ. Sci. 2021, 14, 4712–4739. [Google Scholar] [CrossRef]
- Nykvist, B.; Nilsson, M. Rapidly falling costs of battery packs for electric vehicles. Nat. Clim. Chang. 2015, 5, 329–332. [Google Scholar] [CrossRef]
- Patry, G.; Romagny, A.; Martinet, S.; Froelich, D. Cost modeling of lithium-ion battery cells for automotive applications. Energy Sci. Eng. 2015, 3, 71–82. [Google Scholar] [CrossRef]
- Quinn, J.B.; Waldmann, T.; Richter, K.; Kasper, M.; Wohlfahrt-Mehrens, M. Energy Density of Cylindrical Li-Ion Cells: A Comparison of Commercial 18650 to the 21700 Cells. J. Electrochem. Soc. 2018, 165, A3284–A3291. [Google Scholar] [CrossRef]
- Muenzel, V.; Hollenkamp, A.; Bhatt, A.I.; De Hoog, J.; Brazil, M.; Thomas, D.; Mareels, I. A Comparative Testing Study of Commercial 18650-Format Lithium-Ion Battery Cells. J. Electrochem. Soc. 2015, 162, A1592–A1600. [Google Scholar] [CrossRef]
- Zeng, X.; Li, M.; Abd El-Hady, D.; Alshitari, W.; Al-Bogami, A.S.; Lu, J.; Amine, K. Commercialization of Lithium Battery Technologies for Electric Vehicles. Adv. Energy Mater. 2019, 9, 1900161. [Google Scholar] [CrossRef]
- Safari, M. Battery electric vehicles: Looking behind to move forward. Energy Policy 2018, 115, 54–65. [Google Scholar] [CrossRef]
- Barkholtz, H.M.; Fresquez, A.; Chalamala, B.; Ferreira, S.R. A Database for Comparative Electrochemical Performance of Commercial 18650-Format Lithium-Ion Cells. J. Electrochem. Soc. 2017, 164, A2697–A2706. [Google Scholar] [CrossRef]
- A2Mac1. Benchmarking Data and Tear-Down Reports; A2Mac1: Belleville, MI, USA, 2023; Available online: https://www.a2mac1.com/ (accessed on 3 February 2023).
- B3 Corporation. LIB Materials Market Bulletin E15Q3—Market Reports; B3 Corporation: Tokyo, Japan, 2015. [Google Scholar]
- Nykvist, B.; Sprei, F.; Nilsson, M. Assessing the progress toward lower priced long range battery electric vehicles. Energy Policy 2019, 124, 144–155. [Google Scholar] [CrossRef]
- B3 Corporation. LIB-Equipped Vehicle Market Bulletin E16Q4 and Long-Term LIB Market Forecast; B3 Corporation: Tokyo, Japan, 2016. [Google Scholar]
- B3 Corporation. LIB-Equipped Vehicle Market Bulletin E17Q4 and Long-Term LIB Market Forecast; B3 Corporation: Tokyo, Japan, 2017. [Google Scholar]
- B3 Corporation. LIB-Equipped Vehicle Market Bulletin E18Q4 and Long-Term LIB Market Forecast; B3 Corporation: Tokyo, Japan, 2018. [Google Scholar]
- B3 Corporation. LIB-Equipped Vehicle Market Bulletin E19Q4 and Long-Term LIB Market Forecast; B3 Corporation: Tokyo, Japan, 2019. [Google Scholar]
- B3 Corporation. LIB-Equipped Vehicle Market Bulletin E20Q4 and Long-Term LIB Market Forecast; B3 Corporation: Tokyo, Japan, 2020. [Google Scholar]
- B3 Corporation. LIB-Equipped Vehicle Market Bulletin E21Q4 and Long-Term LIB Market Forecast; B3 Corporation: Tokyo, Japan, 2021. [Google Scholar]
- B3 Corporation. Market Bulletin on LIB-Equipped Vehicle Markets (Q23Q1); B3 Corporation: Tokyo, Japan, 2023. [Google Scholar]
- Berman, K.; Carlson, R.; Dziuba, J.; Jackson, J.; Sklar, P.; Hamilton, C. The Lithium Ion Battery and the eV Market: The Science behind What You Can’t See; BMO: Hong Kong, China, 2018. [Google Scholar]
- Goldman, A.R.; Rotondo, F.S.; Swallow, J.G. Lithium Ion Battery Industrial Base in the U.S. and Abroad; IDA: Alexandria, VA, USA, 2019; IDA Document D-11032. [Google Scholar]
- Curry, C. Lithium-ion Battery Cost and Market; BNEF: New York, NY, USA, 2018; Available online: https://data.bloomberglp.com/bnef/sites/14/2017/07/BNEF-Lithium-ion-battery-costs-and-market.pdf (accessed on 2 February 2023).
