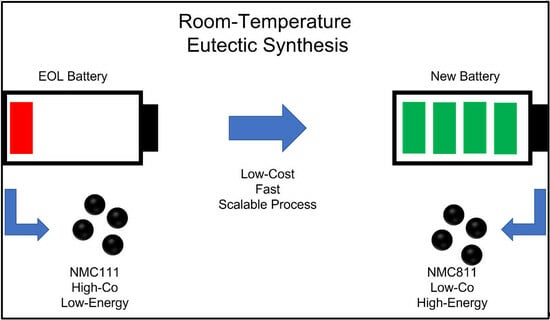
Room-Temperature Eutectic Synthesis for Upcycling of Cathode Materials
Abstract
:1. Introduction
2. Experimental Section
2.1. Eutectic Synthesis
2.2. Material Characterization
2.3. Electrochemical Characterization
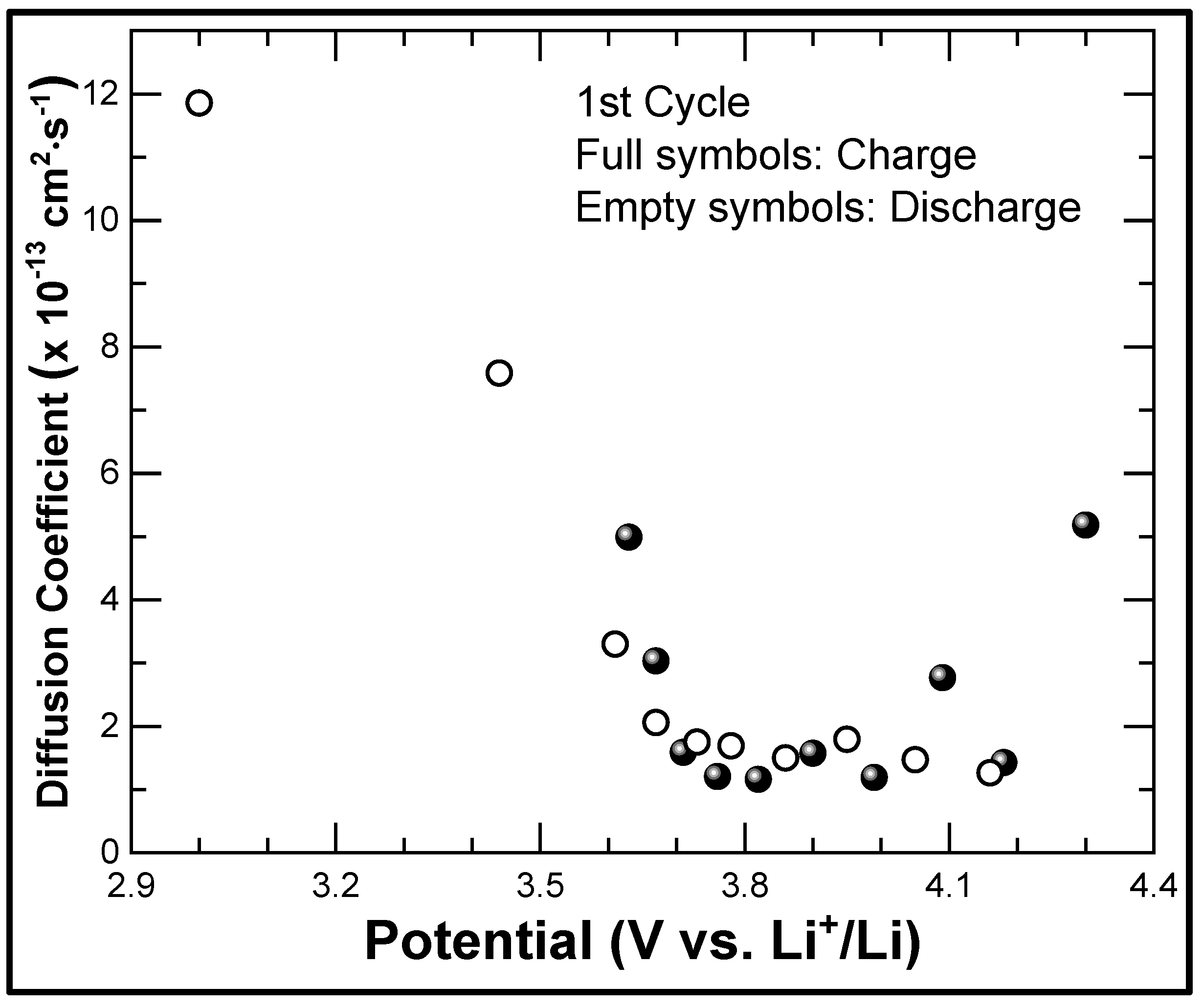
3. Results and Discussion
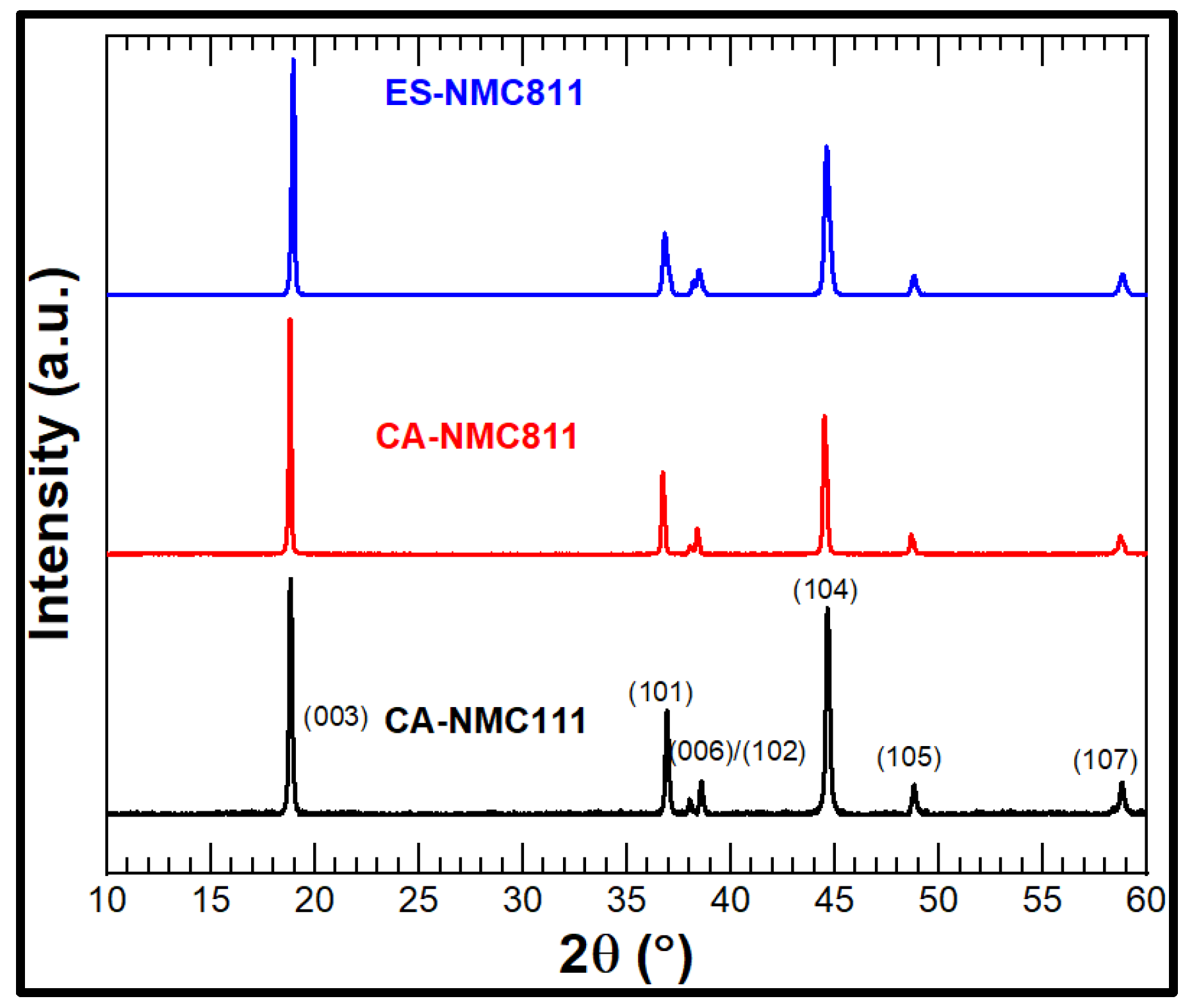
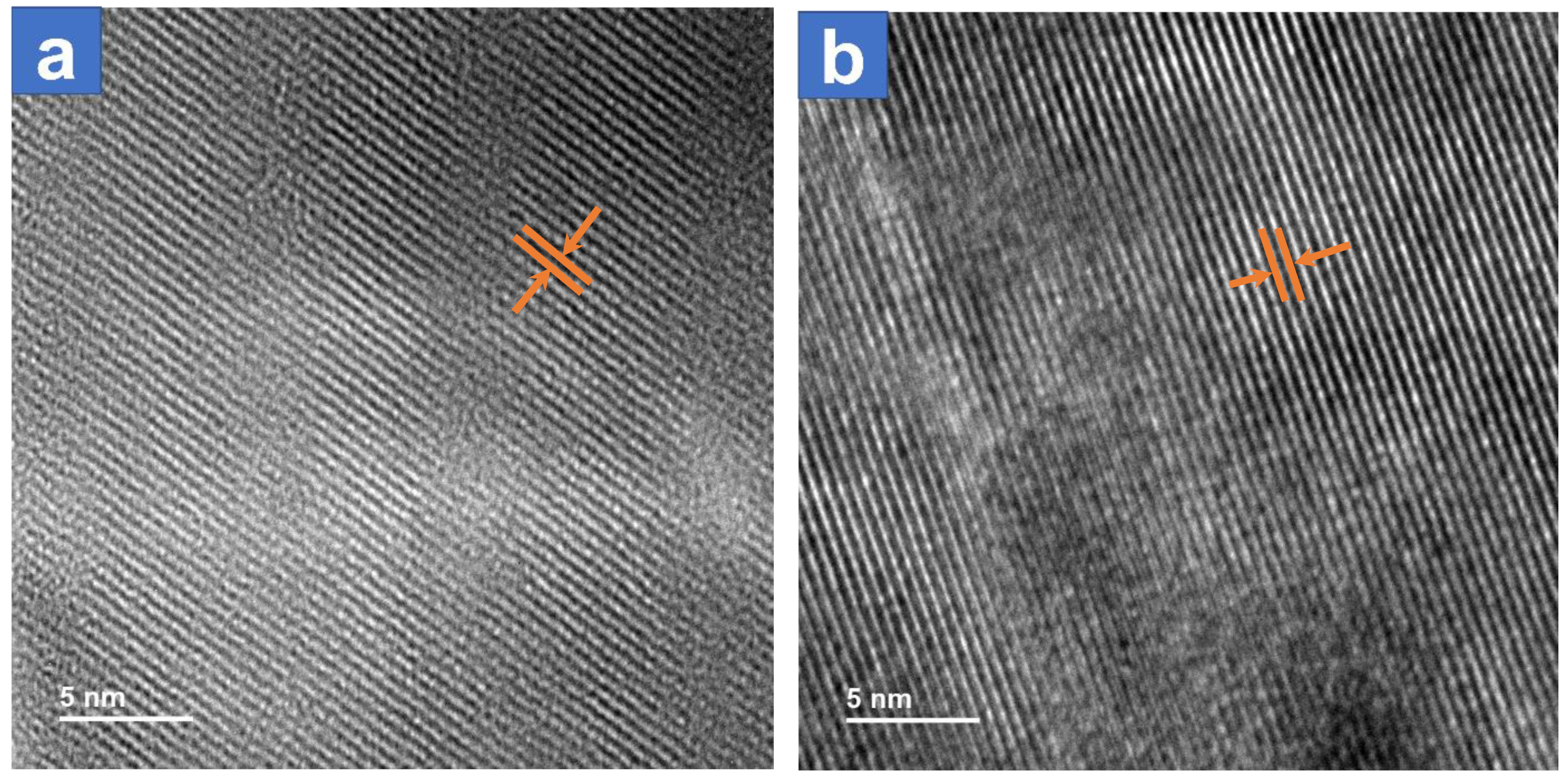
3.1. Material Properties
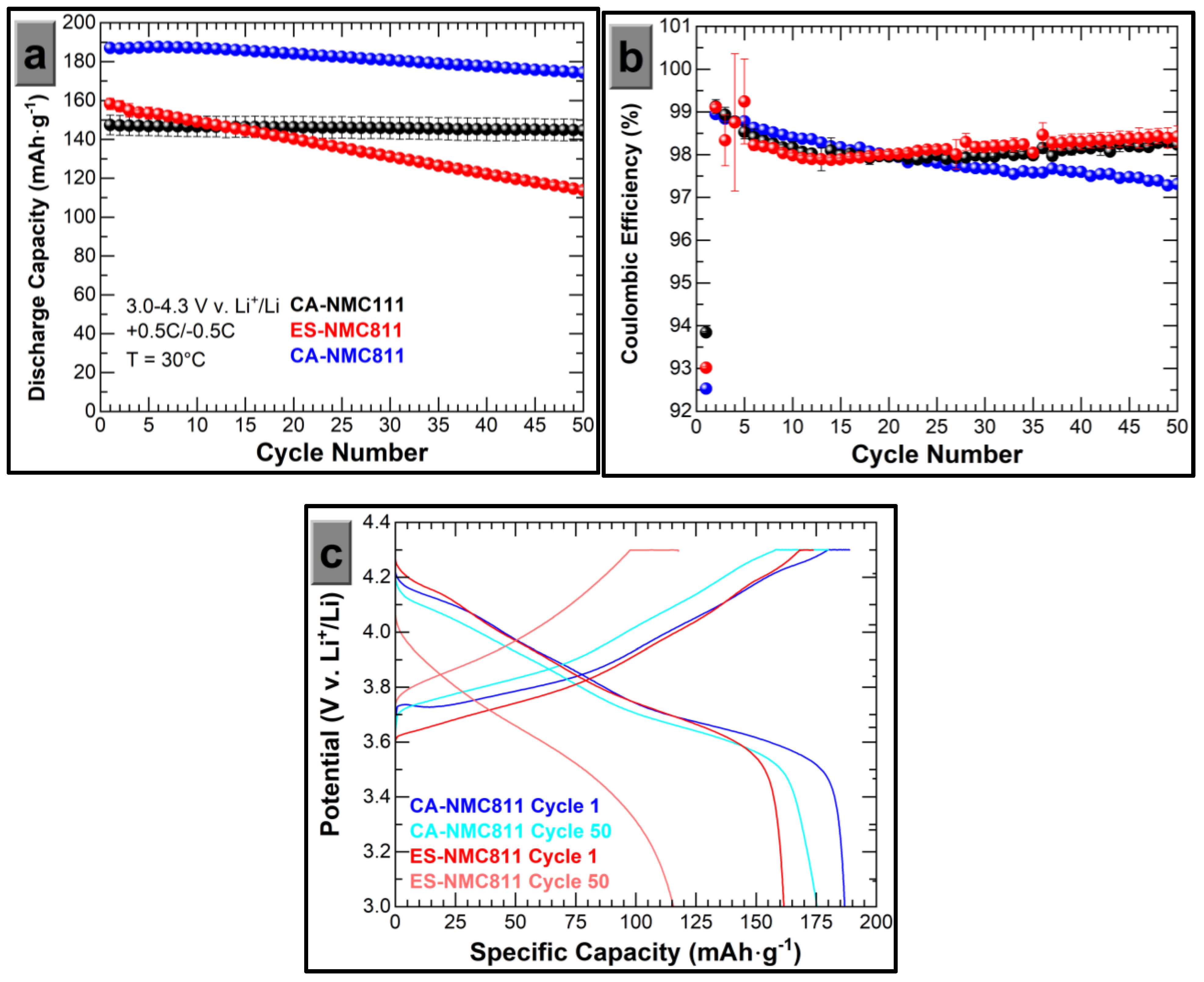
3.2. Electrochemical Properties
3.3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muralidharan, N.; Self, E.C.; Dixit, M.; Du, Z.; Essehli, R.; Amin, R.; Nanda, J.; Belharouak, I. Next-generation cobalt-free cathodes—A prospective solution to the battery industry’s cobalt problem. In Transition Metal Oxides for Electrochemical Energy Storage; Wiley: Hoboken, NJ, USA, 2022; pp. 33–53. [Google Scholar] [CrossRef]
