Abstract
While past recycling efforts have primarily concentrated on extracting valuable metals from discarded cathode materials, the focus is now shifting towards anode materials, particularly graphite, which makes up 10–20% of LIB mass. Escalating prices of battery-grade graphite and environmental considerations surrounding its production highlight the significance of graphite recycling. This review categorizes methods for graphite recovery into three approaches: recovery, recycle, and reuse. Moreover, it explores their potential applications and comparative electrochemical performance analysis, shedding light on the promising prospects of utilizing spent graphite-based functional materials. The review underscores the importance of sustainable recycling practices to address the environmental and economic challenges posed by the proliferation of LIBs and the growing demand for graphite.
1. Introduction
The number of lithium-ion batteries has grown exponentially in recent years, as these secondary batteries have been broadly used in portable electronics, electric vehicles, aerospace, and large-scale electric energy storage systems [1]. It is predicted that the world demand for LIBs will grow up to 3600 GWh by 2030 [2]. Taking into account the fact that the service life of the LIB is limited to 3–10 years, due to the high growth rates of using this technology, the number of end-of-life batteries is also increasing. Therefore, researchers and industries worldwide have paid more attention to the recycling of Li-ion battery components. It is commonly known that LIBs consist of four parts: the cathode, anode, electrolyte, and separator. Cathode materials contain the most precious metals; thus, the recycling of spent LIBs was predominantly focused on the metal’s recovery from waste cathode, and the recycling of the spent anode materials and the remaining parts of the battery received only a partial focus [3,4,5]. In turn, anode materials account for 5–15% of the total cost of Li-ion batteries. Graphite is the most common commercial material used as an anode material because of its long-lasting cycling stability and high values of electroconductivity, small expansion coefficient, high level of crystallinity, low intercalation potential of lithium, and thermal and mechanical stability [6,7]. Nonetheless, the quantity of graphite in LIBs is about 10–20%, which is 11 times more than their lithium content, and currently, from the economic and environmental points of view, studies are being performed on anode recycling in order to recover graphite. To date, several studies on the recycling of graphite have already been published [8,9], but nevertheless, globally, this issue is not yet as popular as that of the processing of cathode materials. The price of battery-grade graphite was USD 1500 per ton in October 2023 [10]. This evidence proves that the recycling of spent graphite can become a high-importance path to produce a source of low-cost graphite. Meanwhile, spent graphite contains some metals, binders, and toxic and flammable electrolytes [11].
Nowadays, graphite used in the Li-ion batteries’ manufacturing can be obtained from two sources: natural and synthetic [12]. The growing demand for this kind of battery has led to the extreme consumption of natural resources, and consequently, these resources will soon be drained if no action is planned to restore them [13]. On the other hand, the usage of synthetic graphite leads to a significant emission of carbon dioxide, since this process requires a large amount of energy. In this context, various graphite extraction methods have been developed [14]. Each of them has its own benefits and drawbacks. According to the existing research, the first step of the recycling of spent graphite is unloading the spent graphite, and then, it is separated using physical methods such as dismantling, crushing, screening, striking, ultrasonicating, and other mechanical processes [15,16,17,18]. Typically, but not surprisingly, LIBs are the source for most of the graphite produced [19]. In this review, the path of recycling of spent graphite is considered as follows: The first R is recovery, and this approach is focused on the regeneration of graphite without using any other materials to improve its structure. The second R is recycle, used here for characterizing the strategy of spent graphite modification using different technologies. Last but not least, the final R is reuse, referring to the synthesis of functional materials based on graphite for energy and environmental applications.
Accordingly, in this review, we focus on newly investigated methods for the recovery, recycling, and reuse of graphite obtained from spent lithium-ion batteries and its potential applications after undergoing different treatment methods. We compare the obtained electrochemical performances of recovered and recycled graphite and analyze the perspectives on spent graphite-based functional materials for reuse.
2. Graphite Separation from End-of-Life LIBs
End-of-life Li-ion batteries regularly have a residual charge, and an explosion may happen during their separation using mechanical and physical methods, such as via the crushing or milling process. Hence, the discharge process is a necessary pretreatment step to guarantee the safety of further graphite separation procedures. The choice of pretreatment process completely depends on the final application of the recycled/recovered components.
Currently, the most common discharge method is the chemical treatment of different solutions, such as the commonly used NaCl [20,21,22], which has a good conductivity and can completely remove the residual charge in a battery. Additionally, NaCl has the lowest price compared to other solutions, for example, MnSO4 and FeSO4 solutions [23,24].
Moreover, various discharge methods have been discussed in existing research, like cryogenic freezing using liquid nitrogen [25], thermal deactivation, which allows the removal of fluorine compounds and flammable organics from LIB waste [26,27], and discharge using solid electrical conductors [24].
After the battery-discharging pretreatment comes to an end, the question is raised of which method should be chosen for further graphite treatment. LIB waste is directly crushed and sieved, and then, the milled particles are separated into various fractions, such as metal shell, polymer, and electrode mass (cathode and anode) [28]. In general, the ways of obtaining the graphite from crushed LIB waste could be divided into two categories: direct (physical and mechanical treatment) and artificial (pyro- and hydrometallurgical) separation.
Via direct separation methods, the separated components are further recycled, and the recovered graphite may be used for various applications. Among the crushed and sieved fractions, there are metallic components (aluminum and copper current collectors) and electrode materials (graphite, LiCoO2, LiMn2O4, LiFePO4, and LiNixCoyMnzO2), which can be separated only based on their size. Apart from this size-based separation method, there are advanced separation techniques used in lithium-ion battery recycling processes, such as eddy current separation [29], electrostatic separation [30,31], and pneumatic separation [32]. Magnetic separation [33,34] and separation by flotation [35] are additional techniques applied for the further liberation of electrode materials from other components during the recycling of LIBs. In flotation, the separation is based on the hydrophobic and hydrophilic properties of the materials. Hydrophobic materials tend to repel water and adhere to air bubbles, while hydrophilic materials have an affinity for water and tend to sink. In the context of battery recycling, during the flotation process, the hydrophilic cathode, which consists of lithium oxides of various chemical compositions, is totally wetted with water and then lowered to the bottom of the tank. At the same time, the hydrophobic graphite anode is attached to the bubbles and then rises to the top layer of foam. It is worth noting that one of the challenges in the recovery of spent graphite anodes is to remove the binder (polyvinylidene fluoride) from electrode materials. To overcome this issue, some modified flotation processes have been proposed. For instance, He et al. [36] used the Fenton reagent to modify the electrode material’s surface in order to remove the binder coating.
In artificial separation, the most common processes are focused on removing the cathode and anode’s active masses from the aluminum and copper foils, respectively. The appearance of metal impurity residues (e.g., Al) has an extreme influence on the quality of the target graphite material. To avoid the presence of aluminum impurities in the recovered graphite, the spent batteries are mostly disassembled manually. After this step, one of the most common ways to split the copper foil and the active anode mass is hydrometallurgical treatment. By placing graphite in solutions of inorganic mineral acids, such as HCl and H2SO4, almost 100% of metal residues can be removed from the recovered graphite. After that, practically all of the copper can be recycled, and most of the organic components, such as conductive additive (for instance, carbon black), polyvinylidene fluoride (PVDF)-binder (known as one of the most commonly used materials for the better adhesion of the anode material to the current collector), and other organic substances in the separated materials, are withdrawn via various types of heat treatment [37,38]. One more efficient way to liberate graphite from copper foil is facile smelting in a nitrogen atmosphere at 1400 °C for 4 h. During the heat treatment, the copper foil transforms into spheroidal particles and is split from the graphite coating. Moreover, residual salts of electrolytes are also removed through heat treatment. In addition to high-temperature treatment, ultrasonic and sieving treatments are also conducted to separate high-purity graphite (99.5%) and copper. Efficient separation through these treatments can be achieved when two fractions, namely, copper and graphite particles, possess distinct particle size distributions. Compared with the hydrometallurgical approach of graphite recovery, facile high-temperature smelting combined with ultrasonic and sieving treatments allows one to obtain pure graphite without the usage of toxic acids or alkalis, hence this way is much more eco-friendly [39].
3. Recovery
The simplest way to recovery spent graphite anodes from the production of LIBs is direct recovery. Bai et al. [40] reported a sustainable solvent-based technique to directly recover graphite anode scraps. The anode coatings are placed in the water to the delaminate anode material from the copper current collector, and then new anodes are manufactured based on slurry, which are obtained from delaminated anode coatings. This method allows one to avoid the binder dissolution step in which it is usually necessary to use hazardous solvents.
The leaching process is the most widely used treatment to liberate and purify the spent graphite anode. This hydrometallurgical strategy allows one to remove the impurities from the recovered graphite, and moreover, this simple operation has a high level of efficiency. Various leaching solutions (e.g., acid, alkali, deionized water) convert metals that are contained in the spent graphite (e.g., Al, Li, Cu, etc.) into metal ions. For instance, the deionized water treatment is able to remove intercalated lithium and solid electrolyte interface layers from waste graphite anodes [16]. It became possible to eliminate lithium impurities from recovered graphite due to the reaction of Li with water. This is an exothermic reaction and, as a result, releases gaseous H2, which contributes to the liberation of the SEI layer from recovered graphite, thus removing unwanted impurities.
