Abstract
Due to a high energy density and satisfactory longevity, lithium-ion batteries (LIBs) have been widely applied in the fields of consumer electronics and electric vehicles. Cathodes, an essential part of LIBs, greatly determine the energy density and total cost of LIBs. In order to make LIBs more competitive, it is urgent to develop low-cost commercial cathode materials. Among all cathode materials, Mn-based cathode materials, such as layered LiNi0.5Mn0.5O2 and Li-rich materials, spinel LiMn2O4 and LiNi0.5Mn1.5O4, olivine-type LiMnPO4 and LiMn0.5Fe0.5PO4, stand out owing to their low cost and high energy density. Herein, from the perspective of industrial application, we calculate the product cost of Mn-based cathode materials, select promising candidates with low cost per Wh, and summarize the structural and electrochemical properties and improvement strategies of these low-cost Mn-based cathode materials. Apart from some common issues for Mn-based cathode materials, such as Jahn–Teller distortions and Mn dissolution, we point out the specific problems of each material and provide corresponding improvement strategies to overcome these drawbacks.
1. Introduction
The widespread application of consumer electronics and the urgent requirements of electrical vehicles have greatly promoted the development of lithium-ion batteries (LIBs) [1,2,3]. Among the main components of LIBs, such as the cathode, anode, electrolyte, and separator, cathode materials, one of the main components of LIBs along with anode materials, liquid electrolytes, and separators, are crucial to the whole energy density and costing of LIBs. This is because of the relatively lower capacity of cathode materials compared with that of anode materials (for example, commercial LiCoO2 cathode: ~180 mAh g−1; commercial graphite anode: ~350 mAh g−1) and their non-negligibly higher cost (about 40–60% of total LIBs). The high cost of cathode materials has caused the high price of LIBs, which drives up the cost of LIB-based products, especially those that need a large quantity of LIBs, such as electrical vehicles, energy storage devices and electrical tools. As lithium resources are limited and unevenly distributed, LIBs are becoming more and more expensive, which has resulted in the emergence of other competitive batteries, including sodium-ion batteries (SIBs), potassium-ion batteries (KIBs) and magnesium-ion batteries (MIBs). Meanwhile, low-cost lead-acid batteries (LAB) still occupy a large part of the battery market. Under the pressure of traditionally commercial batteries (LABs) and emerging batteries (SIBs, KIBs, MIBs etc.), it is urgent to develop new inexpensive commercial cathode materials for LIBs, so that LIBs can be more competitive in the battery market.
To screen low-cost cathode materials, it is necessary to take the cost into account from the elemental standpoint. At present, cobalt (Co), which is included in commercial LiCoO2 and ternary materials (LiNixCoyMn1-x-yO2, NCM; LiNixCoyAl1-x-yO2, NCA etc.), is the primary element of cathode materials. However, the price of Co is extremely high and keeps rising due to its rare reserve and uneven distribution [4]. Another widely used element for cathode materials is Ferrum (Fe), which is included in commercial LiFePO4 for power batteries. However, Fe ions not only have a +2 and +3 valance state, which limits the diversity of Fe-based cathode materials, but also exhibit a low discharge voltage (3.5 V vs. Li/Li+), which greatly limits the discharge capacity of LiFePO4 [5]. By comparison, manganese (Mn) is plentiful in the Earth’s crust and has been utilized extensively in Ferrum and the steel industry, non-ferrous metallurgy, the chemical industry, electronics, batteries, agriculture, medicine and other fields. Additionally, Mn ions have manifold valance states ranging from +2 to +7, which enables them to produce various cathode materials with different types of crystal structures, such as spinel LiMn2O4, layered LiMnO2 and olivine LiMnPO4. Therefore, Mn-based materials should be the main emphasis when seeking next-generation low-cost cathode materials. Nowadays, LiMn2O4 has become a commercial material by virtue of its price advantage and is mainly applied in the two-wheel electric-vehicle industries. Other Mn-based materials, including LiNi0.5Mn0.5O2, LiNi0.5Mn1.5O4, LiMnPO4 and Li-rich Mn-based materials, will potentially be used as commercial cathode materials for LIBs because of their high energy density and inexpensive price.
There exist several excellent reviews on Mn-based materials which normally focus on a specific type of Mn-based material, such as spinel LiMn2O4 [6,7], layered LiMnO2 [8] and Li-rich Mn-based materials [9,10], and collect recent advances in the academic area; however, they ignore practical industrial applications. Hence, the purpose of this review is to offer an essential understanding of the performance and preparation cost of various types of Mn-based materials. By calculating the preparation cost (raw materials and preparation technology), we selected potential low-cost cathode materials whose cost per Wh is lower than that of present NCM materials. The basic structures, electrochemical performance, and improvement strategies are also elucidated systematically, and their industrial-application prospects are also outlined.
2. Estimation of Energy Density and Cost for Mn-Based Materials
For the industrial application of cathode materials in LIBs, the energy density (gravimetric energy density, GED; volumetric energy density, VED) and cost (production cost, cost per Wh) are vital parameters. The theoretical specific capacity of a cathode material is computed according to the formula Q = nF/3.6 Mw, where n is the electron-transfer number, F is the Faraday constant, and Mw is the molecular weight of the cathode material [11]. In order to assess the realistic economic worth of the cathode materials, the data of practical specific capacities, average discharge voltages, and compaction densities of most materials (except LiNi0.5Mn0.5O2, Li1.2Ni0.2Mn0.6O2 and Li1.2Mn0.625Nb0.175O1.95F0.05) are obtained from various suppliers of LIB’s materials. The material cost is computed according to the average price of raw materials on the CBCIE website (www.cbcie.com) in 2021 and the production cost. The raw materials include the transition-metal salts or transition-metal precursor, lithium salt, phosphate salts for phosphate materials, and additives. The production cost not only includes the cost of electricity, packaging and transportation, but also considers the cost of labor and depreciation of equipment. The GED is generated by multiplying practical specific capacity and average discharge voltage. The VED is generated by multiplying GED and compaction density. The final cost per Wh is the result of material cost/GED. Table 1 summarizes the results from the above.
According to Table 1, LiCoO2 has the highest VED, implying its widespread application in consumer electrics that provide limited space for batteries. For electric vehicles, the GED and material cost are more important than VED due to its larger space and use of many more batteries to produce much more energy. Although NCM materials, such as LiNi0.5Co0.2Mn0.3O2 (NCM523) and LiNi0.8Co0.1Mn0.1O2 (NCM811) have a substantially lower VED than LiCoO2, their GED is comparable to that of LiCoO2. NCM is widely used in batteries of electrical vehicles due to its significantly cheaper material cost compared to LiCoO2 [12]. Although LiFePO4 has a relatively lower GED than NCM and LiCoO2, it is still a viable cathode material for electrical vehicles due to its low cost per Wh and extraordinarily extended working life. The cost per Wh of LiFePO4 is 0.15 CNY Wh–1, which is relatively low in present commercial materials, but its energy density is limited to a low level. This paper reviews the low-cost materials with lower cost per Wh than NCM (NCM523: 0.34 CNY Wh–1; NCM811: 0.34 CNY Wh–1). Then, we find that almost all Mn-based materials can meet the aforementioned requirement, and most of them have prices of less than 0.20 CNY Wh–1, with the exception of Li1.2Mn0.625Nb0.175O1.95F0.05. Among these low-price Mn-based materials, spinel LiMn2O4 and LiNi0.5Mn1.5O4 demonstrated the lowest cost per Wh at 0.13 CNY Wh–1. LiMn2O4 has been applied in two-wheel electric vehicles and electric tools that need higher cost performance and tolerance for relative lower energy density and cycle performance, and it has the potential to replace LABs. The layered LiNi0.5Mn0.5O2 has a comparable GED to NCM523 and its cost of Wh (0.19 CNY Wh−1) is much lower than that of NCM523, which has the potential to replace NCM materials by virtue of its low price. The cost per Wh for LiMnPO4 (0.16 CNY Wh–1) and LiMn0.5Fe0.5PO4 (0.15 CNY Wh–1) is similar to that of LiFePO4, which has shown a commendable advantage in price. Meanwhile, the higher GED of these two materials make them more attractive, and some material companies are developing them. Li-rich materials, such as layered Li1.2Ni0.2Mn0.6O2 (0.19 CNY Wh–1, 825.0 Wh kg–1) and rock-salt Li1.2Mn0.625Nb0.175O1.95F0.05 (0.30 CNY Wh–1, 1056 Wh kg–1) are becoming research-and-development hotspots for many material companies. Although they do not have a low cost per Wh, they have the highest GEDs among all materials, which can greatly improve the range of electric vehicles.

Table 1.
The comparison of various Mn-based materials. Most data are obtained from the materials’ suppliers in China except LiNi0.5Mn0.5O2, Li1.2Ni0.2Mn0.6O2 and Li1.2Mn0.625Nb0.175O1.95F0.05. The currency type “CNY” is “Chinese Yuan”.
Table 1.
The comparison of various Mn-based materials. Most data are obtained from the materials’ suppliers in China except LiNi0.5Mn0.5O2, Li1.2Ni0.2Mn0.6O2 and Li1.2Mn0.625Nb0.175O1.95F0.05. The currency type “CNY” is “Chinese Yuan”.
| Materials | Theoretical Specific Capacity (mAh g–1) | Practical Specific Capacity (mAh g–1) @ 0.1C | Initial Coulombic Efficiency | Average Discharge Voltage (V) | Compaction Density (g cm–3) | Gravimetricenergydensity (Wh kg–1) | Volumetricenergydensity (Wh L–1) | Material Cost (CNY kg–1) | Cost per Wh (CNY Wh–1) | Main Drawbacks | Cycling Performance | Air Stability | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LiCoO2 | 274 | 210 | 94% | 3.80 | 3.80 | 798.0 | 3032 | 308.6 | 0.39 | High cost | ★★★☆☆ | ★★★★★ | |
| NCM523 | 276 | 180 | 88% | 3.71 | 3.45 | 667.8 | 2304 | 226.4 | 0.34 | Relatively low energy density | ★★★★☆ | ★★★☆☆ | |
| NCM811 | 275 | 210 | 91% | 3.66 | 3.40 | 768.6 | 2613 | 235.2 | 0.31 | Poor stability, bad safety | ★★★☆☆ | ★★☆☆☆ | |
| LiFePO4 | 170 | 160 | 98% | 3.35 | 2.20 | 536.0 | 1179 | 81.1 | 0.15 | Low energy density | ★★★★★ | ★★★★☆ | |
| Spinel | LiMn2O4 | 148 | 120 | 95% | 3.80 | 3.00 | 456.0 | 1368 | 57.5 | 0.13 | Low energy density | ★★☆☆☆ | ★★★★☆ |
| LiNi0.5Mn1.5O4 | 147 | 133 | 94% | 4.70 | 3.00 | 625.1 | 1875 | 82.7 | 0.13 | High voltage plateau | ★★★☆☆ | ★★★★☆ | |
| Layered | LiNi0.5Mn0.5O2 [13] | 280 | 199 | 96% | 3.40 | / | 676.6 | / | 128.3 | 0.19 | Poor rate capability | ★★☆☆☆ | ★★★☆☆ |
| Li1.2Ni0.2Mn0.6O2 [14] | 378 | 240 | 82% | 3.45 | 2.80 | 845.0 | 2367 | 158.3 | 0.19 | Low initial coulombic efficiency | ★★☆☆☆ | ★★★★☆ | |
| Rock salt | Li1.2Mn0.625Nb0.175O1.95F0.05 [15] | 353 | 330 | 94% | 3.20 | 2.70 | 1056 | 2851 | 319.2 | 0.30 | High production cost | ★☆☆☆☆ | ★★★★☆ |
| Olivine | LiMnPO4 | 171 | 154 | 83% | 3.90 | / | 600.6 | / | 96.5 | 0.16 | Low electronic conductivity | ☆☆☆☆☆ | ★★★★☆ |
| LiMn0.5Fe0.5PO4 | 170 | 160 | 93% | 3.72 | 2.40 | 595.2 | 1428 | 89.1 | 0.15 | Low energy density | ★★★★★ | ★★★★☆ | |
3. Structure, Performance and Improvement Strategies of Mn-Based Materials
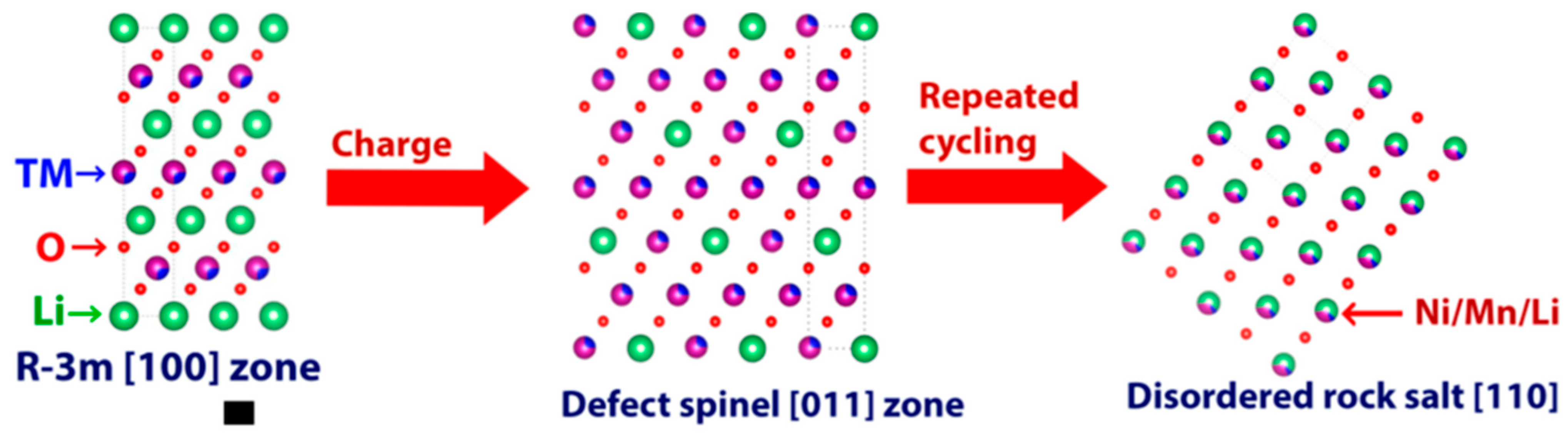
The typical low-cost Mn-based materials listed in Table 1 include layered, spinel, rock-salt and olivine structures. Most layered transition-metal oxides often undergo a transformation during cycling, first becoming layered, then spinel, and, finally, forming a rock-salt structure [16,17] (Figure 1). For the layered structure, lithium ions and transition-metal (TM) ions are situated in two independent layers. When some Li sites are replaced with TM, the layered structure can change into a defect spinel/spinel-like structure. After a long cycling process, all metal ions, including Li+ and TM ions, take up the metal sites randomly, leading to the formation of the rock-salt structure.

Figure 1.
Failure model of layered transition metal oxides (taking Li−rich materials as an example) [16].
