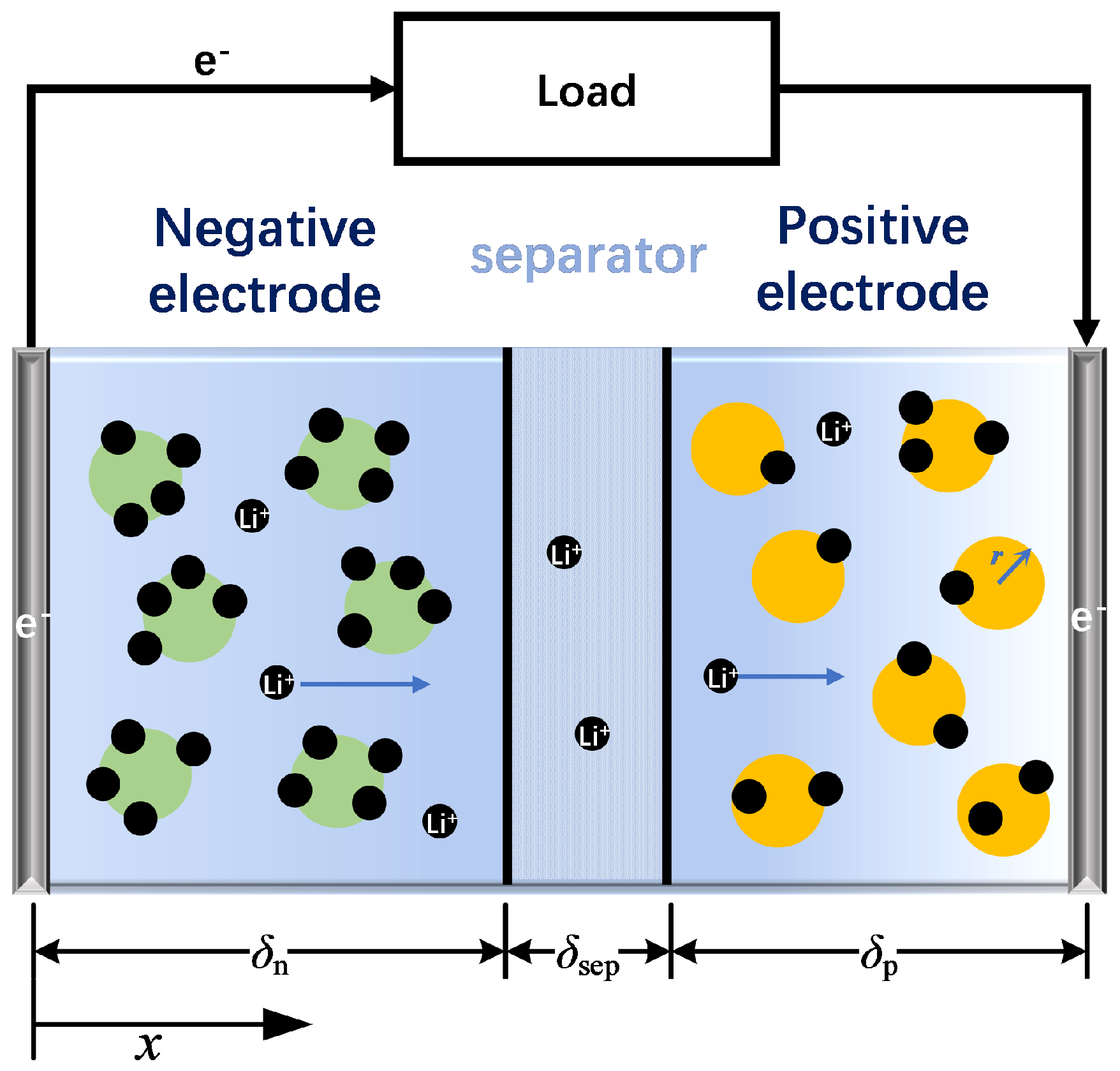
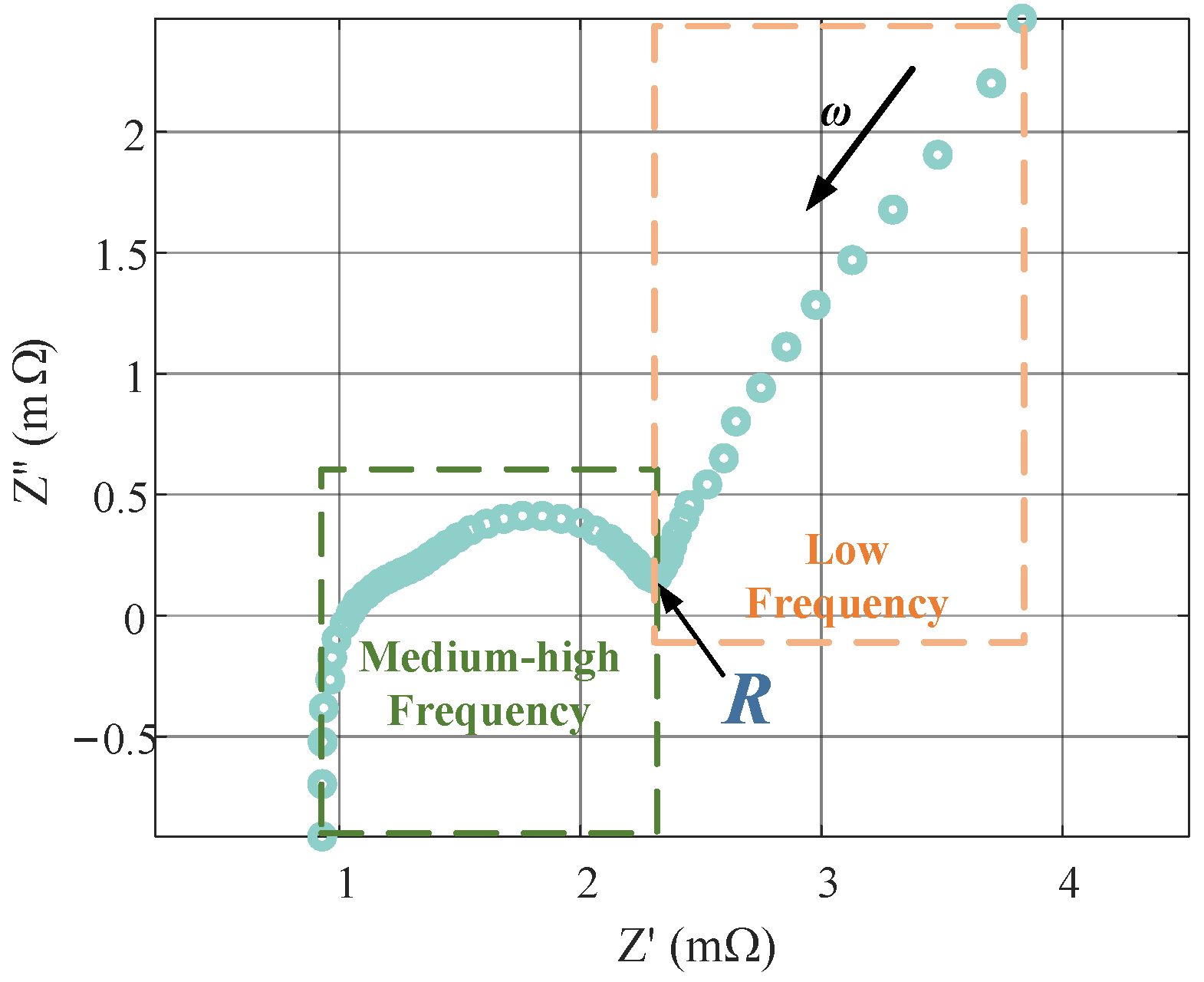
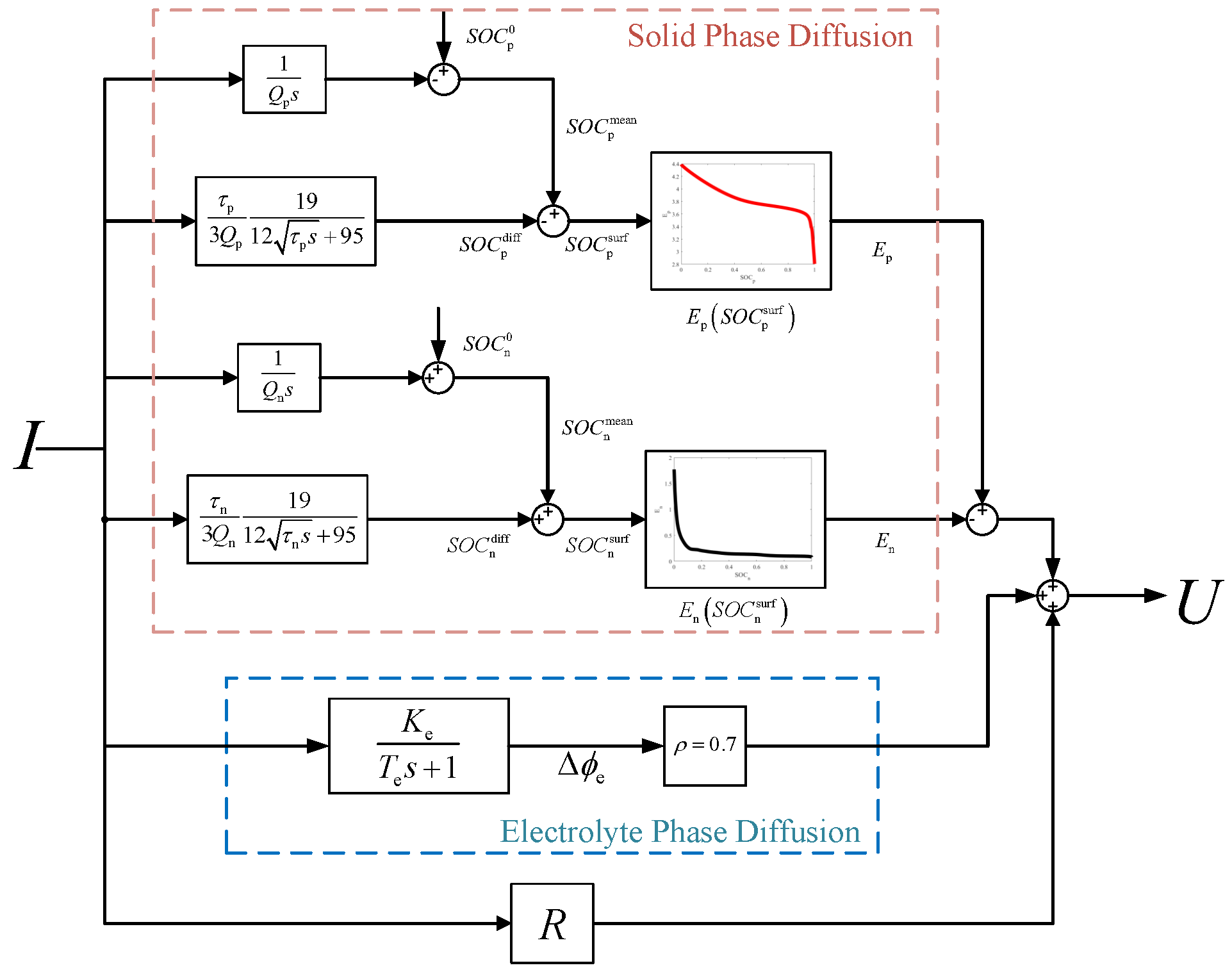
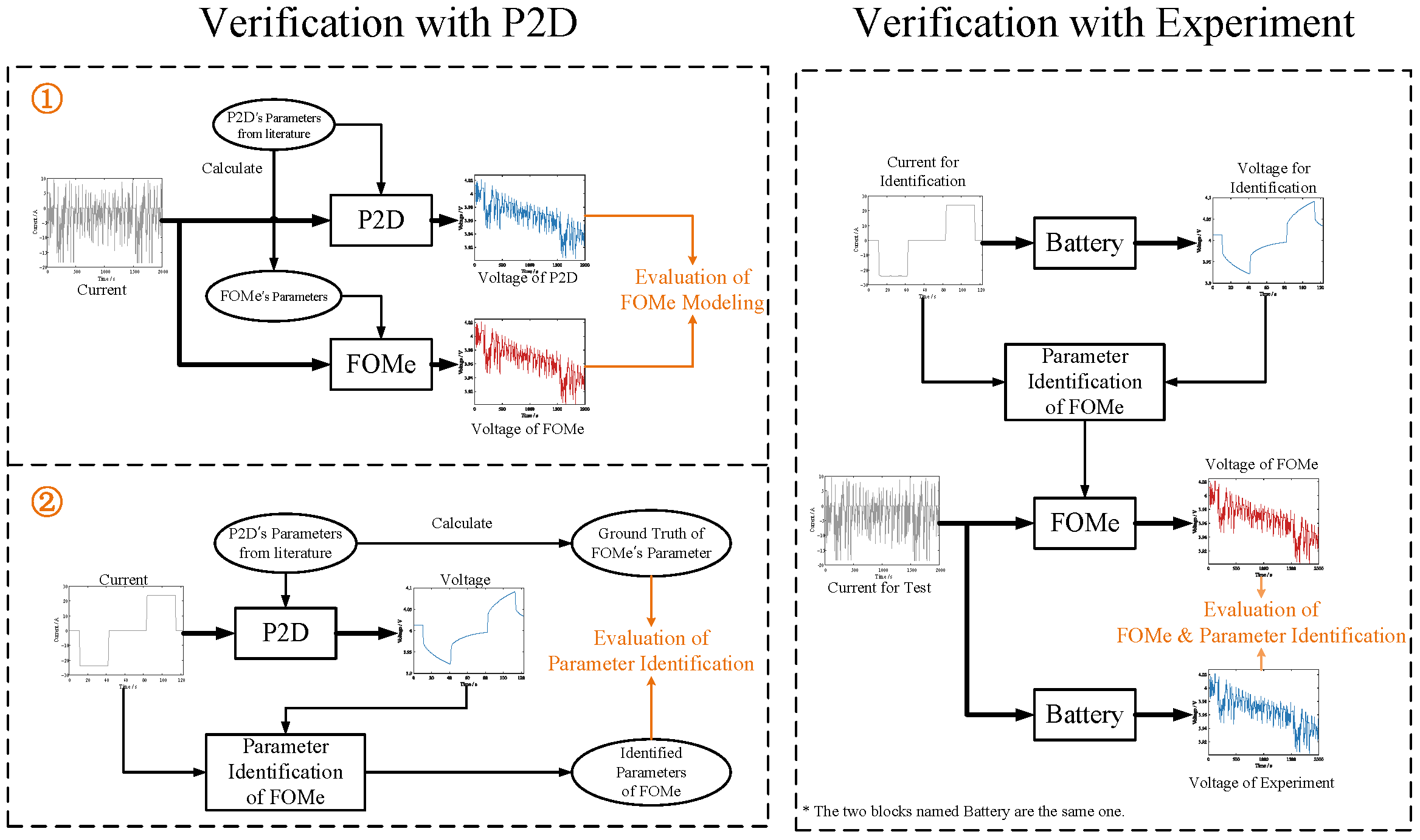
1. Introduction
The electrochemical lithium-ion battery (LiB) model has been widely utilized in LiB research, such as for parameter identification and aging mechanism research [
1,
2,
3,
4]. The electrochemical model has a more precise physical meaning than the equivalent circuit model (ECM) [
5,
6]. The parameters of the electrochemical model can reflect the inner electrochemical status of an LiB, which is more suitable for many applications, such as battery status monitoring. The commonly employed electrochemical models include the pseudo two-dimensional (P2D) model [
7] and single-particle model [
8]. However, these models require solving partial differential equation systems, which are computationally inefficient, have too many parameters and are not easily identifiable. There are many papers that propose reduced-order methods for these models. The main dynamics affecting the voltage in a cell are solid-phase diffusion (SPD) and electrolyte-phase diffusion (EPD). For SPD, the methods for simplification are polynomial approximation [
9], Pade approximation [
10], etc. In particular, the fractional-order Pade approximation employed in the fractional-order model (FOM) proposed by Guo [
11] is more consistent with the fractional-order characteristics exhibited in the electrochemical impedance spectrum (EIS), resulting in higher accuracy and practicality. However, the FOM treats EPD as a medium-high frequency dynamic and models as a lumping model, which limits the accuracy of the model at a higher current rate. It has been noted that EPD tends to be a low-frequency dynamic for certain cells, and its frequency may be lower than that of SPD [
12,
13,
14,
15]. Refs. [
16,
17] revealed that the aging-induced increase in the diffusion impedance of LiB is mainly caused by the aging of EPD. Therefore, it is necessary to add EPD modeling to the FOM. Commonly utilized electrolyte-phase modeling methods include polynomial approximation [
15], equilibrium point polynomial approximation [
13], and Pade approximation [
18]. The EPD model derived by the polynomial approximation method is a first-order inertial component with only two parameters, which is more accessible for application. We combine the FOM with the EPD of the polynomial approximation method to propose a fractional-order model FOMe considering EPD.
The electrochemical model requires an accompanying parameter identification method. Accurate parameter identification is the key to monitoring the battery’s aging and safety status and to enabling the model to better fit a real battery. Considering the usage scenario of batteries, the method based on the input–output data of the battery in the time domain shows a higher application value than the method that requires disassembly [
