Capacity Fading Rules of Lithium-Ion Batteries for Multiple Thermoelectric Aging Paths
Abstract
:1. Introduction
2. Setup of Aging Paths for the Lithium-Ion Battery
3. Experimental Scheme and Platform
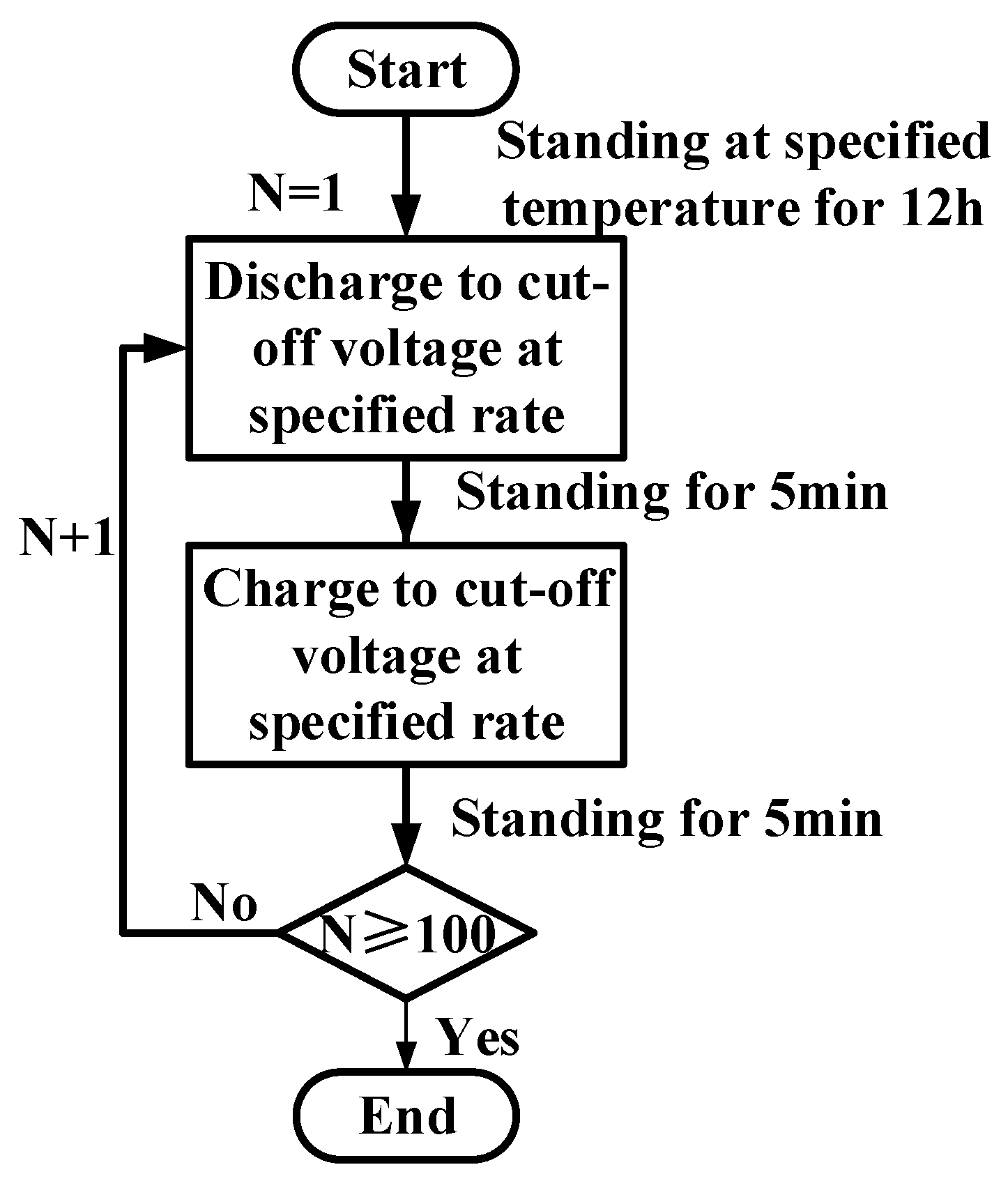
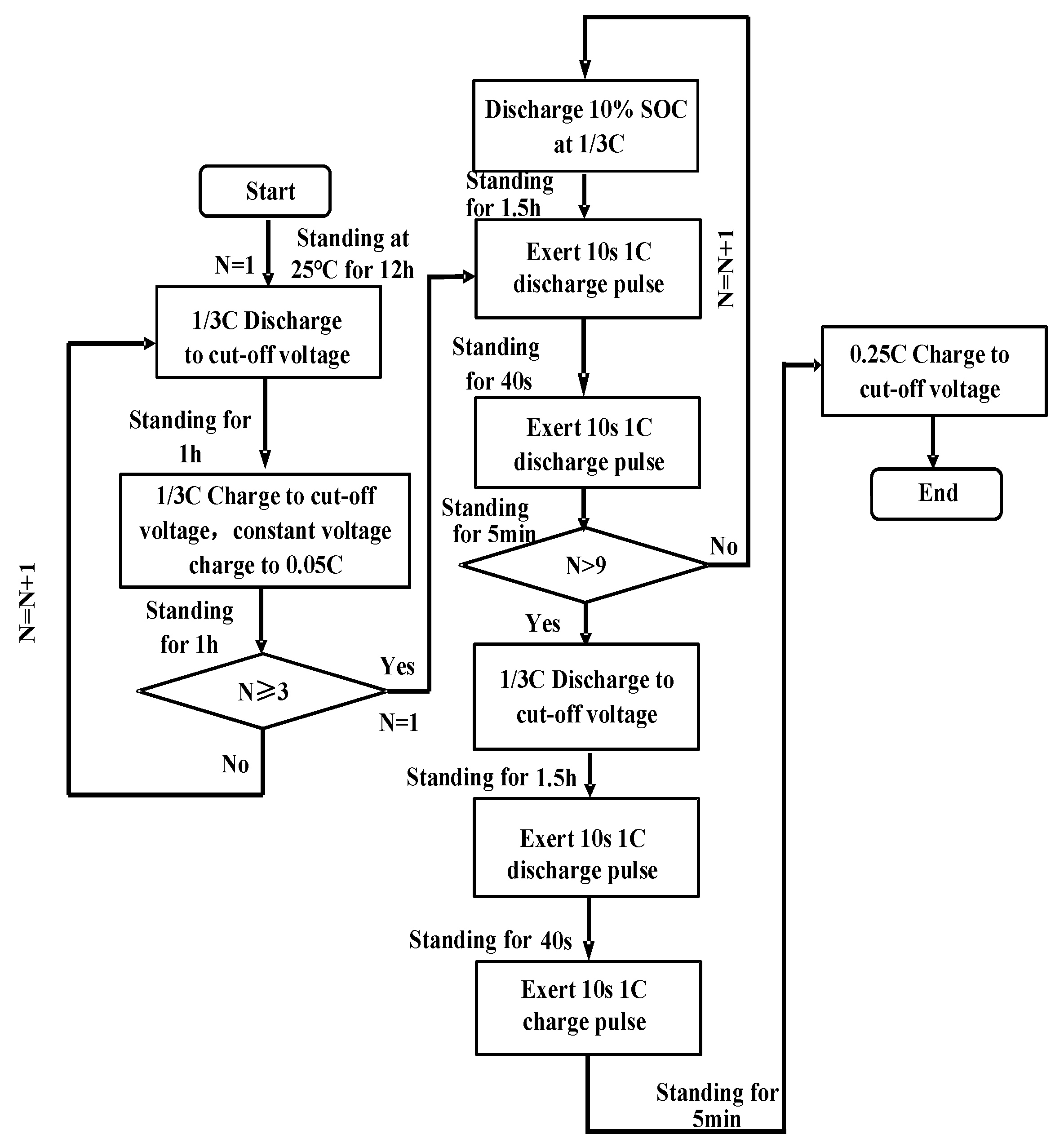
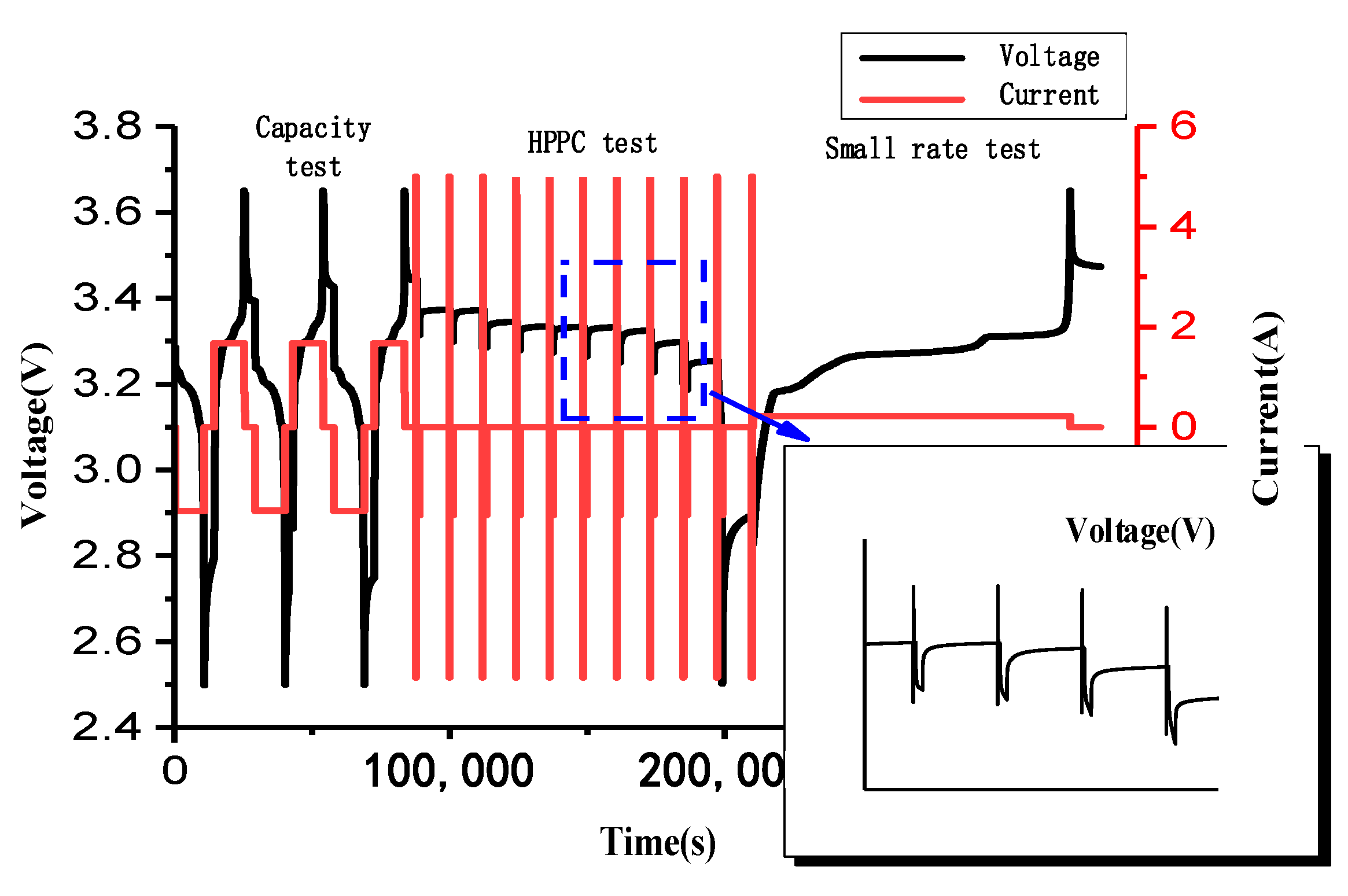
3.1. Basic Performance Tests of the Battery
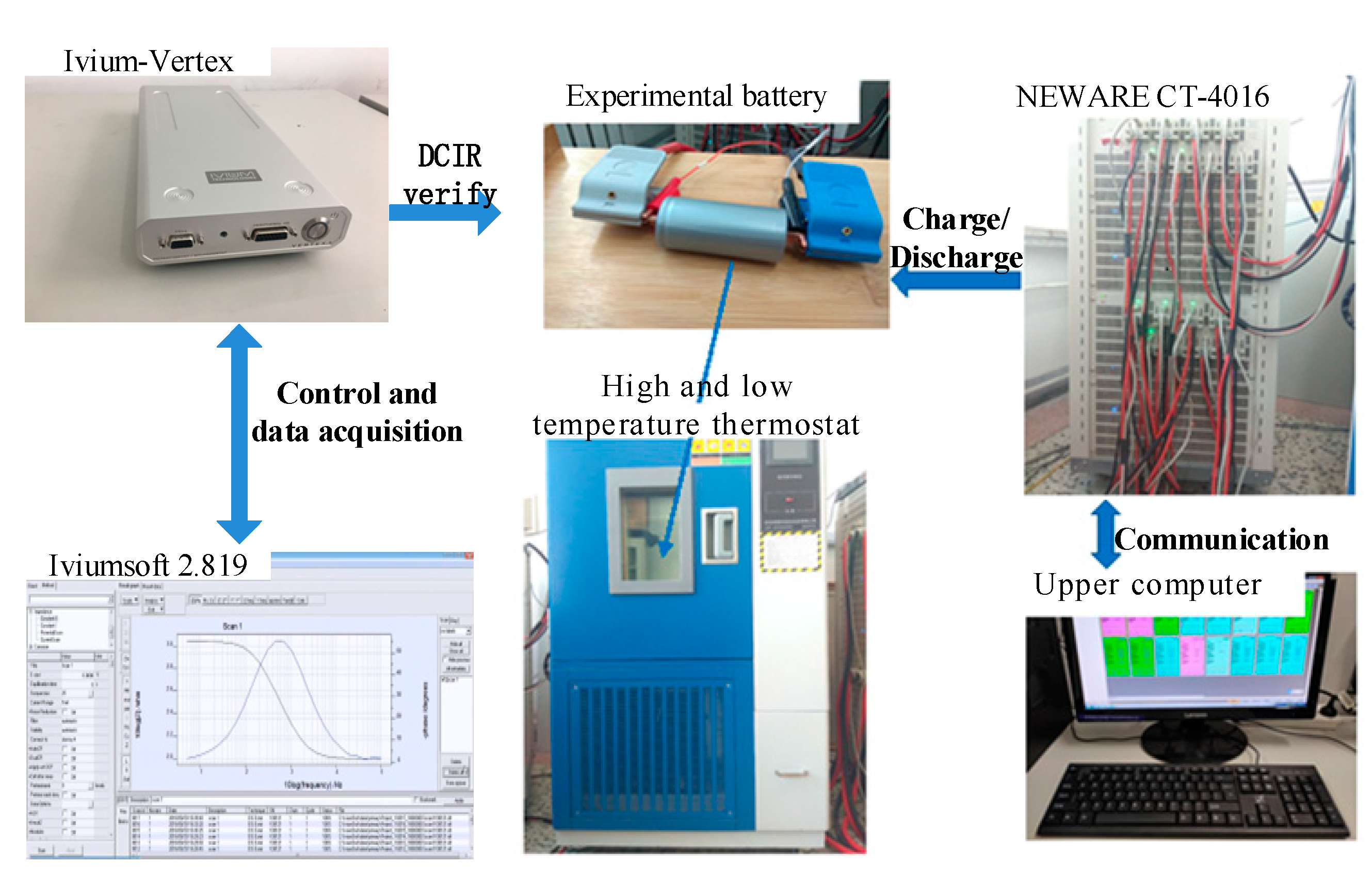
3.2. Construction of the Experimental Platform