3.1. Layered Mn-Based Oxides
LiMnO2 is the primary Mn-based material, from which many other cathode materials are derived, including LiNi0.5Mn0.5O2, LiMn2O4 (Li0.5MnO2), and Li2MnO3 (Li(Li1/3Mn2/3)O2). LiMnO2 has three different types of structures, with space group Pmnm, C2/m and Rm, respectively: orthorhombic, monoclinic and rhombohedral structures [18]. In these, the monoclinic LiMnO2 with a layered structure can be utilized as a cathode material for LIBs owing to its high theoretical capacity of 285 mAh g–1 [19]. However, orthorhombic LiMnO2 is usually produced during the high-temperature solid-state methods [20], indicating that monoclinic LiMnO2 (Figure 2a) has a metastable phase. The monoclinic LiMnO2 is usually synthesized by low-temperature ion-exchange methods [21], which sharply raises the production cost of LiMnO2. At present, LiMnO2 has not been commercialized not only due to the high production cost, but also because of some performance disadvantages, such as Jahn–Teller distortions, Mn dissolution, low electronic conductivity, and structure transformation to spinel phase, which results in low stability [22]. In Mn-based materials, the Jahn–Teller distortion is a common problem which leads to the phase transformation and the disproportionation reaction of Mn3+ which generates Mn2+ ions and results in Mn dissolution. According to the Jahn–Teller theory, nonlinear molecules with a spatially degenerate electronic ground state tend to experience a geometrical distortion to lower the energy of total molecule. For Mn3+ in MO6 octahedrons, four d orbital electrons will be arranged in two eg and three t2g orbitals, forming high-spin t2g3eg1 or low-spin t2g4, which results in an odd number of electrons in d orbitals and Jahn–Teller distortion [18]. To alleviate the Jahn–Teller distortion, various strategies, such as doping, surface modification, and constructing nanostructures with distinctive morphologies have already been used.
3.1.1. LiNi0.5Mn0.5O2
When the Mn cations in LiMnO2 are partially substituted by Ni cations, a LiNixMn1-xO2 solid solution is formed. The structure of LiNixMn1-xO2 is determined by the ratio of Ni/Mn. When the ratio of Ni/Mn < 1, the spinel structure is formed; otherwise, the α-NaFeO2 structure with Rm symmetry (Figure 2b) will be generated [23]. The hexagonal α-NaFeO2-structured LiNi0.5Mn0.5O2 delivers a discharge specific capacity of about 200 mAh g–1, as well as a plateau potential of 3.8 V [24]. As the valence state of Mn and Ni ions in LiNi0.5Mn0.5O2 is +4 and +2, respectively, only the Ni ions undergo the redox reaction from +2 to +4 during the charging and discharging process. The Mn ion keeps the valance state of +4 without the appearance of Mn3+, which can effectively avoid Mn dissolution and the Jahn–Teller distortion [25]. Nevertheless, with the utilization of Ni2+, the Li/Ni cation mixing problem will appear in LiNi0.5Mn0.5O2, which reduces the Li+ diffusion rate and the reversible capacity [26].
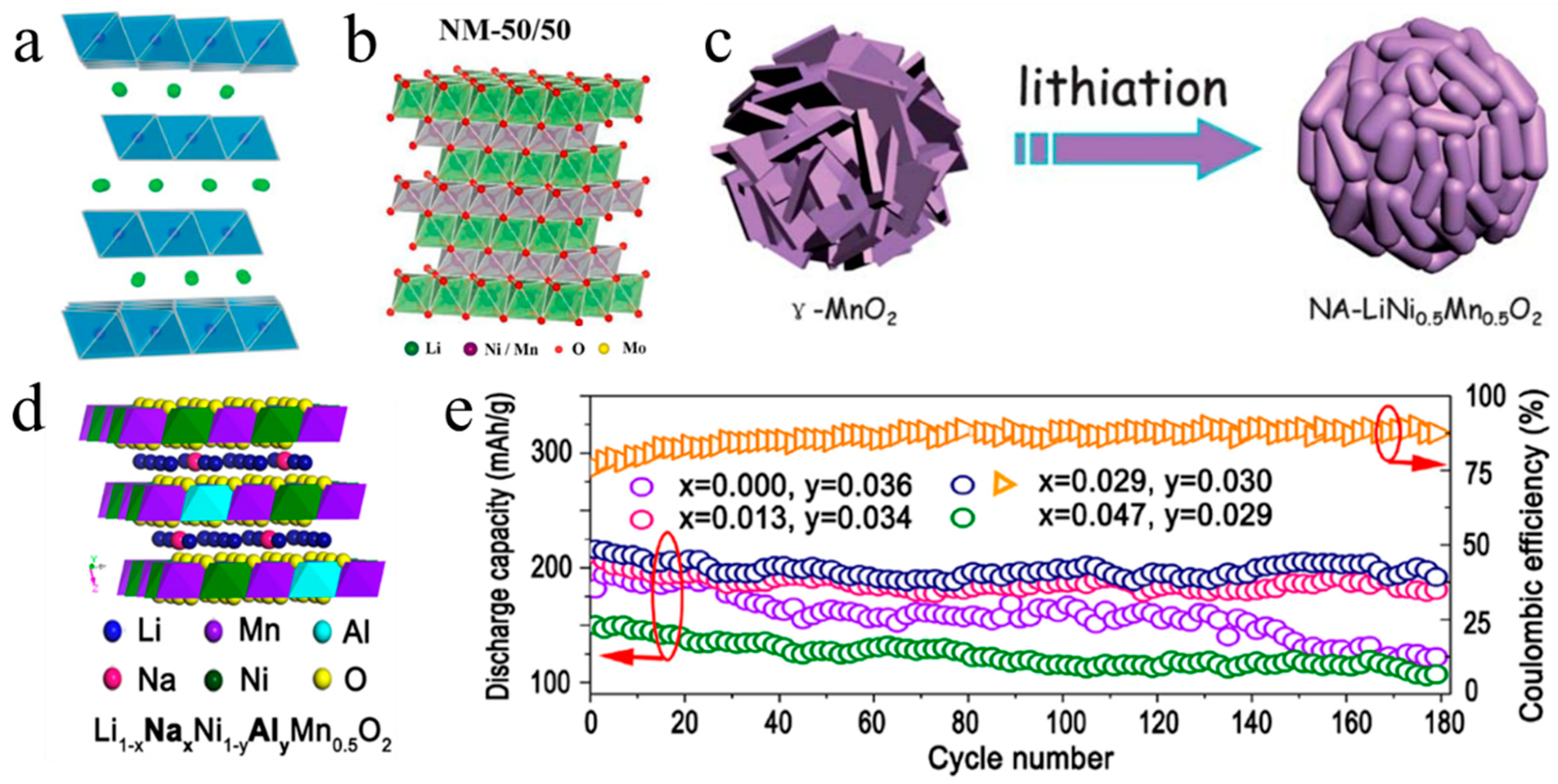
In order to enhance the electrochemical performance of LiNi0.5Mn0.5O2, various strategies were employed, including nanostructure construction, element doping and surface coating. The nano-structured LiNi0.5Mn0.5O2 is mainly synthesized using template methods. Yuan et al. [27] carried out an in-situ conversion from γ-MnO2 hollow nanospheres to LiNi0.5Mn0.5O2 nanoarchitecture spheres (Figure 2c). In comparison to LiNi0.5Mn0.5O2 particles synthesized via a conventional solid-state reaction process, the nanostructured LiNi0.5Mn0.5O2 displays a significant improvement in rate performance (121.9 mAh g–1 at 3.2 C, 70% of the capacity at 0.2 C), because much faster interfacial kinetics and higher Li+ insertion/removal rates are realized by reducing the size of LiNi0.5Mn0.5O2 particles. Later, Yuan et al. [28] utilized cryptomelane-type octahedral molecular sieve manganese dioxide (OMS-2) in the form of dendritic nanostructures as templates to prepare the three-dimensional LiNi0.5Mn0.5O2 nanostructures, which also showed improved rate performance.
Element doping is an efficacious strategy to enhance the electrochemical properties of cathode materials. Numerous elements have been attempted to dope into LiNi0.5Mn0.5O2, such as Al [29,30], Mg [31], Ba [32], Cu [33], Sb [34], Ti [35], Zr [36], Si [37], Mo [38] and F [39]. All these doping ions can suppress the Li/Ni cation mixing in LiNi0.5Mn0.5O2, and further improve the rate capability, specific capacity, and cycling performance. In addition, the special element has particular effects. For instance, LiNi0.5Mn0.5O2 doped with Al element displayed the highest initial specific capacity of 206 mAh g–1 [30] and 215 mAh g–1 [29]. This is because Al doping could narrow the size distribution and decrease Li+ migration resistance with extended lattice parameter of c axle. Ba doping can effectively promote the cycling performance of LiNi0.5Mn0.5O2 because the Ba-O bond has a greater dissociation energy than the Ni-O bond (563 kJ mol–1 vs. 391.6 kJ mol–1), which helps to stabilize the crystal structure [32]. As a result, after 100 cycles, Ba-doped LiNi0.5Mn0.5O2 can still operate at 97% of its initial specific capacity. Cu doping could extend the migration channels of lithium ions in LiNi0.5Mn0.5O2, which, in turn, improves the rate performance effectively [32]. In addition to single-element doping, the double-element co-doping is applied in LiNi0.5Mn0.5O2, which has shown better improvement than single-element doping. Jia et al. [40] realized a Na-Al dual-doped LiNi0.5Mn0.5O2 material, improving the reversible capacity, cycling stability, and the stability of discharge midpoint potential (Figure 2d). Na+ successfully entered into the lithium layer and played a “pillar” role to promote the structural stability, as opposed to hindering the diffusion of Li+. The Al-O bond with a higher bond dissociation energy further reinforced the crystal structure. As a result, the Na-Al dual-doped LiNi0.5Mn0.5O2 displayed a high initial specific capacity of 216 mAh g–1 and a capacity retention of 90.56% after 180 cycles (Figure 2e).

Figure 2.
(a) Crystal models of monoclinic LiMnO2 (blue: MnO6 octahedron, green: Li) [23]. (b) Crystal models of LiNi0.5Mn0.5O2 [35]. (c) Schematic diagram of the synthesizing nanostructured LiNi0.5Mn0.5O2 derived from the in-situ conversion of γ−MnO2 hollow nanospheres [27]. Schematic diagrams (d) and cycling performance (e) of crystal structure of Li1−xNaxNi0.5-yAlyMn0.5O2 [40].
Figure 2.
(a) Crystal models of monoclinic LiMnO2 (blue: MnO6 octahedron, green: Li) [23]. (b) Crystal models of LiNi0.5Mn0.5O2 [35]. (c) Schematic diagram of the synthesizing nanostructured LiNi0.5Mn0.5O2 derived from the in-situ conversion of γ−MnO2 hollow nanospheres [27]. Schematic diagrams (d) and cycling performance (e) of crystal structure of Li1−xNaxNi0.5-yAlyMn0.5O2 [40].
Surface coating can function as a barrier between cathode materials and liquid electrolytes, preventing direct contact and further enhancing the interface stability. Meanwhile, the conductivity of cathode materials can also be promoted by coating a conductive layer. Hashem et al. [41] prepared carbon-coated LiNi0.5Mn0.5O2 using an oxalate coprecipitation method, with table sugar as a carbon source. In comparison to pristine LiNi0.5Mn0.5O2, the as-prepared sample exhibited superior capacity retention (92% after 50 cycles vs. 75% after 30 cycles). Jia et al. [42] coated a stable, 10 nm thick, and lithium super conductive Li2TiO3 layer on the surface of LiNi0.5Mn0.5O2, which effectively enhanced the Li+ diffusion rate, protected the particle morphologies, and helped to maintain a better structure stability, by reducing side reactions between the cathode materials and liquid electrolytes. In order to further improve the electrochemical properties of LiNi0.5Mn0.5O2, element doping and surface coating were combined, such as Sb-doped and Sb2O3-coated LiNi0.5Mn0.5O2 [34], and Zr-doped and Li2ZrO3-coated LiNi0.5Mn0.5O2 [36]. In these, element doping could enhance structural stability by reducing the degree of Li/Ni cation mixing, while surface coating could efficaciously shield the active material from direct contact with liquid electrolytes, and increase the Li+ diffusion rate at the interface between electrode and electrolyte. However, present methods for these materials usually follow two steps: preparation of precursors (without doping agents) and preparation of final materials. Since the doping ion is diffused from surface to bulk, resulting in a low doping amount [36], it is important to investigate how to add a doping agent to the precursor.
3.1.2. Li-Rich Mn-Based Oxides
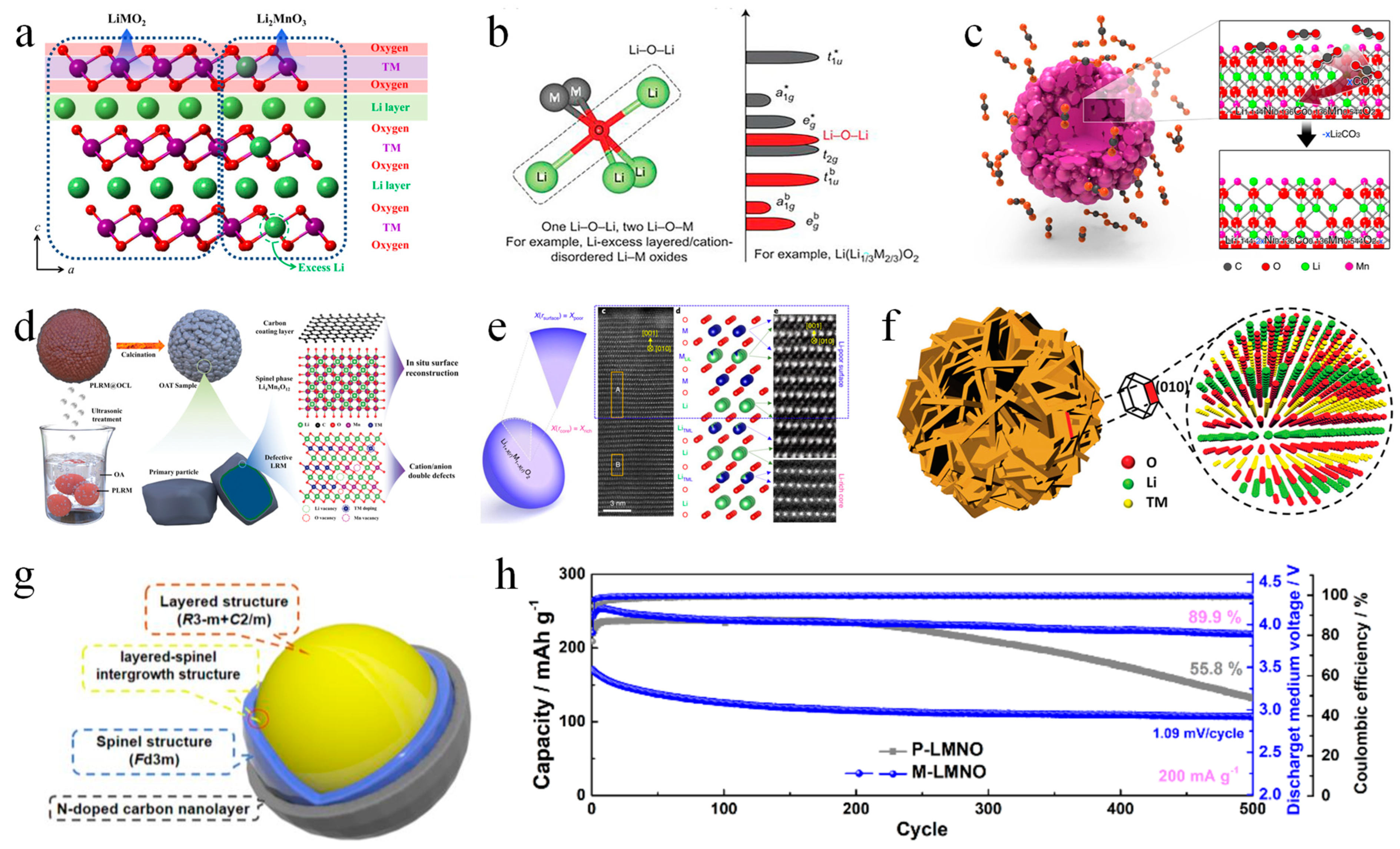
Owing to their high specific capacity (>250 mAh g–1@0.1C), low cost, and outstanding safety, Li-rich Mn-based (LRM) layered oxides are regarded as the most promising candidate of cathode materials for the next-generation high-specific-energy LIBs. The main component of LRM cathode materials is the inexpensive Mn element; low Co content (< 10 mol%) or no Co content is the main way to reduce the material cost of LRM. The low cost of LRMs combined with the high energy density can significantly reduce the cost per Wh. Since Numata et al. [43] reported LiCoO2-Li2MnO3 for the first time in 1997 and Gopukumar et al. [44] originally used Li2MnO3 as a cathode material for LIBs in 1999, numerous studies have been conducted on LRM cathode materials. However, the structure of LRMs has not been figured out yet; there are still two arguments about their structure, one is a two-phase nanodomain (xLi2MnO3 • (1-x) LiNixCoyMnzO2), and the other one is a single-phase solid solution (Li1+xNiyCozMn1-x-y-zO2) [10]. Nevertheless, both nano-domain and solid-solution structures are composed of monoclinic Li2MnO3 (C2/m) and trigonal LiMO2 (Rm) (Figure 3a).
