Building Bridges: Unifying Design and Development Aspects for Advancing Non-Aqueous Redox-Flow Batteries
Abstract
1. Introduction
2. Description of General Redox-Flow Cell Types
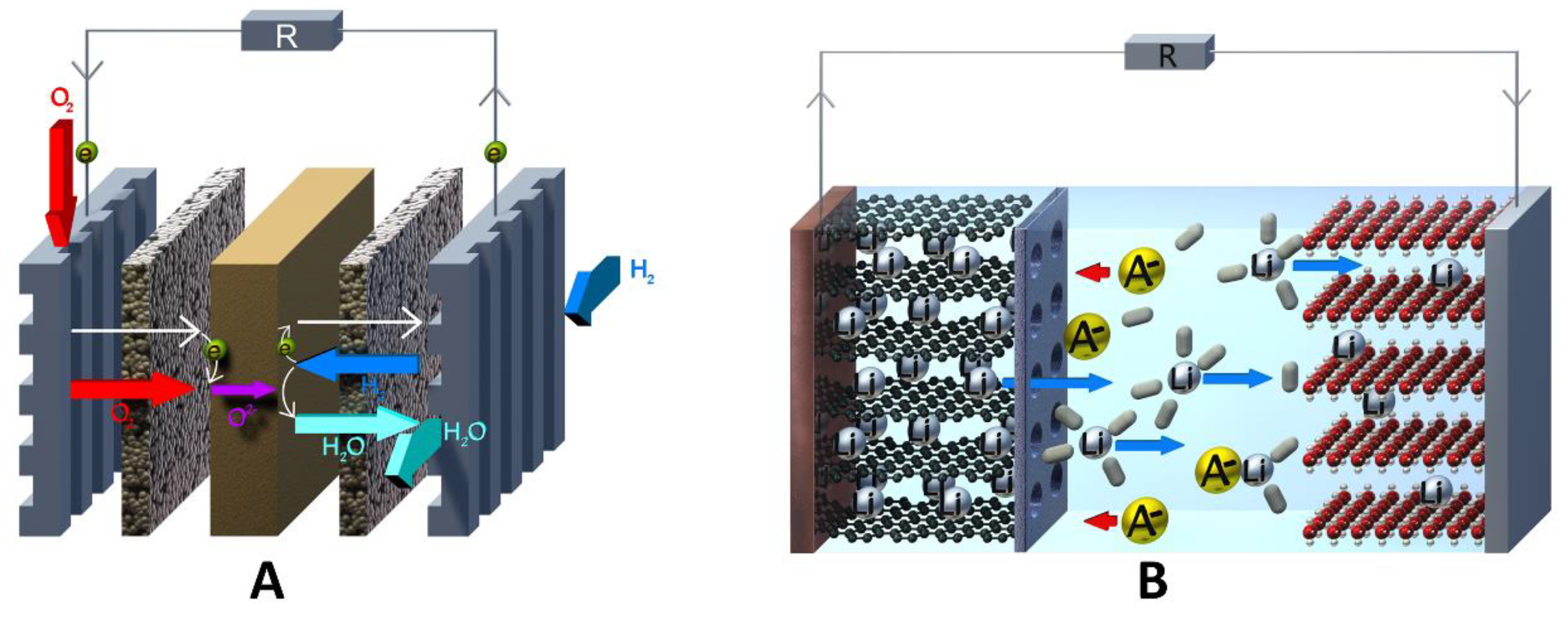
2.1. General Definition of a Redox-Flow Cell
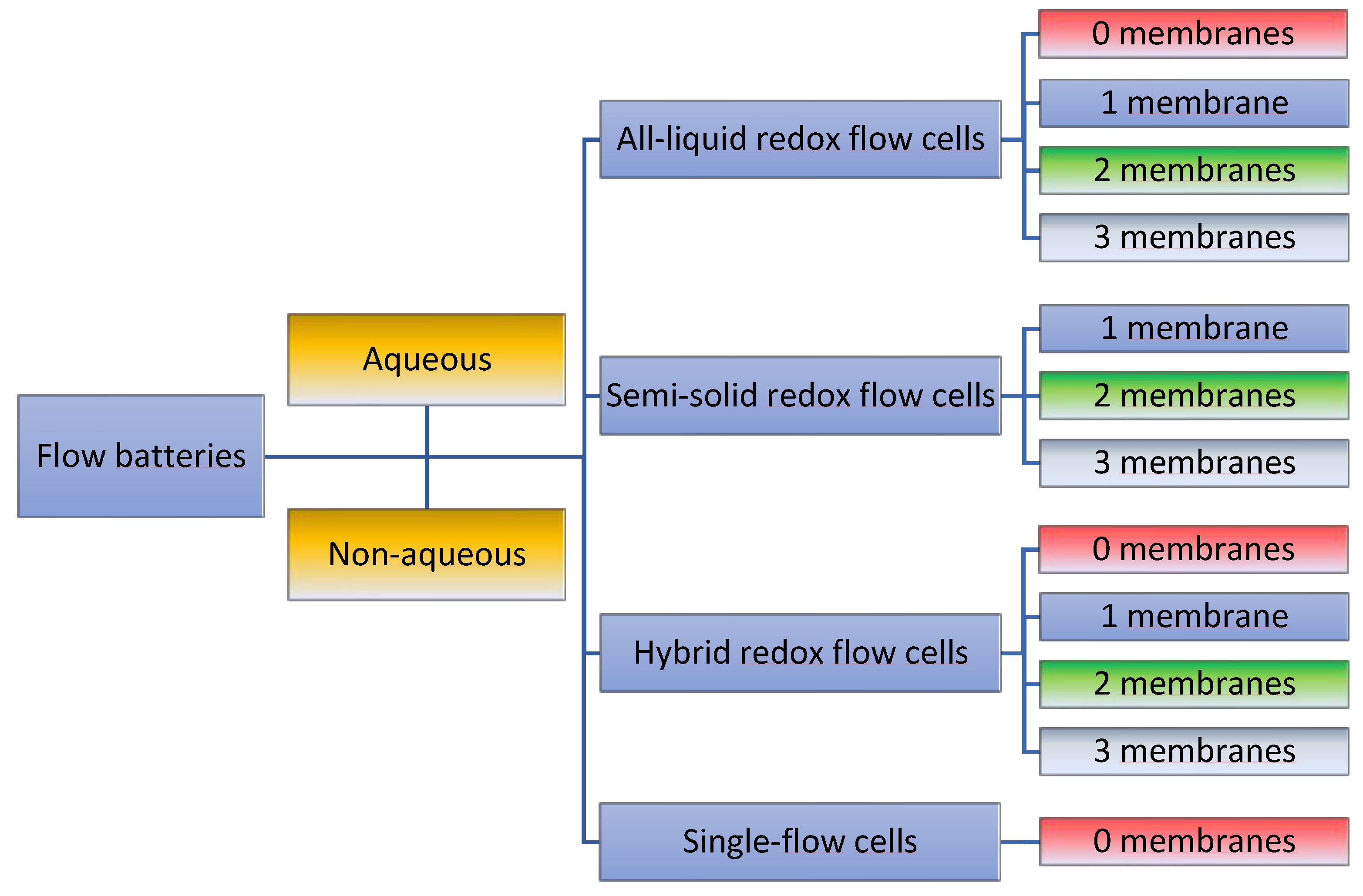
2.2. Definition and Terminology of Possible Redox-Flow-Cell Setups
2.3. General Cell Types
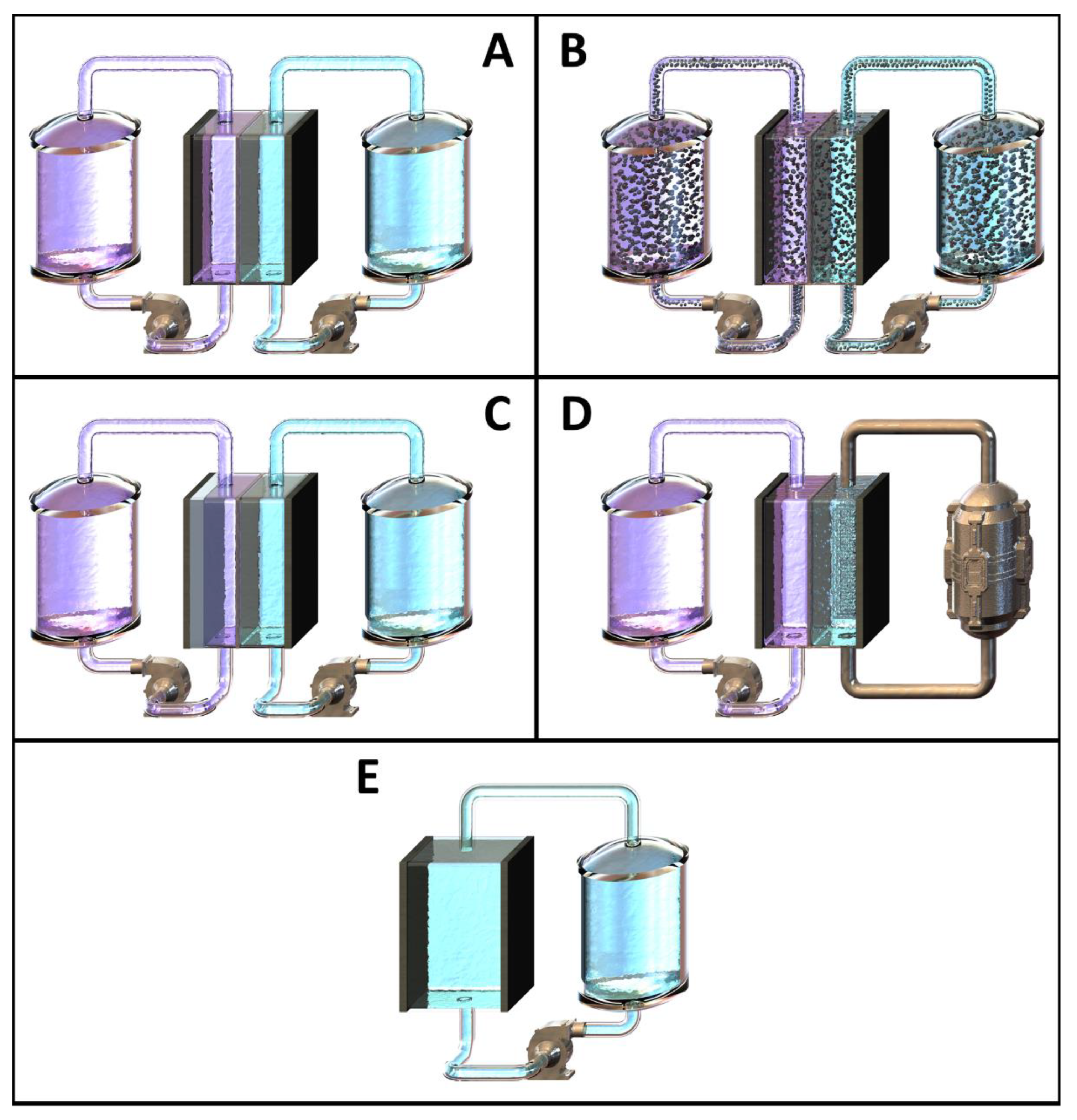
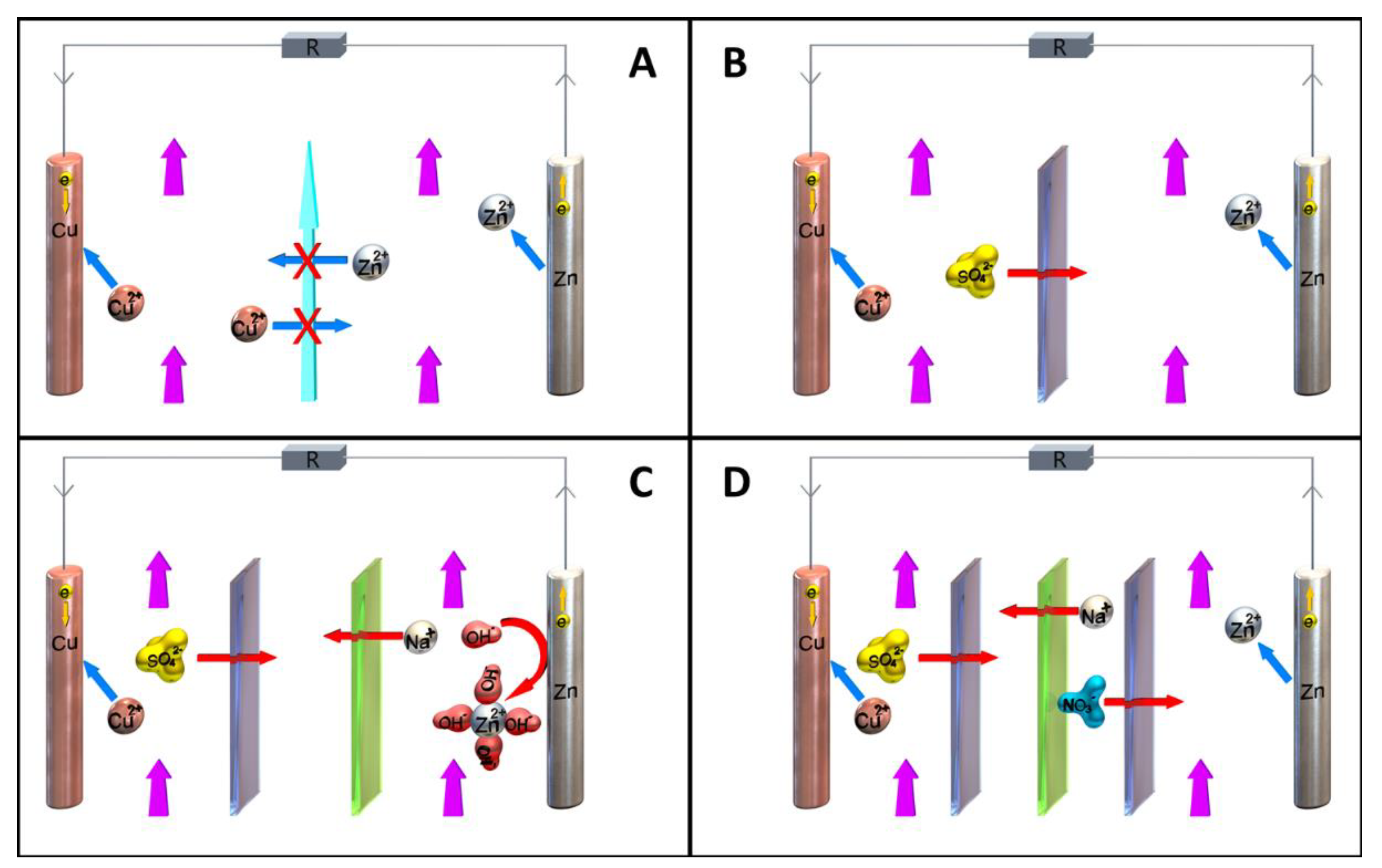
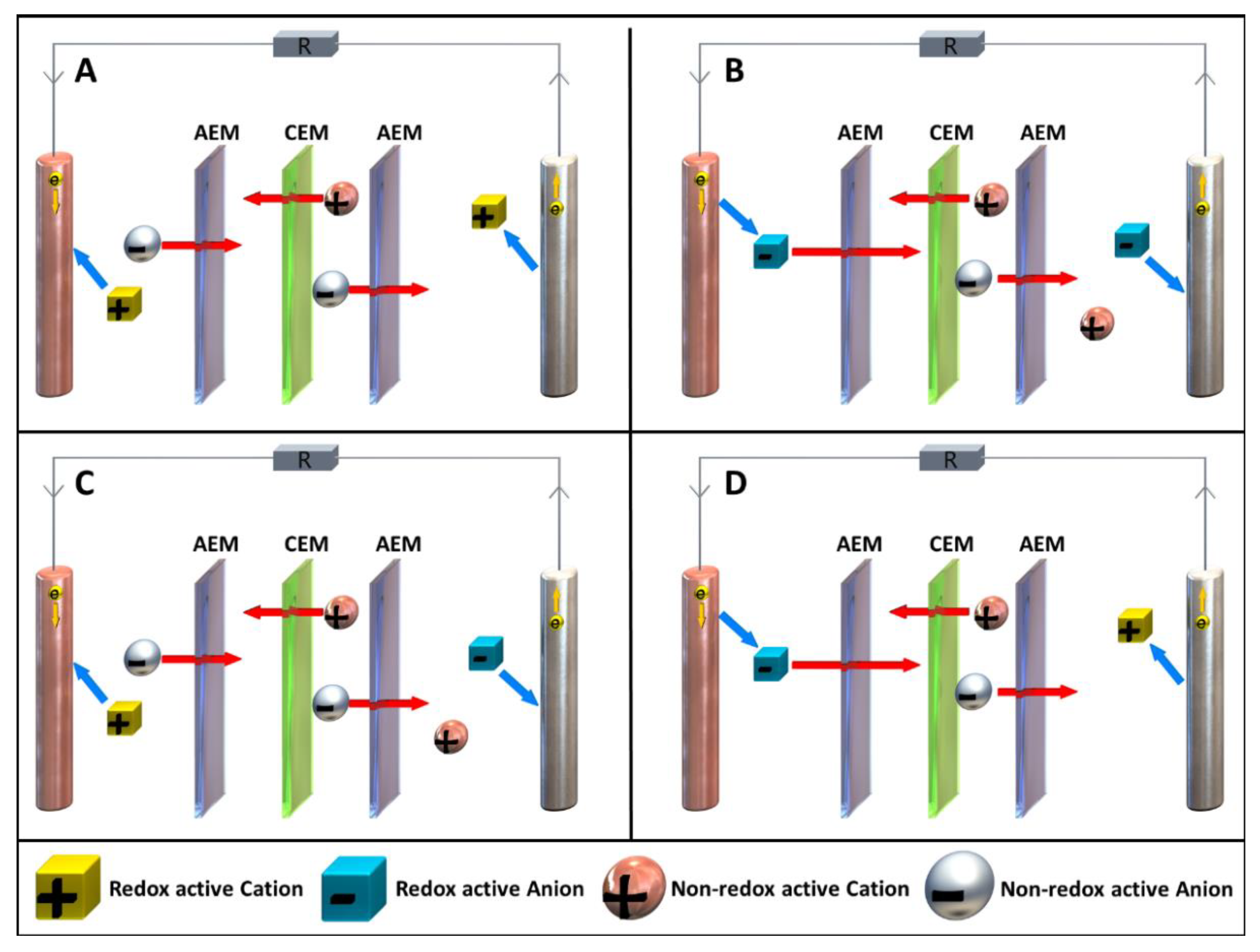
2.4. Cell Separation Techniques and Membrane Configurations
2.5. Summary
- Type and number of membranes.
- The nature, geometry, and surface area of the electrodes.
- Geometry of the cell body, which strongly affects the electrolyte flow.
3. Electrolytes and Electrochemical Behavior
3.1. Introduction