4. Analysis of the Aging Characteristics
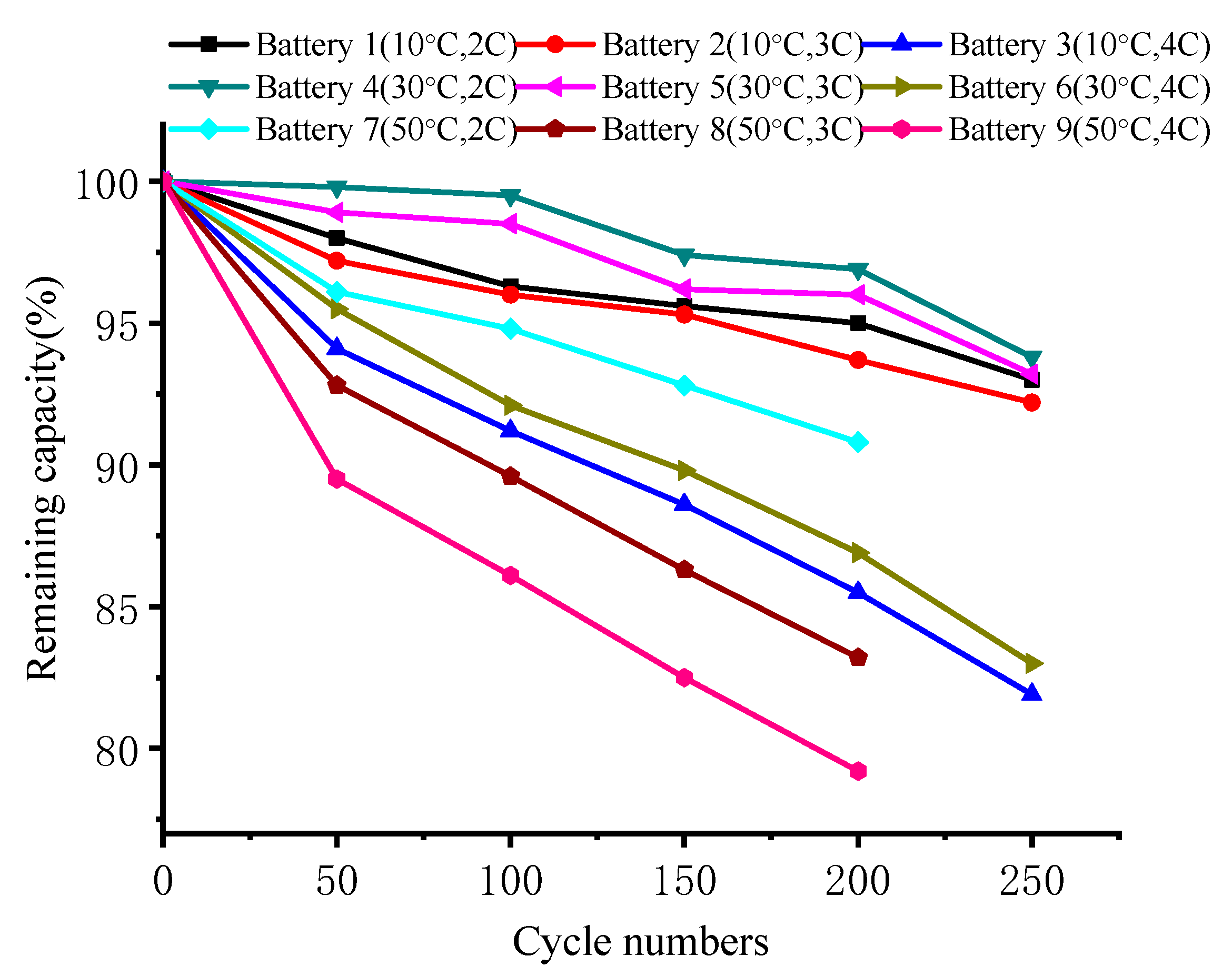
4.1. Capacity Analysis
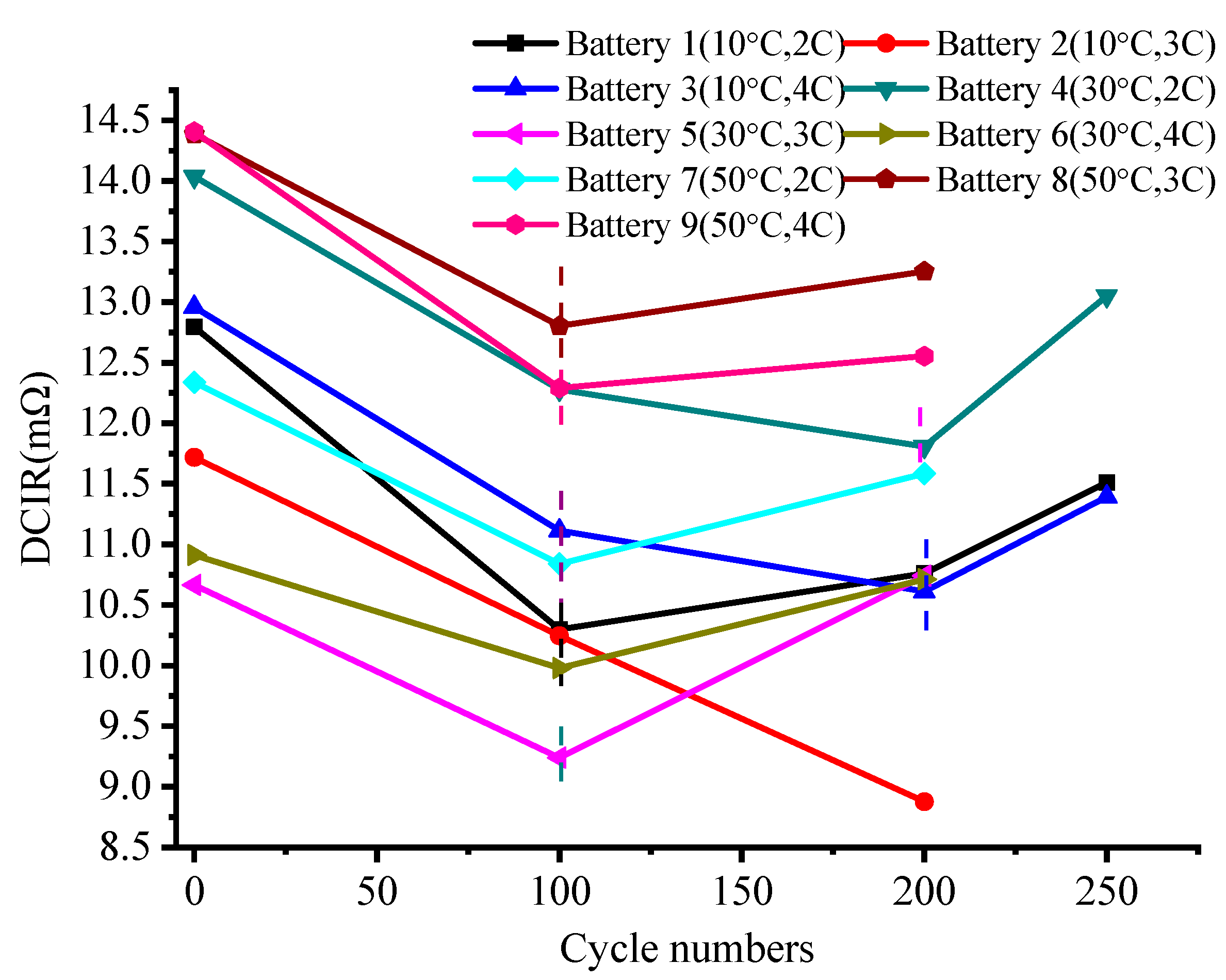
4.2. Impedance Analysis
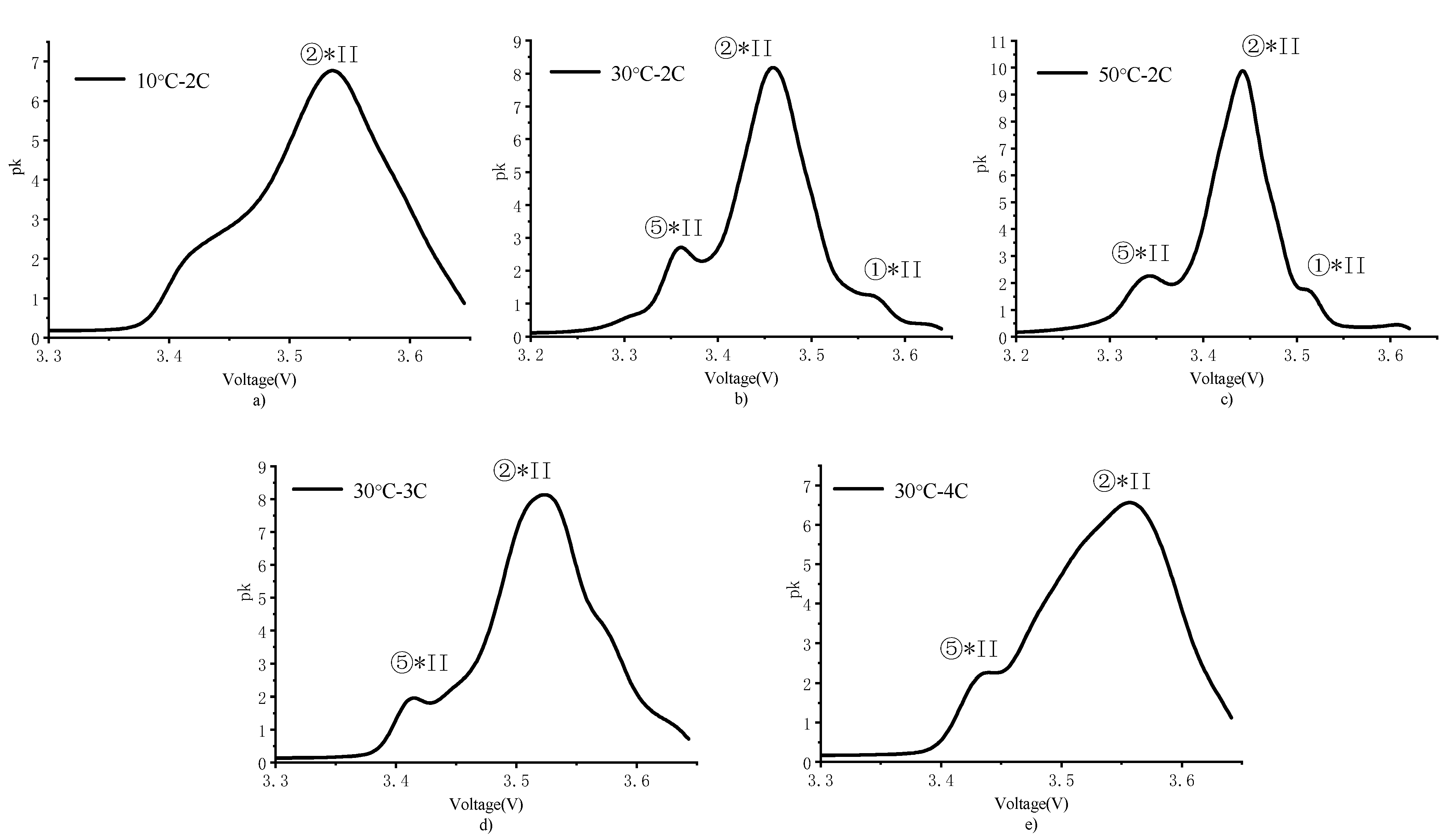
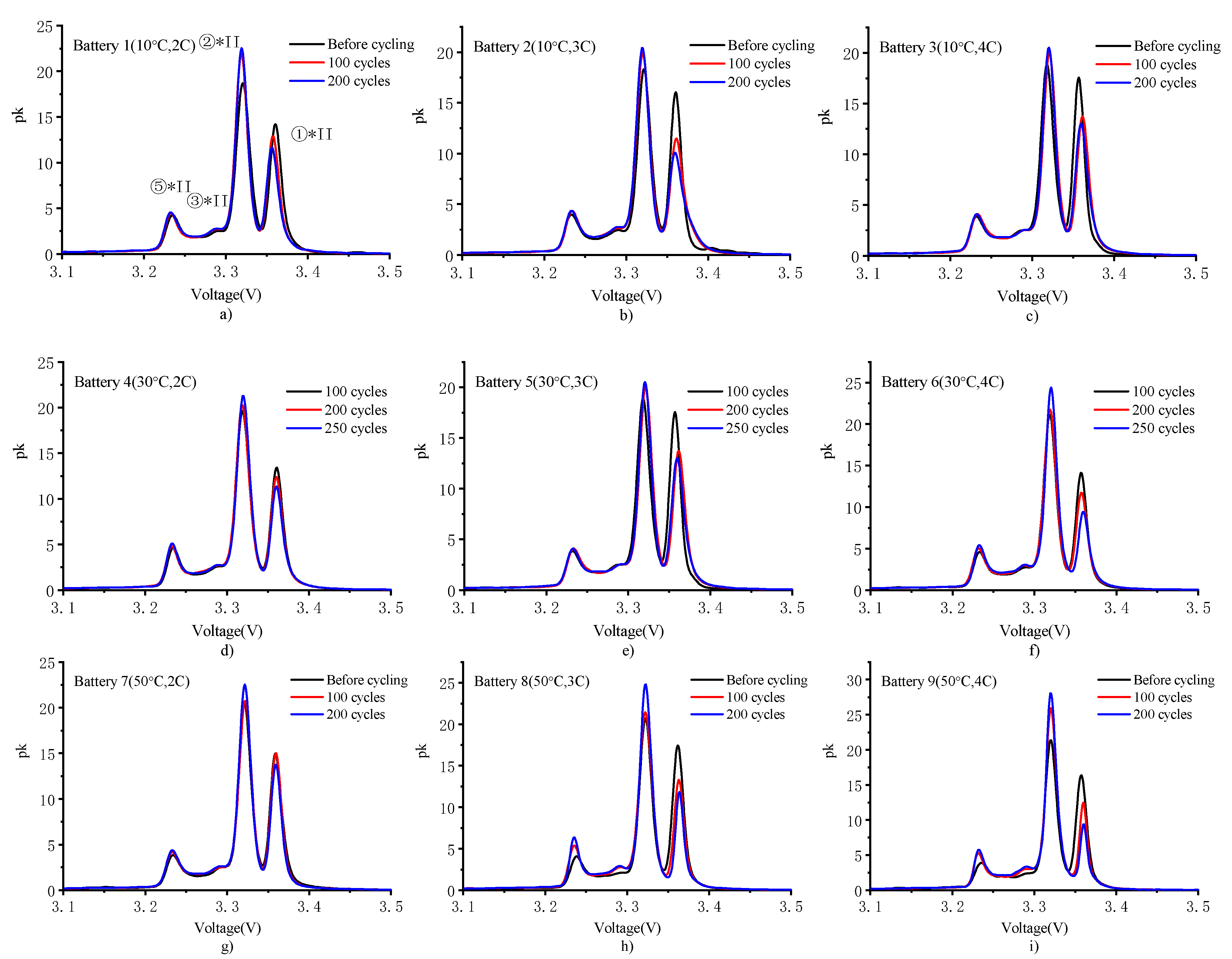
4.3. PDF Analysis
5. Rules for Capacity Deterioration in Different Aging Paths
6. Conclusions
- (1)
- Charge–discharge cycle conditions away from room temperature accelerate the capacity deterioration of the battery. The analysis of the impedance