Localized High-Concentration Electrolyte (LHCE) for Fast Charging Lithium-Ion Batteries
Abstract
1. Introduction
2. Experimental Procedure
2.1. Methods
2.2. Electrochemical Testing
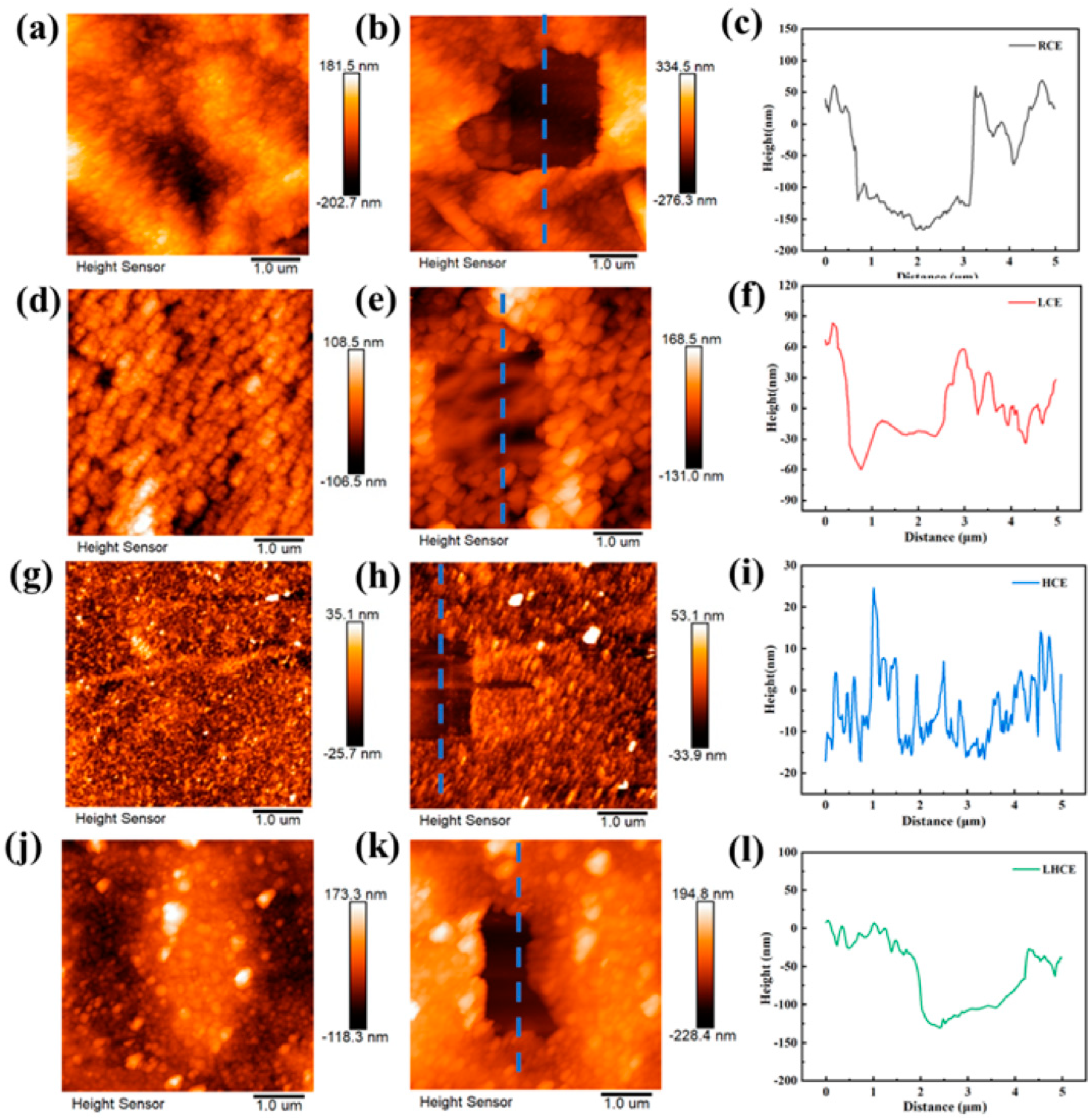
2.3. AFM Measurements
2.4. Materials Characterization
3. Results and Discussion
3.1. CV Tests
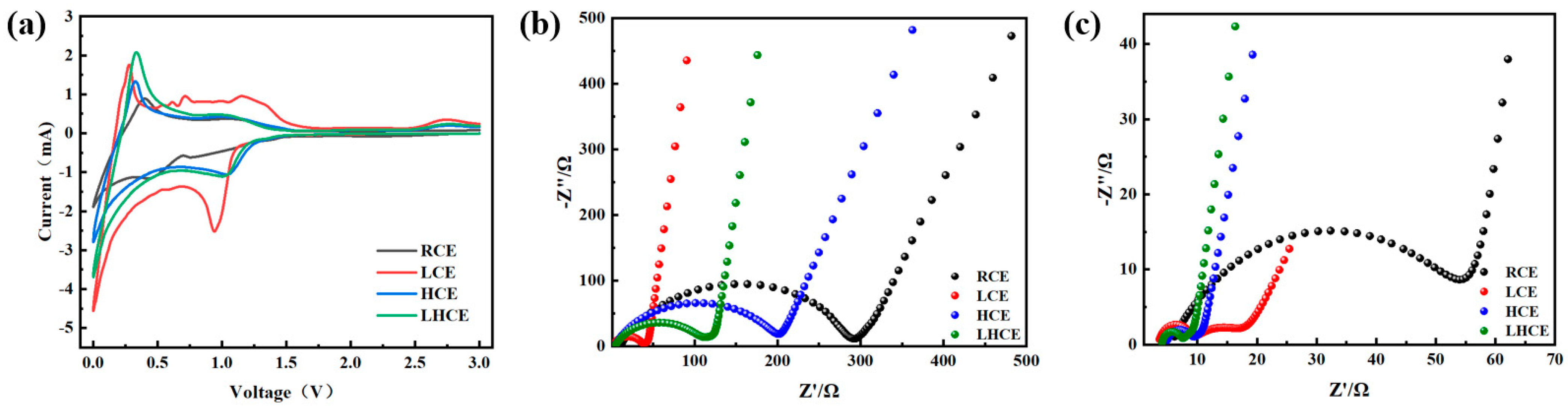
3.2. Electrochemical Impedance Tests
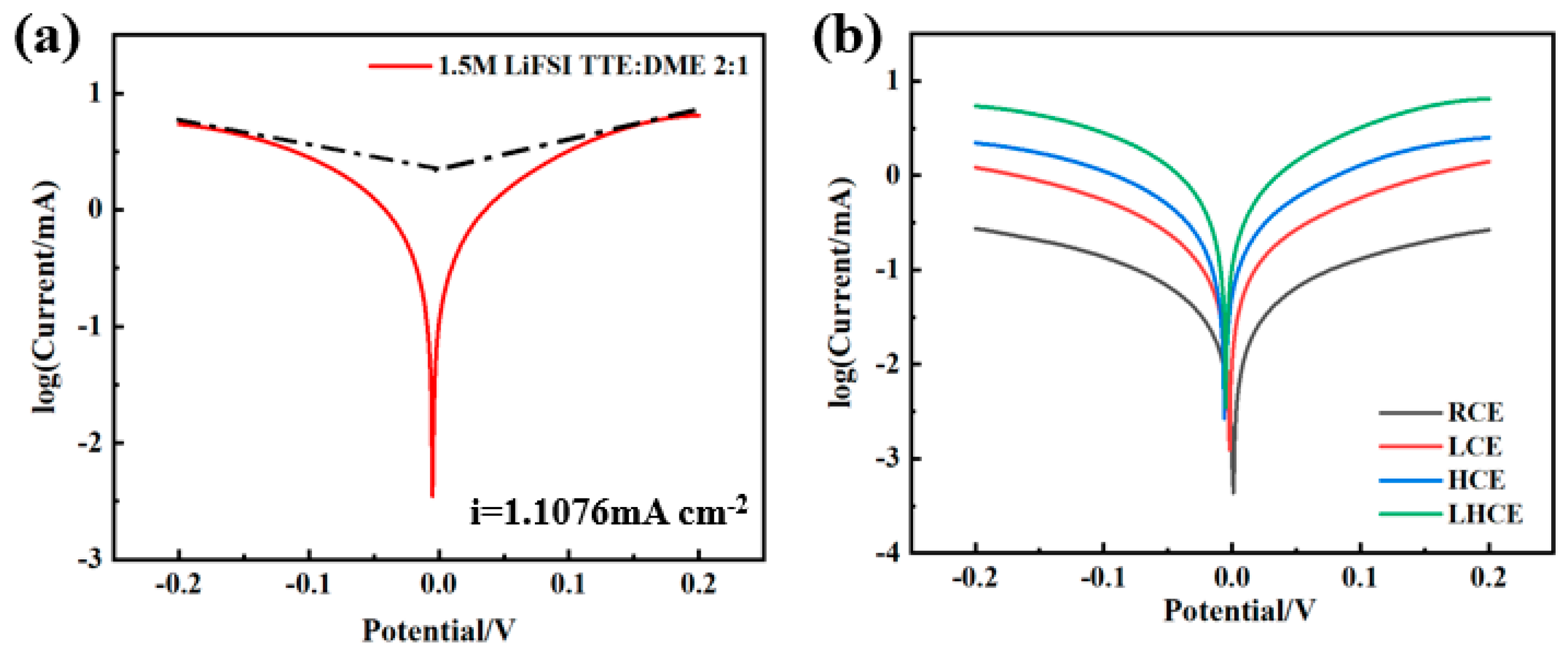
3.3. The Tafel Curve
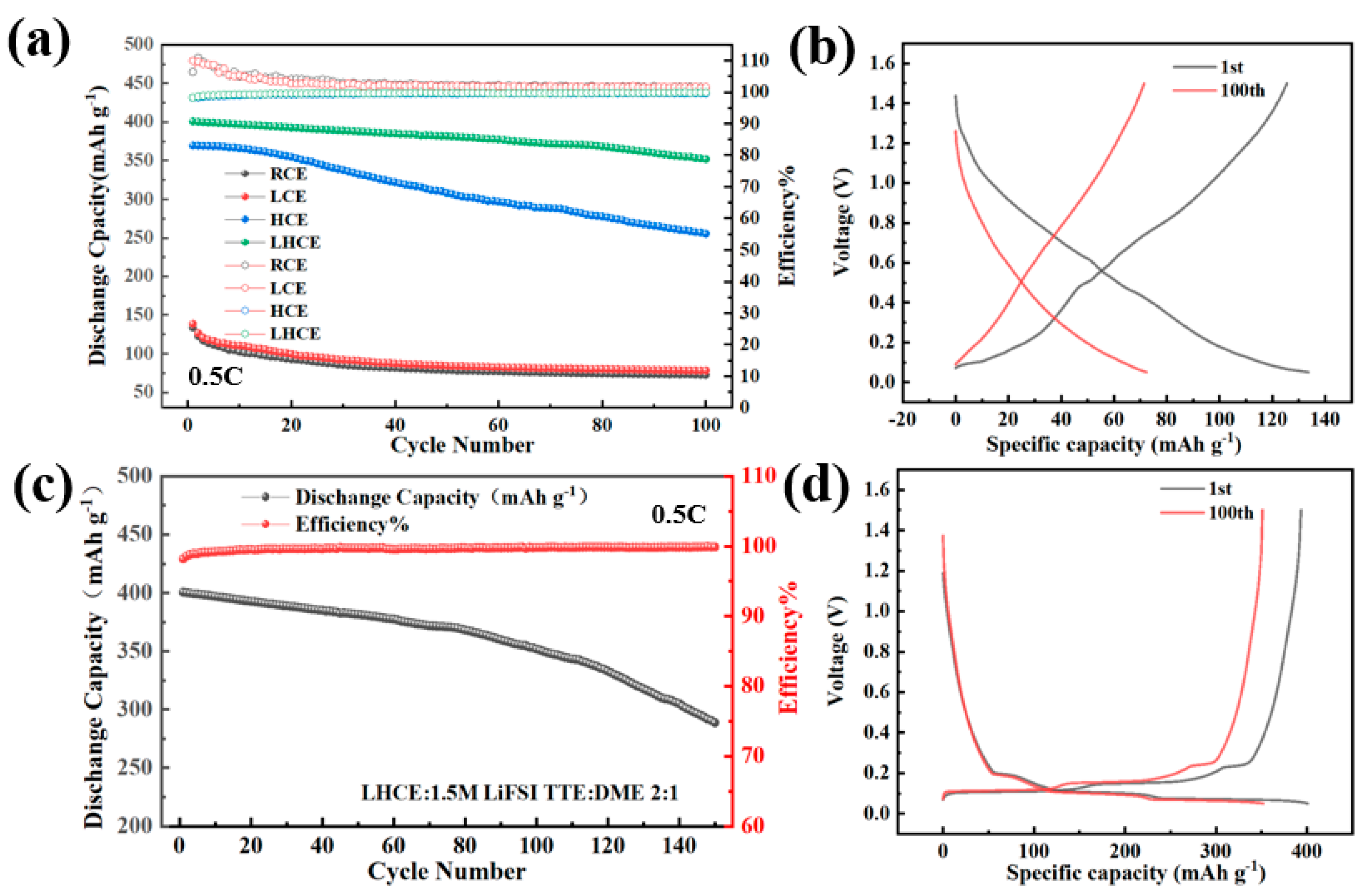
3.4. Cyclic Performance
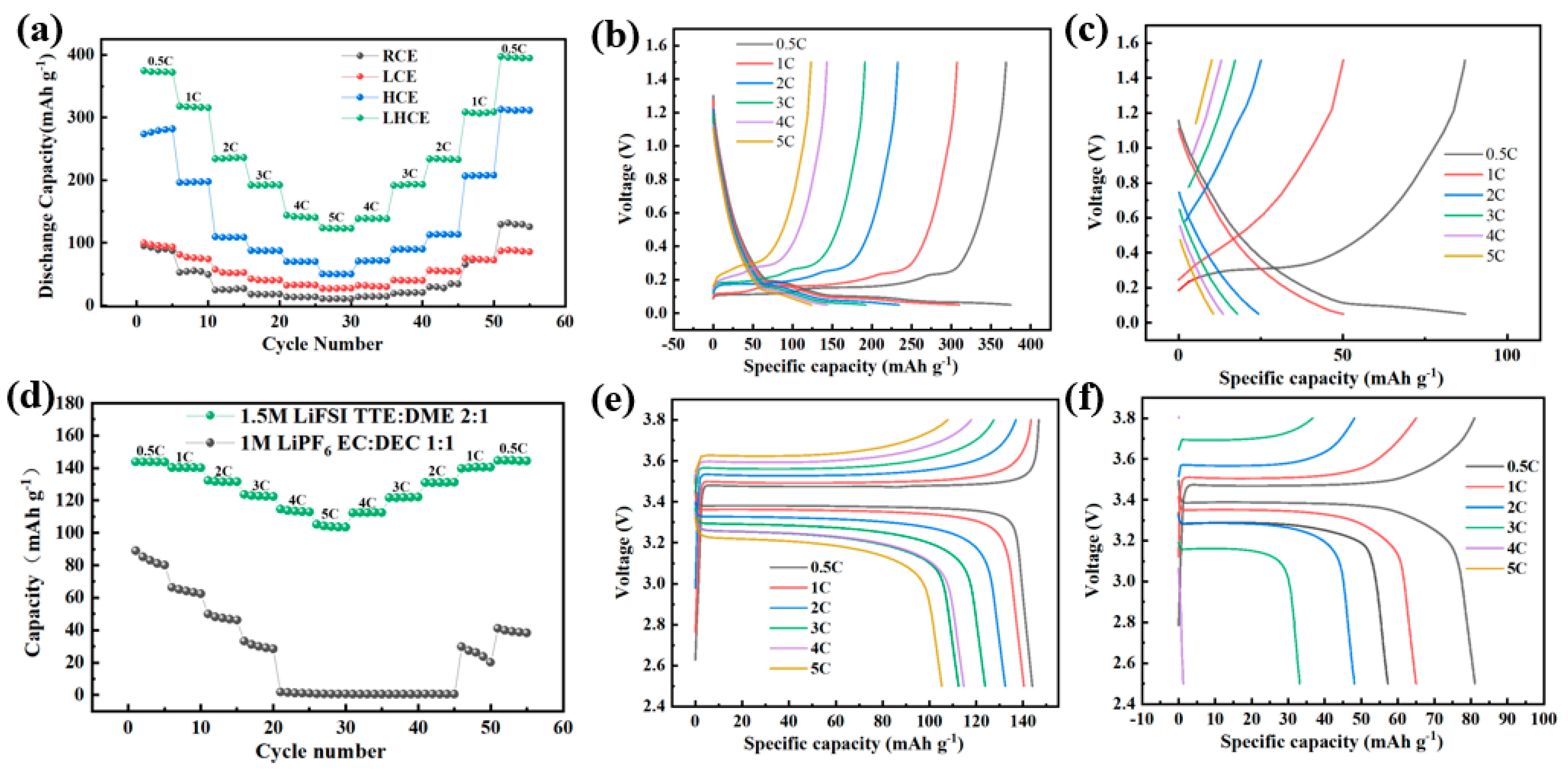
3.5. Rate Performance Tests
3.6. EC-AFM of Different Electrolytes on HOPG
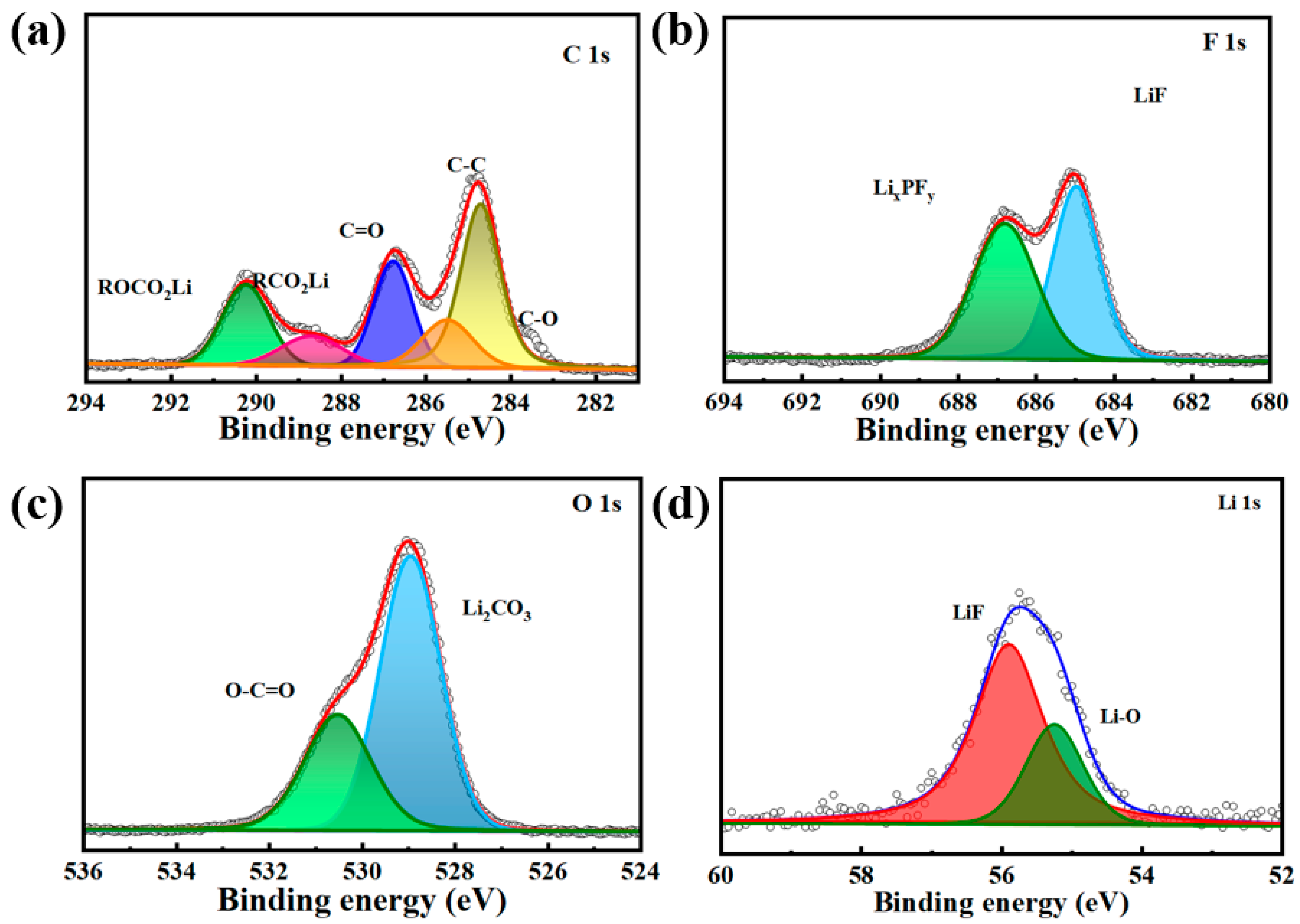
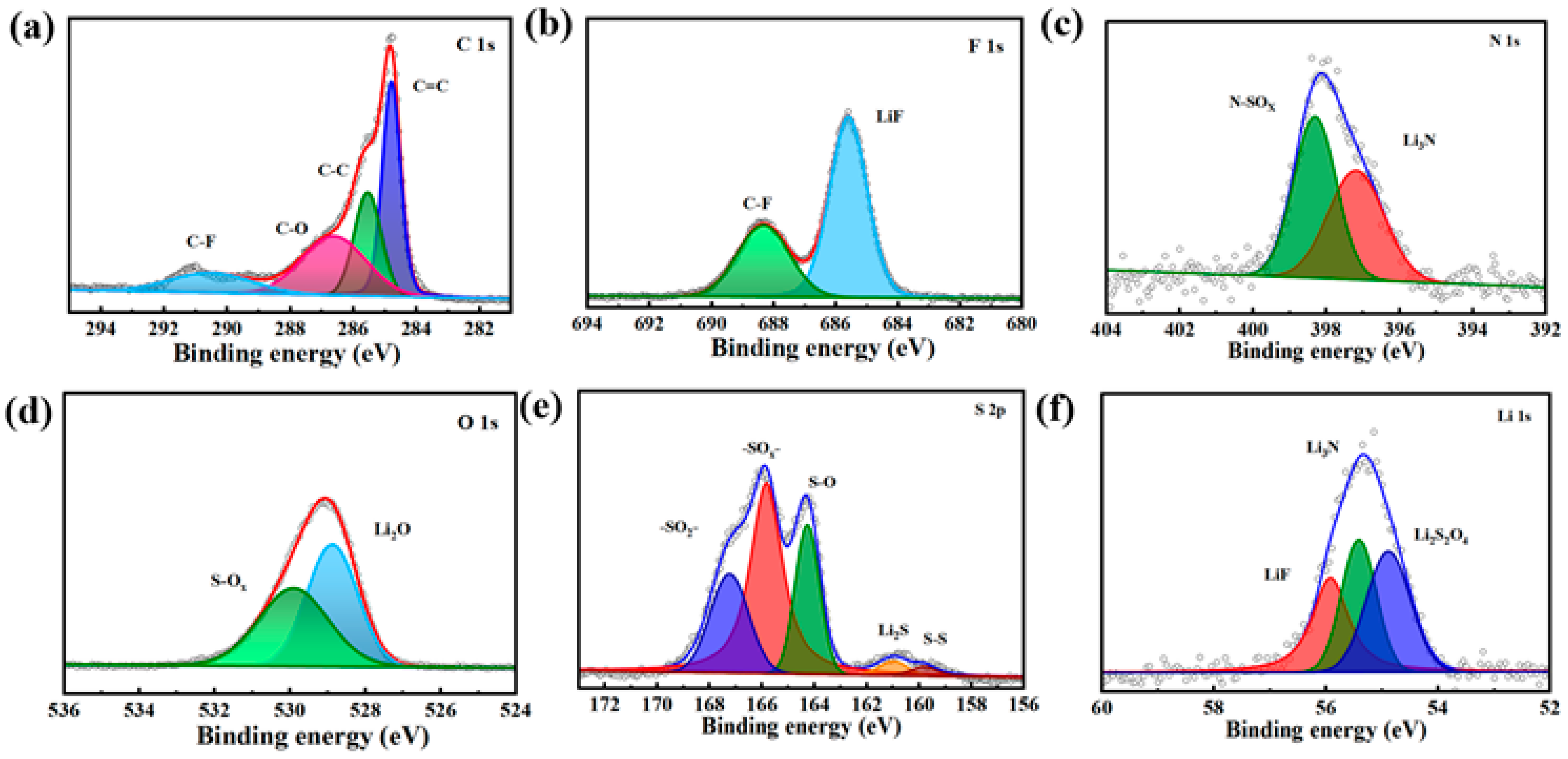
3.7. XPS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cai, W.; Yao, Y.-X.; Zhu, G.-L.; Yan, C.; Jiang, L.-L.; He, C.; Huang, J.-Q.; Zhang, Q. A review on energy chemistry of fast-charging anodes. Chem. Soc. Rev. 2020, 49, 3806–3833. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Chen, X.; Yao, N.; Gao, J.; Xu, G.; Ding, J.; Song, C.; Cai, W.; Yan, C.; Zhang, Q. Unlocking Charge Transfer Limitations for Extreme Fast Charging of Li-Ion Batteries. Angew. Chem. Int. Ed. 2022, 62, e202214828. [Google Scholar]
- Chu, Y.; Shen, Y.; Guo, F.; Zhao, X.; Dong, Q.; Zhang, Q.; Li, W.; Chen, H.; Luo, Z.; Chen, L. Advanced Characterizations of Solid Electrolyte Interphases in Lithium-Ion Batteries. Electrochem. Energy Rev. 2019, 3, 187–219. [Google Scholar] [CrossRef]
- Du, Z.; Wood, D.L., III; Belharouak, I. Enabling fast charging of high energy density Li-ion cells with high lithium ion transport electrolytes. Electrochem. Commun. 2019, 103, 109–113. [Google Scholar] [CrossRef]
