Abstract
Quinone organic materials are promising electrodes for the next lithium-ion batteries (LIBs) owing to their versatile molecular designs, high theoretical capacity, flexibility, sustainability, and environmental friendliness. However, quinone organic electrode materials can easily dissolve in organic electrolytes during the cycling process, which leads to the decay of capacity and poor cycling stability. Here, two metal-organic frames (MOFs), one-dimensional (1D) linear structural anthraquinone-2,3-dicarboxylate zinc coordination polymer (ZnAQDC) and two-dimensional (2D) structural anthraquinone-2,3-dicarboxylate manganese coordination polymer (MnAQDC), are synthesized by using anthraquinone 2,3-dicarboxylic acid, zinc acetate, and manganese acetate in a simple hydrothermal reaction. The formed 1D and 2D structures facilitate the insertion and extraction of lithium ions in and from carbonyl groups of anthraquinone. When MnAQDC is used as cathodes for LIBs, MnAQDC electrodes show an initial discharge capacity of ~63 mAh g−1 at 50 mA g−1. After 200 cycles, the MnAQDC electrode still maintains the specific capacity of ~45 mA h g−1, which exhibits good cycle stability. the ZnAQDC electrode displays a initial discharge capacity of ~85 mA h g−1 at 50 mA g−1, and retains the specific capacity of ~40 mA h g−1 after 200 cycles, showing moderate cyclic performance. The lithium-inserted mechanism shows that lithium ions are inserted and extracted in and from the carbonyl groups, and the valences of the Zn and Mn ions in the two MOFs do not change, and coordination metals do not contribute capacities for the two MOFs electrodes. The strategy of designing and synthesizing MOFs with 1D and 2D structures provides guidance for suppressing the dissolution and improving the electrochemical performance of quinone electrode materials.
1. Introduction
Lithium-ion batteries (LIBs) have been efficiently applied in portable devices, smart grids, and new energy vehicles owing to their high energy and power densities [1,2,3]. However, inorganic electrode materials for commercial LIBs have drawbacks, including low theoretical specific capacity, scarcity of mineral resources, and being unfriendly to the environment, which is difficult when meeting societal development needs. Organic electrode materials, with their designable structure, environmental friendliness, renewable resources, and high theoretical capacity, are regarded as promising electrode materials for next-generation lithium/sodium ion batteries [4,5]. Nevertheless, the active components of organic electrodes are gradually dissolved in organic electrolytes during the cycling process, resulting in a poor cycle life, low capacity, and rate capability. Among organic-based materials, metal–organic frameworks (MOFs) offer many physical and chemical advantages, including gas storage and selection [6,7], molecule recognition [8], enantioselective catalysis [9], and energy storage and conversion (such as application in LIBs) [10,11]. MOFs, as organic-based electrode materials for LIBs, can effectively restrain its solution in organic electrolyte and enhance the cycling stability of organic electrodes [12,13]. In addition, the preparation of MOFs generally involves a simple hydrothermal reaction. Also, higher specific capabilities can be gained by adjusting the active functional groups of the organic ligands. Importantly, the ligand and metal in the MOF structure have a significant impact on its electrochemical performance in the modification of MOFs electrodes for LIBs [14,15]. At the same time, the stability of the coordinated bonds with different coordination metals also influences the chemical and structural stability of the MOFs electrode. Therefore, adjusting the coordinated metal and ligand can well-modify the electrochemical performance of MOFs electrodes for LIBs. Many MOFs electrode materials with good charge and discharge performance for LIBs were synthesized according to the strategy [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36], especially using MOFs as cathodes for LIBs [25,26,27,28,29,30,31,32,33,34,35,36]. For example, a 2D conjugated copper–benzoquinone metal–organic framework with porosity and semiconducting features (Cu-THQ MOFs, THQ: tetrahydroxy-1,4-quinone), used as cathode for LIBs, was obtained by Jiang et al., which achieved a high capacity of 387 mA h g−1 in the second cycle at a current density of 50 mA g−1, corresponding to Coulombic efficiency of 90%, and maintained 340 mA h g−1 over 100 cycles [26]. The capacity contribution of Cu-THQ MOFs was ascribed to the insertion/extraction of lithium ion and the redox of the Cu ion (Supplementary Scheme S1a). Awaga synthesized a redox-active MOF (Cu(2,7-AQDC)) with 2,7-anthraquinonedicarboxylic acid as ligand. The as-prepared Cu(2,7-AQDC) with a 2D structure, used as cathode for LIBs, delivered an initial specific capacity of 147 mA h g−1, the capacity of 105 mA h g−1 could be maintained after 50 cycles at a current density of 1 mA. In addition, the coordinated metal Cu ion caused a redox reaction with a high potential of ~3.1 V and contributed to the capacity [27]. The high BET surface area (631 m2/g) and π–π interaction between the adjacent layers facilitated Li-ion intercalation and the structural stability of Cu(2,7-AQDC). At the same time, a Mn-based MOF (Mn(2,7-AQDC)) with the same ligand was also prepared by the Awaga group. Mn(2,7-AQDC) was used as cathode for LIBs, and demonstrated “bipolar charging” charge and discharge behavior, displaying a high first reversible capacity of 205 mA h g−1 at a current density of 1 mA resulting from the redox reaction of carbonyl groups (-C=O) of anthraquinone and Mn ions. After 50 cycles, only 7% capacity loss of its initial capacity was observed, exhibiting excellent cyclic stability [28]. Sakaushi et al., reported 2D MOFs-based cathode material (bis(diimino)nickel framework (NiDI)) for LIBs. The NiDI exhibited a reversible capacity of 155 mA h g−1 at a current density of 10 mA g−1, while both Li+ and PF6− participated in the insertion/desertion (Scheme S1b, supplementary materials), and exhibited remarkable cycling performance with ~50 mA h g−1 after 300 cycles at a current density of 250 mA g−1 [29]. A novel conductive MOFs, Cu3(2,3,6,7,10,11-hexahydroxytriphenylene)2 (Cu3(HHTP)2) as a cathode was synthesized by the Chen group, and displayed a reversible capacity of ~90 mA h g−1 at a current density of 1C and maintained the reversible capacity of ~60 mA h g−1 after 100 cycles [30]. The highly intrinsic electrical conductivity and two-dimensional porous structure greatly improved the electrochemical performance of the Cu3(HHTP)2 electrode.
