Effect of Si-Based Anode Lithiation on Charging Characteristics of All-Solid-State Lithium-Ion Battery
Abstract
:1. Introduction
2. Experimental
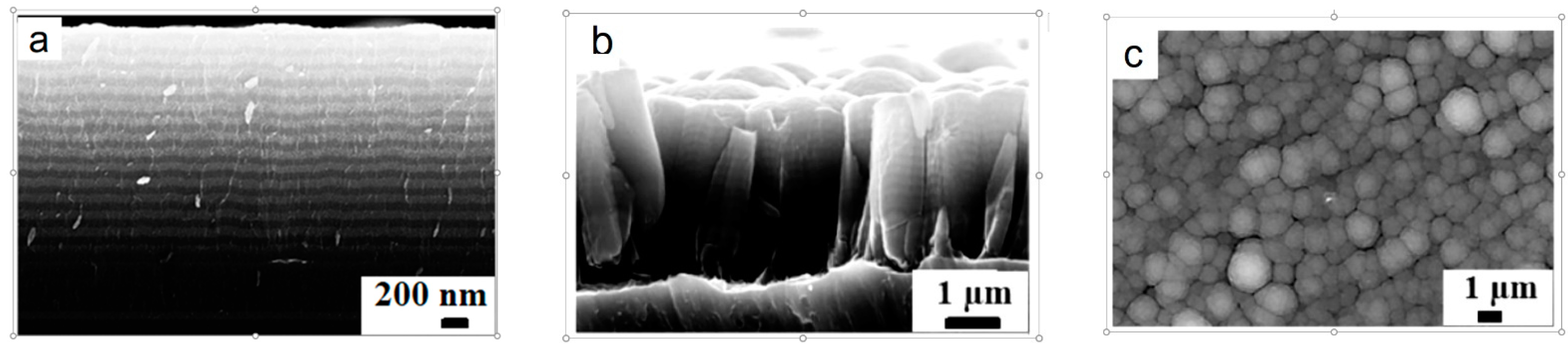
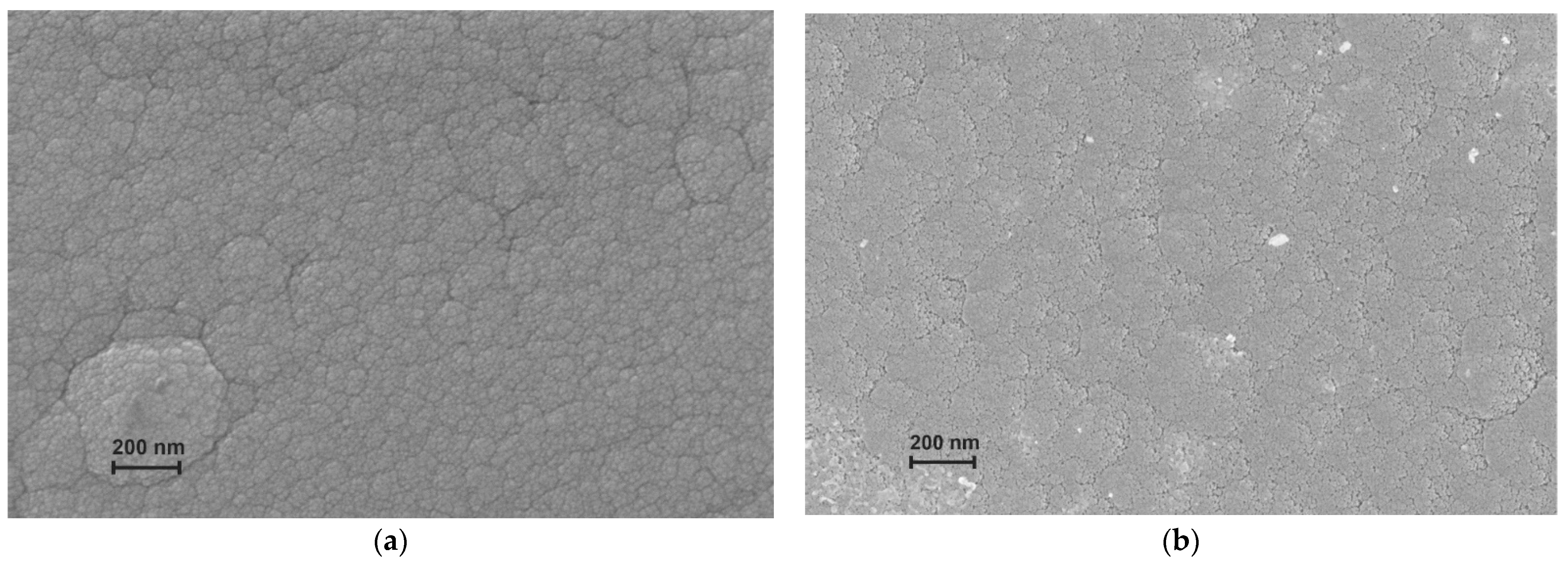
2.1. Si@O@Al Electrode Manufacturing and Characterization
- MVU TM Magna 10 (developed by JSC “Research Institute of Precision Machine Manufacturing”, Zelenograd, Russia), equipped with two magnetrons which were used for separate Si and Al depositions;
- Oratorio 22 system (idem) equipped with four magnetrons connected in pairs.