- Ahmed, S.; Bloom, I.; Jansen, A.N.; Tanim, T.; Dufek, E.J.; Pesaran, A.; Burnham, A.; Carlson, R.B.; Dias, F.; Hardy, K.; et al. Enabling fast charging—A battery technology gap assessment. J. Power Sources 2017, 367, 250–262. [Google Scholar] [CrossRef]
- Cai, W.; Yan, C.; Yao, Y.; Xu, L.; Chen, X.; Huang, J.; Zhang, Q. The Boundary of Lithium Plating in Graphite Electrode for Safe Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2021, 60, 13007–13012. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Cui, Y. Challenges and opportunities towards fast-charging battery materials. Nat. Energy 2019, 4, 540–550. [Google Scholar] [CrossRef]
- Michelbacher, C.; Ahmed, S.; Bloom, I.; Burnham, A.; Carlson, B.; Dias, F.; Dufek, E.J.; Jansen, A.N.; Keyser, M.; Markel, A.; et al. Enabling fast charging—Introduction and overview. J. Power Sources 2017, 367, 214–215. [Google Scholar] [CrossRef]
- Mussa, A.; Klett, M.; Behm, M.; Lindbergh, G.; Lindström, R. Fast-charging to a partial state of charge in lithium-ion bat-teries: A comparative ageing study. J. Energy Storage 2017, 13, 325–333. [Google Scholar] [CrossRef]
- Jiang, L.; Dong, D.; Lu, Y.-C. Design strategies for low temperature aqueous electrolytes. Nano Res. Energy 2022, 1, e9120003. [Google Scholar] [CrossRef]
- Yang, J.; Hou, W.; Pan, R.; Zhou, M.; Zhang, S.; Zhang, Y. The interfacial electronic engineering in polyhedral MOF derived Co-doped NiSe2 composite for upgrading rate and longevity performance of aqueous energy storage. J. Alloys Compd. 2022, 897, 163187. [Google Scholar] [CrossRef]
- Ye, H.; Li, Y. Towards practical lean-electrolyte Li–S batteries: Highly solvating electrolytes or sparingly solvating electro-lytes? Nano Res. Energy 2022, 1, 9120012. [Google Scholar] [CrossRef]
