High-Performance Layered Oxides for Sodium-Ion Batteries Achieved through Combined Aluminum Substitution and Surface Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Al Substitution of Ni in Layered Na2/3[Ni1/2Mn1/2]O2: Concentration Limits
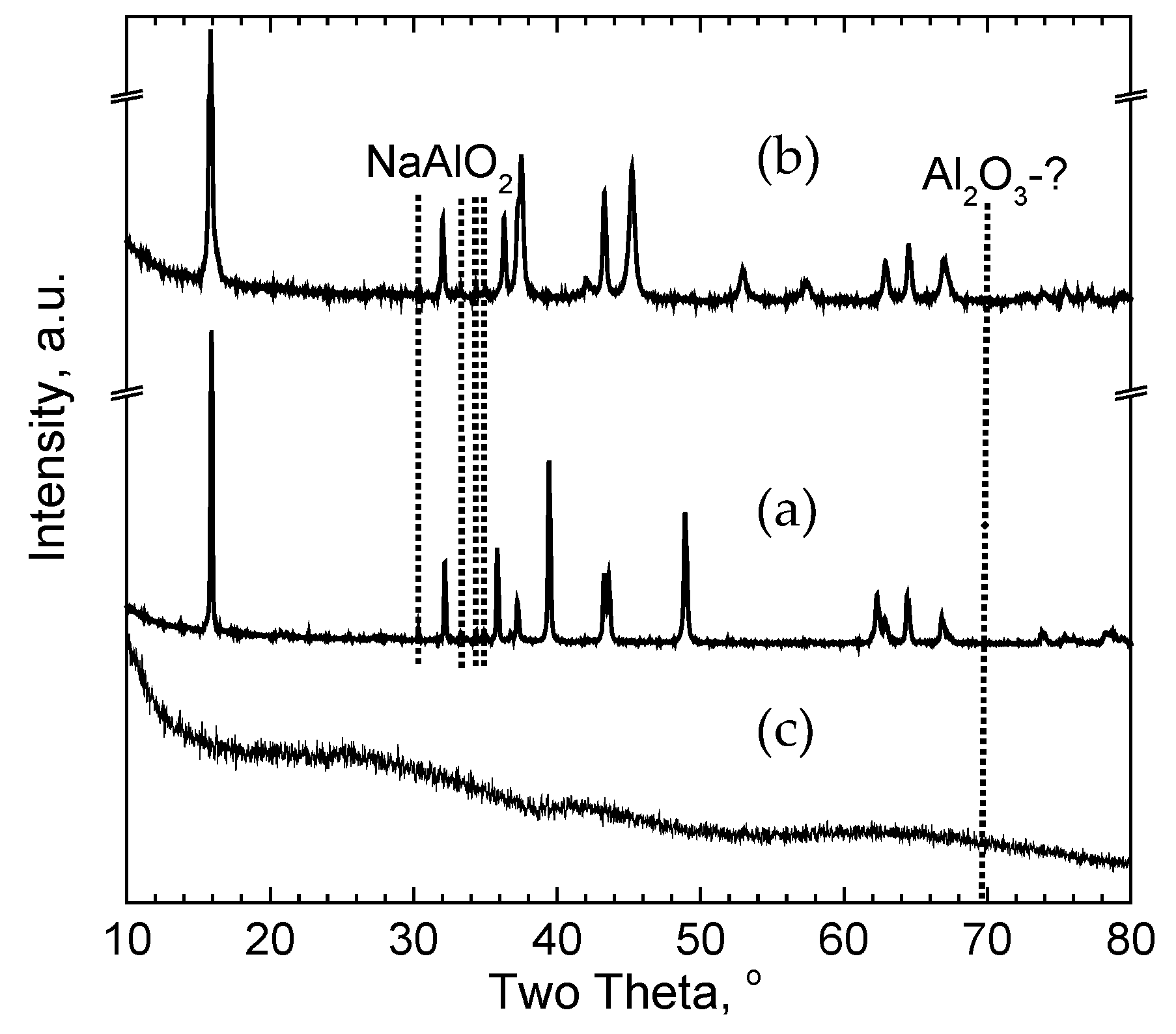
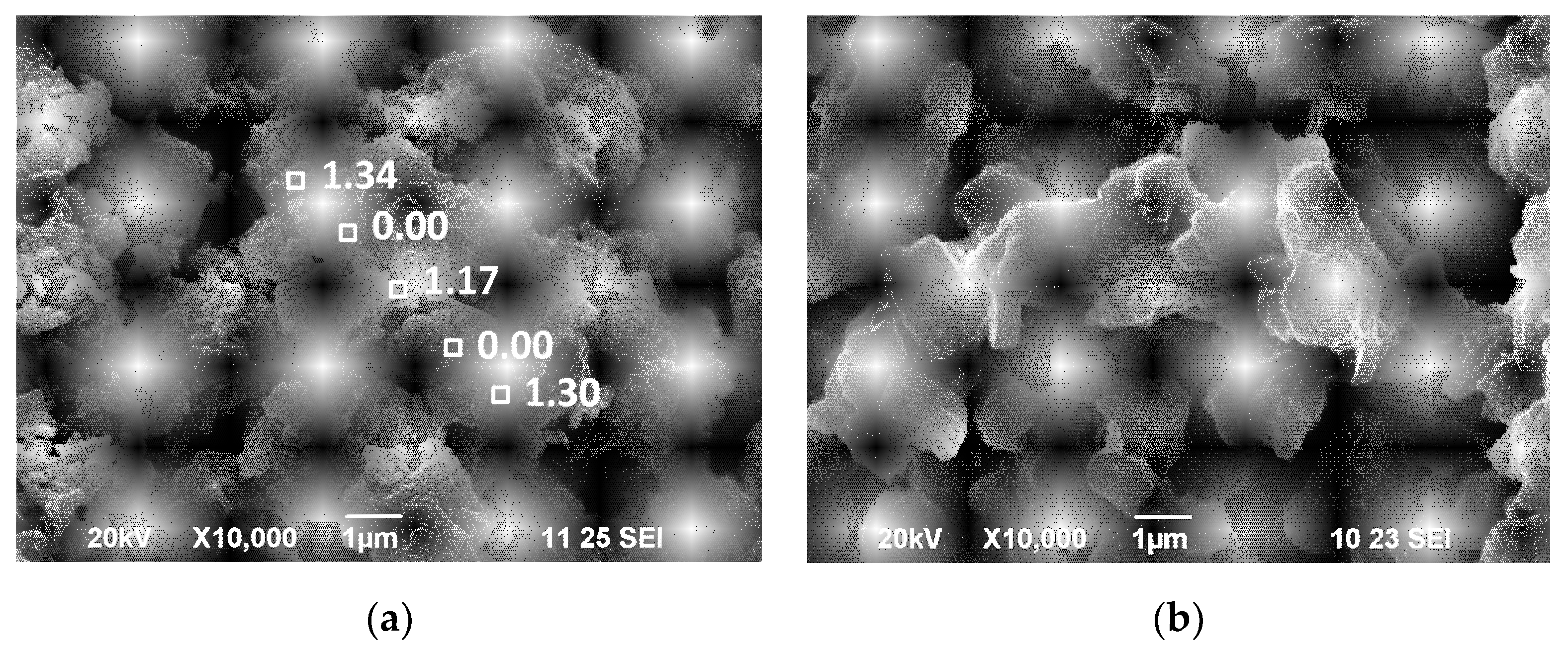
3.2. Treatment of Layered Oxides with Al2O3
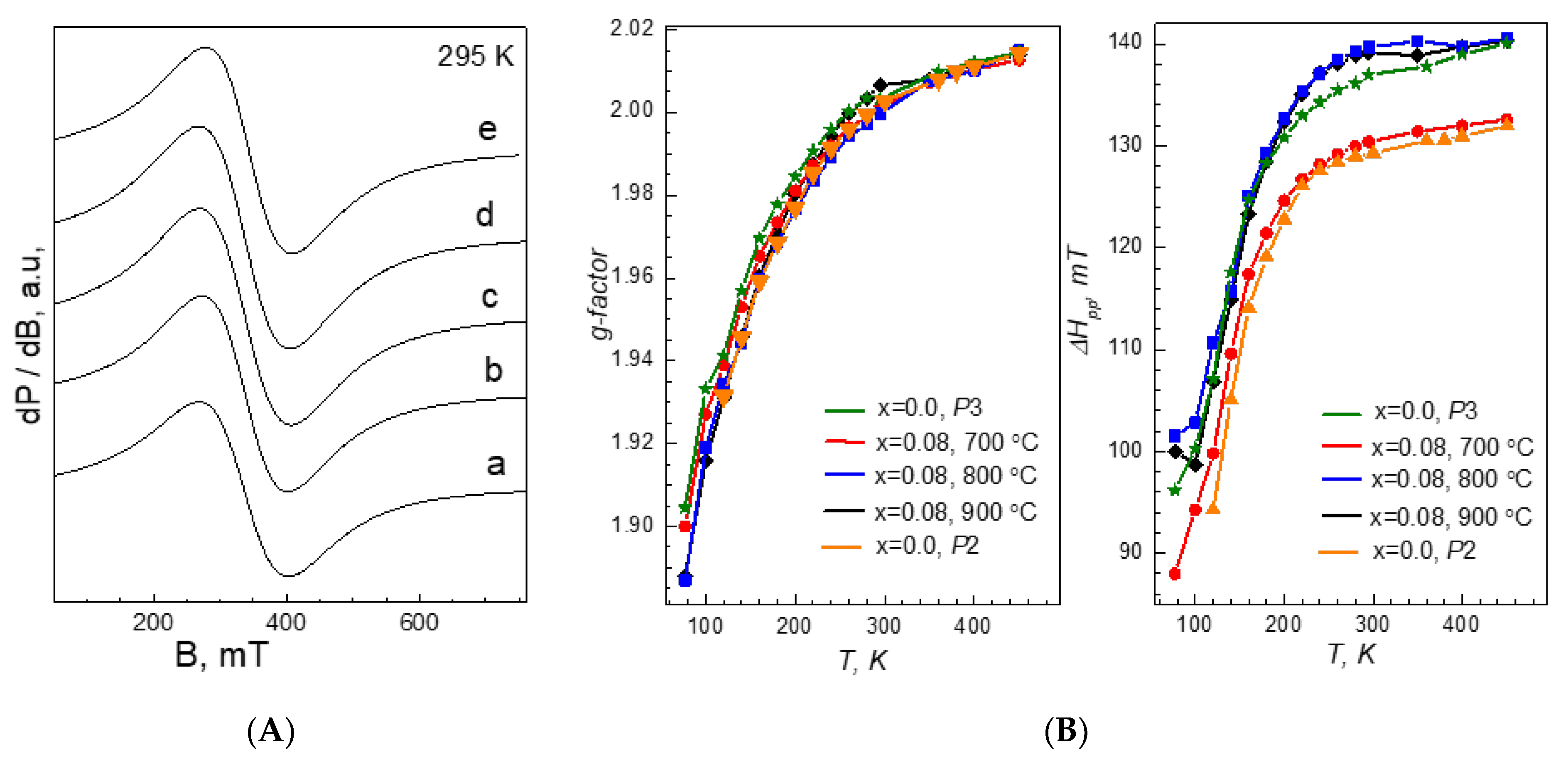
3.3. Na Storage Performance of Al-Substituted and Al2O3-Treated Layered Oxides
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, T.; Zhang, Y.; Jiang, Z.; Zeng, X.; Ji, J.; Li, Z.; Gao, X.; Sun, M.; Lin, Z.; Ling, M.; et al. Exploring competitive features of stationary sodium ion batteries for electrochemical energy storage. Energy Environ. Sci. 2019, 12, 1512–1533. [Google Scholar] [CrossRef]
- Hirsh, H.S.; Li, Y.; Tan, D.H.S.; Zhang, M.; Zhao, E.; Meng, Y.S. Sodium-Ion Batteries Paving the Way for Grid Energy Storage. Adv. Energy Mater. 2020, 10, 2001274. [Google Scholar] [CrossRef]
- Su, H.; Jaffer, S.; Yu, H. Transition metal oxides for sodium-ion batteries. Energy Storage Mater. 2016, 5, 116–131. [Google Scholar] [CrossRef]
- Wei, F.; Zhang, Q.; Zhang, P.; Tian, W.; Dai, K.; Zhang, L.; Mao, J.; Shao, G. Review—Research Progress on Layered Transition Metal Oxide Cathode Materials for Sodium Ion Batteries. J. Electrochem. Soc. 2021, 168, 050524. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, Z.; Li, W.; Zou, C.; Jin, H.; Wang, S.; Chou, S.; Dou, S.-X. Sodium transition metal oxides: The preferred cathode choice for future sodium-ion batteries? Energy Environ. Sci. 2021, 14, 158–179. [Google Scholar] [CrossRef]
