Use of Water-In-Salt Concentrated Liquid Electrolytes in Electrochemical Energy Storage: State of the Art and Perspectives
Abstract
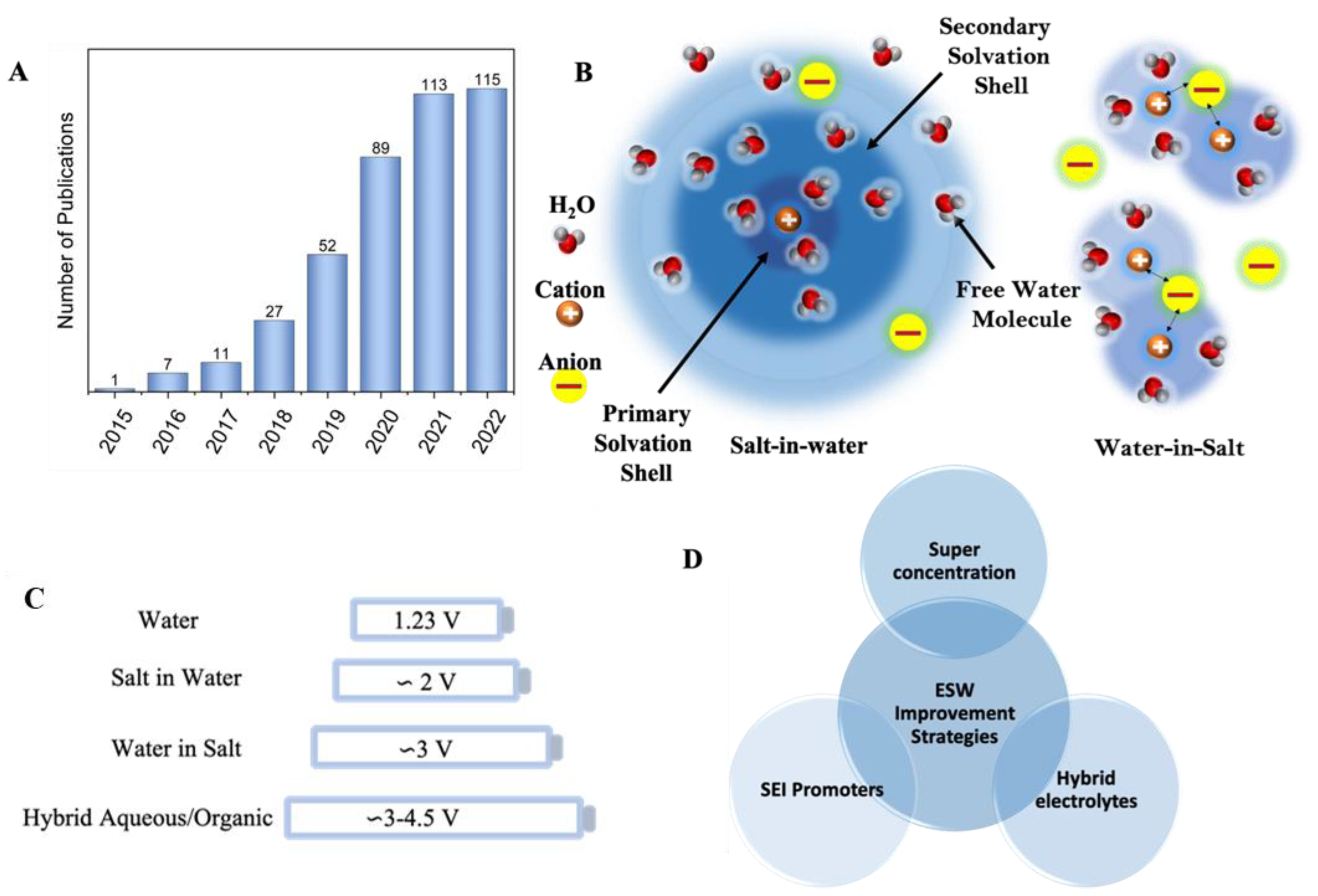
1. Introduction
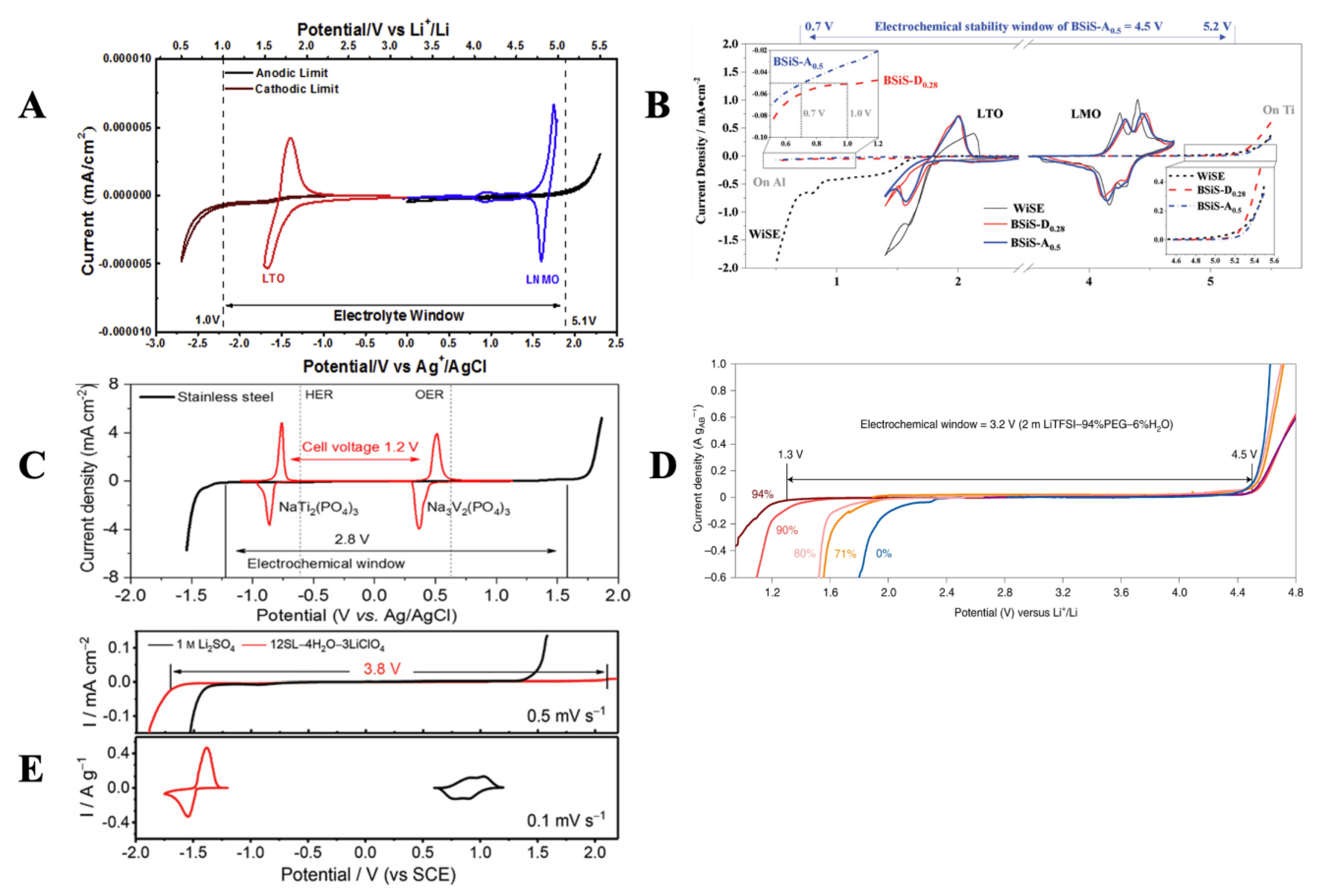
2. Strategies to Improve ESW
2.1. ESW Expansion via SEI Promoters (TFSI, FSI, F-Based Systems)
2.2. ESW Expansion via Water Reduced Activity

2.3. ESW Expansion: The Hybrid Solvents Approach
3. Electrode Interfaces
3.1. Li-Intercalation Systems

3.2. Other Intercalation Chemistries
3.3. Metallic Batteries
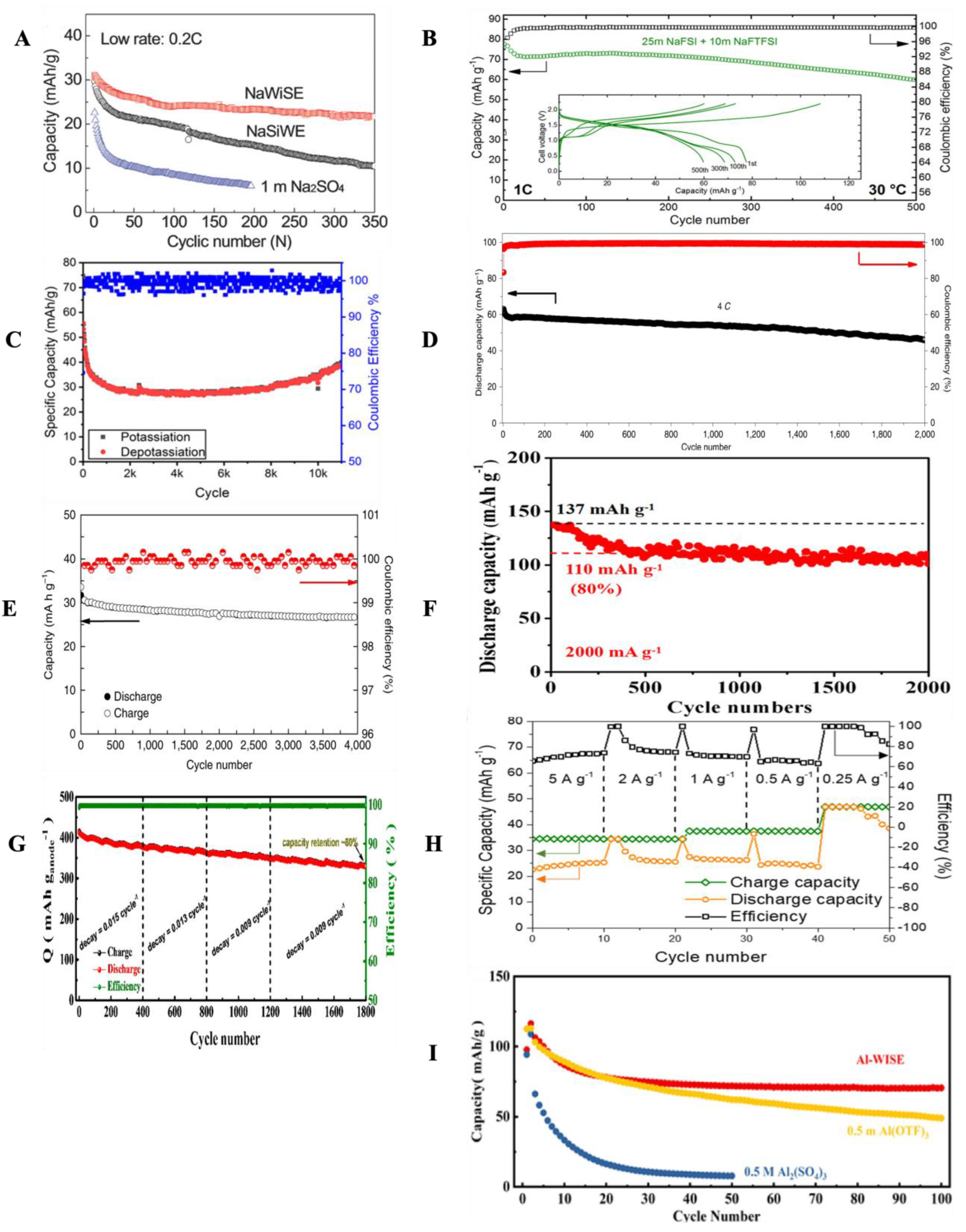
3.4. Supercaps
4. Technological Perspectives
4.1. Portable Electronics (Energy, Safety, Cycles)
4.2. Grid Applications (Quickness, Safety, Cost)
4.3. Power Back-Up (Energy, Quickness, Cost)
4.4. Automotive (Energy/Power Density, Safety, Life Cycle, Cost)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kamat, P.V. Lithium-Ion Batteries and Beyond: Celebrating the 2019 Nobel Prize in Chemistry—A Virtual Issue. ACS Energy Lett. 2019, 4, 2757–2759. [Google Scholar] [CrossRef]
- Xie, J.; Lu, Y.-C. A retrospective on lithium-ion batteries. Nat. Commun. 2020, 11, 2499. [Google Scholar] [CrossRef]
- Vincent, C.A. Lithium batteries: A 50-year perspective, 1959–2009. Solid State Ion. 2000, 134, 159–167. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, L.; Yu, Y.; Sun, J. Progress of enhancing the safety of lithium ion battery from the electrolyte aspect. Nano Energy 2018, 55, 93–114. [Google Scholar] [CrossRef]
- Hou, J.; Yang, M.; Wang, D.; Zhang, J. Fundamentals and Challenges of Lithium Ion Batteries at Temperatures between −40 and 60 °C. Adv. Energy Mater. 2020, 10, 1904152. [Google Scholar] [CrossRef]
- Dey, A.N.; Sullivan, B.P. The Electrochemical Decomposition of Propylene Carbonate on Graphite. J. Electrochem. Soc. 1970, 117, 222–224. [Google Scholar] [CrossRef]
- Peled, E. The Electrochemical Behavior of Alkali and Alkaline Earth Metals in Nonaqueous Battery Systems—The Solid Electrolyte Interphase Model. J. Electrochem. Soc. 1979, 126, 2047–2051. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous Liquid Electrolytes for Lithium-Based Rechargeable Batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar] [CrossRef]
- Kim, H.; Hong, J.; Park, K.-Y.; Kim, H.; Kim, S.-W.; Kang, K. Aqueous Rechargeable Li and Na Ion Batteries. Chem. Rev. 2014, 114, 11788–11827. [Google Scholar] [CrossRef]
- Hu, L.; Xu, K. Nonflammable electrolyte enhances battery safety. Proc. Natl. Acad. Sci. USA 2014, 111, 3205–3206. [Google Scholar] [CrossRef]
- Liang, T.; Hou, R.; Dou, Q.; Zhang, H.; Yan, X. The Applications of Water-in-Salt Electrolytes in Electrochemical Energy Storage Devices. Adv. Funct. Mater. 2021, 31, 2006749. [Google Scholar] [CrossRef]
- Droguet, L.; Grimaud, A.; Fontaine, O.; Tarascon, J. Water-in-Salt Electrolyte (WiSE) for Aqueous Batteries: A Long Way to Practicality. Adv. Energy Mater. 2020, 10, 2002440. [Google Scholar] [CrossRef]
- Mankowski, P.J.; Kanevsky, J.; Bakirtzian, P.; Cugno, S. Cellular phone collateral damage: A review of burns associated with lithium battery powered mobile devices. Burns 2016, 42, e61–e64. [Google Scholar] [CrossRef] [PubMed]
- Ratner, M.A.; Shriver, D.F. Ion transport in solvent-free polymers. Chem. Rev. 1988, 88, 109–124. [Google Scholar] [CrossRef]