- Sun, C.; Ji, X.; Weng, S.; Li, R.; Huang, X.; Zhu, C.; Xiao, X.; Deng, T.; Fan, L.; Chen, L.; et al. 50C Fast-Charge Li-Ion Batteries using a Graphite Anode. Adv. Mater. 2022, 34, e2206020. [Google Scholar] [CrossRef]
- Yang, L.; Cheng, X.; Gao, Y.; Ma, Y.; Zuo, P.; Du, C.; Cui, Y.; Guan, T.; Lou, S.; Wang, F.; et al. Lithium deposition on graphite anode during long-term cycles and the effect on capacity loss. RSC Adv. 2014, 4, 26335–26341. [Google Scholar] [CrossRef]
- Chen, H.; Tu, H.; Hu, C.; Liu, Y.; Dong, D.; Sun, Y.; Dai, Y.; Wang, S.; Qian, H.; Lin, Z.; et al. Cationic Covalent Organic Framework Nanosheets for Fast Li-Ion Conduction. J. Am. Chem. Soc. 2018, 140, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zou, L.; Cui, Z.; Jia, H.; Engelhard, M.H.; Matthews, B.E.; Cao, X.; Xie, Q.; Wang, C.; Manthiram, A.; et al. Stabilizing ultrahigh-nickel layered oxide cathodes for high-voltage lithium metal batteries. Mater. Today 2021, 44, 15–24. [Google Scholar] [CrossRef]
- Sun, Q.; Xi, B.; Li, J.-Y.; Mao, H.; Ma, X.; Liang, J.; Feng, J.; Xiong, S. Nitrogen-Doped Graphene-Supported Mixed Transi-tion-Metal Oxide Porous Particles to Confine Polysulfides for Lithium-Sulfur Batteries. Adv. Energy Mater. 2018, 8, 1800595. [Google Scholar] [CrossRef]
- Okoshi, M.; Yamada, Y.; Yamada, A.; Nakai, H. Theoretical Analysis on De-Solvation of Lithium, Sodium, and Magnesium Cations to Organic Electrolyte Solvents. J. Electrochem. Soc. 2013, 160, A2160–A2165. [Google Scholar] [CrossRef]
- Zhang, S.S. Challenges and Strategies for Fast Charge of Li-Ion Batteries. Chemelectrochem 2020, 7, 3569–3577. [Google Scholar] [CrossRef]
