Solid-State Lithium Batteries with Cathode-Supported Composite Solid Electrolytes Enabling High-Rate Capability and Excellent Cyclic Performance
Abstract
:1. Introduction
2. Experimental Section
2.1. Fabrication of Composite Solid Electrolytes
2.2. Fabrication of LFP-Supported Composite Solid Electrolytes
2.3. Materials and Electrochemical Characterization
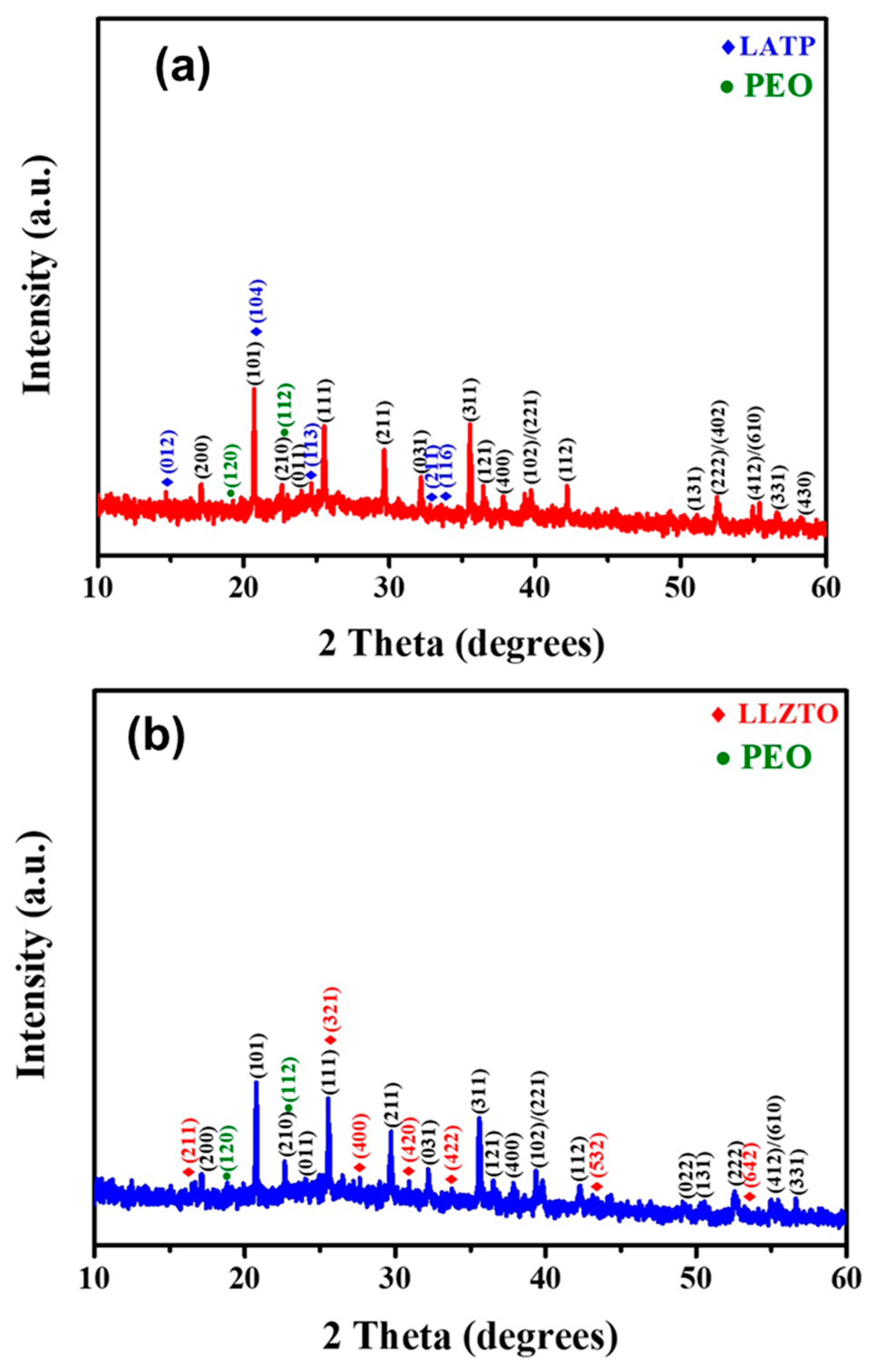
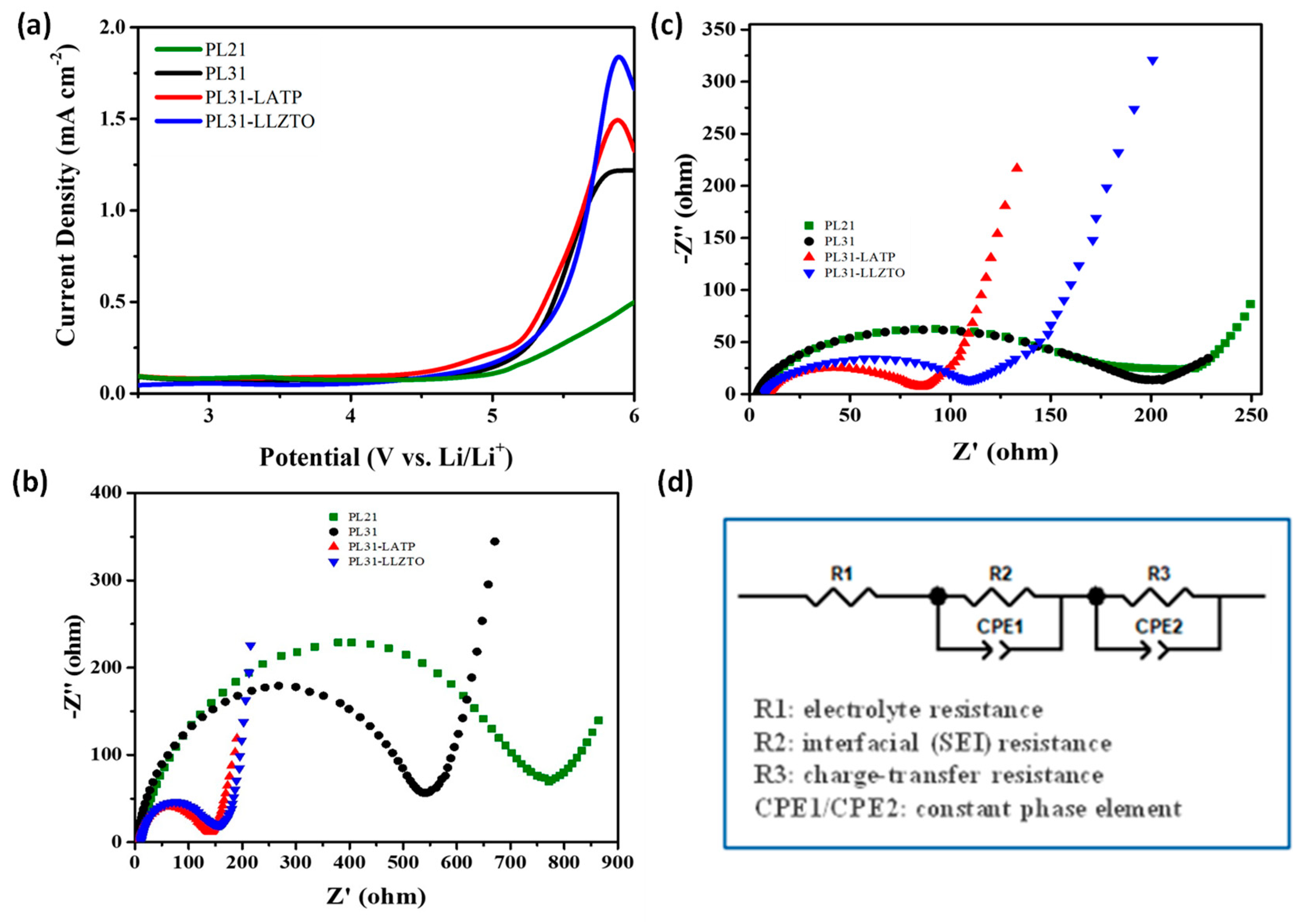
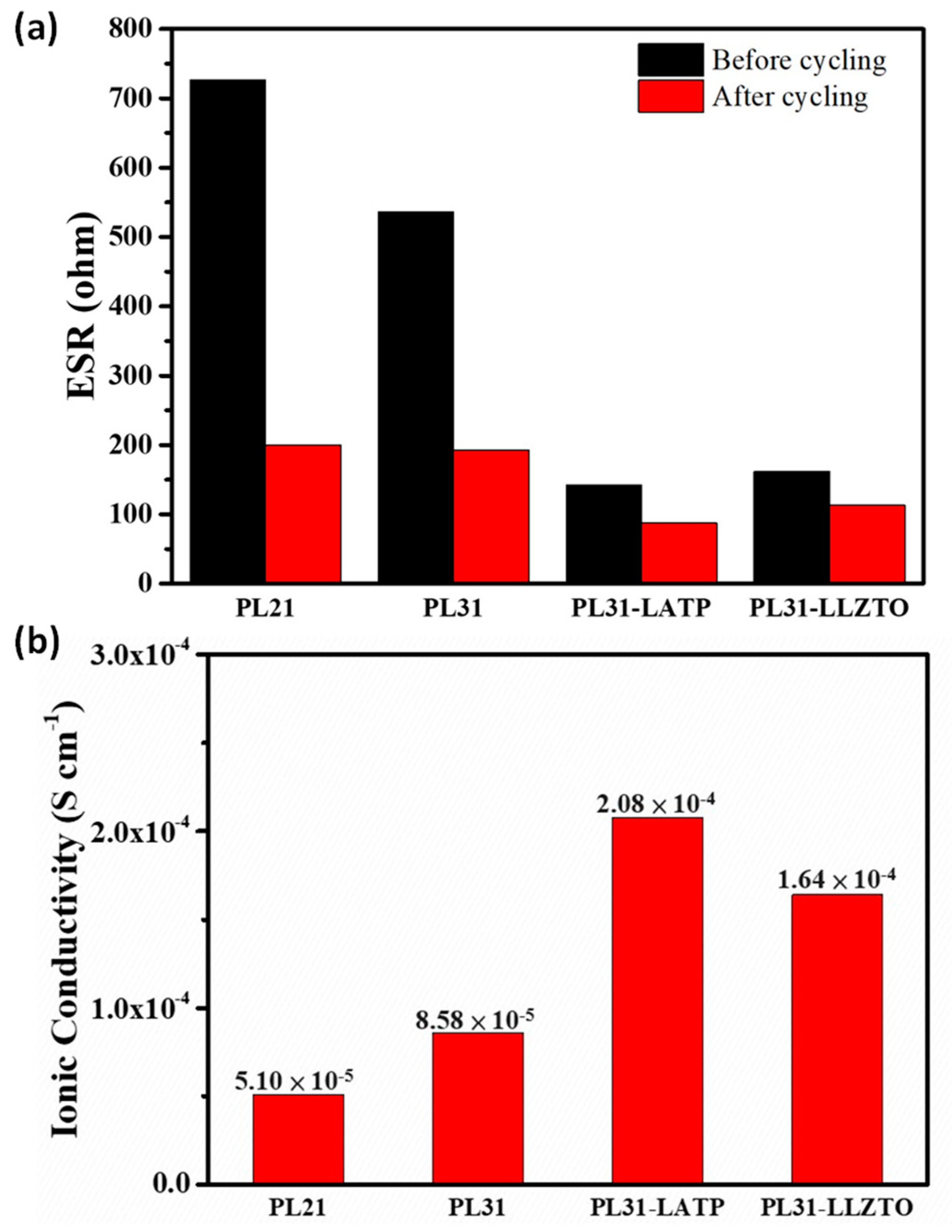
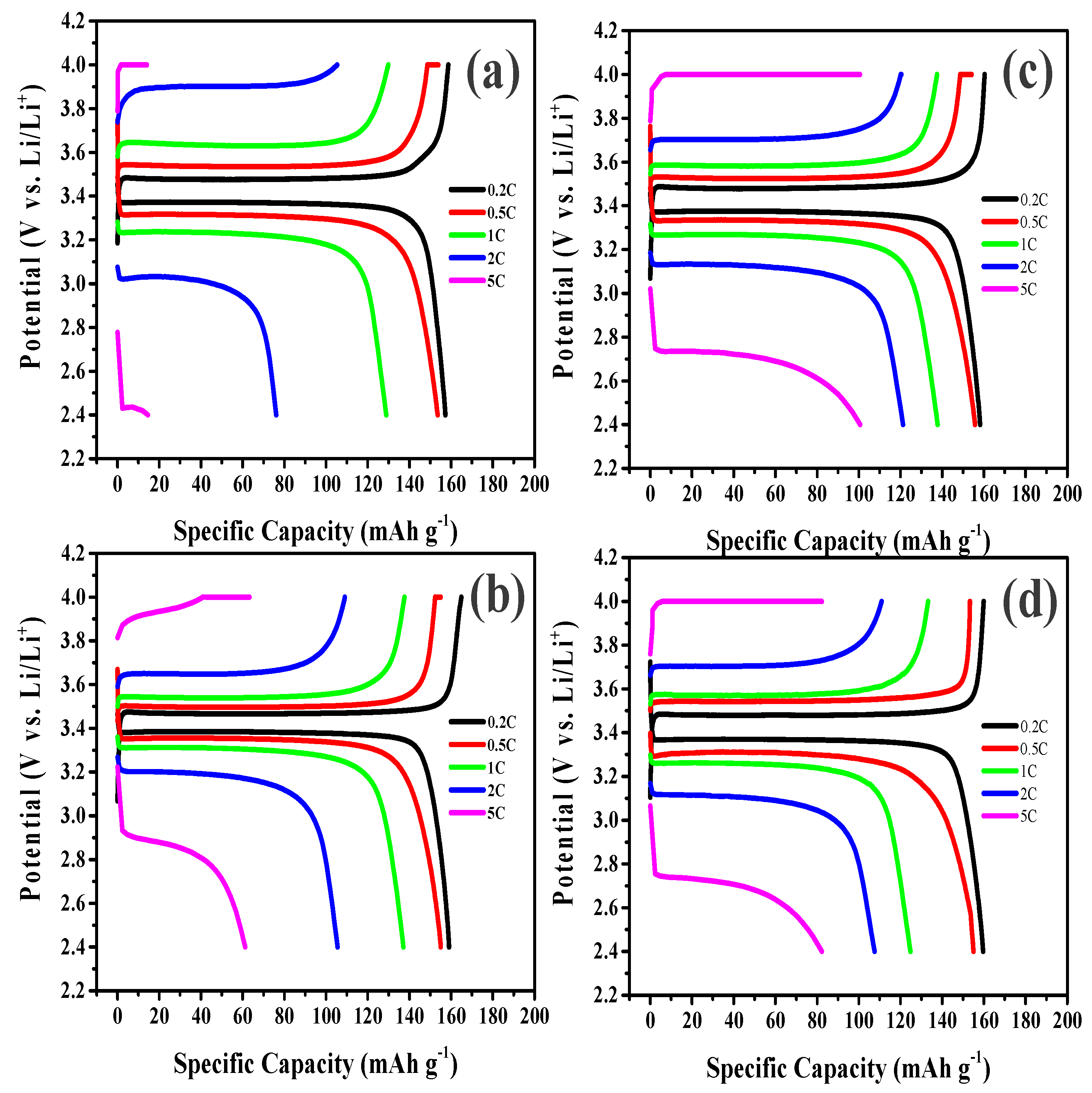
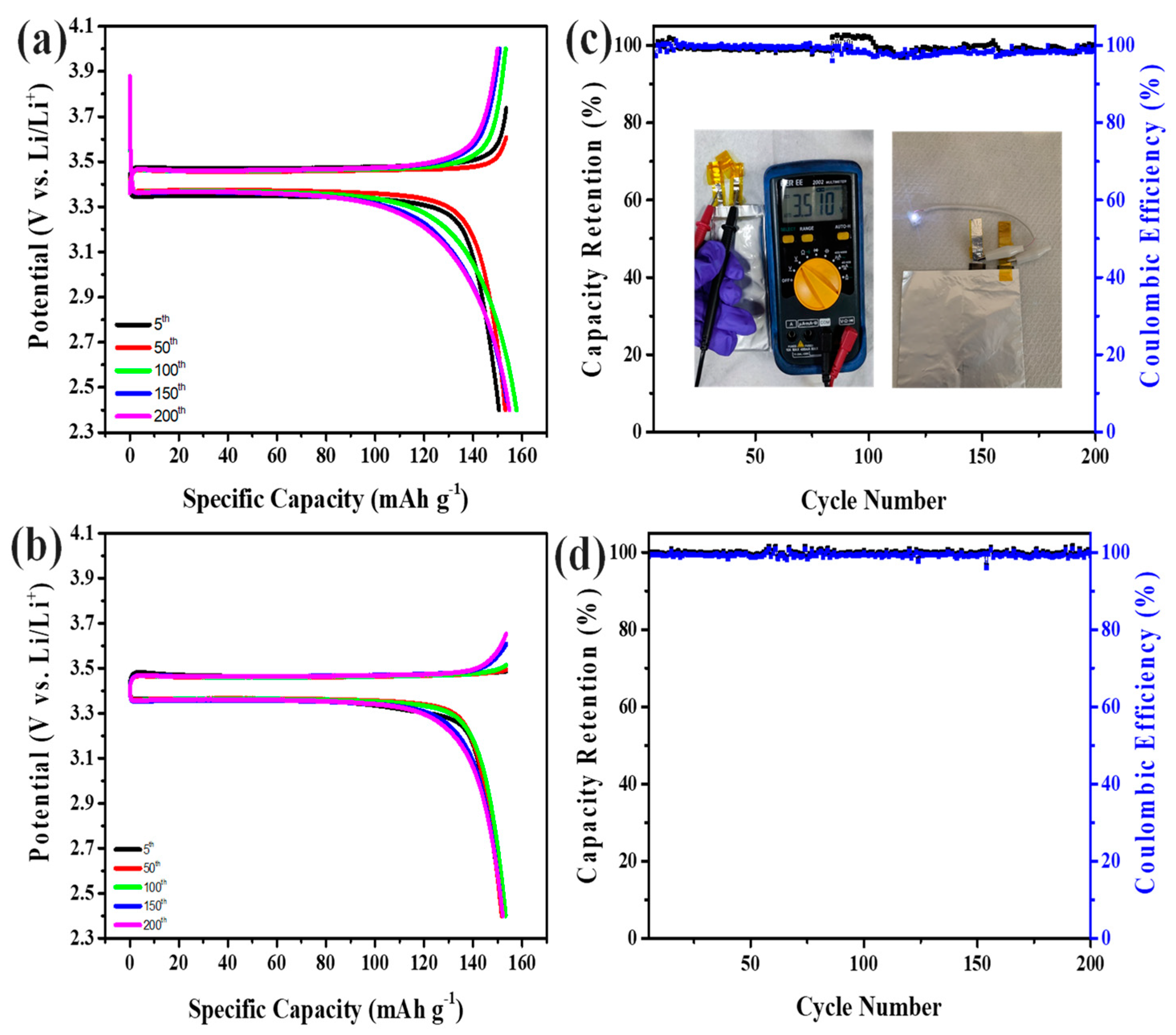
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Janek, J.; Zeier, W.G. A solid future for battery development. Nat. Energy 2016, 1, 16141. [Google Scholar] [CrossRef]
- Xu, K. Electrolytes and interphases in Li-ion batteries and beyond. Chem. Rev. 2014, 114, 11503–11618. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, Q.; Li, Y.; Cui, Z.; Guo, X.; Li, H. Sustainable interfaces between Si anodes and garnet electrolytes for room-temperature solid-state batteries. ACS Appl. Mater. Interfaces 2018, 10, 2185–2190. [Google Scholar] [CrossRef]
- Song, S.W.; Lee, K.C.; Park, H.Y. High-performance flexible all-solid-state micro batteries based on solid electrolyte of lithium boron oxynitride. J. Power Source 2016, 328, 311–317. [Google Scholar] [CrossRef]
- Hammami, A.; Raymond, N.; Armand, M. Lithium-ion batteries: Runaway risk of forming toxic compounds. Nature 2003, 424, 635–636. [Google Scholar] [CrossRef]
- Wang, D.; Zhong, G.; Li, Y.; Gong, Z.; McDonald, M.J.; Mi, J.-X.; Fu, R.; Shi, Z.; Yang, Y. Enhanced ionic conductivity of Li3.5Si0.5P0.5O4 with addition of lithium borate. Solid State Ion. 2015, 283, 109–114. [Google Scholar] [CrossRef]