- Shi, C.; Wang, L.; Chen, X.; Li, J.; Wang, S.; Wang, J.; Jin, H. Challenges of layer-structured cathodes for sodium-ion batteries. Nanoscale Horiz. 2022, 7, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Kalapsazova, M.; Kukeva, R.; Zhecheva, E.; Stoyanova, R. Metal Substitution versus Oxygen-Storage Modifier to Regulate the Oxygen Redox Reactions in Sodium-Deficient Three-Layered Oxides. Batteries 2022, 8, 56. [Google Scholar] [CrossRef]
- Chang, Y.-X.; Yu, L.; Xing, X.; Guo, Y.-J.; Xie, Z.; Xu, S. Ion Substitution Strategy of Manganese-Based Layered Oxide Cathodes for Advanced and Low-Cost Sodium Ion Batteries. Chem. Rec. 2022, 10, e202200122. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, M.; Liu, B.; Zhang, Y.; Liang, X.; Xia, X. Heteroatom Doping: An Effective Way to Boost Sodium Ion Storage. Adv. Energy Mater. 2020, 10, 2000927. [Google Scholar] [CrossRef]
- Kaufman, J.L.; Vinckevičiūtė, J.; Kolli, S.K.; Goiri, J.G.; Van der Ven, A. Understanding intercalation compounds for sodium-ion batteries and beyond. Philos. Trans. A Math. Phys. Eng. Sci. 2019, 377, 20190020. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, H.J. Crystalline Domain Battery Material. Acc. Chem. Res. 2020, 53, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhao, Z.; Xu, H.; Sun, J.; He, X.; Lei, Z.; Liu, Z.-H.; Jiang, R.; Li, Q. A Queue-Ordered Layered Mn-Based Oxides with Al Substitution as High-Rate and High-Stabilized Cathode for Sodium-Ion Batteries. Small 2021, 17, e2006259. [Google Scholar] [CrossRef] [PubMed]
- Abate, I.; Kim, S.Y.; Pemmaraju, C.D.; Toney, M.F.; Yang, W.; Devereaux, T.P.; Chueh, W.C.; Nazar, L.F. The Role of Metal Substitution in Tuning Anion Redox in Sodium Metal Layered Oxides Revealed by X-Ray Spectroscopy and Theory. Angew. Chem. Int. Ed. 2021, 60, 10880–10887. [Google Scholar] [CrossRef] [PubMed]
- Di Lecce, D.; Campanella, D.; Hassoun, J. Insight on the Enhanced Reversibility of a Multi-Metal Layered Oxide for Sodium-Ion Battery. J. Phys. Chem. C 2018, 122, 23925–23933. [Google Scholar] [CrossRef]