- Zhang, S. Identifying rate limitation and a guide to design of fast-charging Li-ion battery. InfoMat 2019, 2, 942–949. [Google Scholar] [CrossRef]
- Zhang, D.; Li, L.; Zhang, W.; Cao, M.; Qiu, H.; Ji, X. Research progress on electrolytes for fast-charging lithium-ion batteries. Chin. Chem. Lett. 2023, 34, 107122. [Google Scholar] [CrossRef]
- Cao, X.; Gao, P.; Ren, X.; Zou, L.; Engelhard, M.H.; Matthews, B.E.; Hu, J.; Niu, C.; Liu, D.; Arey, B.W.; et al. Effects of fluorinated solvents on electrolyte solvation structures and electrode/electrolyte interphases for lithium metal batteries. Proc. Natl. Acad. Sci. USA 2021, 118, e2020357118. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Chen, X.; Zhou, P.; Wang, P.; Zhou, H.; Zhang, W.; Xia, Y.; Liu, K. Regulating the growth of lithium dendrite by coat-ing an ultra-thin layer of gold on separator for improving the fast-charging ability of graphite anode. J. Energy Chem. 2022, 67, 467–473. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, W.; Ming, J.; Li, M.; Xie, L.; He, X.; Wang, J.; Liang, S.; Wu, Y. An Exploration of New Energy Storage System: High Energy Density, High Safety, and Fast Charging Lithium Ion Battery. Adv. Funct. Mater. 2019, 29, 1805978. [Google Scholar] [CrossRef]
- Wassiliadis, N.; Schneider, J.; Frank, A.; Wildfeuer, L.; Lin, X.; Jossen, A.; Lienkamp, M. Review of fast charging strategies for lithium-ion battery systems and their applicability for battery electric vehicles. J. Energy Storage 2021, 44, 103306. [Google Scholar] [CrossRef]
- Tomaszewska, A.; Chu, Z.; Feng, X.; O’Kane, S.; Liu, X.; Chen, J.; Ji, C.; Endler, E.; Li, R.; Liu, L.; et al. Lithium-ion battery fast charging: A review. eTransportation 2019, 1, 100011. [Google Scholar] [CrossRef]