Therefore, the electrochemical performance of MOFs electrodes are significantly influenced by the choice of various coordination metals and ligands. In this work, we select anthraquinone 2,3-dicarboxylic acid (H2AQDC) as the ligand, and zinc or manganese ions as the coordination metal to prepare MOF electrodes for LIBs. The reasons are as follows: first, anthraquinone is a renewable resource, and has high theoretical capacity and environmental friendliness advantages and so on, and is regarded as a promising electrode material for next-generation LIBs. However, the dissolution of anthraquinone as an electrode for LIBs needs to be solved during the charge and discharge process. Second, zinc and manganese ions are transition elements and are easily coordinated by H2AQDC. Therefore, using the advantages of the combination of anthraquinone and transition elements, one-dimensional (1D) linear-structured ZnAQDC MOFs and two-dimensional (2D) structured MnAQDC MOFs were prepared by hydrothermal reaction between H2AQDC and zinc acetate or manganese acetate to assess the effects of coordination metals and the topology structure on the electrochemical performance of MOFs electrodes. Importantly, ZnAQDC and MnAQDC are firstly used as cathodes for LIBs.
2. Materials and Methods
2.1. Synthesis of Anthraquinone 2,3-Dicarboxylate Zinc (or Manganese) Coordination Polymer (ZnAQDC, or MnAQDC)
ZnAQDC and MnAQDC were synthesized according to the reference [37]. Typically, 0.02 mmol Zn(CH3COO)2.2H2O) (or 0.02 mmol (Mn(CH3COO)2.4H2O)) was added in 5 mL of deionized water in a glass bottle. Then, 0.02 mmol anthraquinone-2,3-dicarboxylic acid (H2AQDC) was introduced in the solution and was sealed in a glass bottle and maintained under autogenous pressure at 90 °C for 48 h with a heating rate of 3 °C/min. The solution was cooled to room temperature at a cooling rate of 1 °C/min, and yellow crystals were gained in an output of 28% and 31% for anthraquinone-2,3-dicarboxylate zinc coordination polymer (ZnAQDC) and anthraquinone-2,3-dicarboxylate manganese coordination polymer (MnAQDC), respectively, and were dried in a vacuum-drying oven at 60 °C for 24 h, then, ZnAQDC and MnAQDC were obtained. Elemental analysis calcd (%) for MnAQDC: MnC16H14O10 (421.21): calcd C 45.58, H 3.32; found C 45.45, H 3.42. Elemental analysis calcd (%) for ZnAQDC: ZnC16H14O10 (431.66): calcd C 44.48, H 3.24; found C 44.35, H 3.16.
2.2. Material Characterization
The single-crystal diffraction measurements of ZnAQDC and MnAQDC were conducted by on a Bruker Apex II CCD diffractometer equipped with a sealed tube X-ray source (Mo Ka radiation (λ = 0.71073 Å)). APEX II software was used to collect and reduce single-crystal data [38,39]. The structures of ZnAQDC and MnAQDC were solved by direct methods, and then were refined against |F|2 using the SHELXL software package [38,39].
Morphological information and elemental composition of the samples were collected using field-emission scanning electron microscopy (FESEM, ZEISSUltra 55, 5 kV, Pt spray treatment, Carl Zeiss, Jena, Germany), transmission electron microscopy (TEM, JEM-2100HR, 200 kV, Jeol, Tokyo, Japan), and energy-dispersive X-ray spectroscopy (EDS, IET250), respectively. The crystal structure was detected using X-ray diffraction (XRD) (BRUKER D8 ADVANCE, Cu Ka radiation (λ = 1.5406 Å), Bruker, Billerica, MA, USA). Functional groups of the samples were distinguished using Shimadzu Fourier-transform infrared spectrometer (FTIR Prestige-21, Shimadzu Corp., Tokyo, Japan), while thermal stability of the samples was evaluated by thermogravimetric analysis (TGA) (Perkin-Elmer TGA 7 thermogravimetric analyzer, Perkin-Elmer, Waltham, MA, USA). In addition, solubility of the samples was carried out using a double beam UV−vis spectrophotometer SPECORD210 (Analytik Jena, Jena, Germany).
2.3. Electrochemical Characterization
Electrochemical properties were evaluated using coin-type cells, which were fabricated inside an argon-filled glove-box. The working electrodes were prepared following the procedures: grounding ZnAQDC (or MnAQDC), acetylene black, and polyvinylidene fluoride (PVDF) with a mass ratio of 60:30:10 for 1 h, while N-methyl-2-pyrrolidone (NMP) solution was added dropwise with continued grinding for 0.5 h. The slurry was then coated onto aluminum foil, which was dried in a vacuum oven at 80 °C for 12 h, followed by cutting into discs with an area of 1 cm2. The average load of active material on the electrode was controlled at 1.2∼1.5 mg; 1M LiTFSI-DOL+DME (1/1, v/v) was used as electrolyte. Charge/discharge experiments were carried on Land cell test system (Land CT 2001A), a potential range of 1.2∼3.6 V was used at current densities ranging from 10 to 500 mA g−1. Cyclic voltammetry (CV) was performed on a CHI660D equipment, while a potential range of 1.2∼3.6 V and a scanning rate of 0.1 mV s−1 were used. Electrochemical impedance spectra were collected on an AUT72630 (Autolab) in a frequency range of 0.1∼100,000 Hz.