- Takada, K. Progress in solid electrolytes toward realizing solid-state lithium batteries. J. Power Source 2018, 394, 74–85. [Google Scholar] [CrossRef]
- Jiang, B.; Wei, Y.; Wu, J.; Cheng, H.; Yuan, L.; Li, Z.; Xu, H.; Huang, Y. Recent progress of asymmetric solid-state electrolytes for lithium/sodium-metal batteries. EnergyChem 2021, 3, 100058. [Google Scholar] [CrossRef]
- Chen, S.Y.; Hsieh, C.T.; Zhang, R.S.; Mohanty, D.; Gandomi, Y.A.; Hung, I.M. Hybrid solid state electrolytes blending NASICON-Type Li1+xAlxTi2–x(PO4)3 with poly(vinylidene fluoride-co-hexafluoropropene) for lithium metal batteries. Electrochim. Acta 2022, 427, 140903. [Google Scholar] [CrossRef]
- Wang, S.; Ben, L.; Li, H.; Chen, L. Identifying Li+ ion transport properties of aluminum doped lithium titanium phosphate solid electrolyte at wide temperature range. Solid State Ion. 2014, 268 Pt A, 110–116. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Y.; Chen, S.; Xie, D.; Yao, X.; Cui, P.; Xu, X. An advanced construction strategy of all-solid-state lithium batteries with excellent interfacial compatibility and ultralong cycle life. J. Mater. Chem. A 2017, 5, 16984–16993. [Google Scholar] [CrossRef]
- Kim, D.H.; Oh, D.Y.; Park, K.H.; Choi, Y.E.; Nam, Y.J.; Lee, H.A.; Lee, S.M.; Jung, Y.S. Infiltration of solution-processable solid electrolytes into conventional Li-ion-battery electrodes for all-solid-state Li-ion batteries. Nano Lett. 2017, 17, 3013–3020. [Google Scholar] [CrossRef]