- Rodrigues, M.-T.F.; Kalaga, K.; Trask, S.E.; Dees, D.W.; Shkrob, I.A.; Abraham, D.P. Fast Charging of Li-Ion Cells: Part I. Using Li/Cu Reference Electrodes to Probe Individual Electrode Potentials. J. Electrochem. Soc. 2019, 166, A996–A1003. [Google Scholar] [CrossRef]
- Zhu, G.; Zhao, C.; Huang, J.; He, C.; Zhang, J.; Chen, S.; Xu, L.; Yuan, H.; Zhang, Q. Fast Charging Lithium Batteries: Recent Progress and Future Prospects. Small 2019, 15, e1805389. [Google Scholar] [CrossRef]
- Xu, S.; Peng, B.; Pang, X.; Huang, F. Anionic Activity in Fast-Charging Batteries: Recent Advances, Prospects, and Challenges. ACS Mater. Lett. 2022, 4, 2195–2209. [Google Scholar] [CrossRef]
- Jiang, L.; Yan, C.; Yao, Y.; Cai, W.; Huang, J.; Zhang, Q. Inhibiting Solvent Co-Intercalation in a Graphite Anode by a Localized High-Concentration Electrolyte in Fast-Charging Batteries. Angew. Chem. Int. Ed. 2021, 60, 3402–3406. [Google Scholar] [CrossRef]
- Zhou, X.; Li, X.; Li, Z.; Xie, H.; Fu, J.; Wei, L.; Yang, H.; Guo, X. Hybrid electrolytes with an ultrahigh Li-ion transference number for lithium-metal batteries with fast and stable charge/discharge capability. J. Mater. Chem. A 2021, 9, 18239–18246. [Google Scholar] [CrossRef]
- Cao, X.; Jia, H.; Xu, W.; Zhang, J.-G. Review—Localized High-Concentration Electrolytes for Lithium Batteries. J. Electrochem. Soc. 2021, 168, 010522. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, L.; Chen, Z.; He, X. Challenges of Fast Charging for Electric Vehicles and the Role of Red Phosphorous as Anode Material: Review. Energies 2019, 12, 3897. [Google Scholar] [CrossRef]