3. Results and Discussion
3.1. Structural Characterization of MnAQDC and ZnAQDC
As shown in Supplementary Table S1, showing the crystal structure information of the ZnAQDC and MnAQDC, it can be seen that both ZnAQDC and MnAQDC crystallize in the triclinic space group P-1. As illustrated in Figure 1a, showing the coordination environment diagram of MnAQDC structural units, each Mn2+ is connected by six oxygen atoms, including four oxygen atoms that are from the carboxyl groups of four H2AQDC ligands, while two oxygen atoms are from two H2O molecules. Mn ions are bridged by carboxyl of H2AQDC ligands to form one-dimensional (1D) secondary building units (SBUs); those SBUs are further linked by H2AQDC ligands to form a two-dimensional structure (Figure 1c). Figure 1b shows the coordination environment diagram of the structural units of ZnAQDC. It can be seen from the diagram that each Zn2+ is coordinated by three oxygen atoms from three H2AQDC ligands, and two oxygen atoms from two H2O molecules. The structural unit, which consists of three H2AQDC ligands and one Zn2+, is interconnected to form a one-dimensional linear structure (Figure 1d). Both ZnAQDC and MnAQDC display 1D and 2D structures that facilitate the diffusion and insertion/extraction of lithium ions.
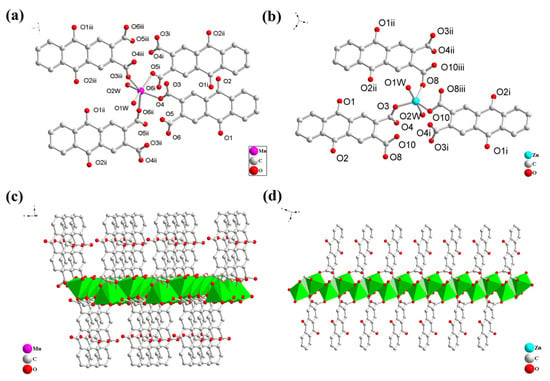
Figure 1.
Structural unit coordination environment diagram of MnAQDC (MnC16H10O8·2(H2O)), symmetry codes: i (1 + x, y, z), ii (1 − x, 2 − y, 2 − z), iii (2 − x, 1 − y, 2 − z) (a) and ZnAQDC (ZnC16H10O8·2(H2O)), symmetry codes: i (1 − x, 1 − y, 1 − z), ii (x, −1 + y, z), iii (1 − x, −y, 1 − z) (b); 2D coordination framework of MnAQDC (c) and 1D ZnAQDC (d) (all H atoms and guest water were omitted for clarity).
To further analyze the structure of the two samples, infrared spectroscopic tests were performed. As shown in Supplementary Figure S1, FT-IR exhibits the vibration of -O-H of -COOH for H2AQDC between 2500∼3200 cm−1, and the peaks of 1136 and 1175 cm−1 correspond to the vibration of -C-O of H2AQDC. After the reactions of H2AQDC and Mn(CH3COO)2 or Zn(CH3COO)2, the characteristic peaks at ~1136 and ~1175 cm−1 and ~2500∼3200 cm−1 disappear, forming O–Mn or O–Zn coordination bonds, which indicate that MnAQDC and ZnAQDC single crystal samples are successfully prepared. It is worth noting that at a new peak at 3200–3500 cm−1 appears, indicating the existence of H2O molecules. To analyze the purity of the as-prepared MnAQDC and ZnAQDC, it can be seen from Supplementary Figure S2, powder XRD patterns, that the characteristic peaks of MnAQDC and ZnAQDC are well-matched with the diffraction peaks of the standard XRD patterns for MnAQDC and ZnAQDC, indicating that the synthesized samples have high purity.
In order to analyze the morphology of MnAQDC and ZnAQDC samples, as can be seen from Supplementary Figure S3, SEM images, it is found that both MnAQDC and ZnAQDC samples are composed of nano-sphere particles, which are aggregated to form micron-level secondary block structures with diameters of 1∼10 µm through grinding. As shown in Supplementary Figure S4, TEM images, both MnAQDC and ZnAQDC have a smooth surface, and the diameter of the two samples are almost consistent with the results of the SEM images in Supplementary Figure S3. Furthermore, the two samples have a set of well-defined spots, indicative of the single-crystallinity of ZnAQDC and MnAQDC (Supplementary Figure S5). To further explore the element composition of MnAQDC and ZnAQDC, the mapping and EDS tests were conducted for MnAQDC and ZnAQDC (Supplementary Figures S6–S8). It is found that the mapping results show the uniform distribution of C, O, and Mn elements in MnAQDC, and C, O, and Zn elements in ZnAQDC (Supplementary Figures S6 and S7). EDS in Supplementary Figure S8 further confirms the presence of C, O, Mn, and Zn elements in the MnAQDC and ZnAQDC samples. TGA curves show MnAQDC and ZnAQDC begin to decompose above 400 °C (Supplementary Figure S9), revealing both MnAQDC and ZnAQDC have good thermal stability. X-ray crystallographic diffraction, IR, power XRD, SEM, mapping and EDS tests show that the 2D MnAQDC and 1D ZnAQDC with high-purity are successfully prepared by H2AQDC and manganese acetate and zinc acetate.
3.2. Electrochemical Performance of MnAQDC and ZnAQDC
To study the lithium-inserted mechanism of MnAQDC and ZnAQDC samples, the initial CV curves of MnAQDC and ZnAQDC electrodes were obtained. From Figure 2a,b, it is found that both MnAQDC and ZnAQDC have a pair of obvious redox peaks, which are ~2.3/~2.5 V and ~2.3/~2.4 V, respectively, corresponding to the intercalation/de-intercalation of lithium ions on the carbonyl groups of MnAQDC and ZnAQDC, respectively (Figure 2c). However, there are some differences between MnAQDC and ZnAQDC. The CV curves of MnAQDC almost overlap from the second to fifth cycles, while the peak current of ZnAQDC tends to increase with the increase in cycle number, which may be the infiltration of electrode material in the electrolyte and an increase in active sites during the cycling process. It can be seen from the initial CV curves of Figure 2 that the insertion/de-insertion of lithium ions are very reversible in MnAQDC and ZnAQDC electrodes. It should be emphasized that the two samples have only one oxidation peak and one reduction peak in the range of 2.2∼2.5 V. However, there is no redox peak in the higher potential range. Therefore, it can be inferred that there is no valence change in the Mn and Zn ions in the two samples. In addition, the initial CV curves of the H2AQDC ligand was also obtained (Supplementary Figure S10). Obviously, the electrochemical behavior of H2AQDC is similar to that of MnAQDC and ZnAQDC during the initial cycling process, including the value of redox peaks and the reversibility of the insertion/extraction of the lithium ion.