- Koerver, R.; Aygün, I.; Leichtweiß, T.; Dietrich, C.; Zhang, W.; Binder, J.O.; Hartmann, P.; Zeier, W.G.; Janek, J. Capacity fade in solid-state batteries: Interphase formation and chemomechanical processes in nickel-rich layered oxide cathodes and lithium thiophosphate solid electrolytes. Chem. Mater. 2017, 29, 5574–5582. [Google Scholar] [CrossRef]
- Zhang, B.; Tan, R.; Yang, L.; Zheng, J.; Zhang, K.; Mo, S.; Lin, Z.; Pan, F. Mechanisms and properties of ion-transport in inorganic solid electrolytes. Energy Storage Mater. 2018, 10, 139–159. [Google Scholar] [CrossRef]
- Zhou, Q.; Ma, J.; Dong, S.; Li, X.; Cui, G. Intermolecular chemistry in solid polymer electrolytes for high-energy-density lithium batteries. Adv. Mater 2019, 31, 1902029. [Google Scholar] [CrossRef]
- Yan, Y.; Ju, J.; Dong, S.; Wang, Y.; Huang, L.; Cui, L.; Jiang, F.; Wang, Q.; Zhang, Y.; Cui, G. In situ polymerization permeated three-dimensional Li+-percolated porous oxide ceramic framework boosting All solid-state lithium metal battery. Adv. Sci. 2021, 8, 2003887. [Google Scholar] [CrossRef]
- Lv, Z.; Zhou, Q.; Zhang, S.; Dong, S.; Wang, Q.; Huang, L.; Chen, K.; Cui, G. Cyano-reinforced in-situ polymer electrolyte enabling long-life cycling for high-voltage lithium metal batteries. Energy Storage Mater. 2021, 37, 215–223. [Google Scholar] [CrossRef]
- Cui, G. Reasonable design of high-energy-density solidstate lithium-metal batteries. Matter 2020, 2, 805–815. [Google Scholar] [CrossRef]
- Nie, K.; Wang, X.; Qiu, J.; Wang, Y.; Yang, Q.; Xu, J.; Yu, X.; Li, H.; Huang, X.; Chen, L. Increasing poly(ethylene oxide) stability to 4.5 V by surface coating of the cathode. ACS Energy Lett. 2020, 5, 826–832. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, N.; Zhang, M.; Li, Y.; Chu, P.K.; Guo, X.; Di, Z.; Wang, X.; Li, H. Flexible and ion-conducting membrane electrolytes for solid-state lithium batteries: Dispersion of garnet nanoparticles in insulating polyethylene oxide. Nano Energy 2016, 28, 447–454. [Google Scholar] [CrossRef]