- Angell, C.A.; Liu, C.; Sanchez, E. Rubbery solid electrolytes with dominant cationic transport and high ambient conductivity. Nature 1993, 362, 137–139. [Google Scholar] [CrossRef]
- McKinnon, W.R.; Dahn, J.R. How to Reduce the Cointercalation of Propylene Carbonate in Li x ZrS2 and Other Layered Compounds. J. Electrochem. Soc. 1985, 132, 364–366. [Google Scholar] [CrossRef]
- Suo, L.; Borodin, O.; Gao, T.; Olguin, M.; Ho, J.; Fan, X.; Luo, C.; Wang, C.; Xu, K. “Water-in-salt” electrolyte enables high-voltage aqueous lithium-ion chemistries. Science 2015, 350, 938–943. [Google Scholar] [CrossRef]
- Dubouis, N.; Lemaire, P.; Mirvaux, B.; Salager, E.; Deschamps, M.; Grimaud, A. The role of the hydrogen evolution reaction in the solid–electrolyte interphase formation mechanism for “Water-in-Salt” electrolytes. Energy Environ. Sci. 2018, 11, 3491–3499. [Google Scholar] [CrossRef]
- Li, M.; Wang, C.; Chen, Z.; Xu, K.; Lu, J. New Concepts in Electrolytes. Chem. Rev. 2020, 120, 6783–6819. [Google Scholar] [CrossRef]
- Yang, C.; Chen, J.; Qing, T.; Fan, X.; Sun, W.; von Cresce, A.; Ding, M.S.; Borodin, O.; Vatamanu, J.; Schroeder, M.; et al. 4.0 V Aqueous Li-Ion Batteries. Joule 2017, 1, 122–132. [Google Scholar] [CrossRef]
- Suo, L.; Oh, D.; Lin, Y.; Zhuo, Z.; Borodin, O.; Gao, T.; Wang, F.; Kushima, A.; Wang, Z.; Kim, H.-C.; et al. How Solid-Electrolyte Interphase Forms in Aqueous Electrolytes. J. Am. Chem. Soc. 2017, 139, 18670–18680. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.; Zhou, W.; Xie, F.; Ye, C.; Li, H.; Jaroniec, M.; Qiao, S.-Z. Roadmap for advanced aqueous batteries: From design of materials to applications. Sci. Adv. 2020, 6, eaba4098. [Google Scholar] [CrossRef] [PubMed]
- Suo, L.; Borodin, O.; Sun, W.; Fan, X.; Yang, C.; Wang, F.; Gao, T.; Ma, Z.; Schroeder, M.; von Cresce, A.; et al. Advanced High-Voltage Aqueous Lithium-Ion Battery Enabled by “Water-in-Bisalt” Electrolyte. Angew. Chem. Int. Ed. 2016, 55, 7136–7141. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, T.; Kubota, K.; Kojima, H.; Komaba, S. Highly concentrated electrolyte solutions for 4 V class potassium-ion batteries. Chem. Commun. 2018, 54, 8387–8390. [Google Scholar] [CrossRef]
- Li, W.; McKinnon, W.R.; Dahn, J.R. Lithium Intercalation from Aqueous Solutions. J. Electrochem. Soc. 1994, 141, 2310–2316. [Google Scholar] [CrossRef]
- Li, W.; Dahn, J.R.; Wainwright, D.S. Rechargeable Lithium Batteries with Aqueous Electrolytes. Science 1994, 264, 1115–1118. [Google Scholar] [CrossRef]
- Li, W.; Dahn, J.R. Lithium-Ion Cells with Aqueous Electrolytes. J. Electrochem. Soc. 1995, 142, 1742–1746. [Google Scholar] [CrossRef]
- Lux, S.F.; Terborg, L.; Hachmöller, O.; Placke, T.; Meyer, H.-W.; Passerini, S.; Winter, M.; Nowak, S. LiTFSI Stability in Water and Its Possible Use in Aqueous Lithium-Ion Batteries: pH Dependency, Electrochemical Window and Temperature Stability. J. Electrochem. Soc. 2013, 160, A1694–A1700. [Google Scholar] [CrossRef]
- Kulkarni, P.; Ghosh, D.; Balakrishna, R.G. Recent progress in ‘water-in-salt’ and ‘water-in-salt’-hybrid-electrolyte-based high voltage rechargeable batteries. Sustain. Energy Fuels 2021, 5, 1619–1654. [Google Scholar] [CrossRef]
- Becker, M.; Kühnel, R.-S.; Battaglia, C. Water-in-salt electrolytes for aqueous lithium-ion batteries with liquidus temperatures below −10 °C. Chem. Commun. 2019, 55, 12032–12035. [Google Scholar] [CrossRef]