- Shkrob, I.; Rodrigues, M.-T.; Abraham, D. Fast Charging of Li-Ion Cells: Part V. Design and Demonstration of Pro-tocols to Avoid Li-Plating. J. Electrochem. Soc. 2021, 168, 010512. [Google Scholar] [CrossRef]
- Ding, X.; Huang, Q.; Xiong, X. Research and Application of Fast-Charging Graphite Anodes for Lithium-Ion Batteries. Acta Phys. Chim. Sin. 2022, 38, 2204057. [Google Scholar] [CrossRef]
- Beheshti, S.H.; Javanbakht, M.; Omidvar, H.; Hosen, S.; Hubin, A.; Van Mierlo, J.; Berecibar, M. Development, retainment, and assessment of the graphite-electrolyte interphase in Li-ion batteries regarding the functionality of SEI-forming additives. iScience 2022, 25, 103862. [Google Scholar] [CrossRef]
- Weiss, M.; Ruess, R.; Kasnatscheew, J.; Levartovsky, Y.; Levy, N.; Minnmann, P.; Stolz, L.; Waldmann, T.; Wohlfahrt-Mehrens, M.; Aurbach, D.; et al. Fast Charging of Lithium-Ion Batteries: A Review of Materials Aspects. Adv. Energy Mater. 2021, 11, 2101126. [Google Scholar] [CrossRef]
- Chen, L.; Shu, J.; Huang, Y.; Shi, Z.; Luo, H.; Liu, Z.; Shen, C. Engineering solid electrolyte interphase for the application of propylene carbonate solvent for graphite anode in low temperate battery. Appl. Surf. Sci. 2022, 598, 153740. [Google Scholar] [CrossRef]
- Chen, M.; Wang, W.; Shi, Z.; Liu, Z.; Shen, C. Revealing the cathode electrolyte interphase on Li- and Mn-rich materials by in-situ electrochemical atomic force microscopy. Appl. Surf. Sci. 2022, 600, 154119. [Google Scholar] [CrossRef]
- Xie, W.; Liu, X.; He, R.; Li, Y.; Gao, X.; Li, X.; Peng, Z.; Feng, S.; Feng, X.; Yang, S. Challenges and opportunities toward fast-charging of lithium-ion batteries. J. Energy Storage 2020, 32, 101837. [Google Scholar] [CrossRef]