Although LRM show great potential as cathode materials for LIBs due to their high specific capacity, their low initial coulombic efficiency, serious voltage fading, bad cycling and rate properties have limited their practical utilization. Several studies have focused on the causes of the LRM’s poor initial coulombic efficiency. Oxygen activation brought about by the Li-O-Li configuration (Figure 3b) is not only the cause of LRMs’ high specific capacity [45], but also the main origin of the problems of oxygen loss and transition metal migration. During the initial charging/discharging process, O2 gas is generated and released due to the oxidation of some active O, causing an amount of irreversible capacity. Meanwhile, some TM ions will transfer from TM sites to Li sites and form an irreversible spinel phase in the first cycle because the irreversible oxygen release reduces the binding energy of TM ions and oxygen, which further increases irreversible capacity loss. The continuous structural transition from layered to spinel phase has been considered as the cause of the voltage decay and poor cycling performance during cycling [46,47]. In addition, some researchers found that the capacity and voltage decay are caused by the decrease in the redox couple of TM ions [48]. The diffusion kinetics of Li+ in the crystal structure often sets a limit on the rate performance of LRMs. The pristine structure of cathode materials is destroyed during the phase transition from the layered to spinel structure, leading to a poor rate performance [49,50]. Interestingly, some scholars have explored how the Li+ content affects the structure of LRM cathode materials. LRMs with lower lithium content had more chemical ordering defects, while the spinel-structured surface had no obvious structural change with the change in lithium content [51]. Some advanced characterization methods are used to reveal the failure mechanism of LRM positive electrode during the cycle [52,53]. Li/Ni and Li/Mn anti-site defects were also discussed in the case of LiNixCoyMn(1-x-y)O2 and Li2MnO3 cathode materials [54]. In order to avoid the anti-site defects, LiNixCoyMn(1-x-y)O2 and Li2MnO3 were prepared under Ni-rich condition and under O-rich and Mn-poor conditions, respectively.
In order to solve the above problems, the main strategies are doping, coating and surface modification. The elements that are usually utilized to dope cations at TM sites have a stronger bond with oxygen than Ni, Co, and Mn, which can efficaciously inhibit the structural shift from layered to spinel [55]. Surface coating is utilized in LRMs to keep the structural stability of the electrode/electrolyte interface and reduce voltage fading [55]. However, doping and coating are unable to address the issue of oxygen loss, which is the essential problem of LRMs. One present effective strategy to reduce the oxygen loss is creating defects/vacancies which is mostly realized by surface modification. For example, Qiu et al. [56] modified the Li[Li0.144Ni0.136Co0.136Mn0.544]O2 with CO2 by gas–solid interface reaction (GSIR) to form a layer of oxygen vacancy with a thickness of 10–20 nm on the surface of material (Figure 3c). Oxygen vacancy can effectively inhibit the release of lattice oxygen; hence, the obtained sample has shown an improved initial coulomb efficiency of 93.2% and cycling performance; Peng et al. [57] treated the LRM with oleic acid to manufacture cation and anion double defects and an in-situ surface reconstruction layer to reduce oxygen release and improve structural stability, which can enable precise control of the ICE from 84.1% to 100.7%. At the same time, this sample presents a high specific capacities of 330 mAh g−1 at 0.1 C with a large energy density of 1143 Wh kg−1, and 276 and 250 mAh g−1 at 1 and 5 C due to the fast kinetics of Li+ and its electron (Figure 3d). In contrast to the surface modification, Zhu et al. [58] developed the method of molten molybdate-assisted LiO extraction to create gradient Li-rich single crystals, which can inhibit oxygen loss. Lithium is rich in the core of the particle, poor on the surface, and continuous in between (Figure 3e). The prepared material shows a good voltage and cycling stability with high discharge specific capacity of 250.4 mAh g−1 and high average voltage of 3.368 V after 200 cycles at 0.2 C.

Figure 3.
(a) Schematic diagram of Li−rich materials [10]. (b) Structural origin of the preferred oxygen oxidation along the Li−O−Li configuration [45]. (c) Schematic diagram of gas−solid interface reaction (GSIR) between Li−rich layered oxides and carbon dioxide [56]. (d) Schematic diagram of oleic acid−assisted interface engineering [57]. (e) Schematic of the Li gradient Li−rich material [58]. (f) Schematic illustration of hierarchical structured Li1.2Mn0.6Ni0.2O2 material [59]. (g) Schematic illustration and cycling performance (h) (blue lines) of Li1.2Mn0.6Ni0.2O2 by three−in−one surface modification [14].
Figure 3.
(a) Schematic diagram of Li−rich materials [10]. (b) Structural origin of the preferred oxygen oxidation along the Li−O−Li configuration [45]. (c) Schematic diagram of gas−solid interface reaction (GSIR) between Li−rich layered oxides and carbon dioxide [56]. (d) Schematic diagram of oleic acid−assisted interface engineering [57]. (e) Schematic of the Li gradient Li−rich material [58]. (f) Schematic illustration of hierarchical structured Li1.2Mn0.6Ni0.2O2 material [59]. (g) Schematic illustration and cycling performance (h) (blue lines) of Li1.2Mn0.6Ni0.2O2 by three−in−one surface modification [14].
Since the price of Cobalt salts has increased sharply in recent years, LRMs with low Co content or without Co are more attractive in industrial application due to their relatively low cost. However, LRMs with less Co (<10%) show poor structure stability and ionic/electron conductivity, such as Li1.2Ni0.2Mn0.6O2. To enhance the electrochemical properties, Chen et al. [59] prepared a hierarchical Li1.2Ni0.2Mn0.6O2 quasi-sphere with a plane-based surface, in which the electrochemical active planes allow for fast Li+ transport kinetics due to efficient ion and electron transport (Figure 3f). The as-prepared Li1.2Ni0.2Mn0.6O2 displayed improved cycling and rate performance. On the surface of the Li1.2Ni0.2Mn0.6O2 microsphere, Ding et al. [14] pyrolyzed urea to form a multifunctional surface modification, which could simultaneously construct oxygen vacancy, integrated spinel phase and N-doped carbon nanolayer (Figure 3g). The integration of oxygen vacancy and spinel phase could not only inhibit the irreversible release of O2 but also promote the diffusion of Li+. Meanwhile, the N-doped carbon nanolayer with high electrical conductivity could promote electron transport and reduce electrolyte corrosion. The electrochemical results show that the surface-modified Li1.2Ni0.2Mn0.6O2 can retain its initial specific capacity up to 89.9% after 500 cycles at 1 C, and its voltage decay rate per cycle is merely 1.09 mV (Figure 3h), which significantly inhibits the capacity and voltage decay.
3.2. Spinel Mn-Based Oxides
Spinel Mn-based oxides show great structural stability and have been widely applied in LIBs, MIBs [60,61], and so on. LiMn2O4 and LiNi0.5Mn1.5O4 are, at present, the main spinel Mn-based oxides for lithium batteries. Although their theoretical specific capacity is only about half that of layered Mn-based oxides, their more stable structures and quicker Li+ diffusion compared with layered forms have attracted much attention in scientific research and industrial application. LiMn2O4 has become the most mature commercial material in Mn-based cathode materials, while LiNi0.5Mn1.5O4 was originally prepared and investigated as one of the metal-substituted LiMn2O4 derivatives. Since the low valance state of Ni2+ can increase the valence state of Mn and further prevent the Jahn–Teller effect and Mn dissolution, Ni2+ doping can stabilize the crystal structure [62]. Similar to the aforementioned LiNi0.5Mn0.5O2, the valance state of Mn in LiNi0.5Mn1.5O4 is +4, so only Ni ions undergo oxidation and reduction during cycling. Compared with LiMn2O4, LiNi0.5Mn1.5O4 has a comparable cost per Wh (0.13 CNY Wh–1), but a higher potential plateau of ~4.7 V as well as a higher energy density of 650 Wh kg–1. When matched with a suitable high-voltage electrolyte, LiNi0.5Mn1.5O4 has great potential to be widely applied.
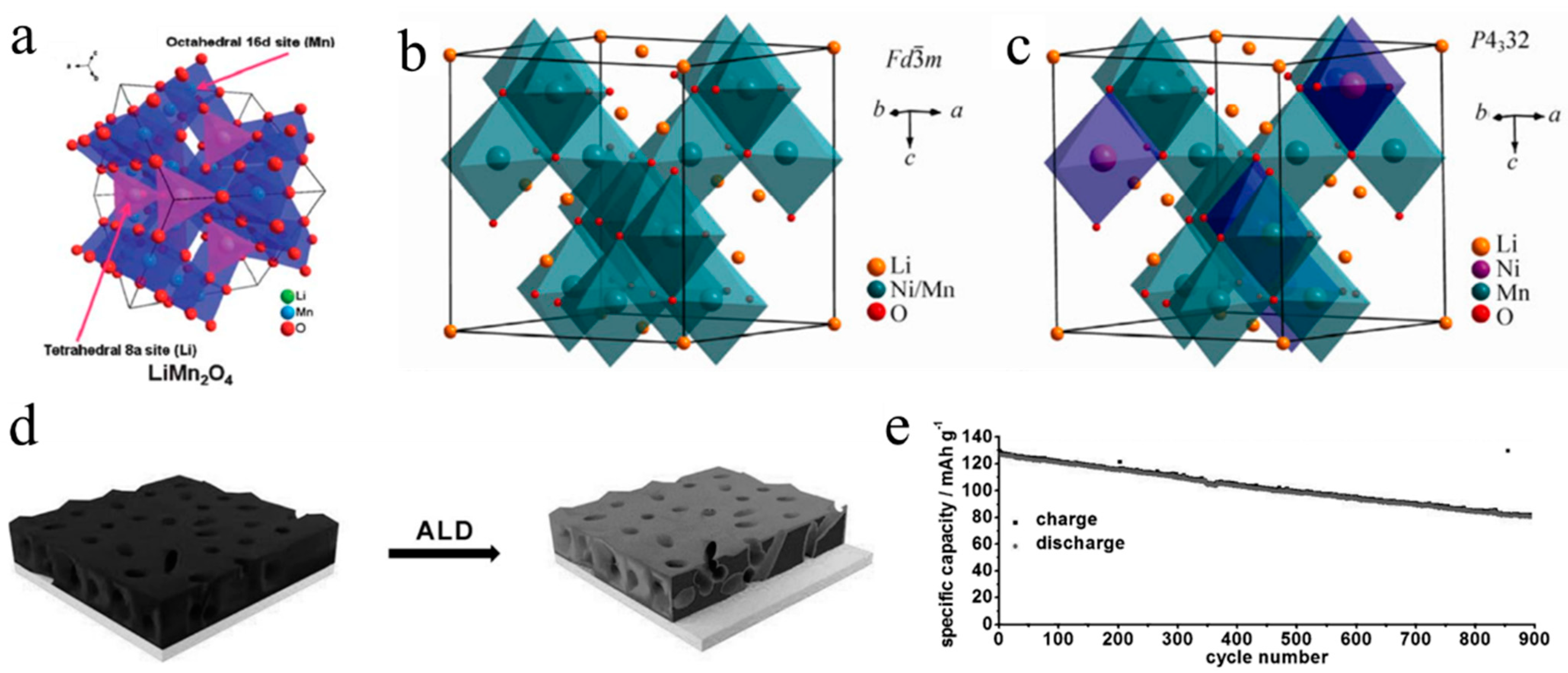
LiMn2O4 is a cubic spinel with an Fd3m space group, and can be synthesized via a simple solid-state reaction in an air atmosphere at a high temperature. In the spinel structure of LiMn2O4, the Li+, Mn ions and O ions are situated at the 8a tetrahedral sites, 16d octahedral sites and 32e positions, respectively. O ions are cubic-close-packed [63] (Figure 4a). The edge-shared MnO6 octahedrons can construct a continuous three-dimensional cubic array, and, further, cause the formation of the robust Mn2O4 spinel framework, in which fast diffusion of Li+ is realized. Since the theoretical specific capacity and average discharge voltage of LiMn2O4 is relatively low (146 mAh g–1, ~3.8 V), it exhibits a relatively low energy density. When the discharge voltage is lower than 3 V, the lithiation of LiMn2O4 occurs, which leads to irreversible phase transformation from spinel to rock-salt (Li2Mn2O4) [63]. Besides the low energy density, another problem of LiMn2O4 is its poor cycling performance due to its Mn dissolution, especially at high temperatures. In LiMn2O4, the average valance state of Mn ions is +3.5, mostly composed of Mn3+ and Mn4+. Mn dissolution has been mainly ascribed to the dissolution of Mn2+, which originates from the disproportionation of Mn3+ (2Mn3+→Mn2++Mn4+) [64,65]. Meanwhile, the currently used LiPF6-based carbonate electrolyte will produce large amounts of hydrofluoric acid (HF) with the presence of trace water, which accelerates the dissolution of Mn4+. Surface coating can also effectively promote the electrochemical performance of LiMn2O4 [66,67,68,69,70]. Coating with solid electrolytes can avoid the Mn reactions of LiMn2O4 in the case of liquid electrolytes. Li6.375La3Zr1.375Nb0.625O12 (LLZNO) electrolytes were used by Bi et al. [71] to coat LiMn2O4, which not only suppressed Mn reactivity but also enhanced the interface between Li6.4La3Zr1.4Ta0.6O12 (LLZTO) and LiMn2O4. After 100 cycles at 0.2C and 55 °C, the solid battery with LMO@LLZNO cathode, LLZTO electrolyte, and Li metal showed a high discharge capacity retention of 81.3%.
LiNi0.5Mn1.5O4, one of the most significant LiMn2O4 derivatives, was originally synthesized in 1996 via substituting part of Mn sites with Ni. LiNi0.5Mn1.5O4 contains two types of spinel crystal structures, one is face-centered cubic (Fd3m) with disordered TM ions, and the other is primitive simple cubic (P4332) with ordered TM ions [72] (Figure 4b,c). In the Fd3m space group, Li+ occupies tetrahedral 8a sites, Mn/Ni ions occupies octahedral 16d sites randomly, and O2- occupies 32e sites. In P4332 space group, Li+ occupies tetrahedral 8a sites, Mn4+ occupies 12d sites, Ni2+ occupies 4b sites, and O2- occupies 24e and 8c sites. It is worth noting that Ni2+ replaces part of Mn4+ sites orderly in the P4332 space group, different from that in the Fd3m space group. Compared with P4332, LiNi0.5Mn1.5O4 with the Fd3m space group exhibits outstanding electrochemical performance and structural reversibility. LiNi0.5Mn1.5O4 has been deemed as one of the most promising cathode materials for LIBs, owing to its high working voltage (4.7 V), high specific capacity (146.7 mAh g–1), high energy density, low cost, and good cycle stability [73,74]. However, in addition to some common obstacles for Mn-based cathode such as TM ions’ dissolution and the Jahn–Teller effect of Mn3+, LiNi0.5Mn1.5O4 also has great side reactions with liquid electrolytes due to its high voltage plateau [75].