- Wang, L.P.; Zhang, X.D.; Wang, T.S.; Yin, Y.X.; Shi, J.L.; Wang, C.R.; Guo, Y.G. Ameliorating the interfacial problems of cathode and solid-state electrolytes by interface modification of functional polymers. Adv. Energy Mater. 2018, 8, 1801528. [Google Scholar] [CrossRef]
- Sung, P.Y.; Lu, M.; Hsieh, C.T.; Gandomi, Y.A.; Gu, S.; Liu, W.R. Sodium super ionic conductor-type hybrid electrolytes for high performance lithium metal batteries. Membranes 2023, 13, 201. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xue, Y.; Zhang, K.; Zhang, Y. Synthesis of FePO4·2H2O nanoplates and their usage for fabricating superior high-rate performance LiFePO4. Electrochim. Acta 2011, 56, 4294–4298. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Pai, C.T.; Chen, Y.F.; Chen, I.L.; Chen, W.Y. Preparation of lithium iron phosphate cathode materials with different carbon contents using glucose additive for Li-ion batteries. J. Taiwan Inst. Chem. Eng. 2014, 45, 1501–1508. [Google Scholar] [CrossRef]
- Polu, A.R.; Kumar, R.; Rhe, H.W. Magnesium ion conducting solid polymer blend electrolyte based on biodegradable polymers and application in solid-state batteries. Ionics 2015, 21, 125–132. [Google Scholar] [CrossRef]
- Marceau, H.; Kim, C.S.; Paolella, A.; Ladouceur, S.; Lagacé, M.; Chaker, M.; Vijh, A.; Guerfi, A.; Julien, C.M.; Mauger, A.; et al. In operando scanning electron microscopy and ultraviolet-visible spectroscopy studies of lithium/sulfur cells using all solid-state polymer electrolyte. J. Power Source 2016, 319, 247–254. [Google Scholar] [CrossRef]
