Improvement on the Use of Se@C in Batteries by Synergistic Effect of Nano-Confinement and C-Se Bond
Abstract
1. Introduction
2. Results
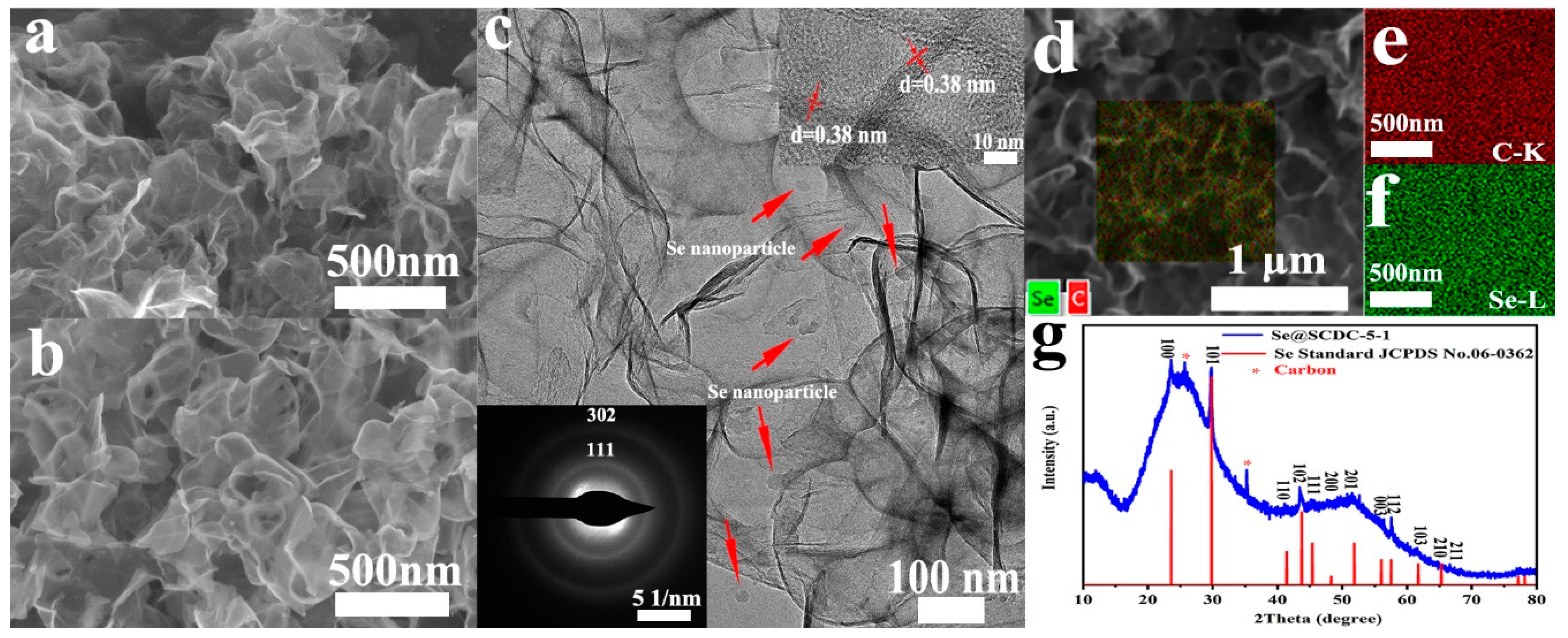
2.1. Characteristic of Morphology of Se@SCDC
2.2. Analysis of Physicochemical Properties of Se@SCDC
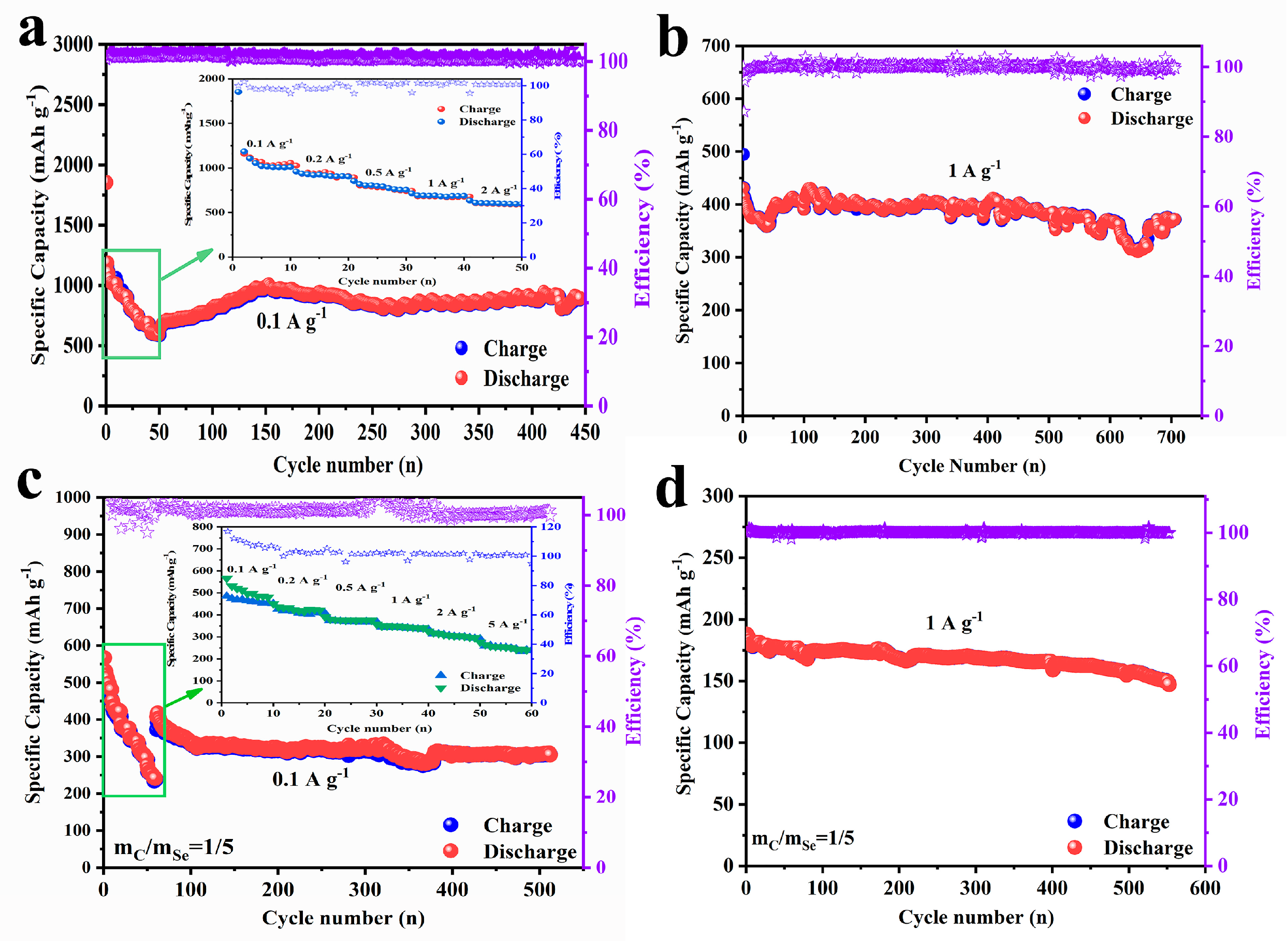
2.3. Comparison of the Electrochemical Performances of Se@SCDC Anode
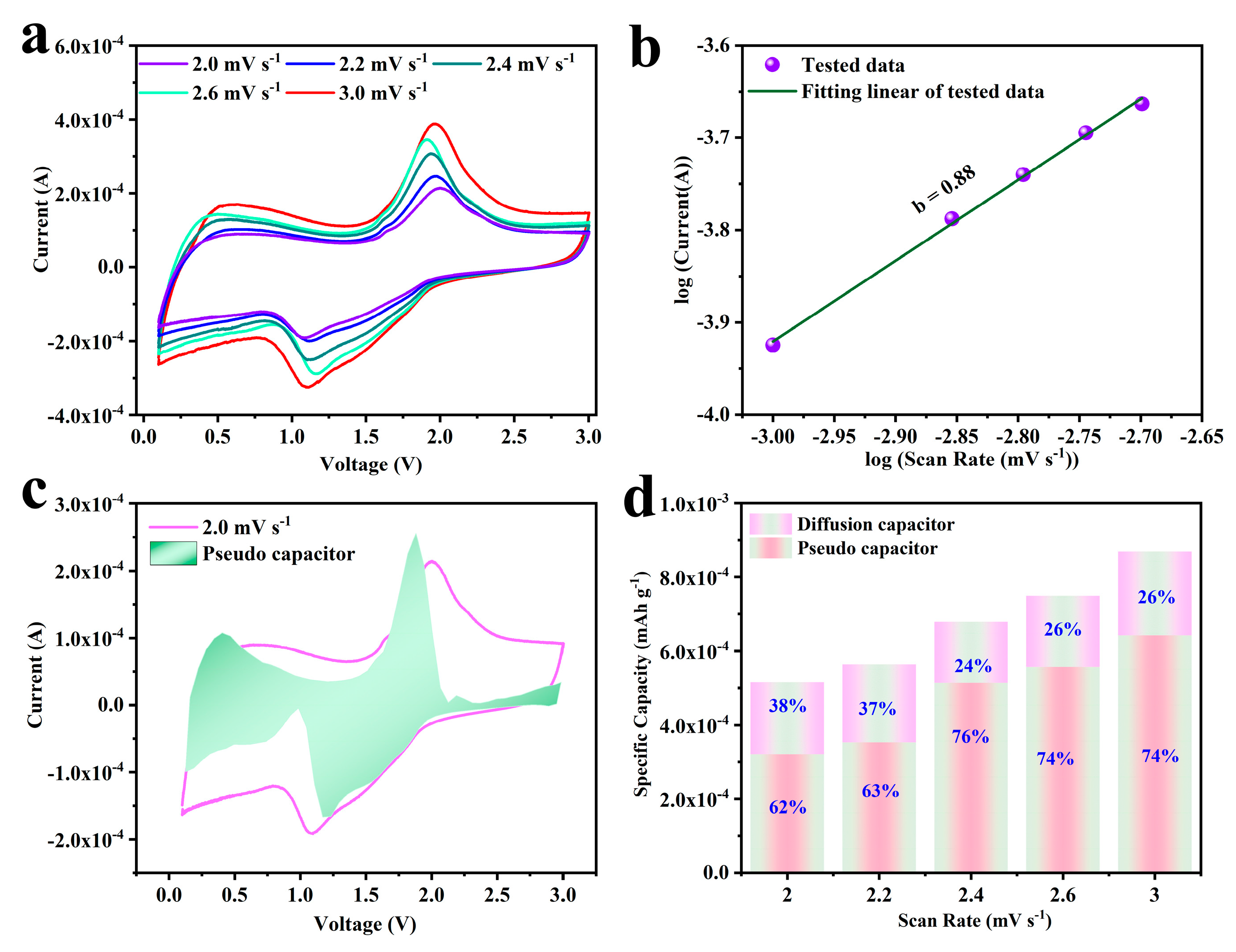
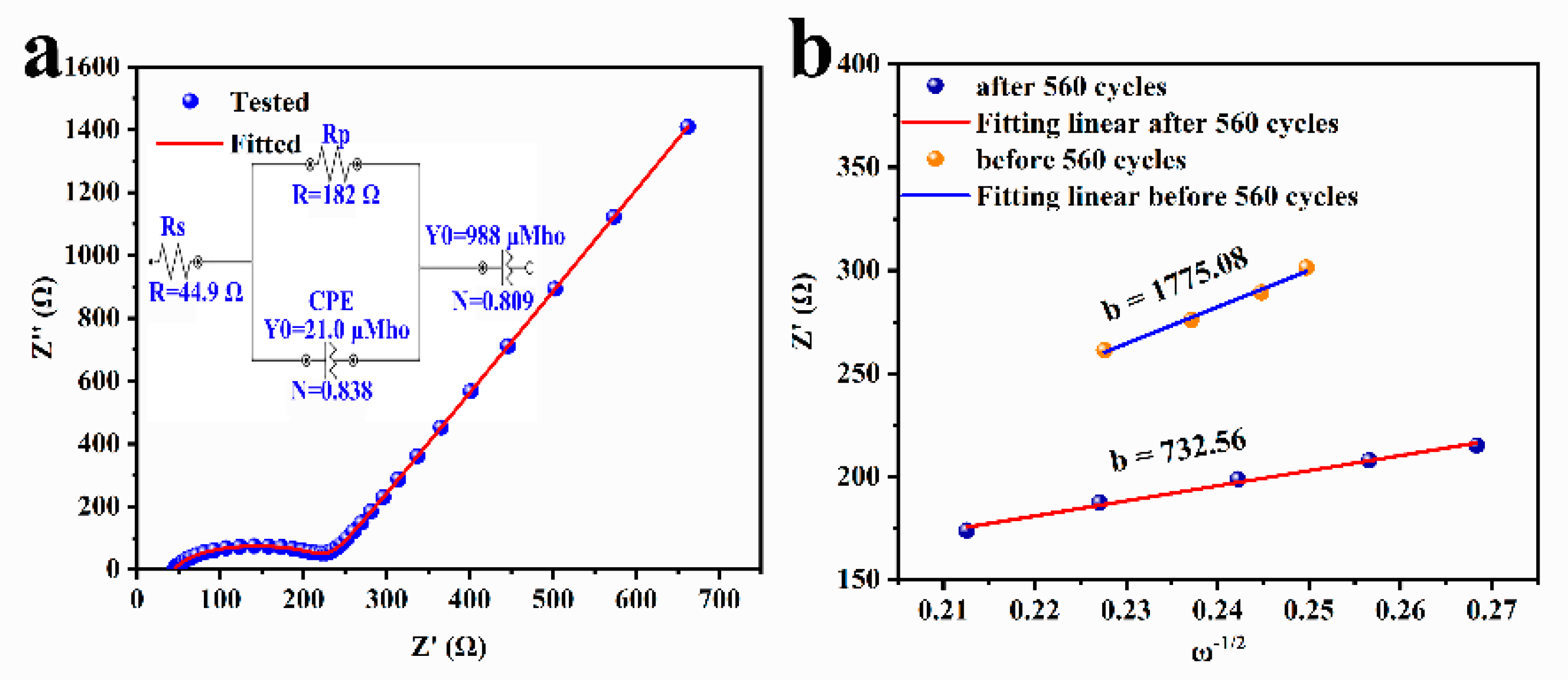
2.4. Electrode Dynamic Analysis of Se@SCDC-5-1
3. Conclusions
4. Materials and Methods
4.1. Preparation of SCDC and Se@SCDC
4.2. Instrumentation and Sample Analysis
4.3. Electrochemical Preparation and Electrochemical Measurements
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Lecce, D.; Verrelli, R.; Hassoun, J. Lithium-Ion Batteries for Sustainable Energy Storage: Recent Advances Towards New Cell Configurations. Green Chem. 2017, 19, 3442–3467. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef]
- Nayak, P.K.; Yang, L.; Brehm, W.; Adelhelm, P. From Lithium-Ion to Sodium-Ion Batteries: Advantages, Challenges, and Surprises. Angew. Chem. Int. Ed. 2018, 57, 102–120. [Google Scholar] [CrossRef] [PubMed]
- Tarascon, J.-M.; Armand, M. Issues and Challenges Facing Rechargeable Lithium Batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Wu, H.B.; Lou, X.W.D. Metal-Organic Frameworks and Their Derived Materials for Electrochemical Energy Storage and Conversion: Promises and Challenges. Sci. Adv. 2017, 3, 9252. [Google Scholar] [CrossRef]
- Turcheniuk, K.; Singhal, V.; Yushin, G. Ten Years Left to Redesign Lithium-Ion Batteries. Nature 2018, 559, 467. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, H.; An, Y.; Fu, L.; An, Q.; Wu, Y. Facile and Scalable Synthesis of a Sulfur, Selenium and Nitrogen Co-Doped Hard Carbon Anode for High Performance Na- and K-Ion Batteries. J. Mate. Chem. A 2020, 8, 14993–15001. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, H.; Du, Y.; Zhang, Y.; Wang, Z.; Zhang, J. Hierarchical Lamellar-Structured MnO2@Graphene for High Performance Li, Na and K Ion Batteries. Chem. Select. 2020, 5, 12481–12486. [Google Scholar] [CrossRef]