To solve the above problems of LiMn2O4 and LiNi0.5Mn1.5O4, various strategies, including element doping, surface coating, an appropriate synthesis method, and electrolyte modification, etc., are proposed to improve their electrochemical performance. Element doping can restrain the Mn3+ disproportionation reaction and Jahn–Teller distortion for spinel Mn-based oxides. Present doping ions can be divided into two categories, cations and anions. Present doping cations include Al, Cr, Co, Ga, Pr, Gd, La, Ce, Nd, Sm, Sc, Y, Tb, Er, B, Fe, Mg, Ti, Ru, Si, Ni, Zn, Cu, Sn, Li, and Na ions, etc., and anions include F, Cl, Br S, and PO3 ions, etc., which have been summarized by Cui et al. [7]. Among the various single cation doping options, Al doping has shown the best improvement effect on the cycling performance of LiMn2O4. Sun et al. [75], Xia et al. [76] and Xiao et al. [77] synthesized the Al-doped samples, which show excellent cycling performances with capacity retentions of 98.5%, 99.34% and 99.3% after 50 cycles at ~50 °C, respectively. The improvement effects can be summarized as: (1) an Al–O bond with a higher bonding energy than Mn–O (512 kJ mol−1 vs. 402 kJ mol−1) could enhance the stability of the spinel structure during insertion/de-insertion of lithium; (2) the smaller lattice parameter of Al-doped LiMn2O4 alleviates the dissolution of the active material and maintains its structural integrity. Meanwhile, a multi-doped strategy with three elements also shows potential to greatly enhance the electrochemical properties of LiMn2O4. Sun et al. prepared the Li, Co, Gd multi-doped LiMn2O4, which shows an outstanding capacity retention of 98.3% after 100 cycles at 25 °C due to its improved structure stability [78]. Manthiram et al. synthesized several Li-M-F (M = Ti, Ni, Cu, Fe, Co, Zn) multi-doped LiMn2O4 with excellent electrochemical performance. In these samples, Li+ and Ni2+ are used to prevent Mn dissolution which is caused by a much smaller lattice-parameter difference between the two cubic phases formed during the charge–discharge process. However, the low-valance-state ion doping will increase the valance state of Mn, causing low specific capacity (<100 mAh g−1). Then, F doping was applied to decrease the valence state of Mn in the Li-Ni co-doped LiMn2O4. Among these materials, LiMn1.85Li0.075Zn0.075O3.85F0.15 displayed a high specific capacity of 113 mAh g−1, good cycling performance with a capacity retention of 94.6% after 50 cycles at 60 °C, and excellent rate capability with a retention of 96% at 4 C of its initial specific capacity at 0.1 C [79]. Anion doping can also inhibit the structural changes during the charging/discharging process, thereby improving the electrochemical properties of LiNi0.5Mn1.5O4. Previous research has shown that doping with F can suppress the generation of NiO impurity, reducing TMs dissolution and improving the rate performance of LiNi0.5Mn1.5O4. It is a pity that doping is unable to prevent undesired side reactions between LiNi0.5Mn1.5O4 and liquid electrolytes [80].
Coating can effectively inhibit side reactions by impeding the direct contact between cathodes and liquid electrolytes. Ideal coating materials for spinel Mn-based oxides should have a good match with the spinel lattice and good diffusion ability of Li+ and electrons. Chong et al. [81] synthesized Li3PO4-coated LiNi0.5Mn1.5O4 by solid-state reaction. With the Li3PO4 layer (<6 nm), coated LiNi0.5Mn1.5O4 had a disordered crystal structure, protected cathode-electrolyte interface, and dramatically enhanced cycling performance. Jang et al. [82] synthesized LiFePO4-modified spinel LiNi0.5Mn1.5O4 through a single-step coating process. LiFePO4 coating greatly improved the thermal stability and high temperature performance, with negligible discharge-capacity reduction. Fang et al. [83] employed atomic layer deposition (ALD) to coat an ultrathin Al2O3 layer onto LiNi0.5Mn1.5O4 particles (Figure 4d). Al2O3 coating protected LiNi0.5Mn1.5O4 from direct exposure to liquid electrolytes, which improved the cycling performance of LiNi0.5Mn1.5O4. The Al2O3-coated LiNi0.5Mn1.5O4 showed 63% capacity retention after 900 cycles (Figure 4e), whereas the bare LiNi0.5Mn1.5O4 maintained 75% of the original capacity after 200 cycles. Apart from inorganic coating, organic materials such as polyimide (PI) [84] and polypyrrole (PPy) [85] can also improve the electrochemical performance of spinel Mn-based oxides. Kim et al. [86] utilized thermal polymerization to produce PI coating from polyamic acid and found that 0.3 wt % PI coated LiNi0.5Mn1.5O4 delivered excellent cycle ability with capacity retention of >90% at 55 °C.

Figure 4.
Crystal structure of LiMn2O4 (a) [62], LiNi0.5Mn1.5O4 with Fd m space group (b) and P4332 space group (c) [72]. Diagram showing (d) and long−term cycling performance (e) of ALD−based Al2O3 coating LiNi0.5Mn1.5O4 [83].
Figure 4.
Crystal structure of LiMn2O4 (a) [62], LiNi0.5Mn1.5O4 with Fd m space group (b) and P4332 space group (c) [72]. Diagram showing (d) and long−term cycling performance (e) of ALD−based Al2O3 coating LiNi0.5Mn1.5O4 [83].
Choosing an appropriate synthesis method can also improve the electrochemical properties of spinel Mn-based oxides, especially LiNi0.5Mn1.5O4. The solid-phase method, sol-gel method, and molten-salt method are the three main methods to synthesize LiNi0.5Mn1.5O4, while each technique has its own advantages and problems. The solid-phase method is a simple, economical and time-saving method [87]. However, the produced LiNi0.5Mn1.5O4 not only has a lot of oxygen defects and impurities such as NiO, but also uneven particle-size distribution and severe agglomeration of particles. Oxygen defects usually lead to the generation of more Mn3+, which will have Jahn–Teller distortion, a disproportionation reaction to producing soluble Mn2+, and, thereby, impair the electrochemical properties of LiNi0.5Mn1.5O4 [88]. Rosedhi et al. [89] synthesized LiNi0.5Mn1.5O4 via ball-milling and following calcination at 750 °C. The as-prepared LiNi0.5Mn1.5O4 displayed an initial discharge capacity of 81 mAh g–1 and 86 mAh g–1 after 100 cycles at 1C. The widely used sol-gel method can synthesize LiNi0.5Mn1.5O4 with good crystallization, small particle size, homogenous dispersion, and outstanding electrochemical performance [90]. In comparison to other synthetic methods, the sol-gel method is much more complicated, time-consuming, and relatively expensive. Cui et al. [91] synthesized nanosized LiNi0.5Mn1.5O4 with an average particle size of 80–100 nm, via a high-oxidation-state manganese sol-gel method. The produced LiNi0.5Mn1.5O4 materials not only have an impurity-free cubic spinel structure, but also exhibit excellent dispersion. Both the solid-phase method and sol-gel method need a high-temperature annealing process, which will easily cause impurities and oxygen defects. The molten-salt method is a simple method for preparing complex oxides with pure phase in a low-melting point flux [92]. Wen et al. [93] synthesized spherical LiNi0.5Mn1.5O4 materials using the molten-salt method. The as-prepared material displayed a high discharge capacity of 129 mAh g–1 in the first cycle and 127 mAh g–1 after 50 cycles. The good retention of capacity is credited to significantly fewer impurity phases than that prepared via a solid-state reaction.
For LiNi0.5Mn1.5O4, the high working voltage at around 4.7 V makes it promising due to its high theoretical energy density (≈690 Wh kg–1 = 147 mAh g–1 × 4.7 V), but it greatly limits the compatibility of LiNi0.5Mn1.5O4 with conventional liquid electrolytes. The mainstream liquid electrolyte is prepared by mixing organic carbonate esters and LiPF6 and will undergo a continuous decomposition above 4.5 V versus Li+/Li, resulting in increased thickness of solid electrolyte interphase (SEI), aggravated dissolution of Mn ions, and destroyed electrode structure [94]. Hence, electrolyte additives, fluorinated electrolytes and solid electrolytes are utilized to improve the compatibility between high-voltage LiNi0.5Mn1.5O4 and electrolytes. Some additives can be polymerized to form a protective layer and thereby suppress the side reaction between material and electrolytes, prior to the decomposition of electrolyte. For example, Li et al. [95] utilized lithium bis(2-methyl-2-fluoromalonato)borate (LiBMFMB) as an electrolyte additive for LiNi0.5Mn1.5O4. With the addition of LiBMFMB, a thinner and stabler SEI was formed to prevent further decomposition of the carbonate solvent. Johannes et al. [96] synthesized a novel electrolyte with γ-Butyrolactone (GBL) as solvent, fluoroethylene carbonate (FEC) as solid electrolyte interphase (SEI) additive, and lithium tetrafluro borate (LiBF4) as electrolyte salt, and its cutoff potential was increased to 4.6 V [97,98]. Apart from the continuous side reactions between electrolytes and cathode materials, the reactions between LiPF6 and trace water will produce HF, which will corrode the cathodes and then cause the TMs dissolution [99]. To solve the above-mentioned problems, additives that have strong bonding with HF, F-, and H+ have been utilized to scavenge the detrimental HF. Li et al. [100] utilized pentafluorophenyltriethoxysilane (TPS) as an additive to improve the cycling stability of LiNi0.5Mn1.5O4. TPS not only constructed an ionically conductive cathode electrolyte interphase (CEI) film, but also captured detrimental species in electrolytes. As a result, after 400 cycles at 1 C, LiNi0.5Mn1.5O4 presented an improved discharge capacity retention from 28% to 85%, with the addition of 1 wt % TPS. In addition, fluorinated electrolytes can promote the capacity of LiNi0.5Mn1.5O4 because of their high oxidation potential. Zhang et al. [101] substituted ethyl methyl carbonate (EMC) and (ethylene carbonate) EC with fluorinated EMC and fluorinated cyclic carbonate (F-AEC) and found that fluorinated electrolytes greatly improve the voltage limits of the electrolyte, and thereby enhance the electrochemical properties of full batteries at elevated temperatures. Developing solid electrolytes can completely solve the decomposition and side reactions of liquid electrolytes, because some solid electrolytes show a wide voltage window beyond 5 V [102]. Li et al. [103] realized high-voltage cycling in solid-state systems using LiNi0.5Mn1.5O4 cathode, Lipon solid electrolyte, and Li anode. The solid-state high-voltage battery delivered a remarkable capacity retention of 90% over 1000 cycles, a high coulombic efficiency of 97% and a round-trip energy efficiency of 97%.
3.3. Cation-Disordered Rock-Salt Mn-Based Li-Rich Materials
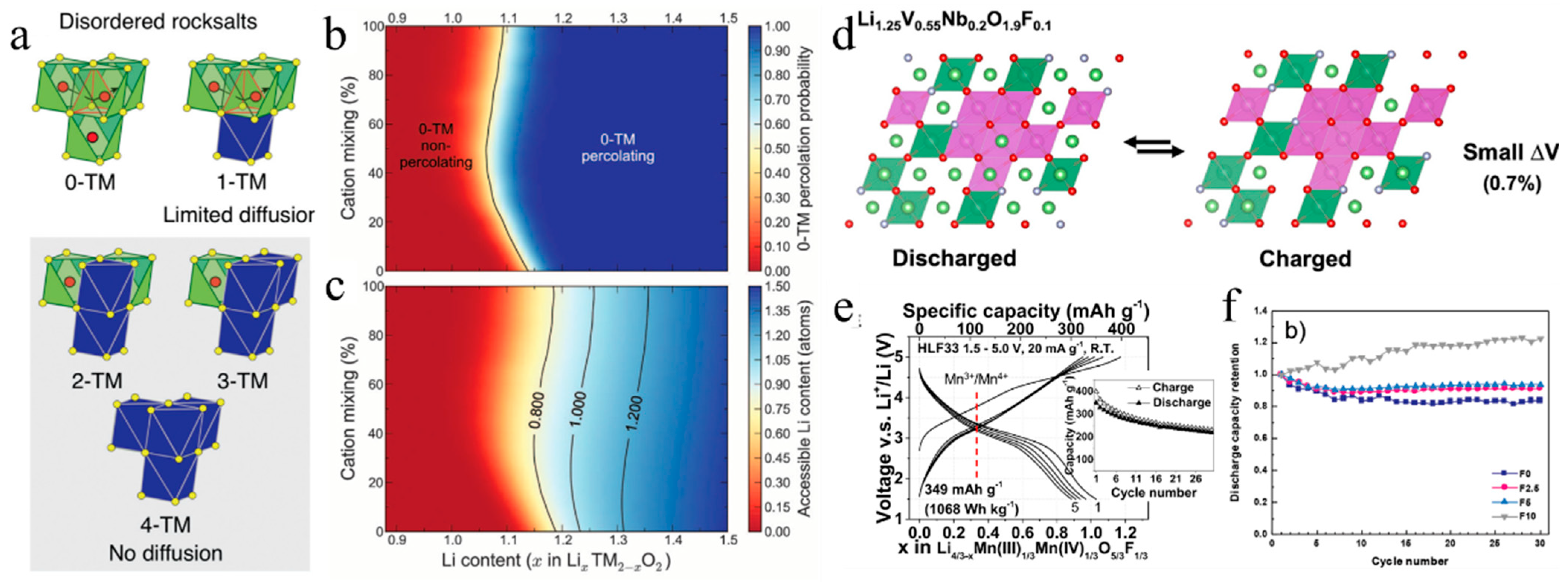
The cation-disordered rock-salt (DRX) structure is mostly considered to be the product of material that has lost its electrochemical activity after cycling. As opposed to the layered structure’s ordered arrangement of metal ions, Li and TM ions of DRXs are randomly mixed in each other’s positions [104]. Therefore, the DRX structure was thought to be harmful to Li+ transport and incapable of providing reversible capacity, until Ceder et al. discovered the electrochemical activity of Li1.211Mo0.467Cr0.3O2 in 2014 [105]. In the DRX structure, the Li+ diffusion between two octahedral (o) sites must undergo an intermediate tetrahedral (Td) site, known as o-t-o diffusion. The intermediate tetrahedral (Td) site has four face-sharing octahedrons that can be filled with Li or M, which forms a “tetrahedral cluster”, generating five possible situations: Li4 (0-TM), Li3M (1-TM), Li2M2 (2-TM), LiM3 (3-TM) and M4 (4-TM) [104] (Figure 5a). The o-t-o Li+ diffusion needs a minimum of two Li+, making a Li4, Li3M, Li2M2 environment possible through Li+ diffusion pathways. However, the Li+ mobility in structures that only contain 1-TM channel or 2-TM channels would be negligible in DRX compounds. Then, in order to guarantee the Li+ diffusion in DRX compounds, sufficient 0-TM channels are required to form the 0-TM percolating network [105], which can be constructed by excess Li+ in a DRX structure (Figure 5b,c). Therefore, the investigations of DRX compounds mainly focus on the Li-rich materials.