- Hummel, P.; Bush, T.; Gong, P.; Yasui, K.; Lee, T.; Radlinger, J.; Langan, C.; Lesne, D.; Takahashi, K.; Jung, E.; et al. UBS Q-Series Tearing Down the Heart of an Electric Car: Can Batteries Provide an Edge, and Who Wins? Report; UBS: New York, NY, USA, 2018. [Google Scholar]
- José Pontes. EV Sales Blog. Available online: www.ev-sales.blogspot.com (accessed on 3 February 2023).
- Adam, A.; Knobbe, E.; Wandt, J.; Kwade, A. Application of the differential charging voltage analysis to determine the onset of lithium-plating during fast charging of lithium-ion cells. J. Power Sources 2021, 495, 229794. [Google Scholar] [CrossRef]
- Ahmed, S.; Trask, S.E.; Dees, D.W.; Nelson, P.A.; Lu, W.; Dunlop, A.R.; Polzin, B.J.; Jansen, A. Cost of automotive lithium-ion batteries operating at high upper cutoff voltages. J. Power Sources 2018, 403, 56–65. [Google Scholar] [CrossRef]
- B3 Corporation. LIB Materials Market Bulletin E16Q1; B3 Corporation: Tokyo, Japan, 2016. [Google Scholar]
- B3 Corporation. LIB Materials Market Bulletin E18Q1; B3 Corporation: Tokyo, Japan, 2018. [Google Scholar]
- B3 Corporation. LIB Materials Market Bulletin E19Q1; B3 Corporation: Tokyo, Japan, 2019. [Google Scholar]
- B3 Corporation. LIB Materials Market Bulletin E20Q1; B3 Corporation: Tokyo, Japan, 2020. [Google Scholar]
- B3 Corporation. LIB Materials Market Bulletin E22Q1; B3 Corporation: Tokyo, Japan, 2022. [Google Scholar]
- Daubinger, P.; Schelter, M.; Petersohn, R.; Nagler, F.; Hartmann, S.; Herrmann, M.; Giffin, G.A. Impact of Bracing on Large Format Prismatic Lithium-Ion Battery Cells during Aging. Adv. Energy Mater. 2022, 12, 2102448. [Google Scholar] [CrossRef]
- Deich, T.; Storch, M.; Steiner, K.; Bund, A. Effects of module stiffness and initial compression on lithium-ion cell aging. J. Power Sources 2021, 506, 230163. [Google Scholar] [CrossRef]
- Dreizler, A.M.; Bohn, N.; Geßwein, H.; Müller, M.; Binder, J.R.; Wagner, N.; Friedrich, K.A. Investigation of the Influence of Nanostructured LiNi0.33Co0.33Mn0.33O2Lithium-Ion Battery Electrodes on Performance and Aging. J. Electrochem. Soc. 2018, 165, A273–A282. [Google Scholar] [CrossRef]
- Du, Z.; Wood, D.L., III; Daniel, C.; Kalnaus, S.; Li, J. Understanding limiting factors in thick electrode performance as applied to high energy density Li-ion batteries. J. Appl. Electrochem. 2017, 47, 405–415. [Google Scholar] [CrossRef]
- DuBeshter, T.; Sinha, P.K.; Sakars, A.; Fly, G.W.; Jorne, J. Measurement of Tortuosity and Porosity of Porous Battery Electrodes. J. Electrochem. Soc. 2014, 161, A599–A605. [Google Scholar] [CrossRef]
- Duffner, F.; Kronemeyer, N.; Tübke, J.; Leker, J.; Winter, M.; Schmuch, R. Post-lithium-ion battery cell production and its compatibility with lithium-ion cell production infrastructure. Nat. Energy 2021, 6, 123–134. [Google Scholar] [CrossRef]