parameters showed that this type of battery breaks and rebuilds the SEI layer at the negative electrode at the beginning of the cycle. This causes the impedance to temporarily decrease, then rise. The change in charge transfer impedance indicates that the cathode material maintains a relatively stable performance for charge–discharge cycles at high temperatures.
- (2)
- The PDF analysis results indicated that the main aging mechanism for high charging rates was not the increase in impedance but the loss of active lithium in the battery. High temperature is beneficial to reduce polarization but aggravates side reactions. The increase in the number of cycles delays the phase change reaction to voltage response, while the phase change reaction involves the acceleration of capacity attenuation.
- (3)
- Compared with the traditional ICA method, the PDF analysis method is less influenced by noise, has no need for complex function fitting, does not result in the ∆V = 0 condition, requires minimal calculation, and uses data that are easy to acquire. However, it has a few limitations: there is a gap between the calculated and actual changes in capacity, and a lack of data on the charging and discharging process can cause drawbacks, such as peak distortion. This method is not recommended when high accuracy is required for the peak voltage or when the charging and discharging process is extremely short.
- (1)
- In terms of aging path design, there are many coupling effects of factors that need to be studied, such as different charging and discharging cycles under different SOCs, different charging modes, and calendar-aging modes (standing). Additional coupling factors should be considered in subsequent studies.
- (2)
- To verify the universality of the aging mechanism of LiFePO4 power batteries, more aging LiFePO4 power batteries should be investigated in subsequent studies.
