Experimental Study on Thermal Runaway Behavior of Lithium-Ion Battery and Analysis of Combustible Limit of Gas Production
Abstract
1. Introduction
2. Experimental Setting
2.1. Battery Sample
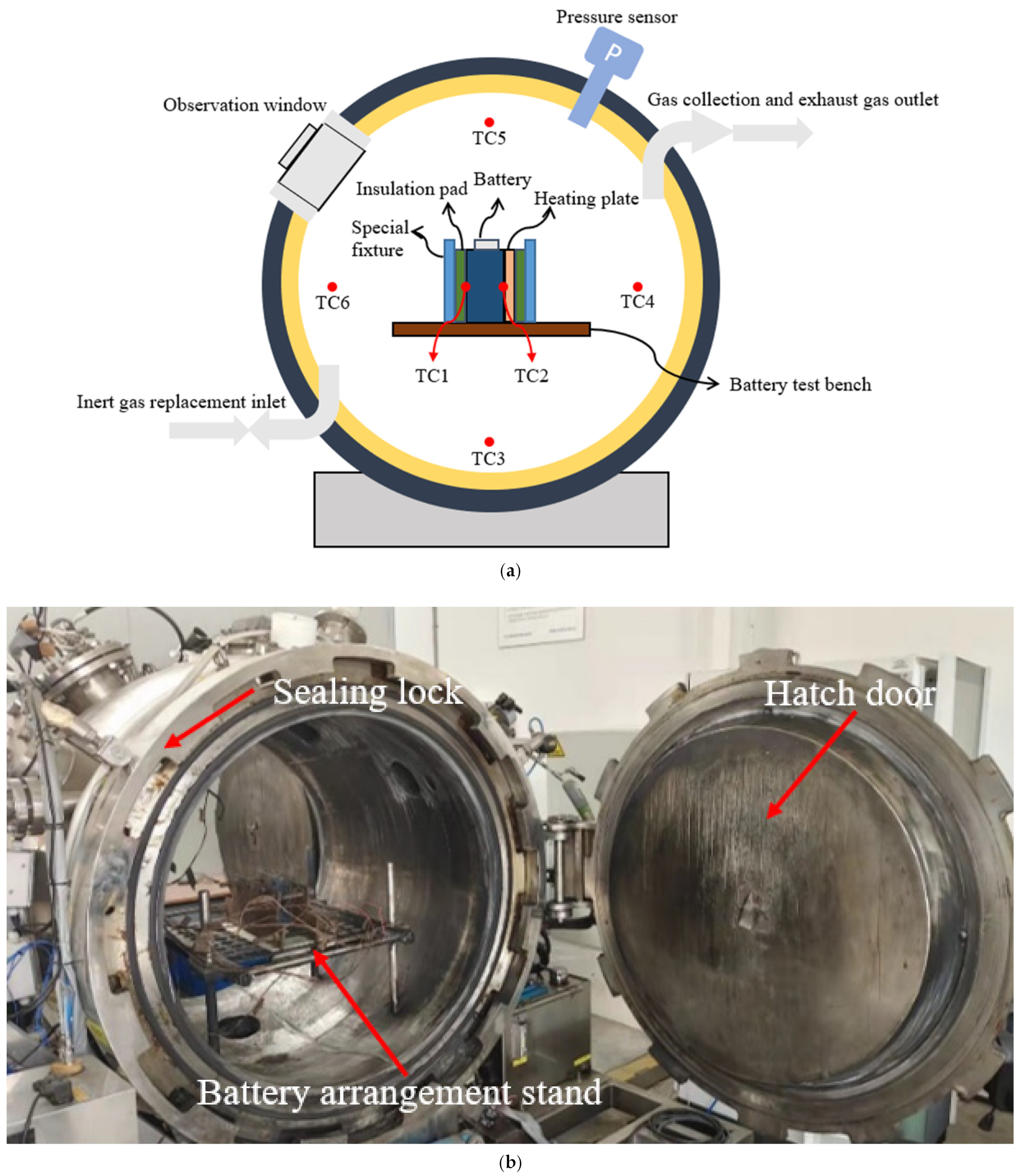
2.2. Experimental Instruments
2.3. Experimental Design
- (1)
- Measure the open circuit voltage of the battery, ensure that the battery is in