Still, not all the residual lithium appears in water-soluble form. In fact, there are also LiF and ROCO2Li, which can be barely removed by water treatment. Therefore, acid leaching by hydrochloric acid has been used to remove almost all lithium salts and metallic residues from recovered graphite in order to achieve graphite of high purity [37]. Yang et al. used HCl leaching for 60 min (leaching conditions of 1.5 mol/L HCl with an S/L ratio of 100 g/L) and obtained graphite exhibiting impressive electrochemical and cycling capacities. The whole recovery process proposed by Yang and co-workers is illustrated in Figure 1 [37]. Additionally, Li et al. [41] used sulfuric and malonic acids as leaching agents for extracting valuable metals from discarded LIBs. In their study, the leaching efficiencies of Li, Ni, Co and Mn were shown to reach 99.79%, 99.46%, 97.24% and 96.88%, respectively, under the optimal leaching conditions within 81 min. Also, another acid solution (5M H2SO4 with 35 wt. % H2O2) was used as a leaching solution to extract metal impurities from both spent cathode and anode materials [42]. Then, purified graphite was sintered with sodium hydroxide at 500 °C. During this process, all the cathode residue was removed from the graphite with almost all the PVDF binders. In addition, an oxidation process took place and led to the expansion of distance between graphite layers, which in turn led to crystal lattice changes and the following raising of the electrochemical performance of graphite [42].
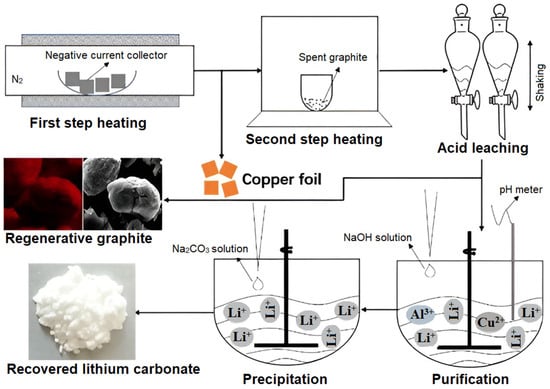
Figure 1.
Schematic representation of the whole (anode graphite, copper, and lithium) recycling process developed by Yang and co-workers. Reprinted with permission from Ref. [37], Copyright 2019, Elsevier.
It is worth noting that using mineral acids, which are often strong and corrosive, as leaching agents poses significant hazards to both human health and the environment. Considering this drawback of inorganic leaching agents, some researchers shifted their focus to studying environment-friendly organic acids (e.g., citric acid [43]) as safer and more sustainable alternatives to mineral acids. In [43], citric acid was chosen as the extraction reagent to recover the lithium element and regenerate spent graphite. A citric acid solution with the concentration of 0.2 mol/L was heated to 90 °C with graphite anode materials from spent LIBs. After that, 97.58% lithium was extracted into leaching solution and high-purity and excellent-performance graphite was obtained via leaching for 50 min [43].
Apart from the acids and alkalis, which are used to extract metals directly, bioleaching is a promising way to obtain acids for the further metal leaching process. The bioleaching mechanism is based on microorganisms producing acids via metabolism to extract target metals from spent graphite. For instance, some types of fungi and bacteria may be used in bioleaching, such as chemolithotrophic and acidophilic ones. Acidithiobacillus ferrooxidans bacteria can use elemental sulfur and Fe2+ to produce H2SO4 and Fe3+ in the leaching medium [44]. The fungus Aspergillus niger has demonstrated fine potential to produce a high concentration of organic acids, and has been proven useful for the bioleaching of spent LIBs [45,46].
Pyrometallurgy, in the context of graphite recycling, is also known as graphitization, and is frequently used to remove various impurities from regenerated graphite and recover its crystal structure in order to increase the electrochemical behavior of obtained graphite. For instance, Yang et al. [47] conducted high-temperature treatment at 2600 °C under an inert atmosphere (argon atmosphere). The obtained material demonstrated uniform graphitization and a slightly expanded layer distance in the crystal lattice. Besides this, in order to clarify the mechanism of structural reorganization of the material during heat treatment, Yu et al. [48] conducted several experiments with the usage of semi-in-situ X-ray diffraction with electron and Raman spectroscopy investigation. In addition, after investigations of various factors’ influence, such as inert gas atmosphere (nitrogen, argon, or helium), temperature value, and time duration, optimal conditions were determined for the process for a high degree of structural reconfiguration of the material, namely, a temperature of 3000 °C and time of 6 h in N2 atmosphere.
The pyro-hydrometallurgy approach is also often used in graphite recycling, as only leaching is not enough for efficient graphite recovery due to the complex composition of waste materials. Mixing the sintering process with acid leaching allows the acquisition of materials of high purity with an outstanding level of structural repair. Gao et al. [49] suggested a combined method consisting of sulfuric acid leaching and high-temperature sintering to recover spent graphite. After acid leaching, the graphite was placed into the furnace at 1500 °C for 2 h in an inert argon atmosphere. Later, the same research team investigated the impact of heat treatment temperature (heat treatment was conducted after the sulfuric acid leaching process) on the structural and morphological characteristics of the obtained graphite and, moreover, on its electrochemical behavior [50]. The analysis of the obtained material demonstrated that even at 900 °C the graphite’s crystal structure had transformed into a good one, and the material showed outstanding performance. Almost the same approach, but with the addition of the catalyst, was applied in the investigation by Chen et al. [51]. They applied the same approach, consisting of H2SO4 leaching followed by heat treatment, but they added Co(NO3)2 as a catalytic additive for the removal of the structural defects in the crystal layer of the obtained graphite without the need to maintain extremely high temperatures during the sintering process. The operating parameters of the process were 900 °C for 4 h under N2 atmosphere.
Moreover, there are a few other technologies that also allow one to obtain an improved crystal structure in spent graphite. For instance, electrolysis is an efficient way to recover graphite anodes from spent LIBs [52]. Gao et al. used a Na2SO4 solution as an electrolyte, an anode from a spent LIB with a copper current collector acted as a negative electrode, and a commercially available graphite plate was used as a positive electrode. Under the current flow, graphite was split from copper foil, and lithium was liberated from the crystal layers of graphite and transferred to the solution. Additionally, there is research focused on the usage of subcritical CO2 [53], which allows to extraction of the electrolyte salts before the following heat treatment. This approach prevents the formation of phosphorous compounds on the recovered graphite, but in turn, it also lowers the crystallinity of the material. Lately, the usage of microwave technology for eliminating the electrolyte salts has attracted much attention due to its significant advantages: less energy consumption and short time of the reaction. Under the action of microwaves, it becomes possible to remove the electrolyte residue and the binder, so 100% of the graphite can be recovered [54]. Microwave irradiation could also reconstruct the graphite structure in order to create open areas for the intercalation and diffusion of Li ions [55].
The mentioned recovery strategies offer various advantages and disadvantages, which are listed in Table 1, and the choice of recovery method should consider factors such as purity requirements, environmental impact, energy efficiency, and the specific impurities present in the spent graphite.

Table 1.
The advantages and disadvantages of various Recovery strategies of spent graphite anodes.
4. Recycle
Unlike the recovery approach, the recycle approach is targeted not only at the extraction of graphite from the spent anode, but also at the improvement and development of its electrochemical performance.
The most interesting approach is the carbon coating of the graphite due to its low cost and efficiency. For instance, Li et al. obtained carbon-coated graphite with the usage of both carboxymethyl cellulose and glucose by a carbonization heating process at 800 °C for 5 h [56]. The modified graphite demonstrated an increasing capacity. Moreover, some other sources of carbon were used for the synthesis of new improved carbon-coated graphite—for instance, pitch [57,58], polyethylene glycol 400 monooleate [59], and phenolic resin [60]. Before starting the process of carbon-coting, often, spent graphite undergoes leaching and pre-treatment sintering to remove residual electrolyte salts and lithium ions. Then, the materials are sintered at high temperatures with various carbon sources for the following structure reconstruction and coating. Furthermore, the doping path was also investigated. Gas phase exfoliation and element doping were used to obtain nitrogen-doped graphite with enhanced layer distance in the crystal structure from acid-pretreated retired graphite and urea as initial materials [61]. Ammonia formation from the urea decomposition took place during the heating process. Formed NH3 was able to etch and exfoliate the upper layer of graphite and subsequently intercalate into layers and exfoliate the graphite. g-C3N4 at 800 °C decomposed and was able to dope graphite, as it was a source of further doping nitrogen.