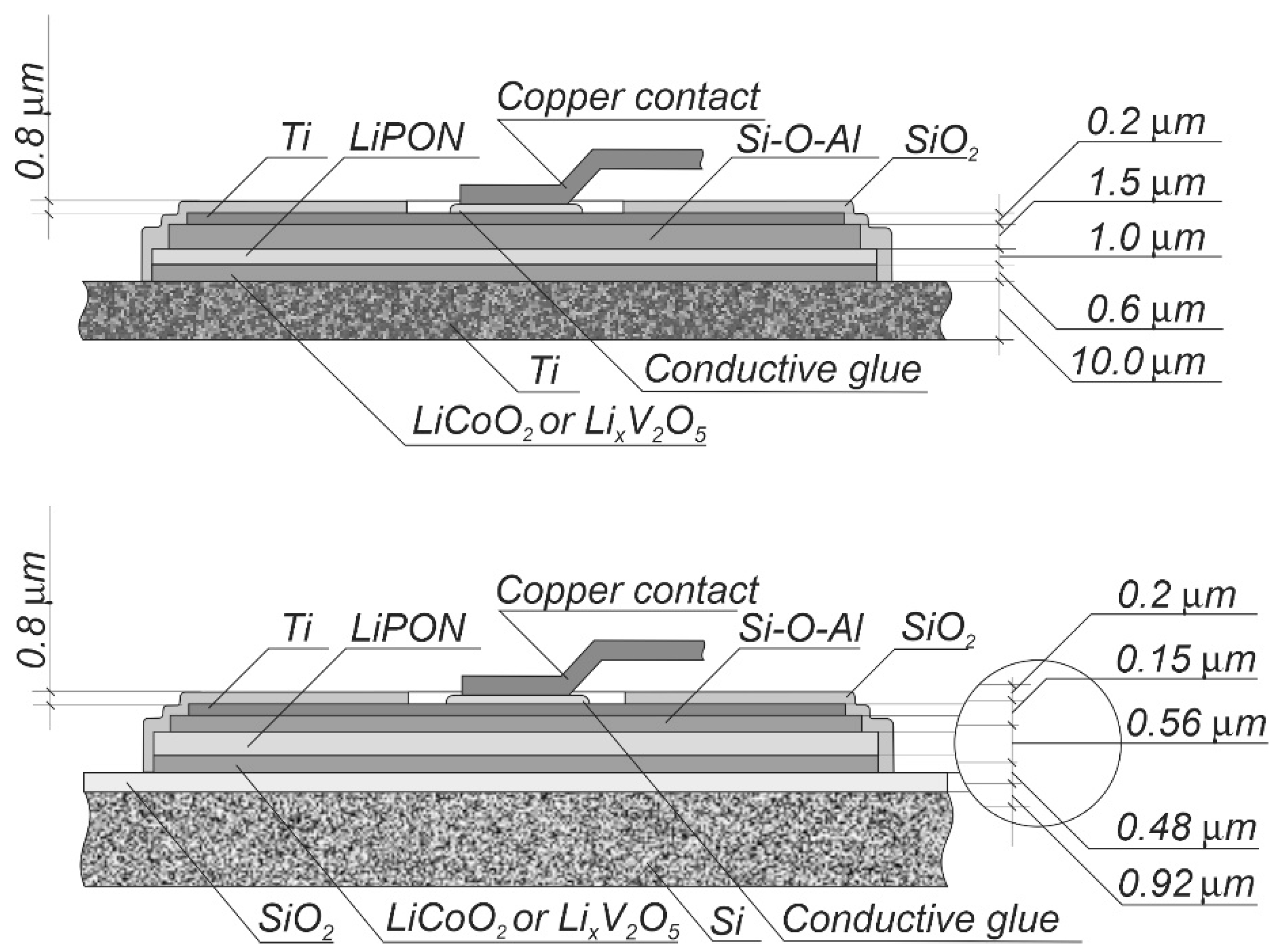
2.2. SSLIB Prototypes Manufacturing
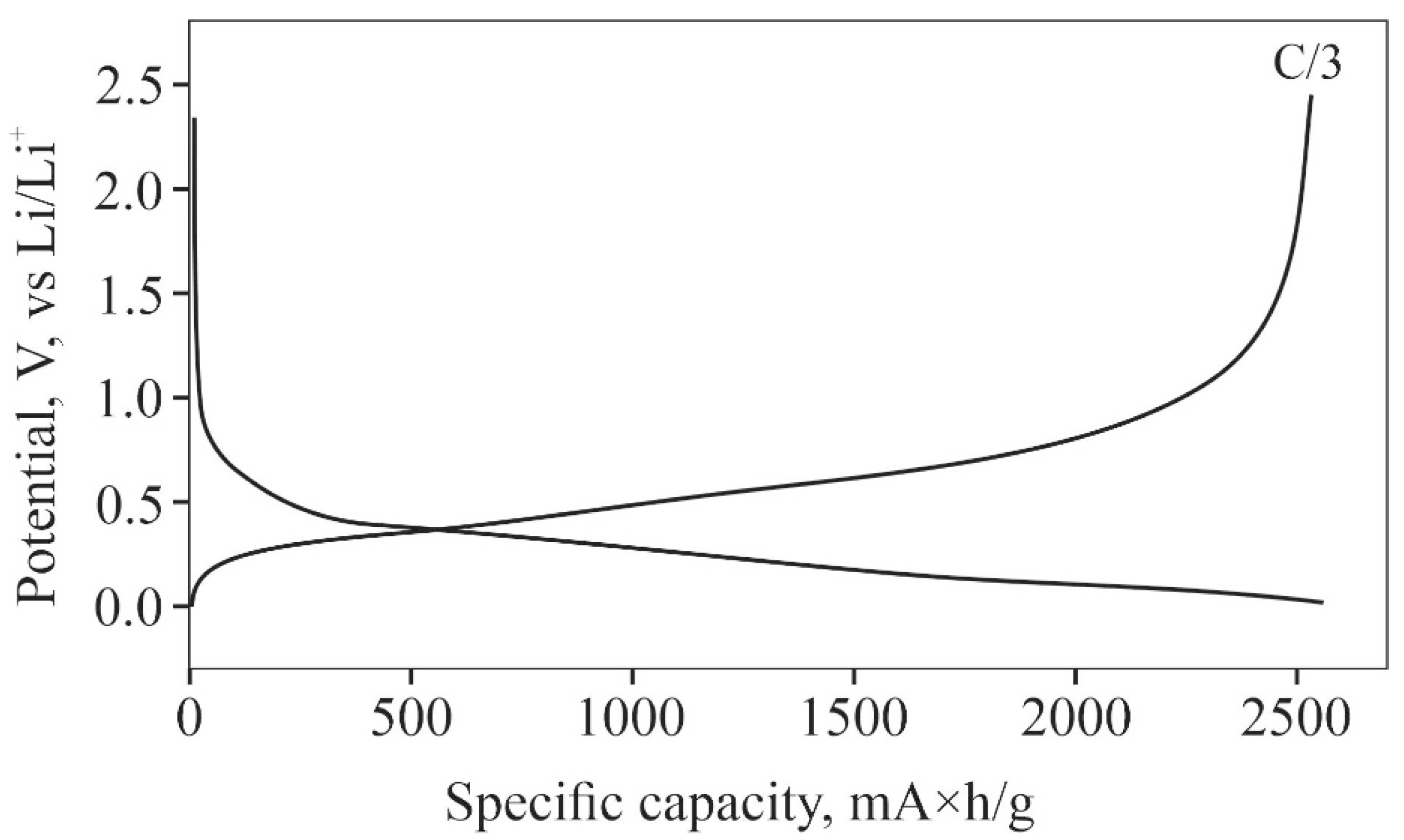
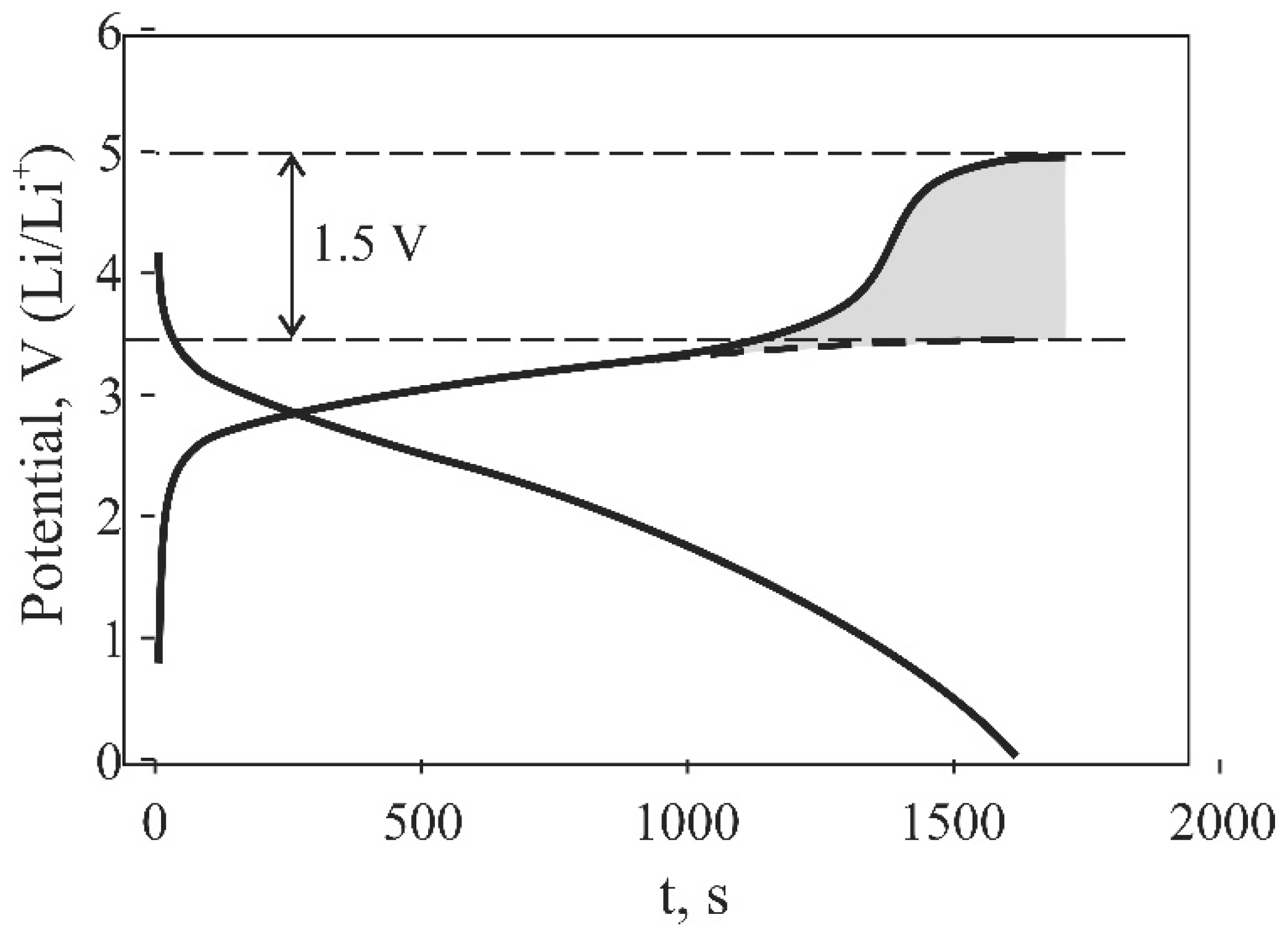
2.3. Features of Si@O@Al Electrodes Charging
3. Discussion
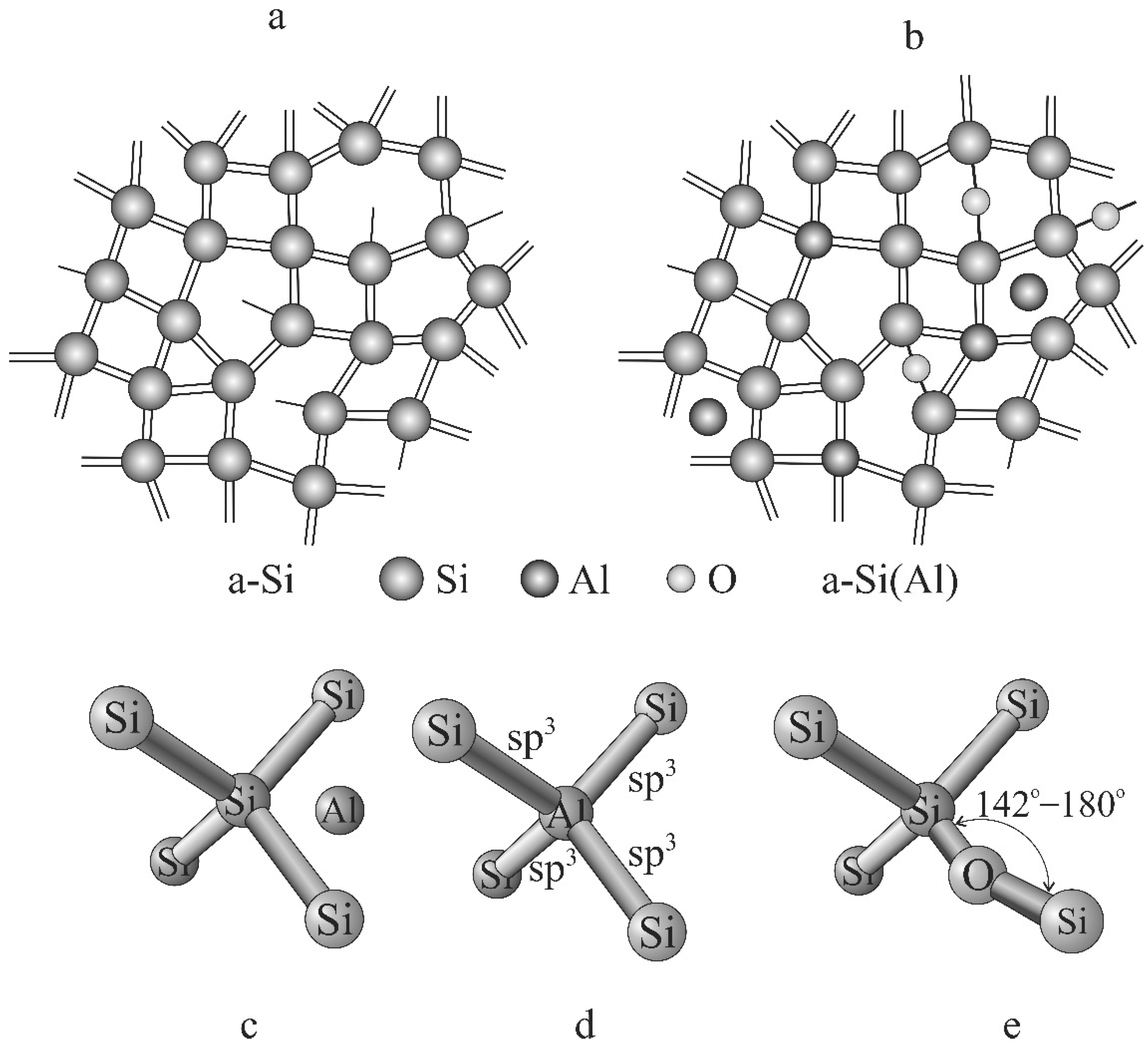
3.1. Structure and Electrical Properties of the Si@O@Al Nanocomposite
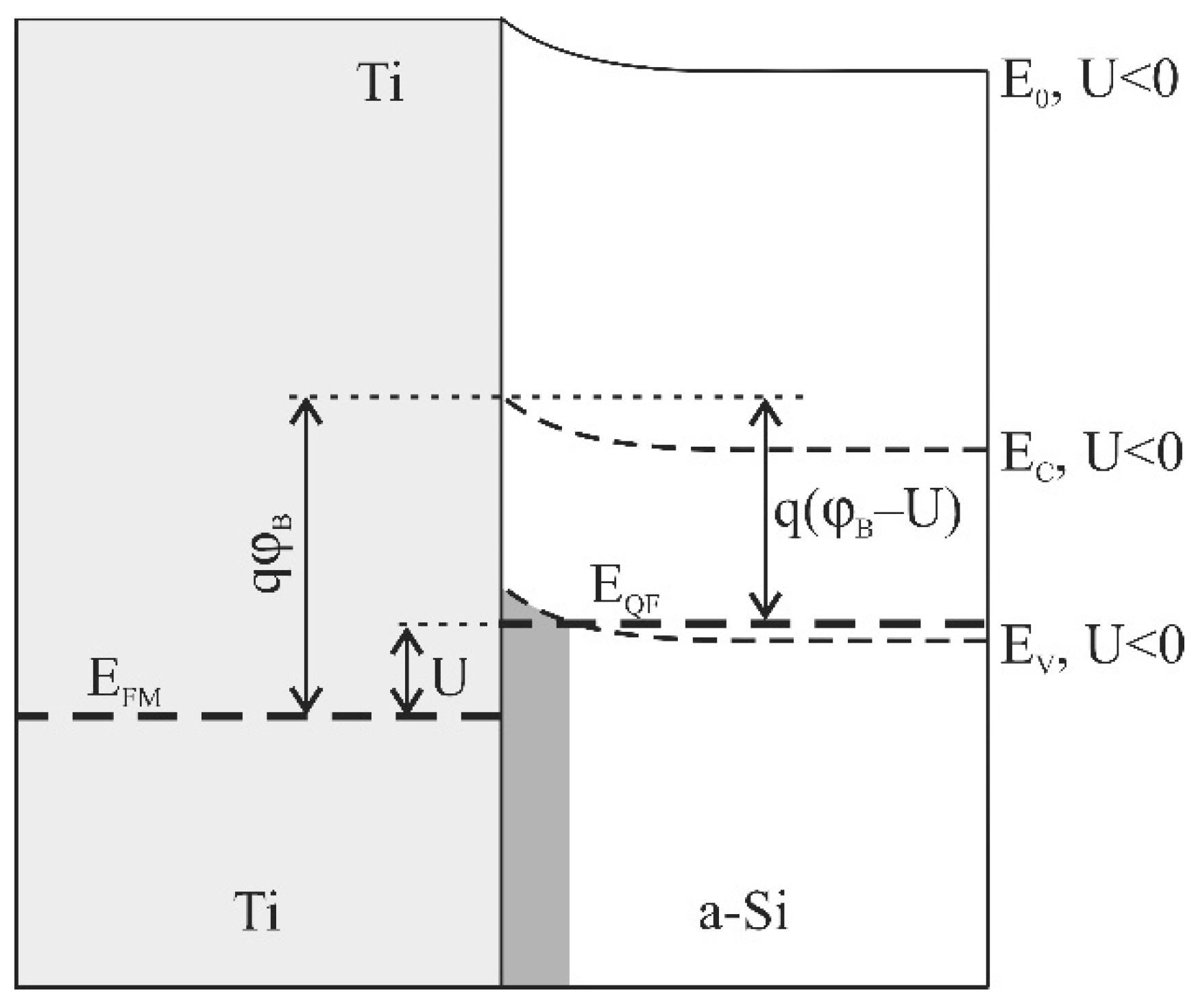
3.2. Model of Hike Formation during Lithiation of the Si@O@Al Electrode
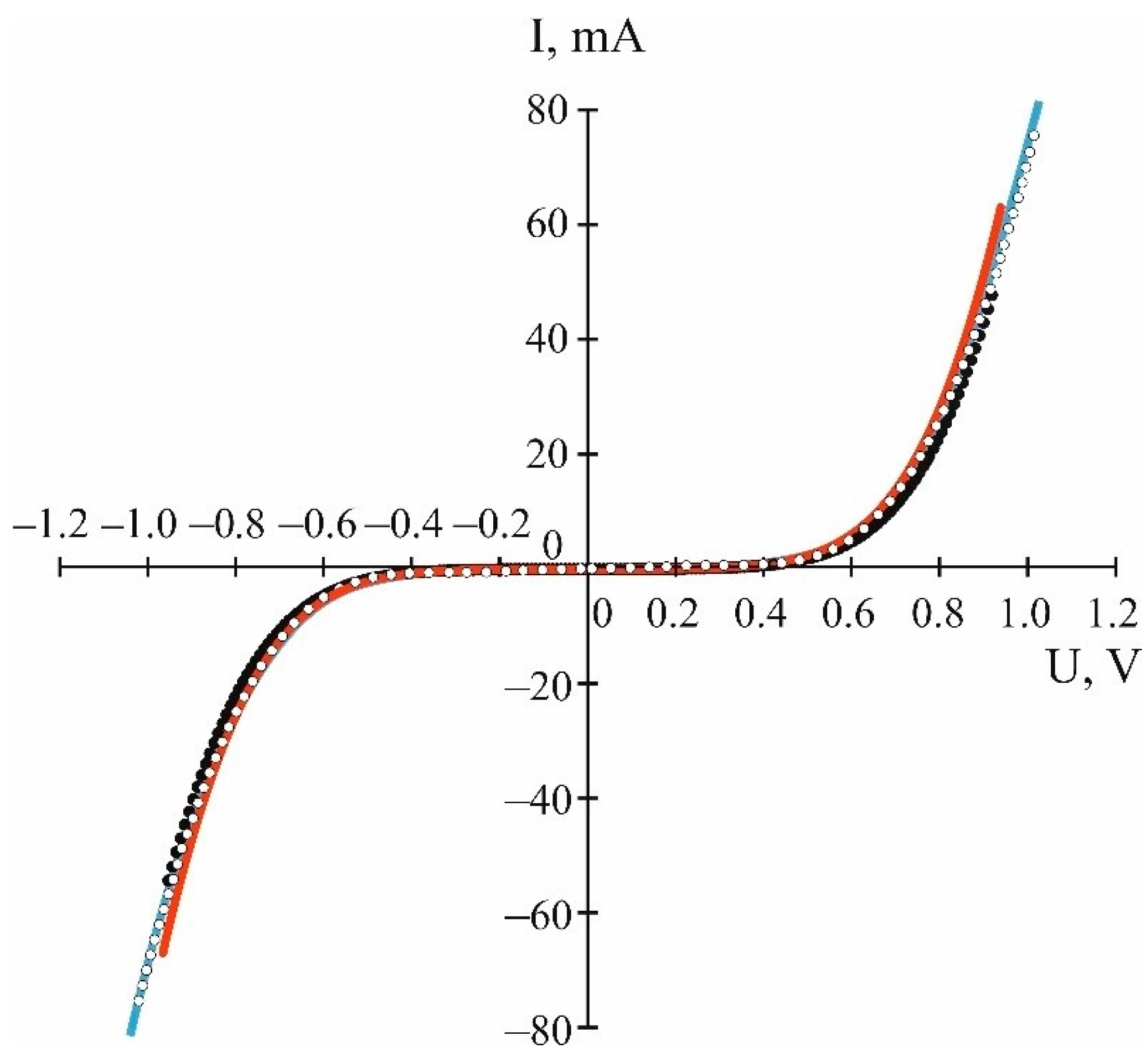
3.3. Experimental Confirmation of the Hole Conductivity of Si@O@Al
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Skundin, A.; Kulova, T.; Rudy, A.; Mironenko, A. All Solid State Thin-Film Lithium-Ion Batteries: Materials, Technology, and Diagnostics, 1st ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Boca Raton, FL, USA, 2021; 214p, ISBN 9780367086824. [Google Scholar]
- Cymbet.com. Available online: https://www.cymbet.com/wp-content/uploads/2019/02/DS-72-41.pdf (accessed on 9 June 2022).
- Jones, S.D.; Akridge, J.R. Thin film rechargeable Li batteries. Solid State Ion. 1994, 69, 357–368. [Google Scholar] [CrossRef]
- Bates, J.B.; Dudney, N.J.; Neudecker, B.; Ueda, A.; Evans, C.D. Thin-film lithium and lithium-ion batteries. Solid State Ion. 2000, 135, 33–45. [Google Scholar] [CrossRef]
- Souquet, J.L.; Duclot, M. Thin film lithium batteries. Solid State Ion. 2002, 148, 375–379. [Google Scholar] [CrossRef]