- (3)
- In future studies, more electrochemical characterization methods should be attempted to elucidate the aging mechanism.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, K.; Qiao, Y.; Xie, L.; Li, J.; Lu, Z.; Yang, H. A fuzzy hierarchical strategy for improving frequency regulation of battery energy storage system. J. Mod. Power Syst. Clean Energy 2021, 9, 689–698. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, L.; Cheng, H.; Liu, D.; Zhang, J.; Li, G. Frequency-constrained Co-planning of Generation and Energy Storage with High-penetration Renewable Energy. J. Mod. Power Syst. Clean Energy 2021, 9, 760–775. [Google Scholar] [CrossRef]
- Martinez-Rico, J.; Zulueta, E.; de Argandoña, I.R.; Fernandez-Gamiz, U.; Armendia, M. Multi-objective optimization of production scheduling using particle swarm optimization algorithm for hybrid renewable power plants with battery energy storage system. J. Mod. Power Syst. Clean Energy 2020, 9, 285–294. [Google Scholar] [CrossRef]
- Aatif, S.; Yang, X.; Hu, H.; Maharjan, S.K.; He, Z. Integration of PV and battery storage for catenary voltage regulation and stray current mitigation in MVDC railways. J. Mod. Power Syst. Clean Energy 2020, 9, 585–594. [Google Scholar] [CrossRef]
- Teixeira, T.P.; Borges, C.L.T. Operation strategies for coordinating battery energy storage with wind power generation and their effects on system reliability. J. Mod. Power Syst. Clean Energy 2020, 9, 190–198. [Google Scholar] [CrossRef]
- Gomes, I.S.F.; Perez, Y.; Suomalainen, E. Coupling small battery and PV generation: A review. Renew. Sustain. Energy Rev. 2020, 126, 109835. [Google Scholar] [CrossRef]
- Pozzi, A.; Torchio, M.; Braatz, R.D.; Raimondo, D.M. Optimal charging of an electric vehicle battery pack: A real-time sensitivity-based model predictive control approach. J. Power Sources 2020, 461, 228133. [Google Scholar] [CrossRef]
- Sufyan, M.; Rahim, N.A.; Muhammad, M.A.; Tan, C.K.; Raihan, S.R.S.; Bakar, A.H.A. Charge coordination and battery lifecycle analysis of electric vehicles with V2G implementation. Electr. Power Syst. Res. 2020, 184, 106307. [Google Scholar] [CrossRef]
- Liu, T.; Yang, X.-G.; Ge, S.; Leng, Y.; Wang, C.-Y. Ultrafast charging of energy-dense lithium-ion batteries for urban air mobility. eTransportation 2021, 7, 100103. [Google Scholar] [CrossRef]
- Wang, K.; Wan, J.J.; Xiang, Y.X.; Zhu, J.P.; Leng, Q.Y.; Wang, M.; Xu, L.M.; Yang, Y. Recent advances and historical developments of high voltage lithium cobalt oxide materials for rechargeable Li-ion battery. J. Power Sources 2020, 460, 228062. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, Y.Z.; Guo, B.J.; Zhu, C.; Zhou, X.; Wang, L.; Cao, J. A novel quantitative electrochemical aging model considering side reactions for lithium-ion battery. Electrochim. Acta 2020, 343, 136070. [Google Scholar] [CrossRef]
- O’Kan, S.E.J.; Campbell, I.D.; Marzook, M.W.J.; Offer, G.J.; Marinescu, M. Physical origin of the differential voltage minimum associated with lithium plating in Li-ion battery. J. Electrochem. Soc. 2020, 167, 090540. [Google Scholar] [CrossRef]
- Li, S.T.; Meng, Q.H.; Fan, M.S.; Yang, K.; Tian, G.Y. Analysis for performance degradation mechanisms of the retired LiFePO4/graphite power cells. Ionics 2020, 26, 4443–4454. [Google Scholar] [CrossRef]
- Xie, X.Y.; Wang, L.; Feng, X.N.; Ren, D.S.; He, X.M. High-temperature aging behavior of commercial Li-ion battery. Int. J. Electrochem. Sci. 2020, 15, 4586–4591. [Google Scholar] [CrossRef]
- Zilberman, I.; Ludwig, S.; Schiller, M.; Jossen, A. Online aging determination in lithium-ion battery module with forced temperature gradient. J. Energy Storage 2020, 28, 101170. [Google Scholar] [CrossRef]
- Naumann, M.; Spingler, F.B.; Jossen, A. Analysis and modeling of cycle aging of a commercial LiFePO4/graphite cell. J. Power Sources 2020, 451, 227666. [Google Scholar] [CrossRef]
- Zhu, J.G.; Darma MS, D.; Knapp, M.; Soresen, D.R.; Heere, M.; Fang, Q.H.; Wang, X.Y.; Dai, H.F.; Mereacre, L.; Senyshyn, A. Investigation of lithium-ion battery degradation mechanisms by combining differential voltage analysis and alternating current impedance. J. Power Sources 2020, 448, 227575. [Google Scholar] [CrossRef]
- Heins, T.P.; Schluter, N.; Ernst, S.T.; Schroder, U. On the interpretation of impedance spectra of large-format lithium-ion battery and its application in aging studies. Energy Technol. 2020, 8, 1900279. [Google Scholar] [CrossRef]
- Rumberg, B.; Epding, B.; Stradtmann, I.; Kwade, A. Identification of Li ion battery cell aging mechanisms by half-cell and full-cell open-circuit-voltage characteristic analysis. J. Energy Storage 2019, 25, 100890. [Google Scholar] [CrossRef]
- Fath, J.P.; Dragicevic, D.; Bittel, L.; Nuhic, A.; Sieg, J.; Hahn, S.; Alsheimer, L.; Spier, B.; Wetzel, T. Quantification of aging mechanisms and inhomogeneity in cycled lithium-ion cells by differential voltage anlysis. J. Energy Storage 2019, 25, 100813. [Google Scholar] [CrossRef]