- Lyu, H.; Sun, X.-G.; Dai, S. Organic cathode materials for lithium-ion batteries: Past, present, and future. Adv. Energy Sustain. Res. 2021, 2, 2000044. [Google Scholar] [CrossRef]
- Li, J.; Sun, X.-G. Polypeptide-based batteries toward sustainable and cyclic manufacturing. Chem 2021, 7, 1705–1707. [Google Scholar] [CrossRef]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y.; Yang, T.; Ge, D.; Wood III, D.L.; Li, Z. Water-based electrode manufacturing and direct recycling of lithium-ion battery electrodes—A green and sustainable manufacturing system. iScience 2020, 23, 101081. [Google Scholar] [CrossRef]
- Wang, T.; Luo, H.; Bai, Y.; Li, J.; Belharouak, I.; Dai, S. Direct recycling of spent NCM cathodes through ionothermal lithiation. Adv. Energy Mater. 2020, 10, 2001204. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Li, M.; Chen, R.; Wu, F.; Amine, K.; Lu, J. The recycling of spent lithium-ion batteries: A review of current processes and technologies. Electrochem. Energy Rev. 2018, 1, 461–482. [Google Scholar] [CrossRef]
- Zheng, R.; Wang, W.; Dai, Y.; Ma, Q.; Liu, Y.; Mu, D.; Li, R.; Ren, J.; Dai, C. A closed-loop process for recycling LiNixCoyMn(1−x−y)O2 from mixed cathode materials of lithium-ion batteries. Green Energy Environ. 2017, 2, 42–50. [Google Scholar] [CrossRef]
- Pagliaro, M.; Meneguzzo, F. Lithium battery reusing and recycling: A circular economy insight. Heliyon 2019, 5, e01866. [Google Scholar] [CrossRef]
- Ji, Y.; Jafvert, C.T.; Zhao, F. Recovery of cathode materials from spent lithium-ion batteries using eutectic system of lithium compounds. Resour. Conserv. Recycl. 2021, 170, 105551. [Google Scholar] [CrossRef]