One more promising path to recycle graphite anode materials from spent LIBs is to composite spent graphite with the silicon anode. Si anodes were in the spotlight due to their extremely high theoretical capacity, up to 4000 mAh/g, and low operating voltage of about 0.4 V. In spite of that, the silicon anode had low electrical conductivity, and cycling volume expansion takes place, which makes it impossible to use it in LIBs [62]. The joining of Si anodes with graphite allows for obtaining low-cost working anodes with outstanding characteristics. Carbon-coated silicon-spent graphite anode (T-SGT/Si@C) was synthesized by sintering at 1000 °C with silicon, pitch, and spent graphite (T-SGT) as raw materials. Because of T-SGT’s high porosity, Si is easily bonded to spent graphite, and consequently, volume expansion could be prevented. Moreover, the silicon–graphite anode was prepared by combining ball milling and carbonization [63]. The matrix of spent graphite better combines with silicon particles due to electrostatic forces; this fact allows one to restrain volume expansion.
Additionally, Ye et al. [64] developed an effective regeneration path with the usage of both electrodes from end-of-life LIBs: cathode and anode materials. The authors synthesized a composite anode CoO/CoFe2O4/expanded graphite. The cathode part was leached by reduction acid treatment to extract Li and Co into the solution, the waste graphite went through calcination under an Ar atmosphere and then the intercalation process took place to obtain oxidized graphite. The next step was a high-temperature treatment to expand the graphite. Then, the expanded graphite and leachate from the cathode material were collected and went through solvothermal treatment to synthesize the target composite anode. The whole recycling strategy of the waste LiCoO2–graphite battery developed by Ye and co-workers is illustrated in Figure 2 [64].
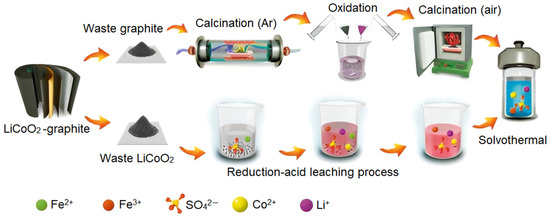
Figure 2.
Schematical representation demonstrating the whole recycling process of end-of-life LiCoO2–graphite batteries proposed by Ye and co-workers [64]. Ref. [64] is an open-access article distributed under the terms of the Creative Commons CC BY license, which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Furthermore, one more research group investigated the gas sulfidation process to obtain metal sulfides/graphite composite anodes [65]. Firstly, Li(NixNiyMnz)O2/Li(NixNiyAlz)O2 (NCM/NCA) chopped cathodes were mixed and pretreated with NaOH to dissolve the aluminum current collector to obtain cathode powder. The spent graphite was manually split from copper foil and went through oxidation–intercalation treatment. Afterward, mechanically mixed cathode, graphite, and sublimed sulfur were annealed at 600 °C for 2 h under an Ar/H2 atmosphere. To synthesize the composite Li(NixNiyMnz)O2 spent carbon–Li(NixNiyAlz)O2 spent carbon) (NCMS/C (NCAS/C), the obtained material underwent deionized water leaching to extract lithium and then was dried. The synthesized composites demonstrated a high electrochemical capacity for Li storage.
To summarize all processes and technologies attributed to recovery and recycle approaches and to compare the electrochemical performance of obtained graphite materials, Table 2 is given below.

Table 2.
The electrochemical performance of obtained graphite materials by recovery and recycle approaches.
The feasibility of industrialization of either the recovery or the recycling approach for obtaining restored graphite from spent graphite anodes depends on several factors, including the specific application, economic considerations, and environmental impact. A comparison of these two approaches in terms of the possibility of their industrial application is given in Table 3.

Table 3.
The main advantages and disadvantages of the recovery and recycle approaches in terms of the feasibility of their industrialization.
The choice between the recovery and recycling approaches depends on the specific goals and constraints of an industrial operation. If the priority is to produce high-purity graphite for demanding applications like lithium-ion batteries, the recovery approach may be more feasible. However, it comes with higher costs and environmental considerations. On the other hand, for applications where high purity is not critical and sustainability is a key concern, the recycling approach may be more practical.
In practice, a hybrid approach may be the most suitable for many industrial applications. This approach combines recovery methods to obtain high-purity graphite for premium applications and recycling methods to produce less pure but environmentally friendly graphite for less demanding uses. The choice ultimately depends on the balance between performance, cost, and sustainability objectives for a particular industrial process.
5. Reuse
The third approach, the reuse approach, is focused on preparing functional materials from the spent LIB graphite, which are possible to use in other applications. In this subsection, such functional materials, such as adsorbents graphite-based capacitors, catalysts, and graphene, are provided. Additionally, there are few publications in which spent graphite is reused in other types of secondary rechargeable batteries, such as sodium/potassium-ion batteries and dual-ion batteries.
5.1. Adsorbents
Graphite from retired batteries is an attractive material due to its surface with large quantities of functional groups and porous morphology. These properties are extremely important for adsorbents, and subsequently, spent graphite may be a promising candidate from the economic and ecological points of view. Three types of graphite materials—extracted from spent LIBs, commercial graphite, and biochar—were compared in terms of sorption ability [66]. Spent graphite demonstrated excellent sorption capacity for metals, such as barium, lead, and cadmium (~43.5 mg/g), and lower values for organic compounds, such as 2,4-dinitrotoluene, hexahydro-1,3,5-trinitro-1,3,5-triazine and 2,4-dichlorphenol (~6.5 mg/g). Presumably, the residual impurities could have an influence on sorption properties. The high values of adsorption are based on the arrangement of graphite layers. In order to develop the specific surface area and adsorption sites, various modification methods have been studied, for instance, spent graphite was treated with KMnO4 to obtain MnO2-loaded graphite (MnO2-AG) [67]. This material exhibited good removal rates of lead, cadmium, and silver from wastewater—99.9%, 79.7%, and 99.8%, respectively. The great removal efficiency can be explained by the ion exchange between functional groups on the surface and the metal ions. Further, Hao et al. [68] obtained amorphous carbon with the coating MnO2 from spent LIB graphite by ball-milling and the hydrothermal method. The ball-milling completely reconstructed the graphite structure and allowed it to enhance the surface area. These changes led to an increase in the sorption capacity of cadmium from 4.88 to 135.81 mg/g. By various analyses, such as XPS, the explanation of excellent adsorption was given as ion exchange, electrostatic attraction, and surface complexation (Figure 3).
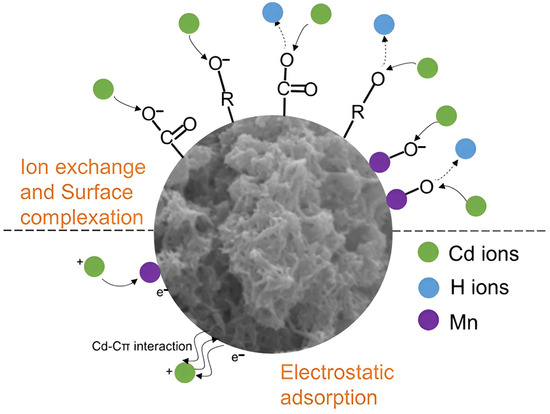
Figure 3.
Schematic depicting the Cd(II) sorption mechanisms on the AG@MnO2 adsorbent prepared from spent graphite anodes from end-of-life LIBs. Reprinted with permission from Ref. [68], copyright 2020, American Chemical Society.
5.2. Capacitors
Graphite from EoL lithium-ion batteries can also be used in capacitors, such as Li-ion and Na-ion capacitors. We systematically studied the probability of spent graphite usage as the negative electrode for Li-ion and Na-ion capacitors. Dual carbon capacitors of different types were assembled with activated carbon, used as the positive electrode. For Li-ion capacitors, the spent graphite went through lithiation in order to form a LiC6 compound for further lithium-ion supply. In a carbonate solution at room temperature, this capacitor delivered 185.84 Wh/kg [69]. Subsequently, this research team investigated the phenomenon of solvated Li ions’ cointercalation into graphite with the usage of LiPF6 in tetraethylene glycol dimethyl ether as the electrolyte. In comparison with carbonate-based electrolytes, the glyme-based Li-ion capacitor displayed better cycling properties and safety, and in addition the capacitor showed an energy density of 46.40 Wh/kg at room temperature [70]. Likewise, in the Na-ion capacitor, Divya et al. [71] used a solvent cointercalation mechanism for high reversibility with NaPF6 in tetraethylene glycol dimethyl ether. The capacitor was assembled in the same way, with activated carbon as positive and pre-sodiated graphite as negative, and this demonstrated an energy density of 59.93 Wh/kg and saved about 98% of capacity after 5000 cycles.
Besides this, some experiments have focused on applying the spent graphite in supercapacitors. For instance, Schiavi et al. [72] regenerated cobalt or cobalt–copper nanowires and graphite from EoL-LIBs, which acted as positive and negative electrodes, respectively, in supercapacitors. Figure 4 depicts the flow chart of the overall process proposed by Schiavi and co-workers. Pure graphite without any metal impurities and mixtures of metal salts was obtained after mechanical–physical treatment and hydrometallurgy. After that, a positive electrode in the form of a nanowire was made via electrodeposition technology with the usage of mixed metal salts as electrolytes. The synthesized supercapacitor demonstrated a specific capacitance of 42 F/g [67].
