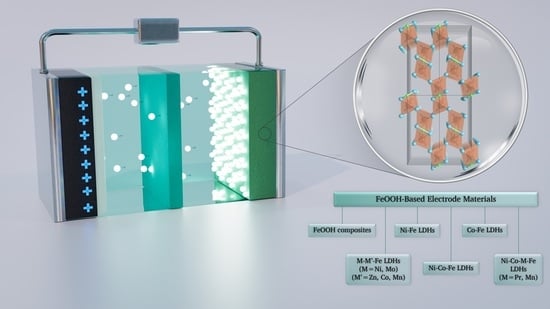
Recent Advances and Prospects of FeOOH-Based Electrode Materials for Supercapacitors
Abstract
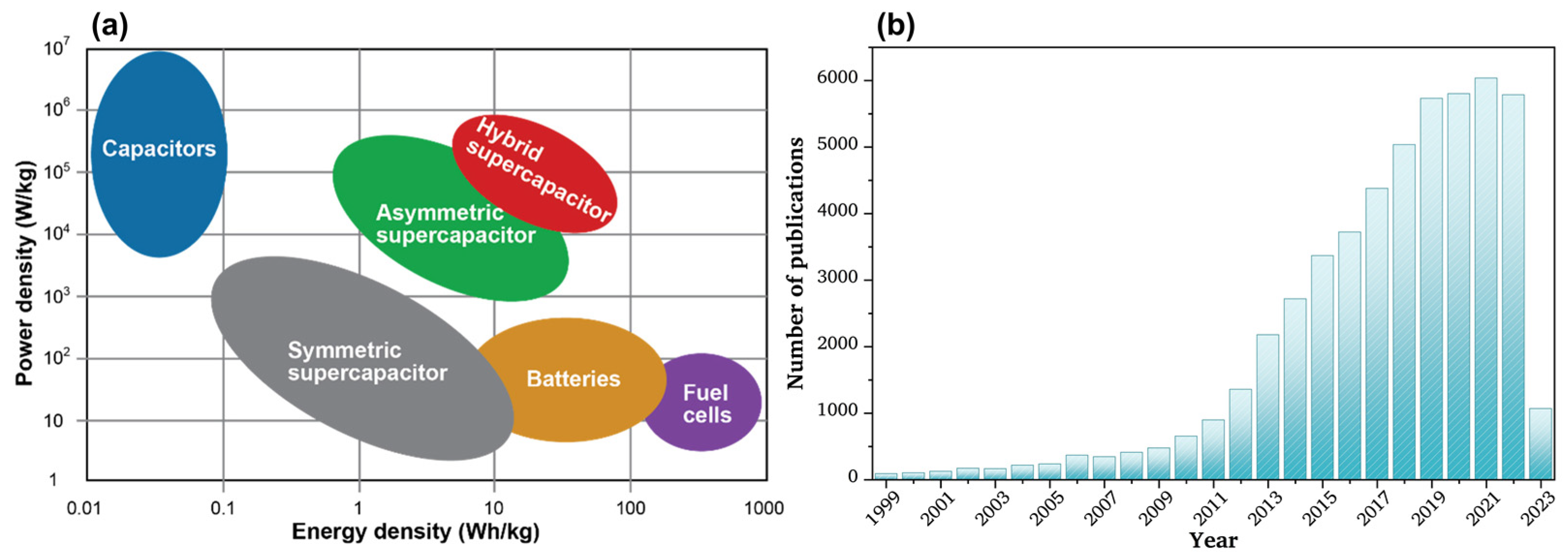
1. Introduction
1.1. Types of Supercapacitors
- Electric double-layer capacitor (EDLC) or so-called non-faradaic EDLC;
- Pseudo-capacitor (PC) or faradaic supercapacitor;
- Hybrid capacitor of hybrid supercapacitor (HSC).
1.2. Iron-Based Materials for Supercapacitors
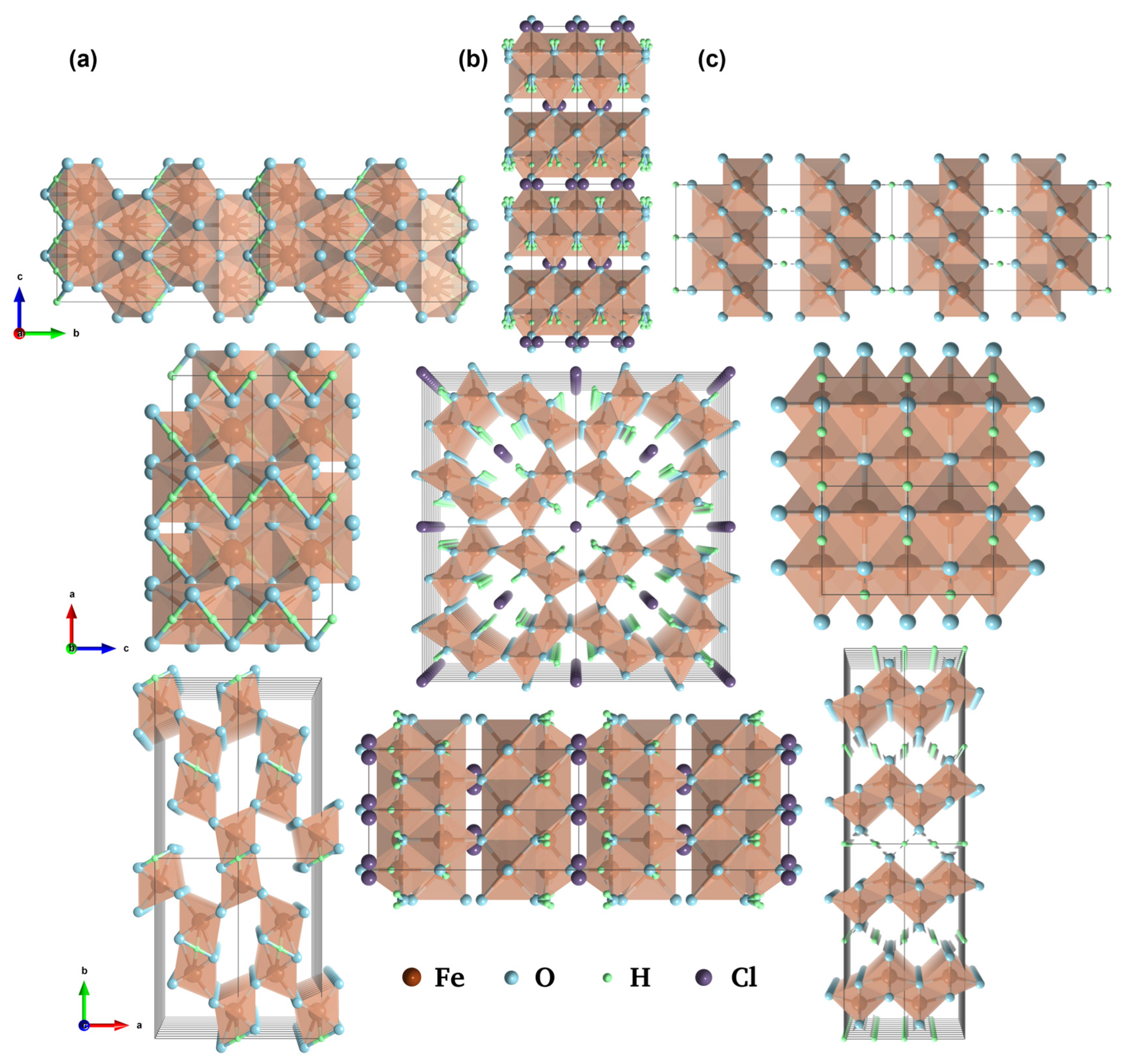
2. FeOOH
2.1. Low-Crystalline and Crystalline FeOOH
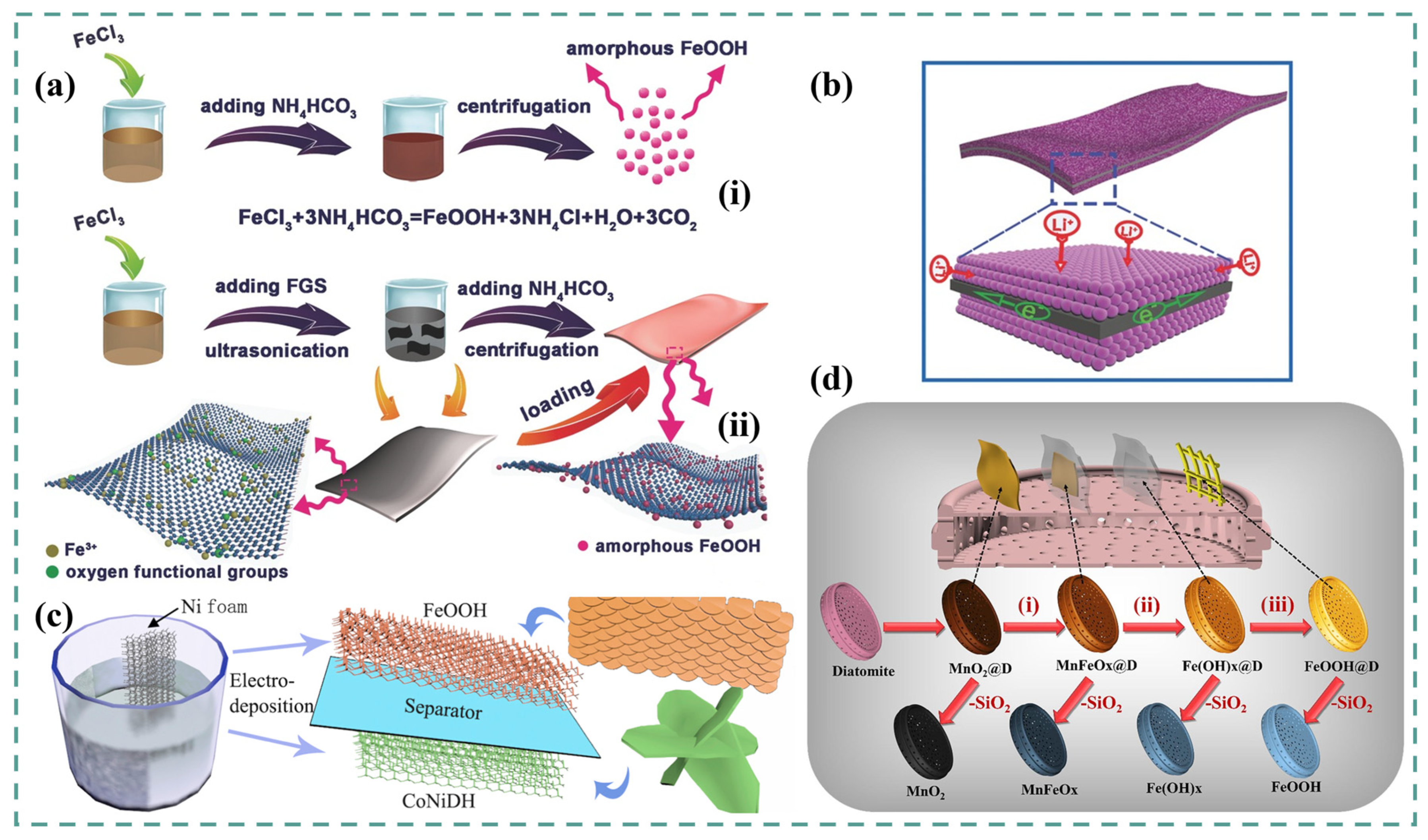
2.2. FeOOH Replica from MnO2
2.3. FeOOH Composites
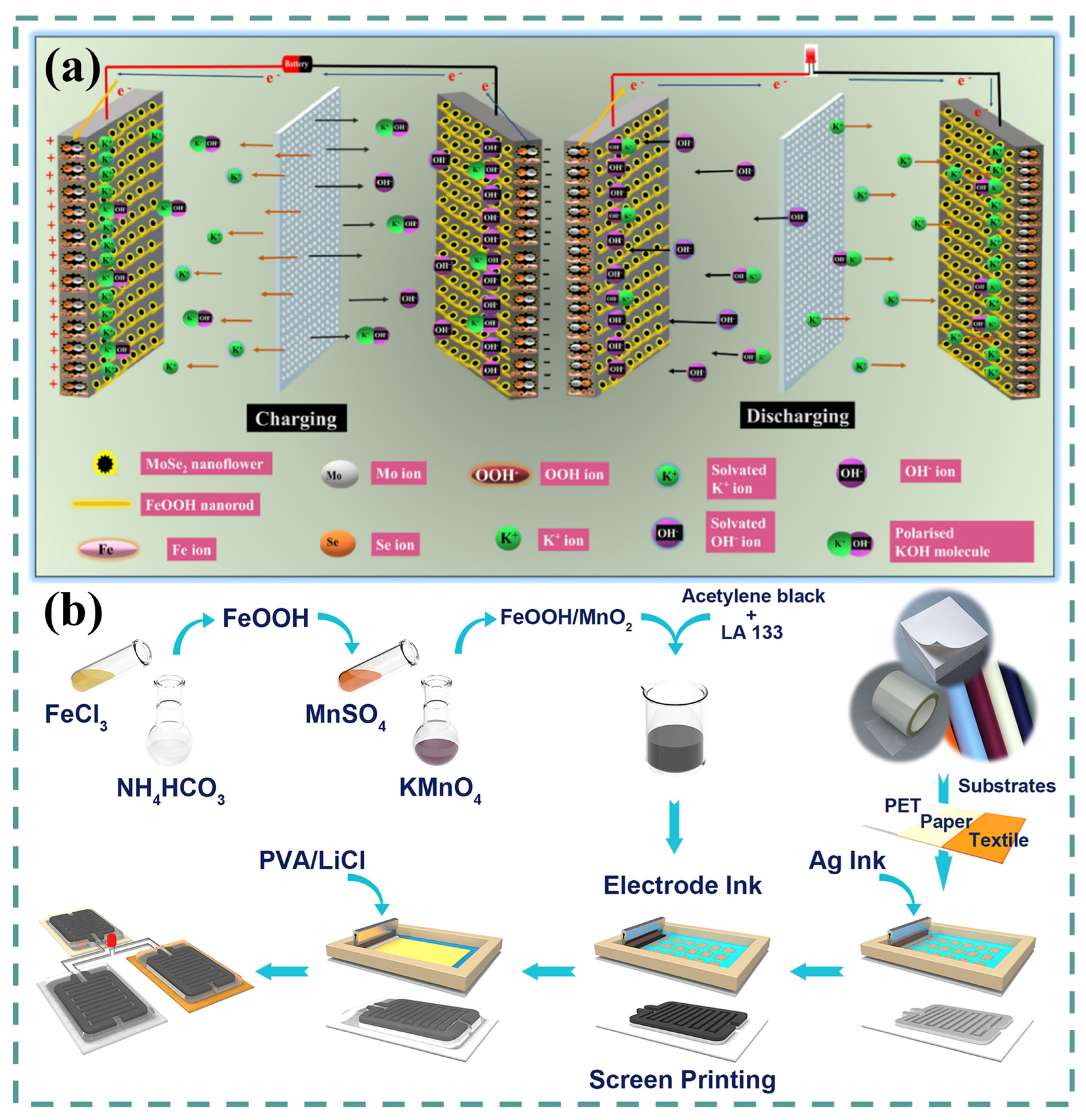