- Yan, J.; Li, H.; Wang, K.; Jin, Q.; Lai, C.; Wang, R.; Cao, S.; Han, J.; Zhang, Z.; Su, J.; et al. Ultrahigh Phosphorus Doping of Carbon for High-Rate Sodium Ion Batteries Anode. Adv. Energy Mater. 2021, 11, 2003911. [Google Scholar] [CrossRef]
- Kim, S.-W.; Seo, D.-H.; Ma, X.; Ceder, G.; Kang, K. Electrode Materials for Rechargeable Sodium-Ion Batteries: Potential Alternatives to Current Lithium-Ion Batteries. Adv. Energy Mater. 2012, 2, 710–721. [Google Scholar] [CrossRef]
- Andersen, H.L.; Djuandhi, L.; Mittal, U.; Sharma, N. Strategies for the Analysis of Graphite Electrode Function. Adv. Energy Mater. 2021, 11, 2102693. [Google Scholar] [CrossRef]
- Chaudhary, M.; Tyagi, S.; Gupta, R.K.; Singh, B.P.; Singhal, R. Surface Modification of Cathode Materials for Energy Storage Devices: A Review. Surf. Coat. Technol. 2021, 412, 127009. [Google Scholar] [CrossRef]
- Luo, W.; Chen, X.; Xia, Y.; Chen, M.; Wang, L.; Wang, Q.; Li, W.; Yang, J. Surface and Interface Engineering of Silicon-Based Anode Materials for Lithium-Ion Batteries. Adv. Energy Mater. 2017, 7, 1701083. [Google Scholar] [CrossRef]
- Jo, C.-H.; Jo, J.-H.; Yashiro, H.; Kim, S.-J.; Sun, Y.-K.; Myung, S.-T. Bioinspired Surface Layer for the Cathode Material of High-Energy-Density Sodium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1702942. [Google Scholar] [CrossRef]
- Piao, J.-Y.; Gu, L.; Wei, Z.; Ma, J.; Wu, J.; Yang, W.; Gong, Y.; Sun, Y.-G.; Duan, S.-Y.; Tao, X.-S.; et al. Phase Control on Surface for the Stabilization of High Energy Cathode Materials of Lithium Ion Batteries. J. Am. Chem. Soc. 2019, 141, 4900–4907. [Google Scholar] [CrossRef]
- Chae, S.; Choi, S.H.; Kim, N.; Sung, J.; Cho, J. Integration of Graphite and Silicon Anodes for the Commercialization of High-Energy Lithium-Ion Batteries. Angew. Chem. Int. Ed. Engl. 2020, 59, 110–135. [Google Scholar] [CrossRef]
- Yu, C.-X.; Li, Y.; Wang, Z.-H.; Wang, X.-R.; Bai, Y.; Wu, C. Surface Engineering Based on in Situ Electro-Polymerization to Boost the Initial Coulombic Efficiency of Hard Carbon Anode for Sodium-Ion Battery. Rare Met. 2022, 41, 1616–1625. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Q.; Pang, H. Recent Advances in Metal Organic Frameworks and Their Composites for Batteries. Nano Futures 2020, 4, 032007. [Google Scholar] [CrossRef]