the specified state of charge, and record the initial quality of the battery before the test;
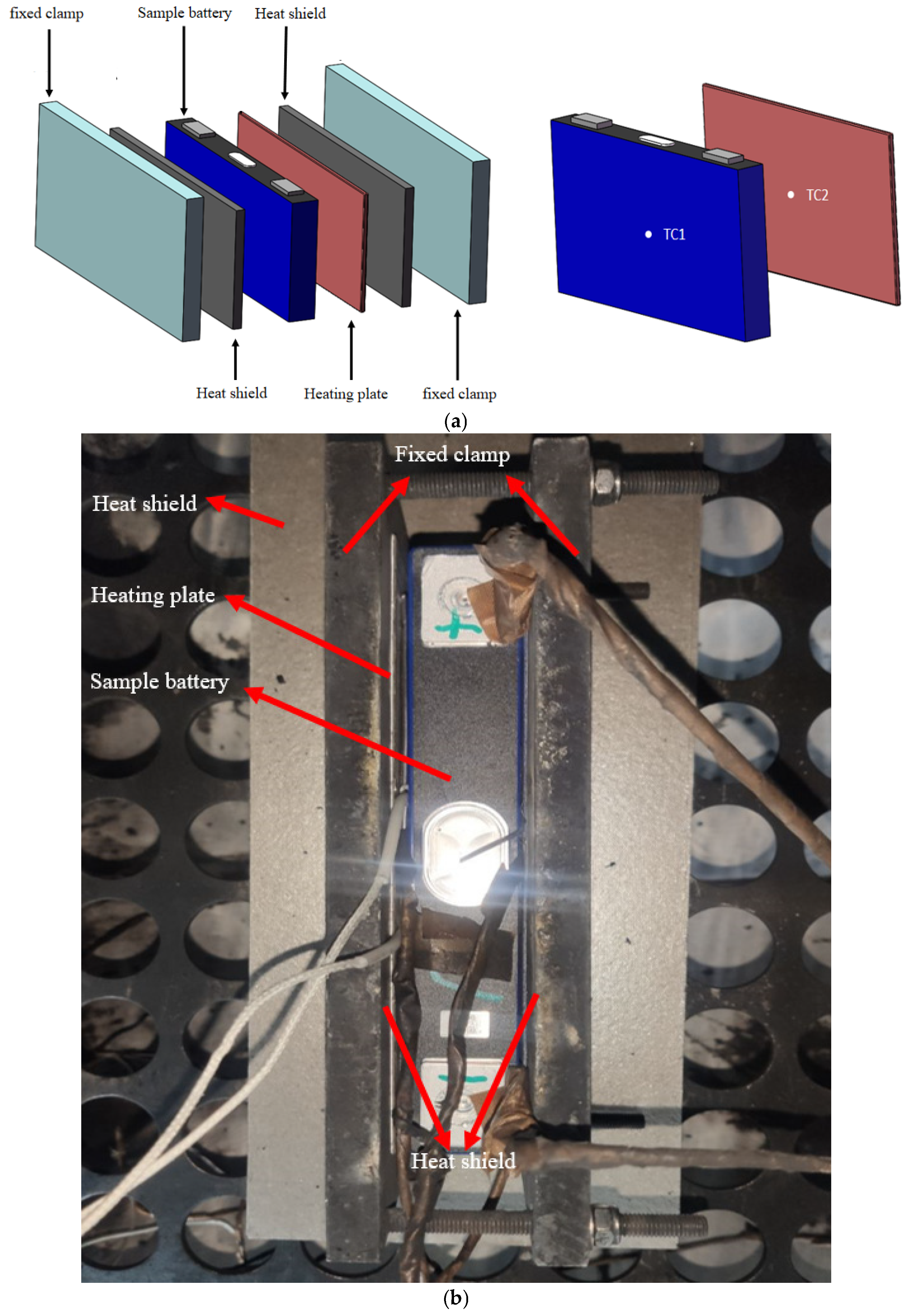
- (2)
- Arrange the battery, heating plate, and heat insulation plate on the battery rack, arrange the temperature measuring thermocouple and fix it with fixed clamps;
- (3)
- Vacuum the test chamber and rinse it with N2 to reach the specified experimental atmosphere;