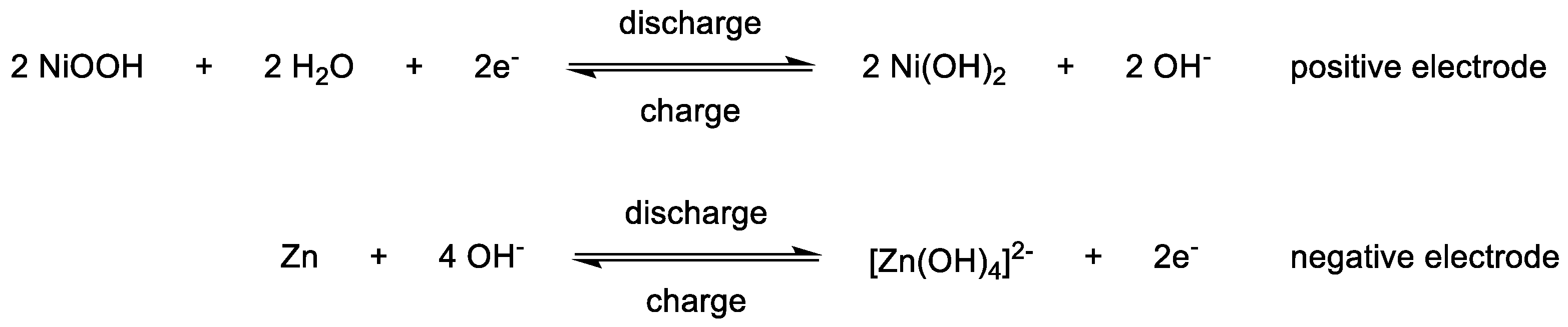
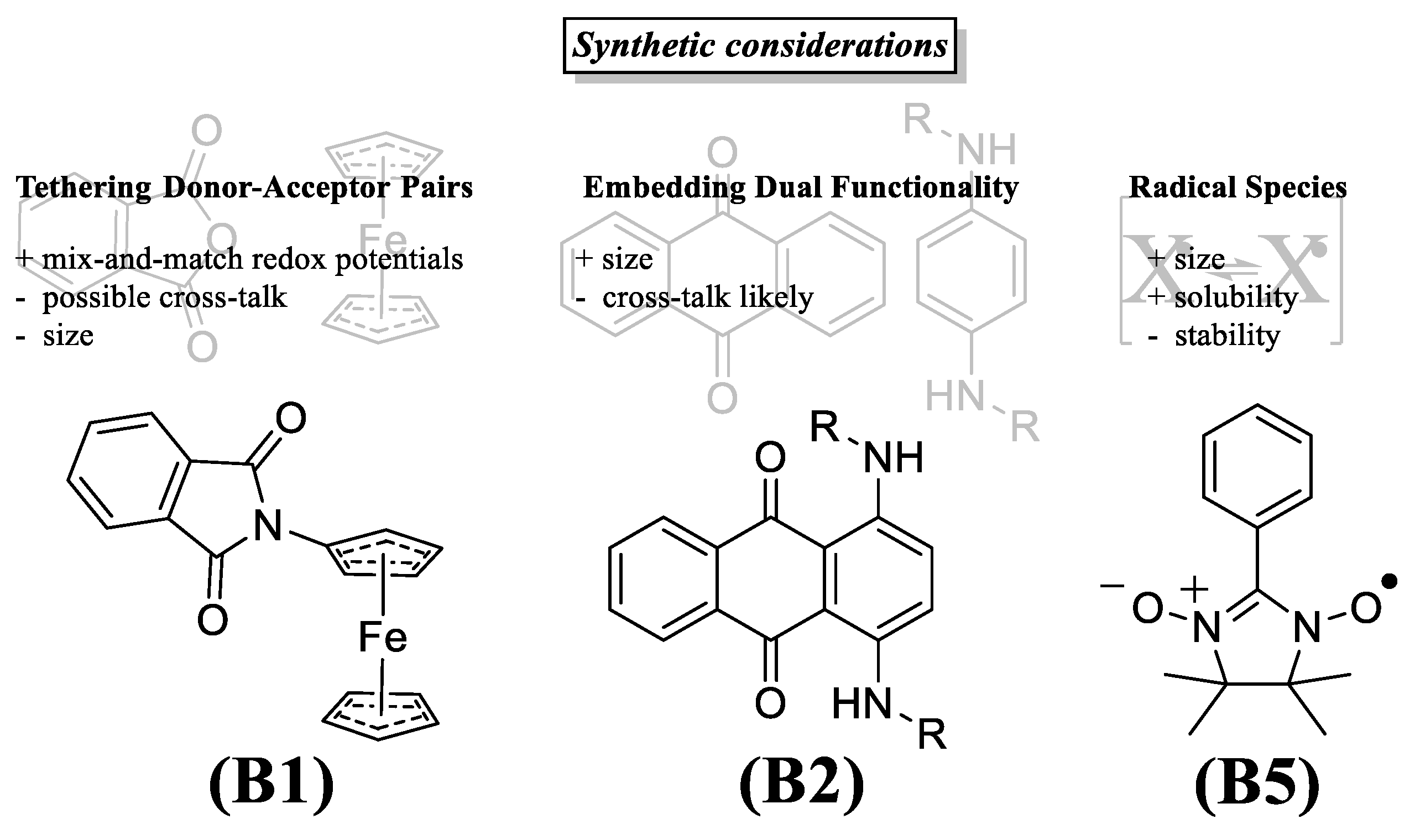
3.2. Catholytes
3.3. Anolytes
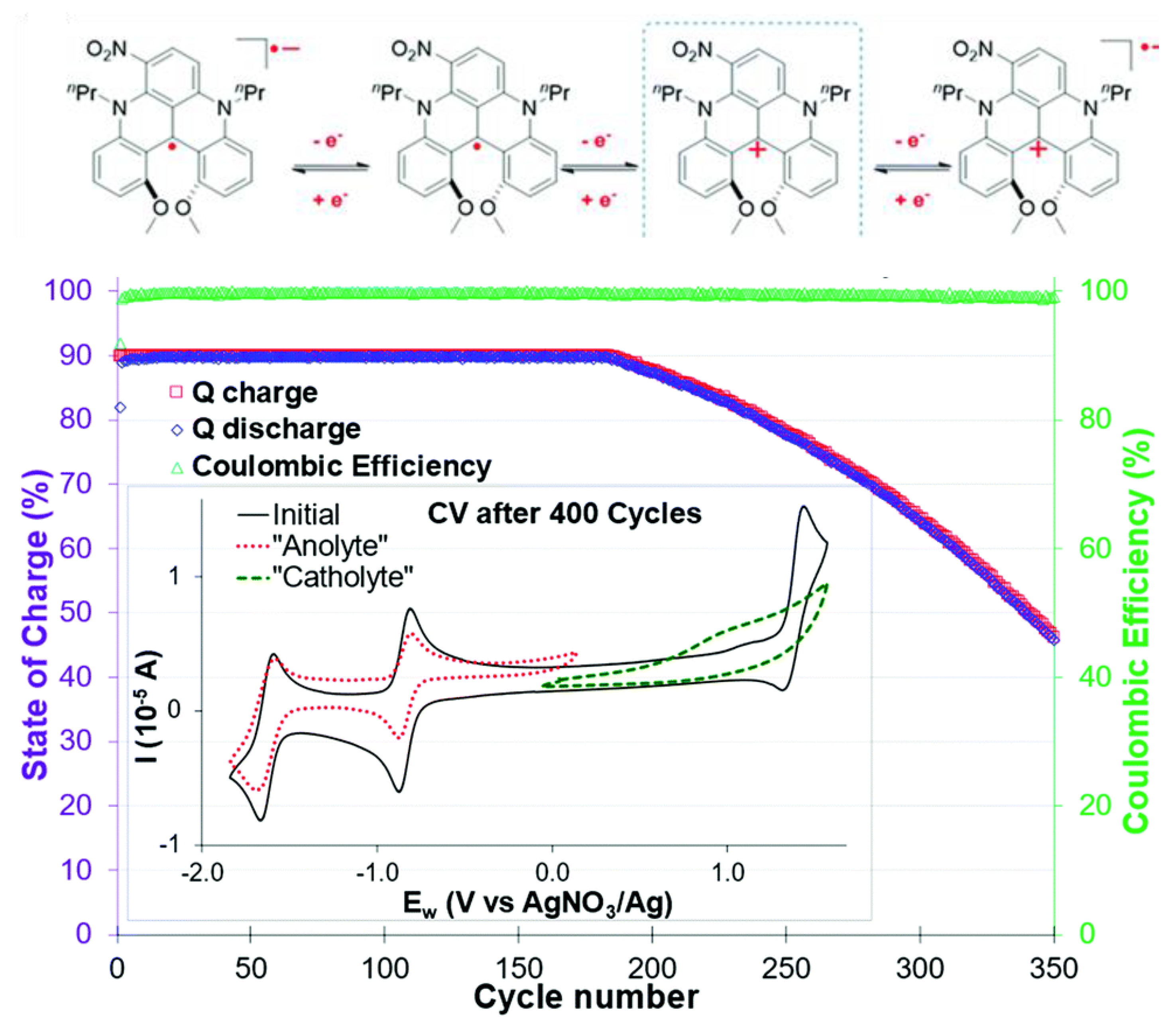
3.4. Bipolar Electrolytes
3.5. Summary
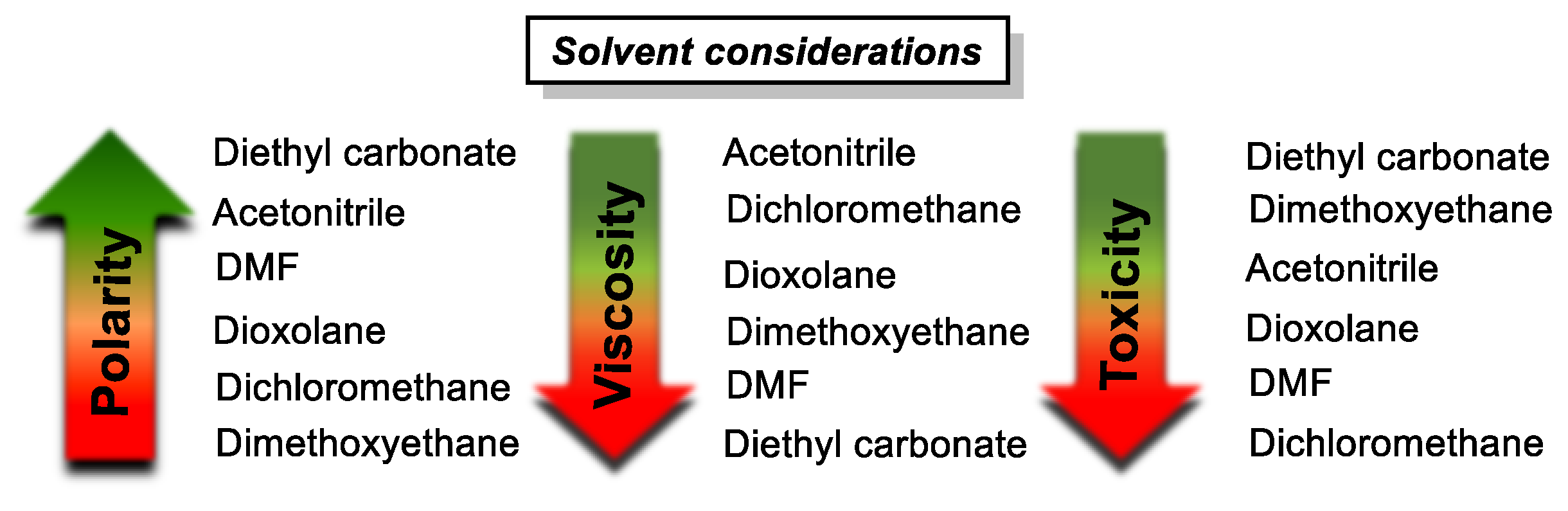
- Acetonitrile is the solvent of choice, given its low viscosity and toxicity, and importantly, its polar nature, which generally contributes to solubilizing charged states also.
- Generally, TFSI− and TEA+ are preferred supporting ions. Alternatively, TBAPF6 is also often applied and performs well. Li+, notably, has exhibited some incompatibility issues in the anolyte compartment.
- Electron-withdrawing substituents on catholytes and electron donating substituents on anolytes can slightly increase the system’s voltage, though a possible negative effect on stability may negate the gain in OCV.