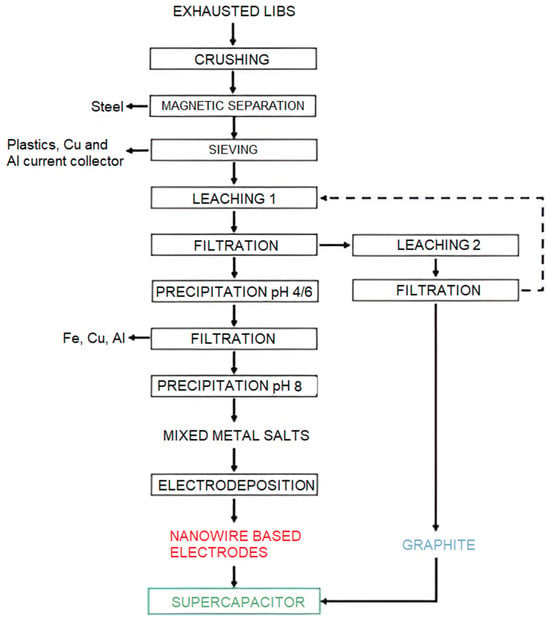
Figure 4.
Schematic showing the overall recycling process developed by Schiavi and co-workers. Reprinted with permission from Ref. [72], copyright 2021, Elsevier.
5.3. Catalysts
The preparation of catalysts based on spent batteries’ graphite has attracted a lot of attention due to its good carbon matrix structure and rich surface. Nowadays, these graphite-based catalysts appear as an outstanding material for use in the redox of organic compound degradation, electrochemical reduction reactions with O2, and trapping and catalytic polysulfides. Nguyen and Oh [66] observed that graphite addition to persulfate oxidation with iron and reduction with dithiothreitol and hydrogen sulfides affects the removal of organics. This relationship can be explained by the presence of oxygen-containing functional groups and the type of graphite structure. These factors simplified the electron transfer during oxidation/reduction reactions. Furthermore, spent graphite after binder removal could also be applied in LiS batteries as a functional interlayer with advanced polysulfide trapping and catalytic behavior [73]. The presence of residual transition metals in spent graphite can influence the polysulfide conversion kinetics. The porous structure and polar functional groups cause the spent graphite to be able to confine polysulfides by physical and chemical adsorption.
Additionally, graphene (LIB-rGO) was obtained from spent graphite for the further catalytic ozonation of organic pollutants [74]. In comparison with that synthesized from commercially available graphite, rGO from spent LIB graphite demonstrated better catalytic behavior due to its defective structure.
Moreover, the application of composite catalysts based on spent graphite was studied in pollutant degradation. Guan et al. [75] prepared zero-valent iron with expanded graphite (ZVI-EG) from iron chloride (FeCl3) and graphite from spent lithium-ion batteries by carbothermic reduction. A similar type of composite was obtained by Chen et al. [76]. In their proposed route (Figure 5), graphite and copper of the spent LIB anode and iron oxide from mill-scale waste went through carbothermic reduction to synthesize the final product—the zero-valent iron-copper bimetallic catalyst (denoted as ZVI-Cu/C). Both these composite catalysts achieved an efficient removal of 4-chlorophenol from water by heterogenous Fenton reactions.
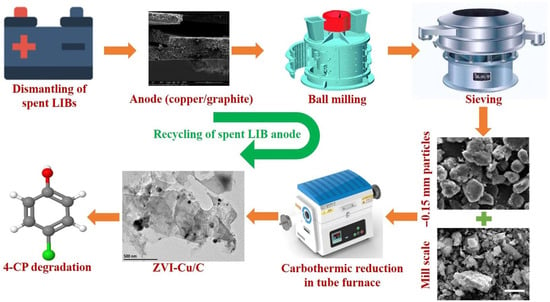
Figure 5.
Preparation of the ZVI–Cu/C composites from exhausted LIB anodes and mill-scale degradation of 4-chlorophenol (4-CP) in water by both reduction and oxidation chemical reactions [76]. Ref. [76] is an open-access article distributed under the terms of the Creative Commons CC BY license, which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Moreover, copper from spent anodes was also applied for graphene oxide/CuO composite synthesis [77]. CuSO4 was obtained through the reaction of copper foils and sulfuric acid, and graphene oxide was synthesized by Hummer’s method from spent graphite. The final composite GO/CuO was prepared by adsorption and bonding reactions. To analyze its properties, GO/CuO was compared to CuO, and the composite demonstrated a higher value of photodegradation on methylene blue. To increase the photodegradation degree, it is possible to apply an electric field.
5.4. Graphene
One of the most attention-grabbing materials nowadays is graphene, with outstanding mechanical, chemical, electric, and thermal characteristics [78]. Graphene comprises a single layer of carbon atoms with sp2 hybridization, which are bonded in a hexagonal lattice. Previously, the synthesis of graphene has been extremely complicated, but many researchers have investigated various methods of obtaining this exceptional material, such as chemical vapor deposition [79], chemical oxidation/reduction [80], mechanical exfoliation [81], and electrochemical exfoliation [82]. By chemical oxidation/reduction and mechanical exfoliation, it became possible to obtain graphene from spent graphite from EoL LIB.
Zhang et al. [83] studied the processes of both calcination and oxidation/reduction for graphene synthesis from spent graphite. Firstly, the spent graphite was calcined at 600 °C for 1 h in order to remove organic residue from the top layer of graphite powder. Then, with the usage of a modified Hummer’s method and ultrasonic exfoliation, graphite oxide was synthesized and went through reduction in N2H4·H2O to obtain reduced graphene oxide. Various impurities in spent graphite, such as LiPF6 salts, PVDF binder, copper oxide, and lithium salts, could be simply removed by Hummer’s method (mixture of KMnO4 and sulfuric acid) [84]. This path allows one to obtain graphene without pre-calcination. Moreover, Zhao et al. [85] proposed a synthesis of soluble graphene oxide from spent graphite by also using a modified Hummer’s process following NaOH–KOH eutectic reduction. Molten NaOH–KOH at 220 °C effectively removed oxygen-containing groups from graphene, while creating additional hydroxyl functional groups, which is explained by the exceptional solubility of rGO in water/ethanol solution. As the cost of the oxidation–reduction compounds is extremely high, some investigations that focused on decreasing the cost were carried out. For instance, Natarajan et al. [86] used metallic cases made from aluminum or stainless steel as reducing agents with concentrated HCl for the following synthesis of graphene. A schematic illustration of the synthesis route of rGO from waste LIBs is reported in Figure 6. Among all the obtained samples, graphene, which was reduced by Al at room temperature, demonstrated the highest capacity as a result of the highest value of reduction and its porous structure.

Figure 6.
Flow sheet for the production of reduced graphene oxide (rGO) using recovered substances from end-of-life LIBs. Reprinted with permission from Ref. [86], copyright 2018, Elsevier.
Chen et al. [87] investigated the exfoliation with sonication of spent graphite into few-layered graphene. Due to the decrease in interlayer force between layers after many cycles of charge/discharge of spent graphite, the effect of exfoliating was higher than that of commercially available graphite. After heat treatment at 500 °C, the conductivity value was raised to 9100 S/m. Additionally, during the process of charging the spent batteries, the lithium intercalated graphite. This intercalation led to the direct splitting of the graphite layers [88]. When the SOC of the LIB reached 50%, the graphite electrode completely transformed into lithium intercalated compounds, such as LiC6 and LiC12. After hydrolysis and ultrasonication, the Li from the intercalated compounds dissolved and was recovered in Li2CO3, and two- to four-layered and one- to two-layered types of graphite were formed. From an economic point of view, using spent graphite in the following synthesis of graphene entails a much lower cost than the commercially developed graphene synthesis process.
5.5. Other Types of the Rechargeable Batteries
Besides the simplest mode of spent graphite application in new LIBs after regeneration, some researchers have investigated and studied the possibility of reusing graphite anodes from LIB in other types of batteries. For instance, Natarajan et al. [89] discovered that spent graphite coated on the copper foil could be transformed into Cu-BTC MOF, which could be further applied not only in LIBs, but also in SIBs. The obtained anode material demonstrated a discharge capacity of 208.9 mAh/g at a current density of 100 mA/g. This reveals further possible processing paths of LIB waste.
Liang et al. [90] proposed a new concept of graphite anode waste reuse. High-temperature heat treatment leads to an increase in interlayer distance and contributes to reducing oxygen content and defects. The recovered electrode was reused as an anode in sodium-ion and potassium-ion batteries, and demonstrated outstanding performance: for SIB 162 mAh/g at 0.2 A/g and for KIB 320 mAh/g at 0.05 A/g.
A unique cation/anion workable DIB was investigated by Meng et al. [91]. The research group offered an innovative approach—to join spent LFP cathodes and graphite anodes in order to obtain one electrode for further use in dual-ion batteries. This unique electrode is able to work together with cations and anions, in which LFP can store Li+ ions and graphite -PF6− ions during the intercalation and deintercalation processes by variations in voltage ranges. The LFP/graphite composite (RLFPG) exhibited a high specific capacity of 117.4 mAh/g at 24 mA/g current density and outstanding cycle life (78% after 1000 cycles at 100 mA/g).