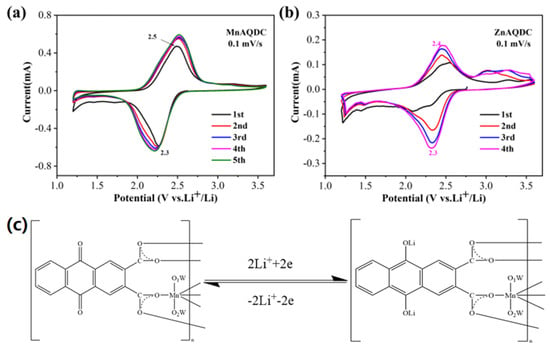
Figure 2.
CV curves of MnAQDC and ZnAQDC at a scan rate of 0.1 mV s−1, MnAQDC (a), ZnAQDC (b); schematic diagram for the proposed insertion/extraction of lithium ion in MnAQDC (c).
Compared with valence state change in Zn, Mn ions easily bring about valence state change. Therefore, X-ray photoelectron spectroscopy (XPS) tests were conducted to analyze the valence state changes in the Mn ion in the charge and discharge states for MnAQDC. Figure 3a shows the overall characteristic peaks of Mn, C, and O for MnAQDC, which is consistent with the elemental composition of EDS (Supplementary Figure S8). Before charge and discharge of MnAQDC electrode (pristine MnAQDC), the spectra of Mn 2p in MnAQDC exhibits the characteristic peaks of Mn 2p1/2 at 653.2 eV and Mn 2p3/2 at 641.3 eV with an energy separation (ΔE) of 11.90 eV, and the satellite peaks at ∼646.6 eV (Figure 3b). These peaks show the +2 valence state of the Mn element in MnAQDC [40]. After three cycles, the peak shift of Mn 2p, as well as the satellite peaks, do not bring about change in the charge and discharge states (Figure 3b). the energy separation (ΔE) of the charge and discharge states are 11.86 and 11.89 eV, respectively, which is close to the ΔE before cycle, indicating the valence state of the Mn ion does not change.
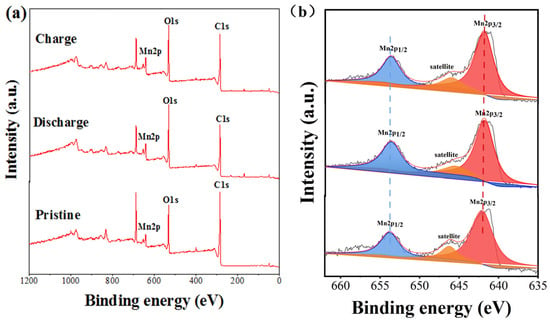
Figure 3.
(a) XPS spectra of MnAQDC before and after charge/discharge states; (b) XPS spectra of Mn 2p of MnAQDC before and after charge/discharge states.
To investigate the cycling performance and rate capability of the MnAQDC, the charging and discharging performance of the MnAQDC was tested at different current densities. As can be seen from Figure 4a, MnAQDC delivers first discharge and charge capacities of ~63 and 38 m Ah g−1 at a current density of 50 mA g−1, respectively, corresponding to a Coulomb efficiency of 60%. The discharge capacity increase gradually and can reach ~58 mA h g−1 after 60 cycles. Then, the capacity decays gradually, and the reversible capacity of MnAQDC maintains ~45 mAh g−1 with a capacity retention of 78% after 200 cycles, corresponding to a Coulomb efficiency of 100% (Figure 4b). The capacity decays gradually between 60 and 140 cycles, which may be the decomposition of electrolyte. It is worth noting that the discharge capacity of MnAQDC does not decay after 200 cycles, exhibiting good cyclic stability, which is better that of the reported references [31,33,34]. The good cycling performance can be ascribed to the 2D structure of MnAQDC. In addition, the discharge and charge platform of MnAQDC with 2.24 V and 2.51 V are exactly consistent with the CV curves (Figure 2a), and the difference between the discharge and charge platforms almost does not change with the increase in cyclic number, which indicates good reversibility of MnAQDC. The rate performance of MnAQDC was also tested at current densities varying from 50 to 500 mA g−1 (Figure 4c). At the current densities of 50, 100, 200, and 500 mA g−1, MnAQDC delivers specific capacities of ~75, ~60, ~50, and ~33 mA h g−1, respectively. To our surprise, the capacity can increase to ~110 mA h g−1 when the current density returns to 50 mA g−1, corresponding to 79% of theoretical capacity (theoretical capacity of C16H10O8Mn: 139 mAh g−1), exhibiting a remarkable rate performance. Figure 4d displays the charge and discharge curves at different current densities of MnAQDC, and it is found that the difference between the charge and discharge platform increase gradually, the polarization enlarges, and the capacities reduce gradually with the increase in current density.
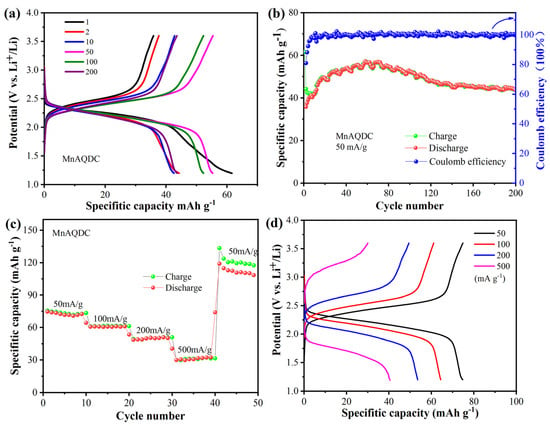
Figure 4.