Since Ni, Co and Mn will not migrate to Li sites when a significant amount of Li is removed, causing further structural change, they are the main elements in the majority of layered cathode materials [106]. In contrast, the DRX Li-rich materials show an important advantage of using a large range of TM species, such as V, Mn, Nb, Mo, Ti, Cr and so on. Another advantage of DRX Li-rich materials is the higher specific capacity (>250 mAh g–1), compared with present NCM materials [104]. Meanwhile, the intrinsic cation disorder can cause a small volume change in the DRX structure which, in principle, is advantageous for the cycling of cathode materials. Even the nearly zero-strain Li-ion cathodes of Li1.3V0.4Nb0.3O2 and Li1.25V0.55Nb0.2O1.9F0.1 have been designed and synthesized by Ceder et al. in order to enhance the cycling of cathode materials [107] (Figure 5d). However, the rate capability and cycling performance of DRX Li-rich materials are extremely poor and far from that of today’s commercial cathode materials. What is worse, only few of the improvement strategies are effective at promoting the electrochemical properties of DRX materials, such as fluorination.
Among various DRX Li-rich cathodes, the Mn-based materials have shown many advantages, including low cost, resource friendliness, and high energy density (about 1000 Wh kg−1) [108,109], demonstrating great potential as low-cost high-energy-density materials. The Mn-based Li-rich Li2Mn2/3Nb1/3O2F and Li2Mn1/2Ti1/2O2F materials were synthesized via high-energy ball-milled methods and exhibited a high specific capacity of >300 mAh g−1 and high energy density of around 1000 Wh kg–1 without the use of O redox [108]. Another series of Mn-based LixMn2-xO2-yFy compounds with a high capacity of 350 mAh g–1 was also synthesized using ball-milling methods [109] (Figure 5e). Although the high-energy ball-milling process is a good method to produce DRX compounds even for materials whose disorder cannot be accessed thermally, its high energy consumption and low productivity make it unsuitable for industrial application. To realize the industrial product of Mn-based DRX compounds, the traditional solid-state method has been employed. However, these Mn-based DRX compounds must contain d0 element to stabilize the disordered structure, and Nb5+ is often used. For instance, Ceder et al. [110] and Tong et al. [15] prepared Li-Mn-Nb-O-F compounds by sintering at 1000 °C under an argon atmosphere for 7h using Li2CO3, MnO2, Nb2O5 and LiF as raw materials. The as-prepared Li1.2Mn0.625Nb0.175O1.95F0.05 showed a high specific capacity of 330 mAh g–1 and high average discharge voltage of 3.2 V. However, to improve its electrochemical performance, fluorinated DRX needs a larger F content since the F solubility in DRX using LiF as the F source is limited at 7.5 at%. Chen et al. [111] synthesized the Li-Mn-Nb-O-F compounds using a solid-state calcination method using poly(tetrafluoroethylene) (PTFE) as an F source; the incorporation of F content was up to 12.5 at%. In this, the Li-Mn-Nb-O-F compound with 10 at% of F substitution displays a reversible discharge capacity of ≈255 mAh g−1 and good cycling performance (123% capacity retention after 30 cycles) (Figure 5f).

Figure 5.
(a) Cation disorder causes the formation of all varieties of tetrahedral clusters (0−TM, 1−TM, 2−TM, 3−TM and 4−TM channels) [104]. (b) Computed probability of discovering a percolating network of 0-TM channels versus Li content (x in LixTM2-xO2) and cation mixing (TMLi layers/TMTM layers × 100%) [105]. (c) Accessible Li content by a percolating 0−TM network versus Li content and cation mixing [105]. (d) Structure change of Li1.25V0.55Nb0.2O1.9F0.1 during charging and discharging [107]. (e) Electrochemical performance of Li1.3333Mn(III)0.3333Mn(IV)0.3333O1.6667F0.3333 (HLF33) [109]. (f) Capacity retention of Li1.2Mn0.6Nb0.2O2 (F0), Li1.2Mn0.625Nb0.175O1.95F0.05 (F2.5), Li1.2Mn0.65Nb0.15O1.9F0.1 (F5), and Li1.2Mn0.7Nb0.1O1.8F0.2 (F10) cathodes [111].
Figure 5.
(a) Cation disorder causes the formation of all varieties of tetrahedral clusters (0−TM, 1−TM, 2−TM, 3−TM and 4−TM channels) [104]. (b) Computed probability of discovering a percolating network of 0-TM channels versus Li content (x in LixTM2-xO2) and cation mixing (TMLi layers/TMTM layers × 100%) [105]. (c) Accessible Li content by a percolating 0−TM network versus Li content and cation mixing [105]. (d) Structure change of Li1.25V0.55Nb0.2O1.9F0.1 during charging and discharging [107]. (e) Electrochemical performance of Li1.3333Mn(III)0.3333Mn(IV)0.3333O1.6667F0.3333 (HLF33) [109]. (f) Capacity retention of Li1.2Mn0.6Nb0.2O2 (F0), Li1.2Mn0.625Nb0.175O1.95F0.05 (F2.5), Li1.2Mn0.65Nb0.15O1.9F0.1 (F5), and Li1.2Mn0.7Nb0.1O1.8F0.2 (F10) cathodes [111].
3.4. Phospho-Olivine Mn-Based Compounds
Since the groundbreaking investigation of Goodenough et al. in 1996 [112], the Phospho-olivine LiMPO4 compound (where M = Fe, Mn, Co or Ni) has been recognized as a viable cathode material for LIBs. Among the olivine phosphate family, LiMnPO4 and LiFexMn1-xFexPO4 are excellent candidates for stable and high-energy-density cathode materials. Phospho-olivine LiMnPO4 is made up of a hexagonal close-packed (hcp) framework of oxygen with Pnma space group, where Mn and Li occupy octahedral Figure 4a,c octahedral sites, and P atom being in Figure 4c tetrahedral site, respectively (Figure 6a). LiFexMn1-xFexPO4 has a similar crystal structure, with Li and Mn/Fe atoms located in the octahedral Figure 4a,c sites, respectively, while the tetrahedral Figure 4c position harbors the P atoms [113]. LiMnPO4 is attractive due to its high theoretical discharge capacity (≈170 mAh g–1), high operating voltage (4.1 V vs. Li/Li+), high structural stability during the charging/discharging process, superior theoretical energy density (≈701 Wh kg–1 = 171 mAh g–1 × 4.1 V), and high safety due to its strong P–O covalent bond. Additionally, LiMnPO4 shows low toxicity, environmental friendliness and low cost. However, the low electronic conductivity (<10–10 S cm–1) and low lithium–ion diffusion (<10–16 cm2 S–1) of LiMnPO4 significantly affects its rate capability and cycling performance at high rates and limits its industrial application [114]. LiFexMn1-xPO4 is a solid-solution material between LiMnPO4 and LiFePO4 with uniform distribution of Mn and Fe elements [115,116,117]. Fe2+ doping can reduce electrochemical polarization, leading to an enhancement in aspects such as the reversibility of electrodes, the ability of de-lithiation/lithiation and the diffusion of Li+. Moreover, Jahn–Teller lattice distortion of Mn3+ ions will hinder the lithium extraction/insertion process in LiMnPO4, which will further cause a large volume change and interface strain between the LiMnPO4 and MnPO4 phases [118]. In order to overcome these problems, several methods have been applied to improve the electrochemical properties of LiMnPO4 and LiFexMn1-xPO4, including particle-size reduction, carbon coating, ion doping, and an optimized synthesis process.
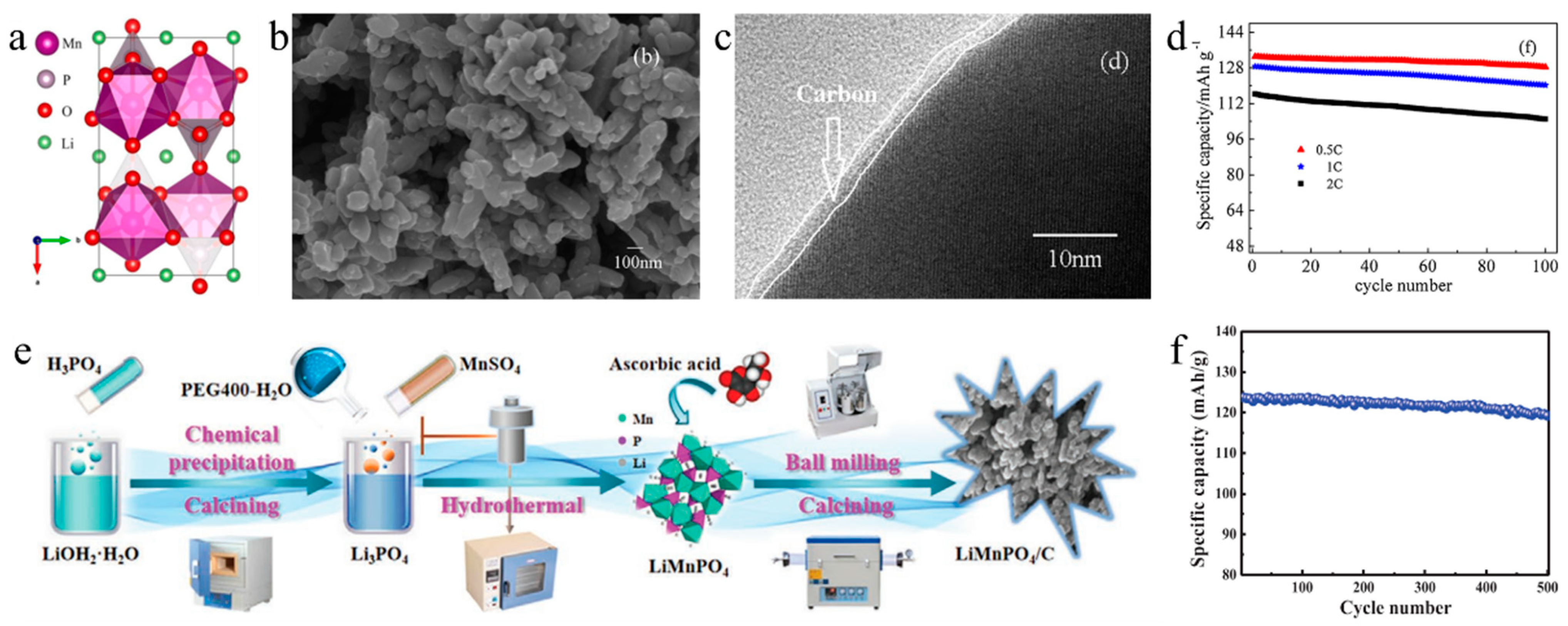
Reducing particle size to a nanometer scale can efficaciously enhance the electrochemical properties of phospho-olivine materials, such as nanoparticles [119] and nanosheets [120,121,122], because nanoparticles can provide shorter diffusion pathways and larger surface-area contact with electrolytes for electron and ion transfer [123]. Particle size and shape may be greatly controlled via synthesis methods. Common preparation methods include the solid-phase method, sol-gel method, hydrothermal/solvothermal method, precipitation method, spray pyrolysis method, polyol synthesis and so on. The solid-phase method is a conventional and economical synthesis method, which heats a mixture of lithium, manganese and phosphorus sources at a high temperature to form olivine-phase products [124] This method can be easily scaled up for commercial use. However, the solid-phase method not only needs a high-temperature environment, but also produces large or agglomerated particles with poor electrochemical performance [125]. In comparison to the solid-phase method, the liquid-phase method is beneficial for controlling particle morphology and synthesizing nanoparticles. For example, powders produced using the sol-gel method have high purity, uniformity, increased crystallinity, accurate stoichiometric control, and minimal size [126]. Sol-gel synthesis is a low-temperature wet chemical method which involves the formation of sols, the gelation of sols into gels, the drying of gels into “xerogels” with reduced volume, and the densification to obtain final powder products [127]. The sol-gel method can adjust the product’s structure and morphology within the nanometric range, by controlling reaction time, pH value, calcination temperature, concentration, viscosity and so on. Kwon et al. [128] studied the influence of calcination temperature on particle size and successfully synthesized LiMnPO4 nanoparticles with a diameter of 130 ± 10 nm via a glycolic-acid-assisted sol-gel approach and this sample showed a reversible capacity of 134 mAh g−1 at 0.1 C. Liu et al. [129] synthesized carbon-coated nano-sized LiMnPO4 and LiMn0.5Fe0.5PO4/C of 100–150 nm in width and 200–400 nm in length using a high-energy ball-milling-assisted sol-gel method (Figure 6b,c). The as-prepared LiMn0.5Fe0.5PO4/C attained considerable electrochemical performance with initial discharge capacities of 128.6 mAhg−1 and capacity retentions of 93.5% and 90.3% after 100 cycles at 1 C and 2 C rates, respectively (Figure 6d). Although nanoparticles provide shorter diffusion lengths and better electrochemical performances, they have low tap density because of their high surface area, which causes low tap density and loading of active material in electrodes [130]. Since spherical particles have the largest tap density, it is crucial to control the particle morphology. The hydrothermal/solvothermal method can produce homogenous and morphology controllable particles. Hydrothermal/solvothermal method involves wet chemical processes that take place in an aqueous solution of mixed precursors above the solvent’s boiling temperature [131]. Cao et al. [132] synthesized LiMnPO4 particles with good sphericity and high tap density using the hydrothermal method with Li3PO4 as the precursor (Figure 6e). This sample exhibited excellent cycling performance with capacity retention of 95.55% after 500 cycles (Figure 6f). Luo et al. [133] synthesized a LiMn0.8Fe0.2PO4/C nanocrystal using a facile solvothermal reaction and studied the transformation law of morphology from nanosheet to nanoellipsoid. By modifying pH value and precursor ions, nanoellipsoid S-2.6 delivers excellent cycling performance and chemical stability.

Figure 6.
(a) Crystallographic structures of LiMnPO4. SEM images (b), TEM images (c) and cycling performance (d) of nano−sized LiMn0.5Fe0.5PO4/C [129]. Process diagram (e) and cycling performance (f) of LiMnPO4/C synthesized by hydrothermal method [132].
Figure 6.
(a) Crystallographic structures of LiMnPO4. SEM images (b), TEM images (c) and cycling performance (d) of nano−sized LiMn0.5Fe0.5PO4/C [129]. Process diagram (e) and cycling performance (f) of LiMnPO4/C synthesized by hydrothermal method [132].
Ion doping at Li, Mn, and O sites can also enhance the electrochemical performances of LiMnPO4. Li-site doping can reduce the charge transfer resistance and broaden the one-dimensional diffusion channels of Li+ [134], but the transition metal in the Li layer will hinder Li+ diffusion to a certain extent [135]. Thus, Mn-site doping has received a lot of attention, such as doping with Fe [136], V [137], Mg [138], Ni [139], Cu [140], Cr [141], and Zn [135]. Oukahou et al. [136] synthesized LiMn1-xMxPO4 (M = Ni, Fe) with improved electronic conductivity and reduced Li+ diffusion energy barrier, by doping Ni2+ and Fe2+ cations at Mn sites. Hu et al. [142] synthesized Fe-doped LiMnPO4 nanoparticles through the solvothermal method, and found that Fe doping can significantly increase the initial reversible capacity, cycle performance and rate capacity. LiMn0.5Fe0.5PO4 exhibits a high discharge capacity of 147 mAh g–1 and nearly 100% capacity retention after 100 cycles at 1 C. Anion doping at O sites can also enhance the electrochemical properties of LiMnPO4 by facilitating Li+ migration of lithium ions in the diffusion channels and enhancing electronic conductivity [134]. Zhang et al. [129] prepared Sulphur-doped LiMn0.5Fe0.5PO4 via a one-step solvothermal process. Since doping Sulphur with a less electronegative atom is advantageous for increasing electronic conductivity, the as-prepared cathode material delivers a high specific discharge capacity of 166.83 mAh g−1 at 0.1 C.