Another attractive approach to spent graphite usage was investigated by Yang et al. [92]. They created an advanced cathode based on the spent graphite from LIBs for dual-ion batteries. The transformation included the recovery of the graphite crystal structure and morphology to restore the regular layer order and layer spacing so that the spent graphite was damaged after long cycling. This recovery increased the anion intercalation in the graphite. Moreover, the SEI’s thermal decomposition leads to the formation of an amorphous carbon layer, which in turn prevents electrode degradation and develops the cycle life of the material. The synthesized material exhibited the capacity of 87 mAh/g at 200 mA/g current density. By this environmentally friendly path, spent graphite could be applied in Li-, Na-, and K-DIBs, effectively reducing resource consumption.
To summarize all processes and technologies attributed to the reuse approach, and to compare the performances and applications of thus-obtained graphite materials, Table 4 is given below.

Table 4.
A comparison table of different functional materials obtained by the reuse approach.
6. Challenges in the Recovery of Spent Graphite Anodes
The recovery of spent graphite anodes presents several challenges that need to be addressed for efficient and sustainable recycling. Some key challenges associated with the recovery of spent graphite are listed below:
- Complex composition—Spent graphite anodes from lithium-ion batteries (LIBs) often have a complex composition, including various impurities, lithium salts, and binders. Removing and separating these components to obtain pure graphite can be a complex and energy-intensive process;
- Environmental impact—Many traditional recovery methods involve the use of strong acids, high-temperature processes, or other chemical treatments. These methods can have negative environmental impacts, including the generation of hazardous waste and emissions;
- Energy consumption—Some recovery methods, such as high-temperature pyrometallurgy, can be energy-intensive. Energy consumption is a concern both in terms of environmental impact and cost-effectiveness;
- Purity requirements—The level of purity required for the recovered graphite depends on the intended application. Meeting high-purity standards, particularly for LIBs, can be challenging and may require more extensive and resource-intensive recovery processes;
- Impurities—Some impurities, such as LiF and ROCO2Li, are challenging to remove using standard recovery methods, leading to the need for more advanced and complex techniques;
- Safety concerns—Handling strong acids and other chemicals in the recovery process can pose safety risks for workers, and the disposal of chemical waste must be managed carefully;
- Energy storage requirements—The recovered graphite’s electrochemical performance, including its capacity and cycling stability, may not match that of newly manufactured graphite. Achieving the same electrochemical properties can be challenging;
- Resource efficiency—Balancing the use of resources (both energy and materials) with the desired level of recovery and purity is an ongoing challenge. Maximizing resource efficiency while achieving acceptable purity levels is critical;
- Recycling infrastructure—Establishing a recycling infrastructure that can efficiently process and recover spent graphite from a growing number of end-of-life batteries is a logistical challenge;
- Cost-effectiveness—Finding cost-effective recovery methods that balance the expenses associated with recovery and recycling against the potential value of the recovered materials is crucial;
- Environmental regulations—Meeting environmental regulations and sustainability goals while recovering and recycling graphite materials is a significant challenge, particularly as regulations may become more stringent.
Addressing these challenges requires ongoing research and development efforts to innovate and optimize recovery methods, reduce environmental impacts, and improve the economic viability of recycling spent graphite. Furthermore, a shift toward more sustainable and eco-friendly recovery methods is essential to meet the growing demand for graphite and the need to minimize its environmental footprint.
7. Conclusions and Promising Prospects for Utilizing Spent Graphite-Based Functional Materials
The recycling of spent graphite from LIBs not only addresses environmental concerns and resource depletion, but also presents promising opportunities for the development of innovative functional materials. Spent graphite, which may contain residual metals, binders, and electrolytes, can be repurposed and transformed into valuable products for various applications. Here, we explore the potential prospects and applications of spent graphite-based functional materials:
- Energy storage systems—Spent graphite can be processed and modified to create high-performance anode materials for the energy storage systems of the future. Advanced treatments and engineering techniques can enhance the electrochemical properties of recycled graphite, allowing it to store and release energy efficiently. These recycled materials could lead to the development of cost-effective and sustainable energy storage solutions, supporting the growing demand for renewable energy integration and grid stability;
- Supercapacitors—Spent graphite-based materials can find applications in supercapacitors, offering rapid charge–discharge capabilities and extended cycling stability. The unique features of graphite, such as its high surface area and electrical conductivity, make it an ideal candidate for supercapacitor electrodes. Recycling graphite for supercapacitors can enhance their energy storage performance while reducing the need for virgin graphite production;
- Advanced composite materials—The incorporation of spent graphite into composite materials can lead to the development of lightweight and high-strength materials. Graphite’s mechanical stability and electrical conductivity can enhance the properties of composites used in aerospace, automotive, and construction industries, reducing the reliance on virgin graphite and promoting sustainability in material production;
- Environmental remediation—Spent graphite can serve as an effective adsorbent for environmental remediation purposes. Its porous structure and affinity for various pollutants make it suitable for the removal of metals, organic contaminants, and hazardous chemicals from water and air. By repurposing spent graphite in environmental applications, we can contribute to cleaner ecosystems and mitigate pollution;
- Electrochemical sensors—Recycled graphite can be tailored for use in electrochemical sensors and analytical devices. Its electrochemical activity, coupled with surface modification techniques, can enable the sensitive detection of analytes, paving the way for improved sensing technologies in fields such as healthcare, environmental monitoring, and diagnostics;
- Thermal management—The high thermal conductivity of graphite makes it valuable in thermal management applications. Spent graphite-based materials can be incorporated into thermal interface materials, heat sinks, and cooling solutions for electronics and electric vehicle batteries, enhancing heat dissipation and system efficiency;
- Construction and infrastructure—Recycled graphite can be employed in construction materials, such as concrete additives and coatings, to improve durability and reduce carbon emissions. Its inclusion can enhance the overall performance and sustainability of infrastructure projects.
In summary, the recycling and repurposing of spent graphite from LIBs offers a multitude of opportunities to create valuable functional materials with a range of applications across industries. These prospects not only promote sustainability and resource conservation, but also contribute to the development of innovative technologies that address the global challenges of energy storage, environmental protection, and advanced materials development. As research and development efforts continue to evolve in this area, the full potential of spent graphite-based functional materials is expected to be realized, speeding up the transition towards a more sustainable and eco-friendly future.
Author Contributions
Conceptualization, A.K. and K.P.; methodology, A.K. and K.P.; formal analysis, A.K.; investigation, A.K., K.P. and A.A.P. (Alexander A. Pavlovskii); resources, A.A.P. (Anatoliy A. Popovich); data curation, A.K. and A.A.P. (Alexander A. Pavlovskii); writing—original draft preparation, A.K.; writing—review and editing, A.A.P. (Alexander A. Pavlovskii); visualization, A.A.P. (Alexander A. Pavlovskii); supervision, P.N.; project administration, P.N. and A.A.P. (Anatoliy A. Popovich); funding acquisition, A.A.P. (Anatoliy A. Popovich) All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the Ministry of Science and Higher Education of the Russian Federation by “Agreement on the grant in the form of subsidies from the federal budget for the implementation of state support for the creation and development of world-class scientific centers, those are performing research and development on the priorities of scientific and technological development” dated 20 April 2022 No. 075-15-2022-311.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to corporate confidentiality requirements.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, Y.; Zhang, R.; Wang, J.; Wang, Y. Current and Future Lithium-Ion Battery Manufacturing. iScience 2021, 24, 102332. [Google Scholar] [CrossRef] [PubMed]
- Heid, B.; Kane, S.; Schaufuss, P. Powering Up Sustainable Energy: Building a More Sustainable Battery Industry. McKinsey Quarterly. 2020. Available online: https://www.mckinsey.com/capabilities/sustainability/our-insights/powering-up-sustainable-energy#section-header-3 (accessed on 15 August 2023).
- Yu, W.; Guo, Y.; Shang, Z.; Zhang, Y.; Xu, S. A Review on Comprehensive Recycling of Spent Power Lithium-Ion Battery in China. eTransportation 2022, 11, 100155. [Google Scholar] [CrossRef]
- Natarajan, S.; Aravindan, V. Recycling Strategies for Spent Li-Ion Battery Mixed Cathodes. ACS Energy Lett. 2018, 3, 2101–2103. [Google Scholar] [CrossRef]
- Wu, X.-L.; Xu, H.-Y. Advances and Challenges on Recycling the Electrode and Electrolyte Materials in Spent Lithium-Ion Batteries. Mater. Lab 2022, 1, 220036. [Google Scholar] [CrossRef]
- Cheng, H.; Shapter, J.G.; Li, Y.; Gao, G. Recent Progress of Advanced Anode Materials of Lithium-Ion Batteries. J. Energy Chem. 2021, 57, 451–468. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Ren, D.; Wang, L.; He, X. Graphite as Anode Materials: Fundamental Mechanism, Recent Progress and Advances. Energy Storage Mater. 2021, 36, 147–170. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhao, H.; Shen, Y.; Li, L.; Rao, Z.; Shao, G.; Lei, Y. Recycling of Graphite Anode from Spent Lithium-ion Batteries: Advances and Perspectives. EcoMat 2023, 5. [Google Scholar] [CrossRef]
- Abdollahifar, M.; Doose, S.; Cavers, H.; Kwade, A. Graphite Recycling from End-of-Life Lithium-Ion Batteries: Processes and Applications. Adv. Mater. Technol. 2023, 8, 2200368. [Google Scholar] [CrossRef]
- Fubao. Available online: http://battery.f139.com/ (accessed on 10 October 2023).