Charge and discharge performance of MnAQDC, (a) charge and discharge performance at a current density of 50 mA g−1; (b) cycling performance at a current density of 50 mA g−1; (c) rate performance; (d) charge-discharge curves under different current density.
Having investigated the charge-discharge performance of MnAQDC, we continued to study the charge/discharge performance of the ZnAQDC electrode. As shown in Figure 5a, the ZnAQDC electrode displays initial discharge and charge capacities of 85/68 mA h g−1, respectively, at a current density of 50 mA g−1, corresponding to a Coulomb efficiency of 80%. The capacity decays gradually during the charge and discharge process and can be observed at ~40 mA h g−1 after 200 cycles (Figure 5b), with capacity retention of 47%, exhibiting moderate cycling stability. The capacity decay for ZnAQDC may be ascribed to the decomposition of the electrolyte and the dissolution of ZnAQDC. It is found that the cycling performance and capacity retention of ZnAQDC is poor compared with that of MnAQDC. Generally, the 2D structure is more stable than the 1D structure. Therefore, the 2D structure of MnAQDC is not easily destroyed in comparison with the 1D structure of ZnAQDC during the process of the insertion/removal of lithium ions to and from the carbonyl groups. It can be seen from Figure 5a that the ZnAQDC electrode displays a discharging plateau of 2.0 V in the first cycle, and then the discharging plateau shifts to 2.30 V during the subsequent cycles, which is in line with the results of above CV curves in Figure 2b. The rate performance of ZnAQDC is further investigated. As shown in Figure 5c, the current density increases from 50 to 200 mA g−1, the discharge capacity can still maintain 80~85 mA h g−1, and achieve ~55 mA h g−1 in a high current density of 500 mA g−1. Interestingly, the reversible capacity increases to ~95 mA h g−1 when the current density reduces to 50 mA g−1, corresponding to 70% of theoretical capacity (theoretical capacity of C16H10O8Zn: 136 mA h g−1). Also, the difference between charge and discharge platform overall increases with the increase in current density (Figure 5d).
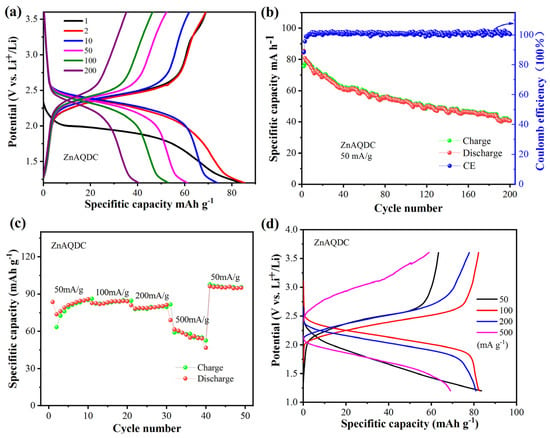
Figure 5.
Charge and discharge performance of ZnAQDC, (a) charge and discharge performance at a current density of 50 mA g−1; (b) cycling performance at a current density of 50 mA g−1; (c) rate performance; (d) charge–discharge curves under different current density.
To confirm the good charge and discharge performance of MnAQDC and ZnAQDC electrodes during the cycling process, electrochemical impedance spectroscopy (EIS) was carried out (Supplementary Figure S11). It can be seen that the charge transfer resistance (Rct) of MnAQDC and ZnAQDC are overall small after 5 and 10 cycles, indicating the 2D structure of MnAQDC and the 1D chain structure ZnAQDC facilitate the uptake and removal of lithium ions. Supplementary Figure S12 shows the solubility test of MnAQDC and ZnAQDC. It is obvious that the color of MnAQDC almost does not change, and does not dissolve in the electrolyte (1M LiTFSI-DOL + DME (1/1, v/v)) after 0, 1, and 7 days. However, the color of the H2AQDC ligand and ZnAQDC significantly change. This reveals that the formation of MnAQDC MOFs with 2D structures can well inhibit the dissolution of H2AQDC in the electrolyte, and that the 2D structure of MnAQDC is more stable than the 1D chain of ZnAQDC. The dissolution of MnAQDC, ZnAQDC, and AQDC in the electrolyte are further analyzed by UV−vis spectroscopy (Supplementary Figure S13). It is found that the absorption peak intensities of MnAQDC and ZnAQDC are lower than that of H2AQDC in the absorption wavelength range from 200 to 400 nm at different times, verifying MnAQDC and ZnAQDC MOFs are difficult to dissolve in the organic electrolyte. However, the absorption peak intensity of ZnAQDC is higher than that of MnAQDC, showing that the 1D chain structure of ZnAQDC is unstable in comparison with MnAQDC with a 2D structure. ZnAQDC is easily dissolved in the organic electrolyte compared with to MnAQDC, which is in line with the results of Supplementary Figure S12 about the solubility test experiment.
The H2AQDC ligand was selected and reacted with zinc acetate and manganese acetate to obtain ZnAQDC and MnAQDC MOFs, respectively. The 1D chain ZnAQDC and 2D structure MnAQDC aid the lithiation and de-lithiation of lithium ions. However, the 2D structure of MnAQDC is more stable than the 1D chain of ZnAQDC. This makes it difficult for MnAQDC to dissolve in organic electrolyte compared with ZnAQDC, and the dissolution investigation of ZnAQDC and MnAQDC in Supplementary Figures S12 and S13 (supplementary materials) also confirms the results. Therefore, the cyclic stability and the capacity retention of the 2D structure MnAQDC are better than that of the 1D chain ZnAQDC during the cycling process. It should be noted the valence of Zn2+ and Mn2+ does not change and does not contribute to the capacity, which is conducive to retaining the stability of the MOFs structure and enhancing its physical and chemical properties during the charge and discharge process. By preparing the MOFs coordination polymer structure with different metal coordination, the dissolution can be well-suppressed and a good charge/discharge performance of anthraquinone can be achieved. However, compared with the theoretical and practical capacities of conventional cathodes (Supplementary Table S2), such as LiCoO2, LiNi0.90Mn0.04Co0.05Mg0.01O2, and LiFePO4, ZnAQDC and MnAQDC present low theoretical and practical capacities. The next work is to introduce -C=O groups and redox transition metal ions to increase the theoretical and practical capacities of anthraquinone derivatives.