2.4. FeOOH for Solid-State Supercapacitors (SSSs)
3. Ni-Fe LDH
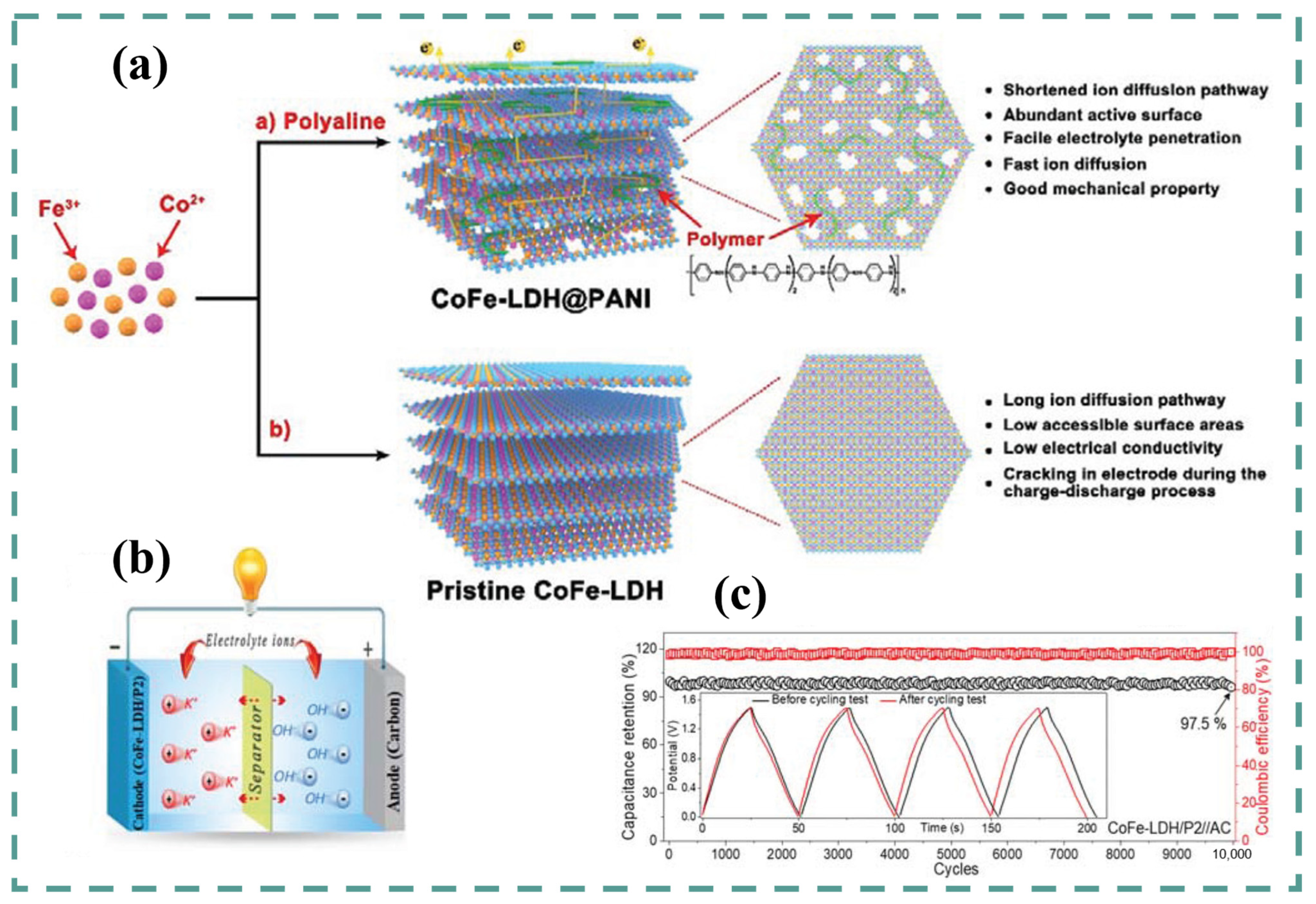
4. Co-Fe LDH
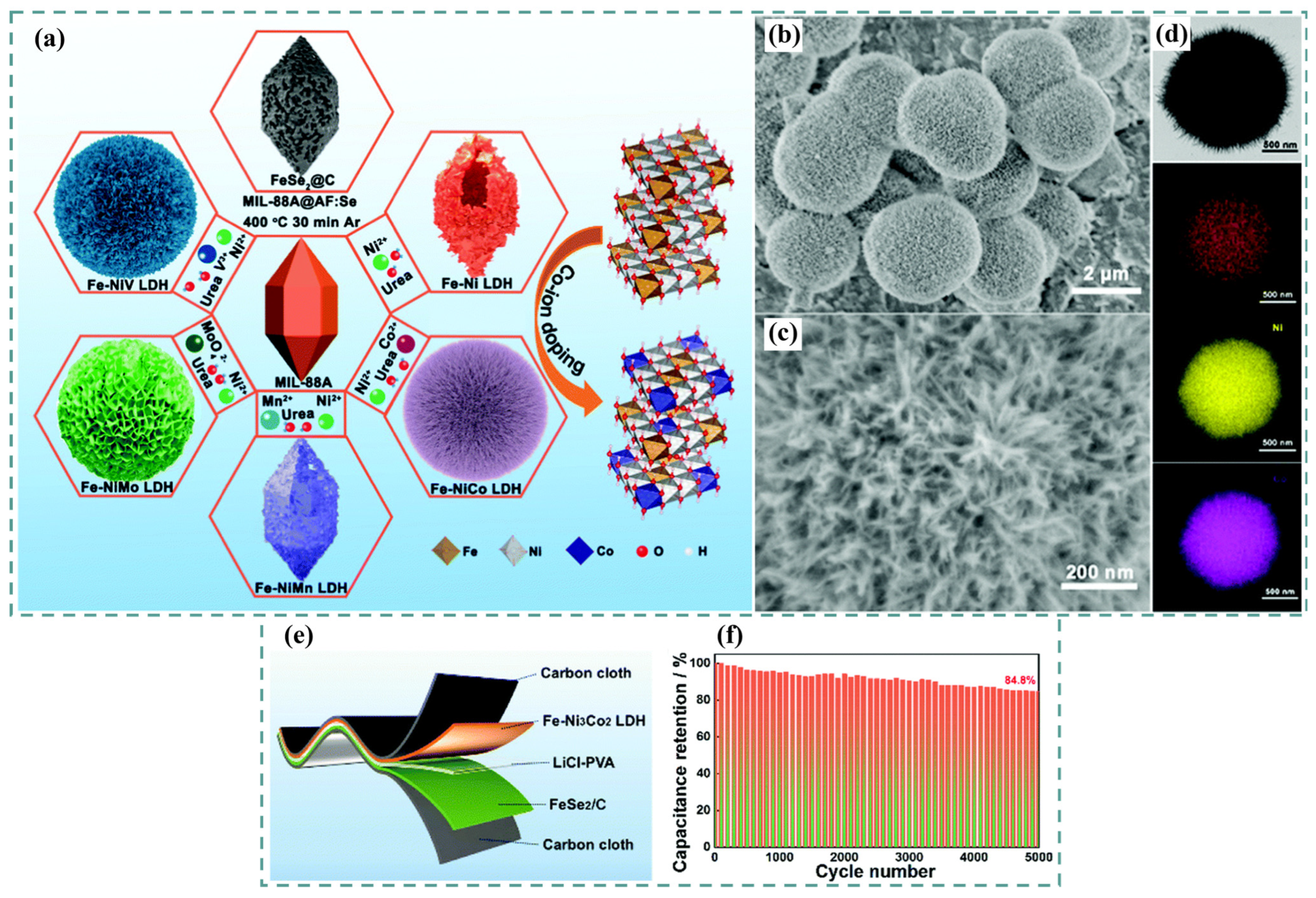
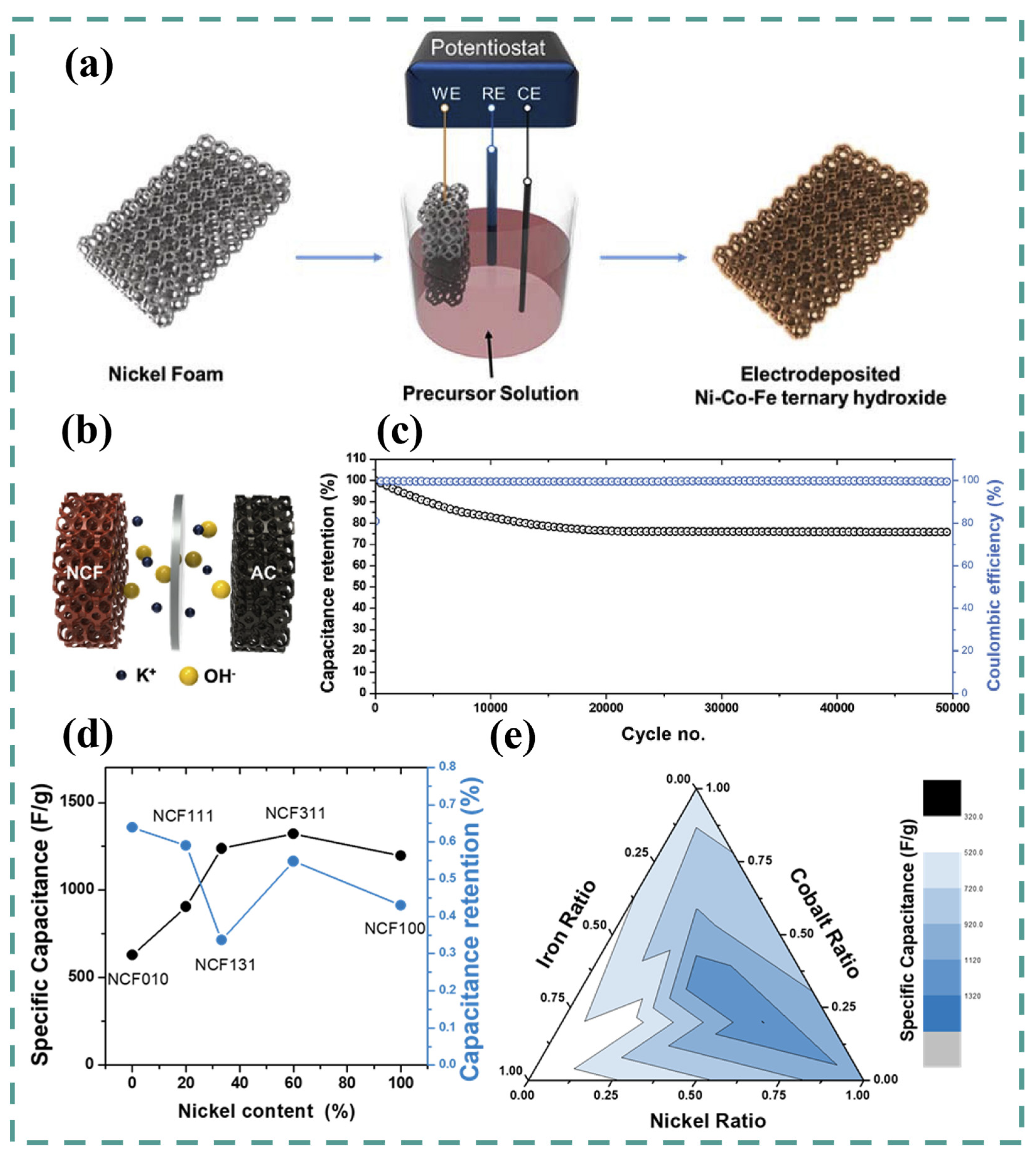
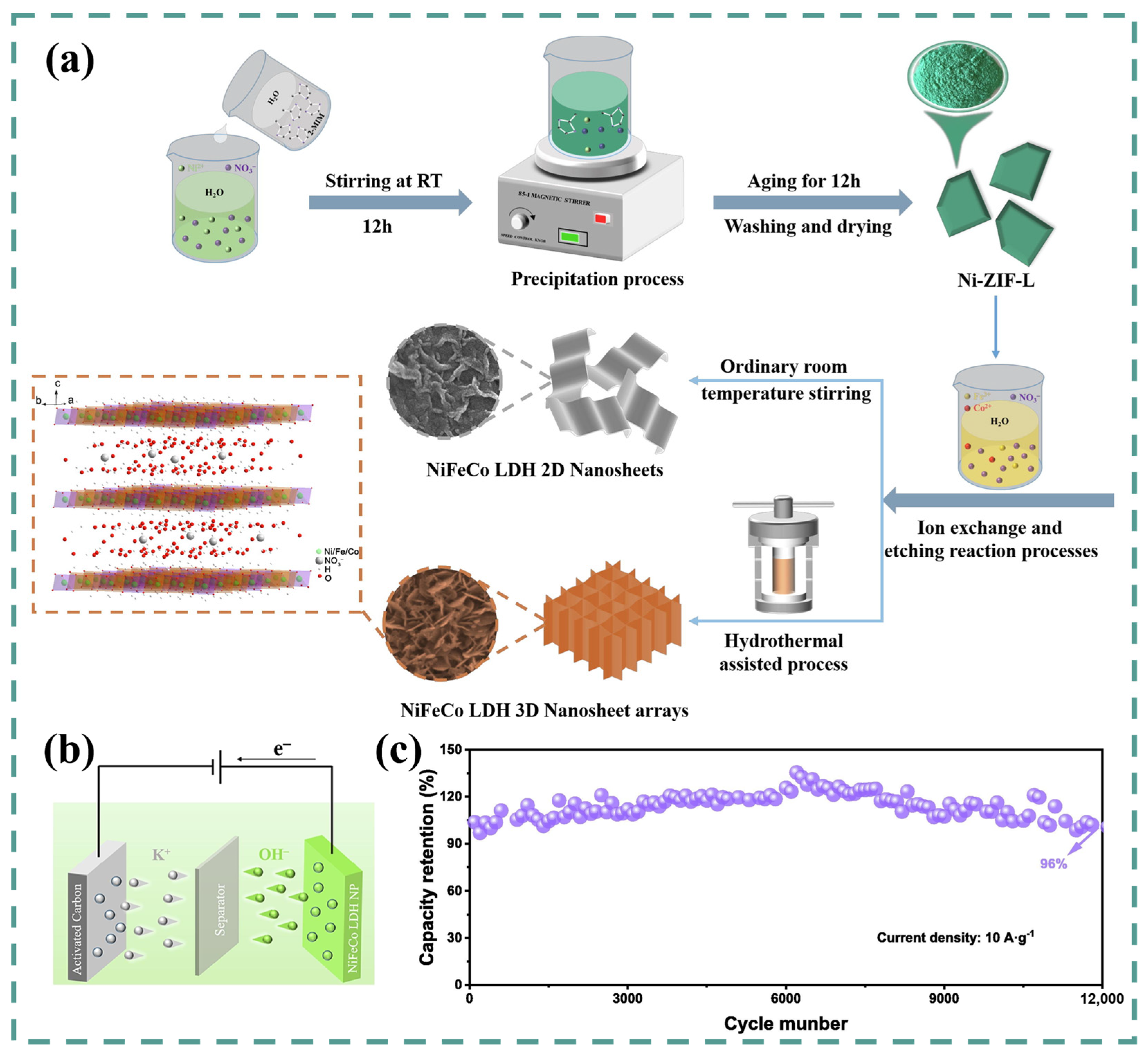
5. Ni-Co-Fe LDH
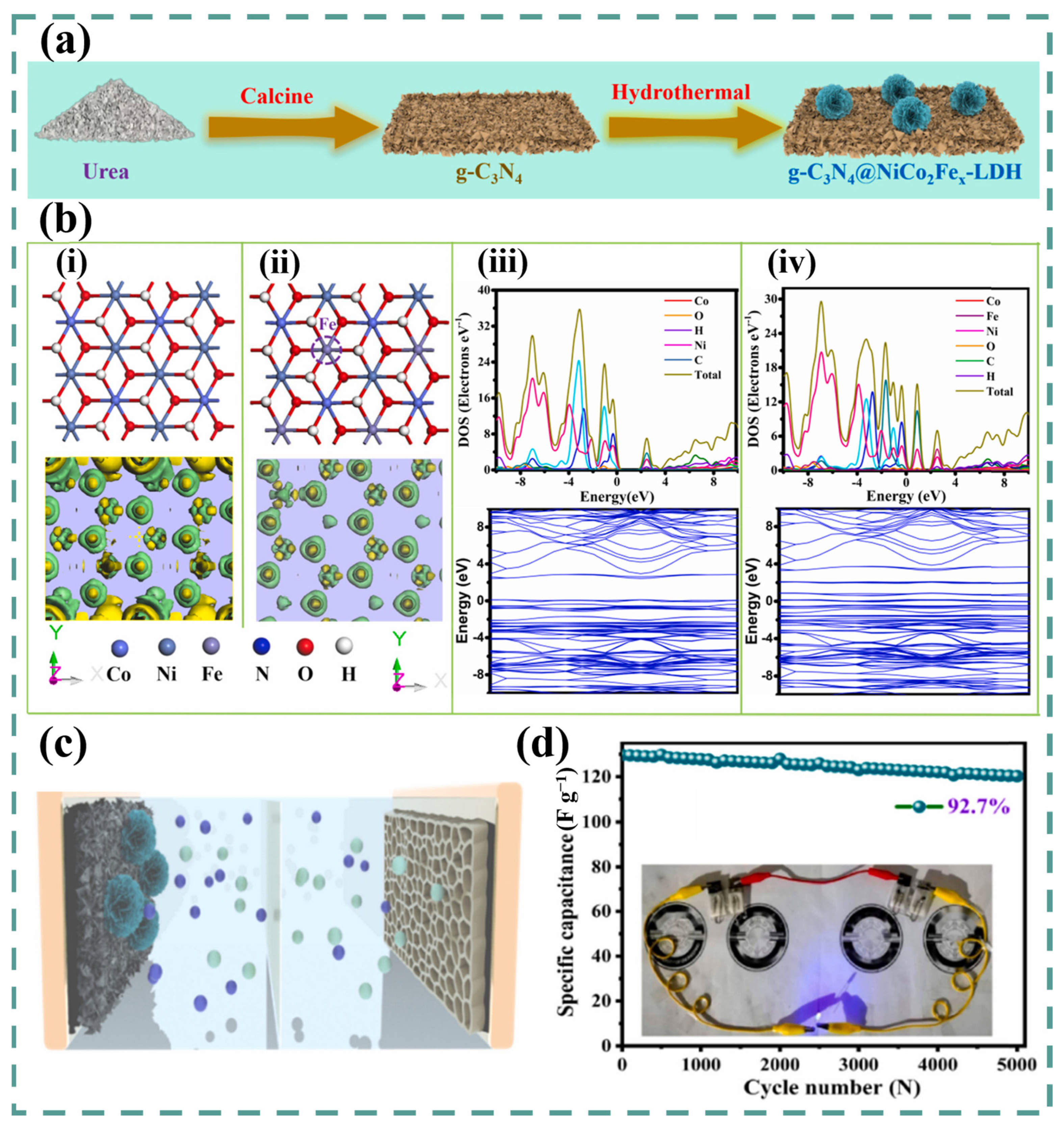
6. Ternary and Quaternary LDH
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lalwani, S.; Karade, S.; Eum, J.-H.; Kim, H. Maximizing Redox Charge Storage via Cation (V)–Anion (S) Dual Doping on Nickel Diselenide Nanodiscs for Hybrid Supercapacitors. ACS Appl. Energy Mater. 2021, 4, 2430–2439. [Google Scholar] [CrossRef]
- Global Energy Crisis—Topics. Available online: https://www.iea.org/topics/global-energy-crisis (accessed on 23 February 2023).
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.M.; Gregor, L.; Hauck, J.; Le Quéré, C.; Luijkx, I.T.; Olsen, A.; Peters, G.P.; et al. Global Carbon Budget 2022. Earth Syst. Sci. Data 2022, 14, 4811–4900. [Google Scholar] [CrossRef]
- Nations, U. Net Zero Coalition. Available online: https://www.un.org/en/climatechange/net-zero-coalition (accessed on 23 February 2023).
- Net Zero by 2050—Analysis. Available online: https://www.iea.org/reports/net-zero-by-2050 (accessed on 23 February 2023).
- The Paris Agreement | UNFCCC. Available online: https://unfccc.int/process-and-meetings/the-paris-agreement (accessed on 23 February 2023).