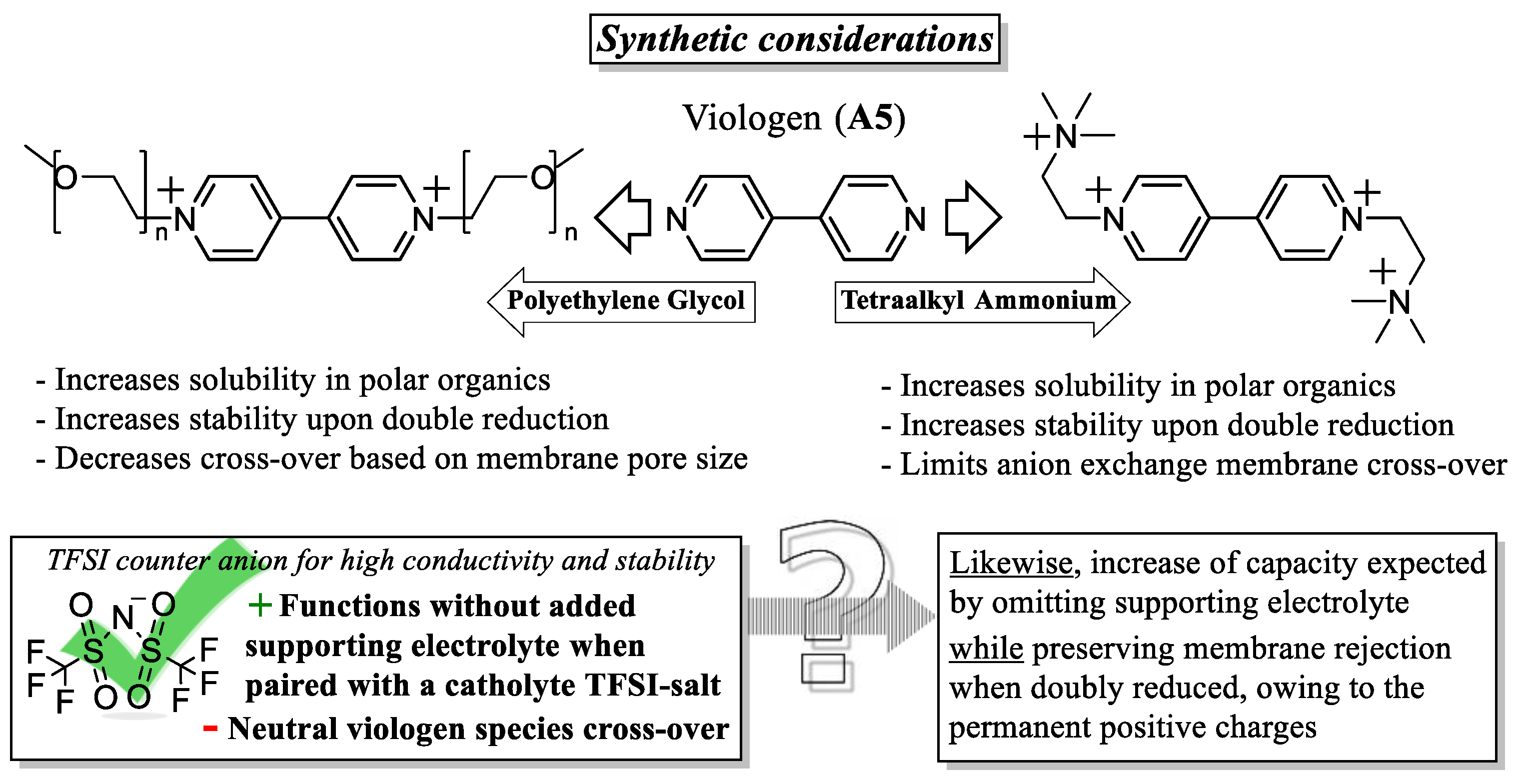
- Appending ethylene glycol (preferably asymmetrically) or tetraalkylammonium chains substantially increases the solubility in acetonitrile and to date seems to be the most efficient way to non-invasively increase energy density.
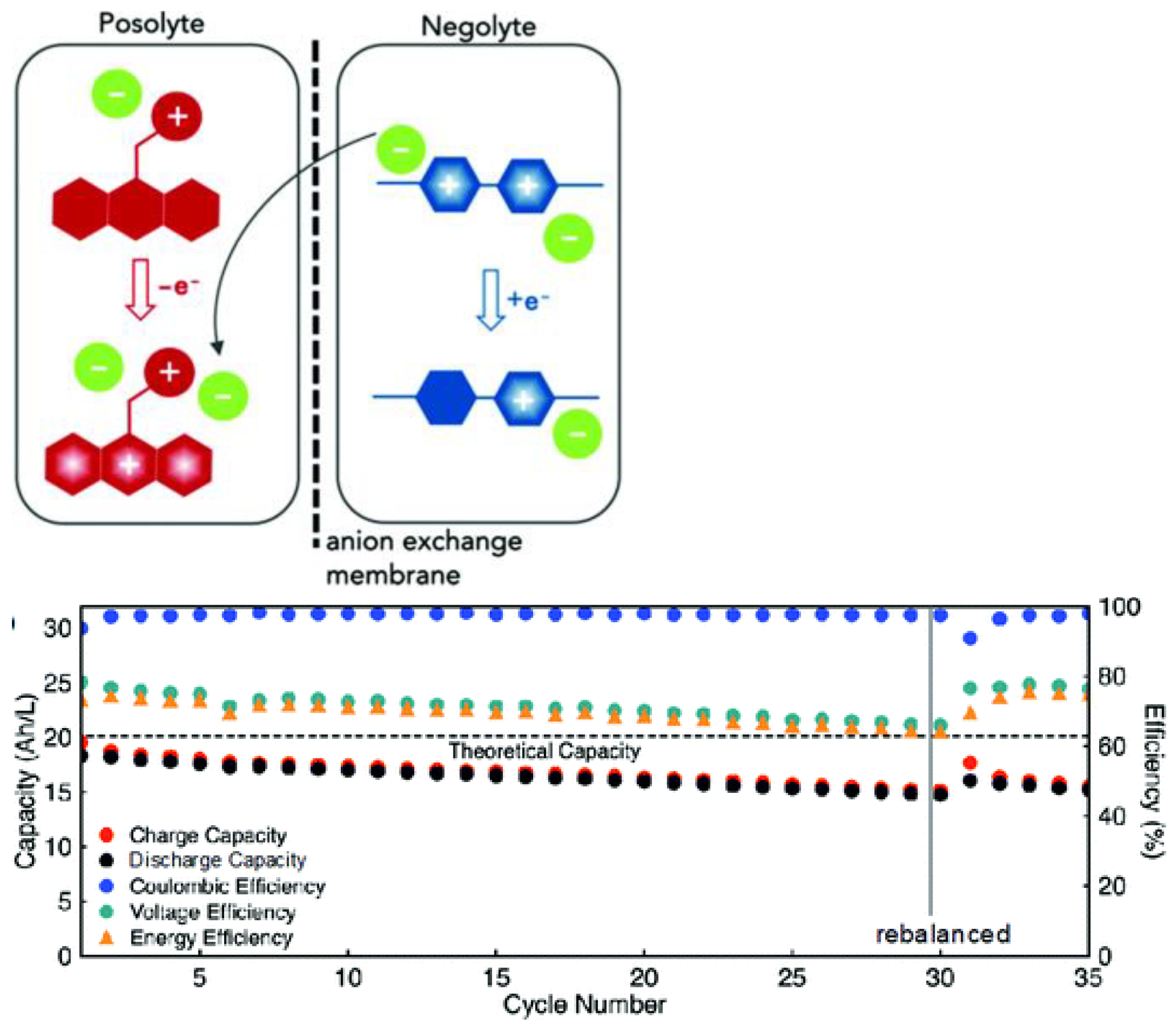
- Imbuing both the catholyte and anolyte with permanent charge, for example, with tetraalkylammonium side-groups, enables the formation of electrolyte salts. This approach is especially promising, as it discards the need for a supporting electrolyte, since solely the counter-ion (e.g., TFSI) needs to traverse the anion exchange membrane, thereby simultaneously maximizing the concentration of active species and combating capacity decay from cross-over of electrolyte.