19] or the method that uses the EIS [
20]. However, parameter identification using only input–output data is also challenging due to the nonlinear characteristics of the cell, low sensitivity of the parameters, and unmodeled dynamics. Refs. [
12,
21] observed the difference in the dynamic frequency bands characterized by different parameters, which led to a parameter identification method that uses frequency segmentation. Therefore, thermodynamic parameters, low-frequency diffusion parameters, and mid-frequency parameters in electrochemical models can be separately identified under different excitations of different frequencies. In particular, the thermodynamic parameters can be identified with small rate constant current discharging [
22], and the ohmic internal resistance can be measured using the HPPC profile [
23]. However, this method does not adequately address the dynamics of similar frequencies, such as SPD and EPD. Some analytical methods for diffusion coefficient determination have been reported in the literature. For example, constant potential titration (GITT) is a classical method for solid-phase diffusion coefficient determination. However, analytical methods often suffer from a lack of accuracy due to the introduction of too many assumptions [
24]. Most studies treat the identification of diffusion parameters as an optimization problem [
20]:
where
is the current profile sequence,
is the actual battery output voltage sequence under excitation,
is the output voltage sequence of the battery model, and
is the parameter to be identified.
is the state of charge (SOC) at the initial moment of the experiment. When the rate of
is low and the duration is short, the range of the battery SOC changes throughout the experiment is small. Thus,
can also be considered the operating point for parameter identification.
Equation (
1) shows that three main factors affect the accuracy of parameter identification: excitation current
, operating point
, and optimization method. Typical optimization algorithms include metaheuristic approaches, such as PSO [
9,
25], genetic algorithms [
26,
27], and neural network methods [
28,
29]. The difference in optimization methods usually only affects whether the optimization solution will fall into a local optimum and the speed of convergence [
30].
and
mainly affect the accuracy of the parameter identification by influencing the sensitivity of the parameters and are the essential objects that need to be designed. The influence of
on the parameter sensitivity is mainly reflected in the frequency spectrum of the excitation current [