- Reber, D.; Figi, R.; Kühnel, R.-S.; Battaglia, C. Stability of aqueous electrolytes based on LiFSI and NaFSI. Electrochim. Acta 2019, 321, 134644. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Feldblyum, J.I.; Mackanic, D.G.; Lissel, F.; Michels, D.L.; Cui, Y.; Bao, Z. Concentrated mixed cation acetate “water-in-salt” solutions as green and low-cost high voltage electrolytes for aqueous batteries. Energy Environ. Sci. 2018, 11, 2876–2883. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, S.J.; Chang, D.; Kim, J.; Moon, S.; Oh, K.; Park, K.-Y.; Seong, W.M.; Park, H.; Kwon, G.; et al. Toward a low-cost high-voltage sodium aqueous rechargeable battery. Mater. Today 2019, 29, 26–36. [Google Scholar] [CrossRef]
- Han, J.; Zhang, H.; Varzi, A.; Passerini, S. Fluorine-Free Water-in-Salt Electrolyte for Green and Low-Cost Aqueous Sodium-Ion Batteries. Chemsuschem 2018, 11, 3704–3707. [Google Scholar] [CrossRef]
- Stigliano, P.L.; Pianta, N.; Bonizzoni, S.; Mauri, M.; Simonutti, R.; Lorenzi, R.; Vigani, B.; Berbenni, V.; Rossi, S.; Mustarelli, P.; et al. A physico-chemical investigation of highly concentrated potassium acetate solutions towards applications in electrochemistry. Phys. Chem. Chem. Phys. 2021, 23, 1139–1145. [Google Scholar] [CrossRef]
- Khalid, S.; Pianta, N.; Bonizzoni, S.; Mustarelli, P.; Ruffo, R. The properties of highly concentrated aqueous CH3COOK/Na binary electrolyte and its use in sodium-ion batteries. ChemRxiv 2021. [Google Scholar] [CrossRef]
- Wang, F.; Borodin, O.; Ding, M.S.; Gobet, M.; Vatamanu, J.; Fan, X.; Gao, T.; Eidson, N.; Liang, Y.; Sun, W.; et al. Hybrid Aqueous/Non-aqueous Electrolyte for Safe and High-Energy Li-Ion Batteries. Joule 2018, 2, 927–937. [Google Scholar] [CrossRef]
- Chen, J.; Vatamanu, J.; Xing, L.; Borodin, O.; Chen, H.; Guan, X.; Liu, X.; Xu, K.; Li, W. Improving Electrochemical Stability and Low-Temperature Performance with Water/Acetonitrile Hybrid Electrolytes. Adv. Energy Mater. 2020, 10, 1902654. [Google Scholar] [CrossRef]
- Zhang, H.; Qin, B.; Han, J.; Passerini, S. Aqueous/Nonaqueous Hybrid Electrolyte for Sodium-Ion Batteries. ACS Energy Lett. 2018, 3, 1769–1770. [Google Scholar] [CrossRef]
- Xie, J.; Liang, Z.; Lu, Y.-C. Molecular crowding electrolytes for high-voltage aqueous batteries. Nat. Mater. 2020, 19, 1006–1011. [Google Scholar] [CrossRef]
- Liu, J.; Yang, C.; Chi, X.; Wen, B.; Wang, W.; Liu, Y. Water/Sulfolane Hybrid Electrolyte Achieves Ultralow-Temperature Operation for High-Voltage Aqueous Lithium-Ion Batteries. Adv. Funct. Mater. 2022, 32, 2106811. [Google Scholar] [CrossRef]
- Liu, D.; Yuan, L.; Li, X.; Chen, J.; Xiong, R.; Meng, J.; Zhu, S.; Huang, Y. Tuning the Electrolyte Solvation Structure via a Nonaqueous Co-Solvent to Enable High-Voltage Aqueous Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 17585–17593. [Google Scholar] [CrossRef] [PubMed]
- Reber, D.; Kühnel, R.-S.; Battaglia, C. Suppressing Crystallization of Water-in-Salt Electrolytes by Asymmetric Anions Enables Low-Temperature Operation of High-Voltage Aqueous Batteries. ACS Mater. Lett. 2019, 1, 44–51. [Google Scholar] [CrossRef]
- Xiao, D.; Dou, Q.; Zhang, L.; Ma, Y.; Shi, S.; Lei, S.; Yu, H.; Yan, X. Optimization of Organic/Water Hybrid Electrolytes for High-Rate Carbon-Based Supercapacitor. Adv. Funct. Mater. 2019, 29, 1904136. [Google Scholar] [CrossRef]