- Singh, G.; Tapia-Ruiz, N.; del Amo, J.M.L.; Maitra, U.; Somerville, J.W.; Armstrong, A.R.; de Ilarduya, J.M.; Rojo, T.; Bruce, P.G. High Voltage Mg-Doped Na0.67Ni0.3–xMgxMn0.7O2 (x = 0.05, 0.1) Na-Ion Cathodes with Enhanced Stability and Rate Capability. Chem. Mater. 2016, 28, 5087–5094. [Google Scholar] [CrossRef]
- Kalapsazova, M.; Markov, P.; Kostov, K.; Zhecheva, E.; Nihtianova, D.; Stoyanova, R. Controlling at Elevated Temperature the Sodium Intercalation Capacity and Rate Capability of P3-Na2/3Ni1/2Mn1/2O2 through the Selective Substitution of Nickel with Magnesium. Batter. Supercaps 2020, 3, 1329–1340. [Google Scholar] [CrossRef]
- Wu, X.; Xu, G.-L.; Zhong, G.; Gong, Z.; McDonald, J.; Zheng, S.; Fu, R.; Chen, Z.; Amine, K.; Yang, Y. Insights into the effects of zinc doping on structural phase transition of P2-type sodium nickel manganese oxide cathodes for high-energy sodium ion batteries. ACS Appl. Mater. Interfaces 2016, 8, 22227–22237. [Google Scholar] [CrossRef]
- Bai, X.; Sathiya, M.; Mendoza-Sanchez, B.; Iadecola, A.; Vergnet, J.; Dedryvere, R.; Saubanere, M.; Abakumov, A.M.; Rozier, P.; Tarascon, J.-M. Anionic Redox Activity in a Newly Zn-Doped Sodium Layered Oxide P2-Na2/3Mn1−yZnyO2 (0 < y < 0.23). Adv. Energy Mater. 2018, 8, 1802379. [Google Scholar]
- Wang, H.; Gao, R.; Li, Z.; Sun, L.; Hu, Z.; Liu, X. Different Effects of Al Substitution for Mn or Fe on the Structure and Electrochemical Properties of Na0.67Mn0.5Fe0.5O2 as a Sodium Ion Battery Cathode Material. Inorg. Chem. 2018, 57, 5249–5257. [Google Scholar] [CrossRef]
- Cheng, C.; Ding, M.; Yan, T.; Jiang, J.; Mao, J.; Feng, X.; Chan, T.-S.; Li, N.; Zhang, L. Anionic Redox Activities Boosted by Aluminum Doping in Layered Sodium-Ion Battery Electrode. Small Methods 2022, 6, 2101524. [Google Scholar] [CrossRef]