- Liang, Y.; Lin, Z.; Qiu, Y.; Zhang, X. Fabrication and characterization of LATP/PAN composite fiber-based lithium-ion battery separators. Electrochim. Acta 2011, 56, 6474–6480. [Google Scholar] [CrossRef]
- Kumar, J.; Kichambare, P.; Rai, A.K.; Bhattacharya, R.; Rodrigues, S.; Subramanyam, G.A. A high performance ceramic-polymer separator for lithium batteries. J. Power Source 2016, 301, 194–198. [Google Scholar] [CrossRef]
- Yoon, S.A.; Oh, N.R.; Yoo, A.R.; Lee, H.G.; Lee, H.C. Preparation and characterization of Ta-substituted Li7La3Zr2-xO12 garnet solid electrolyte by sol-gel processing. J. Korean Ceram. Soc. 2017, 54, 278–284. [Google Scholar] [CrossRef]
- Xie, H.; Li, C.; Kan, W.H.; Avdeev, M.; Zhu, C.; Zhao, Z.; Chu, X.; Mu, D.; Wu, F. Consolidating the grain boundary of the garnet electrolyte LLZTO with Li3BO3 for high-performance LiNi0.8Co0.1Mn0.1O2/LiFePO4 hybrid solid batteries. J. Mater. Chem. A 2019, 7, 20633–20639. [Google Scholar] [CrossRef]
- Prasanna, K.; Subburaj, T.; Lee, W.J.; Lee, C.W. Polyethylene separator: Stretched and coated with porous nickel oxide nanoparticles for enhancement of its efficiency in Li-ion batteries. Electrochim. Acta 2014, 137, 273–279. [Google Scholar] [CrossRef]
- Fan, X.; Chen, L.; Ji, X.; Deng, T.; Hou, S.; Chen, J.; Zheng, J.; Wang, F.; Jiang, J.; Xu, K.; et al. Highly fluorinated interphases enable high-voltage Li-metal batteries. Chem 2018, 4, 174–185. [Google Scholar] [CrossRef]