- Zeng, Z.; Jiang, X.; Li, R.; Yuan, D.; Ai, X.; Yang, H.; Cao, Y. A Safer Sodium-Ion Battery Based on Nonflammable Organic Phosphate Electrolyte. Adv. Sci. 2016, 3, 1600066. [Google Scholar] [CrossRef]
- Xu, Q.; Yang, Y.; Shao, H. Enhanced Cycleability and Dendrite-Free Lithium Deposition by Addition of Sodium Ion in Electrolyte for Lithium Metal Batteries. Electrochim. Acta 2018, 271, 617–623. [Google Scholar] [CrossRef]
- Morales, D.; Ruther, R.E.; Nanda, J.; Greenbaum, S. Ion Transport and Association Study of Glyme-Based Electrolytes with Lithium and Sodium Salts. Electrochim. Acta 2019, 304, 239–245. [Google Scholar] [CrossRef]
- Conway, B.; Jewell, K.; Tanner, C. Flexible Lithium-Ion Conducting Composite Electrolyte. Batter. Supercaps 2020, 3, 653–657. [Google Scholar] [CrossRef]
- Kartha, T.R.; Mallik, B.S. Ionic Conductance and Viscous Drag in Water-in-Salt Electrolytes for Lithium and Sodium Ion Batteries and Supercapacitors. Mater. Today Commun. 2020, 25, 101588. [Google Scholar] [CrossRef]
- Puthirath, A.B.; Tsafack, T.; Patra, S.; Thakur, P.; Chakingal, N.; Saju, S.K.; Baburaj, A.; Kato, K.; Babu, G.; Narayanan, T.N.; et al. Lithium, Sodium and Magnesium Ion Conduction in Solid State Mixed Polymer Electrolytes. Phys. Chem. Chem. Phys. 2020, 22, 19108–19119. [Google Scholar] [CrossRef]
- Allam, O.; Cho, B.W.; Kim, K.C.; Jang, S.S. Application of Dft-Based Machine Learning for Developing Molecular Electrode Materials in Li-Ion Batteries. RSC Adv. 2018, 8, 39414–39420. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Guo, S.; Zhou, H. Exploration of Advanced Electrode Materials for Rechargeable Sodium-Ion Batteries. Adv. Energy Mater. 2018, 9, 1800212. [Google Scholar] [CrossRef]
- Xiao, B.; Soto, F.A.; Gu, M.; Han, K.S.; Song, J.; Wang, H.; Engelhard, M.H.; Murugesan, V.; Mueller, K.T.; Reed, D.; et al. Lithium-Pretreated Hard Carbon as High-Performance Sodium-Ion Battery Anodes. Adv. Energy Mater. 2018, 8, 1801441. [Google Scholar] [CrossRef]