- Dou, Q.; Lu, Y.; Su, L.; Zhang, X.; Lei, S.; Bu, X.; Liu, L.; Xiao, D.; Chen, J.; Shi, S.; et al. A sodium perchlorate-based hybrid electrolyte with high salt-to-water molar ratio for safe 2.5 V carbon-based supercapacitor. Energy Storage Mater. 2019, 23, 603–609. [Google Scholar] [CrossRef]
- Suo, L.; Han, F.; Fan, X.; Liu, H.; Xu, K.; Wang, C. “Water-in-Salt” electrolytes enable green and safe Li-ion batteries for large scale electric energy storage applications. J. Mater. Chem. A 2016, 4, 6639–6644. [Google Scholar] [CrossRef]
- Wang, F.; Suo, L.; Liang, Y.; Yang, C.; Han, F.; Gao, T.; Sun, W.; Wang, C. Spinel LiNi0.5Mn1.5O4 Cathode for High-Energy Aqueous Lithium-Ion Batteries. Adv. Energy Mater. 2017, 7, 1600922. [Google Scholar] [CrossRef]
- Luo, J.-Y.; Xia, Y.-Y. Aqueous Lithium-ion Battery LiTi2(PO4)3/LiMn2O4 with High Power and Energy Densities as well as Superior Cycling Stability**. Adv. Funct. Mater. 2007, 17, 3877–3884. [Google Scholar] [CrossRef]
- Sun, W.; Suo, L.; Wang, F.; Eidson, N.; Yang, C.; Han, F.; Ma, Z.; Gao, T.; Zhu, M.; Wang, C. “Water-in-Salt” electrolyte enabled LiMn2O4/TiS2 Lithium-ion batteries. Electrochem. Commun. 2017, 82, 71–74. [Google Scholar] [CrossRef]
- Ramanujapuram, A.; Gordon, D.; Magasinski, A.; Ward, B.; Nitta, N.; Huang, C.; Yushin, G. Degradation and stabilization of lithium cobalt oxide in aqueous electrolytes. Energy Environ. Sci. 2016, 9, 1841–1848. [Google Scholar] [CrossRef]
- Wang, L.; Chen, B.; Ma, J.; Cui, G.; Chen, L. Reviving lithium cobalt oxide-based lithium secondary batteries-toward a higher energy density. Chem. Soc. Rev. 2018, 47, 6505–6602. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Wu, X.; Wang, K.; Feng, Z.; Cheng, T.; Liu, Y.; Wang, M.; Chen, R.; Xu, L.; Zhou, J.; et al. An Overview on the Advances of LiCoO 2 Cathodes for Lithium-Ion Batteries. Adv. Energy Mater. 2021, 11, 2000982. [Google Scholar] [CrossRef]
- Chen, Z.; Dahn, J. Methods to obtain excellent capacity retention in LiCoO2 cycled to 4.5 V. Electrochim. Acta 2004, 49, 1079–1090. [Google Scholar] [CrossRef]
- Wang, F.; Lin, Y.; Suo, L.; Fan, X.; Gao, T.; Yang, C.; Han, F.; Qi, Y.; Xu, K.; Wang, C. Stabilizing high voltage LiCoO2 cathode in aqueous electrolyte with interphase-forming additive. Energy Environ. Sci. 2016, 9, 3666–3673. [Google Scholar] [CrossRef]
- Yamada, Y.; Usui, K.; Sodeyama, K.; Ko, S.; Tateyama, Y.; Yamada, A. Hydrate-melt electrolytes for high-energy-density aqueous batteries. Nat. Energy 2016, 1, 16129. [Google Scholar] [CrossRef]
- Suo, L.; Borodin, O.; Wang, Y.; Rong, X.; Sun, W.; Fan, X.; Xu, S.; Schroeder, M.A.; Cresce, A.V.; Wang, F.; et al. “Water-in-Salt” Electrolyte Makes Aqueous Sodium-Ion Battery Safe, Green, and Long-Lasting. Adv. Energy Mater. 2017, 7, 1701189. [Google Scholar] [CrossRef]
- Han, J.; Zarrabeitia, M.; Mariani, A.; Jusys, Z.; Hekmatfar, M.; Zhang, H.; Geiger, D.; Kaiser, U.; Behm, R.; Varzi, A.; et al. Halide-free water-in-salt electrolytes for stable aqueous sodium-ion batteries. Nano Energy 2020, 77, 105176. [Google Scholar] [CrossRef]
- Leonard, D.P.; Wei, Z.; Chen, G.; Du, F.; Ji, X. Water-in-Salt Electrolyte for Potassium-Ion Batteries. ACS Energy Lett. 2018, 3, 373–374. [Google Scholar] [CrossRef]