- Hu, B.; Xu, J.; Fan, Z.; Xu, C.; Han, S.; Zhang, J.; Ma, L.; Ding, B.; Zhuang, Z.; Kang, Q.; et al. Covalent Organic Framework Based Lithium–Sulfur Batteries: Materials, Interfaces, and Solid-State Electrolytes. Adv. Energy Mater. 2023, 13, 2203540. [Google Scholar] [CrossRef]
- Li, L.; Cheng, H.; Zhang, J.; Guo, Y.; Sun, C.; Zhou, M.; Li, Q.; Ma, Z.; Ming, J. Quantitative Chemistry in Electrolyte Solvation Design for Aqueous Batteries. ACS Energy Lett. 2023, 8, 1076–1095. [Google Scholar] [CrossRef]
- Javed, M.S.; Najam, T.; Hussain, I.; Idrees, M.; Ahmad, A.; Imran, M.; Shah, S.S.A.; Luque, R.; Han, W. Fundamentals and Scientific Challenges in Structural Design of Cathode Materials for Zinc-Ion Hybrid Supercapacitors. Adv. Energy Mater. 2023, 13, 2202303. [Google Scholar] [CrossRef]
- Zhang, X.; Han, R.; Liu, Y.; Li, H.; Shi, W.; Yan, X.; Zhao, X.; Li, Y.; Liu, B. Porous and Graphitic Structure Optimization of Biomass-Based Carbon Materials from 0D to 3D for Supercapacitors: A Review. Chem. Eng. J. 2023, 460, 141607. [Google Scholar] [CrossRef]
- Eisa, T.; Abdelkareem, M.A.; Jadhav, D.A.; Mohamed, H.O.; Sayed, E.T.; Olabi, A.G.; Castaño, P.; Chae, K.-J. Critical Review on the Synthesis, Characterization, and Application of Highly Efficient Metal Chalcogenide Catalysts for Fuel Cells. Prog. Energy Combust. Sci. 2023, 94, 101044. [Google Scholar] [CrossRef]
- Vinodh, R.; Atchudan, R.; Kim, H.-J.; Yi, M. Recent Advancements in Polysulfone Based Membranes for Fuel Cell (PEMFCs, DMFCs and AMFCs) Applications: A Critical Review. Polymers 2022, 14, 300. [Google Scholar] [CrossRef]
- Zeng, Y.; Yu, M.; Meng, Y.; Fang, P.; Lu, X.; Tong, Y. Iron-Based Supercapacitor Electrodes: Advances and Challenges. Adv. Energy Mater. 2016, 6, 1601053. [Google Scholar] [CrossRef]
- Ma, X.; Jing, Z.; Feng, C.; Qiao, M.; Xu, D. Research and Development Progress of Porous Foam-Based Electrodes in Advanced Electrochemical Energy Storage Devices: A Critical Review. Renew. Sustain. Energy Rev. 2023, 173, 113111. [Google Scholar] [CrossRef]
- Vlad, A.; Singh, N.; Galande, C.; Ajayan, P.M. Design Considerations for Unconventional Electrochemical Energy Storage Architectures. Adv. Energy Mater. 2015, 5, 1402115. [Google Scholar] [CrossRef]
- Zhang, S.; Pan, N. Supercapacitors Performance Evaluation. Adv. Energy Mater. 2015, 5, 1401401. [Google Scholar] [CrossRef]
- Burke, A. Ultracapacitors: Why, How, and Where Is the Technology. J. Power Sources 2000, 91, 37–50. [Google Scholar] [CrossRef]
- Zhi, M.; Xiang, C.; Li, J.; Li, M.; Wu, N. Nanostructured Carbon–Metal Oxide Composite Electrodes for Supercapacitors: A Review. Nanoscale 2013, 5, 72–88. [Google Scholar] [CrossRef] [PubMed]
- Raza, W.; Ali, F.; Raza, N.; Luo, Y.; Kim, K.-H.; Yang, J.; Kumar, S.; Mehmood, A.; Kwon, E.E. Recent Advancements in Supercapacitor Technology. Nano Energy 2018, 52, 441–473. [Google Scholar] [CrossRef]
- Sial, Q.A.; Kalanur, S.S.; Seo, H. Lamellar Flower Inspired Hierarchical Alpha Manganese Vanadate Microflowers for High-Performance Flexible Hybrid Supercapacitors. Ceram. Int. 2022, 48, 24989–24999. [Google Scholar] [CrossRef]
- Kumar, N.; Kim, S.-B.; Lee, S.-Y.; Park, S.-J. Recent Advanced Supercapacitor: A Review of Storage Mechanisms, Electrode Materials, Modification, and Perspectives. Nanomaterials 2022, 12, 3708. [Google Scholar] [CrossRef]
- Krishna Roy, B.; Tahmid, I.; Ur Rashid, T. Chitosan-Based Materials for Supercapacitor Applications: A Review. J. Mater. Chem. A 2021, 9, 17592–17642. [Google Scholar] [CrossRef]
- Chaichi, A.; Venugopalan, G.; Devireddy, R.; Arges, C.; Gartia, M.R. A Solid-State and Flexible Supercapacitor That Operates across a Wide Temperature Range. ACS Appl. Energy Mater. 2020, 3, 5693–5704. [Google Scholar] [CrossRef]
- Li, K.; Liu, X.; Zheng, T.; Jiang, D.; Zhou, Z.; Liu, C.; Zhang, X.; Zhang, Y.; Losic, D. Tuning MnO2 to FeOOH Replicas with Bio-Template 3D Morphology as Electrodes for High Performance Asymmetric Supercapacitors. Chem. Eng. J. 2019, 370, 136–147. [Google Scholar] [CrossRef]
- Qiao, Y.; Liu, G.; Xu, R.; Hu, R.; Liu, L.; Jiang, G.; Demir, M.; Ma, P. SrFe1-XZrxO3-δ Perovskite Oxides as Negative Electrodes for Supercapacitors. Electrochim. Acta 2023, 437, 141527. [Google Scholar] [CrossRef]
- Guo, W.; Yu, C.; Li, S.; Song, X.; Huang, H.; Han, X.; Wang, Z.; Liu, Z.; Yu, J.; Tan, X.; et al. A Universal Converse Voltage Process for Triggering Transition Metal Hybrids In Situ Phase Restruction toward Ultrahigh-Rate Supercapacitors. Adv. Mater. 2019, 31, 1901241. [Google Scholar] [CrossRef] [PubMed]
- Omar, N.; Daowd, M.; Hegazy, O.; Al Sakka, M.; Coosemans, T.; Van den Bossche, P.; Van Mierlo, J. Assessment of Lithium-Ion Capacitor for Using in Battery Electric Vehicle and Hybrid Electric Vehicle Applications. Electrochim. Acta 2012, 86, 305–315. [Google Scholar] [CrossRef]
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications; Springer Science & Business Media: Berlin, Germany, 2013; ISBN 978-1-4757-3058-6. [Google Scholar]
- Reis, S.; Grosso, R.; Kosctiuk, J.; Franchetti, M.; Oliveira, F.; Souza, A.; Gonin, C.; Freitas, H.; Monteiro, R.; Parreira, L.; et al. Effect of Zr4+ on Lithium-Ion Conductivity of Garnet-Type Li5+xLa3(Nb2−xZrx)O12 Solid Electrolytes. Batteries 2023, 9, 137. [Google Scholar] [CrossRef]
- Yang, Z.; Trask, S.E.; Wu, X.; Ingram, B.J. Effect of Si Content on Extreme Fast Charging Behavior in Silicon–Graphite Composite Anodes. Batteries 2023, 9, 138. [Google Scholar] [CrossRef]
- Palneedi, H.; Peddigari, M.; Hwang, G.-T.; Jeong, D.-Y.; Ryu, J. High-Performance Dielectric Ceramic Films for Energy Storage Capacitors: Progress and Outlook. Adv. Funct. Mater. 2018, 28, 1803665. [Google Scholar] [CrossRef]
- El Issmaeli, Y.; Lahrichi, A.; Nzaba Madila, E.E.; Lamcharfi, T.; Abdi, F.; Duong, A. Novel Dielectric Response in B-Site Zirconium-Doped CaCu3Ti4O12 Ceramics: A Structural, Optical and Electrical Study. Solid State Sci. 2022, 134, 107050. [Google Scholar] [CrossRef]
- McCloskey, B.D. Expanding the Ragone Plot: Pushing the Limits of Energy Storage. J. Phys. Chem. Lett. 2015, 6, 3592–3593. [Google Scholar] [CrossRef]
- Zhou, L.; Li, C.; Liu, X.; Zhu, Y.; Wu, Y.; van Ree, T. 7—Metal Oxides in Supercapacitors. In Metal Oxides in Energy Technologies; Wu, Y., Ed.; Metal Oxides; Elsevier: Amsterdam, The Netherlands, 2018; pp. 169–203. ISBN 978-0-12-811167-3. [Google Scholar]
- Vinodh, R.; Babu, R.S.; Sambasivam, S.; Gopi, C.V.V.M.; Alzahmi, S.; Kim, H.-J.; de Barros, A.L.F.; Obaidat, I.M. Recent Advancements of Polyaniline/Metal Organic Framework (PANI/MOF) Composite Electrodes for Supercapacitor Applications: A Critical Review. Nanomaterials 2022, 12, 1511. [Google Scholar] [CrossRef]
- Vandeginste, V. A Review of Fabrication Technologies for Carbon Electrode-Based Micro-Supercapacitors. Appl. Sci. 2022, 12, 862. [Google Scholar] [CrossRef]
- Haider, W.A.; Tahir, M.; He, L.; Yang, W.; Minhas-khan, A.; Owusu, K.A.; Chen, Y.; Hong, X.; Mai, L. Integration of VS2 Nanosheets into Carbon for High Energy Density Micro-Supercapacitor. J. Alloys Compd. 2020, 823, 151769. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, Z.; Chen, W.; Sun, X.; Luo, M.; Zhang, X.; Li, C.; An, Y.; Song, S.; Wang, K.; et al. A Review on Thermal Behaviors and Thermal Management Systems for Supercapacitors. Batteries 2023, 9, 128. [Google Scholar] [CrossRef]
- Dutta, A.; Mitra, S.; Basak, M.; Banerjee, T. A Comprehensive Review on Batteries and Supercapacitors: Development and Challenges since Their Inception. Energy Storage 2023, 5, e339. [Google Scholar] [CrossRef]
- Lee, J.-H.; Yang, G.; Kim, C.-H.; Mahajan, R.L.; Lee, S.-Y.; Park, S.-J. Flexible Solid-State Hybrid Supercapacitors for the Internet of Everything (IoE). Energy Environ. Sci. 2022, 15, 2233–2258. [Google Scholar] [CrossRef]
- Han, J.; Li, Q.; Wang, J.; Ye, J.; Fu, G.; Zhai, L.; Zhu, Y. Heteroatoms (O, N)-Doped Porous Carbon Derived from Bamboo Shoots Shells for High Performance Supercapacitors. J. Mater. Sci. Mater. Electron. 2018, 29, 20991–21001. [Google Scholar] [CrossRef]
- Echeverry-Montoya, N.A.; Prías-Barragán, J.J.; Tirado-Mejía, L.; Agudelo, C.; Fonthal, G.; Ariza-Calderón, H. Fabrication and Electrical Response of Flexible Supercapacitor Based on Activated Carbon from Bamboo. Phys. Status Solidi C 2017, 14, 1600258. [Google Scholar] [CrossRef]
- Fasakin, O.; Dangbegnon, J.K.; Momodu, D.Y.; Madito, M.J.; Oyedotun, K.O.; Eleruja, M.A.; Manyala, N. Synthesis and Characterization of Porous Carbon Derived from Activated Banana Peels with Hierarchical Porosity for Improved Electrochemical Performance. Electrochim. Acta 2018, 262, 187–196. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, L.; Qi, P.; Zhu, M.; Wang, G.; Ma, Y.; Guo, X.; Chen, H.; Zhang, B.; Zhao, Z.; et al. Nitrogen-Doped Banana Peel–Derived Porous Carbon Foam as Binder-Free Electrode for Supercapacitors. Nanomaterials 2016, 6, 18. [Google Scholar] [CrossRef]
- Shang, T.; Xu, Y.; Li, P.; Han, J.; Wu, Z.; Tao, Y.; Yang, Q.-H. A Bio-Derived Sheet-like Porous Carbon with Thin-Layer Pore Walls for Ultrahigh-Power Supercapacitors. Nano Energy 2020, 70, 104531. [Google Scholar] [CrossRef]
- Seo, M.-K.; Park, S.-J. Electrochemical Characteristics of Activated Carbon Nanofiber Electrodes for Supercapacitors. Mater. Sci. Eng. B 2009, 164, 106–111. [Google Scholar] [CrossRef]