- Ramasamy, H.V.; Kaliyappan, K.; Thangavel, R.; Seong, W.M.; Kang, K.; Chen, Z.; Lee, Y.-S. Efficient Method of Designing Stable Layered Cathode Material for Sodium Ion Batteries Using Aluminum Doping. J. Phys. Chem. Lett. 2017, 8, 5021–5030. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.; Silvan, B.; Choi, Y.-S.; Celorrio, V.; Seymour, V.R.; Cibin, G.; Griffin, J.M.; Scanlon, D.O.; Tapia-Ruiz, N. Na2.4 Al 0.4 Mn2.6 O7 anionic redox cathode material for sodium-ion batteries—A combined experimental and theoretical approach to elucidate its charge storage mechanism. J. Mater. Chem. A 2022, 10, 7341–7356. [Google Scholar] [CrossRef]
- Li, J.; Hu, H.; Wang, J.; Xiao, Y. Surface chemistry engineering of layered oxide cathodes for sodium-ion batteries. Carbon Neutralization 2022, 1, 96–116. [Google Scholar] [CrossRef]
- Jung, S.C.; Kim, H.-J.; Choi, J.W.; Han, Y.-K. Sodium Ion Diffusion in Al2O3: A Distinct Perspective Compared with Lithium Ion Diffusion. Nano Lett. 2014, 14, 6559–6563. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.; Qiu, J.; Liu, X.; Zheng, B.; Zhao, Y.; Li, J.; He, H.; Zhou, K.; Xiao, Z.; Li, Q.; et al. Highly-stable P2–Na0.67MnO2 electrode enabled by lattice tailoring and surface engineering. Energy Storage Mater. 2020, 26, 503–512. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Myung, S.-T.; Choi, J.U.; Yoon, C.S.; Yashirod, H.; Sun, Y.-K. Resolving the degradation pathways of the O3-type layered oxide cathode surface through the nano-scale aluminum oxide coating for high-energy density sodium-ion batteries. J. Mater. Chem. A 2017, 5, 23671–23680. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, X.; Zhang, A.; Shen, C.; Liu, Q.; Enaya, H.A.; Zhou, C. Layered P2-Na2/3[Ni1/3Mn2/3]O2 as high-voltage cathode for sodium-ion batteries: The capacity decay mechanism and Al2O3 surface modification. Nano Energy 2016, 27, 27–34. [Google Scholar] [CrossRef]