- López-Ibarra, J.A.; Goitia-Zabaleta, N.; Herrera, V.I.; Gaztanaga, H.; Camblong, H. Battery aging conscious intelligent energy management strategy and sensitivity analysis of the critical factors for plug-in hybrid electric buses. eTransportation 2020, 5, 100061. [Google Scholar] [CrossRef]
- Yang, H.; Wang, P.; An, Y.; Shi, C.; Sun, X.; Wang, K.; Zhang, X.; Wei, T.; Ma, Y. Remaining useful life prediction based on denoising technique and deep neural network for lithium-ion capacitors. eTransportation 2020, 5, 100078. [Google Scholar] [CrossRef]
- Feng, X.; Merla, Y.; Weng, C.; Ouyang, M.; He, X.; Liaw, B.Y.; Santhanagopalan, S.; Li, X.; Liu, P.; Lu, L.; et al. A reliable approach of differentiating discrete sampled-data for battery diagnosis. eTransportation 2020, 3, 100051. [Google Scholar] [CrossRef]
- Yu, W.; Guo, Y.; Shang, Z.; Zhang, Y.; Xu, S. A review on comprehensive recycling of spent power lithium-ion battery in China. eTransportation 2022, 100155. [Google Scholar] [CrossRef]
- Lu, Y.; Li, K.; Han, X.; Feng, X.; Chu, Z.; Lu, L.; Huang, P.; Zhang, Z.; Zhang, Y.; Yin, F.; et al. A method of cell-to-cell variation evaluation for battery packs in electric vehicles with charging cloud data. eTransportation 2020, 6, 100077. [Google Scholar] [CrossRef]
- Zhu, J.; Mathews, I.; Ren, D.; Li, W.; Cogswell, D.A.; Xu, B.; Sedlatschek, T.; Kantareddy, S.N.R.; Yi, M.; Gao, T.; et al. End-of-life or second-life options for retired electric vehicle batteries. Cell Rep. Phys. Sci. 2021, 2, 100537. [Google Scholar] [CrossRef]
- Chung, H.C. Charge and discharge profiles of repurposed LiFePO4 batteries based on the UL 1974 standard. Sci. Data 2021, 8, 1–12. [Google Scholar] [CrossRef]
- Wang, X.; Wei, X.; Zhu, J.; Dai, H.; Zheng, Y.; Xu, X.; Chen, Q. A review of modeling, acquisition, and application of lithium-ion battery impedance for onboard battery management. eTransportation 2021, 7, 100093. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, B.; Zhao, S.; Li, H.; Yu, H. Oxygen anionic redox activated high-energy cathodes: Status and prospects. eTransportation 2021, 8, 100118. [Google Scholar] [CrossRef]
- Chen, Y.; Xia, D. Systematic density functional theory investigations on cubic lithium-rich iron-based Li2FeO3: A multiple electrons cationic and anionic redox cathode material. eTransportation 2021, 10, 100141. [Google Scholar] [CrossRef]
- Yang, X.; Doyle-Davis, K.; Gao, X.; Sun, X. Recent progress and perspectives on designing high-performance thick electrodes for all-solid-state lithium batteries. eTransportation 2021, 11, 100152. [Google Scholar] [CrossRef]
- Xiang, K.; Xing, W.T.; Ravnsbaek, D.B.; Hong, L.; Tang, M.; Li, Z.; Wiaderek, K.M.; Borkiewicz, O.J.; Chapman, K.W.; Chupas, P.J. Accommodating high transformation strains in battery electrodes via the formation of nanoscale intermediate phases: Operando investigation of olivine NaFePO4. Nano Lett. 2017, 17, 1696–1702. [Google Scholar] [CrossRef]
- Dambournet, D.; Chapman, K.W.; Duttine, M.; Borkiewicz, O.; Chupas, P.J.; Groult, H. Lithium insertion mechanism in iron-based oxyfluorides with anionic vacancies probed by PDF analysis. Chemistryopen 2015, 4, 443–447. [Google Scholar] [CrossRef]
- Su, L.; Wu, M.; Li, Z.; Zhang, J. Cycle life prediction of lithium-ion batteries based on data-driven methods. eTransportation 2021, 10, 100137. [Google Scholar] [CrossRef]
- Morales, J.; Trócoli, R.; Franger, S.; Santos-Pena, J. Cycling-induced stress in lithium ion negative electrodes: LiAl/LiFePO4 and Li4Ti5O12/LiFePO4 cells. Electrochim. Acta 2010, 55, 3075–3082. [Google Scholar] [CrossRef]

| 2 C | 3 C | 4 C | |
|---|---|---|---|
| 10 °C | L11 | L21 | L31 |
| 30 °C | L12 | L22 | L32 |
| 50 °C | L13 | L23 | L33 |
| Parameters (Unit) | Numerical Values |
|---|---|
| Working voltage (V) | 3.2 |
| Rated capacity (Ah) | 5.0 |
| Charging cutoff voltage (V) | 3.65 |
| Discharging cutoff voltage (V) | 2.5 |
| Maximum constant charging current (A) | 5 |
| Maximum constant discharging current (A) | 12.5 |
| Weight (g) | 145 |
| Height (mm) | 69.8 ± 0.2 |
| Diameter (mm) | 32.2 ± 0.5 |
| Paths | L11 | L21 | L31 | L12 | L22 | L32 | L13 | L23 | L33 |
|---|---|---|---|---|---|---|---|---|---|
| Battery numbers | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, J.; Wang, W.; Wei, Z.; Hu, F.; Wu, X. Capacity Fading Rules of Lithium-Ion Batteries for Multiple Thermoelectric Aging Paths. Batteries 2023, 9, 3. https://doi.org/10.3390/batteries9010003
Du J, Wang W, Wei Z, Hu F, Wu X. Capacity Fading Rules of Lithium-Ion Batteries for Multiple Thermoelectric Aging Paths. Batteries. 2023; 9(1):3. https://doi.org/10.3390/batteries9010003
Chicago/Turabian StyleDu, Jiuyu, Wenbo Wang, Zhixin Wei, Fangfang Hu, and Xiaogang Wu. 2023. "Capacity Fading Rules of Lithium-Ion Batteries for Multiple Thermoelectric Aging Paths" Batteries 9, no. 1: 3. https://doi.org/10.3390/batteries9010003
APA StyleDu, J., Wang, W., Wei, Z., Hu, F., & Wu, X. (2023). Capacity Fading Rules of Lithium-Ion Batteries for Multiple Thermoelectric Aging Paths. Batteries, 9(1), 3. https://doi.org/10.3390/batteries9010003