- (4)
- Turn on the heating plate for heating until the battery TR is triggered.
3. Experimental Results and Analysis
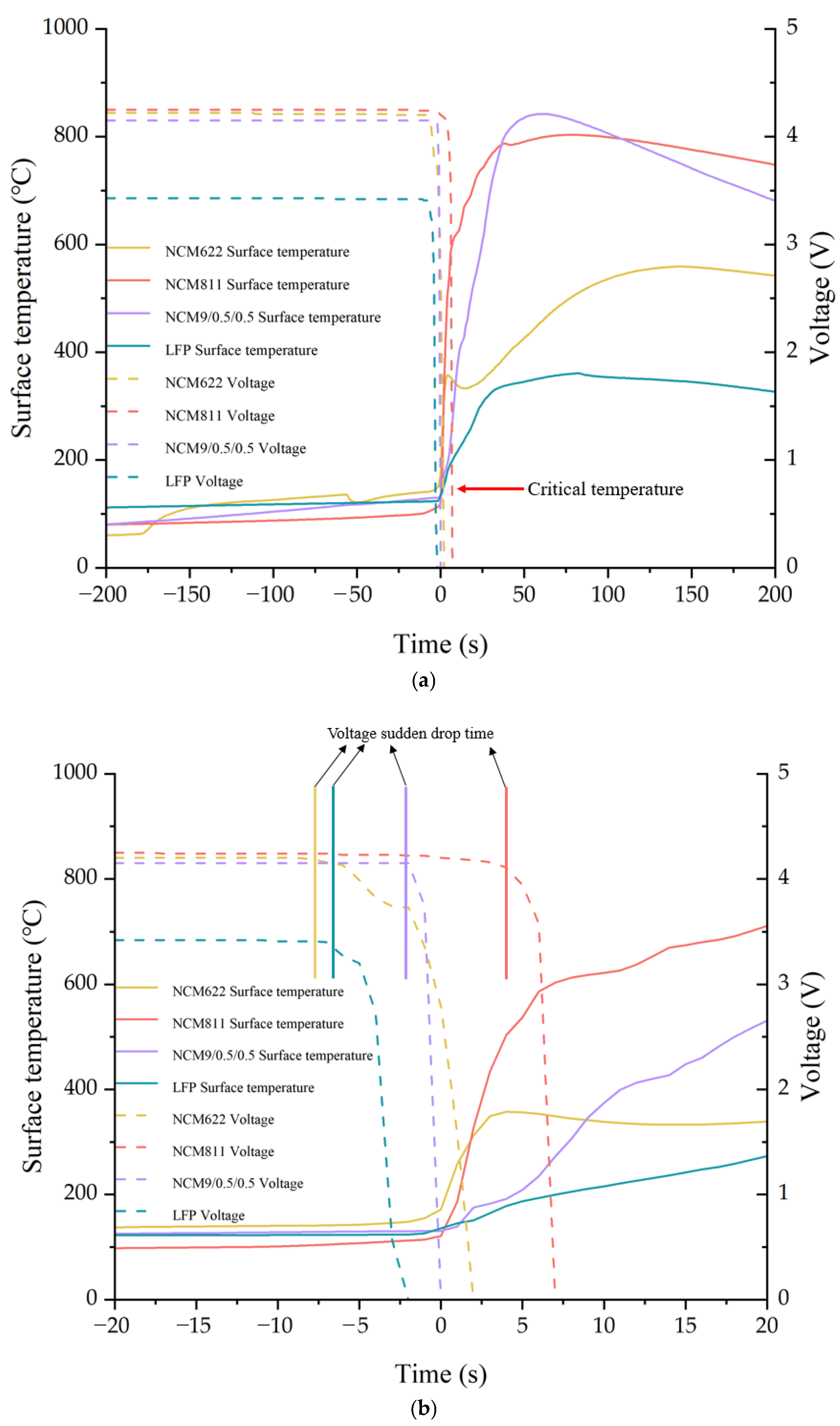
3.1. Battery Temperature and Voltage Changes
3.2. Battery Mass Loss
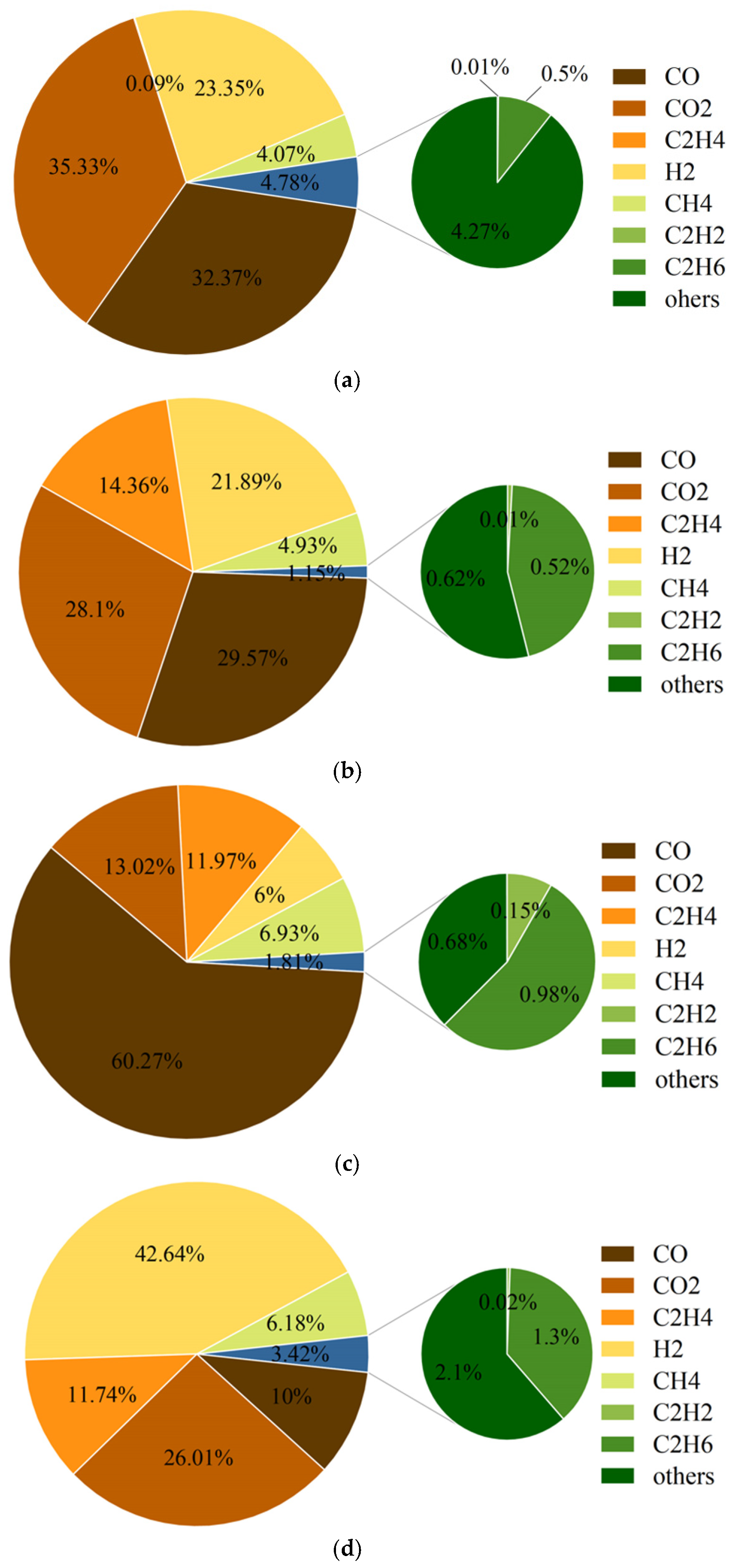
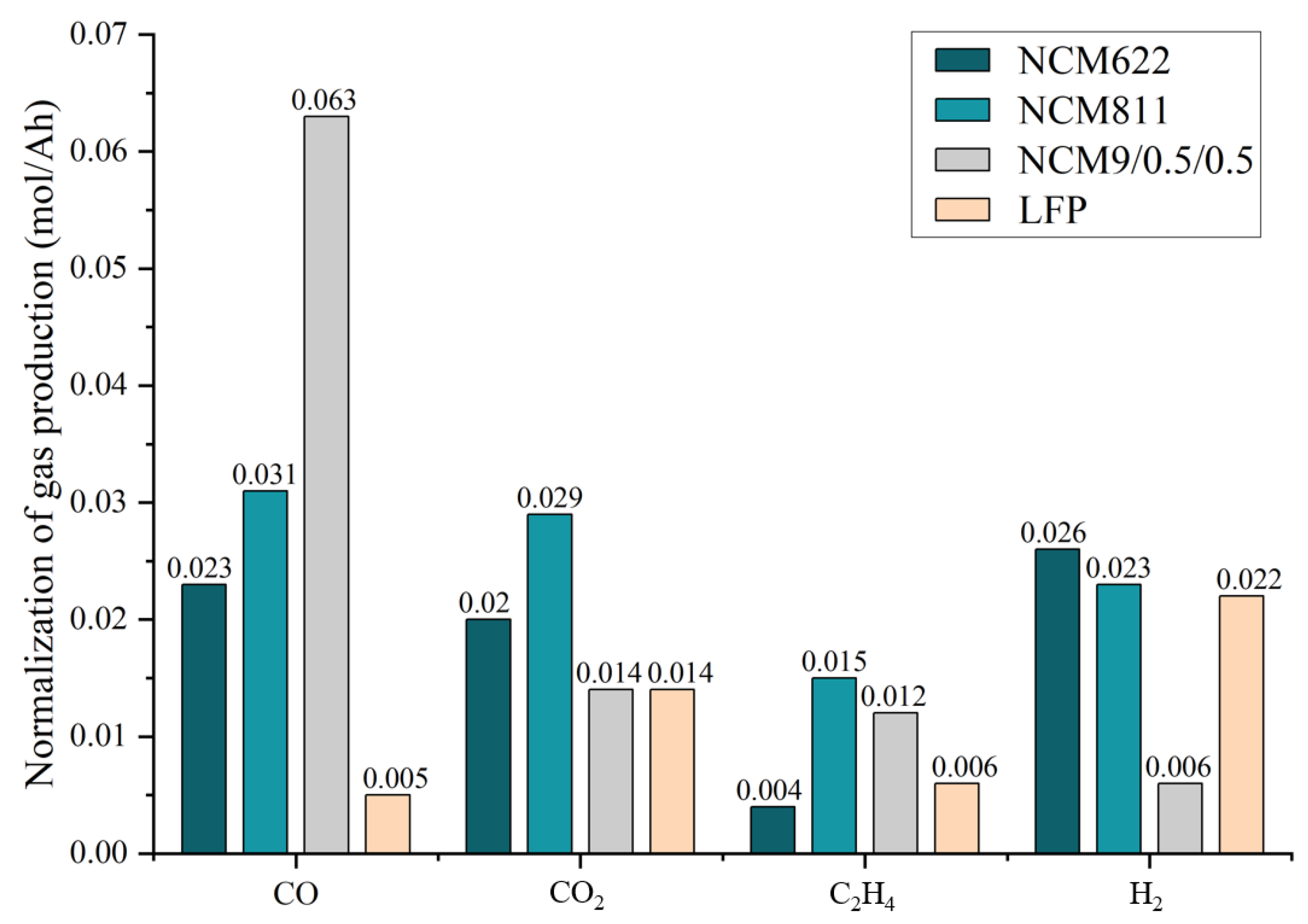
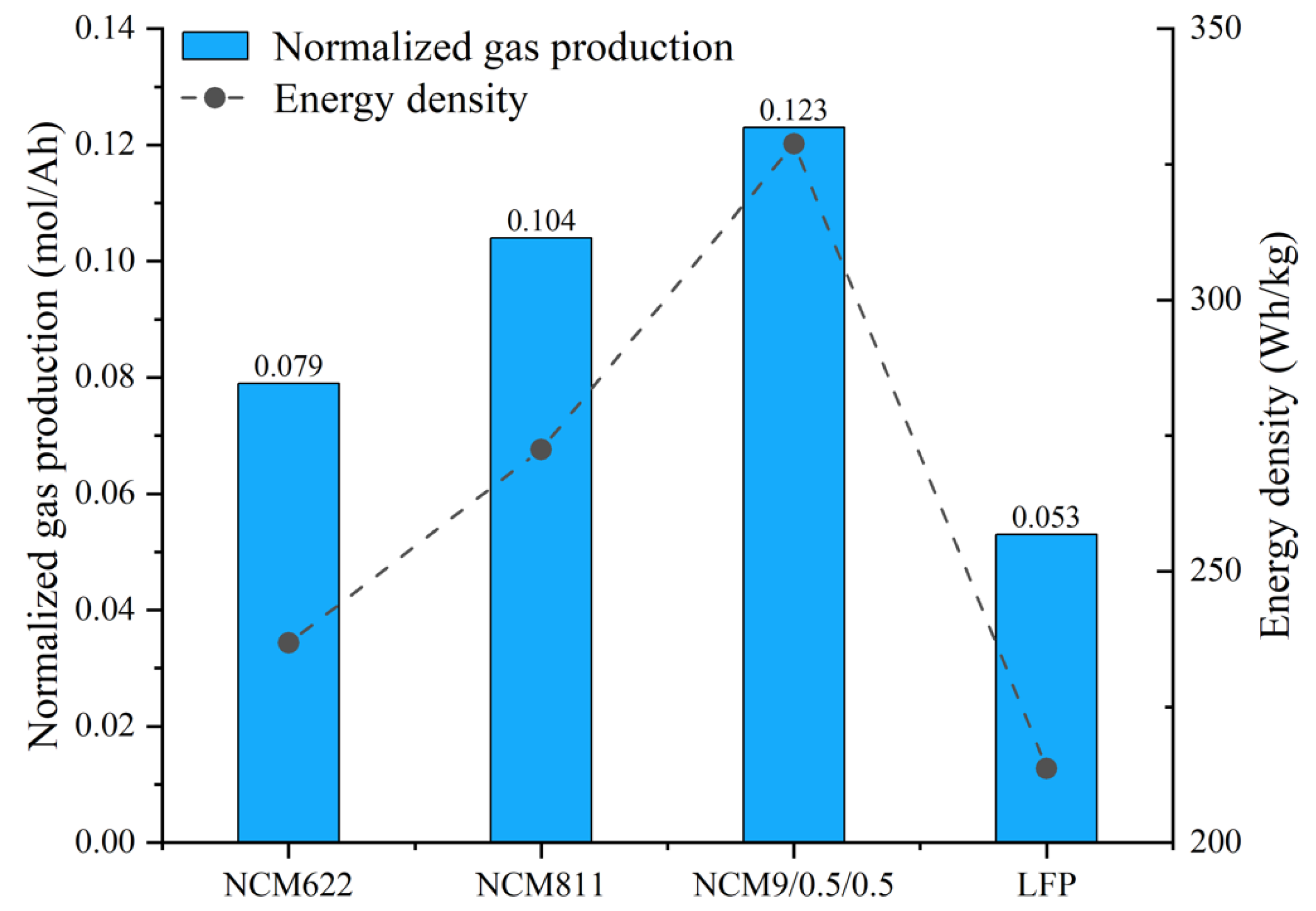