31]. For example, a low-band abundant excitation should be used to identify low-frequency parameters. The influence of the operating point on the sensitivity of the parameter is mainly reflected in the slope of the positive and negative open-circuit potential (OCP): at some operating points, the slope of the positive OCP curve is more significant, while the slope of the negative OCP curve is slight. In such a case, the change in the battery terminal voltage is mainly caused by the positive solid-phase overpotential. These operating points can be regarded as the positive characteristic SOC interval. Similarly, the negative characteristic interval can also be defined. Refs. [
30,
32] use data on a single operating point (or narrow operating SOC segments) to identify all parameters, while certain parameters are not sensitive in these SOC intervals. Although some studies [
13,
33] concluded via sensitivity analysis that positive solid-phase parameters are more sensitive in higher SOC intervals and that negative solid-phase parameters are more sensitive in lower SOC intervals, no specific method for determining the SOC intervals of positive and negative characteristics was given. We aim to address this research gap. On the other hand, it is recommended in this paper that three operating points are used instead of only the positive and negative characteristic intervals to identify the three diffusion parameters, because three different diffusion dynamics (positive SPD, negative SPD, and EPD) are included in FOMe. In addition, some papers [
34,
35,
36] use a very wide working region, an SOC interval of almost 0%–100%, for parameter identification, which leads to too many data and very low efficiency of parameter identification. The calculation required can be significantly reduced by reasonably selecting the SOC operating points for parameter identification.
FOMe and the parameter identification methods cannot be separately verified using only physical experiments because the true parameter values of an actual battery are unknown. For this reason, we propose a two-step validation method based on simulation and experiments. FOMe and the parameter identification methods are separately verified using a P2D model with high accuracy, and then FOMe and the parameter identification methods are simultaneously verified by experiments.
The contributions of the study are summarized as follows:
A fractional-order model considering EPD is proposed by combining the fractional-order model of SPD introduced by Guo with the polynomial approximation model of EPD.
A parameter identification method based on the SOC intervals of positive and negative solid- and electrolyte-phase characteristics is proposed.
A verification framework that combines simulation and experiments is proposed to verify the model’s accuracy and parameter identification methods.
This paper is organized as follows:
Section 2 describes the establishment of FOMe, and
Section 3 presents the parameter identification method of FOMe.
Section 4 designs the parameter identification process with a commercial
(NCM) lithium-ion battery and then analyzes FOMe and its parameter identification process.