4. Conclusions
In summary, anthraquinone-2,3 dicarboxylic acid ligand was used to react with zinc acetate and manganese acetate to obtain 1D ZnAQDC and 2D MnAQDC MOFs. Compared with the ZnAQDC electrode, MnAQDC displays a good cycling performance when used as cathodes for LIBs. Quinones are small molecules that are designed to synthesize MOFs, and the MOFs can effectively inhibit the dissolution of quinone electrode materials in organic electrolyte and enhance the charge and discharge performance of quinone electrodes. The synthesized strategy is simple and effective, and can also applied to improve the electrochemical performance of other organic materials.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/batteries9050247/s1, Table S1: Crystallographic data and structure refinement for MnAQDC and ZnAQDC; Table S2: Crystallographic data and structure refinement for MnAQDC and ZnAQDC; Scheme S1: The mechanism of the insertion/extraction of lithium ion in 2D Cu-THQ MOF electrode; Scheme S2: The mechanism of the insertion/extraction of lithium ion in NiDI electrode; Figure S1: FTIR spectra of MnAQDC, ZnAQDC, and H2AQDC; Figure S2: Single crystal standard XRD patterns and powder XRD patterns of MnAQDC and ZnAQDC; Figure S3: SEM images of MnAQDC and ZnAQDC; Figure S4: TEM images of MnAQDC and ZnAQDC; Figure S5: Selected sample (a) and SAED (b) for ZnAQDC; Selcted sample (c) and SAED (d) for MnAQDC; Figure S6: Mapping images of MnAQDC; Figure S7: Mapping images of ZnAQDC; Figure S8: EDS patterns of MnAQDC and ZnAQDC; Figure S9: TGA curves of ZnAQDC and MnAQDC; Figure S10: The CV curves of H2AQDC; Figure S11: EIS of MnAQDC and ZnAQDC after 0, 5, 10 cycles; Figure S12: The dissolution experiment of MnAQDC and ZnAQDC in the electrolyte composed of 1M LiTFSI-DOL + DME (1/1, v/v) after 0 day, 1 day, and 7 days; Figure S13: UV–vis spectra of AQDC, MnAQDC, and ZnAQDC in the electrolyte (1M LiTFSI-DOL + DME (VDOL:VDME = 1:1)) [41,42,43,44,45,46].
Author Contributions
M.L.: conceptualization, investigation, data curation, wrote draft of manuscript; D.Z.: conceptualization, investigation, wrote draft of manuscript; F.C.: methodology, formal analysis, project administration; X.L.: formal analysis, data curation; A.Q.: methodology, data curation; C.L.: project administration, formal analysis; J.L. (Jiaying Liang): validation, formal analysis; J.L. (Junfeng Liang): validation, visualization; J.L. (Jianhui Li): software, visualization; Q.W.: visualization, software; R.Z.: resources, conceptualization, writing—review and editing, investigation, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 21875076), the Science and Technology Planning Project of Guangzhou City (2023B03J1278), Special Funds for the Cultivation of Guangdong College Students’ Scientific and Technological Innovation (“Climbing Program” Special Funds) (pdjh2022a0128), the Undergraduates’ Innovating Experimentation Project of Guangdong Province (No. S202210574021), and the Undergraduates’ Innovating Experimentation Project of South China Normal University (No. 202324017).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lee, M.J.; Han, J.; Lee, K.; Lee, Y.J.; Kim, B.G.; Jung, K.-N.; Kim, B.J.; Lee, S.W. Elastomeric Electrolytes for High-Energy Solid-State Lithium Batteries. Nature 2022, 601, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-L.; Zhao, X.-X.; Li, W.-H.; Liang, H.-J.; Gu, Z.-Y.; Liu, Y.; Du, M.; Wu, X.-L. Advanced Cathode for Dual-ion Batteries: Waste-to-Wealth Reuse of Spent Graphite from Lithium-Ion Batteries. eScience 2022, 2, 95–101. [Google Scholar] [CrossRef]
- Huang, T.; Gao, H.; Chen, J.; Liu, H.; Wu, D.; Wang, G. A Book-Like Organic Based Electrode with High Areal Capacity for High Performance Flexible Lithium/Sodium-Ion Batteries. Chem. Commun. 2022, 58, 10158–10161. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Dai, H.; Zhu, S.; Wu, Y.; Sun, M.; Chen, Y.; Fan, K.; Zhang, C.; Wang, C. A Branched Dihydrophenazine-Based Polymer as a Cathode Material to Achieve Dual-Ion Batteries with High Energy and Power Density. eScience 2021, 12, 60–68. [Google Scholar] [CrossRef]
- Khetan, A. High-Throughput Virtual Screening of Quinones for Aqueous Redox Flow Batteries: Status and Perspectives. Batteries 2023, 9, 24. [Google Scholar] [CrossRef]
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to Metal–Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef]
- Kosaka, W.; Yamagishi, K.; Hori, A.; Sato, H.; Matsuda, R.; Kitagawa, S.; Takata, M.; Miyasaka, H. Selective NO Trapping in the Pores of Chain-Type Complex Assemblies Based on Electronically Activated Paddlewheel-Type [Ru2II,II]/[Rh2II,II] Dimers. J. Am. Chem. Soc. 2013, 135, 18469–18480. [Google Scholar] [CrossRef]