- Jia, M.; Zhang, C.; Guo, Y.; Peng, L.; Zhang, X.; Qian, W.; Zhang, L.; Zhang, S. Advanced Nonflammable Localized High-Concentration Electrolyte For High Energy Density Lithium Battery. Energy Environ. Mater. 2022, 5, 1294–1302. [Google Scholar] [CrossRef]
- Zhang, C.; Gu, S.; Zhang, D.; Ma, J.; Zheng, H.; Zheng, M.; Lv, R.; Yu, K.; Wu, J.; Wang, X.; et al. Nonflammable, localized high-concentration electrolyte towards a high-safety lithium metal battery. Energy Storage Mater. 2022, 52, 355–364. [Google Scholar] [CrossRef]
- Hou, Z.; Wang, P.-F.; Sun, X.; Li, W.; Sheng, C.; He, P. Protecting Li Metal Anode While Suppressing “Shuttle Effect” of Li-S Battery Through Localized High-Concentration Electrolyte. J. Electron. Mater. 2022, 51, 4772–4779. [Google Scholar] [CrossRef]
- Li, T.; Li, Y.; Sun, Y.; Qian, Z.; Wang, R. New Insights on the Good Compatibility of Ether-Based Localized High-Concentration Electrolyte with Lithium Metal. ACS Mater. Lett. 2021, 3, 838–844. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Chen, Z.; Zhou, Y.; Bai, F.; Li, T. Hybrid diluents enable localized high-concentration electrolyte with balanced performance for high-voltage lithium-metal batteries. Chin. Chem. Lett. 2022, 2022, 107852. [Google Scholar] [CrossRef]
- Guo, F.; Chen, X.; Hou, Y.; Wei, W.; Wang, Z.; Yu, H.; Xu, J. Improved Cycling of Li||NMC811 Batteries under Practical Conditions by a Localized High-Concentration Electrolyte. Small 2023, 2023, e2207290. [Google Scholar] [CrossRef]
- Lin, S.; Zhao, J. Functional Electrolyte of Fluorinated Ether and Ester for Stabilizing Both 4.5 V LiCoO(2) Cathode and Lithium Metal Anode. ACS Appl. Mater. Interfaces 2020, 12, 8316–8323. [Google Scholar] [CrossRef]