- Patil, A.; Patil, V.; Shin, D.W.; Choi, J.-W.; Paik, D.-S.; Yoon, S.-J. Issue and challenges facing rechargeable thin film lithium batteries. Mater. Res. Bull. 2008, 43, 1913–1942. [Google Scholar] [CrossRef]
- Dudney, N. Thin film micro-batteries. Interface 2008, 3, 44–48. [Google Scholar] [CrossRef]
- Sun, C.; Liu, J.; Gong, Y.; Wilkinson, D.P.; Zhang, J. Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy 2017, 33, 363–386. [Google Scholar] [CrossRef]
- Zhou, Y.N.; Xue, M.Z.; Fu, Z.-W. Nanostructured thin film electrodes for lithium storage and all-solid-state thin-film lithium batteries. J. Power Sources 2013, 234, 310–332. [Google Scholar] [CrossRef]
- Zuo, X.; Zhu, J.; Müller-Buschbaum, P.; Cheng, Y.-J. Silicon based lithium-ion battery anodes: A chronicle perspective review. Nano Energy 2017, 31, 113–143. [Google Scholar] [CrossRef]
- Feng, K.; Li, M.; Liu, W.; Kashkooli, A.G.; Xiao, X.; Cai, M.; Chen, Z. Silicon-based anodes for lithium-ion batteries: From fundamentals to practical applications. Small 2018, 14, 1702737. [Google Scholar] [CrossRef]
- Zhang, W.-J. A review of the electrochemical performance of alloy anodes for lithium-ion batteries. J. Power Sources 2011, 196, 13–24. [Google Scholar] [CrossRef]
- Szczech, J.R.; Jin, S. Nanostructured silicon for high capacity lithium battery anodes. Energy Environ. Sci. 2011, 4, 56–72. [Google Scholar] [CrossRef]