Carbon coating is a common method to increase the conductivity of phospho-olivine materials by enhancing the electron conductivity between particles [143] and improving the contact between the active material and liquid electrolyte. The most important thing for carbon coating is to select high-quality and low-cost carbon sources. Mizuno et al. [144] studied the discharge capacities of LiMnPO4 coated with carbon prepared from different carbon sources and found that LiMnPO4 with carbon obtained from carboxymethyl cellulose exhibits the highest discharge capacity of 94 mAh g−1 at 0.01 C. However, this method needs additional heat treatment to form a carbon coating. The polyol method can efficaciously prepare nanosized particles with in-situ carbon coating. The polyol method includes four main steps, in which the polyol acts as solvent, fuel, energy supplier and carbon source, respectively [145]. Long et al. [146] synthesized LiMnPO4 with a carbon coating using a microwave-assisted polyol method at 130 °C for 30 min. The obtained LiMnPO4/C sample contains a 2 nm thick carbon layer and delivers a discharge capacity of 126 mAh g−1 with a capacity retention of ~99.9% after 50 cycles at 1 C. By using a two-step mechanochemically assisted solid-state synthesis, Podgornova et al. [147] synthesized carbon-coated LiFe0.5Mn0.5PO4 cathode materials. They found that the capacity and rate capability of LiFe0.5Mn0.5PO4 improved owing to the good graphitization of the carbon coating and the tight combination between the carbon coating and LiFe0.5Mn0.5PO4. Although these aforementioned methods can enhance the electrochemical performances of LiMnPO4 and LiFe0.5Mn0.5PO4, obtaining all these desired properties is still challenging. To prepare LiMnPO4 and LiFe0.5Mn0.5PO4 cathodes for high-performance lithium-ion batteries, a simple, economic, easy to control, and reliable synthesis technique still needs to be developed.
4. Summary and Perspectives
Mn-based materials have shown advantages in material cost and energy density among LIBs’ cathode materials, due to their richness in the Earth’s crust and wide range of valance states. This has led to the generation of series of potential cathode materials with low cost per Wh, such as spinel LiMn2O4 and LiNi0.5Mn1.5O4, layered LiNi0.5Mn0.5O2 and Li-rich Li1.2Ni0.2Mn0.6O2, rock-salt Li1.2Mn0.625Nb0.175O1.95F0.05, Olivine LiMnPO4 and LiMnFePO4, etc. However, the Mn dissolution and Jahn–Teller effect of Mn-based materials are not negligible, which is detrimental to electrochemical performance. Element doping and surface coating are the major strategies to solve the above problems, which can efficaciously promote the stability and kinetics of Mn-based materials, then improve the cycling performance and rate capability. Some special problems need special improvement strategies; for example, the oxygen release of Li-rich materials can be eliminated by constructing oxygen vacancy and the stability of DRX compounds can be improved by F fluorination.
Among the materials mentioned in this review, spinel LiMn2O4 has been successfully commercialized, while layered LiNi0.5Mn0.5O2, olivine-type LiMnPO4 and LFMP, as well as LRM have the potential to be applied as cathode materials for LIBs in the near future. The applications of LiNi0.5Mn1.5O4 and DRXs, however, are limited due to their high discharge potential and poor cycle performance, respectively. For the Mn-based cathode materials, some prospects are suggested: (1) achieving a uniform coating by simple methods, (2) introducing doping elements into the precursors, (3) producing high-voltage electrolytes with low cost, and (4) analyzing the synergistic effect of multi-element doping and the influences of different components on the cathode’s electrochemical properties, via theoretical calculations.
Author Contributions
H.Y. and Y.L. contributed equally to the literature search and drafted the majority of this review; Y.Q. drafted the chapter on Li-rich Mn-based oxides; X.Z., Y.F. and Z.L. revised the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cheng, F.; Liang, J.; Tao, Z.; Chen, J. Functional materials for rechargeable batteries. Adv. Mater. 2011, 23, 1695–1715. [Google Scholar] [PubMed]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Scrosati, B. History of lithium batteries. J. Solid State Electrochem. 2011, 15, 1623–1630. [Google Scholar] [CrossRef]
- Grey, C.P.; Tarascon, J.M. Sustainability and in situ monitoring in battery development. Nat. Mater. 2016, 16, 45–56. [Google Scholar]
- Zhang, Y.; Huo, Q.Y.; Du, P.P.; Wang, L.Z.; Zhang, A.Q.; Song, Y.H.; Li, G.Y. Advances in new cathode material LiFePO4 for lithium-ion batteries. Synth. Met. 2012, 162, 1315–1326. [Google Scholar]
- Park, O.K.; Cho, Y.; Lee, S.; Yoo, H.-C.; Song, H.-K.; Cho, J. Who will drive electric vehicles, olivine or spinel? Energy Environ. Sci. 2011, 4, 1621–1633. [Google Scholar]
- Xu, G.; Liu, Z.; Zhang, C.; Cui, G.; Chen, L. Strategies for improving the cyclability and thermo-stability of LiMn2O4-based batteries at elevated temperatures. J. Mater. Chem. A 2015, 3, 4092–4123. [Google Scholar]
- Dou, S. Review and prospect of layered lithium nickel manganese oxide as cathode materials for Li-ion batteries. J. Solid State Electrochem. 2013, 17, 911–926. [Google Scholar]
- Zhang, K.; Li, B.; Zuo, Y.; Song, J.; Shang, H.; Ning, F.; Xia, D. Voltage Decay in Layered Li-Rich Mn-Based Cathode Materials. Electrochem. Energy Rev. 2019, 2, 606–623. [Google Scholar]
- Lee, S.H.; Moon, J.S.; Lee, M.S.; Yu, T.H.; Kim, H.; Park, B.M. Enhancing phase stability and kinetics of lithium-rich layered oxide for an ultra-high performing cathode in Li-ion batteries. J. Power Sources 2015, 281, 77–84. [Google Scholar]
- Xiang, X.; Zhang, K.; Chen, J. Recent Advances and Prospects of Cathode Materials for Sodium-Ion Batteries. Adv. Mater. 2015, 27, 5343–5364. [Google Scholar] [PubMed]
- Han, J.; Li, H.; Kong, D.; Zhang, C.; Tao, Y.; Li, H.; Yang, Q.-H.; Chen, L. Realizing High Volumetric Lithium Storage by Compact and Mechanically Stable Anode Designs. ACS Energy Lett. 2020, 5, 1986–1995. [Google Scholar] [CrossRef]
- Meng, X.; Dou, S.; Wang, W.-L. High power and high capacity cathode material LiNi0.5Mn0.5O2 for advanced lithium-ion batteries. J. Power Sources 2008, 184, 489–493. [Google Scholar]
- Ding, X.; Luo, D.; Cui, J.; Xie, H.; Ren, Q.; Lin, Z. An Ultra-Long-Life Lithium-Rich Li1.2Mn0.6Ni0.2O2 Cathode by Three-in-One Surface Modification for Lithium-Ion Batteries. Angew. Chem. Int. Ed. Engl. 2020, 59, 7778–7782. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Li, N.; Li, L.; Foley, E.E.; Fu, Y.; Battaglia, V.S.; Clément, R.J.; Wang, C.; Tong, W. Redox Behaviors in a Li-Excess Cation-Disordered Mn–Nb–O–F Rocksalt Cathode. Chem. Mater. 2020, 32, 4490–4498. [Google Scholar] [CrossRef]
- Zheng, J.; Xu, P.; Gu, M.; Xiao, J.; Browning, N.D.; Yan, P.; Wang, C.; Zhang, J.-G. Structural and Chemical Evolution of Li- and Mn-Rich Layered Cathode Material. Chem. Mater. 2015, 27, 1381–1390. [Google Scholar] [CrossRef]
- Liu, H.; Harris, K.J.; Jiang, M.; Wu, Y.; Goward, G.R.; Botton, G.A. Unraveling the Rapid Performance Decay of Layered High-Energy Cathodes: From Nanoscale Degradation to Drastic Bulk Evolution. ACS Nano 2018, 12, 2708–2718. [Google Scholar] [CrossRef]
- Hao, G.; Lai, Q.; Zhang, H. Nanostructured Mn-based oxides as high-performance cathodes for next generation Li-ion batteries. J. Energy Chem. 2021, 59, 547–571. [Google Scholar]
- Zhao, S.-X.; Liu, H.-X.; Ouyang, S.-X. Synthesis and Performance of LiMnO2 as Cathodes for Li-ion Batteries. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2003, 18, 5–8. [Google Scholar]
- Ceder, G. The Stability of Orthorhombic and Monoclinic-Layered LiMnO2. Electrochem. Solid-State Lett. 1999, 2, 550–552. [Google Scholar] [CrossRef]
- Armstrong, A.R.; Bruce, P.G. Synthesis of layered LiMnO2 as an electrode for rechargeable lithium batteries. Nature 1996, 381, 499–500. [Google Scholar]
- Kim, T.-J.; Son, D.; Cho, J.; Park, B. Enhancement of the electrochemical properties of o-LiMnO2 cathodes at elevated temperature by lithium and fluorine additions. J. Power Sources 2006, 154, 268–272. [Google Scholar]
- He, P.; Yu, H.; Li, D.; Zhou, H. Layered lithium transition metal oxide cathodes towards high energy lithium-ion batteries. J. Mater. Chem. 2012, 22, 3680–3695. [Google Scholar]
- Makimura, Y.; Ohzuku, T. Lithium insertion material of LiNi1/2Mn1/2O2 for advanced lithium-ion batteries. J. Power Sources 2003, 119–121, 156–160. [Google Scholar]
- Islam, M.S.; Davies, R.A.; Gale, J.D. Structural and electronic properties of the layered LiNi0.5Mn0.5O2 lithium battery material. Chem. Mater. 2003, 15, 4280–4286. [Google Scholar]
- Li, H.; Xu, Q.; Shi, X.-X.; Song, D.-W.; Zhang, L.-Q. Electrochemical performance of LiNi0.5Mn0.5O2 with different synthesis methods. Rare Met. 2013, 34, 580–585. [Google Scholar]
- Liu, Y.; Chen, B.; Cao, F.; Zhao, X.; Yuan, J. Synthesis of nanoarchitectured LiNi0.5Mn0.5O2 spheres for high-performance rechargeable lithium-ion batteries via an in situ conversion route. J. Mater. Chem. 2011, 21, 10437–10441. [Google Scholar]
- Liu, Y.; Cao, F.; Chen, B.; Zhao, X.; Suib, S.L.; Chan, H.L.W.; Yuan, J. High performance of LiNi0.5Mn0.5O2 positive electrode boosted by ordered three-dimensional nanostructures. J. Power Sources 2012, 206, 230–235. [Google Scholar] [CrossRef]
- Jia, G.; Liu, S.; Yang, G.; Li, F.; Wu, K.; He, Z.; Shangguan, X. The multiple effects of Al-doping on the structure and electrochemical performance of LiNi0.5Mn0.5O2 as cathode material at high voltage. Ionics 2018, 24, 3705–3715. [Google Scholar]
- Dou, S.; Wang, W.-l.; Li, H.; Xin, X. Synthesis and electrochemical performance of LiNi0.475Mn0.475Al0.05O as cathode material for lithium-ion battery from Ni–Mn–Al–O precursor. J. Solid State Electrochem. 2010, 15, 747–751. [Google Scholar] [CrossRef]
- Nithya, C.; Lakshmi, R.; Gopukumar, S. Effect of Mg Dopant on the Electrochemical Performance of LiNi0.5Mn0.5O2 Cathode Materials for Lithium Rechargeable Batteries. J. Electrochem. Soc. 2012, 159, A1335–A1340. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, H.; Tan, L.; Yuan, S.; Yin, H. Ba-doping to Improve the Cycling Stability of LiNi0.5Mn0.5O2 Cathode Materials for Batteries Operating at High Voltage. Energy Technol. 2018, 6, 1302–1309. [Google Scholar]
- Jia, G.; Shangguan, X.; Liu, S.; He, Z. Improving the rate performance of LiNi0.5Mn0.5O2 material at high voltages by Cu-doping. Ionics 2020, 26, 4969–4976. [Google Scholar]
- Hu, G.; Shi, Y.; Fan, J.; Cao, Y.; Peng, Z.; Zhang, Y.; Zhu, F.; Sun, Q.; Xue, Z.; Liu, Y.; et al. Sb doping and Sb2O3 coating collaboration to improve the electrochemical performance of LiNi0.5Mn0.5O2 cathode material for lithium ion batteries. Electrochim. Acta 2020, 364, 137127. [Google Scholar]
- Darbar, D.; Self, E.C.; Li, L.; Wang, C.; Meyer, H.M.; Lee, C.; Croy, J.R.; Balasubramanian, M.; Muralidharan, N.; Bhattacharya, I.; et al. New synthesis strategies to improve Co-Free LiNi0.5Mn0.5O2 cathodes: Early transition metal d0 dopants and manganese pyrophosphate coating. J. Power Sources 2020, 479, 228591. [Google Scholar]
- Shangguan, X.; Wang, Q.; Yang, G.; Jia, G.; Li, F. New insights into improving electrochemical performances of LiNi0.5Mn0.5O2 cathode material by Li2ZrO3 coating and Zr4+ doping. Ionics 2019, 25, 4547–4556. [Google Scholar]
- Jia, G.; Shangguan, X.; Li, F.; Liu, S.; He, Z. Effect of Si doping on the structure and electrochemical performance of high-voltage LiNi0.5Mn0.5O2 cathode. Ionics 2019, 25, 5259–5267. [Google Scholar]
- Li, L.; Yu, J.; Darbar, D.; Self, E.C.; Wang, D.; Nanda, J.; Bhattacharya, I.; Wang, C. Atomic-Scale Mechanisms of Enhanced Electrochemical Properties of Mo-Doped Co-Free Layered Oxide Cathodes for Lithium-Ion Batteries. ACS Energy Lett. 2019, 4, 2540–2546. [Google Scholar]
- Hu, C.; Guo, J.; Wen, J.; Peng, Y.; Chen, Y. Preparation and electrochemical performance of LiNi0.5Mn0.5O2−xFx (0⩽x⩽0.04) cathode material synthesized with hydroxide co-precipitation for lithium ion batteries. J. Alloy. Compd. 2013, 581, 121–127. [Google Scholar]
- Jia, G.; Li, F.; Wang, J.; Liu, S.; Yang, Y. Dual Substitution Strategy in Co-Free Layered Cathode Materials for Superior Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 18733–18742. [Google Scholar] [CrossRef]