Section 5 presents the conclusion.
3. Parameter Identification Method of FOMe
The FOMe includes nine parameters to be identified: , , , , , , , , and R. These parameters can be divided into three groups according to the time scale of the dynamics that they describe: thermodynamic parameters, including , , , and ; low-frequency dynamics parameters, including , , , and ; and the medium-high frequency dynamics parameter R.
The static-state performance of an LiB is only determined by thermodynamic parameters. In contrast, thermodynamic parameters and low-frequency dynamic parameters influence the transient-state performance. Therefore, first, the static-state performance of an LiB is tested to identify thermodynamic parameters. Second, the low-frequency dynamic parameters are determined based on the transient-state performance of the LiB, and thermodynamic parameters are obtained. The high-frequency dynamic parameter
R can be obtained from the value of the voltage jump edge under HPPC excitation [
23].
3.1. Identification of the Thermodynamic Parameters (, , , )
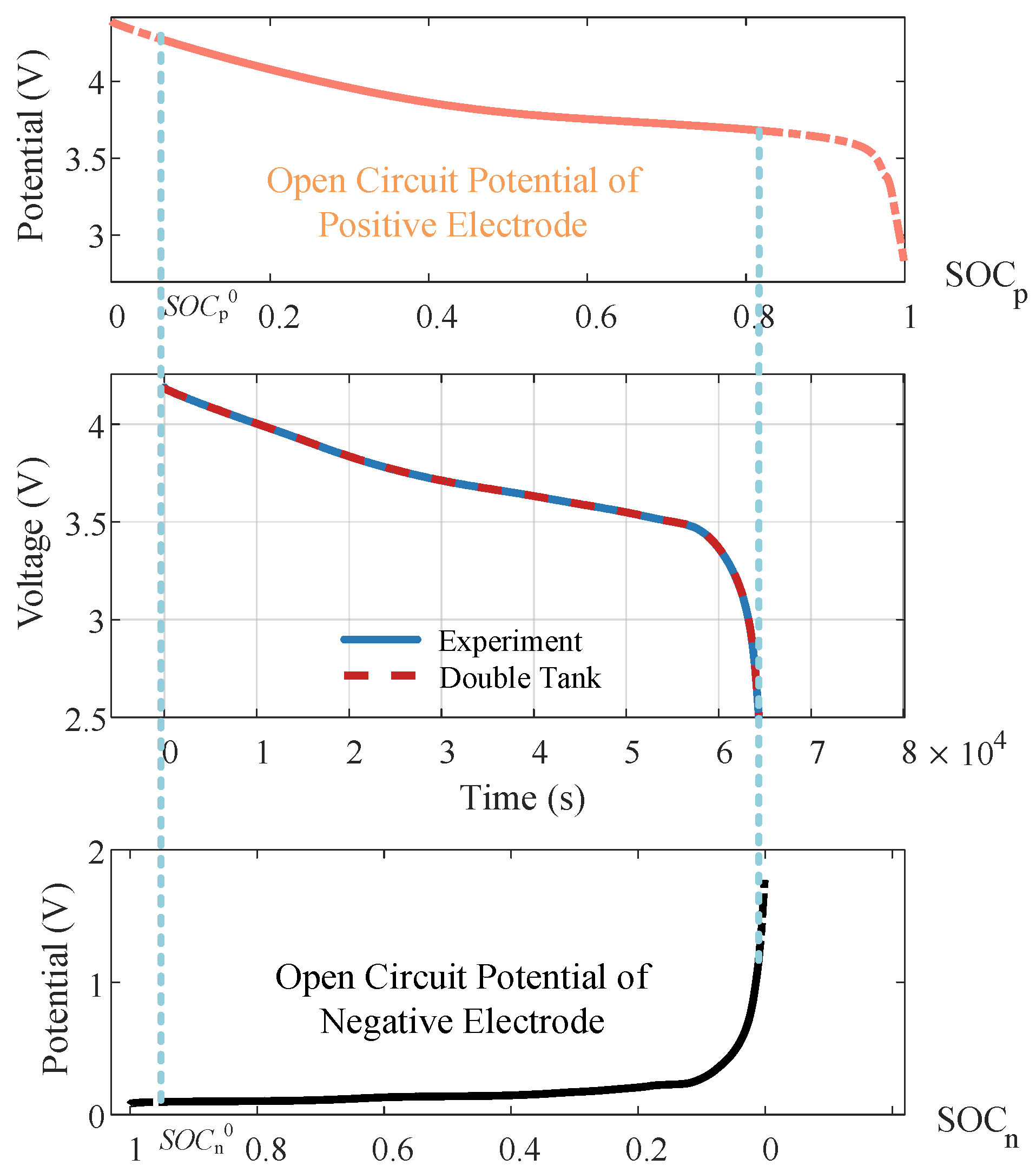
The identification of thermodynamic parameters is based on the double-tank model proposed by Han [
22]. For an LiB, after long-term relaxation, both low-frequency dynamics and medium-high frequency dynamics have reached their steady states, and the polarization overpotential is zero. Under this condition, the terminal voltage is only determined by
,
, and thermodynamic parameters. FOMe can be replaced by the double-tank model,
where
is the open-circuit voltage (OCV) calculated using the double-tank model. The OCV of the battery experiencing long-term relaxation over the entire SOC range is tested and employed for thermodynamic parameter identification:
can be approximately obtained from the voltage of LiB at a low discharging current of 0.05 C.
3.2. Identification of the Diffusion Parameters (, , , ) Using SPD and EPD Characteristic Intervals
There are three low-frequency diffusion dynamics in FOMe: positive SPD, negative SPD, and EPD. Four parameters that describe these dynamics can be identified by solving Equation (
1) with the thermodynamic parameters identified. The HPPC profile is adopted as the
for two reasons: only 120 s are needed, which makes parameter identification more efficient; it has a higher low-frequency component compared with driving profiles such as FUDS.
The contributions of SPDs and EPD to the terminal voltage are influenced by the slope of the positive and negative OCP curves, so they will vary with the operating point. For example, when the SOC is high, the negative SPD has almost no effect on the terminal voltage due to the high and the slight slope of the negative OCP curve. Thus, the terminal voltage is insensitive to the negative SPD time constant. Thus, according to the variation in the slope of positive and negative OCP curves with the SOC, the entire SOC range can be divided into three segments: the positive characteristic interval (PCI), negative characteristic interval (NCI), and positive and negative similar areas. For the operating points in the PCI, the terminal voltage is mainly influenced by the positive SPD and has a high sensitivity to the positive SPD parameters. Voltage is mainly influenced by the negative SPD in the NCI and has a high sensitivity to the negative SPD parameters. For the other operating point, the effects of positive and negative SPD on the terminal voltage are similar, and the SPD parameters are not easily identified. Furthermore, if there is a specific SOC interval such that both positive characteristics and negative characteristics are small, the terminal voltage is mainly determined by the EPD, and the electrolyte-phase characteristic interval (ECI) can be defined.
The parameters of the three dynamics have high sensitivity in the three characteristic intervals. Therefore, the current and voltage data from each of the three different characteristic intervals should be simultaneously employed to identify the dynamic parameters for accuracy. The optimization in Equation (
1) should be rewritten as
where
Particle swarm optimization (PSO) is selected as the optimization method, comprehensively considering the issues of convergence speed, global optimum, and algorithm complexity.