- Gallagher, K.G.; Trask, S.; Bauer, C.; Woehrle, T.; Lux, S.F.; Tschech, M.; Lamp, P.; Polzin, B.J.; Ha, S.; Long, B.; et al. Optimizing Areal Capacities through Understanding the Limitations of Lithium-Ion Electrodes. J. Electrochem. Soc. 2016, 163, A138–A149. [Google Scholar] [CrossRef]
- Greenwood, M.; Wentker, M.; Leker, J. A bottom-up performance and cost assessment of lithium-ion battery pouch cells utilizing nickel-rich cathode active materials and silicon-graphite composite anodes. J. Power Sources Adv. 2021, 9, 100055. [Google Scholar] [CrossRef]
- Günter, F.J.; Wassiliadis, N. State of the Art of Lithium-Ion Pouch Cells in Automotive Applications: Cell Teardown and Characterization. J. Electrochem. Soc. 2022, 169, 030515. [Google Scholar] [CrossRef]
- Heenan, T.M.M.; Jnawali, A.; Kok, M.D.R.; Tranter, T.G.; Tan, C.; Dimitrijevic, A.; Jervis, R.; Brett, D.J.L.; Shearing, P.R. An Advanced Microstructural and Electrochemical Datasheet on 18650 Li-Ion Batteries with Nickel-Rich NMC811 Cathodes and Graphite-Silicon Anodes. J. Electrochem. Soc. 2020, 167, 140530. [Google Scholar] [CrossRef]
- Heubner, C.; Nickol, A.; Seeba, J.; Reuber, S.; Junker, N.; Wolter, M.; Schneider, M.; Michaelis, A. Understanding thickness and porosity effects on the electrochemical performance of LiNi0.6Co0.2Mn0.2O2-based cathodes for high energy Li-ion batteries. J. Power Sources 2019, 419, 119–126. [Google Scholar] [CrossRef]
- Hewawasam, D.; Subasinghe, L.; Karunathilake, H.; Witharana, S. Bottom-up cost modeling of lithium-ion battery cells for electric vehicle applications. In Proceedings of the 2022 Moratuwa Engineering Research Conference (MERCon), Moratuwa, Sri Lanka, 27–29 July 2022; pp. 1–6. [Google Scholar]
- Hille, L.; Noecker, M.P.; Ko, B.; Kriegler, J.; Keilhofer, J.; Stock, S.; Zaeh, M.F. Integration of laser structuring into the electrode manufacturing process chain for lithium-ion batteries. J. Power Sources 2023, 556, 232478. [Google Scholar] [CrossRef]
- Huang, C.; Grant, P.S. Coral-like directional porosity lithium ion battery cathodes by ice templating. J. Mater. Chem. A 2018, 6, 14689–14699. [Google Scholar] [CrossRef]
- Huang, Q.-A.; Shen, Y.; Huang, Y.; Zhang, L.; Zhang, J. Impedance Characteristics and Diagnoses of Automotive Lithium-Ion Batteries at 7.5% to 93.0% State of Charge. Electrochim. Acta 2016, 219, 751–765. [Google Scholar] [CrossRef]
- Huang, W.; Ye, Y.; Chen, H.; Vilá, R.A.; Xiang, A.; Wang, H.; Liu, F.; Yu, Z.; Xu, J.; Zhang, Z.; et al. Onboard early detection and mitigation of lithium plating in fast-charging batteries. Nat. Commun. 2022, 13, 7091. [Google Scholar] [CrossRef]
- Kaiser, J.; Wenzel, V.; Nirschl, H.; Bitsch, B.; Willenbacher, N.; Baunach, M.; Schmitt, M.; Jaiser, S.; Scharfer, P.; Schabel, W. Prozess-und Produktentwicklung von Elektroden für Li-Ionen-Zellen. Chem. Ing. Tech. 2014, 86, 695–706. [Google Scholar] [CrossRef]