- Girard, G.M.A.; Hilder, M.; Nucciarone, D.; Whitbread, K.; Zavorine, S.; Moser, M.; Forsyth, M.; MacFarlane, D.R.; Howlett, P.C. Role of Li concentration and the SEI layer in enabling high performance Li metal electrodes using a phosphonium bis(fluorosulfonyl)imide ionic liquid. J. Phys. Chem. C 2017, 121, 21087–21095. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Pai, C.T.; Chen, Y.F.; Yu, P.Y.; Juang, R.S. Electrochemical performance of lithium iron phosphate cathodes at various temperatures. Electrochim. Acta 2014, 115, 96–102. [Google Scholar] [CrossRef]
- Samsinger, R.F.; Schopf, S.O.; Schuhmacher, J.; Treis, P.; Schneider, M.; Roters, A.; Kwade, A. Influence of the processing on the ionic conductivity of solid-state hybrid electrolytes based on glass-ceramic particles dispersed in PEO with LiTFSI. J. Electrochem. Soc. 2020, 167, 120538. [Google Scholar] [CrossRef]
- Panda, A.; Patra, J.P.; Hsieh, C.T.; Huang, Y.C.; Gandomi, Y.A.; Fu, C.C.; Lin, M.H.; Juang, R.S.; Chang, J.K. Improving high-temperature performance of lithium-rich cathode by roll-to-roll atomic layer deposition of titania nanocoating for lithium-ion batteries. J. Energy Storage 2021, 44, 103348. [Google Scholar] [CrossRef]
- Nien, Y.H.; Carey, J.R.; Chen, J.S. Physical and electrochemical properties of LiFePO4/C composite cathode prepared from various polymer-containing precursors. J. Power Source 2009, 193, 822–827. [Google Scholar] [CrossRef]
- Wu, H.C.; Wu, H.C.; Lee, E.; Wu, N.L. High-temperature carbon-coated aluminum current collector for enhanced power performance of LiFePO4 electrode of Li-ion batteries. Electrochem. Commun. 2010, 12, 488–491. [Google Scholar] [CrossRef]
- Wang, W.; Yi, E.; Fici, A.J.; Laine, R.M.; Kieffer, J. Lithium ion conducting poly(ethylene oxide)-based solid electrolytes contain-ing active or passive ceramic nanoparticles. J. Phys. Chem. C 2017, 121, 2563–2573. [Google Scholar] [CrossRef]