3.3. Gas Production and Composition
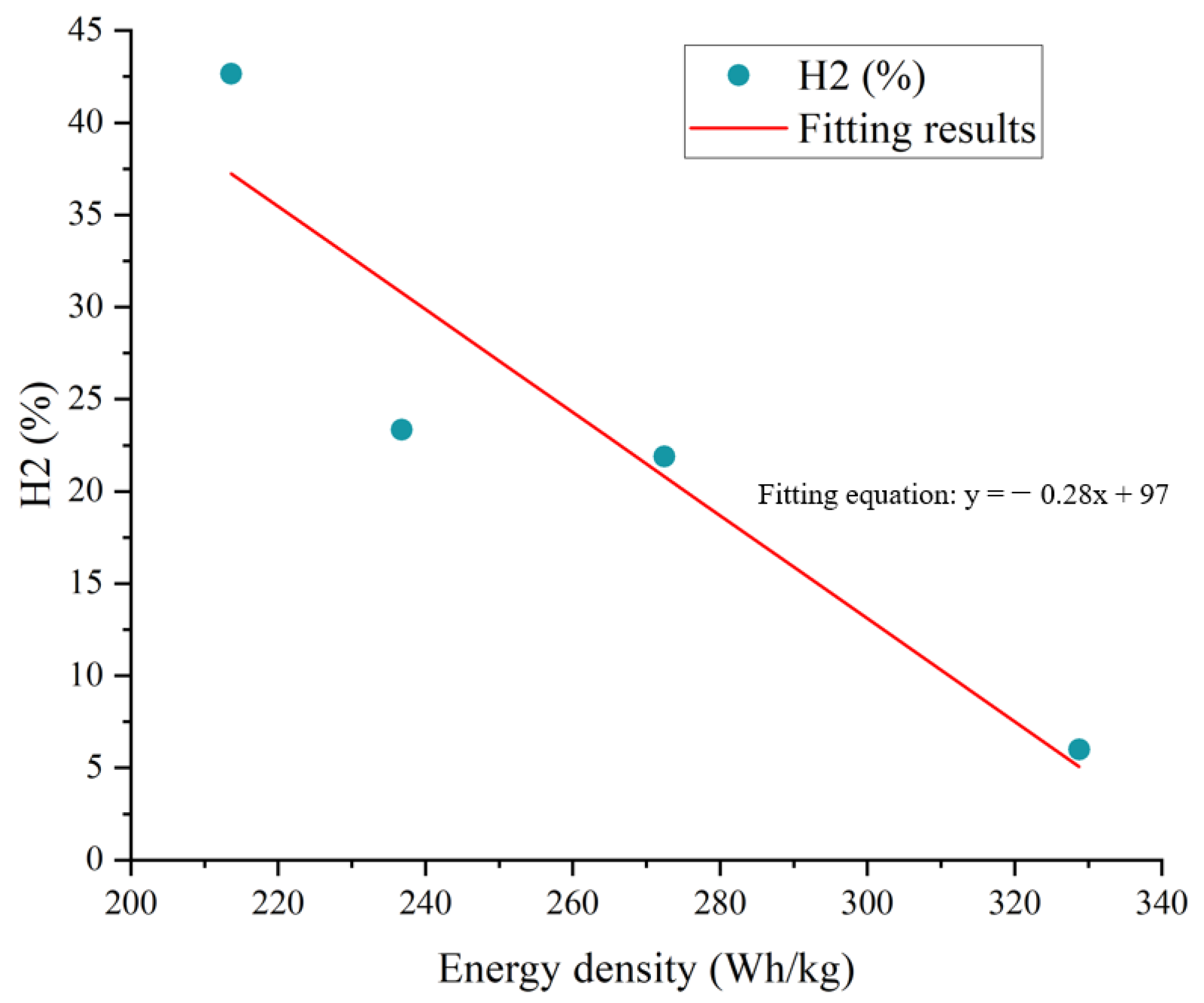
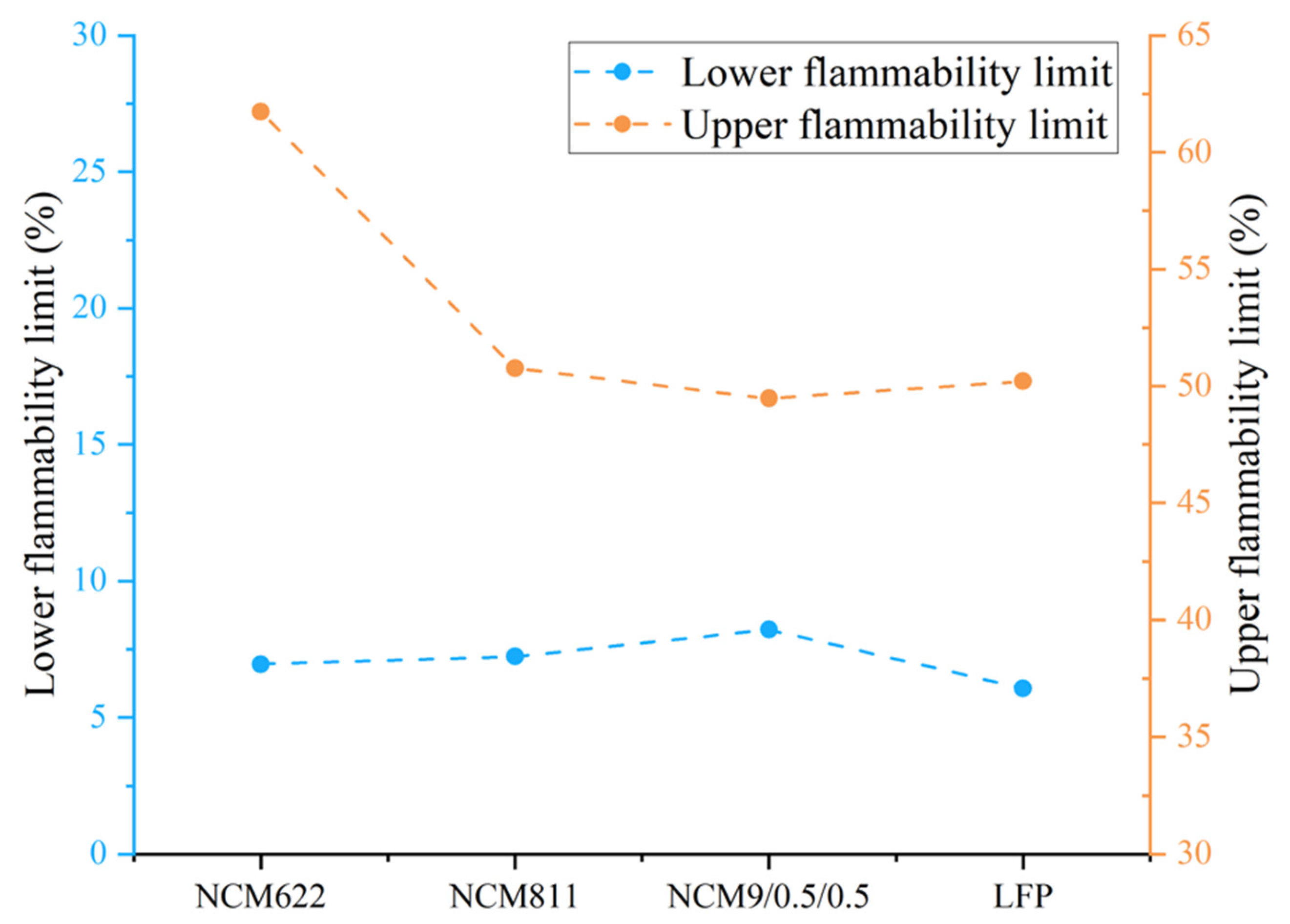
3.4. Gas Production Characteristics and Deflagration Limit
4. Conclusions and Summary
- (1)
- The critical triggering temperature of TR of the ternary high nickel system battery is lower than that of the LFP system, and the maximum temperature of the battery surface is much higher than that of the LFP system. Furthermore, the voltage drop of the battery during heating occurs 2–8 s before TR;
- (2)
- The NCM battery will eject gas and black solid particles during TR, while the LFP battery will eject unreacted electrolytes during TR, and the mass loss rate of the NCM battery during TR is higher than that of the LFP battery.