- Bai, Y.; Muralidharan, N.; Sun, Y.-K.; Passerini, S.; Whittingham, M.S.; Belharouak, I. Energy and environmental aspects in recycling lithium-ion batteries: Concept of Battery Identity Global Passport. Mater. Today 2020, 41, 304–315. [Google Scholar] [CrossRef]
- Zhan, R.; Yang, Z.; Bloom, I.; Pan, L. Significance of a solid electrolyte interphase on separation of anode and cathode materials from spent Li-ion batteries by froth flotation. ACS Sustain. Chem. Eng. 2021, 9, 531–540. [Google Scholar] [CrossRef]
- Xu, P.; Dai, Q.; Gao, H.; Liu, H.; Zhang, M.; Li, M.; Chen, Y.; An, K.; Meng, Y.S.; Liu, P.; et al. Efficient direct recycling of lithium-ion battery cathodes by targeted healing. Joule 2020, 4, 2609–2626. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Li, J.; Fleetwood, J.; Hawley, W.B.; Kays, W. From materials to cell: State-of-the-art and prospective technologies for lithium-ion battery electrode processing. Chem. Rev. 2022, 122, 903–956. [Google Scholar] [CrossRef]
- Hawley, W.B.; Li, J. Electrode manufacturing for lithium-ion batteries—Analysis of current and next generation processing. J. Energy Storage 2019, 25, 100862. [Google Scholar] [CrossRef]
- Blomgren, G.E. The development and future of lithium ion batteries. J. Electrochem. Soc. 2017, 164, A5019–A5025. [Google Scholar] [CrossRef]
- Shahan, Z. LG Chem Has Begun Mass Production of NCM712. 2020. Available online: https://cleantechnica.com/2020/06/11/lg-chem-began-mass-production-of-ncm712-batteries-in-poland-in-q1/ (accessed on 11 January 2022).