- Liang, B.; Liu, Y.; Xu, Y. Silicon-based materials as high capacity anodes for next generation lithium ion batteries. J. Power Sources 2014, 267, 469–490. [Google Scholar] [CrossRef]
- Kulova, T.L.; Skundin, A.M.; Andreev, V.N.; Gryzlov, D.Y.; Mironenko, A.A.; Rudyi, A.S.; Gusev, V.N.; Naumov, V.V. Cyclic Voltammetry Studies of Silicon–Aluminum Thin-Film Electrodes Synthesized in the Presence of Oxygen. Russ. J. Electrochem. 2015, 51, 1157–1161. [Google Scholar] [CrossRef]
- Kulova, T.L.; Mironenko, A.A.; Skundin, A.M.; Rudy, A.S.; Naumov, V.V.; Pukhov, D.E. Study of Silicon Composite for Negative Electrode of Lithium-Ion Battery. Intern. J. Electrochem. Sci. 2016, 11, 1370–1381. [Google Scholar]
- Airapetov, A.A.; Vasiliev, S.V.; Kulova, T.L.; Lebedev, M.E.; Metlitskaya, A.V.; Mironenko, A.A.; Nikol’skaya, N.F.; Odinokov, V.V.; Pavlov, G.Y.; Pukhov, D.E.; et al. Thin Film Negative Electrode Based on Silicon Composite or Lithium-Ion Batteries. Russ. Microelectron. 2016, 45, 285–291. [Google Scholar] [CrossRef]
- Mironenko, A.A.; Fedorov, I.S.; Rudy, A.S.; Andreev, V.N.; Gryzlov, D.Y.; Kulova, T.L.; Skundin, A.M. Charge–discharge performances of the Si–O–Al electrodes. Mon. Chem. Chem. Mon. 2019, 150, 1753–1759. [Google Scholar] [CrossRef]
- Ohara, S.; Junji Suzuki, J.; Kyoichi Sekine, K.; Takamura, T. A thin film silicon anode for Li-ion batteries having a very large specific capacity and long cycle life. J. Power Sources 2004, 136, 303–306. [Google Scholar] [CrossRef]