- Yuan, S.; Fan, W.; Wang, D.; Zhang, L.; Miao, Y.-E.; Lai, F.; Liu, T. 3D Printed Carbon Aerogel Microlattices for Customizable Supercapacitors with High Areal Capacitance. J. Mater. Chem. A 2021, 9, 423–432. [Google Scholar] [CrossRef]
- Yang, X.; Kong, L.; Cao, M.; Liu, X.; Li, X. Porous Nanosheets-Based Carbon Aerogel Derived from Sustainable Rattan for Supercapacitors Application. Ind. Crop. Prod. 2020, 145, 112100. [Google Scholar] [CrossRef]
- Lee, K.; Shabnam, L.; Faisal, S.N.; Hoang, V.C.; Gomes, V.G. Aerogel from Fruit Biowaste Produces Ultracapacitors with High Energy Density and Stability. J. Energy Storage 2020, 27, 101152. [Google Scholar] [CrossRef]
- Long, S.; Feng, Y.; He, F.; Zhao, J.; Bai, T.; Lin, H.; Cai, W.; Mao, C.; Chen, Y.; Gan, L.; et al. Biomass-Derived, Multifunctional and Wave-Layered Carbon Aerogels toward Wearable Pressure Sensors, Supercapacitors and Triboelectric Nanogenerators. Nano Energy 2021, 85, 105973. [Google Scholar] [CrossRef]
- Yao, B.; Peng, H.; Zhang, H.; Kang, J.; Zhu, C.; Delgado, G.; Byrne, D.; Faulkner, S.; Freyman, M.; Lu, X.; et al. Printing Porous Carbon Aerogels for Low Temperature Supercapacitors. Nano Lett. 2021, 21, 3731–3737. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-W.; Park, S.-J. Synthesis of Reduced Graphene Oxide/Thorn-like Titanium Dioxide Nanofiber Aerogels with Enhanced Electrochemical Performance for Supercapacitor. J. Colloid Interface Sci. 2017, 486, 287–295. [Google Scholar] [CrossRef]
- Sathish Kumar, P.; Prakash, P.; Srinivasan, A.; Karuppiah, C. A New Highly Powered Supercapacitor Electrode of Advantageously United Ferrous Tungstate and Functionalized Multiwalled Carbon Nanotubes. J. Power Sources 2021, 482, 228892. [Google Scholar] [CrossRef]
- Awata, R.; Shehab, M.; El Tahan, A.; Soliman, M.; Ebrahim, S. High Performance Supercapacitor Based on Camphor Sulfonic Acid Doped Polyaniline/Multiwall Carbon Nanotubes Nanocomposite. Electrochim. Acta 2020, 347, 136229. [Google Scholar] [CrossRef]
- Ovhal, M.M.; Kumar, N.; Hong, S.-K.; Lee, H.-W.; Kang, J.-W. Asymmetric Supercapacitor Featuring Carbon Nanotubes and Nickel Hydroxide Grown on Carbon Fabric: A Study of Self-Discharging Characteristics. J. Alloys Compd. 2020, 828, 154447. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, W.; Zhou, H.; Zhang, Z.; Zhao, M.; Liu, Q.; Yang, J.; Lu, X. Self-Discharge of Supercapacitors Based on Carbon Nanotubes with Different Diameters. Electrochim. Acta 2020, 357, 136855. [Google Scholar] [CrossRef]
- Kim, M.; Lee, B.; Li, M.; Noda, S.; Kim, C.; Kim, J.; Song, W.-J.; Lee, S.W.; Brand, O. All-Soft Supercapacitors Based on Liquid Metal Electrodes with Integrated Functionalized Carbon Nanotubes. ACS Nano 2020, 14, 5659–5667. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Kim, J.-I.; Park, S.-J. Activated Carbon Nanotubes/Polyaniline Composites as Supercapacitor Electrodes. Energy 2014, 78, 298–303. [Google Scholar] [CrossRef]
- Ni, G.; Qin, F.; Guo, Z.; Wang, J.; Shen, W. Nitrogen-Doped Asphaltene-Based Porous Carbon Fibers as Supercapacitor Electrode Material with High Specific Capacitance. Electrochim. Acta 2020, 330, 135270. [Google Scholar] [CrossRef]
- Heo, Y.-J.; Park, M.; Kang, W.-S.; Rhee, K.Y.; Park, S.-J. Preparation and Characterization of Carbon Black/Pitch-Based Carbon Fiber Paper Composites for Gas Diffusion Layers. Compos. Part B Eng. 2019, 159, 362–368. [Google Scholar] [CrossRef]
- Shimanoe, H.; Mashio, T.; Nakabayashi, K.; Inoue, T.; Hamaguchi, M.; Miyawaki, J.; Mochida, I.; Yoon, S.-H. Manufacturing Spinnable Mesophase Pitch Using Direct Coal Extracted Fraction and Its Derived Mesophase Pitch Based Carbon Fiber. Carbon 2020, 158, 922–929. [Google Scholar] [CrossRef]
- Yang, C.-M.; Kim, B.-H. Highly Conductive Pitch-Based Carbon Nanofiber/MnO2 Composites for High-Capacitance Supercapacitors. J. Alloys Compd. 2018, 749, 441–447. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, Y.; Guo, X.; Yu, G. Conductive Polymers for Stretchable Supercapacitors. Nano Res. 2019, 12, 1978–1987. [Google Scholar] [CrossRef]
- Libich, J.; Máca, J.; Vondrák, J.; Čech, O.; Sedlaříková, M. Supercapacitors: Properties and Applications. J. Energy Storage 2018, 17, 224–227. [Google Scholar] [CrossRef]
- Han, H.; Sial, Q.A.; Kalanur, S.S.; Seo, H. Binder Assisted Self-Assembly of Graphene Oxide/Mn2O3 Nanocomposite Electrode on Ni Foam for Efficient Supercapacitor Application. Ceram. Int. 2020, 46, 15631–15637. [Google Scholar] [CrossRef]
- Owusu, K.A.; Qu, L.; Li, J.; Wang, Z.; Zhao, K.; Yang, C.; Hercule, K.M.; Lin, C.; Shi, C.; Wei, Q.; et al. Low-Crystalline Iron Oxide Hydroxide Nanoparticle Anode for High-Performance Supercapacitors. Nat. Commun. 2017, 8, 14264. [Google Scholar] [CrossRef]
- Gao, L.; Cao, K.; Zhang, H.; Li, P.; Song, J.; Surjadi, J.U.; Li, Y.; Sun, D.; Lu, Y. Rationally Designed Nickel Oxide Ravines@iron Cobalt-Hydroxides with Largely Enhanced Capacitive Performance for Asymmetric Supercapacitors. J. Mater. Chem. A 2017, 5, 16944–16952. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, L.; Yang, S.; Liu, J.; Tian, Q.; Yao, W.; Xue, Q.; Li, M.; Wu, W. Facile Synthesis of Amorphous FeOOH/MnO2 Composites as Screen-Printed Electrode Materials for All-Printed Solid-State Flexible Supercapacitors. J. Power Sources 2017, 361, 31–38. [Google Scholar] [CrossRef]
- Zheng, W.; Sun, S.; Xu, Y.; Yu, R.; Li, H. Facile Synthesis of NiAl-LDH/MnO2 and NiFe-LDH/MnO2 Composites for High-Performance Asymmetric Supercapacitors. J. Alloys Compd. 2018, 768, 240–248. [Google Scholar] [CrossRef]
- Wei, G.; Du, K.; Zhao, X.; Li, C.; Li, J.; Ren, K.; Huang, Y.; Wang, H.; Yao, S.; An, C. Integrated FeOOH Nanospindles with Conductive Polymer Layer for High-Performance Supercapacitors. J. Alloys Compd. 2017, 728, 631–639. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Lin, Y.-G.; Hsu, Y.-K.; Yen, S.-C.; Chen, K.-H.; Chen, L.-C. Novel Iron Oxyhydroxide Lepidocrocite Nanosheet as Ultrahigh Power Density Anode Material for Asymmetric Supercapacitors. Small 2014, 10, 3803–3810. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Shin, D.H.; Jun, J.; Lee, C.; Jang, J. Fe3O4/Carbon Hybrid Nanoparticle Electrodes for High-Capacity Electrochemical Capacitors. ChemSusChem 2014, 7, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Yang, S.; Feng, X. 2D Sandwich-like Sheets of Iron Oxide Grown on Graphene as High Energy Anode Material for Supercapacitors. Adv. Mater. 2011, 23, 5574–5580. [Google Scholar] [CrossRef]
- Lu, X.; Zeng, Y.; Yu, M.; Zhai, T.; Liang, C.; Xie, S.; Balogun, M.-S.; Tong, Y. Oxygen-Deficient Hematite Nanorods as High-Performance and Novel Negative Electrodes for Flexible Asymmetric Supercapacitors. Adv. Mater. 2020, 32, 2003125. [Google Scholar] [CrossRef]
- Zeng, Y.; Han, Y.; Zhao, Y.; Zeng, Y.; Yu, M.; Liu, Y.; Tang, H.; Tong, Y.; Lu, X. Advanced Ti-Doped Fe2O3@PEDOT Core/Shell Anode for High-Energy Asymmetric Supercapacitors. Adv. Energy Mater. 2015, 5, 1402176. [Google Scholar] [CrossRef]
- Yang, P.; Ding, Y.; Lin, Z.; Chen, Z.; Li, Y.; Qiang, P.; Ebrahimi, M.; Mai, W.; Wong, C.P.; Wang, Z.L. Low-Cost High-Performance Solid-State Asymmetric Supercapacitors Based on MnO2 Nanowires and Fe2O3 Nanotubes. Nano Lett. 2014, 14, 731–736. [Google Scholar] [CrossRef]
- Wu, M.-S.; Lee, R.-H. Electrochemical Growth of Iron Oxide Thin Films with Nanorods and Nanosheets for Capacitors. J. Electrochem. Soc. 2009, 156, A737. [Google Scholar] [CrossRef]
- Liu, T.; Ling, Y.; Yang, Y.; Finn, L.; Collazo, E.; Zhai, T.; Tong, Y.; Li, Y. Investigation of Hematite Nanorod–Nanoflake Morphological Transformation and the Application of Ultrathin Nanoflakes for Electrochemical Devices. Nano Energy 2015, 12, 169–177. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, X.; Qian, G.; Watkins, J.J. Additive-Driven Self-Assembly of Well-Ordered Mesoporous Carbon/Iron Oxide Nanoparticle Composites for Supercapacitors. Chem. Mater. 2014, 26, 2128–2137. [Google Scholar] [CrossRef]
- Chaudhari, N.K.; Chaudhari, S.; Yu, J.-S. Cube-like α-Fe2O3 Supported on Ordered Multimodal Porous Carbon as High Performance Electrode Material for Supercapacitors. ChemSusChem 2014, 7, 3102–3111. [Google Scholar] [CrossRef] [PubMed]
- Shivakumara, S.; Penki, T.R.; Munichandraiah, N. Synthesis and Characterization of Porous Flowerlike α-Fe2O3 Nanostructures for Supercapacitor Application. ECS Electrochem. Lett. 2013, 2, A60. [Google Scholar] [CrossRef]
- Chen, L.-F.; Yu, Z.-Y.; Ma, X.; Li, Z.-Y.; Yu, S.-H. In Situ Hydrothermal Growth of Ferric Oxides on Carbon Cloth for Low-Cost and Scalable High-Energy-Density Supercapacitors. Nano Energy 2014, 9, 345–354. [Google Scholar] [CrossRef]
- Barik, R.; Jena, B.K.; Mohapatra, M. Metal Doped Mesoporous FeOOH Nanorods for High Performance Supercapacitors. RSC Adv. 2017, 7, 49083–49090. [Google Scholar] [CrossRef]
- Chen, R.; Puri, I.K.; Zhitomirsky, I. High Areal Capacitance of FeOOH-Carbon Nanotube Negative Electrodes for Asymmetric Supercapacitors. Ceram. Int. 2018, 44, 18007–18015. [Google Scholar] [CrossRef]
- Wei, Y.; Ding, R.; Zhang, C.; Lv, B.; Wang, Y.; Chen, C.; Wang, X.; Xu, J.; Yang, Y.; Li, Y. Facile Synthesis of Self-Assembled Ultrathin α-FeOOH Nanorod/Graphene Oxide Composites for Supercapacitors. J. Colloid Interface Sci. 2017, 504, 593–602. [Google Scholar] [CrossRef]