- Hashem, A.M.; Ghany, A.E.A.; Nikolowski, K.; Ehrenberg, H. Effect of carbon coating process on the structure and electrochemical performance of LiNi0.5Mn0.5O2 used as cathode in Li-ion batteries. Ionics 2009, 16, 305–310. [Google Scholar]
- Jia, G.; Liu, S.; Yang, G.; Li, F.; Wu, K.; He, Z.; Shangguan, X. Effects of Li2TiO3 coating on the structure and the electrochemical properties of LiNi0.5Mn0.5O2 cathode materials at high voltages. Ionics 2019, 25, 399–410. [Google Scholar]
- Numata, K.; Sakaki, C.; Yamanaka, S. Synthesis of solid solutions in a system of LiCoO2-Li2MnO3 for cathode materials of secondary lithium batteries. Chem. Lett. 1997, 8, 725–726. [Google Scholar] [CrossRef]
- Kalyani, P.; Chitra, S.; Mohan, T.; Gopukumar, S. Lithium metal rechargeable cells using Li2MnO3 as the positive electrode. J. Power Sources 1999, 80, 103–106. [Google Scholar] [CrossRef]
- Seo, D.H.; Lee, J.; Urban, A.; Malik, R.; Kang, S.; Ceder, G. The structural and chemical origin of the oxygen redox activity in layered and cation-disordered Li-excess cathode materials. Nat. Chem. 2016, 8, 692–697. [Google Scholar]
- Chong, S.; Chen, Y.; Yan, W.; Guo, S.; Tan, Q.; Wu, Y.; Jiang, T.; Liu, Y. Suppressing capacity fading and voltage decay of Li-rich layered cathode material by a surface nano-protective layer of CoF2 for lithium-ion batteries. J. Power Sources 2016, 332, 230–239. [Google Scholar]
- Xiao, B.; Wang, P.-B.; He, Z.-J.; Yang, Z.; Tang, L.-B.; An, C.-S.; Zheng, J.-C. Effect of MgO and TiO2 Coating on the Electrochemical Performance of Li-Rich Cathode Materials for Lithium-Ion Batteries. Energy Technol. 2019, 7, 1800829. [Google Scholar]
- Hu, E.; Yu, X.; Lin, R.; Bi, X.; Lu, J.; Bak, S.; Nam, K.-W.; Xin, H.L.; Jaye, C.; Fischer, D.A.; et al. Evolution of redox couples in Li- and Mn-rich cathode materials and mitigation of voltage fade by reducing oxygen release. Nat. Energy 2018, 3, 690–698. [Google Scholar] [CrossRef]
- Yu, X.; Lyu, Y.; Gu, L.; Wu, H.; Bak, S.-M.; Zhou, Y.; Amine, K.; Ehrlich, S.N.; Li, H.; Nam, K.-W.; et al. Understanding the Rate Capability of High-Energy-Density Li-Rich Layered Li1.2Ni0.15Co0.1Mn0.55O2 Cathode Materials. Adv. Energy Mater. 2014, 4, 1300950. [Google Scholar]
- Ates, M.N.; Mukerjee, S.; Abraham, K.M. A Li-Rich Layered Cathode Material with Enhanced Structural Stability and Rate Capability for Li-on Batteries. J. Electrochem. Soc. 2014, 161, A355–A363. [Google Scholar] [CrossRef]
- Shukla, A.K.; Ramasse, Q.M.; Ophus, C.; Kepaptsoglou, D.M.; Hage, F.S.; Gammer, C.; Venkatachalam, S. present. Energy Environ. Sci. 2018, 11, 830–840. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Y.; Martha, S.K.; Wu, Z.; Andrews, J.C.; Ice, G.E.; Nanda, J. Nanoscale morphological and chemical changes of high voltage lithium–manganese rich NMC composite cathodes with cycling. Nano Lett. 2014, 14, 4334–4341. [Google Scholar] [PubMed]
- Schmidt, B.; Wetzig, K. Ion Beams in Materials Processing and Analysis; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Park, M.S. First-principles study of native point defects in LiNi1/3Co1/3Mn1/3O2 and Li2MnO3. Phys. Chem. Chem. Phys. 2014, 16, 16798–16804. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Guo, W.; Wu, H.; Lin, L.; Liu, Q.; Han, X.; Xie, Q.; Liu, P.; Zheng, H.; Wang, L.; et al. Challenges and Recent Advances in High Capacity Li-Rich Cathode Materials for High Energy Density Lithium-Ion Batteries. Adv. Mater. 2021, 33, e2005937. [Google Scholar] [PubMed]
- Qiu, B.; Zhang, M.; Wu, L.; Wang, J.; Xia, Y.; Qian, D.; Liu, H.; Hy, S.; Chen, Y.; An, K.; et al. Gas-solid interfacial modification of oxygen activity in layered oxide cathodes for lithium-ion batteries. Nat. Commun. 2016, 7, 12108. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhang, C.; Zhang, Y.; Lin, L.; He, W.; Xie, Q.; Sa, B.; Wang, L.; Peng, D.L. A Universal Strategy toward the Precise Regulation of Initial Coulombic Efficiency of Li-Rich Mn-Based Cathode Materials. Adv. Mater. 2021, 33, e2103173. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Yu, D.; Yang, Y.; Su, C.; Huang, Y.; Dong, Y.; Waluyo, I.; Wang, B.; Hunt, A.; Yao, X.; et al. Gradient Li-rich oxide cathode particles immunized against oxygen release by a molten salt treatment. Nat. Energy 2019, 4, 1049–1058. [Google Scholar]
- Chen, L.; Su, Y.; Chen, S.; Li, N.; Bao, L.; Li, W.; Wang, Z.; Wang, M.; Wu, F. Hierarchical Li1.2Ni0.2Mn0.6O2 nanoplates with exposed 010 planes as high-performance cathode material for lithium-ion batteries. Adv. Mater. 2014, 26, 6756–6760. [Google Scholar] [CrossRef]
- Yokozaki, R.; Kobayashi, H.; Honma, I. Reductive solvothermal synthesis of MgMn2O4 spinel nanoparticles for Mg-ion battery cathodes. Ceram. Int. 2021, 47, 10236–10241. [Google Scholar] [CrossRef]
- Ruiz, R.; Perez-Vicente, C.; Rubio, S.; Stoyanova, R.; Zuo, W.; Yang, Y.; Ortiz, G.F. A Cubic Mg2MnO4 Cathode for non-aqueous Magnesium Batteries. Energy Storage Mater. 2022, 48, 12–19. [Google Scholar] [CrossRef]
- Thackeray, M.M. Manganese oxides for lithium batteries. Prog. Solid State Chem. 1996, 25, 1–71. [Google Scholar] [CrossRef]
- Ohzuku, T.; Kitagawa, M.; Hirai, T. Electrochemistry of manganese dioxide in lithium nonaqueous cell. III. X-ray diffractional study on the reduction of spinel-related manganese dioxide. J. Electrochem. Soc. 1990, 137, 769–775. [Google Scholar] [CrossRef]
- Gummow, R.J.; De Kock, A.; Thackeray, M.M. Improved capacity retention in rechargeable 4 V lithium lithium-manganese oxide (spinel) cells. Solid State Ion. 1994, 69, 59–67. [Google Scholar] [CrossRef]
- Jang, D.H.; Shin, Y.J.; Oh, S.M. Dissolution of Spinel Oxides and Capacity Losses in 4 V Li/LixMn2O4 Cells. J. Electrochem. Soc. 1996, 143, 2204–2211. [Google Scholar] [CrossRef]
- Yi, T.F.; Zhu, Y.R.; Zhu, X.D.; Shu, J.; Yue, C.B.; Zhou, A.N. A review of recent developments in the surface modification of LiMn2O4 as cathode material of power lithium-ion battery. Ionics 2009, 15, 779–784. [Google Scholar] [CrossRef]
- Young, M.J.; Letourneau, S.; Warburton, R.E.; Dose, W.M.; Johnson, C.; Greeley, J.; Elam, J.W. High-Rate Spinel LiMn2O4 (LMO) Following Carbonate Removal and Formation of Li-Rich Interface by ALD Treatment. J. Phys. Chem. C 2019, 123, 23783–23790. [Google Scholar] [CrossRef]
- Scivetti, I.; Teobaldi, G. (Sub) surface-promoted disproportionation and absolute band alignment in high-power LiMn2O4 cathodes. J. Phys. Chem. C 2015, 119, 21358–21368. [Google Scholar] [CrossRef]
- Zhai, H.; Du, X.; Wang, F.; Wang, D.; Li, Y.; Chen, K. Improvement of electrochemical performance of Li1.96Mg0.02MnO3 by surface treatment. Ionics 2020, 26, 2129–2137. [Google Scholar]
- Abbas, S.M.; Hashem, A.M.; Abdel-Ghany, A.E.; Ismail, E.H.; Kotlár, M.; Winter, M.; Julien, C.M. Ag-modified LiMn2O4 cathode for lithium-ion batteries: Coating functionalization. Energies 2020, 13, 5194. [Google Scholar] [CrossRef]
- Bi, Z.; Zhao, N.; Ma, L.; Shi, C.; Fu, Z.; Xu, F.; Guo, X. Surface coating of LiMn2O4 cathodes with garnet electrolytes for improving cycling stability of solid lithium batteries. J. Mater. Chem. A 2020, 8, 4252–4256. [Google Scholar] [CrossRef]
- Yang, J.; Han, X.; Zhang, X.; Cheng, F.; Chen, J. Spinel LiNi0.5Mn1.5O4 cathode for rechargeable lithiumion batteries: Nano vs. micro, ordered phase (P4332) vs. disordered phase (Fd3m). Nano Res. 2013, 6, 679–687. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Z.; Zeng, W.; Cao, F.; Ma, J.; Tian, W.; Mu, S. Synthesis, Modification, and Lithium-Storage Properties of Spinel LiNi0.5Mn1.5O4. ChemElectroChem 2021, 8, 608–624. [Google Scholar]
- Xu, X.; Deng, S.; Wang, H.; Liu, J.; Yan, H. Research Progress in Improving the Cycling Stability of High-Voltage LiNi0.5Mn1.5O4 Cathode in Lithium-Ion Battery. Nanomicro. Lett. 2017, 9, 22. [Google Scholar] [PubMed]
- Sun, Y.K.; Yoon, C.S.; Kim, C.K.; Youn, S.G.; Lee, Y.S.; Yoshio, M.; Oh, I.H. Degradation mechanism of spinel LiAl0.2Mn1.8O4 cathode materials on high temperature cycling. J. Mater. Chem. 2001, 11, 2519–2522. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, H.; Zhang, Q.; Nakamura, H.; Noguchi, H.; Yoshio, M. Oxygen deficiency, a key factor in controlling the cycle performance of Mn-spinel cathode for lithium-ion batteries. J. Power Sources 2007, 166, 485–491. [Google Scholar] [CrossRef]
- Xiao, L.; Zhao, Y.; Yang, Y.; Cao, Y.; Ai, X.; Yang, H. Enhanced electrochemical stability of Al-doped LiMn2O4 synthesized by a polymer-pyrolysis method. Electrochim. Acta 2008, 54, 545–550. [Google Scholar] [CrossRef]
- Sun, X.; Hu, X.; Shi, Y.; Li, S.; Zhou, Y. The study of novel multi-doped spinel Li1.15Mn1.96Co0.03Gd0.01O4+δ as cathode material for Li-ion rechargeable batteries. Solid State Ion. 2009, 180, 377–380. [Google Scholar] [CrossRef]
- Choi, W.; Manthiram, A. Factors Controlling the Fluorine Content and the Electrochemical Performance of Spinel Oxyfluoride Cathodes. J. Electrochem. Soc. 2007, 154, A792–A797. [Google Scholar] [CrossRef]
- Kong, F.; Liang, C.; Longo, R.C.; Yeon, D.-H.; Zheng, Y.; Park, J.-H.; Doo, S.-G.; Cho, K. Conflicting Roles of Anion Doping on the Electrochemical Performance of Li-Ion Battery Cathode Materials. Chem. Mater. 2016, 28, 6942–6952. [Google Scholar] [CrossRef]
- Chong, J.; Xun, S.; Zhang, J.; Song, X.; Xie, H.; Battaglia, V.; Wang, R. Li3PO4-coated LiNi0.5Mn1.5O4: A stable high-voltage cathode material for lithium-ion batteries. Chemistry 2014, 20, 7479–7485. [Google Scholar] [CrossRef]
- Jang, W.H.; Kim, M.C.; Kim, S.H.; Aravindan, V.; Kim, W.S.; Yoon, W.S.; Lee, Y.S. Understanding the exceptional elevated temperature performance of high voltage LiNi0.5Mn1.5O4 cathodes by LiFePO4 modification. Electrochim. Act 2014, 137, 404–410. [Google Scholar] [CrossRef]
- Fang, X.; Ge, M.; Rong, J.; Che, Y.; Aroonyadet, N.; Wang, X.; Liu, Y.; Zhang, A.; Zhou, C. Ultrathin Surface Modification by Atomic Layer Deposition on High Voltage Cathode LiNi0.5Mn1.5O4 for Lithium Ion Batteries. Energy Technol. 2014, 2, 159–165. [Google Scholar] [CrossRef]
- Fang, X.; Ge, M.; Rong, J.; Zhou, C. Graphene-oxide-coated LiNi0.5Mn1.5O4 as high voltage cathode for lithium ion batteries with high energy density and long cycle life. J. Mater. Chem. A 2013, 1, 4083–4088. [Google Scholar] [CrossRef]
- Gao, X.-W.; Deng, Y.-F.; Wexler, D.; Chen, G.-H.; Chou, S.-L.; Liu, H.-K.; Shi, Z.-C.; Wang, J.-Z. Improving the electrochemical performance of the LiNi0.5Mn1.5O4 spinel by polypyrrole coating as a cathode material for the lithium-ion battery. J. Mater. Chem. A 2015, 3, 404–411. [Google Scholar] [CrossRef]
- Kim, M.C.; Kim, S.H.; Aravindan, V.; Kim, W.S.; Lee, S.Y.; Lee, Y.S. Ultrathin Polyimide Coating for a Spinel LiNi0.5Mn1.5O4 Cathode and Its Superior Lithium Storage Properties under Elevated Temperature Conditions. J. Electrochem. Soc. 2013, 160, A1003–A1008. [Google Scholar] [CrossRef]
- Fang, H.-S.; Wang, Z.-X.; Li, X.-H.; Guo, H.-J.; Peng, W.-J. Exploration of high capacity LiNi0.5Mn1.5O4 synthesized by solid-state reaction. J. Power Sources 2006, 153, 174–176. [Google Scholar] [CrossRef]
- Wang, Z.; Su, Q.; Deng, H.; Fu, Y. Oxygen Deficiency and Defect Chemistry in Delithiated Spinel LiNi0.5Mn1.5O4 Cathodes for Li-Ion Batteries. ChemElectroChem 2015, 2, 1182–1186. [Google Scholar] [CrossRef]
- Rosedhi, N.D.; Idris, N.H. Electrochemical performance of LiNi0.5Mn1.5O4 synthesised via ball-milling for Li-ion batteries. Ionics 2018, 25, 2069–2076. [Google Scholar] [CrossRef]
- Santhanam, R.; Rambabu, B. Research progress in high voltage spinel LiNi0.5Mn1.5O4 material. J. Power Sources 2010, 195, 5442–5451. [Google Scholar] [CrossRef]
- Cui, X.-L.; Li, Y.-L.; Li, S.-Y.; Li, L.-X.; Liu, J.-L. Nanosized LiNi0.5Mn1.5O4 spinels synthesized by a high-oxidation-state manganese sol–gel method. Ionics 2013, 19, 1489–1494. [Google Scholar] [CrossRef]
- Chen, W.; Kume, S.; Watari, K. Molten salt synthesis of 0.94Na1/2Bi1/2TiO3–0.06BaTiO3 powder. Mater. Lett. 2005, 59, 3238–3240. [Google Scholar] [CrossRef]
- Wen, L.; Lu, Q.; Xu, G. Molten salt synthesis of spherical LiNi0.5Mn1.5O4 cathode materials. Electrochim. Acta 2006, 51, 4388–4392. [Google Scholar] [CrossRef]