- Park, M.S.; Wang, G.X.; Liu, H.K.; Dou, S.X. Electrochemical properties of Si thin film prepared by pulsed laser deposition for lithium ion micro-batteries. Electrochim. Acta 2006, 51, 5246–5249. [Google Scholar] [CrossRef]
- Uehara, M.; Suzuki, J.; Tamura, K.; Sekine, K.; Takamura, T. Thick vacuum deposited silicon films suitable for the anode of Li-ion battery. J. Power Sources 2005, 146, 441–444. [Google Scholar] [CrossRef]
- Zhao, L.; Dvorak, D.J.; Obrovac, M.N. Layered amorphous silicon as negative electrodes in lithium-ion batteries. J. Power Sources 2016, 332, 290–298. [Google Scholar] [CrossRef]
- Reuter, F.; Baasner, A.; Pampel, J.; Piwko, M.; Dörfler, S.; Althues, H.; Kaskel, S. Importance of Capacity Balancing on The Electrochemical Performance of Li[Ni0.8Co0.1Mn0.1]O2 (NCM811)/Silicon Full Cells. J. Electrochem. Soc. 2019, 166, A3265–A3271. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Tae Lee, J.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Smets, A.H.M. Growth Related Material Properties of Hydrogenated Amorphous Silicon. Ph.D. Thesis, Technische Universiteit Eindhoven, Eindhoven, The Netherlands, 30 May 2002. [Google Scholar] [CrossRef]
- Moore, A.R. Electron and hole drift mobility in amorphous silicon. Appl. Phys. Lett. 1977, 31, 762. [Google Scholar] [CrossRef]
- Goldstein, B. Li-defect interactions in electron-irradiated n-type silicon by EPR measurements. Radiat. Eff. 1971, 8, 229–237. [Google Scholar] [CrossRef]