- (3)
- The batteries of the NCM system and the LFP system will produce CO, CO2, H2, CH4, C2H4, and other gases in the process of TR. The higher the energy density of the battery, the greater the concentration of CO gas produced, and the smaller the concentration of H2 gas. The normalized gas production of the NCM9/0.5/0.5 battery is the highest, and the normalized gas production of the LFP battery is the lowest.
- (4)
- The deflagration limit of gas generated by the TR of LIBs is related to the battery energy density. The higher the energy density, the lower the deflagration risk of gas generated. Among the four battery samples used in this study, the lower flammability limit of gas produced by the TR was that of the LFP battery.
5. Prospect Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Q.S.; Ping, P.; Zhao, X.J.; Chu, G.Q.; Sun, J.H.; Chen, C.H. Thermal runaway caused fire and explosion of lithium ion battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, G.; Zhu, J.; Feng, X.; Wei, X.; Ouyang, M.; Dai, H. Multi-objective optimization design and experimental investigation for a parallel liquid cooling-based Lithium-ion battery module under fast charging. Appl. Therm. Eng. 2022, 211, 118503. [Google Scholar] [CrossRef]
- Ou, H.; Huang, J.; Zhou, Y.; Zhu, J.; Fang, G.; Cao, X.; Li, J.; Liang, S. Surface-dominated ultra-stable sodium and potassium storage enabled by N/P/O tri-doped porous carbon. Chem. Eng. J. 2022, 450, 138444. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J. An overview of modification strategies to improve LiNi0·8Co0 1Mn0·1O2 (NCM811) cathode performance for automotive lithium-ion batteries. eTransportation 2021, 7, 100105. [Google Scholar] [CrossRef]
- Chen, J.M. Carbon neutrality, Toward a sustainable future. Innovation 2021, 2, 100127. [Google Scholar] [CrossRef]
- Fachrizal, R.; Shepero, M.; Van Der Meer, D.; Munkhammar, J.; Widen, J. Smart charging of electric vehicles considering photovoltaic power production and electricity consumption: A review. eTransportation 2020, 4, 100056. [Google Scholar] [CrossRef]
- Lyu, P.Z.; Liu, X.J.; Qu, J.; Zhao, J.T.; Huo, Y.T.; Qu, Z.G.; Rao, Z.H. Recent advances of thermal safety of lithium ion battery for energy storage. Energy Storage Mater. 2020, 31, 195–220. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wei, X.Z.; Zhu, J.G.; Dai, H.F.; Zheng, Y.J.; Xu, X.M.; Chen, Q.J. A review of modeling, acquisition, and application of lithium-ion battery impedance for onboard battery management. eTransportation 2021, 7, 100093. [Google Scholar] [CrossRef]
- Wu, C.J.; Wu, Y.; Xu, X.D.; Ren, D.S.; Li, Y.L.; Chang, R.Z.; Deng, T.; Feng, X.N.; Ouyang, M.G. Synergistic Dual-Salt Electrolyte for Safe and High-Voltage LiNi0.8Co0.1Mn0.1O2//Graphite Pouch Cells. ACS Appl. Mater. Interfaces 2022, 14, 10467–10477. [Google Scholar] [CrossRef]
- Amano, K.O.A.; Hahn, S.K.; Tschirschwitz, R.; Rappsilber, T.; Krause, U. An Experimental Investigation of Thermal Runaway and Gas Release of NMC Lithium-Ion Pouch Batteries Depending on the State of Charge Level. Batteries 2022, 8, 41. [Google Scholar] [CrossRef]
- Yu, W.; Guo, Y.; Shang, Z.; Zhang, Y.; Xu, S. A review on comprehensive recycling of spent power lithium-ion battery in China. eTransportation 2022, 11, 100155. [Google Scholar] [CrossRef]
- Marinaro, M.; Bresser, D.; Beyer, E.; Faguy, P.; Hosoi, K.; Li, H.; Sakovica, J.; Amine, K.; Wohlfahrt-Mehrens, M.; Passerini, S. Bringing forward the development of battery cells for automotive applications, Perspective of R&D activities in China, Japan, the EU and the USA. J. Power Sources 2020, 459, 228073. [Google Scholar]
- Dixon, J.; Bell, K. Electric vehicles, battery capacity, charger power, access to charging and the impacts on distribution networks. eTransportation 2020, 4, 100059. [Google Scholar] [CrossRef]
- Liao, C. Electrolytes and additives for batteries Part I: Fundamentals and insights on cathode degradation mechanisms. eTransportation 2020, 5, 100068. [Google Scholar] [CrossRef]
- Meng, X.D.; Li, S.; Fu, W.D.; Chen, Y.W.; Duan, Q.L.; Wang, Q.S. Experimental study of intermittent spray cooling on suppression for lithium iron phosphate battery fires. eTransportation 2022, 11, 100142. [Google Scholar] [CrossRef]
- Huang, W.S.; Feng, X.N.; Han, X.B.; Zhang, W.F.; Jiang, F.C. Questions and Answers Relating to Lithium-Ion Battery Safety Issues. Cell Rep. Phys. Sci. 2021, 2, 100285. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Qiu, Y.; Jiang, F. A review on passive and active strategies of enhancing the safety of lithium-ion batteries. Int. J. Heat Mass Transfer 2022, 184, 122288. [Google Scholar] [CrossRef]