- Jiang, L.; Lu, Y.; Zhao, C.; Liu, L.; Zhang, J.; Zhang, Q.; Shen, X.; Zhao, J.; Yu, X.; Li, H.; et al. Building aqueous K-ion batteries for energy storage. Nat. Energy 2019, 4, 495–503. [Google Scholar] [CrossRef]
- Liu, T.; Tang, L.; Luo, H.; Cheng, S.; Liu, M. A promising water-in-salt electrolyte for aqueous based electrochemical energy storage cells with a wide potential window: Highly concentrated HCOOK. Chem. Commun. 2019, 55, 12817–12820. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Z.; Wei, Z.; Chen, G.; Yang, X.; Wang, C.; Du, F. Use of a water-in-salt electrolyte to avoid organic material dissolution and enhance the kinetics of aqueous potassium ion batteries. Sustain. Energy Fuels 2019, 4, 128–131. [Google Scholar] [CrossRef]
- Liang, G.; Gan, Z.; Wang, X.; Jin, X.; Xiong, B.; Zhang, X.; Chen, S.; Wang, Y.; He, H.; Zhi, C. Reconstructing Vanadium Oxide with Anisotropic Pathways for a Durable and Fast Aqueous K-Ion Battery. ACS Nano 2021, 15, 17717–17728. [Google Scholar] [CrossRef] [PubMed]
- Ponrouch, A.; Bitenc, J.; Dominko, R.; Lindahl, N.; Johansson, P.; Palacin, M. Multivalent rechargeable batteries. Energy Storage Mater. 2019, 20, 253–262. [Google Scholar] [CrossRef]
- Fang, G.; Zhou, J.; Pan, A.; Liang, S. Recent Advances in Aqueous Zinc-Ion Batteries. ACS Energy Lett. 2018, 3, 2480–2501. [Google Scholar] [CrossRef]
- Wang, F.; Borodin, O.; Gao, T.; Fan, X.; Sun, W.; Han, F.; Faraone, A.; Dura, J.A.; Xu, K.; Wang, C. Highly reversible zinc metal anode for aqueous batteries. Nat. Mater. 2018, 17, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Yan, M.; Zhu, T.; Wang, X.; Wei, X.; Li, J.; Zhou, L.; Li, Z.; Chen, L.; Mai, L. Zn/V2O5 Aqueous Hybrid-Ion Battery with High Voltage Platform and Long Cycle Life. ACS Appl. Mater. Interfaces 2017, 9, 42717–42722. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, Y.; Peng, X.; Dong, S.; Ma, J.; Cui, G.; Chen, L. High-voltage Zn/LiMn0.8Fe0.2PO4 aqueous rechargeable battery by virtue of “water-in-salt” electrolyte. Electrochem. Commun. 2016, 69, 6–10. [Google Scholar] [CrossRef]
- Zhang, C.; Holoubek, J.; Wu, X.; Daniyar, A.; Zhu, L.; Chen, C.; Leonard, D.P.; Rodríguez-Pérez, I.A.; Jiang, J.-X.; Fang, C.; et al. A ZnCl2 water-in-salt electrolyte for a reversible Zn metal anode. Chem. Commun. 2018, 54, 14097–14099. [Google Scholar] [CrossRef]
- Lee, C.; Jeong, S.-K. A Novel Superconcentrated Aqueous Electrolyte to Improve the Electrochemical Performance of Calcium-ion Batteries. Chem. Lett. 2016, 45, 1447–1449. [Google Scholar] [CrossRef]
- Adil; Ghosh, A.; Mitra, S. Water-in-Salt Electrolyte-Based Extended Voltage Range, Safe, and Long-Cycle-Life Aqueous Calcium-Ion Cells. ACS Appl. Mater. Interfaces 2022, 14, 25501–25515. [Google Scholar] [CrossRef]
- Leong, K.W.; Pan, W.; Wang, Y.; Luo, S.; Zhao, X.; Leung, D.Y.C. Reversibility of a High-Voltage, Cl–-Regulated, Aqueous Mg Metal Battery Enabled by a Water-in-Salt Electrolyte. ACS Energy Lett. 2022, 7, 2657–2666. [Google Scholar] [CrossRef]
- Zhou, A.; Jiang, L.; Yue, J.; Tong, Y.; Zhang, Q.; Lin, Z.; Liu, B.; Wu, C.; Suo, L.; Hu, Y.-S.; et al. Water-in-Salt Electrolyte Promotes High-Capacity FeFe(CN)6 Cathode for Aqueous Al-Ion Battery. ACS Appl. Mater. Interfaces 2019, 11, 41356–41362. [Google Scholar] [CrossRef]