- Kalapsazova, M.; Kostov, K.; Kukeva, R.; Zhecheva, E.; Stoyanova, R. Oxygen-Storage Materials to Stabilize the Oxygen Redox Activity of Three-Layered Sodium Transition Metal Oxides. J. Phys. Chem. Lett. 2021, 12, 7804–7811. [Google Scholar] [CrossRef]
- Zhang, Q.; Gu, Q.-F.; Li, Y.; Fan, H.-N.; Luo, W.-B.; Liu, H.-K.; Dou, S.-X. Surface Stabilization of O3-type Layered Oxide Cathode to Protect the Anode of Sodium Ion Batteries for Superior Lifespan. Iscience 2019, 19, 244–254. [Google Scholar] [CrossRef]
- Feng, Y.-H.; Cheng, Z.; Xu, C.-L.; Yu, L.; Si, D.; Yuan, B.; Liu, M.; Zhao, B.; Wang, P.-F.; Han, X. Low-Cost Al-Doped Layered Cathodes with Improved Electrochemical Performance for Rechargeable Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 23465–23473. [Google Scholar] [CrossRef]
- Nayak, D.; Jha, P.K.; Ghosh, S.; Adyam, V. Aluminium substituted β–type NaMn1-xAlxO2: A stable and enhanced electrochemical kinetic sodium-ion battery cathode. J. Power Sources 2019, 438, 227025. [Google Scholar] [CrossRef]
- Delmas, C.; Fouassier, C.; Hagenmuller, P. Structural Classification and Properties of the Layered Oxides. Phys. B+C 1980, 99, 81–85. [Google Scholar] [CrossRef]
- Kalapsazova, M.; Zhecheva, E.; Tyuliev, G.; Nihtianova, D.; Mihaylov, L.; Stoyanova, R. Effects of the Particle Size Distribution and of the Electrolyte Salt on the Intercalation Properties of P3-Na2/3Ni1/2Mn1/2O2. J. Phys. Chem. C 2017, 121, 5931–5940. [Google Scholar] [CrossRef]
- Pang, W.-L.; Zhang, X.-H.; Guo, J.-Z.; Li, J.-Y.; Yan, X.; Hou, B.-H.; Guan, H.-Y.; Wu, X.-L. P2-type Na2/3Mn1-xAlxO2 cathode material for sodium-ion batteries: Al-doped enhanced electrochemical properties and studies on the electrode kinetics. J. Power Sources 2017, 356, 80–88. [Google Scholar] [CrossRef]