- Wildfeuer, L.; Lienkamp, M. Quantifiability of inherent cell-to-cell variations of commercial lithium-ion batteries. eTransportation 2021, 9, 100129. [Google Scholar] [CrossRef]
- Lee, E.-P. Analysis of car fire cases related to a lithium battery and cause investigation technique. Fire Sci. Eng. 2019, 33, 98–106. [Google Scholar] [CrossRef]
- Meng, J.; Guo, H.; Niu, C.; Zhao, Y.; Xu, L.; Li, Q.; Mai, L. Advances in structure and property optimizations of battery electrode materials. Joule 2017, 1, 522–547. [Google Scholar] [CrossRef]
- Singh, A.K.; Cao, L.; Ma, J.; Seo, J.; Bakis, C.E.; Zhang, Y.C.; Hickner, M.A.; Rahn, C.D. Design, manufacture and test of a novel structural battery based on sandwich construction. J. Sandw. Struct. Mater. 2015, 17, 666–690. [Google Scholar] [CrossRef]
- Yin, H.F.; Ma, S.; Li, H.G.; Wen, G.L.; Santhanagopalan, S.; Zhang, C. Modeling strategy for progressive failure prediction in lithium-ion batteries under mechanical abuse. eTransportation 2021, 7, 100098. [Google Scholar] [CrossRef]
- Kovachev, G.; Ellersdorfer, C.; Gstrein, G.; Hanzu, L.; Wilkening, H.M.R.; Werling, T.; Schauwecker, F.; Sinz, W. Safety assessment of electrically cycled cells at high temperatures under mechanical crush loads. eTransportation 2020, 6, 100087. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Bai, J. Influences of multi factors on thermal runaway induced by overcharging of lithium-ion battery. J. Energy Chem. 2022, 70, 531–541. [Google Scholar] [CrossRef]
- Zhu, Y.; Xie, J.; Pei, A.; Liu, B.; Wu, Y.; Lin, D.; Li, J.; Wang, H.; Chen, H.; Xu, J.; et al. Fast lithium growth and short circuit induced by localized-temperature hotspots in lithium batteries. Nat. Commun. 2019, 10, 2067. [Google Scholar] [CrossRef]
- Dong, H.B.; Xian, X.L.; Ma, J.Q.; Yi, C.Y. Research on thermal runaway characteristics of lithium manganate battery. Fire Sci. Technol. 2022, 41, 21–25. [Google Scholar]
- Weng, J.; Ouyang, D.; Liu, Y.; Chen, M.; Li, Y.; Huang, X.; Wang, J. Alleviation on battery thermal runaway propagation, effects of oxygen level and dilution gas. J. Power Sources 2021, 509, 230340. [Google Scholar] [CrossRef]
- Liu, J.; Duan, Q.; Ma, M.; Zhao, C.; Sun, J.; Wang, Q. Aging mechanisms and thermal stability of aged commercial 18,650 lithium-ion battery induced by slight overcharging cycling. J. Power Sources 2020, 445, 227263. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, L.; Ju, X.; Liao, B.; Ye, K.; Li, L.; Cao, B.; Ni, Y. A comprehensive investigation on the thermal and toxic hazards of large format lithium-ion batteries with LiFePO4 cathode. J. Hazard. Mater. 2020, 381, 120916. [Google Scholar] [CrossRef]
- Koch, S.; Fill, A.; Birke, K.P. Comprehensive gas analysis on large scale automotive lithium-ion cells in thermal runaway. J. Power Sources 2018, 398, 106–112. [Google Scholar] [CrossRef]
- Yuan, L.M.; Dubaniewicz, T.; Zlochower, I.; Thomas, R.; Rayyan, N. Experimental study on thermal runaway and vented gases of lithium-ion cells. Process Saf. Environ. Prot. 2020, 144, 186–192. [Google Scholar] [CrossRef]
- Wu, T.Q.; Chen, H.D.; Wang, Q.S.; Sun, J.H. Comparison analysis on the thermal runaway of lithium-ion battery under two heating modes. J. Hazard. Mater. 2018, 344, 733–741. [Google Scholar] [CrossRef]
- Wang, H.; Xu, H.; Zhang, Z.; Wang, Q.; Jin, C.; Wu, C.; Xu, C.; Hao, J.; Sun, L.; Du, Z.; et al. Fire and explosion characteristics of vent gas from lithium-ion batteries after thermal runaway: A comparative study. eTransportation 2022, 13, 100190. [Google Scholar] [CrossRef]
- Baird, A.R.; Archibald, E.J.; Marr, K.C.; Ezekoye, O.A. Explosion hazards from lithium-ion battery vent gas. J. Power Sources 2020, 446, 227257. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Wang, H.W.; Li, W.F.; Li, C. Quantitative identification of emissions from abused prismatic Ni-rich lithium-ion batteries. eTransportation 2019, 2, 100031. [Google Scholar] [CrossRef]
- Zhong, G.; Mao, B.; Wang, C.; Jiang, L.; Xu, K.; Sun, J.; Wang, Q. Thermal runaway and fire behavior investigation of lithium-ion batteries using modified cone calorimeter. J. Therm. Anal. Calorim. 2019, 135, 2879–2889. [Google Scholar] [CrossRef]
- Bugryniec, P.J.; Davidson, J.N.; Brown, S.F. Assessment of thermal runaway in commercial lithium iron phosphate cells due to overheating in an oven test. 3rd Annual Conference on Energy Storage and its Applications (CDT-ESA-AC). Energy Procedia 2018, 151, 74–78. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Z.; Yan, W. Identification and characteristic analysis of powder ejected from a lithium-ion battery during thermal runaway at elevated temperatures. J. Hazard. Mater. 2020, 400, 123169. [Google Scholar] [CrossRef]