- Chen, L.-F.; Yu, Z.-Y.; Wang, J.-J.; Li, Q.-X.; Tan, Z.-Q.; Zhu, Y.-W.; Yu, S.-H. Metal-like Fluorine-Doped β-FeOOH Nanorods Grown on Carbon Cloth for Scalable High-Performance Supercapacitors. Nano Energy 2015, 11, 119–128. [Google Scholar] [CrossRef]
- Nguyen, T.; Montemor, M.F. γ-FeOOH and Amorphous Ni–Mn Hydroxide on Carbon Nanofoam Paper Electrodes for Hybrid Supercapacitors. J. Mater. Chem. A 2018, 6, 2612–2624. [Google Scholar] [CrossRef]
- Chen, J.; Xu, J.; Zhou, S.; Zhao, N.; Wong, C.-P. Amorphous Nanostructured FeOOH and Co–Ni Double Hydroxides for High-Performance Aqueous Asymmetric Supercapacitors. Nano Energy 2016, 21, 145–153. [Google Scholar] [CrossRef]
- Nie, Y.; Yang, T.; Luo, D.; Liu, Y.; Ma, Q.; Yang, L.; Yao, Y.; Huang, R.; Li, Z.; Akinoglu, E.M.; et al. Tailoring Vertically Aligned Inorganic-Polymer Nanocomposites with Abundant Lewis Acid Sites for Ultra-Stable Solid-State Lithium Metal Batteries. Adv. Energy Mater. 2023, 13, 2204218. [Google Scholar] [CrossRef]
- Ma, J.; Guo, Z.; Han, X.; Guo, K.; Li, H.; Fang, P.; Wang, X.; Xin, J. Reduced Graphene Oxide/FeOOH-Based Asymmetric Evaporator for the Simultaneous Generation of Clean Water and Electrical Power. Carbon 2023, 201, 318–327. [Google Scholar] [CrossRef]
- Yang, P.-Q.; Ko, T.-E.; Tseng, C.-M.; Wang, W.-H.; Huang, C.-C.; Tsai, J.-E.; Fu, Y.-C.; Li, Y.-Y. FeOOH-Carbon Nanotube-FeCo/Nitrogen-Doped Porous Carbon as an Excellent Bifunctional Catalyst for Achieving High Power Performance in Rechargeable Zinc-Air Batteries. J. Ind. Eng. Chem. 2023, 121, 338–347. [Google Scholar] [CrossRef]
- Tang, M.; Liu, X.; Ali, A.; He, Y.; Shen, P.; Ouyang, Y. Operando Spectroscopies Capturing Surface Reconstruction and Interfacial Electronic Regulation by FeOOH@Fe2O3@Ni(OH)2 Heterostructures for Robust Oxygen Evolution Reaction. J. Colloid Interface Sci. 2023, 636, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Vishwanathan, S.; Moolayadukkam, S.; Gangaiah, V.K.; Matte, H.S.S.R. Amorphous MnO2-Modified FeOOH Ternary Composite with High Pseudocapacitance As Anode for Lithium-Ion Batteries. ACS Appl. Energy Mater. 2023, 6, 2022–2030. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, M.; Tian, Y.; Zhao, G.; Huang, J.; Xu, X. In-Situ Growth of 3D Hierarchical γ-FeOOH/Ni3S2 Heterostructure as High Performance Electrocatalyst for Overall Water Splitting. J. Colloid Interface Sci. 2023, 639, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ren, X.; Chen, J.; Xu, W.; He, Q.; Wang, H.; Zhan, F.; Chen, L. Recent Advances of Emerging Oxyhydroxide for Electrochemical Energy Storage Applications. J. Power Sources 2023, 554, 232309. [Google Scholar] [CrossRef]
- Mohapatra, M.; Anand, S. Synthesis and Applications of Nano-Structured Iron Oxides/Hydroxides—A Review. Int. J. Eng. Sci. Technol. 2010, 2, 127–146. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, M.; Shi, X.; Zeng, H.; Xia, H. Amorphous FeOOH Quantum Dots Assembled Mesoporous Film Anchored on Graphene Nanosheets with Superior Electrochemical Performance for Supercapacitors. Adv. Funct. Mater. 2016, 26, 919–930. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.; Li, J.; Du, G. One-Pot Hydrothermal Synthesis of Amorphous FeOOH on Ni Foam for High Performance Supercapacitors. J. Alloys Compd. 2018, 763, 134–140. [Google Scholar] [CrossRef]
- Wang, T.; Li, K.; Le, Q.; Zhu, S.; Guo, X.; Jiang, D.; Zhang, Y. Tuning Parallel Manganese Dioxide to Hollow Parallel Hydroxyl Oxidize Iron Replicas for High-Performance Asymmetric Supercapacitors. J. Colloid Interface Sci. 2021, 594, 812–823. [Google Scholar] [CrossRef]
- Tanwar, S.; Arya, A.; Sharma, A.L. MoSe2-FeOOH Nanocomposite as Hybrid Electrode Material for High-Performance Symmetric Supercapacitor. Mater. Res. Bull. 2023, 160, 112144. [Google Scholar] [CrossRef]
- Sun, C.; Pan, W.; Zheng, D.; Zheng, Y.; Zhu, J.; Liu, C. Low-Crystalline FeOOH Nanoflower Assembled Mesoporous Film Anchored on MWCNTs for High-Performance Supercapacitor Electrodes. ACS Omega 2020, 5, 4532–4541. [Google Scholar] [CrossRef]
- Li, N.; Zhi, C.; Zhang, H. High-Performance Transparent and Flexible Asymmetric Supercapacitor Based on Graphene-Wrapped Amorphous FeOOH Nanowire and Co(OH)2 Nanosheet Transparent Films Produced at Air-Water Interface. Electrochim. Acta 2016, 220, 618–627. [Google Scholar] [CrossRef]
- Li, N.; Huang, X.; Zhang, H. High Energy Density Transparent and Flexible Asymmetric Supercapacitor Based on a Transparent Metal Hydroxides@graphene Micro-Structured Film via a Scalable Gas-Liquid Diffusion Method. J. Alloys Compd. 2017, 712, 194–203. [Google Scholar] [CrossRef]
- Parveen, S.; Kavyashree; Sharma, S.K.; Pandey, S.N. High Performance Solid State Symmetric Supercapacitor Based on Reindeer Moss-like Structured Al(OH)3/MnO2/FeOOH Composite Electrode for Energy Storage Applications. Energy 2021, 224, 120137. [Google Scholar] [CrossRef]
- Gao, X.; Lv, H.; Li, Z.; Xu, Q.; Liu, H.; Wang, Y.; Xia, Y. Low-Cost and High-Performance of a Vertically Grown 3D Ni–Fe Layered Double Hydroxide/Graphene Aerogel Supercapacitor Electrode Material. RSC Adv. 2016, 6, 107278–107285. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, B.; Fang, L.; Ling, F.; Wu, F.; Hu, B.; Meng, F.; Niu, K.; Lin, F.; Zheng, H. An Investigation of Ultrathin Nickel-Iron Layered Double Hydroxide Nanosheets Grown on Nickel Foam for High-Performance Supercapacitor Electrodes. J. Alloys Compd. 2017, 714, 63–70. [Google Scholar] [CrossRef]
- Sanati, S.; Rezvani, Z. Ultrasound-Assisted Synthesis of NiFe- Layered Double Hydroxides as Efficient Electrode Materials in Supercapacitors. Ultrason. Sonochem. 2018, 48, 199–206. [Google Scholar] [CrossRef]
- Wang, F.; Wang, T.; Sun, S.; Xu, Y.; Yu, R.; Li, H. One-Step Synthesis of Nickle Iron-Layered Double Hydroxide/Reduced Graphene Oxide/Carbon Nanofibres Composite as Electrode Materials for Asymmetric Supercapacitor. Sci. Rep. 2018, 8, 8908. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Wang, S.; Li, J.; Yang, N.; Li, W.; Xiang, P.; Jiang, L.; Tan, X. Sulfidation of NiFe-Layered Double Hydroxides as Novel Negative Electrodes for Supercapacitors with Enhanced Performance. J. Alloys Compd. 2018, 768, 635–643. [Google Scholar] [CrossRef]
- Li, M.; Jijie, R.; Barras, A.; Roussel, P.; Szunerits, S.; Boukherroub, R. NiFe Layered Double Hydroxide Electrodeposited on Ni Foam Coated with Reduced Graphene Oxide for High-Performance Supercapacitors. Electrochim. Acta 2019, 302, 1–9. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, A.; Liu, R.; Huang, W.; Yuan, Z.; Zheng, R.; Wei, D.; Liu, J. Preparation of CoS2 Supported Flower-like NiFe Layered Double Hydroxides Nanospheres for High-Performance Supercapacitors. J. Colloid Interface Sci. 2020, 579, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wu, F.; Fang, L.; Hu, J.; Luo, H.; Guan, T.; Hu, B.; Zhou, M. Layered NiFe-LDH/MXene Nanocomposite Electrode for High-Performance Supercapacitor. Int. J. Hydrogen Energy 2020, 45, 13080–13089. [Google Scholar] [CrossRef]
- Shakir, I.; Almutairi, Z.; Shar, S.S. Fabrication of Carbon Cloth Supported Ni@Fe Double Hydroxide Based Electrode for Flexible Supercapacitor Applications. Ceram. Int. 2021, 47, 17427–17434. [Google Scholar] [CrossRef]
- Hsiao, C.; Lee, C.; Tai, N. High Retention Supercapacitors Using Carbon Nanomaterials/Iron Oxide/Nickel-Iron Layered Double Hydroxides as Electrodes. J. Energy Storage 2022, 46, 103805. [Google Scholar] [CrossRef]
- Shao, W.; Wang, Q.; Huang, C.; Zhang, D. High Valence State Metal-Ion Doped Fe–Ni Layered Double Hydroxides for Oxygen Evolution Electrocatalysts and Asymmetric Supercapacitors. Mater. Adv. 2022, 3, 1816–1824. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Zhang, M.; Liu, Y.; Luo, H.; Yan, K. Green Fabrication of Nickel-Iron Layered Double Hydroxides Nanosheets Efficient for the Enhanced Capacitive Performance. Green Energy Environ. 2022, 7, 1053–1061. [Google Scholar] [CrossRef]
- Ma, K.; Cheng, J.P.; Zhang, J.; Li, M.; Liu, F.; Zhang, X. Dependence of Co/Fe Ratios in Co-Fe Layered Double Hydroxides on the Structure and Capacitive Properties. Electrochim. Acta 2016, 198, 231–240. [Google Scholar] [CrossRef]
- Wu, Y.-J.; Zhang, W.-T.; Liu, Z.-W.; Chen, R.-F. Synthesis of Co1−xFex Hydroxide Nanoplatelets and Its Electrochemical Performances as Supercapacitor Electrode Materials. Electrochemistry 2016, 84, 2–6. [Google Scholar] [CrossRef]
- Jiang, L.; Sui, Y.; Qi, J.; Chang, Y.; He, Y.; Meng, Q.; Wei, F.; Sun, Z.; Jin, Y. Structure Dependence of Fe-Co Hydroxides on Fe/Co Ratio and Their Application for Supercapacitors. Part. Part. Syst. Charact. 2017, 34, 1600239. [Google Scholar] [CrossRef]
- Fang, X.; Han, S.; Liu, D.; Zhu, Y. Two-Dimensional CoFe Hydroxides Nanostructure as Positive Material for Asymmetric Supercapacitor. Chem. Phys. Lett. 2020, 746, 137282. [Google Scholar] [CrossRef]
- Jo, S.; Jayababu, N.; Kim, D. Rational Design of Cobalt-Iron Bimetal Layered Hydroxide on Conductive Fabric as a Flexible Battery-Type Electrode for Enhancing the Performance of Hybrid Supercapacitor. J. Alloys Compd. 2022, 904, 164082. [Google Scholar] [CrossRef]