4. Introducing a RAS from Basic Lab-Scale Identification towards in-Flow RFB Measurements
4.1. Main RFB-Cell Components
4.1.1. Reference Electrode
4.1.2. Current Collector, Flow Field, and Bipolar Plate
4.1.3. Separator
4.2. Practical Difficulties of RAS Transversion and Their Theoretical Background
4.2.1. Cell Setup and Processing Impacts on Mass Transport, Conversion, and Overpotential
4.2.2. Membrane’s Impacts on Mass Transport, Conversion, and Overpotential
4.2.3. Viscosity’s Impacts on Mass Transport, Conversion, and Overpotential
4.3. Summary
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dühnen, S.; Betz, J.; Kolek, M.; Schmuch, R.; Winter, M.; Placke, T. Toward Green Battery Cells: Perspective on Materials and Technologies. Small Methods 2020, 4, 2000039. [Google Scholar] [CrossRef]
- Winter, M.; Barnett, B.; Xu, K. Before Li Ion Batteries. Chem. Rev. 2018, 118, 11433–11456. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, S. A review on energy management, operation control and application methods for grid battery energy storage systems. CSEE JPES 2019, 7, 1026–1040. [Google Scholar] [CrossRef]
- Alotto, P.; Guarnieri, M.; Moro, F. Redox flow batteries for the storage of renewable energy: A review. Renew. Sustain. Energy Rev. 2014, 29, 325–335. [Google Scholar] [CrossRef]
- Darling, R.M.; Gallagher, K.G.; Kowalski, J.A.; Ha, S.; Brushett, F.R. Pathways to low-cost electrochemical energy storage: A comparison of aqueous and nonaqueous flow batteries. Energy Environ. Sci. 2014, 7, 3459–3477. [Google Scholar] [CrossRef]
- Noack, J.; Roznyatovskaya, N.; Herr, T.; Fischer, P. The Chemistry of Redox-Flow Batteries. Angew. Chem. Int. Ed. 2015, 54, 9776–9809. [Google Scholar] [CrossRef] [PubMed]
- Arenas, L.F.; Ponce de León, C.; Walsh, F.C. Redox flow batteries for energy storage: Their promise, achievements and challenges. Curr. Opin. Electrochem. 2019, 16, 117–126. [Google Scholar] [CrossRef]
- Sánchez-Díez, E.; Ventosa, E.; Guarnieri, M.; Trovò, A.; Flox, C.; Marcilla, R.; Soavi, F.; Mazur, P.; Aranzabe, E.; Ferret, R. Redox flow batteries: Status and perspective towards sustainable stationary energy storage. J. Power Sources 2021, 481, 228804. [Google Scholar] [CrossRef]
- Thaller, L. Energy Storage System. 1976. Available online: https://patents.google.com/patent/US3996064A/en (accessed on 15 December 2022).
- Kangro, W. Method for Storing Electrical Energy. Available online: https://patents.google.com/patent/DE914264C/en (accessed on 15 December 2022).
- Skyllas-Kazacos, M.; Rychcik, M.; Robins, R.G.; Fane, A.G.; Green, M.A. New All-Vanadium Redox Flow Cell. J. Electrochem. Soc. 1986, 133, 1057–1058. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; Rychick, M.; Robins, R. All-Vanadium Redox Battery. U.S. Patent 4786567, 22 November 1988. [Google Scholar]
- Ke, X.; Prahl, J.M.; Alexander, J.I.D.; Wainright, J.S.; Zawodzinski, T.A.; Savinell, R.F. Rechargeable redox flow batteries: Flow fields, stacks and design considerations. Chem. Soc. Rev. 2018, 47, 8721–8743. [Google Scholar] [CrossRef]
- Lucas, A.; Chondrogiannis, S. Smart grid energy storage controller for frequency regulation and peak shaving, using a vanadium redox flow battery. Int. J. Electr. Power Energy Syst. 2016, 80, 26–36. [Google Scholar] [CrossRef]
- Kear, G.; Shah, A.A.; Walsh, F.C. Development of the all-vanadium redox flow battery for energy storage: A review of technological, financial and policy aspects. Int. J. Energy Res. 2012, 36, 1105–1120. [Google Scholar] [CrossRef]
- Aramendia, I.; Fernandez-Gamiz, U.; Martinez-San-Vicente, A.; Zulueta, E.; Lopez-Guede, J.M. Vanadium Redox Flow Batteries: A Review Oriented to Fluid-Dynamic Optimization. Energies 2021, 14, 176. [Google Scholar] [CrossRef]
- Gür, T.M. Review of electrical energy storage technologies, materials and systems: Challenges and prospects for large-scale grid storage. Energy Environ. Sci. 2018, 11, 2696–2767. [Google Scholar] [CrossRef]
- Arenas, L.F.; Ponce de León, C.; Walsh, F.C. Engineering aspects of the design, construction and performance of modular redox flow batteries for energy storage. J. Energy Storage 2017, 11, 119–153. [Google Scholar] [CrossRef]
- Hameer, S.; van Niekerk, J.L. A review of large-scale electrical energy storage. Int. J. Energy Res. 2015, 39, 1179–1195. [Google Scholar] [CrossRef]
- Wang, W.; Luo, Q.; Li, B.; Wei, X.; Li, L.; Yang, Z. Recent Progress in Redox Flow Battery Research and Development. Adv. Funct. Mater. 2013, 23, 970–986. [Google Scholar] [CrossRef]
- Leung, P.; Shah, A.A.; Sanz, L.; Flox, C.; Morante, J.R.; Xu, Q.; Mohamed, M.R.; Ponce de León, C.; Walsh, F.C. Recent developments in organic redox flow batteries: A critical review. J. Power Sources 2017, 360, 243–283. [Google Scholar] [CrossRef]
- Cao, J.; Tian, J.; Xu, J.; Wang, Y. Organic Flow Batteries: Recent Progress and Perspectives. Energy Fuels 2020, 34, 13384–13411. [Google Scholar] [CrossRef]
- Xu, F.; Li, H.; Liu, Y.; Jing, Q. Advanced redox flow fuel cell using ferric chloride as main catalyst for complete conversion from carbohydrates to electricity. Sci. Rep. 2017, 7, 5142. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What are batteries, fuel cells, and supercapacitors? Chem. Rev. 2004, 104, 4245–4269. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Gong, Y.; Tricker, A.; Wu, G.; Liu, C.; Chao, Z.; Deng, Y. Fundamental Study toward Improving the Performance of a High-Moisture Biomass-Fueled Redox Flow Fuel Cell. Ind. Eng. Chem. Res. 2020, 59, 4817–4828. [Google Scholar] [CrossRef]
- Cho, K.T.; Tucker, M.C.; Weber, A.Z. A Review of Hydrogen/Halogen Flow Cells. Energy Technol. 2016, 4, 655–678. [Google Scholar] [CrossRef]
- Lyu, Y.; Xie, J.; Wang, D.; Wang, J. Review of cell performance in solid oxide fuel cells. J. Mater. Sci. 2020, 55, 7184–7207. [Google Scholar] [CrossRef]
- Brett, D.J.L.; Atkinson, A.; Brandon, N.P.; Skinner, S.J. Intermediate temperature solid oxide fuel cells. Chem. Soc. Rev. 2008, 37, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ruiz Diaz, D.F.; Chen, K.S.; Wang, Z.; Adroher, X.C. Materials, technological status, and fundamentals of PEM fuel cells—A review. Mater. Today 2020, 32, 178–203. [Google Scholar] [CrossRef]
- Mitlitsky, F.; Myers, B.; Weisberg, A.H.; Molter, T.M.; Smith, W.F. Reversible (unitised) PEM fuel cell devices. Fuel Cells Bull. 1999, 2, 6–11. [Google Scholar] [CrossRef]
- Soloveichik, G.L. Flow Batteries: Current Status and Trends. Chem. Rev. 2015, 115, 11533–11558. [Google Scholar] [CrossRef]
- Hamelet, S.; Tzedakis, T.; Leriche, J.-B.; Sailler, S.; Larcher, D.; Taberna, P.-L.; Simon, P.; Tarascon, J.-M. Non-Aqueous Li-Based Redox Flow Batteries. J. Electrochem. Soc. 2012, 159, A1360–A1367. [Google Scholar] [CrossRef]
- Chakrabarti, M.H.; Dryfe, R.; Roberts, E. Evaluation of electrolytes for redox flow battery applications. Electrochim. Acta 2007, 52, 2189–2195. [Google Scholar] [CrossRef]
- Shin, S.-H.; Yun, S.-H.; Moon, S.-H. A review of current developments in non-aqueous redox flow batteries: Characterization of their membranes for design perspective. RSC Adv. 2013, 3, 9095. [Google Scholar] [CrossRef]
- Noh, C.; Shin, M.; Kwon, Y. A strategy for lowering cross-contamination of aqueous redox flow batteries using metal-ligand complexes as redox couple. J. Power Sources 2022, 520, 230810. [Google Scholar] [CrossRef]
- Korshunov, A.; Milner, M.J.; Grünebaum, M.; Studer, A.; Winter, M.; Cekic-Laskovic, I. An oxo-verdazyl radical for a symmetrical non-aqueous redox flow battery. J. Mater. Chem. A 2020, 8, 22280–22291. [Google Scholar] [CrossRef]
- Sikukuu Nambafu, G. Organic molecules as bifunctional electroactive materials for symmetric redox flow batteries: A mini review. Electrochem. Commun. 2021, 127, 107052. [Google Scholar] [CrossRef]
- Rychcik, M.; Skyllas-Kazacos, M. Evaluation of electrode materials for vanadium redox cell. J. Power Sources 1987, 19, 45–54. [Google Scholar] [CrossRef]
- Rychcik, M.; Skyllas-Kazacos, M. Characteristics of a new all-vanadium redox flow battery. J. Power Sources 1988, 22, 59–67. [Google Scholar] [CrossRef]
- Kaneko, H.; Nozaki, K.; Wada, Y.; Aoki, T.; Negishi, A.; Kamimoto, M. Vanadium redox reactions and carbon electrodes for vanadium redox flow battery. Electrochim. Acta 1991, 36, 1191–1196. [Google Scholar] [CrossRef]
- Mohammadi, T.; Kazacos, M. Modification of anion-exchange membranes for vanadium redox flow battery applications. J. Power Sources 1996, 63, 179–186. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, H.; Zhou, H.; Chen, J.; Gao, S.; Yi, B. Characteristics and performance of 10kW class all-vanadium redox-flow battery stack. J. Power Sources 2006, 162, 1416–1420. [Google Scholar] [CrossRef]
- Liao, J.B.; Lu, M.Z.; Chu, Y.Q.; Wang, J.L. Ultra-low vanadium ion diffusion amphoteric ion-exchange membranes for all-vanadium redox flow batteries. J. Power Sources 2015, 282, 241–247. [Google Scholar] [CrossRef]
- Jia, C.; Liu, J.; Yan, C. A significantly improved membrane for vanadium redox flow battery. J. Power Sources 2010, 195, 4380–4383. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, Z.; Lv, Y.; Tang, A.; Dai, L.; Wang, L.; He, Z. Perovskite enables high performance vanadium redox flow battery. Chem. Eng. J. 2022, 443, 136341. [Google Scholar] [CrossRef]
- Xu, W.; Long, J.; Liu, J.; Luo, H.; Duan, H.; Zhang, Y.; Li, J.; Qi, X.; Chu, L. A novel porous polyimide membrane with ultrahigh chemical stability for application in vanadium redox flow battery. Chem. Eng. J. 2022, 428, 131203. [Google Scholar] [CrossRef]
- Duduta, M.; Ho, B.; Wood, V.C.; Limthongkul, P.; Brunini, V.E.; Carter, W.C.; Chiang, Y.-M. Semi-Solid Lithium Rechargeable Flow Battery. Adv. Energy Mater. 2011, 1, 511–516. [Google Scholar] [CrossRef]
- Chen, H.; Lu, Y.-C. A High-Energy-Density Multiple Redox Semi-Solid-Liquid Flow Battery. Adv. Energy Mater. 2016, 6, 1502183. [Google Scholar] [CrossRef]