- Kang, J.; Atwair, M.; Nam, I.; Lee, C.-J. Experimental and numerical investigation on effects of thickness of NCM622 cathode in Li-ion batteries for high energy and power density. Energy 2023, 263, 125801. [Google Scholar] [CrossRef]
- Keppeler, M.; Roessler, S.; Braunwarth, W. Production Research as Key Factor for Successful Establishment of Battery Production on the Example of Large-Scale Automotive Cells Containing Nickel-Rich LiNi0.8Mn0.1Co0.1O2 Electrodes. Energy Technol. 2020, 8, 2000183. [Google Scholar] [CrossRef]
- Keyser, M.; Pesaran, A.; Li, Q.; Santhanagopalan, S.; Smith, K.; Wood, E.; Ahmed, S.; Bloom, I.; Dufek, E.; Shirk, M.; et al. Enabling fast charging—Battery thermal considerations. J. Power Sources 2017, 367, 228–236. [Google Scholar] [CrossRef]
- Kim, H.-J.; Krishna, T.; Zeb, K.; Rajangam, V.; Gopi, C.V.V.M.; Sambasivam, S.; Raghavendra, K.V.G.; Obaidat, I.M. A Comprehensive Review of Li-Ion Battery Materials and Their Recycling Techniques. Electronics 2020, 9, 1161. [Google Scholar] [CrossRef]
- Kisu, K.; Aoyagi, S.; Nagatomo, H.; Iwama, E.; Reid, M.T.H.; Naoi, W.; Naoi, K. Internal resistance mapping preparation to optimize electrode thickness and density using symmetric cell for high-performance lithium-ion batteries and capacitors. J. Power Sources 2018, 396, 207–212. [Google Scholar] [CrossRef]
- Kovachev, G.; Schröttner, H.; Gstrein, G.; Aiello, L.; Hanzu, I.; Wilkening, H.M.R.; Foitzik, A.; Wellm, M.; Sinz, W.; Ellersdorfer, C. Analytical Dissection of an Automotive Li-Ion Pouch Cell. Batteries 2019, 5, 67. [Google Scholar] [CrossRef]
- Krsmanovic and Dejana. Development of a Property Forecast Tool for Flexible Compositions of Li-Ion Batteries, Including Raw Material Availability and Price Forming; Uppsala University: Uppsala, Sweden, 2019. [Google Scholar]
- Lee, D.-C.; Lee, K.-J.; Kim, C.-W. Optimization of a Lithium-Ion Battery for Maximization of Energy Density with Design of Experiments and Micro-genetic Algorithm. Int. J. Precis. Eng. Manuf. Technol. 2020, 7, 829–836. [Google Scholar] [CrossRef]
- Lei, C.; Aldous, I.; Hartley, J.M.; Thompson, D.L.; Scott, S.; Hanson, R.; Anderson, P.A.; Kendrick, E.; Sommerville, R.; Ryder, K.S.; et al. Lithium ion battery recycling using high-intensity ultrasonication. Green Chem. 2021, 23, 4710–4715. [Google Scholar] [CrossRef]
- Li, D.; Lv, Q.; Zhang, C.; Zhou, W.; Guo, H.; Jiang, S.; Li, Z. The Effect of Electrode Thickness on the High-Current Discharge and Long-Term Cycle Performance of a Lithium-Ion Battery. Batteries 2022, 8, 101. [Google Scholar] [CrossRef]
- Liu, B.; Duan, X.; Yuan, C.; Wang, L.; Li, J.; Finegan, D.P.; Feng, B.; Xu, J. Quantifying and modeling of stress-driven short-circuits in lithium-ion batteries in electrified vehicles. J. Mater. Chem. A 2021, 9, 7102–7113. [Google Scholar] [CrossRef]