- Kalapsazova, M.; Ortiz, G.F.; Tirado, J.L.; Dolotko, O.; Zhecheva, E.; Nihtianova, D.; Mihaylov, L.; Stoyanova, R. P3-Type Layered Sodium-Deficient Nickel-Manganese Oxides: A Flexible Structural Matrix for Reversible Sodium and Lithium Intercalation. ChemPlusChem 2015, 80, 1642–1656. [Google Scholar] [CrossRef] [PubMed]
- Kalapsazova, M.; Ivanova, S.; Kukeva, R.; Simova, S.; Wegner, S.; Zhecheva, E.; Stoyanova, R. Combined use of EPR and Na-23 MAS NMR spectroscopy for assessing the properties of the mixed cobalt-nickel-manganese layers of P3-NayCo1-2xNixMnxO2. Phys. Chem. Chem. Phys. 2017, 19, 27065–27073. [Google Scholar] [CrossRef]
- Kim, E.J.; Ma, L.A.; Duda, L.C.; Pickup, D.M.; Chadwick, A.V.; Younesi, R.; Irvine, J.T.S.; Armstrong, A.R. Oxygen Redox Activity through a Reductive Coupling Mechanism in the P3-Type Nickel-Doped Sodium Manganese Oxide. ACS Appl. Energy Mater. 2020, 3, 184–191. [Google Scholar] [CrossRef]
- Lee, D.H.; Xu, J.; Meng, Y.S. An advanced cathode for Na-ion batteries with high rate and excellent structural stability. Phys. Chem. Chem. Phys. 2013, 15, 3304–3312. [Google Scholar] [CrossRef]
- Xu, G.-L.; Amine, R.; Xu, Y.-F.; Liu, J.; Gim, J.; Ma, T.; Ren, Y.; Sun, C.-J.; Liu, Y.; Zhang, X.; et al. Insights into the structural effects of layered cathode materials for high voltage sodium-ion batteries. Energy Environ. Sci. 2017, 10, 1677–1693. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, M.; Zhang, L.; Qi, S.; Feng, Y.; He, P.; Ji, X.; Wang, P.; Zhou, L.; Chen, S.; et al. Shear-resistant interface of layered oxide cathodes for sodium ion batteries. Energy Storage Mater. 2022, 45, 389–398. [Google Scholar] [CrossRef]