- Biendicho, J.J.; Flox, C.; Sanz, L.; Morante, J.R. Static and Dynamic Studies on LiNi1/3 Co1/3 Mn1/3 O2 -Based Suspensions for Semi-Solid Flow Batteries. ChemSusChem 2016, 9, 1938–1944. [Google Scholar] [CrossRef]
- Hamelet, S.; Larcher, D.; Dupont, L.; Tarascon, J.-M. Silicon-Based Non Aqueous Anolyte for Li Redox-Flow Batteries. J. Electrochem. Soc. 2013, 160, A516–A5202013. [Google Scholar] [CrossRef]
- Guillon, O. (Ed.) Advanced Ceramics for Energy Conversion and Storage; Elsevier: Amsterdam, The Netherlands; Kidlington, UK; Oxford, UK; Cambridge, MA, USA, 2020; ISBN 978-0081027264. [Google Scholar]
- Yan, W.; Wang, C.; Tian, J.; Zhu, G.; Ma, L.; Wang, Y.; Chen, R.; Hu, Y.; Wang, L.; Chen, T.; et al. All-polymer particulate slurry batteries. Nat. Commun. 2019, 10, 2513. [Google Scholar] [CrossRef]
- Xing, X.; Liu, Q.; Li, J.; Han, Z.; Wang, B.; Lemmon, J.P. A nonaqueous all organic semisolid flow battery. Chem. Commun. 2019, 55, 14214–14217. [Google Scholar] [CrossRef]
- Wang, X.; Chai, J.; Jiang, J. Redox flow batteries based on insoluble redox-active materials. A review. Nano Mater. Sci. 2021, 3, 17–24. [Google Scholar] [CrossRef]
- Hatzell, K.B.; Boota, M.; Gogotsi, Y. Materials for suspension (semi-solid) electrodes for energy and water technologies. Chem. Soc. Rev. 2015, 44, 8664–8687. [Google Scholar] [CrossRef] [PubMed]
- Yufit, V.; Hale, B.; Matian, M.; Mazur, P.; Brandon, N.P. Development of a Regenerative Hydrogen-Vanadium Fuel Cell for Energy Storage Applications. J. Electrochem. Soc. 2013, 160, A856–A861. [Google Scholar] [CrossRef]
- Alon, M.; Blum, A.; Peled, E. Feasibility study of hydrogen/iron redox flow cell for grid-storage applications. J. Power Sources 2013, 240, 417–420. [Google Scholar] [CrossRef]
- Cho, K.T.; Ridgway, P.; Weber, A.Z.; Haussener, S.; Battaglia, V.; Srinivasan, V. High Performance Hydrogen/Bromine Redox Flow Battery for Grid-Scale Energy Storage. J. Electrochem. Soc. 2012, 159, A1806–A1815. [Google Scholar] [CrossRef]
- Rubio-Garcia, J.; Kucernak, A.; Zhao, D.; Li, D.; Fahy, K.; Yufit, V.; Brandon, N.; Gomez-Gonzalez, M. Hydrogen/manganese hybrid redox flow battery. J. Phys. Energy 2019, 1, 15006. [Google Scholar] [CrossRef]
- Hewa Dewage, H.; Wu, B.; Tsoi, A.; Yufit, V.; Offer, G.; Brandon, N. A novel regenerative hydrogen cerium fuel cell for energy storage applications. J. Mater. Chem. A 2015, 3, 9446–9450. [Google Scholar] [CrossRef]
- Rubio-Garcia, J.; Kucernak, A.; Parra-Puerto, A.; Liu, R.; Chakrabarti, B. Hydrogen/functionalized benzoquinone for a high-performance regenerative fuel cell as a potential large-scale energy storage platform. J. Mater. Chem. A 2020, 8, 3933–3941. [Google Scholar] [CrossRef]
- Rubio-Garcia, J.; Cui, J.; Parra-Puerto, A.; Kucernak, A. Hydrogen/Vanadium Hybrid Redox Flow Battery with enhanced electrolyte concentration. Energy Storage Mater. 2020, 31, 1–10. [Google Scholar] [CrossRef]
- Walsh, F.C.; Ponce de Léon, C.; Berlouis, L.; Nikiforidis, G.; Arenas-Martínez, L.F.; Hodgson, D.; Hall, D. The Development of Zn-Ce Hybrid Redox Flow Batteries for Energy Storage and Their Continuing Challenges. ChemPlusChem 2015, 80, 288–311. [Google Scholar] [CrossRef]
- Jeena, C.B.; Elsa, P.J.; Moly, P.P.; Ambily, K.J.; Joy, V.T. A dendrite free Zn-Fe hybrid redox flow battery for renewable energy storage. Energy Storage 2022, 4, e275. [Google Scholar] [CrossRef]
- Wang, Y.; He, P.; Zhou, H. Li-Redox Flow Batteries Based on Hybrid Electrolytes: At the Cross Road between Li-ion and Redox Flow Batteries. Adv. Energy Mater. 2012, 2, 770–779. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Du, Y.; Jia, C.; Zhou, M.; Li, F.; Wang, X.; Wang, Q. Unleashing the Power and Energy of LiFePO4-Based Redox Flow Lithium Battery with a Bifunctional Redox Mediator. J. Am. Chem. Soc. 2017, 139, 6286–6289. [Google Scholar] [CrossRef] [PubMed]
- Nariyama, H.; Ito, S.; Okada, Y.; Inatomi, Y.; Ichikawa, K.; Masumoto, Y.; Fujimoto, M. High energy density 3V-class redox flow battery using LiFePO4 and graphite with organic bifunctional redox mediators. Electrochim. Acta 2022, 409, 139915. [Google Scholar] [CrossRef]
- Jia, C.; Pan, F.; Zhu, Y.G.; Huang, Q.; Lu, L.; Wang, Q. High-energy density nonaqueous all redox flow lithium battery enabled with a polymeric membrane. Sci. Adv. 2015, 1, e15008862. [Google Scholar] [CrossRef]
- Pan, F.; Yang, J.; Huang, Q.; Wang, X.; Huang, H.; Wang, Q. Batteries: Redox Targeting of Anatase TiO2 for Redox Flow Lithium-Ion Batteries. Adv. Energy Mater. 2014, 4, 1400567. [Google Scholar] [CrossRef]
- Huang, Q.; Li, H.; Grätzel, M.; Wang, Q. Reversible chemical delithiation/lithiation of LiFePO4: Towards a redox flow lithium-ion battery. Phys. Chem. Chem. Phys. 2013, 15, 1793–1797. [Google Scholar] [CrossRef]
- Zou, T.; Shi, X.; Yu, L. Study on energy loss of 35 kW all vanadium redox flow battery energy storage system under closed-loop flow strategy. J. Power Sources 2021, 490, 229514. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, L.; Yang, Y.-S.; Wen, Y.-H.; Cao, G.-P.; Wang, X.-D. Preliminary study of single flow zinc–nickel battery. Electrochem. Commun. 2007, 9, 2639–2642. [Google Scholar] [CrossRef]
- Im, Y.; Kim, J.; Park, K.S.; Cho, T.W.; Jeon, J.; Chung, K.; Eguchi, K.; Kang, M. Influence of small amount of Mg incorporated into hexagonal ZnO crystal on cell performance in membrane free Zinc–Nickel redox battery. J. Ind. Eng. Chem. 2018, 64, 318–327. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, H.; Lai, Q.; Li, X.; Zheng, Q.; Xi, X.; Ding, C. Effect of temperature on the performances and in situ polarization analysis of zinc–nickel single flow batteries. J. Power Sources 2014, 249, 435–439. [Google Scholar] [CrossRef]
- Turney, D.E.; Shmukler, M.; Galloway, K.; Klein, M.; Ito, Y.; Sholklapper, T.; Gallaway, J.W.; Nyce, M.; Banerjee, S. Development and testing of an economic grid-scale flow-assisted zinc/nickel-hydroxide alkaline battery. J. Power Sources 2014, 264, 49–58. [Google Scholar] [CrossRef]
- Amit, L.; Naar, D.; Gloukhovski, R.; La O’, G.J.; Suss, M.E. A Single-Flow Battery with Multiphase Flow. ChemSusChem 2021, 14, 1068–1073. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Wen, Y.; Cheng, J.; Pan, J.; Bai, S.; Yang, Y. Evaluation of substrates for zinc negative electrode in acid PbO2–Zn single flow batteries. Chin. J. Chem. Eng. 2016, 24, 529–534. [Google Scholar] [CrossRef]
- Xie, C.; Liu, Y.; Lu, W.; Zhang, H.; Li, X. Highly stable zinc–iodine single flow batteries with super high energy density for stationary energy storage. Energy Environ. Sci. 2019, 12, 1834–1839. [Google Scholar] [CrossRef]
- Gu, S.; Gong, K.; Yan, E.Z.; Yan, Y. A multiple ion-exchange membrane design for redox flow batteries. Energy Environ. Sci. 2014, 7, 2986–2998. [Google Scholar] [CrossRef]
- Bamgbopa, M.O.; Almheiri, S.; Sun, H. Prospects of recently developed membraneless cell designs for redox flow batteries. Renew. Sustain. Energy Rev. 2017, 70, 506–518. [Google Scholar] [CrossRef]
- Von Doenhoff, A.E.; Braslow, A.L. The effect of distributed surface roughness on laminar flow. In Boundary Layer and Flow Control; Lachmann, G.V., Ed.; Elsevier Science: Amsterdam, The Netherlands, 1961; pp. 657–681. ISBN 9781483213231. [Google Scholar]
- Skyllas-Kazacos, M.; Grossmith, F. Efficient Vanadium Redox Flow Cell. J. Electrochem. Soc. 1987, 134, 2950–2953. [Google Scholar] [CrossRef]
- Schulte, D.; Drillkens, J.; Schulte, B.; Sauer, D.U. Nafion Hybrid Membranes for Use in Vanadium Redox Flow Batteries. ECS Trans. 2010, 25, 247–255. [Google Scholar] [CrossRef]
- Jiang, B.; Wu, L.; Yu, L.; Qiu, X.; Xi, J. A comparative study of Nafion series membranes for vanadium redox flow batteries. J. Membr. Sci. 2016, 510, 18–26. [Google Scholar] [CrossRef]
- Maghsoudy, S.; Rahimi, M.; Dehkordi, A.M. Investigation on various types of ion-exchange membranes in vanadium redox flow batteries: Experiment and modeling. J. Energy Storage 2022, 54, 105347. [Google Scholar] [CrossRef]
- Peng, S.; Zhang, L.; Zhang, C.; Ding, Y.; Guo, X.; He, G.; Yu, G. Gradient-Distributed Metal-Organic Framework-Based Porous Membranes for Nonaqueous Redox Flow Batteries. Adv. Energy Mater. 2018, 8, 1802533. [Google Scholar] [CrossRef]
- Hendriks, K.H.; Robinson, S.G.; Braten, M.N.; Sevov, C.S.; Helms, B.A.; Sigman, M.S.; Minteer, S.D.; Sanford, M.S. High-Performance Oligomeric Catholytes for Effective Macromolecular Separation in Nonaqueous Redox Flow Batteries. ACS Cent. Sci. 2018, 4, 189–196. [Google Scholar] [CrossRef]
- Chen, R. Redox flow batteries for energy storage: Recent advances in using organic active materials. Curr. Opin. Electrochem. 2020, 21, 40–45. [Google Scholar] [CrossRef]
- Gong, K.; Fang, Q.; Gu, S.; Li, S.F.Y.; Yan, Y. Nonaqueous redox-flow batteries: Organic solvents, supporting electrolytes, and redox pairs. Energy Environ. Sci. 2015, 8, 3515–3530. [Google Scholar] [CrossRef]
- Tang, L.; Leung, P.; Xu, Q.; Mohamed, M.R.; Dai, S.; Zhu, X.; Flox, C.; Shah, A.A. Future perspective on redox flow batteries: Aqueous versus nonaqueous electrolytes. Curr. Opin. Chem. Eng. 2022, 37, 100833. [Google Scholar] [CrossRef]
- Singh, P. Application of non-aqueous solvents to batteries. J. Power Sources 1984, 11, 135–142. [Google Scholar] [CrossRef]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry, 4th ed.; Wiley-VCH: Weinheim, Germany, 2011; ISBN 9783527324736. [Google Scholar]
- Smallwood, I. Handbook of Organic Solvent Properties; Elsevier Science: Burlington, MA, USA, 1996; ISBN 0340645784. [Google Scholar]
- Jonsson, M.; Houmam, A.; Jocys, G.; Wayner, D.D.M. Solvent effects on redox properties of radical ions 1. J. Chem. Soc. Perkin Trans. 1999, 2, 425–430. [Google Scholar] [CrossRef]
- Catalán, J. Toward a generalized treatment of the solvent effect based on four empirical scales: Dipolarity (SdP, a new scale), polarizability (SP), acidity (SA), and basicity (SB) of the medium. J. Phys. Chem. B 2009, 113, 5951–5960. [Google Scholar] [CrossRef]