- Lu, X.; Daemi, S.R.; Bertei, A.; Kok, M.D.; O’regan, K.B.; Rasha, L.; Park, J.; Hinds, G.; Kendrick, E.; Brett, D.J.; et al. Microstructural Evolution of Battery Electrodes During Calendering. Joule 2020, 4, 2746–2768. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, X.; Tan, C.; Heenan, T.M.M.; Lagnoni, M.; O’Regan, K.; Daemi, S.; Bertei, A.; Jones, H.G.; Hinds, G.; et al. Multi-length scale microstructural design of lithium-ion battery electrodes for improved discharge rate performance. Energy Environ. Sci. 2021, 14, 5929–5946. [Google Scholar] [CrossRef]
- Lybbert, M.; Ghaemi, Z.; Balaji, A.; Warren, R. Integrating life cycle assessment and electrochemical modeling to study the effects of cell design and operating conditions on the environmental impacts of lithium-ion batteries. Renew. Sustain. Energy Rev. 2021, 144, 111004. [Google Scholar] [CrossRef]
- Ma, R.; Deng, Y. The electrochemical model coupled parameterized life cycle assessment for the optimized design of EV battery pack. Int. J. Life Cycle Assess. 2022, 27, 267–280. [Google Scholar] [CrossRef]
- Mai, W.; Usseglio-Viretta, F.; Colclasure, A.M.; Smith, K. Enabling fast charging of lithium-ion batteries through secondary-/dual-pore network: Part II—Numerical model. Electrochim. Acta 2020, 341, 136013. [Google Scholar] [CrossRef]
- Mei, W.; Chen, H.; Sun, J.; Wang, Q. The effect of electrode design parameters on battery performance and optimization of electrode thickness based on the electrochemical–thermal coupling model. Sustain. Energy Fuels 2019, 3, 148–165. [Google Scholar] [CrossRef]
- Meyer, C.; Bockholt, H.; Haselrieder, W.; Kwade, A. Characterization of the calendering process for compaction of electrodes for lithium-ion batteries. J. Mater. Process. Technol. 2017, 249, 172–178. [Google Scholar] [CrossRef]
- Myung, S.-T.; Maglia, F.; Park, K.-J.; Yoon, C.S.; Lamp, P.; Kim, S.-J.; Sun, Y.-K. Nickel-Rich Layered Cathode Materials for Automotive Lithium-Ion Batteries: Achievements and Perspectives. ACS Energy Lett. 2017, 2, 196–223. [Google Scholar] [CrossRef]
- Parikh, D.; Christensen, T.; Li, J. Correlating the influence of porosity, tortuosity, and mass loading on the energy density of LiNi0.6Mn0.2Co0.2O2 cathodes under extreme fast charging (XFC) conditions. J. Power Sources 2020, 474, 228601. [Google Scholar] [CrossRef]
- Rahe, C.; Kelly, S.T.; Rad, M.N.; Sauer, D.U.; Mayer, J.; Figgemeier, E. Nanoscale X-ray imaging of ageing in automotive lithium ion battery cells. J. Power Sources 2019, 433, 126631. [Google Scholar] [CrossRef]
- Rieger, B.; Schlueter, S.; Erhard, S.; Schmalz, J.; Reinhart, G.; Jossen, A. Multi-scale investigation of thickness changes in a commercial pouch type lithium-ion battery. J. Energy Storage 2016, 6, 213–221. [Google Scholar] [CrossRef]
- Sakti, A.; Michalek, J.J.; Fuchs, E.R.; Whitacre, J.F. A techno-economic analysis and optimization of Li-ion batteries for light-duty passenger vehicle electrification. J. Power Sources 2015, 273, 966–980. [Google Scholar] [CrossRef]