To determine the ranges of the PCI, NCI, and ECI, the slope of the OCP curve with SOC needs to be plotted. Since the correspondence among , and full-cell SOC is affected by the thermodynamic parameters, these parameters must be identified to further determine the three characteristic intervals.
On the other hand, since the battery operating point will deviate from the initial operating point during
excitation, this may lead to the following situation. Although the initial operating point of the battery is located within the characteristic interval, it may leave the characteristic interval during excitation. Therefore, the shift in the operating point should be considered to keep the battery in the characteristic interval during the experiment. This shift, according to FOMe, is
which is related to the parameter value and current magnitude. Considering the range of values of the parameters, the maximum value of this offset is approximately 4% when
is the HPPC.
Therefore, the process of determining the characteristic interval is as follows:
The slopes of the positive and negative OCP curves are plotted versus SOC without considering the SOC shift, and three characteristic intervals are calculated, denoted as , , and .
is increased by 4% and is decreased by 4%; the three intervals are recalculated, and are denoted as , , .
These are intersected with , , and to obtain the real PCI, NCI, and ECI.
4. Verification and Discussion
FOMe and the parameter identification method are verified with the P2D model and experiments for a commercial NCM battery. The test environment for the battery is listed in
Section 4.1.
Two dependent parts need to be verified: the modeling of FOMe and the parameter identification method. However, the two parts cannot be separately verified for an actual battery since the true parameter values in the actual battery are unknown. However, the P2D model with known parameters can be treated as a digital twin of the real battery [
39,
40], allowing the two parts to be independently verified. Therefore, first, the P2D model is employed for verification. The modeling accuracy is verified by comparing the output voltage of the P2D model and FOMe with the same parameter configuration and excitation. Second, the output voltage of the P2D model is treated as the actual voltage, and the parameters of the FOMe are estimated using the proposed parameter identification method. Last, a comparison of the identification results with the actual values of the parameters in the P2D model is carried out to verify the effectiveness of the parameter identification method. A schematic is shown in
Figure 4. This part will be elaborated in
Section 4.4.
The verification procedures based on the actual battery data are presented as follows: First, all the parameters of the actual battery are identified. Second, the results are loaded into FOMe, and the voltage error between FOMe and the experimental battery under multiple profiles is compared. The EIS error between FOMe and the actual battery is analyzed.
The verification methods based on the P2D model and the actual battery require knowledge of the positive and negative characteristic intervals. Therefore, the results for identifying thermodynamic parameters and determining the PCI, NCI, and ECI will be provided in
Section 4.2 and
Section 4.3.
4.1. Experiment
A commercial NCM battery, whose parameters are given in
Table 1, is tested. The experiment consists of three parts: a time domain experiment, EIS test, and half-cell test.
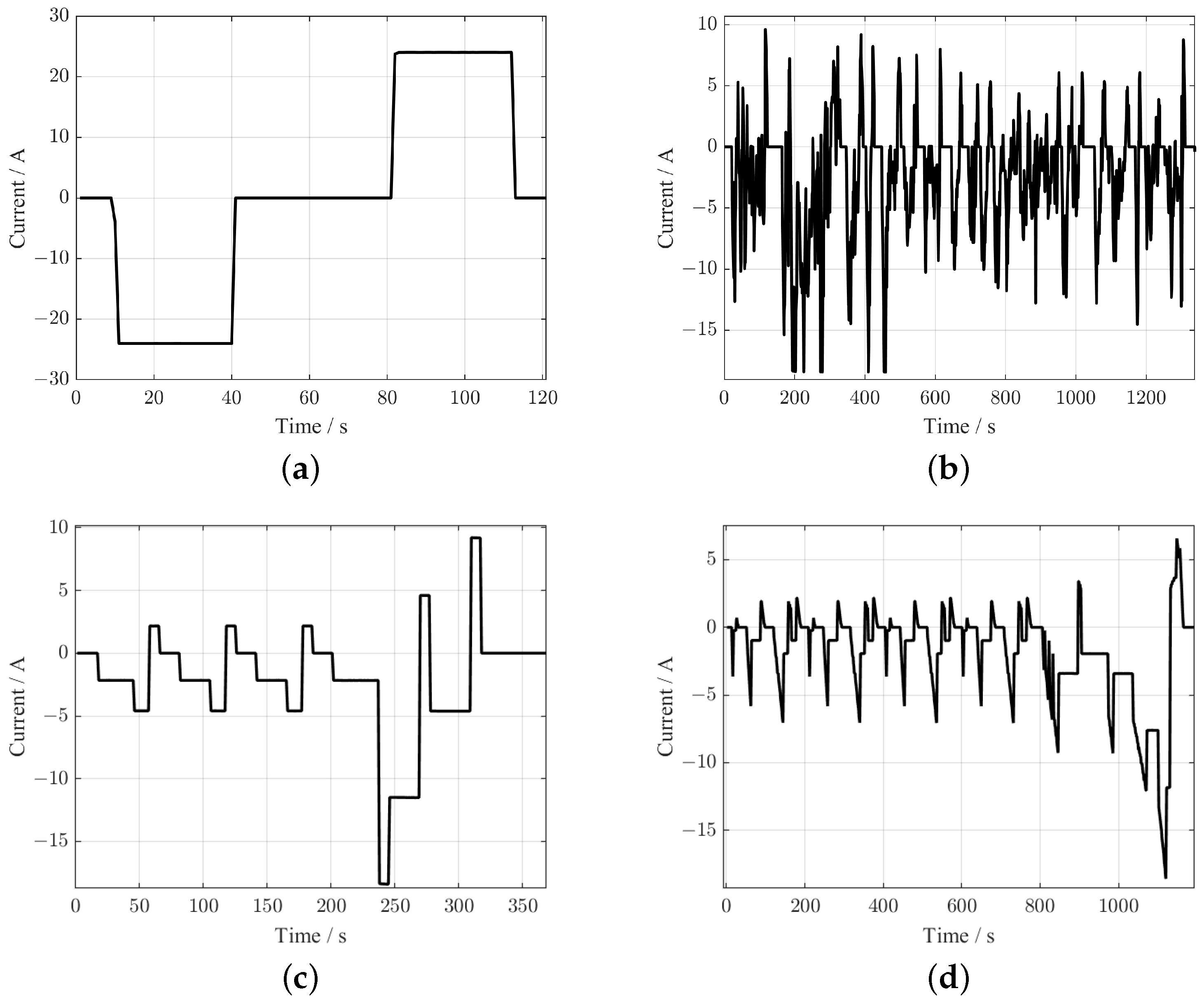
The time-domain experiments are performed with the Neware CT-4008-5V100A-NTFA, whose voltage sampling accuracy is ±0.05%FS. The sampling period is 1 s. The DGBELL BTH-150C thermal chamber maintains an ambient temperature of 25
. The time-domain experiment means obtaining the response voltage of the battery under different excitation current profiles, including a discharging test with a current of 0.05C, an HPPC test, a FUDS test, a DST test, and an NEDC test. The currents of these four profiles are shown in
Figure 5. The EIS test is implemented to capture battery performance in the frequency domain. The applied current amplitude of the EIS test is 0.5 A, and the frequency range is 2 mHz–2 kHz.
To measure the positive and negative OCP, the positive and negative electrodes of the battery are retrieved and used to assemble two half-cells. The details of the half-cell test are presented in Ref. [
11].