- Mahmood, A.; Zhao, B.; Javed, M.S.; He, D.; Cheong, W.-C.; Han, D.; Niu, L. Unprecedented Dual Role of Polyaniline for Enhanced Pseudocapacitance of Cobalt–Iron Layered Double Hydroxide. Macromol. Rapid Commun. 2022, 43, 2100905. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, L.; Chai, H.; Zhang, Y.; Wang, R.; Xie, M.; Xu, Y.; Chen, J.; Jiao, Y. Electrochemical Activation Strategy Assisted Morphology Engineering Co-Fe Layered Double Hydroxides for Oxygen Hydrogen Evolution and Supercapacitor. J. Colloid Interface Sci. 2023, 632, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Sun, S.; Xu, Y.; Wang, T.; Yu, R.; Li, H. High Performance Asymmetric Supercapacitor Based on Cobalt Nickle Iron-Layered Double Hydroxide/Carbon Nanofibres and Activated Carbon. Sci. Rep. 2017, 7, 4707. [Google Scholar] [CrossRef]
- Pourfarzad, H.; Shabani-Nooshabadi, M.; Ganjali, M.R.; Kashani, H. Synthesis of Ni–Co-Fe Layered Double Hydroxide and Fe2O3/Graphene Nanocomposites as Actively Materials for High Electrochemical Performance Supercapacitors. Electrochim. Acta 2019, 317, 83–92. [Google Scholar] [CrossRef]
- Lee, S.C.; Liu, S.; Shinde, P.A.; Chung, K.Y.; Chan Jun, S. A Systematic Approach to Achieve High Energy Density Hybrid Supercapacitors Based on Ni–Co–Fe Hydroxide. Electrochim. Acta 2020, 353, 136578. [Google Scholar] [CrossRef]
- Ren, X.; Zhou, Y.; Du, Y.; Jiang, Y.; Chen, Y.; Wan, J.; Ma, F. Facile Ion Exchange to Construct Ni-Fe-Co Sulfides and Hydroxides Ultrathin Nanosheets with Rich Interfaces for Advanced All-Solid-State Asymmetric Supercapacitors. Appl. Surf. Sci. 2020, 514, 145951. [Google Scholar] [CrossRef]
- Rohit, R.C.; Jagadale, A.D.; Shinde, S.K.; Kim, D.-Y.; Kumbhar, V.S.; Nakayama, M. Hierarchical Nanosheets of Ternary CoNiFe Layered Double Hydroxide for Supercapacitors and Oxygen Evolution Reaction. J. Alloys Compd. 2021, 863, 158081. [Google Scholar] [CrossRef]
- Liao, F.; Yang, G.; Cheng, Q.; Mao, L.; Zhao, X.; Chen, L. Rational Design and Facile Synthesis of Ni-Co-Fe Ternary LDH Porous Sheets for High-Performance Aqueous Asymmetric Supercapacitor. Electrochim. Acta 2022, 428, 140939. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, Z.; Guo, Z.; Mu, J.; Che, H.; Zhang, Z.; Tian, T.; Xiaoliang, Z.; Li, S.; Wang, Y.; et al. Fe Incorporated Ternary Layered Double Hydroxides with Remarkably Improved Electrochemical Performance towards Asymmetric Supercapacitors. Ceram. Int. 2022, 48, 27369–27378. [Google Scholar] [CrossRef]
- Wang, H.; He, Q.; Zhan, F.; Chen, L. Fe, Co-Codoped Layered Double Hydroxide Nanosheet Arrays Derived from Zeolitic Imidazolate Frameworks for High-Performance Aqueous Hybrid Supercapacitors and Zn-Ni Batteries. J. Colloid Interface Sci. 2023, 630, 286–296. [Google Scholar] [CrossRef]
- Zhang, D.; Peng, L.; Yang, Z.; Yang, Y.; Li, H. Gold-Supported Nanostructured NiFeCoPr Hydroxide as a High-Performance Supercapacitor Electrode and Electrocatalyst toward the Oxygen Evolution Reaction. Inorg. Chem. 2019, 58, 15841–15852. [Google Scholar] [CrossRef]
- Elgendy, A.; El Basiony, N.M.; El-Taib Heakal, F.; Elkholy, A.E. Mesoporous Ni-Zn-Fe Layered Double Hydroxide as an Efficient Binder-Free Electrode Active Material for High-Performance Supercapacitors. J. Power Sources 2020, 466, 228294. [Google Scholar] [CrossRef]
- Yang, Y.J.; Chen, S.; Jiang, C.; Yang, P.; Wang, N.; Cheng, Y.; Liu, M. Hierarchical Web-like NiFeMn Ternary Hydroxides Microstructure Assembled on Reduced Graphene Oxide for Binder-Free Supercapacitor Electrode. Diam. Relat. Mater. 2022, 125, 109009. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Nakate, U.T.; Chen, J.; Tran, D.T.; Park, S. Evolution of Novel Nanostructured MoCoFe-Based Hydroxides Composites toward High-Performance Electrochemical Applications: Overall Water Splitting and Supercapacitor. Compos. Part B Eng. 2023, 252, 110528. [Google Scholar] [CrossRef]
- Zhao, N.; Feng, Y.; Chen, H. Exquisite Microstructure Design of Quaternary Nickel Cobalt Manganese Iron Layered Double Hydroxides for High Performance Hybrid Supercapacitors. Electrochim. Acta 2023, 441, 141756. [Google Scholar] [CrossRef]
- Li, H.B.; Yu, M.H.; Wang, F.X.; Liu, P.; Liang, Y.; Xiao, J.; Wang, C.X.; Tong, Y.X.; Yang, G.W. Amorphous Nickel Hydroxide Nanospheres with Ultrahigh Capacitance and Energy Density as Electrochemical Pseudocapacitor Materials. Nat. Commun. 2013, 4, 1894. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gao, Y.; Wang, C.; Yang, G. A Simple Electrochemical Route to Access Amorphous Mixed-Metal Hydroxides for Supercapacitor Electrode Materials. Adv. Energy Mater. 2015, 5, 1401767. [Google Scholar] [CrossRef]
- Ragni, R.; Cicco, S.R.; Vona, D.; Farinola, G.M. Multiple Routes to Smart Nanostructured Materials from Diatom Microalgae: A Chemical Perspective. Adv. Mater. 2018, 30, 1704289. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.W.; Zhang, Y.X.; Losic, D. Diatom Silica, an Emerging Biomaterial for Energy Conversion and Storage. J. Mater. Chem. A 2017, 5, 8847–8859. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The Rise of Graphene. Nat. Mater 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhai, P.; Zhang, Z.; Zhou, Y.; Ju, J.; Shi, Z.; Ma, D.; Han, R.P.S.; Huang, F. Synthesis of Highly Stable Graphene-Encapsulated Iron Nanoparticles for Catalytic Syngas Conversion. Part. Part. Syst. Charact. 2015, 32, 29–34. [Google Scholar] [CrossRef]
- Amiri, A.; Bruno, A.; Polycarpou, A.A. Configuration-Dependent Stretchable All-Solid-State Supercapacitors and Hybrid Supercapacitors. Carbon Energy 2023, e320. [Google Scholar] [CrossRef]
- Transparent, Flexible, and Solid-State Supercapacitors Based on Graphene Electrodes: APL Materials: Vol 1, No 1. Available online: https://aip.scitation.org/doi/full/10.1063/1.4808242 (accessed on 9 March 2023).
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.-S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Ri Kim, H.; Song, Y.I.; et al. Roll-to-Roll Production of 30-Inch Graphene Films for Transparent Electrodes. Nat. Nanotech 2010, 5, 574–578. [Google Scholar] [CrossRef]
- Yoo, J.J.; Balakrishnan, K.; Huang, J.; Meunier, V.; Sumpter, B.G.; Srivastava, A.; Conway, M.; Mohana Reddy, A.L.; Yu, J.; Vajtai, R.; et al. Ultrathin Planar Graphene Supercapacitors. Nano Lett. 2011, 11, 1423–1427. [Google Scholar] [CrossRef]
- Xu, P.; Kang, J.; Choi, J.-B.; Suhr, J.; Yu, J.; Li, F.; Byun, J.-H.; Kim, B.-S.; Chou, T.-W. Laminated Ultrathin Chemical Vapor Deposition Graphene Films Based Stretchable and Transparent High-Rate Supercapacitor. ACS Nano 2014, 8, 9437–9445. [Google Scholar] [CrossRef]
- Li, N.; Yang, G.; Sun, Y.; Song, H.; Cui, H.; Yang, G.; Wang, C. Free-Standing and Transparent Graphene Membrane of Polyhedron Box-Shaped Basic Building Units Directly Grown Using a NaCl Template for Flexible Transparent and Stretchable Solid-State Supercapacitors. Nano Lett. 2015, 15, 3195–3203. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Zhou, W.; Chen, J.; Feng, G.; Li, H.; Hu, Y.; Ma, W.; Dong, H.; Li, J.; Xie, S. A Repeated Halving Approach to Fabricate Ultrathin Single-Walled Carbon Nanotube Films for Transparent Supercapacitors. Small 2013, 9, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhao, Q.; Li, B.; Xue, H.; Pang, H.; Chen, C. Recent Progress in Layered Double Hydroxide Based Materials for Electrochemical Capacitors: Design, Synthesis and Performance. Nanoscale 2017, 9, 15206–15225. [Google Scholar] [CrossRef]
- Mohapatra, L.; Parida, K. A Review on the Recent Progress, Challenges and Perspective of Layered Double Hydroxides as Promising Photocatalysts. J. Mater. Chem. A 2016, 4, 10744–10766. [Google Scholar] [CrossRef]
- Abellán, G.; Carrasco, J.A.; Coronado, E.; Romero, J.; Varela, M. Alkoxide-Intercalated CoFe-Layered Double Hydroxides as Precursors of Colloidal Nanosheet Suspensions: Structural, Magnetic and Electrochemical Properties. J. Mater. Chem. C 2014, 2, 3723–3731. [Google Scholar] [CrossRef]
- Wang, L.; Wang, D.; Dong, X.Y.; Zhang, Z.J.; Pei, X.F.; Chen, X.J.; Chen, B.; Jin, J. Layered Assembly of Graphene Oxide and Co–Al Layered Double Hydroxide Nanosheets as Electrode Materials for Supercapacitors. Chem. Commun. 2011, 47, 3556–3558. [Google Scholar] [CrossRef]
- Li, M.; Cheng, J.P.; Fang, J.H.; Yang, Y.; Liu, F.; Zhang, X.B. NiAl-Layered Double Hydroxide/Reduced Graphene Oxide Composite: Microwave-Assisted Synthesis and Supercapacitive Properties. Electrochim. Acta 2014, 134, 309–318. [Google Scholar] [CrossRef]
- Rohit, R.C.; Jagadale, A.D.; Lee, J.; Lee, K.; Shinde, S.K.; Kim, D.-Y. Tailoring the Composition of Ternary Layered Double Hydroxides for Supercapacitors and Electrocatalysis. Energy Fuels 2021, 35, 9660–9668. [Google Scholar] [CrossRef]









| Material/Substrate | Synthesis Method | Structure/Morphology | Electrolyte | Potential Window | Specific Capacitance or Storage Capacity/Current Density or Scan Rate | Cycling Performance (N° of Cycles, Current Density) | Energy Density | Power Density | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| FeOOH QDs/FGS composite/Ti foil | Facile chemical reaction | Heterostructure/self-assembled FeOOH mesoporous nanofilm tightly anchored on FGS | 1 M Li2SO4 | −0.8–0 V vs. Ag/AgCl | 365 F g−1/1 A g−1 | 89.7% (20,000th, 4 A g−1) | - | - | [97] |
| FeOOH/NF | One-step electrodeposition | Amorphous nanostructured/fish-scale-like | 3 M KOH | −1.1–−0.3 V vs. Hg/HgO | 867 F g−1 at 5 mV s−1 | 92.3% (200th) | 86.4 Wh kg−1 | 11.6 kW kg−1 | [88] |
| FeOOH/NF | One-pot hydrothermal route | 3D porous structure/nanoflakes | 1 M KOH | −0.1–0.5 V vs. Hg/HgO | 1300 F g−1/2 A g−1 | 91% (2000th, 4 A g−1) | - | - | [98] |
| FeOOH/CFC | Hydrothermal growth + subsequent electrochemical transformation of α-Fe2O3 | Nanoparticles | 2 M KOH | −1.2–0 V vs. SCE | 1066 F g−1/1 A g−1 | 91% (10,000th, 30 A g−1) | 104.3 Wh kg−1 | 1.27 kW kg−1 | [66] |