- Inokuma, Y.; Yoshioka, S.; Ariyoshi, J.; Arai, T.; Hitora, Y.; Takada, K.; Matsunaga, S.; Rissanen, K.; Fujita, M. X-ray Analysis on the Nanogram to Microgram Scale Using Porous Complexes. Nature 2013, 495, 461–466. [Google Scholar] [CrossRef]
- Cho, S.-H.; Ma, B.; Nguyen, S.B.T.; Hupp, J.T.; Albrecht-Schmitt, T.E. a Metal–Organic Framework Material That Functions as an Enantioselective Catalyst for Olefin Epoxidation. Chem. Commun. 2006, 24, 2563–2565. [Google Scholar] [CrossRef]
- Li, X.; Cheng, F.; Zhang, S.; Chen, J. Shape-Controlled Synthesis and Lithium-Storage Study of Metal-Organic Frameworks Zn4O(1,3,5-benzenetribenzoate)2. J. Power Sources 2006, 160, 542–547. [Google Scholar] [CrossRef]
- Combarieu, G.D.; Morcrette, M.; Millange, F.; Guillou, N.; Cabana, J.; Grey, C.; Margiolaki, I.; Férey, G.; Tarascon, J. Influence of the Benzoquinone Sorption on the Structure and Electrochemical Performance of the MIL-53(Fe) Hybrid Porous Material in a Lithium-Ion Battery. Chem. Mater. 2009, 21, 1602–1611. [Google Scholar] [CrossRef]
- Reddy, R.C.K.; Lin, J.; Chen, Y.; Zeng, C.; Lin, X.; Cai, Y.; Su, C.-Y. Progress of Nanostructured Metal Oxides Derived from Metal–Organic Frameworks as Anode Materials for Lithium–Ion Batteries. Coord. Chem. Rev. 2020, 420, 213434. [Google Scholar] [CrossRef]
- Baumann, A.E.; Burns, D.A.; Liu, B.; Thoi, V.S. Metal-Organic Framework Functionalization and Design Strategies for Advanced Electrochemical Energy Storage Devices. Commun. Chem. 2019, 2, 86. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, Z.; Zou, R.; Xu, Q. Metal-Organic Frameworks for Batteries. Joule 2018, 2, 2235–2259. [Google Scholar] [CrossRef]
- Huang, Y.; Fang, C.; Zeng, R.; Liu, Y.; Zhang, W.; Wang, Y.; Liu, Q.; Huang, Y. In Situ-Formed Hierarchical Metal–Organic Flexible Cathode for High-Energy Sodium-Ion Batteries. ChemSusChem 2017, 10, 4704–4708. [Google Scholar] [CrossRef]
- An, T.; Wang, Y.; Tang, J.; Wang, Y.; Zhang, L.; Zheng, G. a Flexible Ligand-Based Wavy Layered Metal–Organic Framework for Lithium-Ion Storage. J. Colloid Interface Sci. 2015, 445, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cheng, F.; Shi, W.; Chen, J.; Cheng, P. Transition-Metal-Triggered High-Efficiency Lithium Ion Storage via Coordination Interactions with Redox-Active Croconate in One-Dimensional Metal–Organic Anode Materials. ACS Appl. Mater. Interfaces 2018, 10, 6398–6406. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, M.; Luo, Y.; Pang, B.; Su, X.; Zhou, M.; Han, L. Redox Active Azo-Based Metal–Organic Frameworks as Anode Materials for Lithium-Ion Batteries. New J. Chem. 2019, 43, 1710–1715. [Google Scholar] [CrossRef]
- Huang, Q.; Wei, T.; Zhang, M.; Dong, L.-Z.; Zhang, A.-M.; Li, S.-L.; Liu, W.-J.; Liu, J.; Lan, Y.-Q. A Highly Stable Polyoxometalate-Based Metal–Organic Framework with π–π Stacking for Enhancing Lithium Ion Battery Performance. J. Mater. Chem. A 2017, 5, 8477–8483. [Google Scholar] [CrossRef]
- Sun, L.; Xie, J.; Chen, Z.; Wu, J.; Li, L. Reversible Lithium Storage In a Porphyrin-Based MOF (PCN-600) with Exceptionally High Capacity and Stability. Dalton Trans. 2018, 47, 9989–9993. [Google Scholar] [CrossRef]
- Wang, P.; Lou, X.; Li, C.; Hu, X.; Yang, Q.; Hu, B. One-Pot Synthesis of Co-Based Coordination Polymer Nanowire for Li-Ion Batteries with Great Capacity and Stable Cycling Stability. Nanomicro Lett. 2017, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.; Pramanik, A.; Manju, U.; Mahanty, S. Reversible Lithium Storage in Manganese 1,3,5-Benzenetricarboxylate Metal-Organic Framework with High Capacity and Rate Performance. ACS Appl. Mater. Interfaces 2015, 7, 16357–16363. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Shen, L.; Wang, J.; Wang, C. Reversible Lithiation–Delithiation Chemistry in Cobalt Based Metal Organic Framework Nanowire Electrode Engineering for Advanced Lithium-Ion Batteries. J. Mater. Chem. A 2016, 4, 15411–15419. [Google Scholar] [CrossRef]
- Gan, Q.; He, H.; Zhao, K.; He, Z.; Liu, S. Morphology-Dependent Electrochemical Performance of Ni-1,3,5-Benzenetricarboxylate Metal-Organic Frameworks as an Anode Material for Li-ion Batteries. J. Colloid Interface Sci. 2018, 530, 127–136. [Google Scholar] [CrossRef]
- Meng, C.; Chen, T.; Fang, C.; Huang, Y.; Hu, P.; Tong, Y.; Bian, T.; Zhang, J.; Wang, Z.; Yuan, A. Multiple Active Sites: Lithium Storage Mechanism of Cu-TCNQ as an Anode Material for Lithium-Ion Batteries. Chem. Asian J. 2019, 14, 4289–4295. [Google Scholar] [CrossRef]
- Jiang, Q.; Xiong, P.; Liu, J.; Xie, Z.; Wang, Q.; Yang, X.-Q.; Hu, E.; Cao, Y.; Sun, J.; Xu, Y.; et al. a Redox-Active 2D Metal–Organic Framework for Efficient Lithium Storage with Extraordinary High Capacity. Angew. Chem. Int. Ed. 2020, 59, 5273–5277. [Google Scholar] [CrossRef]