- Krause, O.; Ryssel, H.; Pichler, P. Determination of aluminum diffusion parameters in silicon. J. Appl. Phys. 2002, 91, 5645. [Google Scholar] [CrossRef]
- Steinfink, H.; Post, B.; Fankuchen, I. The Crystall Structure of Octamethyl Cyclotetrasiloxane. Acta Cryst. 1955, 8, 420–424. [Google Scholar] [CrossRef]
- Kulova, T.L.; Mazaletskii, L.A.; Mironenko, A.A.; Rudyi, A.S.; Skundin, A.M.; Tortseva, Y.S.; Fedorov, I.S. Experimental Study of the Influence of the Porosity of Thin-Film Silicon-Based Anodes on Their Charge-Discharge Characteristics. Russ. Microelectron. 2021, 50, 45–53. [Google Scholar] [CrossRef]
- Rudy, A.S.; Mironenko, A.A.; Naumov, V.V.; Churilov, A.B. Schottky Barrier in Si–M Structures of Solid-State Lithium-Ion batteries. Tech. Phys. Lett. 2022, 48, 32–35. [Google Scholar]
- Kerimov, M.K.; Kurbanov, M.A.; Sultanahmedova, I.S.; Faradzhzade, I.A.; Tatardar, F.N.; Aliyev, H.S.; Yahyaev, F.F.; Yusifova, U.V. Varistor effect in polymer-semiconductor composites. Semiconductors 2010, 44, 904–911. [Google Scholar] [CrossRef]
- Thomas, P.; Flachet, J. Conductivity measurements on UHV deposited amorphous silicon. J. Phys. Colloq. 1981, 42, C4-151–C4-154. [Google Scholar] [CrossRef]



| Cleaning and Deposition Parameters | MVU TM Magna 10 | Oratorio 22 | |
|---|---|---|---|
| Cleaning | |||
| Ion source current, mA | 63 | 63 | |