- Arshad, F.; Li, L.; Amin, K.; Fan, E.; Manurkar, N.; Ahmad, A.; Yang, J.; Wu, F.; Chen, R. A Comprehensive Review of the Advancement in Recycling the Anode and Electrolyte from Spent Lithium Ion Batteries. ACS Sustain. Chem. Eng. 2020, 8, 13527–13554. [Google Scholar] [CrossRef]
- Surovtseva, D.; Crossin, E.; Pell, R.; Stamford, L. Toward a Life Cycle Inventory for Graphite Production. J. Ind. Ecol. 2022, 26, 964–979. [Google Scholar] [CrossRef]
- Pinegar, H.; Smith, Y.R. Recycling of End-of-Life Lithium Ion Batteries, Part I: Commercial Processes. J. Sustain. Metall. 2019, 5, 402–416. [Google Scholar] [CrossRef]
- Liu, K.; Yang, S.; Luo, L.; Pan, Q.; Zhang, P.; Huang, Y.; Zheng, F.; Wang, H.; Li, Q. From Spent Graphite to Recycle Graphite Anode for High-Performance Lithium Ion Batteries and Sodium Ion Batteries. Electrochim. Acta 2020, 356, 136856. [Google Scholar] [CrossRef]
- Wang, H.; Huang, Y.; Huang, C.; Wang, X.; Wang, K.; Chen, H.; Liu, S.; Wu, Y.; Xu, K.; Li, W. Reclaiming Graphite from Spent Lithium Ion Batteries Ecologically and Economically. Electrochim. Acta 2019, 313, 423–431. [Google Scholar] [CrossRef]
- Natarajan, S.; Boricha, A.B.; Bajaj, H.C. Recovery of Value-Added Products from Cathode and Anode Material of Spent Lithium-Ion Batteries. Waste Manag. 2018, 77, 455–465. [Google Scholar] [CrossRef]
- Yang, L.; Yang, L.; Xu, G.; Feng, Q.; Li, Y.; Zhao, E.; Ma, J.; Fan, S.; Li, X. Separation and Recovery of Carbon Powder in Anodes from Spent Lithium-Ion Batteries to Synthesize Graphene. Sci. Rep. 2019, 9, 9823. [Google Scholar] [CrossRef]
- Niu, B.; Xiao, J.; Xu, Z. Advances and Challenges in Anode Graphite Recycling from Spent Lithium-Ion Batteries. J. Hazard. Mater. 2022, 439, 129678. [Google Scholar] [CrossRef]
- Wu, M.; Liao, J.; Yu, L.; Lv, R.; Li, P.; Sun, W.; Tan, R.; Duan, X.; Zhang, L.; Li, F.; et al. 2020 Roadmap on Carbon Materials for Energy Storage and Conversion. Chem.—Asian J. 2020, 15, 995–1013. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, N.; Hu, F.; Ye, L.; Xi, Y.; Yang, S. Thermal Treatment and Ammoniacal Leaching for the Recovery of Valuable Metals from Spent Lithium-Ion Batteries. Waste Manag. 2018, 75, 469–476. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, T.; He, Y.; Zhao, Y.; Wang, S.; Zhang, G.; Zhang, Y.; Feng, Y. Recovery of Valuable Materials from Spent Lithium-Ion Batteries by Mechanical Separation and Thermal Treatment. J. Clean. Prod. 2018, 185, 646–652. [Google Scholar] [CrossRef]
- Fu, Y.; He, Y.; Qu, L.; Feng, Y.; Li, J.; Liu, J.; Zhang, G.; Xie, W. Enhancement in Leaching Process of Lithium and Cobalt from Spent Lithium-Ion Batteries Using Benzenesulfonic Acid System. Waste Manag. 2019, 88, 191–199. [Google Scholar] [CrossRef]
- Xiao, J.; Guo, J.; Zhan, L.; Xu, Z. A Cleaner Approach to the Discharge Process of Spent Lithium Ion Batteries in Different Solutions. J. Clean. Prod. 2020, 255, 120064. [Google Scholar] [CrossRef]
- Yao, L.P.; Zeng, Q.; Qi, T.; Li, J. An Environmentally Friendly Discharge Technology to Pretreat Spent Lithium-Ion Batteries. J. Clean. Prod. 2020, 245, 118820. [Google Scholar] [CrossRef]
- Yu, D.; Huang, Z.; Makuza, B.; Guo, X.; Tian, Q. Pretreatment Options for the Recycling of Spent Lithium-Ion Batteries: A Comprehensive Review. Miner. Eng. 2021, 173, 107218. [Google Scholar] [CrossRef]
- Makuza, B.; Tian, Q.; Guo, X.; Chattopadhyay, K.; Yu, D. Pyrometallurgical Options for Recycling Spent Lithium-Ion Batteries: A Comprehensive Review. J. Power Sources 2021, 491, 229622. [Google Scholar] [CrossRef]
- He, Y.; Yuan, X.; Zhang, G.; Wang, H.; Zhang, T.; Xie, W.; Li, L. A Critical Review of Current Technologies for the Liberation of Electrode Materials from Foils in the Recycling Process of Spent Lithium-Ion Batteries. Sci. Total Environ. 2021, 766, 142382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, N.; He, J.; Chen, R.; Li, X. Lithiation-Aided Conversion of End-of-Life Lithium-Ion Battery Anodes to High-Quality Graphene and Graphene Oxide. Nano Lett. 2019, 19, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Zhu, H.; Zu, L.; Bai, Y.; Gao, S.; Gao, Y. A New Model of Trajectory in Eddy Current Separation for Recovering Spent Lithium Iron Phosphate Batteries. Waste Manag. 2019, 100, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Silveira, A.V.M.; Santana, M.P.; Tanabe, E.H.; Bertuol, D.A. Recovery of Valuable Materials from Spent Lithium Ion Batteries Using Electrostatic Separation. Int. J. Miner. Process. 2017, 169, 91–98. [Google Scholar] [CrossRef]
- Widijatmoko, S.D.; Fu, G.; Wang, Z.; Hall, P. Recovering Lithium Cobalt Oxide, Aluminium, and Copper from Spent Lithium-Ion Battery via Attrition Scrubbing. J. Clean. Prod. 2020, 260, 120869. [Google Scholar] [CrossRef]
- Zhang, G.; He, Y.; Wang, H.; Feng, Y.; Xie, W.; Zhu, X. Removal of Organics by Pyrolysis for Enhancing Liberation and Flotation Behavior of Electrode Materials Derived from Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2020, 8, 2205–2214. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, J.; Gan, T.; Lu, D.; Wang, Y.; Zheng, X. High-Intensity Magnetic Separation for Recovery of LiFePO4 and Graphite from Spent Lithium-Ion Batteries. Sep. Purif. Technol. 2022, 297, 121486. [Google Scholar] [CrossRef]
- Mennik, F.; Dinç, N.İ.; Burat, F. Selective Recovery of Metals from Spent Mobile Phone Lithium-Ion Batteries through Froth Flotation Followed by Magnetic Separation Procedure. Results Eng. 2023, 17, 100868. [Google Scholar] [CrossRef]
- Zhan, R.; Yang, Z.; Bloom, I.; Pan, L. Significance of a Solid Electrolyte Interphase on Separation of Anode and Cathode Materials from Spent Li-Ion Batteries by Froth Flotation. ACS Sustain. Chem. Eng. 2021, 9, 531–540. [Google Scholar] [CrossRef]
- He, Y.; Zhang, T.; Wang, F.; Zhang, G.; Zhang, W.; Wang, J. Recovery of LiCoO2 and Graphite from Spent Lithium-Ion Batteries by Fenton Reagent-Assisted Flotation. J. Clean. Prod. 2017, 143, 319–325. [Google Scholar] [CrossRef]
- Yang, Y.; Song, S.; Lei, S.; Sun, W.; Hou, H.; Jiang, F.; Ji, X.; Zhao, W.; Hu, Y. A Process for Combination of Recycling Lithium and Regenerating Graphite from Spent Lithium-Ion Battery. Waste Manag. 2019, 85, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; He, Y.; Wang, H.; Feng, Y.; Xie, W.; Zhu, X. Application of Mechanical Crushing Combined with Pyrolysis-Enhanced Flotation Technology to Recover Graphite and LiCoO2 from Spent Lithium-Ion Batteries. J. Clean. Prod. 2019, 231, 1418–1427. [Google Scholar] [CrossRef]
- Yi, C.; Yang, Y.; Zhang, T.; Wu, X.; Sun, W.; Yi, L. A Green and Facile Approach for Regeneration of Graphite from Spent Lithium Ion Battery. J. Clean. Prod. 2020, 277, 123585. [Google Scholar] [CrossRef]