- Li, Z.; Li, S.; Liu, S.; Huang, K.; Fang, D.; Wang, F.; Peng, S. Electrochemical Properties of an All-Organic Redox Flow Battery Using 2,2,6,6-Tetramethyl-1-Piperidinyloxy and N-Methylphthalimide. Electrochem. Solid State Lett. 2011, 14, A171. [Google Scholar] [CrossRef]
- Daub, N.; Janssen, R.A.J.; Hendriks, K.H. Imide-Based Multielectron Anolytes as High-Performance Materials in Nonaqueous Redox Flow Batteries. ACS Appl. Energy Mater. 2021, 4, 9248–9257. [Google Scholar] [CrossRef]
- Brushett, F.R.; Vaughey, J.T.; Jansen, A.N. An All-Organic Non-aqueous Lithium-Ion Redox Flow Battery. Adv. Energy Mater. 2012, 2, 1390–1396. [Google Scholar] [CrossRef]
- Huang, J.; Yang, Z.; Vijayakumar, M.; Duan, W.; Hollas, A.; Pan, B.; Wang, W.; Wei, X.; Zhang, L. A Two-Electron Storage Nonaqueous Organic Redox Flow Battery. Adv. Sustain. Syst. 2018, 2, 1700131. [Google Scholar] [CrossRef]
- Huo, Y.; Xing, X.; Zhang, C.; Wang, X.; Li, Y. An all organic redox flow battery with high cell voltage. RSC Adv. 2019, 9, 13128–13132. [Google Scholar] [CrossRef] [PubMed]
- Nagarjuna, G.; Hui, J.; Cheng, K.J.; Lichtenstein, T.; Shen, M.; Moore, J.S.; Rodríguez-López, J. Impact of redox-active polymer molecular weight on the electrochemical properties and transport across porous separators in nonaqueous solvents. J. Am. Chem. Soc. 2014, 136, 16309–16316. [Google Scholar] [CrossRef]
- Hu, B.; Liu, T.L. Two electron utilization of methyl viologen anolyte in nonaqueous organic redox flow battery. J. Energy Chem. 2018, 27, 1326–1332. [Google Scholar] [CrossRef]
- Zhang, L.; Qian, Y.; Feng, R.; Ding, Y.; Zu, X.; Zhang, C.; Guo, X.; Wang, W.; Yu, G. Reversible redox chemistry in azobenzene-based organic molecules for high-capacity and long-life nonaqueous redox flow batteries. Nat. Commun. 2020, 11, 3843. [Google Scholar] [CrossRef]
- Wang, X.; Chai, J.; Lashgari, A.; Jiang, J.J. Azobenzene-Based Low-Potential Anolyte for Nonaqueous Organic Redox Flow Batteries. ChemElectroChem 2021, 8, 83–89. [Google Scholar] [CrossRef]
- Hwang, S.; Kim, H.; Ryu, J.H.; Oh, S.M. N-ferrocenylphthalimide; A single redox couple formed by attaching a ferrocene moiety to phthalimide for non-aqueous flow batteries. J. Power Sources 2018, 395, 60–65. [Google Scholar] [CrossRef]
- Potash, R.A.; McKone, J.R.; Conte, S.; Abruña, H.D. On the Benefits of a Symmetric Redox Flow Battery. J. Electrochem. Soc. 2016, 163, A338–A344. [Google Scholar] [CrossRef]
- Geysens, P.; Li, Y.; Vankelecom, I.; Fransaer, J.; Binnemans, K. Highly Soluble 1,4-Diaminoanthraquinone Derivative for Nonaqueous Symmetric Redox Flow Batteries. ACS Sustain. Chem. Eng. 2020, 8, 3832–3843. [Google Scholar] [CrossRef]
- Etkind, S.I.; Lopez, J.; Zhu, Y.G.; Fang, J.-H.; Ong, W.J.; Shao-Horn, Y.; Swager, T.M. Thianthrene-Based Bipolar Redox-Active Molecules Toward Symmetric All-Organic Batteries. ACS Sustain. Chem. Eng. 2022, 10, 11739–11750. [Google Scholar] [CrossRef]
- Wylie, L.; Blesch, T.; Freeman, R.; Hatakeyama-Sato, K.; Oyaizu, K.; Yoshizawa-Fujita, M.; Izgorodina, E.I. Reversible Reduction of the TEMPO Radical: One Step Closer to an All-Organic Redox Flow Battery. ACS Sustain. Chem. Eng. 2020, 8, 17988–17996. [Google Scholar] [CrossRef]
- Duan, W.; Vemuri, R.S.; Milshtein, J.D.; Laramie, S.; Dmello, R.D.; Huang, J.; Zhang, L.; Hu, D.; Vijayakumar, M.; Wang, W.; et al. A symmetric organic-based nonaqueous redox flow battery and its state of charge diagnostics by FTIR. J. Mater. Chem. A 2016, 4, 5448–5456. [Google Scholar] [CrossRef]
- Hagemann, T.; Winsberg, J.; Häupler, B.; Janoschka, T.; Gruber, J.J.; Wild, A.; Schubert, U.S. A bipolar nitronyl nitroxide small molecule for an all-organic symmetric redox-flow battery. NPG Asia Mater. 2017, 9, e340. [Google Scholar] [CrossRef]
- Steen, J.S.; Nuismer, J.L.; Eiva, V.; Wiglema, A.E.T.; Daub, N.; Hjelm, J.; Otten, E. Blatter Radicals as Bipolar Materials for Symmetrical Redox-Flow Batteries. J. Am. Chem. Soc. 2022, 144, 5051–5058. [Google Scholar] [CrossRef]
- Moutet, J.; Veleta, J.M.; Gianetti, T.L. Symmetric, Robust, and High-Voltage Organic Redox Flow Battery Model Based on a Helical Carbenium Ion Electrolyte. ACS Appl. Energy Mater. 2021, 4, 9–14. [Google Scholar] [CrossRef]
- Moutet, J.; Mills, D.; Hossain, M.M.; Gianetti, T.L. Increased performance of an all-organic redox flow battery model via nitration of the [4]helicenium DMQA ion electrolyte. Mater. Adv. 2022, 3, 216–223. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Redfern, P.C.; Curtiss, L.A.; Amine, K. Molecular engineering towards safer lithium-ion batteries: A highly stable and compatible redox shuttle for overcharge protection. Energy Environ. Sci. 2012, 5, 8204. [Google Scholar] [CrossRef]
- Wang, X.; Xing, X.; Huo, Y.; Zhao, Y.; Li, Y. A systematic study of the co-solvent effect for an all-organic redox flow battery. RSC Adv. 2018, 8, 24422–24427. [Google Scholar] [CrossRef]
- Wei, X.; Xu, W.; Huang, J.; Zhang, L.; Walter, E.; Lawrence, C.; Vijayakumar, M.; Henderson, W.A.; Liu, T.; Cosimbescu, L.; et al. Radical Compatibility with Nonaqueous Electrolytes and Its Impact on an All-Organic Redox Flow Battery. Angew. Chem. Int. Ed. Engl. 2015, 54, 8684–8687. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Z.; Shkrob, I.A.; Assary, R.S.; Tung, S.O.; Silcox, B.; Duan, W.; Zhang, J.; Su, C.C.; Hu, B.; et al. Annulated Dialkoxybenzenes as Catholyte Materials for Non-aqueous Redox Flow Batteries: Achieving High Chemical Stability through Bicyclic Substitution. Adv. Energy Mater. 2017, 7, 1701272. [Google Scholar] [CrossRef]
- Huang, J.; Cheng, L.; Assary, R.S.; Wang, P.; Xue, Z.; Burrell, A.K.; Curtiss, L.A.; Zhang, L. Liquid Catholyte Molecules for Nonaqueous Redox Flow Batteries. Adv. Energy Mater. 2015, 5, 1401782. [Google Scholar] [CrossRef]
- Ding, Y.; Zhao, Y.; Li, Y.; Goodenough, J.B.; Yu, G. A high-performance all-metallocene-based, non-aqueous redox flow battery. Energy Environ. Sci. 2017, 10, 491–497. [Google Scholar] [CrossRef]
- Park, K.; Cho, J.H.; Shanmuganathan, K.; Song, J.; Peng, J.; Gobet, M.; Greenbaum, S.; Ellison, C.J.; Goodenough, J.B. New battery strategies with a polymer/Al2O3 separator. J. Power Sources 2014, 263, 52–58. [Google Scholar] [CrossRef]
- Zhao, Y.; Ding, Y.; Song, J.; Li, G.; Dong, G.; Goodenough, J.B.; Yu, G. Sustainable electrical energy storage through the ferrocene/ferrocenium redox reaction in aprotic electrolyte. Angew. Chem. Int. Ed. Engl. 2014, 53, 11036–11040. [Google Scholar] [CrossRef]
- Romadina, E.I.; Volodin, I.A.; Stevenson, K.J.; Troshin, P.A. New highly soluble triarylamine-based materials as promising catholytes for redox flow batteries. J. Mater. Chem. A 2021, 9, 8303–8307. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Charlton, G.D.; Barbon, S.M.; Gilroy, J.B.; Dyker, C.A. A bipolar verdazyl radical for a symmetric all-organic redox flow-type battery. J. Energy Chem. 2019, 34, 52–56. [Google Scholar] [CrossRef]
- Navalpotro, P.; Sierra, N.; Trujillo, C.; Montes, I.; Palma, J.; Marcilla, R. Exploring the Versatility of Membrane-Free Battery Concept Using Different Combinations of Immiscible Redox Electrolytes. ACS Appl. Mater. Interfaces 2018, 10, 41246–41256. [Google Scholar] [CrossRef]
- Peljo, P.; Bichon, M.; Girault, H.H. Ion transfer battery: Storing energy by transferring ions across liquid-liquid interfaces. Chem. Commun. 2016, 52, 9761–9764. [Google Scholar] [CrossRef]
- Cekic-Laskovic, I.; von Aspern, N.; Imholt, L.; Kaymaksiz, S.; Oldiges, K.; Rad, B.R.; Winter, M. Synergistic Effect of Blended Components in Nonaqueous Electrolytes for Lithium Ion Batteries. In Electrochemical Energy Storage; Eichel, R.-A., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–64. ISBN 978-3-030-26128-3. [Google Scholar]
- Von Aspern, N.; Röschenthaler, G.-V.; Winter, M.; Cekic-Laskovic, I. Fluorine and Lithium: Ideal Partners for High-Performance Rechargeable Battery Electrolytes. Angew. Chem. Int. Ed. 2019, 58, 15978–16000. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, J.A.; Carney, T.J.; Huang, J.; Zhang, L.; Brushett, F.R. An investigation on the impact of halidization on substituted dimethoxybenzenes. Electrochim. Acta 2020, 335, 135580. [Google Scholar] [CrossRef]
- Cong, G.; Zhou, Y.; Li, Z.; Lu, Y.-C. A Highly Concentrated Catholyte Enabled by a Low-Melting-Point Ferrocene Derivative. ACS Energy Lett. 2017, 2, 869–875. [Google Scholar] [CrossRef]
- Astruc, D. Why is Ferrocene so Exceptional? Eur. J. Inorg. Chem. 2017, 2017, 6–29. [Google Scholar] [CrossRef]
- Chen, H.; Niu, Z.; Ye, J.; Zhang, C.; Zhang, X.; Zhao, Y. Multicore Ferrocene Derivative as a Highly Soluble Cathode Material for Nonaqueous Redox Flow Batteries. ACS Appl. Energy Mater. 2021, 4, 855–861. [Google Scholar] [CrossRef]
- Wei, X.; Cosimbescu, L.; Xu, W.; Hu, J.Z.; Vijayakumar, M.; Feng, J.; Hu, M.Y.; Deng, X.; Xiao, J.; Liu, J.; et al. Towards High-Performance Nonaqueous Redox Flow Electrolyte Via Ionic Modification of Active Species. Adv. Energy Mater. 2015, 5, 1400678. [Google Scholar] [CrossRef]
- Cosimbescu, L.; Wei, X.; Vijayakumar, M.; Xu, W.; Helm, M.L.; Burton, S.D.; Sorensen, C.M.; Liu, J.; Sprenkle, V.; Wang, W. Anion-Tunable Properties and Electrochemical Performance of Functionalized Ferrocene Compounds. Sci. Rep. 2015, 5, 14117. [Google Scholar] [CrossRef]
- Kim, H.; Yoon, T.; Kim, Y.; Hwang, S.; Ryu, J.H.; Oh, S.M. Increase of both solubility and working voltage by acetyl substitution on ferrocene for non-aqueous flow battery. Electrochem. Commun. 2016, 69, 72–75. [Google Scholar] [CrossRef]
- Tricot, Y.M.; Porat, Z.; Manassen, J. Photoinduced and redox-induced transmembrane processes with vesicle-stabilized colloidal cadmium sulfide and multicharged viologen derivatives. J. Phys. Chem. 1991, 95, 3242–3248. [Google Scholar] [CrossRef]