- Xu, G.; Li, F.; Tao, Z.; Wei, X.; Liu, Y.; Li, X.; Ren, Z.; Shen, G.; Han, G. Monodispersed LiFePO4@C core–shell nanostructures for a high power Li-ion battery cathode. J. Power Source 2014, 246, 696–702. [Google Scholar] [CrossRef]
- Yu, S.; Schmidt, R.D.; Garcia-Mendez, R.; Herbert, E.; Dudney, N.J.; Wolfenstine, J.B.; Sakamoto, J.; Siegel, D.J. Elastic properties of the solid electrolyte Li7La3Zr2O12 (LLZO). Chem. Mater. 2015, 28, 197–206. [Google Scholar] [CrossRef]
- Yan, G.; Malzbender, J.; Fu, S.; Gross, J.P.; Yu, S.; Eichel, R.-A.; Schwaiger, R. Fracture behavior of solid electrolyte LATP material based on micro-pillar splitting method. J. Eur. Ceram. Soc. 2021, 41, 5240–5247. [Google Scholar] [CrossRef]
- Huo, H.; Chen, Y.; Luo, J.; Yang, X.; Guo, X.; Sun, X. Rational design of hierarchical “ceramic-in-polymer” and “polymer-in-ceramic” electrolytes for dendrite-free solid-state batteries. Adv. Energy Mater. 2019, 9, 1804004. [Google Scholar] [CrossRef]
- Tornheim, A.; O’Hanlon, D.C. What do coulombic efficiency and capacity retention truly measure? A deep dive into cyclable lithium inventory, limitation type, and redox side reactions. J. Electrochem. Soc. 2020, 167, 110520. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, Z.; Chen, S.; Chen, B.; Yang, J.; Zhang, Q.; Ding, F.; Chen, Y.; Xu, X. A promising PEO/LAGP hybrid electrolyte prepared by a simple method for all-solid-state lithium batteries. Solid State Ionics 2016, 295, 65–71. [Google Scholar] [CrossRef]