- Zhang, M.; Makino, S.; Mochizuki, D.; Sugimoto, W. High-performance hybrid supercapacitors enabled by protected lithium negative electrode and “water-in-salt” electrolyte. J. Power Sources 2018, 396, 498–505. [Google Scholar] [CrossRef]
- Bu, X.; Su, L.; Dou, Q.; Lei, S.; Yan, X. A low-cost “water-in-salt” electrolyte for a 2.3 V high-rate carbon-based supercapacitor. J. Mater. Chem. A 2019, 7, 7541–7547. [Google Scholar] [CrossRef]
- Tribbia, M.; Pianta, N.; Brugnetti, G.; Lorenzi, R.; Ruffo, R. A new double layer super-capacitor made by free-standing activated carbon membranes and highly concentrated potassium acetate solutions. Electrochim. Acta 2020, 364, 137323. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Shen, Z. “Water-in-salt” electrolyte enhanced high voltage aqueous supercapacitor with all-pseudocapacitive metal-oxide electrodes. J. Power Sources 2019, 414, 479–485. [Google Scholar] [CrossRef]
- Guo, J.; Ma, Y.; Zhao, K.; Wang, Y.; Yang, B.; Cui, J.; Yan, X. High-Performance and Ultra-Stable Aqueous Supercapacitors Based on a Green and Low-Cost Water-In-Salt Electrolyte. Chemelectrochem 2019, 6, 5433–5438. [Google Scholar] [CrossRef]
- Zhang, L.; Rodríguez-Pérez, I.A.; Jiang, H.; Zhang, C.; Leonard, D.P.; Guo, Q.; Wang, W.; Han, S.; Wang, L.; Ji, X. ZnCl 2 “Water-in-Salt” Electrolyte Transforms the Performance of Vanadium Oxide as a Zn Battery Cathode. Adv. Funct. Mater. 2019, 29, 1902653. [Google Scholar] [CrossRef]
- Pan, W.; Wang, Y.; Zhang, Y.; Kwok, Y.H.; Wu, M.; Zhao, X.; Leung, D.Y.C. A low-cost and dendrite-free rechargeable aluminium-ion battery with superior performance. J. Mater. Chem. A 2019, 7, 17420–17425. [Google Scholar] [CrossRef]
- Choi, J.W.; Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1, 16013. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, C.Z.; Yuan, H.; Chen, Y.; Zhang, W.; Huang, J.Q.; Yu, D.; Liu, Y.; Titirici, M.M.; Chueh, Y.L.; et al. A review of rechargeable batteries for portable electronic devices. InfoMat 2019, 1, 6–32. [Google Scholar] [CrossRef]
- Köhler, U.; Antonius, C.; Bäuerlein, P. Advances in alkaline batteries. J. Power Sources 2004, 127, 45–52. [Google Scholar] [CrossRef]
- Yoshino, A. The Birth of the Lithium-Ion Battery. Angew. Chem. Int. Ed. 2012, 51, 5798–5800. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.W.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical Energy Storage for Green Grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Choi, J.W. Opportunities and Reality of Aqueous Rechargeable Batteries. Adv. Energy Mater. 2020, 10, 2001386. [Google Scholar] [CrossRef]
- Fan, X.; Liu, B.; Liu, J.; Ding, J.; Han, X.; Deng, Y.; Lv, X.; Xie, Y.; Chen, B.; Hu, W.; et al. Battery Technologies for Grid-Level Large-Scale Electrical Energy Storage. Trans. Tianjin Univ. 2020, 26, 92–103. [Google Scholar] [CrossRef]
- Bashir, N.; Sardar, H.S.; Nasir, M.; Hassan, N.U.; Khan, H.A. Lifetime maximization of lead-acid batteries in small scale UPS and distributed generation systems. In Proceedings of the 2017 IEEE Manchester PowerTech, Manchester, UK, 18–22 June 2017. [Google Scholar] [CrossRef]
- EV Battery Supply Chain: Trends, Risks and Opportunities in a Fast-Evolving Sector. Available online: https://store.fitchsolutions.com/all-products/ev-battery-supply-chain-trends-risks-and-opportunities-in-a-fast-evolving-sector (accessed on 29 November 2022).