- Bai, Y.; Li, M.; Jafta, C.J.; Dai, Q.; Essehli, R.; Polzin, B.J.; Belharouak, I. Direct Recycling and Remanufacturing of Anode Scraps. Sustain. Mater. Technol. 2023, 35, e00542. [Google Scholar] [CrossRef]
- Li, P.; Luo, S.; Su, F.; Zhang, L.; Yan, S.; Lei, X.; Mu, W.; Wang, Q.; Zhang, Y.; Liu, X.; et al. Optimization of Synergistic Leaching of Valuable Metals from Spent Lithium-Ion Batteries by the Sulfuric Acid-Malonic Acid System Using Response Surface Methodology. ACS Appl. Mater. Interfaces 2022, 14, 11359–11374. [Google Scholar] [CrossRef]
- Ma, X.; Chen, M.; Chen, B.; Meng, Z.; Wang, Y. High-Performance Graphite Recovered from Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2019, 7, 19732–19738. [Google Scholar] [CrossRef]
- Yang, J.; Fan, E.; Lin, J.; Arshad, F.; Zhang, X.; Wang, H.; Wu, F.; Chen, R.; Li, L. Recovery and Reuse of Anode Graphite from Spent Lithium-Ion Batteries via Citric Acid Leaching. ACS Appl. Energy Mater. 2021, 4, 6261–6268. [Google Scholar] [CrossRef]
- Xin, B.; Zhang, D.; Zhang, X.; Xia, Y.; Wu, F.; Chen, S.; Li, L. Bioleaching Mechanism of Co and Li from Spent Lithium-Ion Battery by the Mixed Culture of Acidophilic Sulfur-Oxidizing and Iron-Oxidizing Bacteria. Bioresour. Technol. 2009, 100, 6163–6169. [Google Scholar] [CrossRef] [PubMed]
- Horeh, N.B.; Mousavi, S.M.; Shojaosadati, S.A. Bioleaching of Valuable Metals from Spent Lithium-Ion Mobile Phone Batteries Using Aspergillus Niger. J. Power Sources 2016, 320, 257–266. [Google Scholar] [CrossRef]
- Bahaloo-Horeh, N.; Mousavi, S.M. Enhanced Recovery of Valuable Metals from Spent Lithium-Ion Batteries through Optimization of Organic Acids Produced by Aspergillus Niger. Waste Manag. 2017, 60, 666–679. [Google Scholar] [CrossRef]
- Yang, K.; Zhao, Z.; Xin, X.; Tian, Z.; Peng, K.; Lai, Y. Graphitic Carbon Materials Extracted from Spent Carbon Cathode of Aluminium Reduction Cell as Anodes for Lithium Ion Batteries: Converting the Hazardous Wastes into Value-Added Materials. J. Taiwan Inst. Chem. Eng. 2019, 104, 201–209. [Google Scholar] [CrossRef]
- Yu, H.; Dai, H.; Zhu, Y.; Hu, H.; Zhao, R.; Wu, B.; Chen, D. Mechanistic Insights into the Lattice Reconfiguration of the Anode Graphite Recycled from Spent High-Power Lithium-Ion Batteries. J. Power Sources 2021, 481, 229159. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, C.; Zhang, J.; Jing, Q.; Ma, B.; Chen, Y.; Zhang, W. Graphite Recycling from the Spent Lithium-Ion Batteries by Sulfuric Acid Curing–Leaching Combined with High-Temperature Calcination. ACS Sustain. Chem. Eng. 2020, 8, 9447–9455. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, J.; Jin, H.; Liang, G.; Ma, L.; Chen, Y.; Wang, C. Regenerating Spent Graphite from Scrapped Lithium-Ion Battery by High-Temperature Treatment. Carbon 2022, 189, 493–502. [Google Scholar] [CrossRef]
- Chen, Q.; Huang, L.; Liu, J.; Luo, Y.; Chen, Y. A New Approach to Regenerate High-Performance Graphite from Spent Lithium-Ion Batteries. Carbon 2022, 189, 293–304. [Google Scholar] [CrossRef]
- Cao, N.; Zhang, Y.; Chen, L.; Chu, W.; Huang, Y.; Jia, Y.; Wang, M. An Innovative Approach to Recover Anode from Spent Lithium-Ion Battery. J. Power Sources 2021, 483, 229163. [Google Scholar] [CrossRef]
- Rothermel, S.; Evertz, M.; Kasnatscheew, J.; Qi, X.; Grützke, M.; Winter, M.; Nowak, S. Graphite Recycling from Spent Lithium-Ion Batteries. ChemSusChem 2016, 9, 3473–3484. [Google Scholar] [CrossRef] [PubMed]
- Yuwen, C.; Liu, B.; Zhang, H.; Tian, S.; Zhang, L.; Guo, S.; Zhou, B. Efficient Recovery and Regeneration of Waste Graphite through Microwave Stripping from Spent Batteries Anode for High-Performance Lithium-Ion Batteries. J. Clean. Prod. 2022, 333, 130197. [Google Scholar] [CrossRef]
- Hou, D.; Guo, Z.; Wang, Y.; Hou, X.; Yi, S.; Zhang, Z.; Hao, S.; Chen, D. Microwave-Assisted Reconstruction of Spent Graphite and Its Enhanced Energy-Storage Performance as LIB Anodes. Surf. Interfaces 2021, 24, 101098. [Google Scholar] [CrossRef]
- Li, Z.; Li, S.; Wang, T.; Yang, K.; Zhou, Y.; Tian, Z. Facile Fabrication of High-Performance Li-Ion Battery Carbonaceous Anode from Li-Ion Battery Waste. J. Electrochem. Soc. 2021, 168, 090513. [Google Scholar] [CrossRef]
- Da, H.; Gan, M.; Jiang, D.; Xing, C.; Zhang, Z.; Fei, L.; Cai, Y.; Zhang, H.; Zhang, S. Epitaxial Regeneration of Spent Graphite Anode Material by an Eco-Friendly In-Depth Purification Route. ACS Sustain. Chem. Eng. 2021, 9, 16192–16202. [Google Scholar] [CrossRef]
- Ruan, D.; Wu, L.; Wang, F.; Du, K.; Zhang, Z.; Zou, K.; Wu, X.; Hu, G. A Low-Cost Silicon-Graphite Anode Made from Recycled Graphite of Spent Lithium-Ion Batteries. J. Electroanal. Chem. 2021, 884, 115073. [Google Scholar] [CrossRef]
- Ma, Z.; Zhuang, Y.; Deng, Y.; Song, X.; Zuo, X.; Xiao, X.; Nan, J. From Spent Graphite to Amorphous Sp2 +sp3 Carbon-Coated Sp2 Graphite for High-Performance Lithium Ion Batteries. J. Power Sources 2018, 376, 91–99. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Song, D.; Miao, Y.; Song, J.; Zhang, L. Effective Regeneration of Anode Material Recycled from Scrapped Li-Ion Batteries. J. Power Sources 2018, 390, 38–44. [Google Scholar] [CrossRef]
- Xu, C.; Ma, G.; Yang, W.; Che, S.; Li, Y.; Jia, Y.; Liu, H.; Chen, F.; Zhang, G.; Liu, H.; et al. One-Step Reconstruction of Acid Treated Spent Graphite for High Capacity and Fast Charging Lithium-Ion Batteries. Electrochim. Acta 2022, 415, 140198. [Google Scholar] [CrossRef]
- Zuo, X.; Zhu, J.; Müller-Buschbaum, P.; Cheng, Y.-J. Silicon Based Lithium-Ion Battery Anodes: A Chronicle Perspective Review. Nano Energy 2017, 31, 113–143. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Q.; Chen, D.; Zhong, Y.; Wu, Z.; Song, Y.; Wang, G.; Liu, Y.; Zhong, B.; Guo, X. Silicon/Graphite Composite Anode with Constrained Swelling and a Stable Solid Electrolyte Interphase Enabled by Spent Graphite. Green Chem. 2021, 23, 4531–4539. [Google Scholar] [CrossRef]
- Ye, L.; Wang, C.; Cao, L.; Xiao, H.; Zhang, J.; Zhang, B.; Ou, X. Effective Regeneration of High-Performance Anode Material Recycled from the Whole Electrodes in Spent Lithium-Ion Batteries via a Simplified Approach. Green Energy Environ. 2021, 6, 725–733. [Google Scholar] [CrossRef]
- Xiao, Z.; Gao, L.; Su, S.; Li, D.; Cao, L.; Ye, L.; Zhang, B.; Ming, L.; Ou, X. Efficient Fabrication of Metal Sulfides/Graphite Anode Materials Derived from Spent Lithium-Ion Batteries by Gas Sulfidation Process. Mater. Today Energy 2021, 21, 100821. [Google Scholar] [CrossRef]
- Nguyen, T.-V.; Tuong, V.D.; Tran, A.-T.; Truong, T.P.; Lakew, D.S.; Lee, C.; Lee, Y.; Cho, S. User-Preference-Based Proactive Caching in Edge Networks. In Proceedings of the 2021 International Conference on Information Networking (ICOIN), Jeju Island, Republic of Korea, 13–16 January 2021; pp. 755–757. [Google Scholar]