- Wan, Z.; Lei, D.; Yang, W.; Liu, C.; Shi, K.; Hao, X.; Shen, L.; Lv, W.; Li, B.; Yang, Q.-H.; et al. Low resistance–integrated all-solid-state battery achieved by Li7La3Zr2O12 nanowire upgrading polyethylene oxide (PEO) composite electrolyte and PEO cathode binder. Adv. Funct. Mater. 2019, 29, 1–10. [Google Scholar] [CrossRef]
- Zhu, L.; Zhu, P.H.; Fang, Q.X.; Jing, M.X.; Shen, X.Q.; Yang, L.Z. A novel solid PEO/LLTO-nanowires polymer composite electrolyte for solid-state lithium-ion battery. Electrochim. Acta 2018, 292, 718–726. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, K.-F.; Panda, P.K.; Hsieh, C.-T.; Yang, P.-C.; Kataria, N.; Khoo, K.S. Solid-State Lithium Batteries with Cathode-Supported Composite Solid Electrolytes Enabling High-Rate Capability and Excellent Cyclic Performance. Batteries 2023, 9, 490. https://doi.org/10.3390/batteries9100490
Chang K-F, Panda PK, Hsieh C-T, Yang P-C, Kataria N, Khoo KS. Solid-State Lithium Batteries with Cathode-Supported Composite Solid Electrolytes Enabling High-Rate Capability and Excellent Cyclic Performance. Batteries. 2023; 9(10):490. https://doi.org/10.3390/batteries9100490
Chicago/Turabian StyleChang, Kang-Feng, Pradeep Kumar Panda, Chien-Te Hsieh, Po-Chih Yang, Navish Kataria, and Kuan Shiong Khoo. 2023. "Solid-State Lithium Batteries with Cathode-Supported Composite Solid Electrolytes Enabling High-Rate Capability and Excellent Cyclic Performance" Batteries 9, no. 10: 490. https://doi.org/10.3390/batteries9100490
APA StyleChang, K.-F., Panda, P. K., Hsieh, C.-T., Yang, P.-C., Kataria, N., & Khoo, K. S. (2023). Solid-State Lithium Batteries with Cathode-Supported Composite Solid Electrolytes Enabling High-Rate Capability and Excellent Cyclic Performance. Batteries, 9(10), 490. https://doi.org/10.3390/batteries9100490