- Sivajee Ganesh, K.; Purusottam Reddy, B.; Jeevan Kumar, P.; Hussain, O.M. Influence of Zr Dopant on Microstructural and Electrochemical Properties of LiCoO2 Thin Film Cathodes by Rf Sputtering. J. Electroanal. Chem. 2018, 828, 71–79. [Google Scholar] [CrossRef]
- Pitcheri, R.; Theophile, N.; Sangaraju, S.; Haekyoung, K. Graphene based magnetite carbon nanofiber composites as anodes for high-performance Li-ion batteries. New J. Chem. 2023, 47, 482–490. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, Z.; Yu, H.; Zhang, X.; Liu, T.; Xia, M.; Zheng, R.; Shui, M.; Shu, J. Heteroatom-Doped Carbon-Based Materials for Lithium and Sodium Ion Batteries. Energy Storage Mater. 2020, 32, 65–90. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, Y.; Ming, H.; Cao, G.; Zhang, W.; Ming, J.; Chen, R. Recent Advances in Nanostructured Carbon for Sodium-Ion Batteries. J. Mater. Chem. A 2020, 8, 1604–1630. [Google Scholar] [CrossRef]
- Zhao, X.; Yin, L.; Zhang, T.; Zhang, M.; Fang, Z.; Wang, C.; Wei, Y.; Chen, G.; Zhang, D.; Sun, Z.; et al. Heteroatoms Dual-Doped Hierarchical Porous Carbon-Selenium Composite for Durable Li-Se and Na-Se Batteries. Nano Energy 2018, 49, 137–146. [Google Scholar] [CrossRef]
- Li, Y.; Chen, M.; Liu, B.; Zhang, Y.; Liang, X.; Xia, X. Heteroatom Doping: An Effective Way to Boost Sodium Ion Storage. Adv. Energy Mater. 2020, 10, 2000927. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, X.; Li, J. SnSe/Carbon Nanocomposite Synthesized by High Energy Ball Milling as an Anode Material for Sodium-Ion and Lithium-Ion Batteries. Electrochim. Acta 2015, 176, 1296–1301. [Google Scholar] [CrossRef]
- Balogun, M.-S.; Luo, Y.; Qiu, W.; Liu, P.; Tong, Y. A Review of Carbon Materials and Their Composites with Alloy Metals for Sodium Ion Battery Anodes. Carbon 2016, 98, 162–178. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Z. Carbon-Coated Vanadium Selenide as Anode for Lithium-Ion Batteries and Sodium-Ion Batteries with Enhanced Electrochemical Performance. Mater. Lett. 2017, 189, 152–155. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.P.; Sougrati, M.T.; Feng, Z.; Leconte, Y.; Fisher, A.; Srinivasan, M.; Xu, Z. A Review on Design Strategies for Carbon Based Metal Oxides and Sulfides Nanocomposites for High Performance Li and Na Ion Battery Anodes. Adv. Energy Mater. 2017, 7, 1601424. [Google Scholar] [CrossRef]