- Bhutada, G. Breaking Down the Cost of an EV Battery Cell. Visual Capitalist. 2022. Available online: https://www.visualcapitalist.com/breaking-down-the-cost-of-an-ev-battery-cell/ (accessed on 6 November 2022).
- Houache, M.S.E.; Yim, C.-H.; Karkar, Z.; Abu-Lebdeh, Y. On the Current and Future Outlook of Battery Chemistries for Electric Vehicles—Mini Review. Batteries 2022, 8, 70. [Google Scholar] [CrossRef]




 [75],
[75],  [67],
[67],  [17],
[17],  [46],
[46],  [49],
[49],  ,
,  [77].
[77].
 [75],
[75],  [67],
[67],  [17],
[17],  [46],
[46],  [49],
[49],  ,
,  [77].
[77].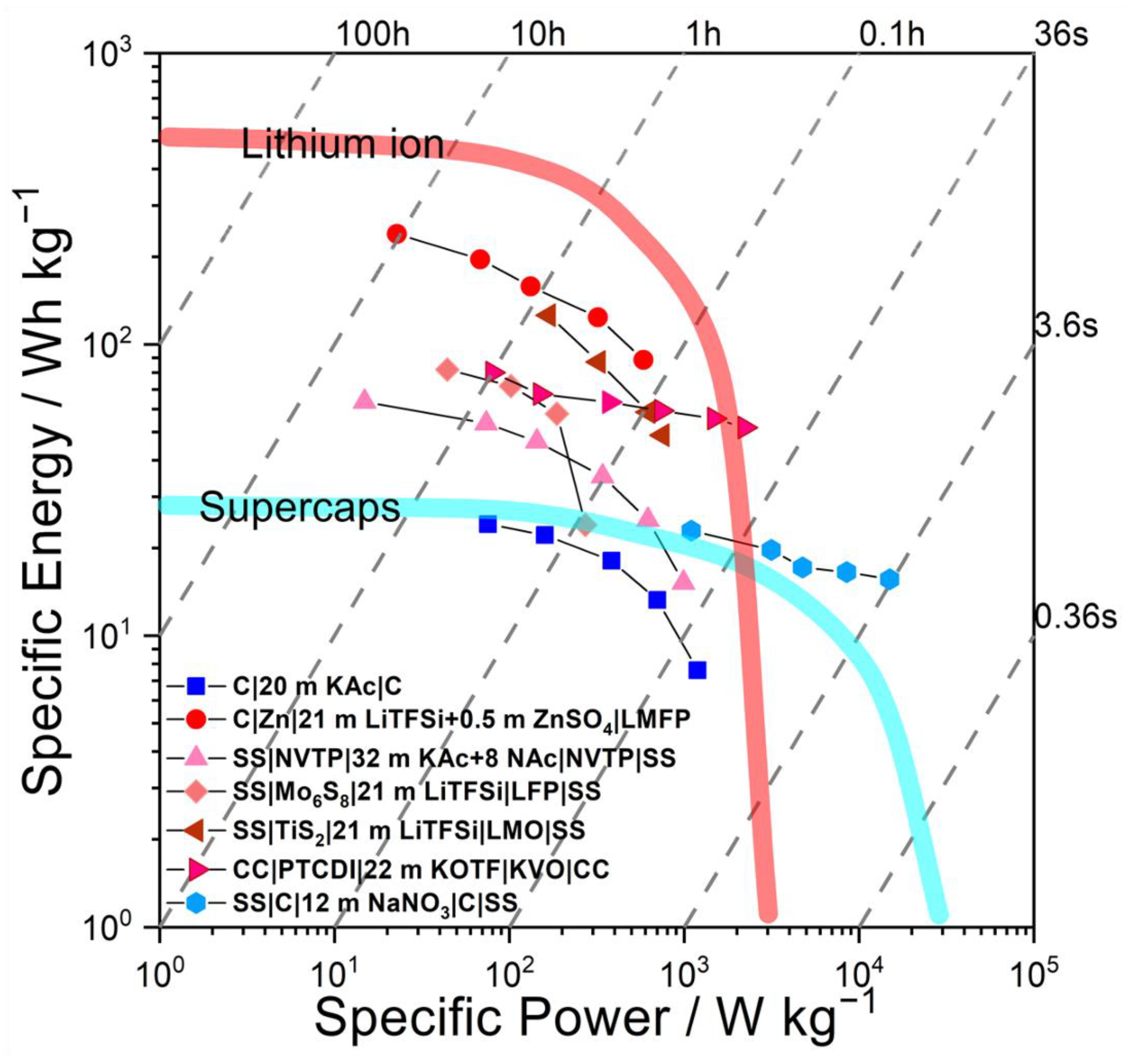
| NFPA 704 Ratings * | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Electrolyte | ESW (V) | Ionic Conductivity (mS cm−1) | Voltage Range vs. (Li+/Li) | Current Collectors | Ref. | Cost ($ g−1) | Health | Instability/ Reactivity | Flammability |
| 21 m LiTFSI | 3 | 10 | 1.9–4.9 | Stainless Steel | [17] | 3.5 | 3 | 0 | 1 |
| 35 m Lise | ∼2.5 | ∼11.2 | −2.4–4.97 | Au | [31] | 16.2 | 3 | 0 | 1 |
| 21 m LiTFSI + 7 m LiOTF | 3.07 | ∼4.4 | 1.83–4.9 | Stainless stee | [23] | 3.3 | 3 | 0 | 1 |
| 32 m KOAc + 8 m LiOAc | 2.7 | 5.3 | 1.97–4.7 | Pt | [32] | 0.32 | 2 | 1 | 1 |
| 32 m KAc + 8 m NaAc | >3 | 27 | n.a. | Al/Ti | [34] | 0.23 | 1 | 1 | 1 |
| 25 m NaFSI +10 m NaTFSI | 2.7 | 11.8 | n.a. | n.a. | [43] | 68.2 | 3 | 0 | 1 |
| LiTFSI/H2O/(ACN)3.5 | 3.26 | 13.2 | n.a. | Stainless steel | [44] | 0.92 | 3 | 0 | 0 |
| 21 m LiTFSI-H2O/9.25 m LiTFSI in DMC (1:1) | 4 | 3 | 1–5.1 | Al/Ti | [37] | 3.5 | 3 | 0 | 0 |
| 8 m NaClO4/(H2O)1.5 (ACN)2.4 | 3.16 | 42.1 | 1.81–4.95 | Stainless steel | [45] | 0.54 | 2 | 1 | 0 |
| LiTFSI + 94%PEG + 6%H2O | 3.2 | 0.8 | 1.3–4.5 | Coated Al | [40] | 2.37 | 3 | 0 | 0 |
| 12SL–4H2O–3LiClO4 | 3.8 | n.a. | 1.68–5.08 | Ti | [41] | 0.64 | 3 | 2 | 1 |
| SL:H2O:LiTFSI (1.3:0.9:1) | 5.1 | 0.8 | 1–5 | Al/Ti | [42] | 1.53 | 3 | 0 | 0 |
| 7 m NaOTF (H2O) + 8 m NaOTF (PC) | 2.8 | ∼25 | 2.02–4.77 | [39] | n.a. | 1 | 1 | 0 | |
| Electrode Couple | Electrolyte | ESW (V) | Collector Used | Cell Voltage (V) | Specific Energy (Wh kg−1) | Performance: Capacity Retention (CR) Capacity Decay (CD) | Ref. |
|---|---|---|---|---|---|---|---|
| LiMn2O4/Mo6S8 | 21 m LiTFSI | 3 | Stainless steel | 2.3 | 100 | 68% CR at 4.5 C over 1000 cycles | [17] |
| LiMn2O4/C–TiO2 | 21 m LiTFSI + 7 m LiOTF | 3.07 | Stainless steel | 2.1 | 100 | 40 mAhg−1 after 100 cycles | [23] |