The P2D model is established in COMSOL Multiphysics ver 5.4. FOMe, and the parameter identification method is programmed and simulated in MATLAB R2020a. All simulations are carried out with a Lenovo Legion R7000 computer.
4.2. Identification of Thermodynamic Parameters
Figure 6 shows the OCP accessed through the half-cell test. The estimated results for the thermodynamic parameters are presented in
Table 2. The simulated and measured voltage curves under 0.05C are shown in
Figure 6, with a root mean square error (RMSE) of 0.61 mV.
4.3. Determination of PCI, NCI, and ECI
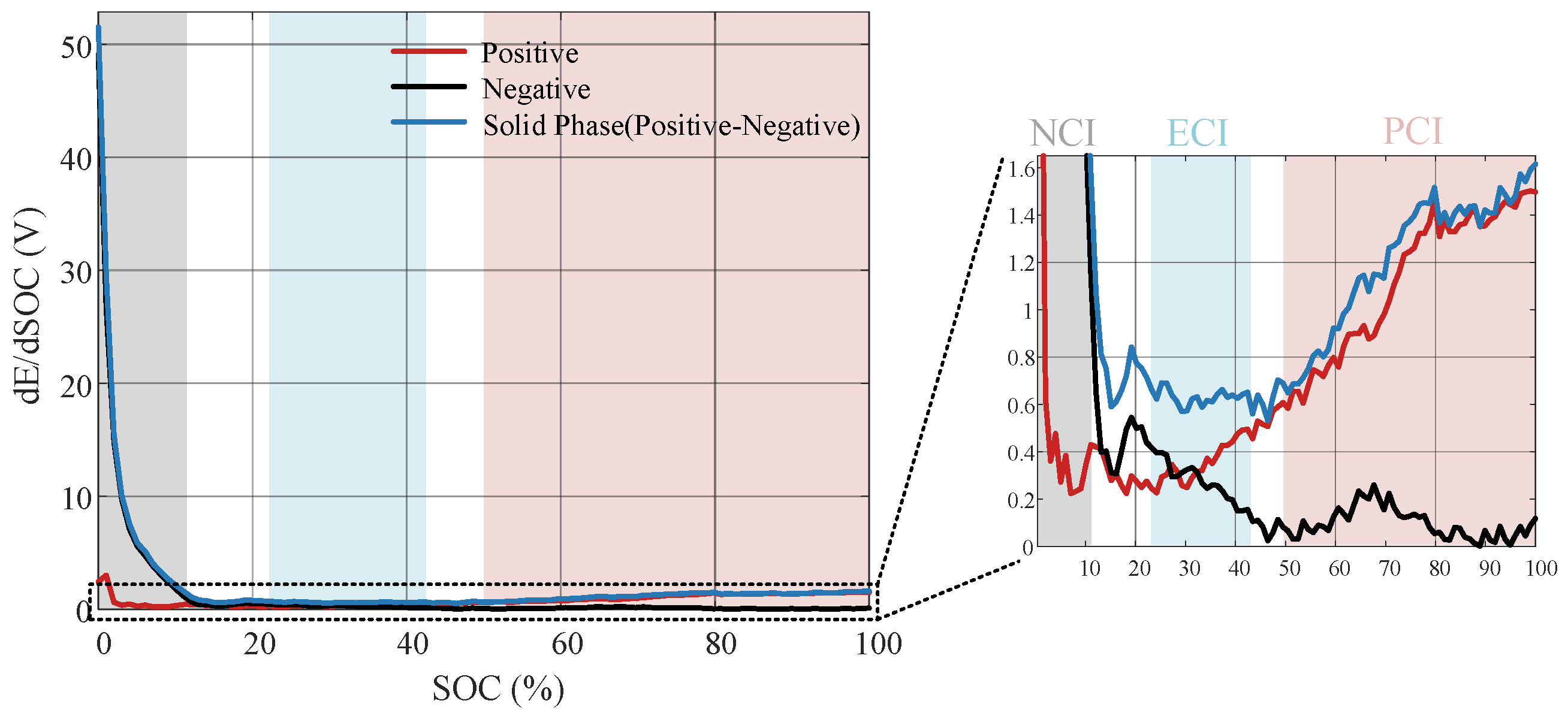
According to the method proposed in
Section 3.2, the slope of the OCP curve is plotted with respect to the SOC, as shown in
Figure 7. To quantify three characteristic intervals, we define the PCI as an SOC interval, where the positive characteristic is five times larger than the negative characteristic, and similarly defines the NCI. The interval where the difference between the slope of the positive OCP curve and the negative OCP curve is slight is defined as the ECI. Selecting the intersection of each characteristic interval before and after considering the SOC shift, we determine that the PCI ranges from 50% to 99%, that the NCI ranges from 0% to 10%, and that the ECI ranges from 23% to 42%.
4.4. Verification with the P2D Model
4.4.1. Verification of FOMe Modeling
The voltages under the FUDS of FOMe and P2D are compared. The parameters of P2D are listed in
Table A1. The FOMe parameters are listed in
Table 2, which are calculated according to the corresponding parameters of P2D. The parameters in
Table A1 are derived from measurements (geometric parameters) or studies, and some parameters are estimated using the experimental results (thermodynamic parameters). The ranges of certain parameters of P2D are also given in
Table A1; most of them are derived from Ref. [
41]. The ranges of the P2D parameters are used to determine the upper and lower limits of the FOMe parameters in the PSO algorithm.