- Zhang, Z.; Yoshikawa, H.; Awaga, K. Monitoring the solid-state electrochemistry of Cu(2,7-AQDC) (AQDC = anthraquinone dicarboxylate) in a lithium battery: Coexistence of metal and ligand redox activities in a metal-organic framework. J. Am. Chem. Soc. 2014, 136, 16112–16115. [Google Scholar] [CrossRef]
- Zhang, Z.; Yoshikawa, H.; Awaga, K. Discovery of a “Bipolar Charging” Mechanism in the Solid-State Electrochemical Process of a Flexible Metal–Organic Framework. Chem. Mater. 2016, 28, 1298–1303. [Google Scholar] [CrossRef]
- Wada, K.; Sakaushi, K.; Sasaki, S.; Nishihara, H. Multielectron-Transfer-Based Rechargeable Energy Storage of Two-Dimensional Coordination Frameworks with Non-Innocent Ligands. Angew. Chem. Int. Ed. 2018, 57, 8886–8890. [Google Scholar] [CrossRef]
- Gu, S.; Bai, Z.; Majumder, S.; Huang, B.; Chen, G. Conductive Metal–Organic Framework with Redox Metal Center as Cathode for High Rate Performance Lithium Ion Battery. J. Power Sources 2019, 429, 22–29. [Google Scholar] [CrossRef]
- Shin, J.; Kim, M.; Cirera, J.; Chen, S.; Halder, G.J.; Yersak, T.A.; Paesani, F.; Cohen, S.M.; Meng, Y.S. MIL-101(Fe) as a Lithium-ion Battery Electrode Material: Relaxation and Intercalation Mechanism During Lithium Insertion. J. Mater. Chem. A 2015, 3, 4738–4744. [Google Scholar] [CrossRef]
- Férey, G.; Millange, F.; Morcrette, M.; Serre, C.; Doublet, M.-L.; Grenèche, J.-M.; Tarascon, J.-M. Mixed-Valence Li/Fe-Based Metal-Organic Frameworks with Both Reversible Redox and Sorption Properties. Angew. Chem. Int. Ed. 2007, 119, 3323–3327. [Google Scholar] [CrossRef]
- Tian, B.; Ning, G.H.; Gao, Q.; Tan, L.M.; Tang, W.; Chen, Z.; Su, C.; Loh, K.P. Crystal Engineering of Naphthalenediimide-Based Metal-Organic Frameworks: Structure-Dependent Lithium Storage. ACS Appl. Mater. Interfaces 2016, 8, 31067–31075. [Google Scholar] [CrossRef]
- Fateeva, A.; Horcajada, P.; Devic, T.; Serre, C.; Marrot, J.; Grenèche, J.M.; Morcrette, M.; Tarascon, J.M.; Maurin, G.; Férey, G. Synthesis, Structure, Characterization, and Redox Properties of the Porous Mil-68(Fe) Solid. Eur. J. Inorg. Chem. 2010, 2010, 3789–3794. [Google Scholar] [CrossRef]
- Yamada, T.; Shiraishi, K.; Kitagawa, H.; Kimizuka, N. Applicability of Mil-101(Fe) as a Cathode of Lithium Ion Batteries. Chem. Commun. 2017, 53, 8215–8218. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Yi, X.; Liu, Z.; Shang, J.; Wang, D. Triphenylamine-Based Metal-Organic Frameworks as Cathode Materials in Lithium-Ion Batteries with Coexistence of Redox Active Sites, High Working Voltage, and High Rate Stability. ACS Appl. Mater. Interfaces 2016, 8, 14578–14585. [Google Scholar] [CrossRef] [PubMed]
- Furman, J.D.; Burwood, R.P.; Tang, M.; Mikhailovsky, A.A.; Cheetham, A.K. Understanding Ligand-Centred Photoluminescence through Flexibility and Bonding of Anthraquinone Inorganic–Organic Frameworks. J. Mater. Chem. 2011, 21, 6595–6601. [Google Scholar] [CrossRef]
- Bruker AXS Inc. APEXII Software, Version 6.12; Bruker AXS Inc.: Madison, WI, USA, 2004. [Google Scholar]
- Sheldrick, G.M. SHELXL-97, Program for X-ray Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Maiti, S.; Pramanik, A.; Dhawa, T.; Sreemany, M.; Mahanty, S. Bi-Metal Organic Framework Derived Nickel Manganese Oxide Spinel for Lithium-Ion Battery Anode. Mater. Sci. Eng. B 2018, 229, 27–36. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Z.; Hu, X.; Tian, H.; Zhang, Y.; Yang, X. Realization of Ti Doping by Electrostatic Assembly to Improve the Stability of LiCoO2 Cycled to 4.5 V. J. Electrochem. Soc. 2019, 166, A1793–A1798. [Google Scholar] [CrossRef]
- Gomez-Martin, A.; Reissig, F.; Frankenstein, L.; Heidbüchel, M.; Winter, M.; Placke, T.; Schmuch, R. Magnesium Substitution in Ni-Rich NMC Layered Cathodes for High-Energy Lithium Ion Batteries. Adv. Energy Mater. 2022, 12, 2103045. [Google Scholar] [CrossRef]
- Zhao, M.; Fu, Y.; Xu, N.; Li, G.; Wu, M.; Gao, X.J. High Performance LiMnPO4/C Prepared by a Crystallite Size Control Method. J. Mater. Chem. A 2014, 2, 15070–15077. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, Y.; Liu, Y.; Luo, C.; Wang, C. Comparison of Electrochemical Performances of Olivine Nafepo4 in Sodium-Ion Batteries And Olivine Lifepo4 In Lithium-Ion Batteries. Nanoscale 2013, 5, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Pagot, G.; Bandiera, M.; Vezzù, K.; Migliori, A.; Bertoncello, R.; Negro, E.; Morandi, V.; Di Noto, V. High Valence Transition Metal-Doped Olivine Cathodes for Superior Energy and Fast Cycling Lithium Batteries. J. Mater. Chem. A 2020, 8, 25727–25738. [Google Scholar] [CrossRef]
- Amine, K. Olivine LiCoPO4 as 4.8 V Electrode Material for Lithium Batteries. Electrochem. Solid-State Lett. 1999, 3, 178. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).