- DeBruler, C.; Hu, B.; Moss, J.; Liu, X.; Luo, J.; Sun, Y.; Liu, T.L. Designer Two-Electron Storage Viologen Anolyte Materials for Neutral Aqueous Organic Redox Flow Batteries. Chem 2017, 3, 961–978. [Google Scholar] [CrossRef]
- Liu, Y.; Goulet, M.-A.; Tong, L.; Liu, Y.; Ji, Y.; Wu, L.; Gordon, R.G.; Aziz, M.J.; Yang, Z.; Xu, T. A Long-Lifetime All-Organic Aqueous Flow Battery Utilizing TMAP-TEMPO Radical. Chem 2019, 5, 1861–1870. [Google Scholar] [CrossRef]
- Buhrmester, C.; Moshurchak, L.; Wang, R.L.; Dahn, J.R. Phenothiazine Molecules. J. Electrochem. Soc. 2006, 153, A288. [Google Scholar] [CrossRef]
- Attanayake, N.H.; Kowalski, J.A.; Greco, K.V.; Casselman, M.D.; Milshtein, J.D.; Chapman, S.J.; Parkin, S.R.; Brushett, F.R.; Odom, S.A. Tailoring Two-Electron-Donating Phenothiazines to Enable High-Concentration Redox Electrolytes for Use in Nonaqueous Redox Flow Batteries. Chem. Mater. 2019, 31, 4353–4363. [Google Scholar] [CrossRef]
- Kaur, A.P.; Casselman, M.D.; Elliott, C.F.; Parkin, S.R.; Risko, C.; Odom, S.A. Overcharge protection of lithium-ion batteries above 4 V with a perfluorinated phenothiazine derivative. J. Mater. Chem. A 2016, 4, 5410–5414. [Google Scholar] [CrossRef]
- Milshtein, J.D.; Kaur, A.P.; Casselman, M.D.; Kowalski, J.A.; Modekrutti, S.; Zhang, P.L.; Harsha Attanayake, N.; Elliott, C.F.; Parkin, S.R.; Risko, C.; et al. High current density, long duration cycling of soluble organic active species for non-aqueous redox flow batteries. Energy Environ. Sci. 2016, 9, 3531–3543. [Google Scholar] [CrossRef]
- Ergun, S.; Casselman, M.D.; Kaur, A.P.; Attanayake, N.H.; Parkin, S.R.; Odom, S.A. Improved synthesis of N -ethyl-3,7-bis(trifluoromethyl)phenothiazine. N. J. Chem. 2020, 44, 11349–11355. [Google Scholar] [CrossRef]
- Kowalski, J.A.; Casselman, M.D.; Kaur, A.P.; Milshtein, J.D.; Elliott, C.F.; Modekrutti, S.; Attanayake, N.H.; Zhang, N.; Parkin, S.R.; Risko, C.; et al. A stable two-electron-donating phenothiazine for application in nonaqueous redox flow batteries. J. Mater. Chem. A 2017, 5, 24371–24379. [Google Scholar] [CrossRef]
- Chai, J.; Lashgari, A.; Wang, X.; Williams, C.K.; Jiang, J. All-PEGylated redox-active metal-free organic molecules in non-aqueous redox flow battery. J. Mater. Chem. A 2020, 8, 15715–15724. [Google Scholar] [CrossRef]
- Attanayake, N.H.; Liang, Z.; Wang, Y.; Kaur, A.P.; Parkin, S.R.; Mobley, J.K.; Ewoldt, R.H.; Landon, J.; Odom, S.A. Dual function organic active materials for nonaqueous redox flow batteries. Mater. Adv. 2021, 2, 1390–1401. [Google Scholar] [CrossRef]
- Romadina, E.I.; Komarov, D.S.; Stevenson, K.J.; Troshin, P.A. New phenazine based anolyte material for high voltage organic redox flow batteries. Chem. Commun. 2021, 57, 2986–2989. [Google Scholar] [CrossRef]
- Zhang, C.; Niu, Z.; Ding, Y.; Zhang, L.; Zhou, Y.; Guo, X.; Zhang, X.; Zhao, Y.; Yu, G. Highly Concentrated Phthalimide-Based Anolytes for Organic Redox Flow Batteries with Enhanced Reversibility. Chem 2018, 4, 2814–2825. [Google Scholar] [CrossRef]
- Zhang, C.; Qian, Y.; Ding, Y.; Zhang, L.; Guo, X.; Zhao, Y.; Yu, G. Biredox Eutectic Electrolytes Derived from Organic Redox-Active Molecules: High-Energy Storage Systems. Angew. Chem. Int. Ed. Engl. 2019, 58, 7045–7050. [Google Scholar] [CrossRef] [PubMed]
- Biso, M.; Mastragostino, M.; Montanino, M.; Passerini, S.; Soavi, F. Electropolymerization of poly(3-methylthiophene) in pyrrolidinium-based ionic liquids for hybrid supercapacitors. Electrochim. Acta 2008, 53, 7967–7971. [Google Scholar] [CrossRef]
- Xing, X.; Liu, Q.; Wang, B.; Lemmon, J.P.; Xu, W.Q. A low potential solvent-miscible 3-methylbenzophenone anolyte material for high voltage and energy density all-organic flow battery. J. Power Sources 2020, 445, 227330. [Google Scholar] [CrossRef]
- Montoto, E.C.; Nagarjuna, G.; Moore, J.S.; Rodríguez-López, J. Redox Active Polymers for Non-Aqueous Redox Flow Batteries: Validation of the Size-Exclusion Approach. J. Electrochem. Soc. 2017, 164, A1688–A1694. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, S.; Zhu, Y.; Qian, Y.; Niu, Z.; Ye, J.; Zhao, Y.; Zhang, X. Pyridyl group design in viologens for anolyte materials in organic redox flow batteries. RSC Adv. 2018, 8, 18762–18770. [Google Scholar] [CrossRef]
- Chai, J.; Lashgari, A.; Cao, Z.; Williams, C.K.; Wang, X.; Dong, J.; Jiang, J.J. PEGylation-Enabled Extended Cyclability of a Non-aqueous Redox Flow Battery. ACS Appl. Mater. Interfaces 2020, 12, 15262–15270. [Google Scholar] [CrossRef]
- Antoni, P.W.; Golz, C.; Hansmann, M.M. Organic Four-Electron Redox Systems Based on Bipyridine and Phenanthroline Carbene Architectures. Angew. Chem. Int. Ed. Engl. 2022, 61, e202203064. [Google Scholar] [CrossRef]
- Makarova, M.V.; Akkuratov, A.V.; Sideltsev, M.E.; Stevenson, K.J.; Romadina, E.I. Novel Ethylene Glycol Substituted Benzoxadiazole and Benzothiadiazole as Anolytes for Nonaqueous Organic Redox Flow Batteries. ChemElectroChem 2022, 9, e202200483. [Google Scholar] [CrossRef]
- Pianowski, Z.L. Recent Implementations of Molecular Photoswitches into Smart Materials and Biological Systems. Chem. Eur. J. 2019, 25, 5128–5144. [Google Scholar] [CrossRef]
- Li, M.; Case, J.; Minteer, S.D. Bipolar Redox-Active Molecules in Non-Aqueous Organic Redox Flow Batteries: Status and Challenges. ChemElectroChem 2021, 8, 1215–1232. [Google Scholar] [CrossRef]
- Hendriks, K.H.; Sevov, C.S.; Cook, M.E.; Sanford, M.S. Multielectron Cycling of a Low-Potential Anolyte in Alkali Metal Electrolytes for Nonaqueous Redox Flow Batteries. ACS Energy Lett. 2017, 2, 2430–2435. [Google Scholar] [CrossRef]
- Li, M.; Agarwal, G.; Shkrob, I.A.; VanderLinden, R.T.; Case, J.; Prater, M.; Rhodes, Z.; Assary, R.S.; Minteer, S.D. Critical role of structural order in bipolar redox-active molecules for organic redox flow batteries. J. Mater. Chem. A 2021, 9, 23563–23573. [Google Scholar] [CrossRef]
- Meister, P.; Jia, H.; Li, J.; Kloepsch, R.; Winter, M.; Placke, T. Best Practice: Performance and Cost Evaluation of Lithium Ion Battery Active Materials with Special Emphasis on Energy Efficiency. Chem. Mater. 2016, 28, 7203–7217. [Google Scholar] [CrossRef]
- Herse, C.; Bas, D.; Krebs, F.C.; Bürgi, T.; Weber, J.; Wesolowski, T.; Laursen, B.W.; Lacour, J. A highly configurationally stable [4]heterohelicenium cation. Angew. Chem. Int. Ed. Engl. 2003, 42, 3162–3166. [Google Scholar] [CrossRef]
- Esan, O.C.; Shi, X.; Pan, Z.; Huo, X.; An, L.; Zhao, T.S. Modeling and Simulation of Flow Batteries. Adv. Energy Mater. 2020, 10, 2000758. [Google Scholar] [CrossRef]
- Langner, J.; Melke, J.; Ehrenberg, H.; Roth, C. Determination of Overpotentials in All Vanadium Redox Flow Batteries. ECS Trans. 2014, 58, 1–7. [Google Scholar] [CrossRef]
- Ventosa, E.; Skoumal, M.; Vázquez, F.J.; Flox, C.; Morante, J.R. Operando studies of all-vanadium flow batteries: Easy-to-make reference electrode based on silver–silver sulfate. J. Power Sources 2014, 271, 556–560. [Google Scholar] [CrossRef]
- Cecchetti, M.; Casalegno, A.; Zago, M. Local potential measurement through reference electrodes in vanadium redox flow batteries: Evaluation of overpotentials and electrolytes imbalance. J. Power Sources 2018, 400, 218–224. [Google Scholar] [CrossRef]
- Huang, Q.; Li, B.; Song, C.; Jiang, Z.; Platt, A.; Fatih, K.; Bock, C.; Jang, D.; Reed, D. In Situ Reliability Investigation of All-Vanadium Redox Flow Batteries by a Stable Reference Electrode. J. Electrochem. Soc. 2020, 167, 160541. [Google Scholar] [CrossRef]
- Amini, K.; Pritzker, M.D. In situ polarization study of zinc–cerium redox flow batteries. J. Power Sources 2020, 471, 228463. [Google Scholar] [CrossRef]
- Duan, Z.; Qu, Z.; Ren, Q.; Zhang, J. Review of Bipolar Plate in Redox Flow Batteries: Materials, Structures, and Manufacturing. Electrochem. Energy Rev. 2021, 4, 718–756. [Google Scholar] [CrossRef]
- Ressel, S.; Laube, A.; Fischer, S.; Chica, A.; Flower, T.; Struckmann, T. Performance of a vanadium redox flow battery with tubular cell design. J. Power Sources 2017, 355, 199–205. [Google Scholar] [CrossRef]
- Gurieff, N.; Keogh, D.F.; Baldry, M.; Timchenko, V.; Green, D.; Koskinen, I.; Menictas, C. Mass Transport Optimization for Redox Flow Battery Design. Appl. Sci. 2020, 10, 2801. [Google Scholar] [CrossRef]
- Percin, K.; Rommerskirchen, A.; Sengpiel, R.; Gendel, Y.; Wessling, M. 3D-printed conductive static mixers enable all-vanadium redox flow battery using slurry electrodes. J. Power Sources 2018, 379, 228–233. [Google Scholar] [CrossRef]
- Ye, R.; Henkensmeier, D.; Yoon, S.J.; Huang, Z.; Kim, D.K.; Chang, Z.; Kim, S.; Chen, R. Redox Flow Batteries for Energy Storage: A Technology Review. J. Electrochem. Energy Convers. Storage 2018, 15. [Google Scholar] [CrossRef]
- Arenas, L.F.; de León, C.P.; Walsh, F.C. Mass transport and active area of porous Pt/Ti electrodes for the Zn-Ce redox flow battery determined from limiting current measurements. Electrochim. Acta 2016, 221, 154–166. [Google Scholar] [CrossRef]
- Cervantes-Alcalá, R.; Miranda-Hernández, M. Flow distribution and mass transport analysis in cell geometries for redox flow batteries through computational fluid dynamics. J. Appl. Electrochem. 2018, 48, 1243–1254. [Google Scholar] [CrossRef]
- Kumar, S.; Jayanti, S. Effect of flow field on the performance of an all-vanadium redox flow battery. J. Power Sources 2016, 307, 782–787. [Google Scholar] [CrossRef]
- Houser, J.; Pezeshki, A.; Clement, J.T.; Aaron, D.; Mench, M.M. Architecture for improved mass transport and system performance in redox flow batteries. J. Power Sources 2017, 351, 96–105. [Google Scholar] [CrossRef]
- Agar, E.; Dennison, C.R.; Knehr, K.W.; Kumbur, E.C. Identification of performance limiting electrode using asymmetric cell configuration in vanadium redox flow batteries. J. Power Sources 2013, 225, 89–94. [Google Scholar] [CrossRef]