- Li, B.; Zhao, J.; Zhang, Z.; Zhao, C.; Sun, P.; Bai, P.; Yang, J.; Zhou, Z.; Xu, Y. Electrolyte-Regulated Solid-Electrolyte Interphase Enables Long Cycle Life Performance in Organic Cathodes for Potassium-Ion Batteries. Adv. Funct. Mater. 2019, 29, 1807137. [Google Scholar] [CrossRef]
- Li, Y.; Wan, S.; Veith, G.M.; Unocic, R.R.; Paranthaman, M.P.; Dai, S.; Sun, X.-G. A Novel Electrolyte Salt Additive for Lithium-Ion Batteries with Voltages Greater than 4.7 V. Adv. Energy Mater. 2017, 7, 1601397. [Google Scholar]
- Kasnatscheew, J.; Schmitz, R.W.; Wagner, R.; Winter, M.; Schmitz, R. Fluoroethylene carbonate as an additive for γ-butyrolactone based electrolytes. J. Electrochem. Soc. 2013, 160, A1369. [Google Scholar] [CrossRef]
- Mauger, A.; Julien, C.M.; Paolella, A.; Armand, M.; Zaghib, K. A comprehensive review of lithium salts and beyond for rechargeable batteries: Progress and perspectives. Mater. Sci. Eng. R Rep. 2018, 134, 1–21. [Google Scholar]
- Yao, Y.; Guo, B.; Ji, L.; Jung, K.H.; Lin, Z.; Alcoutlabi, M.; Zhang, X. Highly proton conductive electrolyte membranes: Fiber-induced long-range ionic channels. Electrochem. Commun. 2011, 13, 1005–1008. [Google Scholar] [CrossRef]
- Asl, H.Y.; Manthiram, A. Reining in dissolved transition-metal ions. Science 2020, 369, 140–141. [Google Scholar] [CrossRef]
- Li, Y.; Wang, K.; Chen, J.; Zhang, W.; Luo, X.; Hu, Z. Stabilized high-voltage cathodes via an f-rich and si-containing electrolyte additive. ACS Appl. Mater. Interfaces 2020, 12, 28169–28178. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, L.; Wu, H.; Weng, W.; Koh, M.; Redfern, P.C.; Curtiss, L.A.; Amine, K. Fluorinated electrolytes for 5 V lithium-ion battery chemistry. Energy Environ. Sci. 2013, 6, 1806–1810. [Google Scholar] [CrossRef]
- Xiao, D.-L.; Tong, J.; Feng, Y.; Zhong, G.-H.; Li, W.-J.; Yang, C.-L. Improved performance of all-solid-state lithium batteries using LiPON electrolyte prepared with Li-rich sputtering target. Solid State Ion. 2018, 324, 202–206. [Google Scholar] [CrossRef]
- Li, J.; Ma, C.; Chi, M.; Liang, C.; Dudney, N.J. Solid Electrolyte: The Key for High-Voltage Lithium Batteries. Adv. Energy Mater. 2015, 5, 1401408. [Google Scholar] [CrossRef]
- Clément, R.J.; Lun, Z.; Ceder, G. Cation-disordered rocksalt transition metal oxides and oxyfluorides for high energy lithium-ion cathodes. Energy Environ. Sci. 2020, 13, 345–373. [Google Scholar]
- Lee, J.; Urban, A.; Li, X.; Su, D.; Hautier, G.; Ceder, G. Unlocking the Potential of Cation-Disordered Oxides for Rechargeable Lithium Batteries. Science 2014, 343, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.; Ceder, G. Role of Electronic Structure in the Susceptibility of Metastable Transition-Metal Oxide Structures to Transformation. Chem. Rev. 2004, 104, 4513–4534. [Google Scholar] [CrossRef]
- Zhao, X.; Tian, Y.; Lun, Z.; Cai, Z.; Chen, T.; Ouyang, B.; Ceder, G. Design principles for zero-strain Li-ion cathodes. Joule 2022, 6, 1654–1671. [Google Scholar]
- Lee, J.; Kitchaev, D.A.; Kwon, D.H.; Lee, C.W.; Papp, J.K.; Liu, Y.S.; Lun, Z.; Clement, R.J.; Shi, T.; McCloskey, B.D.; et al. Reversible Mn(2+)/Mn(4+) double redox in lithium-excess cathode materials. Nature 2018, 556, 185–190. [Google Scholar] [CrossRef]
- Lun, Z.; Ouyang, B.; Cai, Z.; Clément, R.J.; Kwon, D.-H.; Huang, J.; Papp, J.K.; Balasubramanian, M.; Tian, Y.; McCloskey, B.D.; et al. Design Principles for High-Capacity Mn-Based Cation-Disordered Rocksalt Cathodes. Chem 2020, 6, 153–168. [Google Scholar]
- Lun, Z.; Ouyang, B.; Kitchaev, D.A.; Clément, R.J.; Papp, J.K.; Balasubramanian, M.; Tian, Y.; Lei, T.; Shi, T.; McCloskey, B.D.; et al. Improved Cycling Performance of Li-Excess Cation-Disordered Cathode Materials upon Fluorine Substitution. Adv. Energy Mater. 2018, 9, 1802959. [Google Scholar] [CrossRef]
- Ahn, J.; Chen, D.; Chen, G. A Fluorination Method for Improving Cation-Disordered Rocksalt Cathode Performance. Adv. Energy Mater. 2020, 10, 2001671. [Google Scholar] [CrossRef]
- Padhi, A.; Nanjundaswamy, K.S.; Goodenough, J. Phospho-olivines as Positive-Electrode Materials for Rechargeable Lithium Batteries. J. Electrochem. Soc. 1997, 144, 1188. [Google Scholar] [CrossRef]
- Nwachukwu, I.M.; Nwanya, A.C.; Ekwealor, A.B.C.; Ezema, F.I. Recent progress in Mn and Fe-rich cathode materials used in Li-ion batteries. J. Energy Storage 2022, 54, 105248. [Google Scholar]
- Wang, Y.; Wu, C.-Y.; Yang, H.; Duh, J.-G. Rational design of a synthetic strategy, carburizing approach and pore-forming pattern to unlock the cycle reversibility and rate capability of micro-agglomerated LiMn0.8Fe0.2PO4 cathode materials. J. Mater. Chem. A 2018, 6, 10395–10403. [Google Scholar] [CrossRef]
- Gardiner, G.R.; Islam, M.S. Anti-site defects and ion migration in the LiFe0. 5Mn0. 5PO4 mixed-metal cathode material. Chem. Mater. 2010, 22, 1242–1248. [Google Scholar] [CrossRef]
- Paolella, A.; Bertoni, G.; Marras, S.; Dilena, E.; Colombo, M.; Prato, M.; George, C. Etched colloidal LiFePO4 nanoplatelets toward high-rate capable Li-ion battery electrodes. Nano Lett. 2014, 14, 6828–6835. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Hou, M.; Yuan, S.; Yu, H.; Wang, Y.; Wang, C.; Xia, Y. An additional discharge plateau of Mn3+ in LiFe0.5Mn0.5PO4 at high current rates. Electrochem. Commun. 2015, 55, 6–9. [Google Scholar] [CrossRef]
- Bao, L.; Chen, Y.; Xu, G.; Yang, T.; Ji, Z. Hydrothermal Synthesis of Monodispersed LiMnPO4 (010) Nanobelts and [001] Nanorods and Their Applications in Lithium-Ion Batteries. Eur. J. Inorg. Chem. 2018, 2018, 1533–1539. [Google Scholar] [CrossRef]
- Chen, L.; Dilena, E.; Paolella, A.; Bertoni, G.; Ansaldo, A.; Colombo, M.; Monaco, S. Relevance of LiPF6 as etching agent of LiMnPO4 colloidal nanocrystals for high rate performing Li-ion battery cathodes. ACS Appl. Mater. Interfaces 2016, 8, 4069–4075. [Google Scholar] [CrossRef]
- Han, J.; Yang, J.; Xu, Z.; Li, H.; Wang, J. Dramatic improvement in high-rate capability of LiMnPO4 nanosheets via crystallite size regulation. J. Alloy. Compd. 2022, 894, 162510. [Google Scholar] [CrossRef]
- Min, H.; Shu, H.; Zhou, Y.; Liang, Q.; Li, X.; Ren, Q.; Wang, X. LiMnPO4 nanoplates with optimal crystal orientation in situ anchored on the expanded graphite for high-rate and long-life lithium ion batteries. Electrochim. Acta 2020, 359, 136945. [Google Scholar]
- Bao, L.; Xu, G.; Wang, J.; Zong, H.; Li, L.; Zhao, R.; Han, G. Hydrothermal synthesis of flower-like LiMnPO4 nanostructures self-assembled with (010) nanosheets and their application in Li-ion batteries. CrystEngComm 2015, 17, 6399–6405. [Google Scholar] [CrossRef]
- Ni, J.; Morishita, M.; Kawabe, Y.; Watada, M.; Takeichi, N.; Sakai, T. Hydrothermal preparation of LiFePO4 nanocrystals mediated by organic acid. J. Power Sources 2010, 195, 2877–2882. [Google Scholar] [CrossRef]
- Cheng, G.; Zuo, P.; Wang, L.; Shi, W.; Ma, Y.; Du, C.; Cheng, X.; Gao, Y.; Yin, G. High-performance carbon-coated LiMnPO4 nanocomposites by facile two-step solid-state synthesis for lithium-ion battery. J. Solid State Electrochem. 2014, 19, 281–288. [Google Scholar]
- Satyavani, T.V.S.L.; Kumar, A.S.; Rao, P.S.V.S. Methods of synthesis and performance improvement of lithium iron phosphate for high rate Li-ion batteries: A review. Eng. Sci. Technol. Int. J. 2016, 19, 178–188. [Google Scholar]
- Fu, L.; Liu, H.; Li, C.; Wu, Y.; Rahm, E.; Holze, R.; Wu, H. Electrode materials for lithium secondary batteries prepared by sol–gel methods. Prog. Mater. Sci. 2005, 50, 881–928. [Google Scholar] [CrossRef]
- Chen, G.; Shukla, A.K.; Song, X.; Richardson, T.J. Improved kinetics and stabilities in Mg-substituted LiMnPO4. J. Mater. Chem. 2011, 21, 10126–10133. [Google Scholar] [CrossRef]
- Kwon, N.H.; Drezen, T.; Exnar, I.; Teerlinck, I.; Isono, M.; Graetzel, M. Enhanced Electrochemical Performance of Mesoparticulate LiMnPO4 for Lithium Ion Batteries. Electrochem. Solid-State Lett. 2006, 32, 14–18. [Google Scholar]
- Liu, L.; Chen, G.; Du, B.; Cui, Y.; Ke, X.; Liu, J.; Guo, Z.; Shi, Z.; Zhang, H.; Chou, S. Nano-sized cathode material LiMn0.5Fe0.5PO4/C synthesized via improved sol-gel routine and its magnetic and electrochemical properties. Electrochim. Acta 2017, 255, 205–211. [Google Scholar]
- Kwon, N.H.; Yin, H.; Vavrova, T.; Lim, J.H.W.; Steiner, U.; Grobéty, B.; Fromm, K.M. Nanoparticle shapes of LiMnPO4, Li+ diffusion orientation and diffusion coefficients for high volumetric energy Li+ ion cathodes. J. Power Sources 2017, 342, 231–240. [Google Scholar] [CrossRef]
- Devaraju, M.K.; Honma, I. Hydrothermal and Solvothermal Process Towards Development of LiMPO4 (M = Fe, Mn) Nanomaterials for Lithium-Ion Batteries. Adv. Energy Mater. 2012, 2, 284–297. [Google Scholar]
- Cao, S.Y.; Chang, L.J.; Luo, S.H.; Bi, X.L.; Wei, A.L.; Liu, J.N. Investigations on Preparation and Electrochemical Performance Optimization of LiMnPO4/C Composites with High Tap Density. Part. Part. Syst. Charact. 2021, 39, 2100203. [Google Scholar] [CrossRef]
- Luo, T.; Zeng, T.-T.; Chen, S.-L.; Li, R.; Fan, R.-Z.; Chen, H.; Han, S.-C.; Fan, C.-L. Structure, performance, morphology and component transformation mechanism of LiMn0.8Fe0.2PO4/C nanocrystal with excellent stability. J. Alloy. Compd. 2020, 834, 155143. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, L.; Liu, Y.; Li, L. Review—Recent Developments in the Doped LiFePO4 Cathode Materials for Power Lithium Ion Batteries. J. Electrochem. Soc. 2016, 163, A2600–A2610. [Google Scholar] [CrossRef]
- Yi, H.; Hu, C.; He, X.; Xu, H. Electrochemical performance of LiMnPO4 by Fe and Zn co-doping for lithium-ion batteries. Ionics 2014, 21, 667–671. [Google Scholar] [CrossRef]
- Oukahou, S.; Elomrani, A.; Maymoun, M.; Sbiaai, K.; Hasnaoui, A. Investigation of LiMn1-xMxPO4 (M = Ni, Fe) as cathode materials for Li-ion batteries using density functional theory. Comput. Mater. Sci. 2022, 202, 111006. [Google Scholar]
- Bedoya-Lora, F.E.; Vásquez-Salgado, V.; Vásquez, F.A.; Calderón, J.A. Stable V-doped LiMnPO4/C cathode material for Li-ion batteries produced by a fast and facile microwave-assisted synthesis. J. Alloy. Compd. 2023, 938, 168445. [Google Scholar] [CrossRef]
- Rao, B.N.; Narsimulu, D.; Satyanarayana, N. Effect of Mg doping on the electrical, dielectric and relaxation properties of LiMnPO4 nanoparticles. Indian J. Phys. 2022, 96, 1017–1023. [Google Scholar]
- Oukahou, S.; Maymoun, M.; Elomrani, A.; Sbiaai, K.; Hasnaoui, A. Enhancing the Electrochemical Performance of Olivine LiMnPO4 as Cathode Materials for Li-Ion Batteries by Ni–Fe Codoping. ACS Appl. Energy Mater. 2022, 5, 10591–10603. [Google Scholar] [CrossRef]
- Ni, J.; Gao, L. Effect of copper doping on LiMnPO4 prepared via hydrothermal route. J. Power Sources 2011, 196, 6498–6501. [Google Scholar] [CrossRef]
- Gan, Y.; Chen, C.; Liu, J.; Bian, P.; Hao, H.; Yu, A. Enhancing the performance of LiMnPO4/C composites through Cr doping. J. Alloy. Compd. 2015, 620, 350–357. [Google Scholar] [CrossRef]
- Hu, L.; Qiu, B.; Xia, Y.; Qin, Z.; Qin, L.; Zhou, X.; Liu, Z. Solvothermal synthesis of Fe-doping LiMnPO4 nanomaterials for Li-ion batteries. J. Power Sources 2014, 248, 246–252. [Google Scholar] [CrossRef]
- Liu, S.; Fang, H.; Dai, E.; Yang, B.; Yao, Y.; Ma, W.; Dai, Y. Effect of carbon content on properties of LiMn0.8Fe0.19Mg0.01PO4/C composite cathode for lithium ion batteries. Electrochim. Acta 2014, 116, 97–102. [Google Scholar] [CrossRef]
- Mizuno, Y.; Kotobuki, M.; Munakata, H.; Kanamura, K. Effect of carbon source on electrochemical performance of carbon coated LiMnPO4 cathode. J.-Ceram. Soc. Jpn. 2009, 117, 1225–1228. [Google Scholar] [CrossRef]
- Mathew, V.; Gim, J.; Kim, E.; Alfaruqi, M.H.; Song, J.; Ahn, D.; Im, W.B.; Paik, Y.; Kim, J. A rapid polyol combustion strategy towards scalable synthesis of nanostructured LiFePO4/C cathodes for Li-ion batteries. J. Solid State Electrochem. 2014, 18, 1557–1567. [Google Scholar] [CrossRef]
- Long, Y.; Zhang, Z.; Wu, Z.; Su, J.; Lv, X.; Wen, Y. Microwave-assisted polyol synthesis of LiMnPO4/C and its use as a cathode material in lithium-ion batteries. Particuology 2017, 33, 42–49. [Google Scholar] [CrossRef]
- Podgornova, O.A.; Volfkovich, Y.M.; Sosenkin, V.E.; Kosova, N.V. Increasing the efficiency of carbon coating on olivine-structured cathodes by choosing a carbon precursor. J. Electroanal. Chem. 2022, 907, 116059. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).