- Chen, C.; Ding, Z.; Han, Z.; Liang, C.; Lan, H.; Wang, P.; Gao, P.; Wei, W. Unravelling Atomically Irreversible Cation Migration in Sodium Layered Oxide Cathodes. J. Phys. Chem. Lett. 2020, 11, 5464–5470. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.P.P.; Hayes, W.; Stevenson, R.H.W.; Wilkens, J. Investigation of the Bonding of Iron-Group Ions in Fluoride Crystals. I. J. Chem. Phys. 1963, 38, 1977. [Google Scholar] [CrossRef]
- Zhan, C.; Wu, T.; Lu, J.; Amine, K. Dissolution, migration, and deposition of transition metal ions in Li-ion batteries exemplified by Mn-based cathodes—A critical review. Energy Environ. Sci. 2018, 11, 243–257. [Google Scholar] [CrossRef]
- Jung, R.; Linsenmann, F.; Thomas, R.; Wandt, J.; Solchenbach, S.; Maglia, F.; Stinner, C.; Tromp, M.; Gasteiger, H.A. Nickel, Manganese, and Cobalt Dissolution from Ni-Rich NMC and Their Effects on NMC622-Graphite Cells. J. Electrochem. Soc. 2019, 166, A378–A389. [Google Scholar] [CrossRef]
- Pang, P.; Wang, Z.; Tan, X.; Deng, Y.; Nan, J.; Xing, Z.; Li, H. LiCoO2@LiNi0.45Al0.05Mn0.5O2 as high-voltage lithium-ion battery cathode materials with improved cycling performance and thermal stability. Electrochim. Acta 2019, 327, 135018. [Google Scholar] [CrossRef]



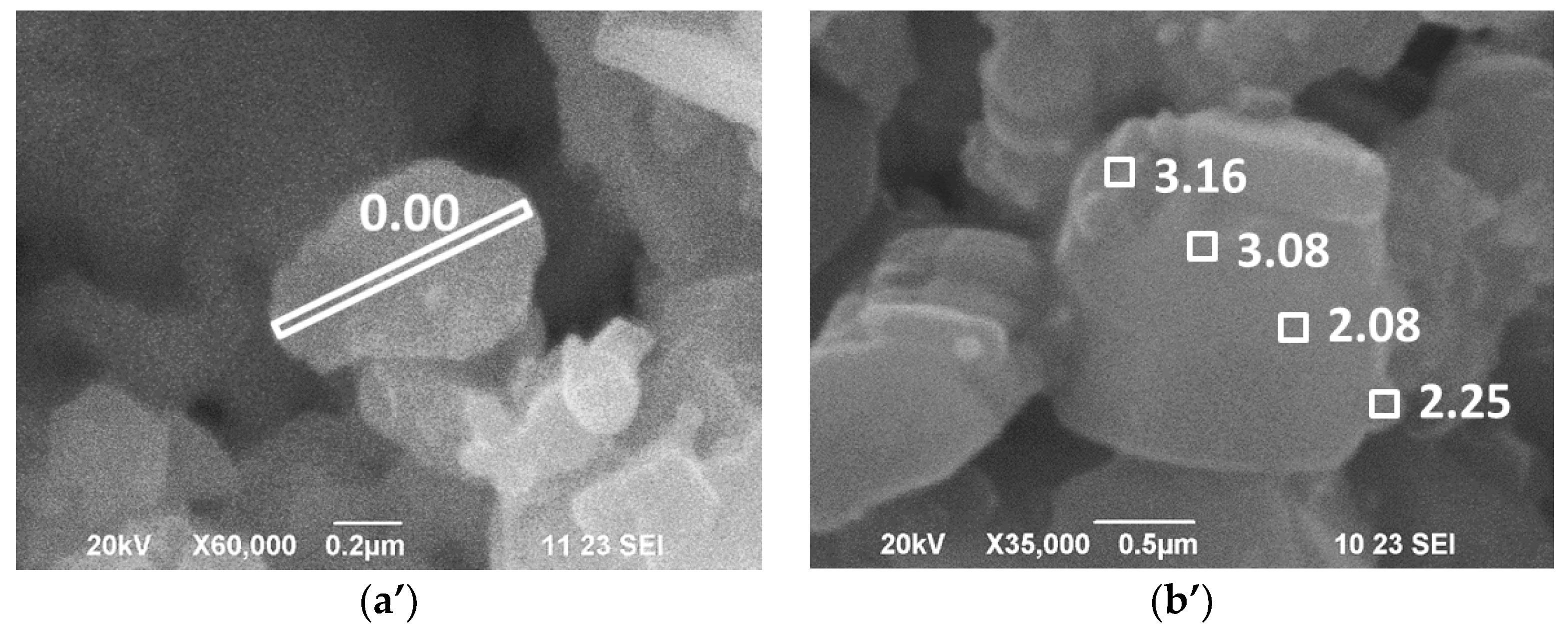
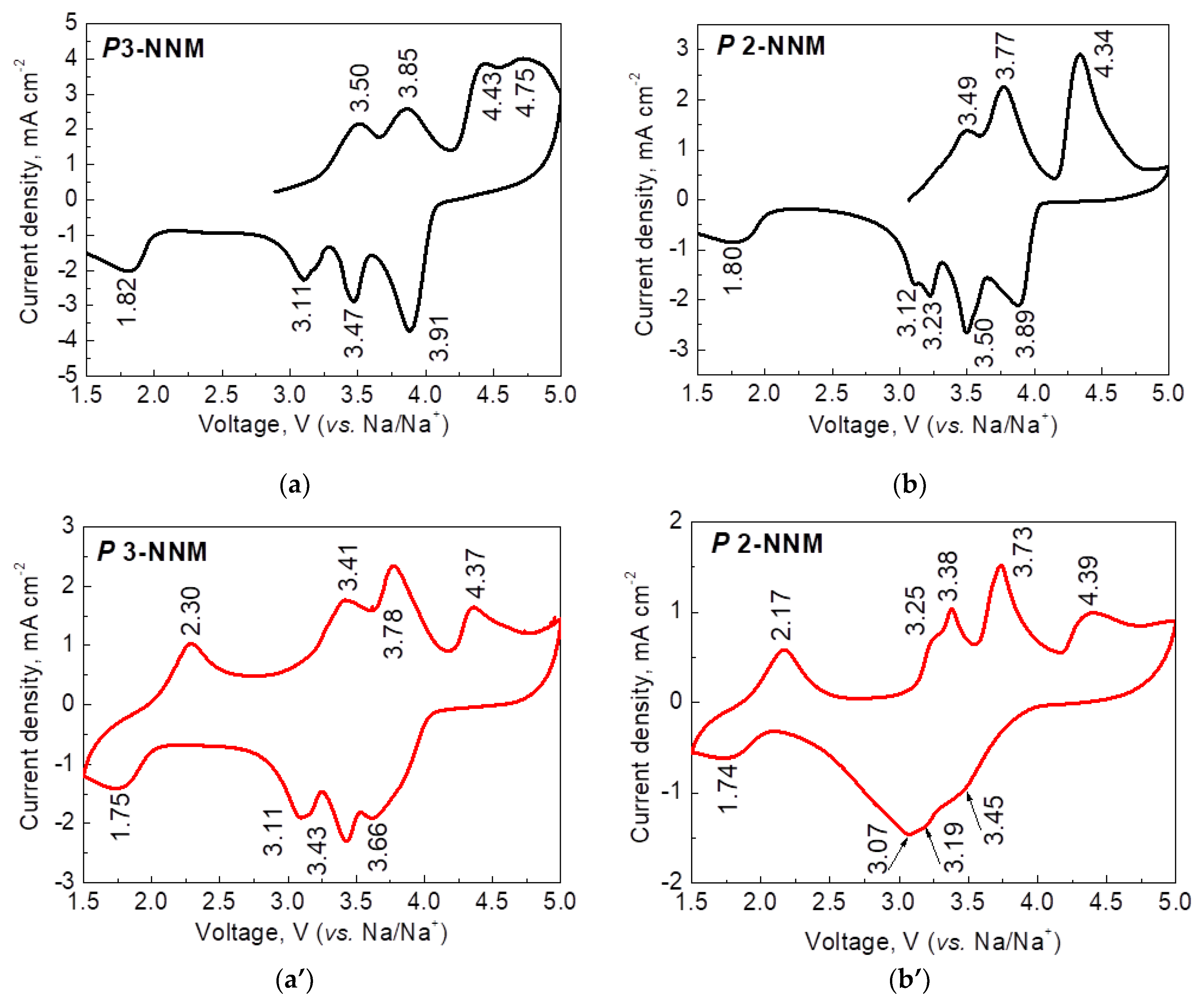
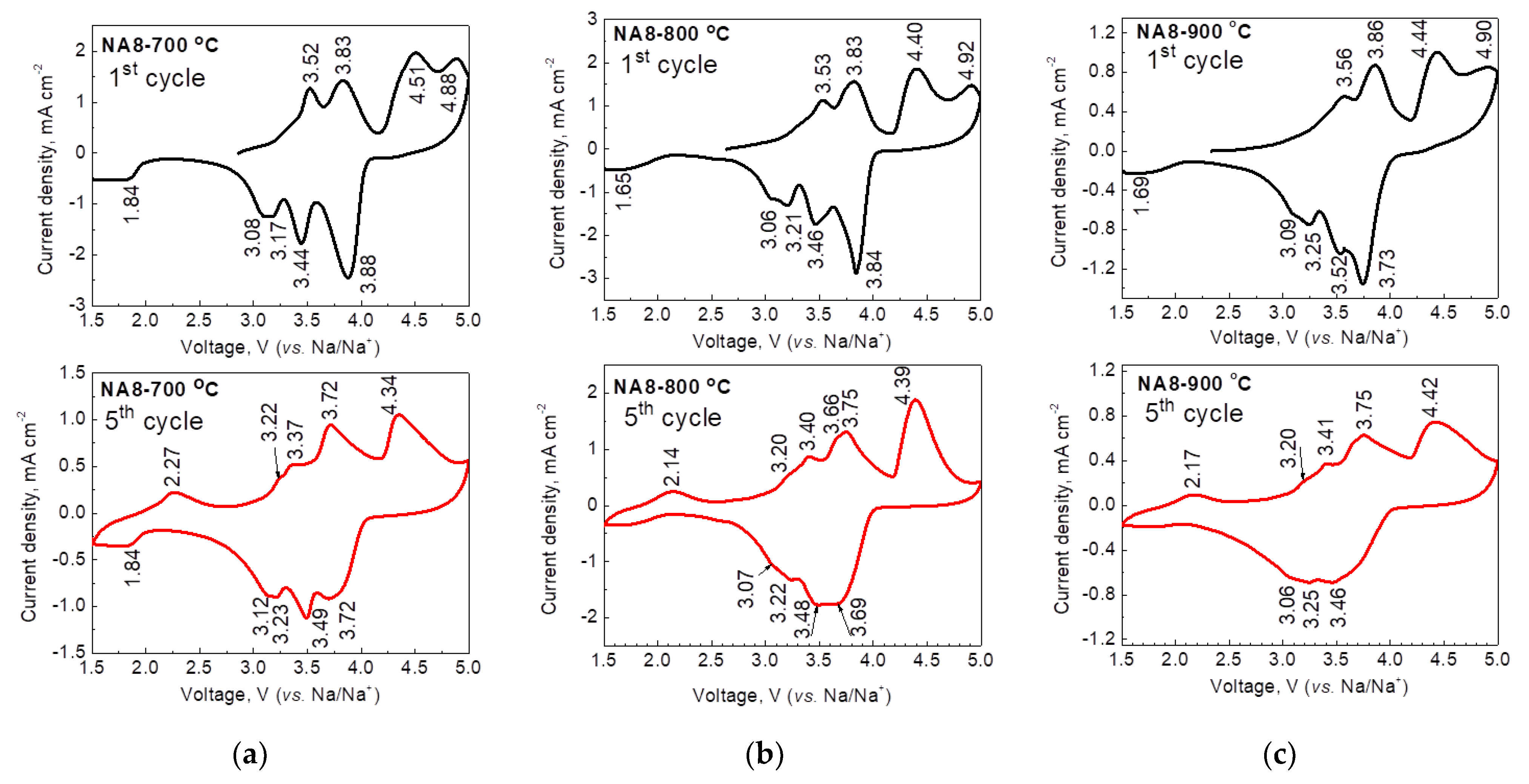
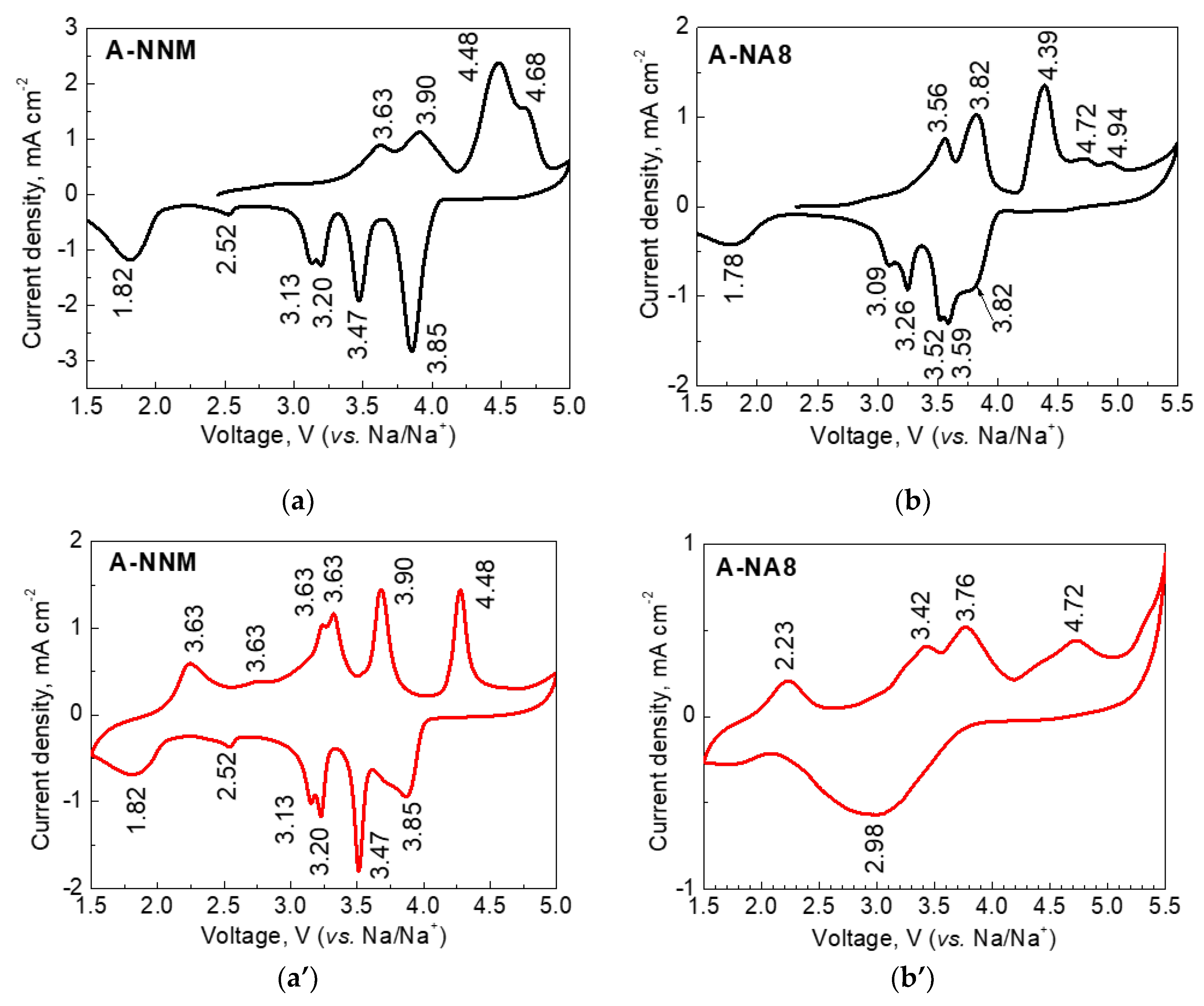
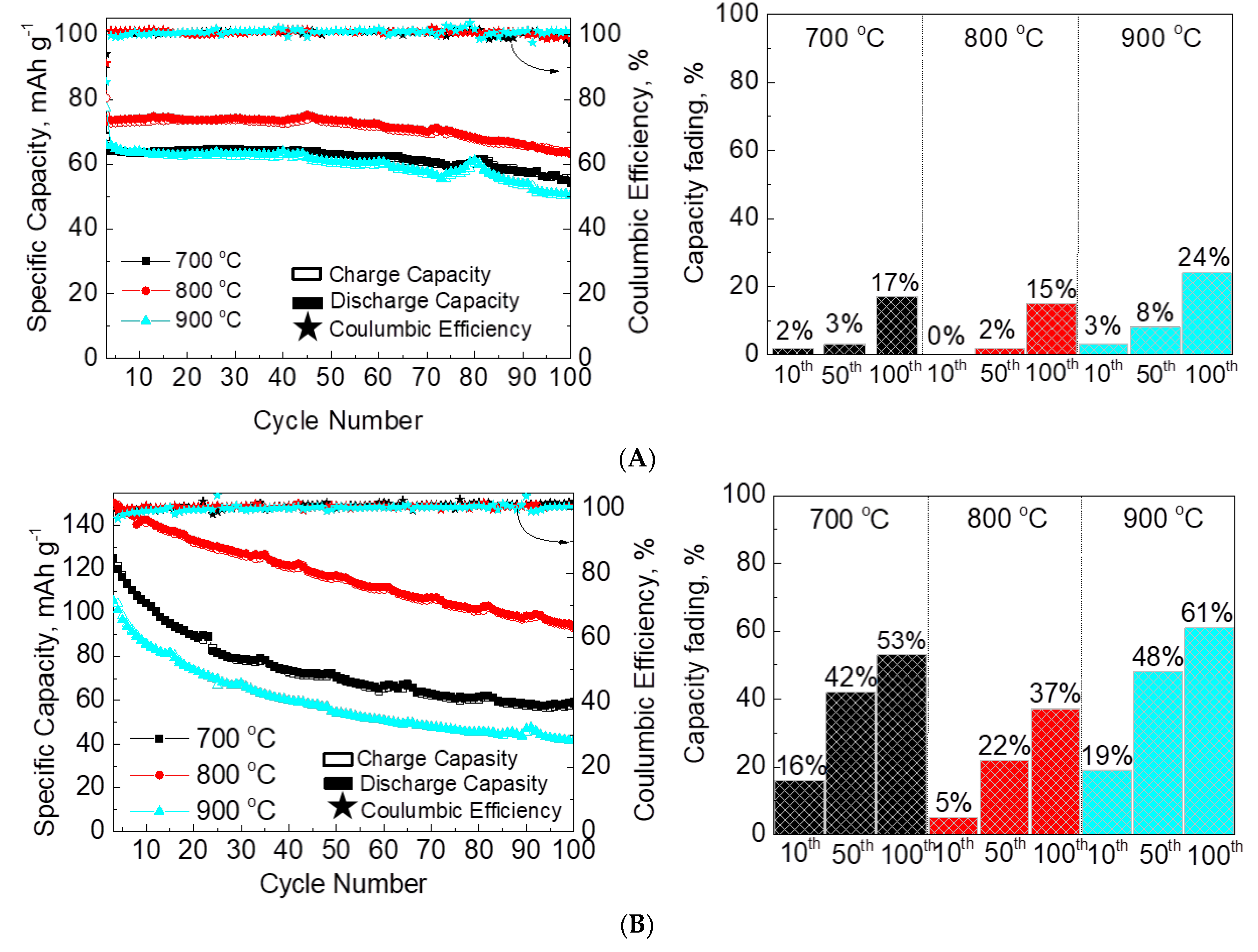
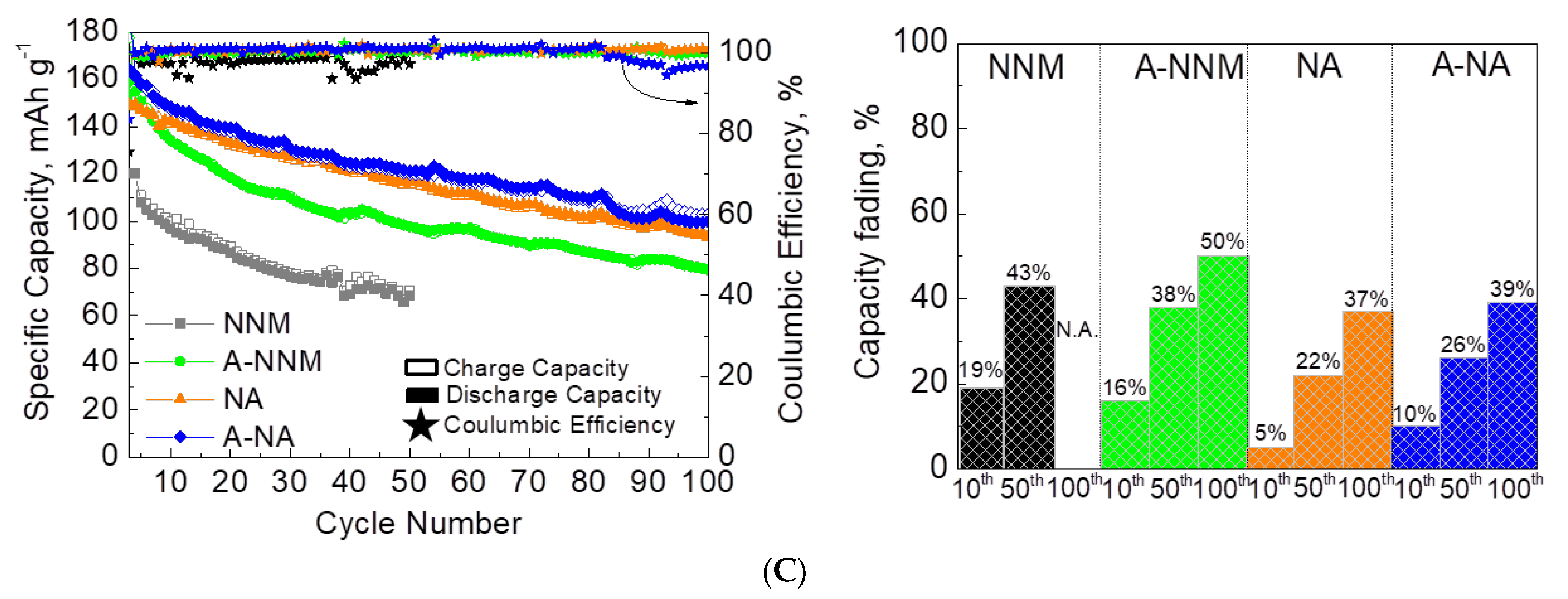
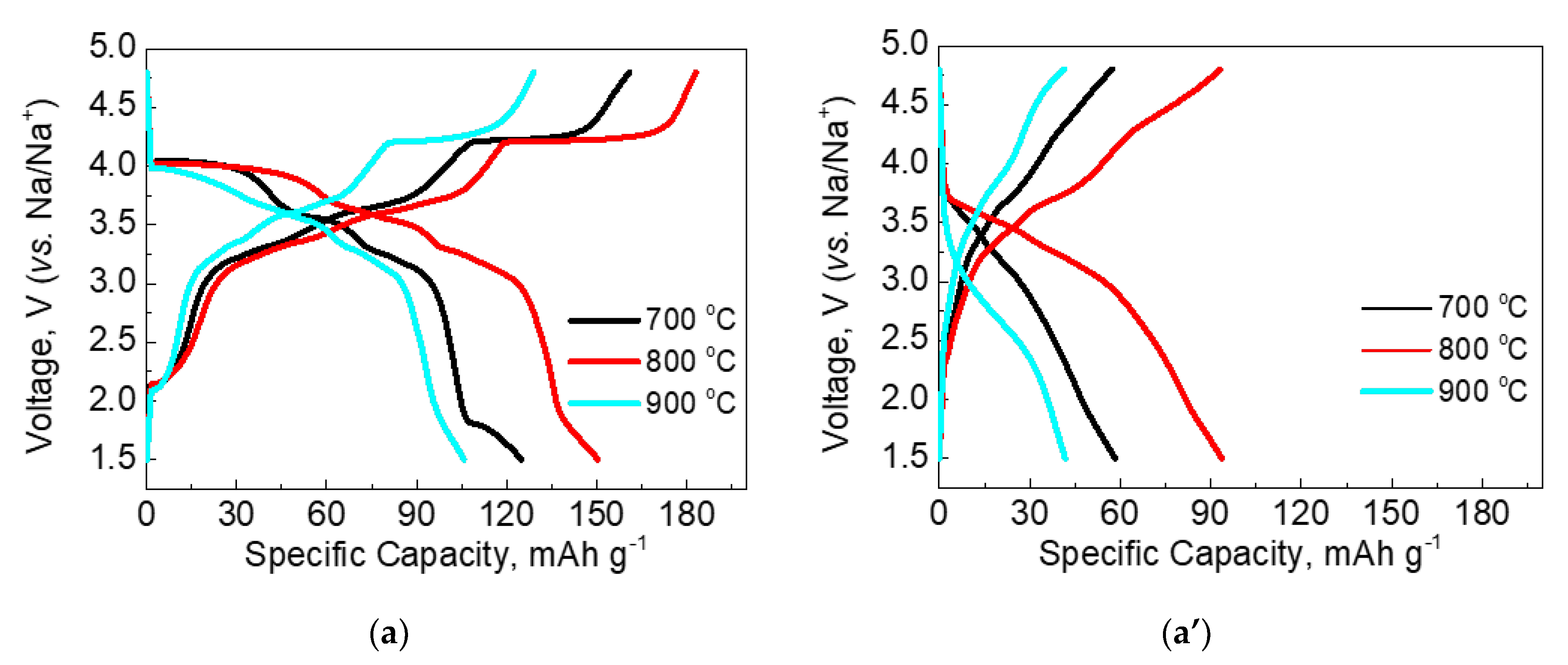

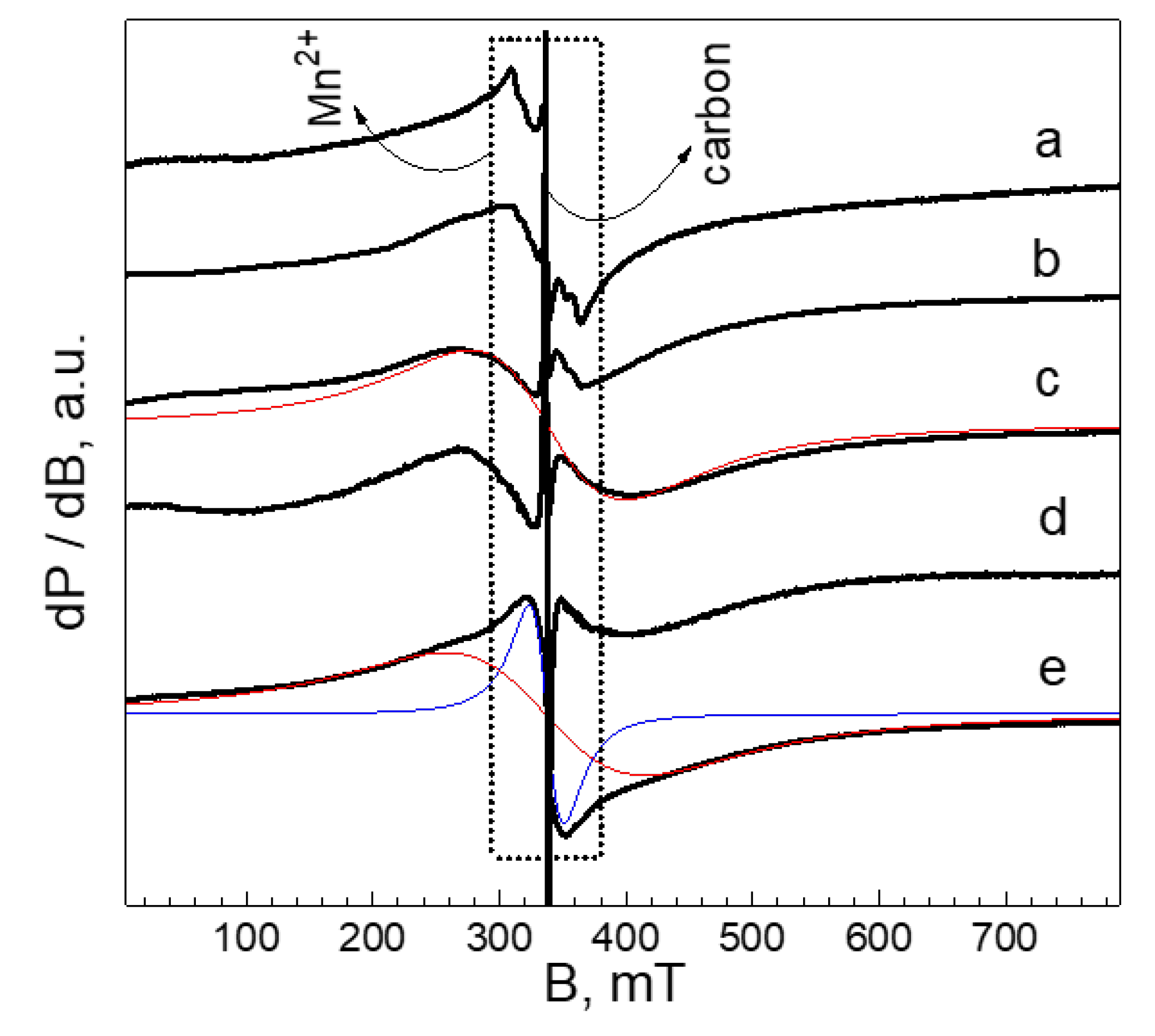
| Samples | Structure | a, Å | c, Å | V, Å3 | wt. % |
|---|---|---|---|---|---|
| P3-Na2/3Ni1/2Mn1/2O2 700 °C | P3 | 2.8890 | 16.7230 | 120.88 | |
| x = 0.08, 700 °C | P3 | 2.8890 | 16.7230 | 120.88 | 88 |
| P2 | 2.8905 | 11.1254 | 80.49 | 12 | |
| x = 0.08, 800 °C | P2 | 2.8903 | 11.0865 | 80.21 | |
| x = 0.08, 900 °C | P2 | 2.8888 | 11.0986 | 80.20 | |
| P2-Na2/3Ni1/3Mn2/3O2 900 °C | P2 | 2.8889 | 11.1562 | 80.63 |