| Ar flow, sccm | 15 | 15 | |
| Heating, °C | 200 | 200 | |
| Heating time, s | 60 | 60 | |
| Deposition | |||
| Pressure, Pa | 1.5 | 0.18 | |
| Ar flow, sccm | 40 | 20 | |
| O2 flow, sccm | 0.7 | 0.6 | |
| Power, M1 * (Si0.9Al0.1), W | 600 | 500 | |
| M2 * (Al) Power switching limits, W | mode 1 mode 2 | 200 50 | - |
| Deposition time, min | 45 | 16 | |
| Sample Nos. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | 3.18 | 3.12 | 3.18 | 3.48 | 3.14 | 3.53 | 2.82 | 3.03 | 1.64 | 1.21 | 0.58 | 1.4 | 2.42 | 1.56 | 2.41 |
| O | 30.3 | 31.4 | 31.0 | 32.2 | 32.3 | 33.3 | 32.61 | 13.3 | 12.3 | 12.5 | 16.4 | 22.3 | 22.9 | 15.20 | 13.2 |
| Al | 6.02 | 5.45 | 5.68 | 5.17 | 5.23 | 5.40 | 5.27 | 4.15 | 4.58 | 4.32 | 3.73 | 3.39 | 3.3 | 7.20 | 7.03 |
| Si | 59.5 | 58.03 | 57.14 | 55.15 | 54.33 | 51.77 | 52.30 | 71.52 | 72.48 | 71.97 | 68.29 | 60.91 | 58.38 | 62.04 | 62.36 |
| Film thickness, μm | 0.81 | 0.80 | 0.86 | 0.75 | 0.64 | 0.69 | 0.63 | 1.81 | 1.76 | 1.78 | 2.00 | 2.35 | 2.60 | 1.90 | 1.90 |
| Target | Main Substance Content,% | Gas | Pressure, µbar | Magnetron Power, W | Deposition Rate, nm/min | Thickness, nm |
|---|---|---|---|---|---|---|
| Ti | 99.95 | Ar | 2 | 300 | 20 | 200 (bottom and top layers) |
| LiCoO2 | 99.9 | 20Ar + 5O2 | 10 | 200 | 4.5 | 500 |
| LixV2O5 | 99.9 | 10Ar + O2 | 2.2 | 200 | 2.7 | 500 |
| Li3PO4 | 99.9 | N2 | 2 | 150 | 5.6 | 600−800 |
| 99.99 | 200Ar + 0.6O2 | 17.5 | 400 | 50 | 200 |