- Aguiló-Aguayo, N.; Drozdzik, T.; Bechtold, T. The role of electrode orientation to enhance mass transport in redox flow batteries. Electrochem. Commun. 2020, 111, 106650. [Google Scholar] [CrossRef]
- Tang, A.; Bao, J.; Skyllas-Kazacos, M. Studies on pressure losses and flow rate optimization in vanadium redox flow battery. J. Power Sources 2014, 248, 154–162. [Google Scholar] [CrossRef]
- Zhou, X.L.; Zhao, T.S.; An, L.; Zeng, Y.K.; Wei, L. Critical transport issues for improving the performance of aqueous redox flow batteries. J. Power Sources 2017, 339, 1–12. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Q.; Lv, Y.; Li, Y.; Zhou, X. Binder-free carbon nano-network wrapped carbon felt with optimized heteroatom doping for vanadium redox flow batteries. J. Mater. Chem. A 2019, 7, 25132–25141. [Google Scholar] [CrossRef]
- Cecchetti, M.; Messaggi, M.; Donazzi, A.; Facibeni, A.; Russo, V.; Casari, C.S.; Bassi, A.L.; Casalegno, A.; Zago, M. A combined morphological and electrochemical characterization of carbon electrodes in vanadium redox flow batteries: Insights into positive and negative electrode performance. Electrochim. Acta 2020, 329, 135143. [Google Scholar] [CrossRef]
- Goulet, M.-A.; Habisch, A.; Kjeang, E. In Situ Enhancement of Flow-through Porous Electrodes with Carbon Nanotubes via Flowing Deposition. Electrochim. Acta 2016, 206, 36–44. [Google Scholar] [CrossRef]
- Dennison, C.R.; Agar, E.; Akuzum, B.; Kumbur, E.C. Enhancing Mass Transport in Redox Flow Batteries by Tailoring Flow Field and Electrode Design. J. Electrochem. Soc. 2016, 163, A5163–A5169. [Google Scholar] [CrossRef]
- Friedrich, J.M.; Ponce-de-León, C.; Reade, G.W.; Walsh, F.C. Reticulated vitreous carbon as an electrode material. J. Electroanal. Chem. 2004, 561, 203–217. [Google Scholar] [CrossRef]
- Crothers, A.R.; Darling, R.M.; Kushner, D.I.; Perry, M.L.; Weber, A.Z. Theory of Multicomponent Phenomena in Cation-Exchange Membranes: Part III. Transport in Vanadium Redox-Flow-Battery Separators. J. Electrochem. Soc. 2020, 167, 13549. [Google Scholar] [CrossRef]
- Darling, R.; Gallagher, K.; Xie, W.; Su, L.; Brushett, F. Transport Property Requirements for Flow Battery Separators. J. Electrochem. Soc. 2016, 163, A5029–A5040. [Google Scholar] [CrossRef]
- You, D.; Zhang, H.; Sun, C.; Ma, X. Simulation of the self-discharge process in vanadium redox flow battery. J. Power Sources 2011, 196, 1578–1585. [Google Scholar] [CrossRef]
- Schwenzer, B.; Zhang, J.; Kim, S.; Li, L.; Liu, J.; Yang, Z. Membrane development for vanadium redox flow batteries. ChemSusChem 2011, 4, 1388–1406. [Google Scholar] [CrossRef] [PubMed]
- Gubler, L. Membranes and separators for redox flow batteries. Curr. Opin. Electrochem. 2019, 18, 31–36. [Google Scholar] [CrossRef]
- Perry, M.L.; Saraidaridis, J.D.; Darling, R.M. Crossover mitigation strategies for redox-flow batteries. Curr. Opin. Electrochem. 2020, 21, 311–318. [Google Scholar] [CrossRef]
- Takechi, K.; Kato, Y.; Hase, Y. A highly concentrated catholyte based on a solvate ionic liquid for rechargeable flow batteries. Adv. Mater. 2015, 27, 2501–2506. [Google Scholar] [CrossRef]
- Bamgbopa, M.O.; Shao-Horn, Y.; Hashaikeh, R.; Almheiri, S. Cyclable membraneless redox flow batteries based on immiscible liquid electrolytes: Demonstration with all-iron redox chemistry. Electrochim. Acta 2018, 267, 41–50. [Google Scholar] [CrossRef]
- Yao, Y.; Lei, J.; Shi, Y.; Ai, F.; Lu, Y.-C. Assessment methods and performance metrics for redox flow batteries. Nat. Energy 2021, 6, 582–588. [Google Scholar] [CrossRef]
- Wang, H.; Sayed, S.Y.; Luber, E.J.; Olsen, B.C.; Shirurkar, S.M.; Venkatakrishnan, S.; Tefashe, U.M.; Farquhar, A.K.; Smotkin, E.S.; McCreery, R.L.; et al. Redox Flow Batteries: How to Determine Electrochemical Kinetic Parameters. ACS Nano 2020, 14, 2575–2584. [Google Scholar] [CrossRef]
- Li, M.; Odom, S.A.; Pancoast, A.R.; Robertson, L.A.; Vaid, T.P.; Agarwal, G.; Doan, H.A.; Wang, Y.; Suduwella, T.M.; Bheemireddy, S.R.; et al. Experimental Protocols for Studying Organic Non-aqueous Redox Flow Batteries. ACS Energy Lett. 2021, 6, 3932–3943. [Google Scholar] [CrossRef]
- Streeter, I.; Wildgoose, G.G.; Shao, L.; Compton, R.G. Cyclic voltammetry on electrode surfaces covered with porous layers: An analysis of electron transfer kinetics at single-walled carbon nanotube modified electrodes. Sens. Actuators B Chem. 2008, 133, 462–466. [Google Scholar] [CrossRef]
- Tichter, T.; Schneider, J.; Andrae, D.; Gebhard, M.; Roth, C. Universal Algorithm for Simulating and Evaluating Cyclic Voltammetry at Macroporous Electrodes by Considering Random Arrays of Microelectrodes. Chemphyschem 2020, 21, 428–441. [Google Scholar] [CrossRef] [PubMed]
- Tichter, T.; Andrae, D.; Mayer, J.; Schneider, J.; Gebhard, M.; Roth, C. Theory of cyclic voltammetry in random arrays of cylindrical microelectrodes applied to carbon felt electrodes for vanadium redox flow batteries. Phys. Chem. Chem. Phys. 2019, 21, 9061–9068. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Gao, Y.; Guo, S.; Tang, H. A coupled three dimensional model of vanadium redox flow battery for flow field designs. Energy 2014, 74, 886–895. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, H.; Sun, C.; Zou, Y.; Zhang, T. An optimal strategy of electrolyte flow rate for vanadium redox flow battery. J. Power Sources 2012, 203, 153–158. [Google Scholar] [CrossRef]
- Liang, Z.; Attanayake, N.H.; Greco, K.V.; Neyhouse, B.J.; Barton, J.L.; Kaur, A.P.; Eubanks, W.L.; Brushett, F.R.; Landon, J.; Odom, S.A. Comparison of Separators vs Membranes in Nonaqueous Redox Flow Battery Electrolytes Containing Small Molecule Active Materials. ACS Appl. Energy Mater. 2021, 4, 5443–5451. [Google Scholar] [CrossRef]
- Machado, C.A.; Brown, G.O.; Yang, R.; Hopkins, T.E.; Pribyl, J.G.; Epps, T.H. Redox Flow Battery Membranes: Improving Battery Performance by Leveraging Structure–Property Relationships. ACS Energy Lett. 2021, 6, 158–176. [Google Scholar] [CrossRef]
- Yuan, J.; Pan, Z.-Z.; Jin, Y.; Qiu, Q.; Zhang, C.; Zhao, Y.; Li, Y. Membranes in non-aqueous redox flow battery: A review. J. Power Sources 2021, 500, 229983. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, C.; Zhen, Y.; Zhao, Y.; Li, Y. Enhancing the performance of an all-organic non-aqueous redox flow battery. J. Power Sources 2019, 443, 227283. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, Z.; Lu, W.; Li, X.; Zhang, H. The porous membrane with tunable performance for vanadium flow battery: The effect of charge. J. Power Sources 2017, 342, 327–334. [Google Scholar] [CrossRef]
- Barton, J.L.; Milshtein, J.D.; Hinricher, J.J.; Brushett, F.R. Quantifying the impact of viscosity on mass-transfer coefficients in redox flow batteries. J. Power Sources 2018, 399, 133–143. [Google Scholar] [CrossRef]
- Barton, J.L.; Milshtein, J.D.; Brushett, F.R. Impact of Electrolyte Viscosity on Redox Flow Battery Performance. Meet. Abstr. 2017, MA2017-02, 560. [Google Scholar] [CrossRef]
- Song, Y.; Li, X.; Yan, C.; Tang, A. Unraveling the viscosity impact on volumetric transfer in redox flow batteries. J. Power Sources 2020, 456, 228004. [Google Scholar] [CrossRef]
- Einstein, A. Über die von der molekularkinetischen Theorie der Wärme geforderte Bewegung von in ruhenden Flüssigkeiten suspendierten Teilchen. Ann. Phys. 1905, 322, 549–560. [Google Scholar] [CrossRef]
- Von Smoluchowski, M. Zur kinetischen Theorie der Brownschen Molekularbewegung und der Suspensionen. Ann. Phys. 1906, 326, 756–780. [Google Scholar] [CrossRef]
- Zhang, J.; Corman, R.E.; Schuh, J.K.; Ewoldt, R.H.; Shkrob, I.A.; Zhang, L. Solution Properties and Practical Limits of Concentrated Electrolytes for Nonaqueous Redox Flow Batteries. J. Phys. Chem. C 2018, 122, 8159–8172. [Google Scholar] [CrossRef]
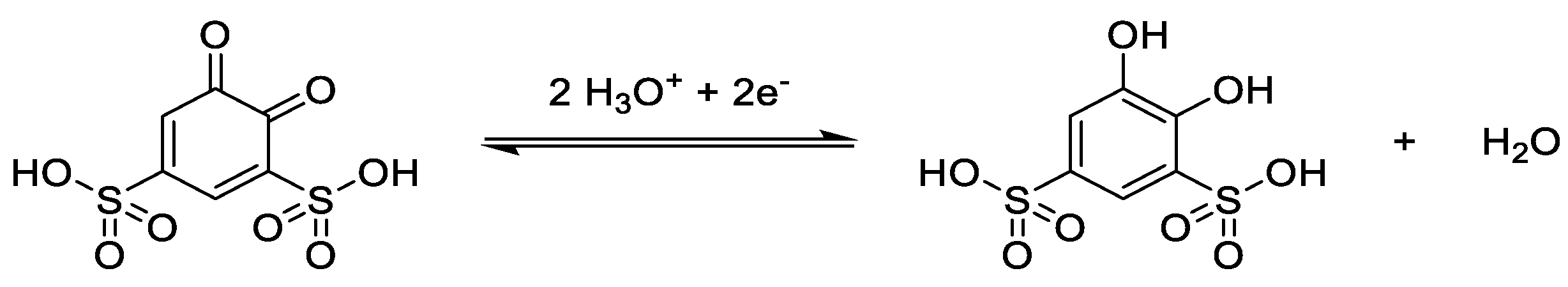
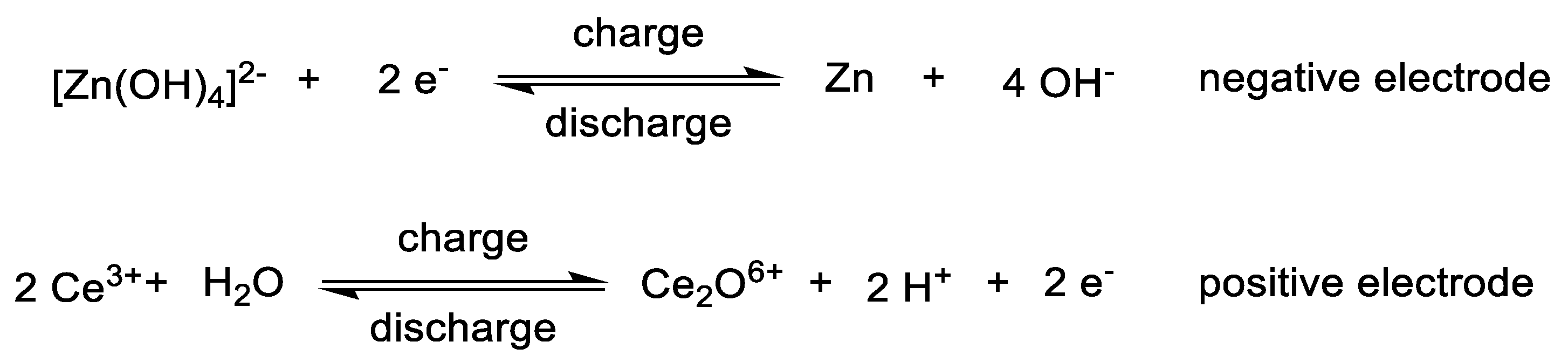





| 3 | Sign | Unit | Note |
|---|---|---|---|
| Theoretical capacity | Q | mAh | |
| State-of-charge | SOC | % | Charged capacity versus theoretical capacity |
| Current | I | A | |
| Current density | J | mA cm−2 | Current per membrane area |
| Open Circuit potential | OCV | V | Cell potential at zero current (also called open circuit voltage (OCV) and 50% SOC |
| Cell potential | U | V | |
| Coulombic efficiency | CE | % | Ratio of charge efficiency output per input |
| Voltage efficiency | VE | % | Ratio of mean discharge and charge voltage |
| Energy efficiency | EE | % | Ratio of mean discharge and charge energy |
| Name | Equation | Variables | |||
|---|---|---|---|---|---|
| Hagen-Poiseuille | Dynamic viscosity | Diffusion coefficient | |||
| Volume flow | Boltzmann constant | ||||
| Stokes-Einstein | Pressure loss | Molecule mobility | |||
| Inner radius of the pipe | Temperature | ||||
| Nernst-Einstein | Length of the pipe | Charge | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kortekaas, L.; Fricke, S.; Korshunov, A.; Cekic-Laskovic, I.; Winter, M.; Grünebaum, M. Building Bridges: Unifying Design and Development Aspects for Advancing Non-Aqueous Redox-Flow Batteries. Batteries 2023, 9, 4. https://doi.org/10.3390/batteries9010004
Kortekaas L, Fricke S, Korshunov A, Cekic-Laskovic I, Winter M, Grünebaum M. Building Bridges: Unifying Design and Development Aspects for Advancing Non-Aqueous Redox-Flow Batteries. Batteries. 2023; 9(1):4. https://doi.org/10.3390/batteries9010004
Chicago/Turabian StyleKortekaas, Luuk, Sebastian Fricke, Aleksandr Korshunov, Isidora Cekic-Laskovic, Martin Winter, and Mariano Grünebaum. 2023. "Building Bridges: Unifying Design and Development Aspects for Advancing Non-Aqueous Redox-Flow Batteries" Batteries 9, no. 1: 4. https://doi.org/10.3390/batteries9010004
APA StyleKortekaas, L., Fricke, S., Korshunov, A., Cekic-Laskovic, I., Winter, M., & Grünebaum, M. (2023). Building Bridges: Unifying Design and Development Aspects for Advancing Non-Aqueous Redox-Flow Batteries. Batteries, 9(1), 4. https://doi.org/10.3390/batteries9010004