- Yoo, D.; Yang, S.; Kim, K.; Choi, J. Fluorinated Aromatic Diluent for High-Performance Lithium Metal Batteries. Angew Chem. Int. Ed. Engl. 2020, 59, 14869–14876. [Google Scholar] [CrossRef]
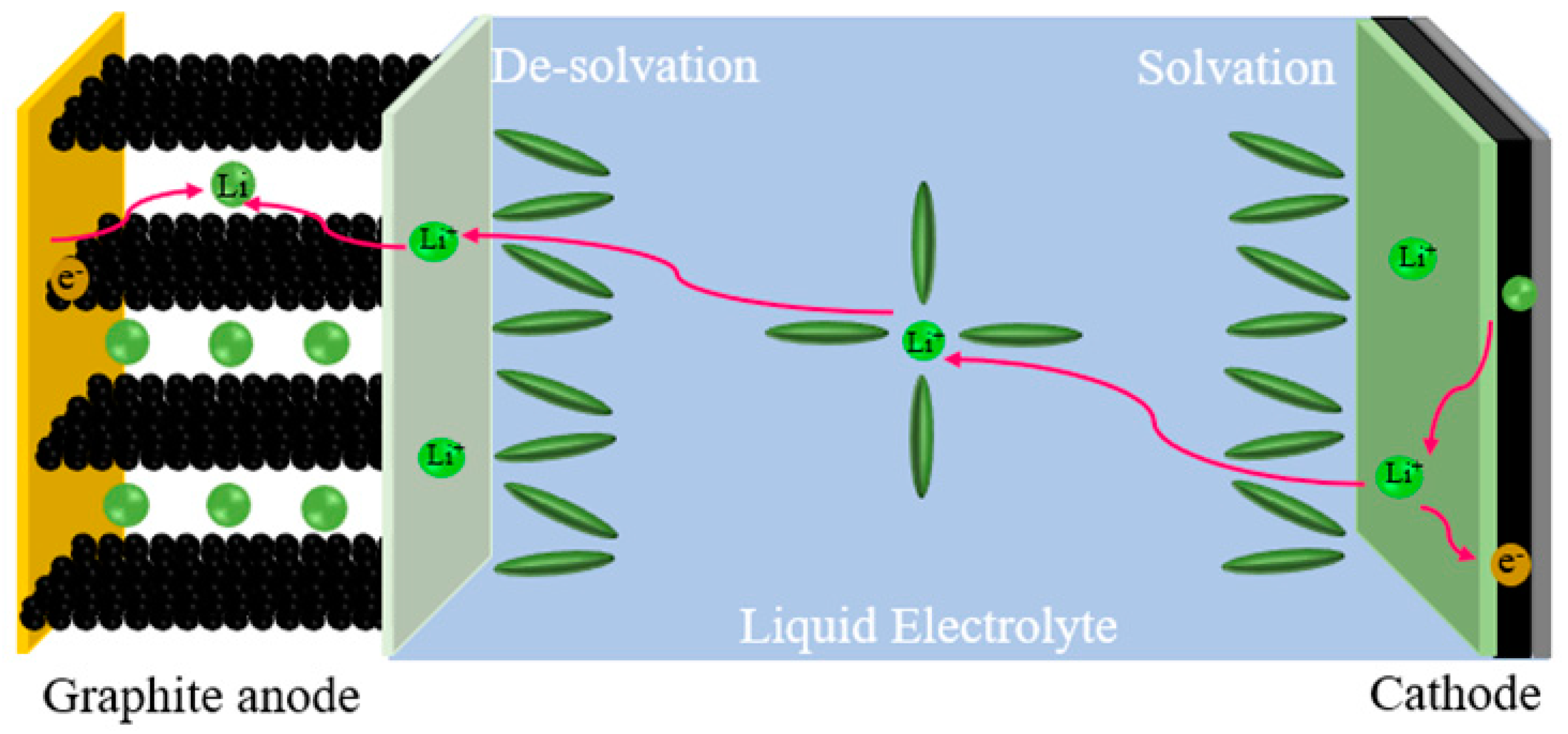
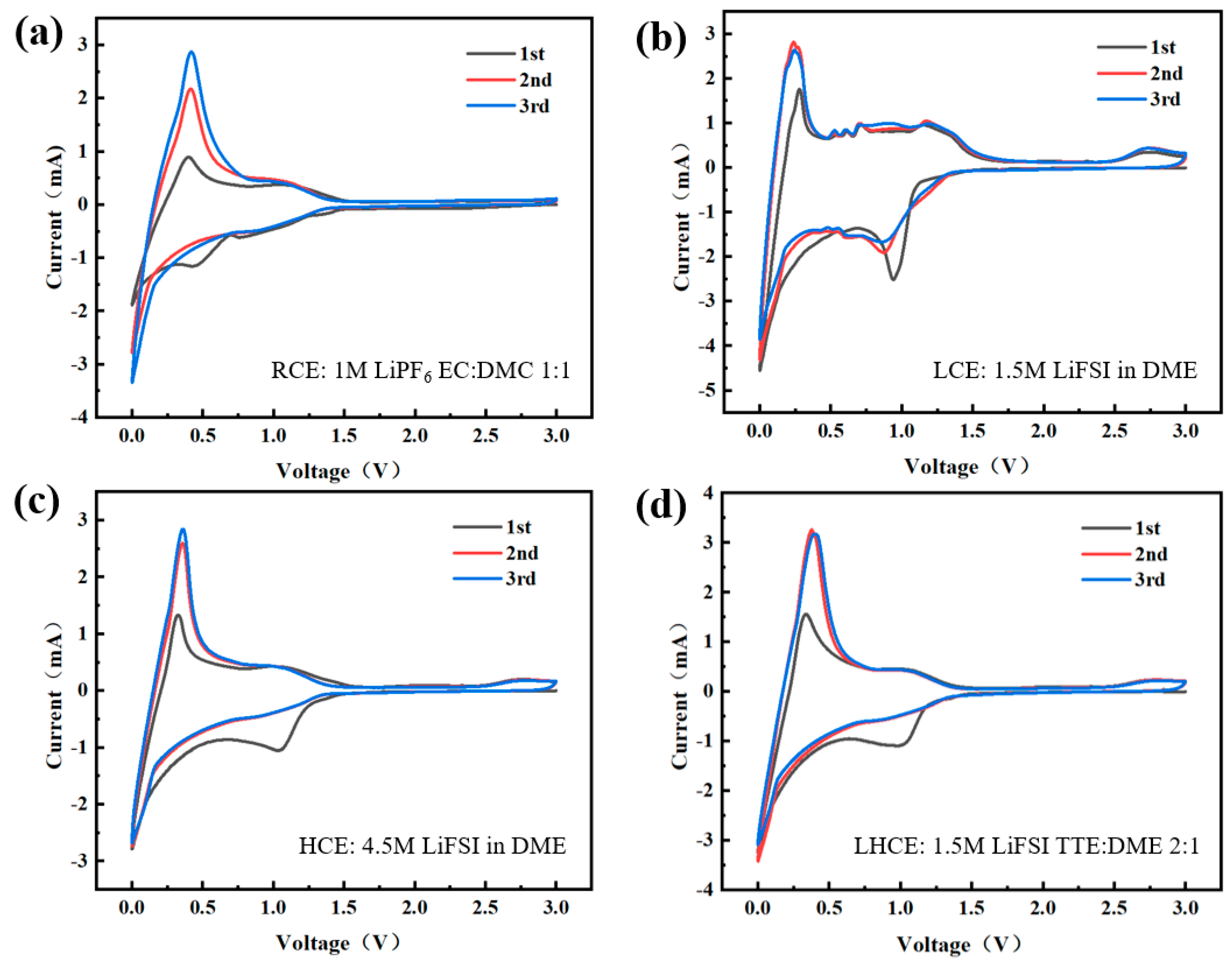
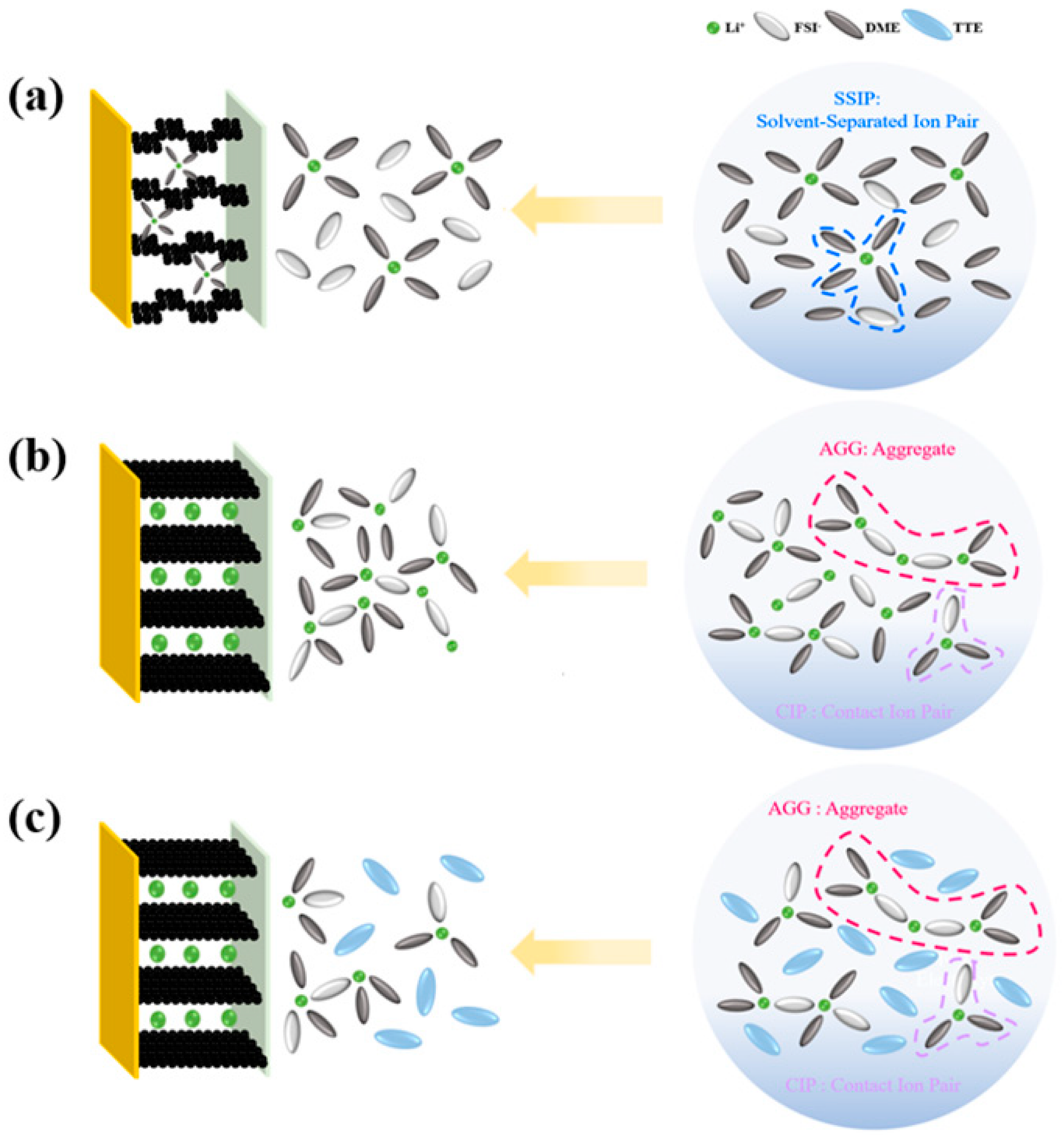
| Name | Electrolyte Components |
|---|---|
| RCE (routine electrolyte) | 1 M LiPF6 EC:DMC = 1:1 |
| LCE (low-concentration ether electrolyte) | 1.5 M LiFSI in DME |
| HCE (high-concentration electrolyte) LHCE (localized high-concentration electrolyte) | 4.5 M LiFSI in DME 1.5 M LiFSI TTE:DME = 2:1 |
| Electrolyte | RSEI/Ω | RSEI’/Ω |
|---|---|---|
| RCE | 291.8 | 53.9 |
| LCE | 39.2 | 8.9 |
| HCE | 199.2 | 9.8 |
| LHCE | 117.4 | 7.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Shi, X.; Wang, W.; Liu, Z.; Shen, C. Localized High-Concentration Electrolyte (LHCE) for Fast Charging Lithium-Ion Batteries. Batteries 2023, 9, 155. https://doi.org/10.3390/batteries9030155
Yang J, Shi X, Wang W, Liu Z, Shen C. Localized High-Concentration Electrolyte (LHCE) for Fast Charging Lithium-Ion Batteries. Batteries. 2023; 9(3):155. https://doi.org/10.3390/batteries9030155
Chicago/Turabian StyleYang, Jingru, Xixiu Shi, Wenyang Wang, Zhaoping Liu, and Cai Shen. 2023. "Localized High-Concentration Electrolyte (LHCE) for Fast Charging Lithium-Ion Batteries" Batteries 9, no. 3: 155. https://doi.org/10.3390/batteries9030155
APA StyleYang, J., Shi, X., Wang, W., Liu, Z., & Shen, C. (2023). Localized High-Concentration Electrolyte (LHCE) for Fast Charging Lithium-Ion Batteries. Batteries, 9(3), 155. https://doi.org/10.3390/batteries9030155