- Schneider, S.F.; Bauer, C.; Novák, P.; Berg, E.J. A modeling framework to assess specific energy, costs and environmental impacts of Li-ion and Na-ion batteries. Sustain. Energy Fuels 2019, 3, 3061–3070. [Google Scholar] [CrossRef]
- Yu, S.; Kim, S.; Kim, T.Y.; Nam, J.H.; Cho, W.I. Model Prediction and Experiments for the Electrode Design Optimization of LiFePO4/Graphite Electrodes in High Capacity Lithium-ion Batteries. Bull. Korean Chem. Soc. 2013, 34, 79–88. [Google Scholar] [CrossRef]
- Sheng, Y.; Fell, C.R.; Son, Y.K.; Metz, B.M.; Jiang, J.; Church, B.C. Effect of Calendering on Electrode Wettability in Lithium-Ion Batteries. Front. Energy Res. 2014, 2, 56. [Google Scholar] [CrossRef]
- AL-Shroofy, M.N. Understanding and Improving Manufacturing Processes for Making Lithium-Ion Battery Electrodes; University of Kentucky Libraries: Lexington, KY, USA, 2017. [Google Scholar]
- Son, B.; Ryou, M.-H.; Choi, J.; Kim, S.-H.; Ko, J.M.; Lee, Y.M. Effect of cathode/anode area ratio on electrochemical performance of lithium-ion batteries. J. Power Sources 2013, 243, 641–647. [Google Scholar] [CrossRef]
- Storch, M.; Fath, J.P.; Sieg, J.; Vrankovic, D.; Krupp, C.; Spier, B.; Riedel, R. Temperature and Lithium Concentration Gradient Caused Inhomogeneous Plating in Large-format Lithium-ion Cells. J. Energy Storage 2021, 41, 102887. [Google Scholar] [CrossRef]
- Sturm, J.; Rheinfeld, A.; Zilberman, I.; Spingler, F.; Kosch, S.; Frie, F.; Jossen, A. Modeling and simulation of inhomogeneities in a 18650 nickel-rich, silicon-graphite lithium-ion cell during fast charging. J. Power Sources 2019, 412, 204–223. [Google Scholar] [CrossRef]
- Ue, M.; Sakaushi, K.; Uosaki, K. Basic knowledge in battery research bridging the gap between academia and industry. Mater. Horizons 2020, 7, 1937–1954. [Google Scholar] [CrossRef]
- Ulvestad, A. A Brief Review of Current Lithium Ion Battery Technology and Potential Solid State Battery Technologies. arXiv 2018, arXiv:1803.04317. [Google Scholar]
- Vishnugopi, B.S.; Verma, A.; Mukherjee, P.P. Fast Charging of Lithium-ion Batteries via Electrode Engineering. J. Electrochem. Soc. 2020, 167, 090508. [Google Scholar] [CrossRef]
- Waldmann, T.; Scurtu, R.-G.; Richter, K.; Wohlfahrt-Mehrens, M. 18650 vs. 21700 Li-ion cells—A direct comparison of electrochemical, thermal, and geometrical properties. J. Power Sources 2020, 472, 228614. [Google Scholar] [CrossRef]
- Wang, F.; Tang, M. A Quantitative Analytical Model for Predicting and Optimizing the Rate Performance of Battery Cells. Cell Rep. Phys. Sci. 2020, 1, 100192. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Li, W.; Li, C.; Ouyang, M. Particles Released by Abused Prismatic Ni-rich Automotive Lithium-ion Batteries. WSEAS Trans. Syst. Control. 2020, 15, 30–38. [Google Scholar] [CrossRef]