- Jia, Z.; Qin, P.; Li, Z.; Wei, Z.; Jin, K.; Jiang, L.; Wang, Q. Analysis of gas release during the process of thermal runaway of lithium-ion batteries with three different cathode materials. J. Energy Storage 2022, 50, 104302. [Google Scholar] [CrossRef]
- Xiang, H.F.; Wang, H.; Chen, C.H.; Ge, X.W.; Guo, S.; Sun, J.H.; Hu, W.Q. Thermal stability of LiPF6-based electrolyte and effect of contact with various delithiated cathodes of Li-ion batteries. J. Power Sources 2009, 191, 575–581. [Google Scholar] [CrossRef]
- Huang, Z.H.; Li, H.; Mei, W.X.; Zhao, C.P.; Sun, J.H.; Wang, Q.S. Thermal Runaway Behavior of Lithium Iron Phosphate Battery During Penetration. Fire Technol. 2020, 56, 2405–2426. [Google Scholar] [CrossRef]
- Sturk, D.; Rosell, L.; Blomqvist, P.; Tidblad, A.A. Analysis of Li-ion battery gases vented in an inert atmosphere thermal test chamber. Batteries 2019, 5, 61. [Google Scholar] [CrossRef]
- Jiang, J.W.; Fortier, H.; Reimers, J.N.; Dahn, J.R. Thermal stability of 18,650 size Li-ion cells containing LiBOB electrolyte salt. J. Electrochem. Soc. 2004, 151, A609–A613. [Google Scholar] [CrossRef]
- Yang, H.; Zhuang, G.V.; Ross, P.N. Thermal stability of LiPF6 salt and Li-ion battery electrolytes containing LiPF6. J. Power Sources 2006, 161, 573–579. [Google Scholar] [CrossRef]
- Li, W.F.; Wang, H.W.; Zhang, Y.J.; Ouyang, M.G. Flammability characteristics of the battery vent gas: A case of NCA and LFP lithium-ion batteries during external heating abuse. J. Energy Storage 2019, 24, 100775. [Google Scholar] [CrossRef]
- Luo, Z.; Liang, H.; Wang, T.; Cheng, F.; Su, B.; Liu, L.; Liu, B. Evaluating the effect of multiple flammable gases on the flammability limit of CH4: Experimental study and theoretical calculation. Process Saf. Environ. Prot. 2021, 146, 369–376. [Google Scholar] [CrossRef]
- Hu, X.; Yu, Q.; Sun, N.; Qin, Q. Experimental study of flammability limits of oxy-methane mixture and calculation based on thermal theory. Int. J. Hydrog. Energy 2014, 39, 9527–9533. [Google Scholar] [CrossRef]
- Wang, T.; Liang, H.; Luo, Z.; Su, B.; Liu, L.; Su, Y.; Wang, X.; Cheng, F.; Deng, J. Near flammability limits behavior of methane-air mixtures with influence of flammable gases and nitrogen: An experimental and numerical research. Fuel 2021, 294, 120550. [Google Scholar] [CrossRef]
- Huang, L.; Pei, S.; Wang, Y.; Zhang, L.; Ren, S.; Zhang, Z.; Xiao, Y. Assessment of flammability and explosion risks of natural gas-air mixtures at high pressure and high temperature. Fuel 2019, 247, 47–56. [Google Scholar] [CrossRef]



| Sample Name | Nominal Voltage (V) | Rated Capacity (Ah) | Energy Density (Wh/kg) | Battery Quality (g) | State of Charge (%) |
|---|---|---|---|---|---|
| NCM622 | 4.3 | 50 | 236.78 | 908 | 100 |
| NCM811 | 4.3 | 115 | 272.45 | 1815 | 100 |
| NCM9/0.5/0.5 | 4.3 | 165 | 328.78 | 2158 | 100 |
| LFP | 3.65 | 196 | 213.62 | 3349 | 100 |
| Sample Name | Thermal Runaway Trigger Temperature (°C) | Thermal Runaway Maximum Temperature (°C) | Mass Loss Rate (%) | Exhaust Time (s) | Thermal Runaway Product |
|---|---|---|---|---|---|
| NCM622 | 154.7 | 559.1 | 38.12 | 76 | Gas and solid particles |
| NCM811 | 120.8 | 803.4 | 37.80 | 84 | Gas and solid particles |
| NCM9/0.5/0.5 | 130.8 | 842.1 | 72.89 | 62 | Gas and solid particles |
| LFP | 135.4 | 360.9 | 22.80 | 408 | Gas and unreacted electrolyte |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Wang, H.; Li, M.; Li, Y.; Li, C.; Zhang, Y.; Chen, S.; Shen, H.; Qian, F.; Feng, X.; et al. Experimental Study on Thermal Runaway Behavior of Lithium-Ion Battery and Analysis of Combustible Limit of Gas Production. Batteries 2022, 8, 250. https://doi.org/10.3390/batteries8110250
Yang X, Wang H, Li M, Li Y, Li C, Zhang Y, Chen S, Shen H, Qian F, Feng X, et al. Experimental Study on Thermal Runaway Behavior of Lithium-Ion Battery and Analysis of Combustible Limit of Gas Production. Batteries. 2022; 8(11):250. https://doi.org/10.3390/batteries8110250
Chicago/Turabian StyleYang, Xinwei, Hewu Wang, Minghai Li, Yalun Li, Cheng Li, Yajun Zhang, Siqi Chen, Hengjie Shen, Feng Qian, Xuning Feng, and et al. 2022. "Experimental Study on Thermal Runaway Behavior of Lithium-Ion Battery and Analysis of Combustible Limit of Gas Production" Batteries 8, no. 11: 250. https://doi.org/10.3390/batteries8110250
APA StyleYang, X., Wang, H., Li, M., Li, Y., Li, C., Zhang, Y., Chen, S., Shen, H., Qian, F., Feng, X., & Ouyang, M. (2022). Experimental Study on Thermal Runaway Behavior of Lithium-Ion Battery and Analysis of Combustible Limit of Gas Production. Batteries, 8(11), 250. https://doi.org/10.3390/batteries8110250