| LiMn2O4/C–TiO2 | 32 m KOAc + 8 m LiOAc | 3 | Ti | 2.5 | n.a. | 90% CR after 100 cycles | [32] |
| LiNi0.5Mn1.5O4/Mo6S8 | 21 m LiTFSI | 4 | 3 | 2.9 | 126 | n.a. | [47] |
| LiNi0.5Mn1.5O4/Li4Ti5O12 | 14 M LiTFSI + DMC | 4.1 | Al/Ti | 3.2 | 165 | 0.024% CD for 1000 cycles (6 C) | [37] |
| Li4Ti5O12/LiMn2O4 | LiTFSI + 94%PEG + 6%H2O | 3.2 | Coated Al | 2.25 | 110 | 68% CR after 300 cycles. | [40] |
| NaTi2(PO4)3/Na3(VOPO4)2F | 25 m NaFSI +10 m NaTFSI | 2.7 | n.a. | 2 | 64 | 77% CR after 500 cycles at 1 C | [43] |
| Na0.66[Mn0.66Ti0.34]O2/NaTi2(PO4)3 | 9.26 m NaCF3SO3 | >2.5 | Stainless steel | 1 | 31 | 92.7% CR after 1200 cycles | [56] |
| Na3V2(PO4)3/NaTi2(PO4)3 | 7 m NaOTF (H2O) and 8 m NaOTF (propylene carbonate) | 2.8 | n.a. | 1.2 | 45 | 20 mA h g−1 after 150 cycles (10 C) | [39] |
| Na4Fe3(PO4)2(P2O7)/NaTi2(PO4)3 | 17 m NaClO4 | 2.7 | n.a. | 2.0 | 36 | 65 mA h g−1 after 200 cycles (1 C) | [33] |
| Zn/LiMn2O4 hybrid battery | 1 m Zn(TFSI)2 +20 m LiTFSI | n.a | n.a | 1.4 | 70 | 85% CR after 4000 cycles | [65] |
| Zn/LiMn0.8Fe0.2PO4 | 21 m of LiTFSI and 0.5 m of ZnSO4 | 3.7 | Stainless steel | 1.8 | 183 | 110 mA h g−1 after 150 cycles (0.3 C) | [67] |
| Zn/Ca0.20V2O5·0.8H2O | 30 m ZnCl2 | 1.8 | n.a. | 1.8 | 206 | 70% CR over 1000 cycles (1.6 A g−1) | [78] |
| Al/graphite | AlCl3·6H2O | 4 | n..a | 1.4 | 220 | 99% CR after 1000 cycles | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalid, S.; Pianta, N.; Mustarelli, P.; Ruffo, R. Use of Water-In-Salt Concentrated Liquid Electrolytes in Electrochemical Energy Storage: State of the Art and Perspectives. Batteries 2023, 9, 47. https://doi.org/10.3390/batteries9010047
Khalid S, Pianta N, Mustarelli P, Ruffo R. Use of Water-In-Salt Concentrated Liquid Electrolytes in Electrochemical Energy Storage: State of the Art and Perspectives. Batteries. 2023; 9(1):47. https://doi.org/10.3390/batteries9010047
Chicago/Turabian StyleKhalid, Shahid, Nicolò Pianta, Piercarlo Mustarelli, and Riccardo Ruffo. 2023. "Use of Water-In-Salt Concentrated Liquid Electrolytes in Electrochemical Energy Storage: State of the Art and Perspectives" Batteries 9, no. 1: 47. https://doi.org/10.3390/batteries9010047
APA StyleKhalid, S., Pianta, N., Mustarelli, P., & Ruffo, R. (2023). Use of Water-In-Salt Concentrated Liquid Electrolytes in Electrochemical Energy Storage: State of the Art and Perspectives. Batteries, 9(1), 47. https://doi.org/10.3390/batteries9010047