- Wentker, M.; Greenwood, M.; Leker, J. A Bottom-Up Approach to Lithium-Ion Battery Cost Modeling with a Focus on Cathode Active Materials. Energies 2019, 12, 504. [Google Scholar] [CrossRef]
- Wood, D.L., III; Li, J.; Daniel, C. Prospects for reducing the processing cost of lithium ion batteries. J. Power Sources 2015, 275, 234–242. [Google Scholar] [CrossRef]
- Xu, M.; Reichman, B.; Wang, X. Modeling the effect of electrode thickness on the performance of lithium-ion batteries with experimental validation. Energy 2019, 186, 115864. [Google Scholar] [CrossRef]
- Yole Market Studies. Lithium Ion Battery Market & Technology Trends 2020: Market and Technology Report 2020; Yole Market Studies: Villeurbanne, France, 2020. [Google Scholar]
- Zhang, X.; He, J.; Zhou, J.; Chen, H.; Song, W.; Fang, D. Thickness evolution of commercial Li-ion pouch cells with silicon-based composite anodes and NCA cathodes. Sci. China Technol. Sci. 2021, 64, 83–90. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Li, W.; Li, C.; Ouyang, M. Size distribution and elemental composition of vent particles from abused prismatic Ni-rich automotive lithium-ion batteries. J. Energy Storage 2019, 26, 100991. [Google Scholar] [CrossRef]
- Zhao, Y.; Diaz, L.B.; Patel, Y.; Zhang, T.; Offer, G.J. How to Cool Lithium Ion Batteries: Optimising Cell Design using a Thermally Coupled Model. J. Electrochem. Soc. 2019, 166, A2849–A2859. [Google Scholar] [CrossRef]
- Zheng, H.; Li, J.; Song, X.; Liu, G.; Battaglia, V.S. A comprehensive understanding of electrode thickness effects on the electrochemical performances of Li-ion battery cathodes. Electrochim. Acta 2012, 71, 258–265. [Google Scholar] [CrossRef]
- Zheng, H.; Tan, L.; Liu, G.; Song, X.; Battaglia, V.S. Calendering effects on the physical and electrochemical properties of Li[Ni1/3Mn1/3Co1/3]O2 cathode. J. Power Sources 2012, 208, 52–57. [Google Scholar] [CrossRef]
- Zhu, P.; Slater, P.R.; Kendrick, E. Insights into architecture, design and manufacture of electrodes for lithium-ion batteries. Mater. Des. 2022, 223, 111208. [Google Scholar] [CrossRef]
- Thiel, C.; Schmidt, J.; Van Zyl, A.; Schmid, E. Cost and well-to-wheel implications of the vehicle fleet CO2 emission regulation in the European Union. Transp. Res. Part A Policy Pract. 2014, 63, 25–42. [Google Scholar] [CrossRef]
- Löbberding, H.; Wessel, S.; Offermanns, C.; Kehrer, M.; Rother, J.; Heimes, H.; Kampker, A. From Cell to Battery System in BEVs: Analysis of System Packing Efficiency and Cell Types. World Electr. Veh. J. 2020, 11, 77. [Google Scholar] [CrossRef]
- IEA. Electric Vehicles Initiative, and Clean Energy Minisatrial. In Global Supply Chains of EV Batteries; IEA: Paris, France, 2021; Available online: https://iea.blob.core.windows.net/assets/4eb8c252-76b1-4710-8f5e-867e751c8dda/GlobalSupplyChainsofEVBatteries.pdf (accessed on 10 December 2022).