| Samples | T = 295 K | T = 100 K | ||
|---|---|---|---|---|
| Pristine Oxide, ΔHpp (mT) | Cycled Oxide, ΔHpp (mT) | Pristine Oxide, ΔHpp (mT) | Cycled Oxide, ΔHpp (mT) | |
| Al-substituted oxide annealed at 700 °C (x = 0.08) | 130 | 137 | 94 | 117 |
| Al-substituted oxide annealed at 800 °C (x = 0.08) | 140 | 146 | 103 | 110 |
| Al-substituted oxide annealed at 900 °C (x = 0.08) | 140 | 158 | 99 | 123 |
| Al2O3-treated oxide, annealed at 700 °C | 130 | 139 | 95 | 105 |
| Al2O3-treated Al-substituted oxide, annealed at 800 °C | 137 | 142 | 98 | 104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalapsazova, M.; Kukeva, R.; Harizanova, S.; Markov, P.; Nihtianova, D.; Zhecheva, E.; Stoyanova, R. High-Performance Layered Oxides for Sodium-Ion Batteries Achieved through Combined Aluminum Substitution and Surface Treatment. Batteries 2023, 9, 144. https://doi.org/10.3390/batteries9020144
Kalapsazova M, Kukeva R, Harizanova S, Markov P, Nihtianova D, Zhecheva E, Stoyanova R. High-Performance Layered Oxides for Sodium-Ion Batteries Achieved through Combined Aluminum Substitution and Surface Treatment. Batteries. 2023; 9(2):144. https://doi.org/10.3390/batteries9020144
Chicago/Turabian StyleKalapsazova, Mariya, Rositsa Kukeva, Sonya Harizanova, Pavel Markov, Diana Nihtianova, Ekaterina Zhecheva, and Radostina Stoyanova. 2023. "High-Performance Layered Oxides for Sodium-Ion Batteries Achieved through Combined Aluminum Substitution and Surface Treatment" Batteries 9, no. 2: 144. https://doi.org/10.3390/batteries9020144
APA StyleKalapsazova, M., Kukeva, R., Harizanova, S., Markov, P., Nihtianova, D., Zhecheva, E., & Stoyanova, R. (2023). High-Performance Layered Oxides for Sodium-Ion Batteries Achieved through Combined Aluminum Substitution and Surface Treatment. Batteries, 9(2), 144. https://doi.org/10.3390/batteries9020144