- Sheng, W.; Zhang, P.; Li, W.; Zhang, T.; Tan, D.; Li, Y.; Wang, F.; Zhuang, X.; Feng, X.; Jordan, R. Mussel-Inspired Nitrogen-Doped Porous Carbon as Anode Materials for Sodium-Ion Batteries. Energy Technol. 2019, 7, 1800763. [Google Scholar] [CrossRef]
- Gomez-Martin, A.; Martinez-Fernandez, J.; Ruttert, M.; Winter, M.; Placke, T.; Ramirez-Rico, J. An Electrochemical Evaluation of Nitrogen-Doped Carbons as Anodes for Lithium Ion Batteries. Carbon 2020, 164, 261–271. [Google Scholar] [CrossRef]
- Huang, J.; Lin, Y.; Ji, M.; Cong, G.; Liu, H.; Yu, J.; Yang, B.; Li, C.; Zhu, C.; Xu, J. Nitrogen-Doped Porous Carbon Derived from Foam Polystyrene as an Anode Material for Lithium-Ion Batteries. Appl. Surf. Sci. 2020, 504, 144398. [Google Scholar] [CrossRef]
- Fu, Y.; Wei, Q.; Zhang, G.; Sun, S. Advanced Phosphorus-Based Materials for Lithium/Sodium-Ion Batteries: Recent Developments and Future Perspectives. Adv. Energy Mater. 2018, 8, 1703058. [Google Scholar] [CrossRef]
- Liu, W.; Zhi, H.; Yu, X. Recent Progress in Phosphorus Based Anode Materials for Lithium/Sodium Ion Batteries. Energy Storage Mater. 2019, 16, 290–322. [Google Scholar] [CrossRef]
- Liu, Y.; Qiao, Y.; Wei, G.; Li, S.; Lu, Z.; Wang, X.; Lou, X. Sodium Storage Mechanism of N, S Co-Doped Nanoporous Carbon: Experimental Design and Theoretical Evaluation. Energy Storage Mater. 2018, 11, 274–281. [Google Scholar] [CrossRef]
- Wan, H.; Ju, X.; He, T.; Chen, T.; Zhou, Y.; Zhang, C.; Wang, J.; Xu, Y.; Yao, B.; Zhuang, W.; et al. Sulfur-Doped Porous Carbon as High-Capacity Anodes for Lithium and Sodium Ions Batteries. J. Alloys Compd. 2021, 863, 158078. [Google Scholar] [CrossRef]
- Ma, G.; Ning, G.; Wei, Q. S-Doped Carbon Materials: Synthesis, Properties and Applications. Carbon 2022, 195, 328–340. [Google Scholar] [CrossRef]
- Kim, J.K.; Kang, Y.C. Encapsulation of Se into Hierarchically Porous Carbon Microspheres with Optimized Pore Structure for Advanced Na-Se and K-Se Batteries. ACS Nano 2020, 14, 13203–13216. [Google Scholar] [CrossRef]