- Schröder, R.; Glodde, A.; Aydemir, M.; Bach, G. Process to Increase the Output of Z-Folded Separators for the Manufacturing of Lithium-Ion Batteries. Appl. Mech. Mater. 2015, 794, 19–26. [Google Scholar] [CrossRef]
- Fink, H.; Fetzer, J.; Hore, S. Method for Producing an Electrode Stack for a Battery Cell and Battery Cell. U.S. Patent US10651508B2, 12 May 2020. [Google Scholar]
- Joong, K.C.; Bum, K.S.; Noh, L.S.; Chan, P.H. Method for stacking cells inside secondary battery and cell stack manufactured using same. Patent WO 2014/042424 A 1, 20 March 2014. [Google Scholar]
- Chang, T.-Y.; Chang, C.-C.; Kuo, H.C. High durability lithium-ion cells. U.S. Patent US8163421B2, 24 April 2012. [Google Scholar]
- Ueno, Y.; Mizuno, T.; Yamamoto, K.; Ohira, J.; Inoue, K.; Fujita, H.; Sano, H. Lithium-ion secondary battery and manufacturing method thereof. U.S. Patent US10424816B2, 24 September 2019. [Google Scholar]
- Kim, J.K.; Saito, A.; Kim, Y.K. Jelly-roll type electrode assembly, lithium secondary battery having the same, and method for manufacturing the same. U.S. Patent US7442465B2, 28 October 2008. [Google Scholar]
- Tran, H.Y.; Täubert, C.; Fleischhammer, M.; Axmann, P.; Küppers, L.; Wohlfahrt-Mehrens, M. LiMn2O4 Spinel/LiNi0.8Co0.15Al0.05O2 Blends as Cathode Materials for Lithium-Ion Batteries. J. Electrochem. Soc. 2011, 158, A556–A561. [Google Scholar] [CrossRef]
- Wang, J.; Yu, Y.; Li, B.; Zhang, P.; Huang, J.; Wang, F.; Zhao, S.; Gan, C.; Zhao, J. Thermal Synergy Effect between LiNi0.5Co0.2Mn0.3O2 and LiMn2O4 Enhances the Safety of Blended Cathode for Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 20147–20156. [Google Scholar] [CrossRef]
- Wang, X.; Ding, Y.; Deng, Y.; Chen, Z. Ni-Rich/Co-Poor Layered Cathode for Automotive Li-Ion Batteries: Promises and Challenges. Adv. Energy Mater. 2020, 10, 1903864. [Google Scholar] [CrossRef]
- Andre, D.; Kim, S.-J.; Lamp, P.; Lux, S.F.; Maglia, F.; Paschos, O.; Stiaszny, B. Future generations of cathode materials: An automotive industry perspective. J. Mater. Chem. A 2015, 3, 6709–6732. [Google Scholar] [CrossRef]
- Zuo, X.; Zhu, J.; Müller-Buschbaum, P.; Cheng, Y.-J. Silicon based lithium-ion battery anodes: A chronicle perspective review. Nano Energy 2017, 31, 113–143. [Google Scholar] [CrossRef]
- Su, X.; Wu, Q.; Li, J.; Xiao, X.; Lott, A.; Lu, W.; Sheldon, B.W.; Wu, J. Silicon-Based Nanomaterials for Lithium-Ion Batteries: A Review. Adv. Energy Mater. 2014, 4, 1300882. [Google Scholar] [CrossRef]
- Herberz, M.; Hahnel, U.J.J.; Brosch, T. Counteracting electric vehicle range concern with a scalable behavioural intervention. Nat. Energy 2022, 7, 503–510. [Google Scholar] [CrossRef]
- Wu, F.; Maier, J.; Yu, Y. Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chem. Soc. Rev. 2020, 49, 1569–1614. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Link, S.; Neef, C.; Wicke, T. Trends in Automotive Battery Cell Design: A Statistical Analysis of Empirical Data. Batteries 2023, 9, 261. https://doi.org/10.3390/batteries9050261
Link S, Neef C, Wicke T. Trends in Automotive Battery Cell Design: A Statistical Analysis of Empirical Data. Batteries. 2023; 9(5):261. https://doi.org/10.3390/batteries9050261
Chicago/Turabian StyleLink, Steffen, Christoph Neef, and Tim Wicke. 2023. "Trends in Automotive Battery Cell Design: A Statistical Analysis of Empirical Data" Batteries 9, no. 5: 261. https://doi.org/10.3390/batteries9050261
APA StyleLink, S., Neef, C., & Wicke, T. (2023). Trends in Automotive Battery Cell Design: A Statistical Analysis of Empirical Data. Batteries, 9(5), 261. https://doi.org/10.3390/batteries9050261