| Capacity | Si@O@Al in the Test Cell | Si@O@Al in SSLIB with LiCoO2 Cathode | Si@O@Al in SSLIB with V2O5 Cathode |
|---|---|---|---|
| CV, A·h/cm3 | 5 | 0.11 | 0.058 |
| CV, C/cm3 | 18,000 | 396 | 209 |
| CLi, cm−3 | 11 × 1022 * | 0.25 × 1022 | 0.13 × 1022 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudy, A.S.; Kurbatov, S.V.; Mironenko, A.A.; Naumov, V.V.; Skundin, A.M.; Egorova, Y.S. Effect of Si-Based Anode Lithiation on Charging Characteristics of All-Solid-State Lithium-Ion Battery. Batteries 2022, 8, 87. https://doi.org/10.3390/batteries8080087
Rudy AS, Kurbatov SV, Mironenko AA, Naumov VV, Skundin AM, Egorova YS. Effect of Si-Based Anode Lithiation on Charging Characteristics of All-Solid-State Lithium-Ion Battery. Batteries. 2022; 8(8):87. https://doi.org/10.3390/batteries8080087
Chicago/Turabian StyleRudy, Alexander S., Sergei V. Kurbatov, Alexander A. Mironenko, Victor V. Naumov, Alexander M. Skundin, and Yulia S. Egorova. 2022. "Effect of Si-Based Anode Lithiation on Charging Characteristics of All-Solid-State Lithium-Ion Battery" Batteries 8, no. 8: 87. https://doi.org/10.3390/batteries8080087
APA StyleRudy, A. S., Kurbatov, S. V., Mironenko, A. A., Naumov, V. V., Skundin, A. M., & Egorova, Y. S. (2022). Effect of Si-Based Anode Lithiation on Charging Characteristics of All-Solid-State Lithium-Ion Battery. Batteries, 8(8), 87. https://doi.org/10.3390/batteries8080087