- Yang, B.; Liu, S.; Fedoseeva, Y.V.; Okotrub, A.V.; Makarova, A.A.; Jia, X.; Zhou, J. Engineering Selenium-Doped Nitrogen-Rich Carbon Nanosheets as Anode Materials for Enhanced Na-Ion Storage. J. Power Sources 2021, 493, 229700. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, M.; Wang, T.; Li, S.; Zhang, Q.; Zhang, M.; Xu, H.; Liu, J.; Liang, J.; Zhu, J.; et al. Covalent Selenium Embedded in Hierarchical Carbon Nanofibers for Ultra-High Areal Capacity Li-Se Batteries. iScience 2020, 23, 100919. [Google Scholar] [CrossRef]
- Tian, H.; Tian, H.; Wang, S.; Chen, S.; Zhang, F.; Song, L.; Liu, H.; Liu, J.; Wang, G. High-Power Lithium–Selenium Batteries Enabled by Atomic Cobalt Electrocatalyst in Hollow Carbon Cathode. Nat. Commun. 2020, 11, 5025. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Guo, K.; Fan, J.; Min, Y.; Xu, Q. Confined Selenium in N-Doped Mesoporous Carbon Nanospheres for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 16558–16566. [Google Scholar] [CrossRef]
- Rajamanickam, D.; Dhatshanamurthi, P.; Shanthi, M. Preparation and characterization of SeO2/TiO2 composite photocatalyst with excellent performance for Sunset Yellow azo dye degradation under natural sunlight illumination. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 138, 389–498. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, S.; Sudhakar, S.; David, L. XPS Study of Some Selected Selenium Compounds. J. Electron Spectrosc. 1986, 40, 329–337. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, S.; Zhang, Y.; Yu, B.; Hou, L.; Su, G.; Ma, S.; Zou, J.; Huang, H. Hollow carbon nanospheres with extremely small size as anode material in lithium-ion batteries with outstanding cycling stability. J. Phys. Chem. C 2016, 120, 3139–3144. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, T.; Zhang, D.; Zhang, W.; Zhang, H.; Liu, R.; Yao, M.; Liu, B. Remarkable cycle-activated capacity increasing in onion-like carbon nanospheres as lithium battery anode material. Nanotechnology 2016, 28, 035704. [Google Scholar] [CrossRef]