| γ-FeOOH/CNFP | Galvanostatic electrodeposition | Fluffy nanoflakes/porous morphology | 1 M KOH | −1.5–0 V vs. SCE | 3.48 C cm−2 at 10 mA cm−2 | 92% (3000th, 50 mA cm−2) | 1515 mW h cm−2 | 9 mW cm−2 | [87] |
| α-FeOOH/NF | Two-step hydrothermal method + etching process | Nanorods | 1 M Na2SO4 | −1.0–0 V vs. SCE | 224.6 F g−1/1 A g−1 | 92.5% (4000th, 5 A g−1) | 51.5 Wh kg−1 | 9.1 kW kg−1 | [24] |
| FeOOH/NF | Dropwise wet chemistry | Parallel and hollow | 1 M Na2SO4 | −0.8–0 V vs. SCE | 186.8 F g−1/0.5 A g−1 | 93.1% (4000th, 5 A g−1) | 20.7 Wh kg−1 | 10 kW kg−1 | [99] |
| α-FeOOH nanorods/GO composite/NF | One-pot hydrothermal method | Nanorods | 1 M KOH | −0.9–0 V vs. Hg/HgO | 127 F g−1/10 A g−1 | 85% (2000th, 5 A g−1) | - | - | [85] |
| FeOOH@MWCNT | Facile synthesis method | Nanofilm/Nanoflowers | 0.5 M Na2SO4 | −0.85–0 V vs. Ag/AgCl | 345 F g−1/1 A g−1 | 76.4% (5000th, 1 A g−1) | - | - | [101] |
| α-FeOOH-MWCNT composite/NF | PELLI strategies | Particles | 0.5 M Na2SO4 | −0.8–0 V vs. SCE | 5.86 F cm−2 at 2 mV s−1 | - | - | - | [84] |
| FeOOH/MoSe2/NF | Hydrothermal method + chemical blending technique | Nanorods | 6 M KOH | 0–1.0 V vs. Ag/AgCl (for device) | 132 F g−1/1 A g−1 (for device) | 100% (3000th) (for device) | 18.3 Wh kg−1 | 1174 W kg−1 | [100] |
| FeOOH@Gr | Bioinspired method at the air-solution interface | Nanowires | 2 M KOH | −1.2–0 V vs. Hg/HgO | 25.5 mF cm−2 at 0.1 V s−1 (for device) | 83.5% (10,000th) (for device) | 1.04 mWh cm−3 | 0.445 W cm−3 | [102] |
| FeOOH@Gr | Gas-liquid diffusion method at the air-solution interface | Nanowires | 2 M KOH | −1.25–0 V vs. Hg/HgO | 1150.3 F g−1/3.0 A g−1 | ~100% (1000th) | 0.67 mWh cm−3 | 41.7 mW cm−3 | [103] |
| FeOOH/MnO2/NF | Facile chemical reaction | Film/nanospheres | 1 M Na2SO4 | 0–0.8 V vs. Ag/AgCl | 350.2 F g−1/0.5 A g−1 | 95.6% (10,000th, 15 A g−1) | 5 × 10−4 mWh cm−2 | 0.04 mW cm−2 | [68] |
| Al(OH)3/MnO2/FeOOH/SS | Layer by Layer method | Mesoporous reindeer moss-like | 1 M Na2SO4 | −1.1–1.05 V vs. Ag/AgCl | 2557 C g−1 at 5 mV s−1 | ~70% (1000th, 5 mA) | 443.67 Wh kg−1 | 13.53 kW kg−1 | [104] |
| NiFe LDH/NF | One-step hydrothermal method | Porous nanostructure/Nanosheet arrays | 1 M KOH | 0–0.7 V vs. SCE | 2708 F g−1/5 A g−1 | 42.6% (500th, 10 A g−1) | 50.2 Wh kg−1 | 800 W kg−1 | [106] |
| NiFe-LDH/NF | Two-step hydrothermal method + sulfidation modification | Nanosheets | 1 M KOH | −1.0–0 V vs. Hg/HgO | 992 mF cm−2 at 2 mA cm−2 | 64.5% (2000th, 4 mA cm−2) | 39.9 Wh kg−1 | 211.4 W kg−1 | [109] |
| NiFe-LDH/Glassy carbon electrode | Sonochemical route | Spherical nanostructures | 6 M KOH | 0–0.4 V vs. SCE | 168 F g−1/1.5 Ag−1 | - | - | - | [107] |
| NiFe-LDH | Ultrasonication and mechanical stirring | Nanosheets | 6 M KOH | −0.2–0.5 V vs. SCE | 1923 F g−1 at 3 A g−1 | 98% (1000th, 10 A g−1) | 49.13 Wh kg−1 | 400 W kg−1 | [116] |
| NiFe-LDH/MXene | One-step hydrothermal method | Interconnected network structure | 1 M KOH | −0.4–1.0 V vs. Calomel E | 720.2 F g−1/1 A g−1 | 86% (1000th) | 42.4 Wh kg−1 | 758.27 W kg−1 | [112] |
| NiFe-LDH/CC | One-step hydrothermal approach | Interconnected nanoflakes | 3 M KOH | 0–0.6 V vs. Ag/AgCl | 984 F g−1/1 A g−1 | 87.6% (7000th, 12 A g−1) | - | - | [113] |
| NiFe LDH/GHA/NF | Hydrothermal method + freeze-drying treatment | Hexagonal platelets | 6 M KOH | 0–0.5 V vs. Hg/HgO | 1196 F g−1/1 A g−1 | 80% (2000th, 10 A g−1) | 17.6 Wh kg−1 | 650 W kg−1 | [105] |
| NiFe-LDH/RGO/CNFs/NF | One-step hydrothermal method | Nanoplates | 6 M KOH | 0–0.57 V vs. Hg/HgO | 1330.2 F g−1/1 A g−1 | 97.1% (2500th, 8 A g−1) (for device) | 33.7 Wh kg−1 | 785.8 W kg−1 | [108] |
| NiFe-LDH/rGO/NF | Two-step electrodeposition method | Nanosheets | 2 M KOH | 0–0.5 V vs. SCE | 1462.5 F g−1/5 A g−1 | 64.7% (2000th, 15 A g−1) | 17.71 Wh kg−1 | 348.49 W kg−1 | [110] |
| rGO-FeO-CNT-NiFeLDH | Hydrothermal + CVD + ECD | Thin layer | 2M KOH | −1.2–0 V vs. Ag/AgCl | 411.9 F g−1 at 5 mV s−1 | 144.89% (3200th) (for device) | 41.4 Wh kg−1 | 5600 W kg−1 | [114] |
| NiFe-LDH@CoS2@Ni | Two-step hydrothermal method | Flower-like nanospheres | 6 M KOH | 0–0.7 V vs. Hg/HgO | 3880 F g−1/1.17 A g−1 | 81.9% (10,000th, 20 mA cm−2) | 15.84 Wh kg−1 | 375.16 W kg−1 | [111] |
| CoFe-LDH/NF | Co-precipitation + I2 partial oxidation process | Hydrotalcite-like/layered plate-like | 2 M KOH | −0.15–0.45 V vs. Ag/AgCl | 728 F g−1/1 A g−1 | 65.3% (5000th, 2 A g−1) | 27.3 W h kg−1 | 823.5 W kg−1 | [117] |
| CoFe-LDHs/NF | Co-precipitation | Layer hexagonal/Nanoflakes | 1 M KOH | −0.2–0.5 V vs. Hg/HgO | 869 F g−1/1 g−1 | 99.5% (1000th, 1 A g−1) | - | - | [118] |
| CoFe-LDH/NFa | ECD | Nanosheets | 1 M KOH | 0–0.55 V vs. Ag/AgCl | 70 µF cm−2 at 2 mA cm−2 (for device) | 91% (2000th, 3 mA cm−2) (for device) | 1.6 mWh cm−2 | 0.09 mW cm−2 | [121] |
| CoFe Hydroxides | One-step ECD | Frame-like structure | 1 M KOH | 0–0.7 V vs. SCE | 2255.6 F g−1/1 A g−1 | 73.5% (2000th, 10 A g−1) | - | - | [119] |
| CoFe hydroxides | One-step liquid-phase reflux | Hexagonal plate-like structure | 6 M KOH | 0–0.45 V vs. SCE | 2358.4 F g−1/0.5 A g−1 | 83% (1400th, 0.5 A g−1) | 28.3 Wh kg−1 | 512 W kg−1 | [120] |
| CoFe-LDH/NF | Hydrothermal + CV | Microflowers | 3 M KOH | 0–0.6 V vs. Hg/HgO | 4662.2 mF cm−2 | 133.8% (10,000th) | - | - | [123] |
| CoFe-LDH/P | Hydrothermal | Nanosheets | 6 M KOH | −0.1–0.7 V vs. Ag/AgCl | 1686 F g−1/1 A g−1 | 98% (10,000th) | 75.9 Wh kg−1 | 1124 W kg−1 | [122] |
| NiO@CoFe-LDH/NF | Acid corrosion route + electrodeposition coating process | 3D open porous structure/uniform wrinkled cross-linked | 2 M KOH | 0–1.5 V vs. SCE (for device) | 205 F g−1/1 A g−1 (for device | 90% (3000th, 1 A g−1) (for device) | 64.1 W h kg−1 | 15 kW kg−1 | [67] |
| NiCoFe-LDH | One-pot self-template method | Nanospheres | 3 M LiCl | 0–1.0 V vs. SCE | 83.9 mF cm−2 at 0.3 mA (for device) | 116.6% (5000th) | 22.3 μWh cm−2 | 2076 μW cm−2 | [115] |
| NiCoFe-LDH/NF | Cyclic voltametric method | Layer structure | 3 M KOH | 0–0.4 V vs. Ag/AgCl | 3130 F g−1/1 A g−1 | 82.5% (5000th) | 101 Wh kg−1 | 91.5 kW kg−1 | [125] |
| NiCoFe-LDH/SS | Electrodeposition | Nanosheets | 2 M KOH | −0.2–0.4 V vs. Ag/AgCl | 360 C g−1/0.4 A g−1 | 84% (2000th) | - | - | [128] |
| NiCoFe-LDH/CNFs | In situ growth approach with hydrothermal | Nanosheets | 6 M KOH | 0–0.6 V vs. Hg/HgO | 1203 F g−1/1 A g−1 | 94.4% (1000th, 20 A g−1) | 30.2 W h kg−1 | 800.1 W kg−1 | [124] |
| NiCoFe-LDH/NF | Hydrothermal | Two-dimensional porous nanosheets | 2 M KOH | 0–0.6 V vs. SCE | 425.56 mAh g−1/1 A g−1 | 94.52% (8000th, 10 A g−1) | 51.81 Wh kg−1 | 1.26 Wh kg−1 | [129] |
| NiCoFe hydroxide | Electrodeposition | Nanosheets | 1 M KOH | 0–0.6 V vs. Hg/HgO | 1321 F g–1/1 A g–1 | 88.57% (10,000th, 10 A g–1) | 73.07 Wh kg–1 | 1.07 kW kg–1 | [126] |
| NiCoFe-LDH NA/NF | Ion exchange + etching reaction under hydrothermal conditions | 2D/3D porous structure/Nanosheets | 2 M KOH | 0–0.6 V vs. SCE | 1495 C g−1/1 A g−1 | 89% (10,000th, 10 A g−1) | 34.4 W h kg−1 | 935.5 W kg−1 | [131] |
| NiFeCo-S@NiFeCo-TH/NIF | Electro-oxidation + sulfuration + controllable Co2+ exchange process | Nanosheets | 2 M KOH | −0.1–0.6 V vs. SCE | 174 mAh g−1 at 10 mA cm−2 | 90.1% (4000th) (for device) | 56.3 Wh kg−1 | 543 W kg−1 | [127] |
| NiCoFe-LDH@g-C3N4/NF | Hydrothermal | Nanosheets | 6 M KOH | 0–0.5 V vs. SCE | 1550 F g−1/1 A g−1 | 92.7% (5000th) (for device) | 35 Wh kg−1 | 701 W kg−1 | [130] |
| Ni-Zn-Fe LDH | SILAR method | Ash-like | 6 M KOH | 0–0.45 V vs. Ag/AgCl | 1452.3 F g−1 at 5 mV s−1 | 112.5% (1000th) | 14.9 Wh kg−1 | 1077.6 W kg−1 | [133] |
| MoCoFe hydroxide | Single-step electrodeposition technique | Nanosheet-like | 1 M KOH | 0–0.5 V vs. Ag/AgCl | 3354.7 mF cm−2 at 1.0 mA cm−2 | 91% (3000th, 10 mA cm−2) | 1.27 × 10−3 Wh cm−3 | 3.75 W cm−3 | [135] |
| NiFeMn hydroxide/rGO/NF | One-pot two-step hydrothermal method | Hierarchical web-like microstructure | 2 M KOH | 0–0.5 V vs. Hg/HgO | 2121 F g−1/0.61 A g−1 | 81.1% (5000th, 0.91 A g−1) | 40.73 W h m−2 | 79.38 W m−2 | [134] |
| NiFeCoPrO | Electrodeposition | Ultrathin nanostructure/amorphous nature | 3 M KOH | −0.2–0.4 V vs. Ag/AgCl | 1792 F g−1/10 A g−1 | 99.5% (30,000th) | - | - | [132] |
| NiCoMnFe-LDH | Electrodeposition | Thin film | 2 M KOH | 0–0.5 V vs. SCE | 1836 mC cm−2 at 3 mA cm−2 | 98.3% (5000th) | 31.3 Wh kg−1 | 375 W kg−1 | [136] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Issmaeli, Y.; Lahrichi, A.; Kalanur, S.S.; Natarajan, S.K.; Pollet, B.G. Recent Advances and Prospects of FeOOH-Based Electrode Materials for Supercapacitors. Batteries 2023, 9, 259. https://doi.org/10.3390/batteries9050259
El Issmaeli Y, Lahrichi A, Kalanur SS, Natarajan SK, Pollet BG. Recent Advances and Prospects of FeOOH-Based Electrode Materials for Supercapacitors. Batteries. 2023; 9(5):259. https://doi.org/10.3390/batteries9050259
Chicago/Turabian StyleEl Issmaeli, Youness, Amina Lahrichi, Shankara S. Kalanur, Sadesh Kumar Natarajan, and Bruno G. Pollet. 2023. "Recent Advances and Prospects of FeOOH-Based Electrode Materials for Supercapacitors" Batteries 9, no. 5: 259. https://doi.org/10.3390/batteries9050259
APA StyleEl Issmaeli, Y., Lahrichi, A., Kalanur, S. S., Natarajan, S. K., & Pollet, B. G. (2023). Recent Advances and Prospects of FeOOH-Based Electrode Materials for Supercapacitors. Batteries, 9(5), 259. https://doi.org/10.3390/batteries9050259