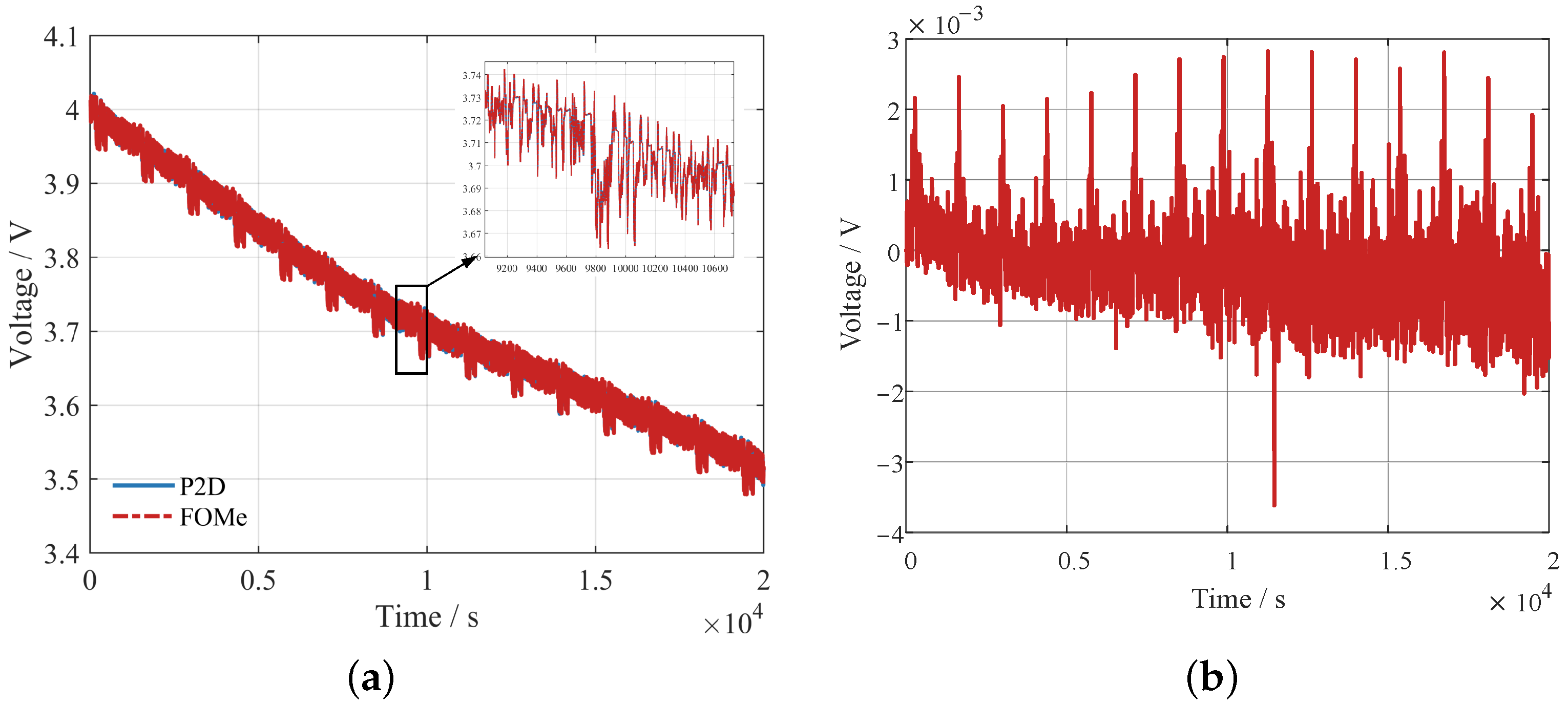
The voltages of P2D and FOMe are shown in
Figure 8. The RMSE between the two voltages is 0.55 mV, and the maximum error is 3.6 mV. The results indicate that FOMe provides an adequate approximation of P2D. For the computation time, for the 20,000 s FUDS profile shown in
Figure 8, the P2D model takes 21 min to solve, while FOMe needs only 7 s.
4.4.2. Verification of the Parameter Identification
First, the HPPC voltage response data of the P2D at the initial SOCs of 90%, 30%, and 10% are obtained through simulation with the parameter configuration in
Table A1. Second, the voltage data are considered the output voltage of a virtual cell, and its corresponding FOMe parameters are identified using the parameter identification method described in
Section 3.
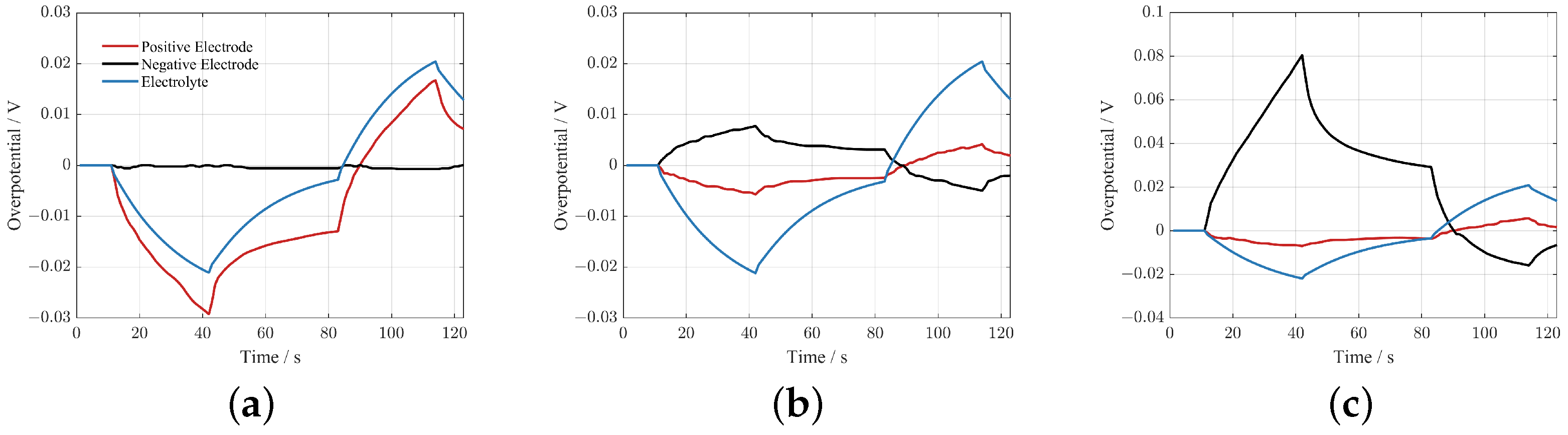
Figure 9 gives the positive SPD overpotential, negative SPD overpotential, and EPD overpotential for the P2D model at three operating points under the HPPC profile. The ratio of the three overpotentials varies, and the three operating points belong to three different characteristic intervals.
The results from comparing the identified values with the true values in the P2D model are shown in
Table 3, which shows that this method can accurately estimate the parameters of the P2D model.
4.5. Verification with Experiment
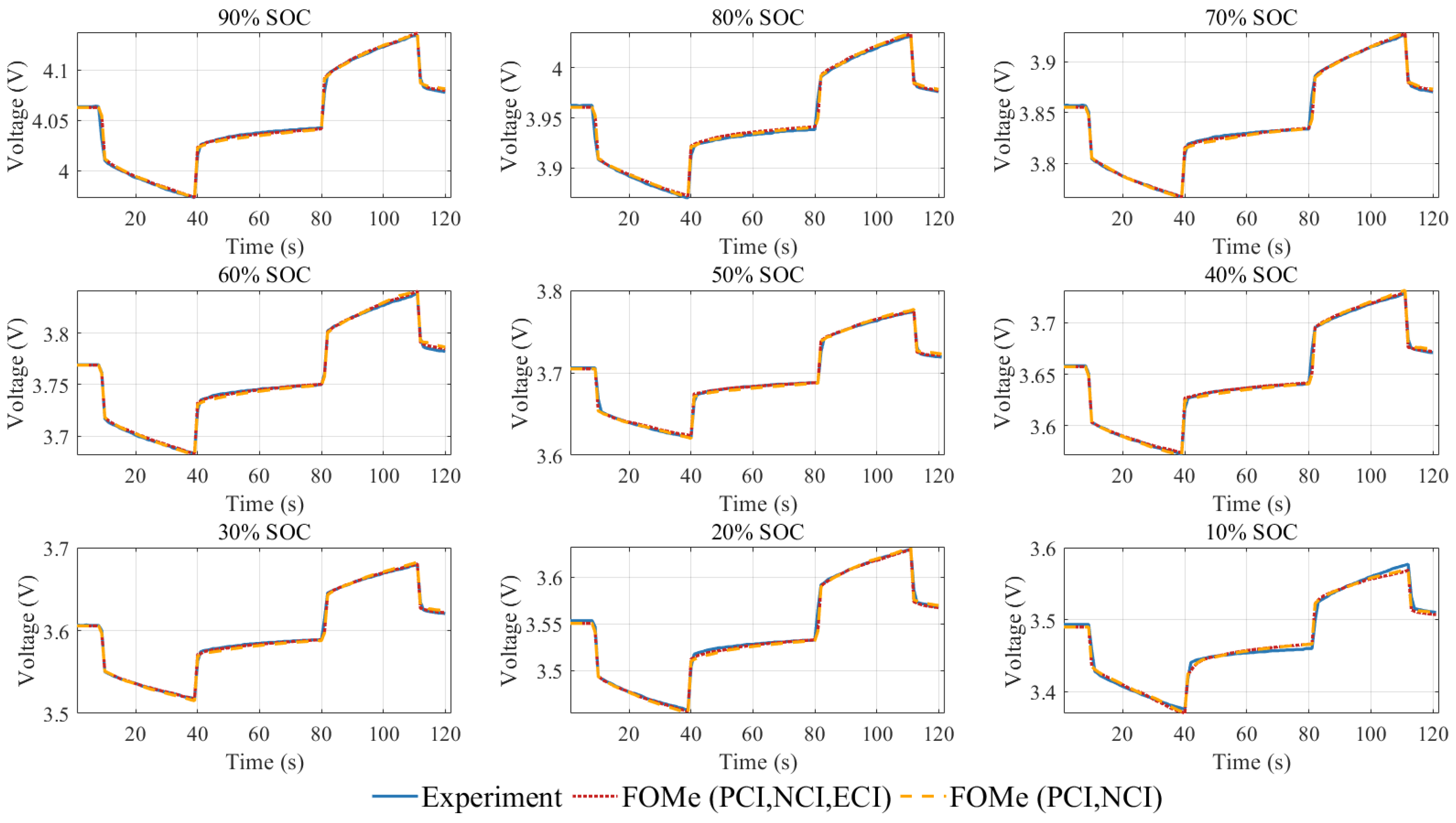
First, HPPC experiments are conducted on the NCM battery at every 10% SOC from 90% to 10% SOC, and a total of nine HPPC experimental data are obtained. Second, the data of 90% SOC, 30% SOC, and 10% SOC are selected for diffusion parameter identification. The results are shown in
Table 4.