- Zhao, T.; Yao, Y.; Wang, M.; Chen, R.; Yu, Y.; Wu, F.; Zhang, C. Preparation of MnO2-Modified Graphite Sorbents from Spent Li-Ion Batteries for the Treatment of Water Contaminated by Lead, Cadmium, and Silver. ACS Appl. Mater. Interfaces 2017, 9, 25369–25376. [Google Scholar] [CrossRef]
- Hao, J.; Meng, X.; Fang, S.; Cao, H.; Lv, W.; Zheng, X.; Liu, C.; Chen, M.; Sun, Z. MnO2-Functionalized Amorphous Carbon Sorbents from Spent Lithium-Ion Batteries for Highly Efficient Removal of Cadmium from Aqueous Solutions. Ind. Eng. Chem. Res. 2020, 59, 10210–10220. [Google Scholar] [CrossRef]
- Divya, M.L.; Natarajan, S.; Lee, Y.-S.; Aravindan, V. Achieving High-Energy Dual Carbon Li-Ion Capacitors with Unique Low- and High-Temperature Performance from Spent Li-Ion Batteries. J. Mater. Chem. A 2020, 8, 4950–4959. [Google Scholar] [CrossRef]
- Divya, M.L.; Lee, Y.; Aravindan, V. Li-ion Capacitor via Solvent-Co-Intercalation Process from Spent Li-ion Batteries. Batter. Supercaps 2021, 4, 671–679. [Google Scholar] [CrossRef]
- Divya, M.L.; Natarajan, S.; Lee, Y.; Aravindan, V. Highly Reversible Na-Intercalation into Graphite Recovered from Spent Li–Ion Batteries for High-Energy Na-Ion Capacitor. ChemSusChem 2020, 13, 5654–5663. [Google Scholar] [CrossRef] [PubMed]
- Schiavi, P.G.; Altimari, P.; Zanoni, R.; Pagnanelli, F. Full Recycling of Spent Lithium Ion Batteries with Production of Core-Shell Nanowires//Exfoliated Graphite Asymmetric Supercapacitor. J. Energy Chem. 2021, 58, 336–344. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Y.; Shi, X.; Zhong, Y.; Wu, Z.; Song, Y.; Wang, G.; Liu, Y.; Zhong, B.; Guo, X. The Direct Application of Spent Graphite as a Functional Interlayer with Enhanced Polysulfide Trapping and Catalytic Performance for Li–S Batteries. Green Chem. 2021, 23, 942–950. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, H.; Chen, L.; Chen, C.; Duan, X.; Xie, Y.; Song, W.; Sun, H.; Wang, S. Tailored Synthesis of Active Reduced Graphene Oxides from Waste Graphite: Structural Defects and Pollutant-Dependent Reactive Radicals in Aqueous Organics Decontamination. Appl. Catal. B Environ. 2018, 229, 71–80. [Google Scholar] [CrossRef]
- Guan, J.; Li, Z.; Chen, S.; Gu, W. Zero-Valent Iron Supported on Expanded Graphite from Spent Lithium-Ion Battery Anodes and Ferric Chloride for the Degradation of 4-Chlorophenol in Water. Chemosphere 2022, 290, 133381. [Google Scholar] [CrossRef]
- Chen, S.; Long, F.; Gao, G.; Belver, C.; Li, Z.; Li, Z.; Guan, J.; Guo, Y.; Bedia, J. Zero-Valent Iron-Copper Bimetallic Catalyst Supported on Graphite from Spent Lithium-Ion Battery Anodes and Mill Scale Waste for the Degradation of 4-Chlorophenol in Aqueous Phase. Sep. Purif. Technol. 2022, 286, 120466. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Z.; Xu, C.; He, W.; Li, G.; Huang, J.; Zhu, H. Preparing Graphene Oxide–Copper Composite Material from Spent Lithium Ion Batteries and Catalytic Performance Analysis. Res. Chem. Intermed. 2018, 44, 5075–5089. [Google Scholar] [CrossRef]
- Sun, X.; Huang, C.; Wang, L.; Liang, L.; Cheng, Y.; Fei, W.; Li, Y. Recent Progress in Graphene/Polymer Nanocomposites. Adv. Mater. 2021, 33, 2001105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Zhou, C. Review of Chemical Vapor Deposition of Graphene and Related Applications. Acc. Chem. Res. 2013, 46, 2329–2339. [Google Scholar] [CrossRef] [PubMed]
- Kurian, M. Recent Progress in the Chemical Reduction of Graphene Oxide by Green Reductants–A Mini Review. Carbon Trends 2021, 5, 100120. [Google Scholar] [CrossRef]
- Yi, M.; Shen, Z. A Review on Mechanical Exfoliation for the Scalable Production of Graphene. J. Mater. Chem. A 2015, 3, 11700–11715. [Google Scholar] [CrossRef]
- Liu, F.; Wang, C.; Sui, X.; Riaz, M.A.; Xu, M.; Wei, L.; Chen, Y. Synthesis of Graphene Materials by Electrochemical Exfoliation: Recent Progress and Future Potential. Carbon Energy 2019, 1, 173–199. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Z.; Xia, J.; Li, F.; He, W.; Li, G.; Huang, J. Preparing Graphene from Anode Graphite of Spent Lithium-Ion Batteries. Front. Environ. Sci. Eng. 2017, 11, 6. [Google Scholar] [CrossRef]
- Yu, J.; Lin, M.; Tan, Q.; Li, J. High-Value Utilization of Graphite Electrodes in Spent Lithium-Ion Batteries: From 3D Waste Graphite to 2D Graphene Oxide. J. Hazard. Mater. 2021, 401, 123715. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, X.; Wan, C.; Ye, X.; Wu, F. Soluble Graphene Nanosheets from Recycled Graphite of Spent Lithium Ion Batteries. J. Mater. Eng. Perform. 2018, 27, 875–880. [Google Scholar] [CrossRef]
- Natarajan, S.; Rao Ede, S.; Bajaj, H.C.; Kundu, S. Environmental Benign Synthesis of Reduced Graphene Oxide (RGO) from Spent Lithium-Ion Batteries (LIBs) Graphite and Its Application in Supercapacitor. Colloids Surf. A Physicochem. Eng. Asp. 2018, 543, 98–108. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, Y.; Peng, W.; Li, Y.; Zhang, G.; Zhang, F.; Fan, X. Direct Exfoliation of the Anode Graphite of Used Li-Ion Batteries into Few-Layer Graphene Sheets: A Green and High Yield Route to High-Quality Graphene Preparation. J. Mater. Chem. A 2017, 5, 5880–5885. [Google Scholar] [CrossRef]
- He, K.; Zhang, Z.-Y.; Zhang, F.-S. Synthesis of Graphene and Recovery of Lithium from Lithiated Graphite of Spent Li-Ion Battery. Waste Manag. 2021, 124, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.; Bhattarai, R.M.; Sudhakaran, M.S.P.; Mok, Y.S.; Kim, S.J. Recycling of Spent Graphite and Copper Current Collector for Lithium-Ion and Sodium-Ion Batteries. J. Power Sources 2023, 577, 233170. [Google Scholar] [CrossRef]
- Liang, H.-J.; Hou, B.-H.; Li, W.-H.; Ning, Q.-L.; Yang, X.; Gu, Z.-Y.; Nie, X.-J.; Wang, G.; Wu, X.-L. Staging Na/K-Ion de-/Intercalation of Graphite Retrieved from Spent Li-Ion Batteries: In Operando X-Ray Diffraction Studies and an Advanced Anode Material for Na/K-Ion Batteries. Energy Environ. Sci. 2019, 12, 3575–3584. [Google Scholar] [CrossRef]
- Meng, Y.-F.; Liang, H.-J.; Zhao, C.-D.; Li, W.-H.; Gu, Z.-Y.; Yu, M.-X.; Zhao, B.; Hou, X.-K.; Wu, X.-L. Concurrent Recycling Chemistry for Cathode/Anode in Spent Graphite/LiFePO4 Batteries: Designing a Unique Cation/Anion-Co-Workable Dual-Ion Battery. J. Energy Chem. 2022, 64, 166–171. [Google Scholar] [CrossRef]
- Yang, J.-L.; Zhao, X.-X.; Li, W.-H.; Liang, H.-J.; Gu, Z.-Y.; Liu, Y.; Du, M.; Wu, X.-L. Advanced Cathode for Dual-Ion Batteries: Waste-to-Wealth Reuse of Spent Graphite from Lithium-Ion Batteries. eScience 2022, 2, 95–101. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).