- Cheng, L.; Ma, C.; Lu, W.; Wang, X.; Yue, H.; Zhang, D.; Xing, Z. A Graphitized Hierarchical Porous Carbon as an Advanced Cathode Host for Alkali Metal-Selenium Batteries. Chem. Eng. 2022, 433, 133527. [Google Scholar] [CrossRef]
- Brezesinski, T.; Wang, J.; Tolbert, S.H.; Dunn, B. Ordered Mesoporous Alpha-MoO3 with Iso-Oriented Nanocrystalline Walls for Thin-Film Pseudocapacitors. Nat. Mater. 2010, 9, 146–151. [Google Scholar] [CrossRef]
- Wu, L.; Yang, J.O.; Guo, S.; Yao, L.; Li, H.; Zhang, S.; Yue, H.; Cai, K.; Zhang, C.; Yang, C.; et al. Pseudocapacitive Trimetal Fe0.8CoMnO4 Nanoparticles@Carbon Nanofibers as High-Performance Sodium Storage Anode with Self-Supported Mechanism. Adv. Funct. Mater. 2020, 30, 2001718. [Google Scholar] [CrossRef]
- Yang, B.; Chen, J.; Lei, S.; Guo, R.; Li, H.; Shi, S.; Yan, X. Spontaneous growth of three-dimensional framework carbon from sodium citrate for high energy- and power-density and long-life sodium-ion hybrid capacitors. Adv. Energy Mater. 2018, 8, 1702409. [Google Scholar] [CrossRef]
- Hong, Y.; Kang, Y. Selenium-Impregnated Hollow Carbon Microspheres as Efficient Cathode Materials for Lithium-Selenium Batteries. Carbon 2017, 111, 198–206. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, L.; Qiu, M.; Zhang, N. Amorphous Se Restrained by Biomass-Derived Defective Carbon for Stable Na-Se Batteries. ACS Appl. Energy Mater. 2021, 4, 7219–7225. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, J.; Xia, Z.; Fan, M.; Lv, C.; Guang, L.; Tian, G.; Li, X. Polyaniline-coated selenium/carbon composites encapsulated in graphene as efficient cathodes for Li-Se batteries. Nano Res. 2018, 11, 2460–2469. [Google Scholar] [CrossRef]
- Mukkabla, R.; Deshagani, S.; Meduri, P.; Deepa, M.; Ghosal, P. Selenium/Graphite Platelet Nanofiber Composite for Durable Li-Se Batteries. ACS Energy Lett. 2017, 2, 1288–1295. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, W.; Xin, S.; Ye, H.; Yin, Y.; Guo, Y. Graphitic Nanocarbon-Selenium Cathode with Favorable Rate Capability for Li-Se Batteries. ACS Appl. Mater. Interfaces 2017, 9, 8759–8765. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.; Xiang, K.; Xiao, L.; Chen, W.; Zhu, Y.; Liao, H.; Chen, H. Se/CNTs microspheres as improved performance for cathodes in Li-Se batteries. J. Alloys Compd. 2019, 786, 537–543. [Google Scholar] [CrossRef]