- Armand, M.; Axmann, P.; Bresser, D.; Copley, M.; Edstrom, K.; Ekberg, C.; Guyomard, D.; Lestriez, B.; Novak, P.; Petranikova, M.; et al. Lithium-ion batteries—Current state of the art and anticipated developments. J. Power Sources 2020, 479, 228708. [Google Scholar] [CrossRef]
- Lin, J.; Fan, E.; Zhang, X.; Li, Z.; Dai, Y.; Chen, R.; Wu, F.; Li, L. Sustainable upcycling of spent lithium-ion batteries cathode materials: Stabilization by in situ Li/Mn disorder. Adv. Energy Mater. 2022, 12, 2201174. [Google Scholar] [CrossRef]
- Wang, T.; Luo, H.; Fan, J.; Thapaliya, B.P.; Bai, Y.; Belharouak, I.; Dai, S. Flux upcycling of spent NMC111 to nickel-rich NMC cathodes in reciprocal ternary molten salts. iScience 2022, 25, 103801. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Fang, X.; Lu, Y.; Ding, N.; Feng, X.Y.; Liu, C.; Chen, C.H. Electrochemical properties of nano- and micro-sized LiNi0.5Mn1.5O4 synthesized via thermal decomposition of a ternary eutectic Li-Ni-Mn acetate. Electrochim. Acta 2010, 55, 832–837. [Google Scholar] [CrossRef]
- Li, M.; Wood, D.L.; Bai, Y.; Essehli, R.; Amin, R.; Jafta, C.; Muralidharan, N.; Li, J.; Belharouak, I. Eutectic synthesis of the P2-type NaxFe1/2Mn1/2O2 cathode with improved cell design for sodium-ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 23951–23958. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zheng, J.; Cui, S.; Song, X.; Su, Y.; Deng, W.; Wu, Z.; Wang, X.; Wang, W.; Rao, M.; et al. Kinetics tuning of Li-ion diffusion in layered Li(NixMnyCoz)O2. J. Am. Chem. Soc. 2015, 137, 8364–8367. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, W.; Zhu, M.; Li, Y.; Li, W.; Zheng, F.; Shen, L.; Dang, M.; Zhang, J. Coating ultra-thin TiN layer onto LiNi0.8Co0.1Mn0.1O2 cathode material by atomic layer deposition for high-performance lithium-ion batteries. J. Alloys Compd. 2021, 888, 161594. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Qin, X.; Gong, J.; Guo, J.; Zong, B.; Zhou, M.; Wang, L.; Liang, G. Synthesis and performance of LiNi0.5Mn1.5O4 cathode materials with different particle morphologies and sizes for lithium-ion battery. J. Alloys Compd. 2019, 786, 240–249. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Kim, U.-H.; Yoon, C.S.; Sun, Y.-K. Enhanced cycling stability of Sn-doped Li[Ni0.90Co0.05Mn0.05]O2 via optimization of particle shape and orientation. Chem. Eng. J. 2021, 405, 126887. [Google Scholar] [CrossRef]
- Muller, M.; Schneider, L.; Bohn, N.; Binder, J.R.; Bauer, W. Effect of nanostructured and open-porous particle morphology on electrode processing and electrochemical performance of Li-ion batteries. ACS Appl. Energy Mater. 2021, 4, 1993–2003. [Google Scholar] [CrossRef]
- Li, W.; Liu, X.; Celio, H.; Smith, P.; Dolocan, A.; Chi, M.; Manthiram, A. Mn versus Al in layered oxide cathodes in lithium-ion batteries: A comprehensive evaluation on long-term cyclability. Adv. Energy Mater. 2018, 8, 1703154. [Google Scholar] [CrossRef]
- Ue, M.; Sakaushi, K.; Uosaki, K. Basic knowledge in battery research bridging the gap between academia and industry. Mater. Horiz. 2020, 7, 1937–1954. [Google Scholar] [CrossRef]
- Wang, Y.; Roller, J.; Maric, R. Morphology-controlled one-step synthesis of nanostructrued LiNi1/3Mn1/3Co1/3O2 electrodes for Li-ion batteries. ACS Omega 2018, 3, 3966–3973. [Google Scholar] [CrossRef] [PubMed]
- Hietaniemi, M.; Hu, T.; Valikangas, J.; Niittykoski, J.; Lassi, U. Effect of precursor particle size and morphology on lithiation of Ni0.6Mn0.2Co0.2(OH)2. J. Appl. Electrochem. 2021, 51, 1545–1557. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hawley, W.B.; Li, M.; Li, J. Room-Temperature Eutectic Synthesis for Upcycling of Cathode Materials. Batteries 2023, 9, 498. https://doi.org/10.3390/batteries9100498
Hawley WB, Li M, Li J. Room-Temperature Eutectic Synthesis for Upcycling of Cathode Materials. Batteries. 2023; 9(10):498. https://doi.org/10.3390/batteries9100498
Chicago/Turabian StyleHawley, W. Blake, Mengya Li, and Jianlin Li. 2023. "Room-Temperature Eutectic Synthesis for Upcycling of Cathode Materials" Batteries 9, no. 10: 498. https://doi.org/10.3390/batteries9100498
APA StyleHawley, W. B., Li, M., & Li, J. (2023). Room-Temperature Eutectic Synthesis for Upcycling of Cathode Materials. Batteries, 9(10), 498. https://doi.org/10.3390/batteries9100498