- Dong, W.; Wang, C.; Li, C.; Xia, F.; Yu, W.; Wu, L.; Mohamed, H.; Hu, Z.; Liu, J.; Chen, L.; et al. The free-standing N-doped Murray carbon framework with the engineered quasi-optimal Se/C interface for high-Se-loading Li/Na-Se batteries at elevated temperature. Mater. Today Energy 2021, 21, 100808. [Google Scholar] [CrossRef]
- Wu, X.; Chen, X.; Wu, H.; Xie, B.; Wang, D.; Wang, R.; Zhang, X.; Piao, Y.; Diao, G.; Chen, M. Encapsulation of Se in dual-wall hollow carbon spheres: Physical confinement and chemisorption for superior Na-Se and K-Se batteries. Carbon 2022, 187, 354–364. [Google Scholar] [CrossRef]
- Kim, J.; Kang, Y. Deliberate introduction of mesopores into microporous activated carbon toward efficient Se cathode of Na-Se batteries. Int. J. Energy Res. 2022, 46, 3396–3408. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Guo, S.; Yue, H.; Li, H.; Li, W.; Yao, C.; Li, P.; Fa, W.; Song, B.; Li, K.; et al. Improvement on the Use of Se@C in Batteries by Synergistic Effect of Nano-Confinement and C-Se Bond. Batteries 2023, 9, 143. https://doi.org/10.3390/batteries9030143
Wu L, Guo S, Yue H, Li H, Li W, Yao C, Li P, Fa W, Song B, Li K, et al. Improvement on the Use of Se@C in Batteries by Synergistic Effect of Nano-Confinement and C-Se Bond. Batteries. 2023; 9(3):143. https://doi.org/10.3390/batteries9030143
Chicago/Turabian StyleWu, Lijun, Shoujie Guo, Hongwei Yue, Hao Li, Wei Li, Chuan Yao, Pinjiang Li, Wenjun Fa, Burong Song, Kai Li, and et al. 2023. "Improvement on the Use of Se@C in Batteries by Synergistic Effect of Nano-Confinement and C-Se Bond" Batteries 9, no. 3: 143. https://doi.org/10.3390/batteries9030143
APA StyleWu, L., Guo, S., Yue, H., Li, H., Li, W., Yao, C., Li, P., Fa, W., Song, B., Li, K., Zhou, B., Yu, Q., Xu, Y., Yang, C., Zheng, Z., & Gao, Y. (2023). Improvement on the Use of Se@C in Batteries by Synergistic Effect of Nano-Confinement and C-Se Bond. Batteries, 9(3), 143. https://doi.org/10.3390/batteries9030143