Substituting the results into FOMe, the comparison between FOMe and the actual voltages at nine operating points is shown in
Figure 10. The RMSE over the nine operating points is 2.75 mV.
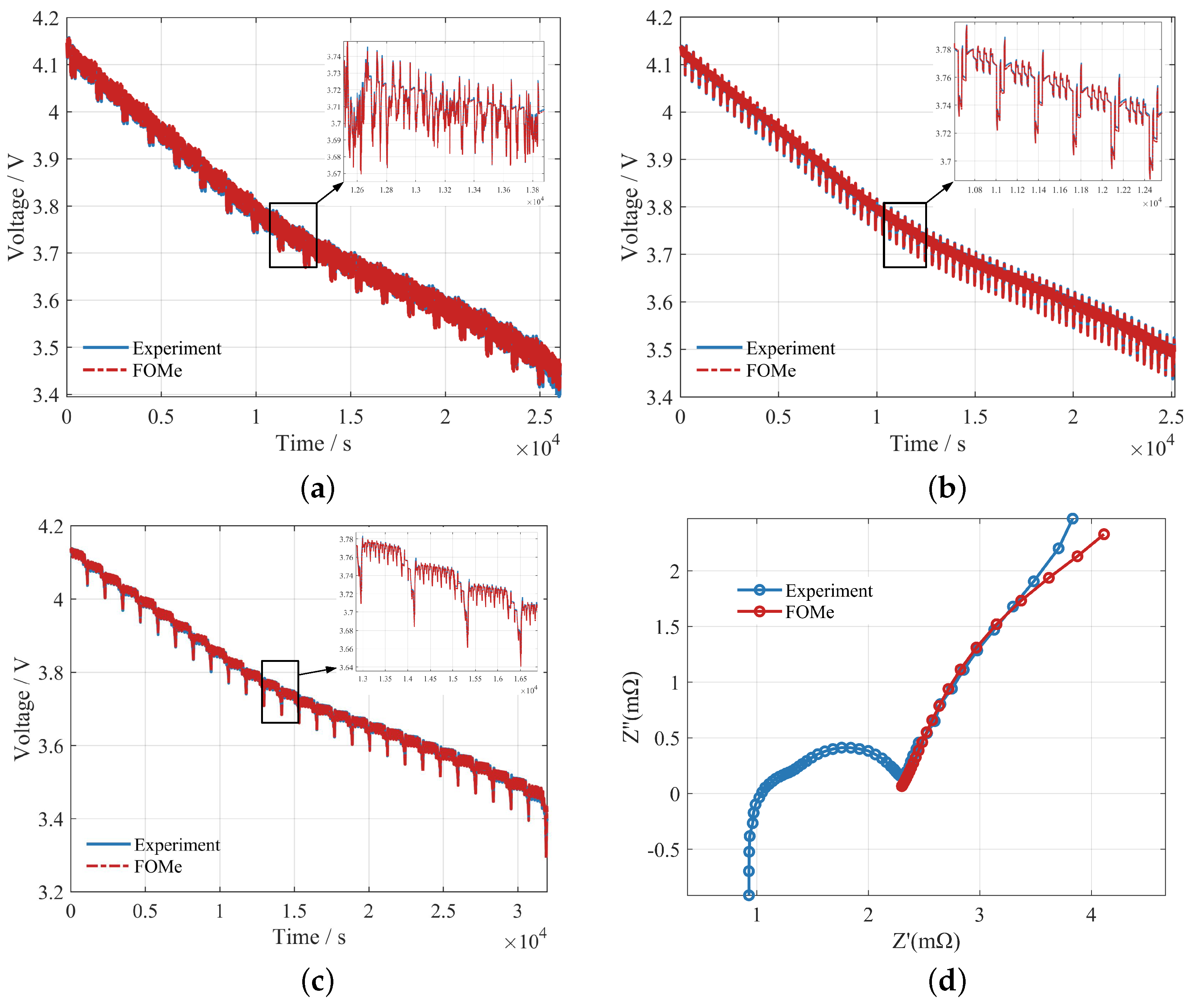
Furthermore, comparisons of the FOMe voltage with experiments under the FUDS, DST, and NEDC profiles are given. As shown in
Figure 11, the statistics of errors are shown in
Table 5. In addition, in the frequency domain, the simulated EIS of FOMe is compared with the measured EIS, as shown in
Figure 11. The mean absolute percentage error (MAPE) in the low-frequency part is 2.03%. In summary, FOMe and its parameter identification method demonstrated high accuracy for the investigated battery.
Figure 11d shows a slight difference between the simulated EIS and the measured EIS in the very low frequency band. The measured EIS tends to be a straight line perpendicular to the real axis in this band, which indicates that the measured EIS is dominated by the Coulomb counting in this band. In contrast, the simulated EIS suggests that the influence of the solid- and electrolyte-phase diffusion dynamics remains significant in this band [
6,
42]. It indicates that the diffusion time constants obtained from our identification are slightly larger than those exhibited by the measured EIS, which is attributed to the subtle inconsistency between the time-domain test and the frequency domain test due to the nonlinearity of the battery.
4.6. Discussion on the Influence of the Operating Point on Identification Results
The proposed parameter identification method uses the HPPC experimental data of three operating points in PCI, NCI, and ECI to simultaneously identify the four diffusion parameters. The following part provides a comparison of this method with the method that uses only PCI and NCI data and the method that uses all nine operating points (denoted as ACI).
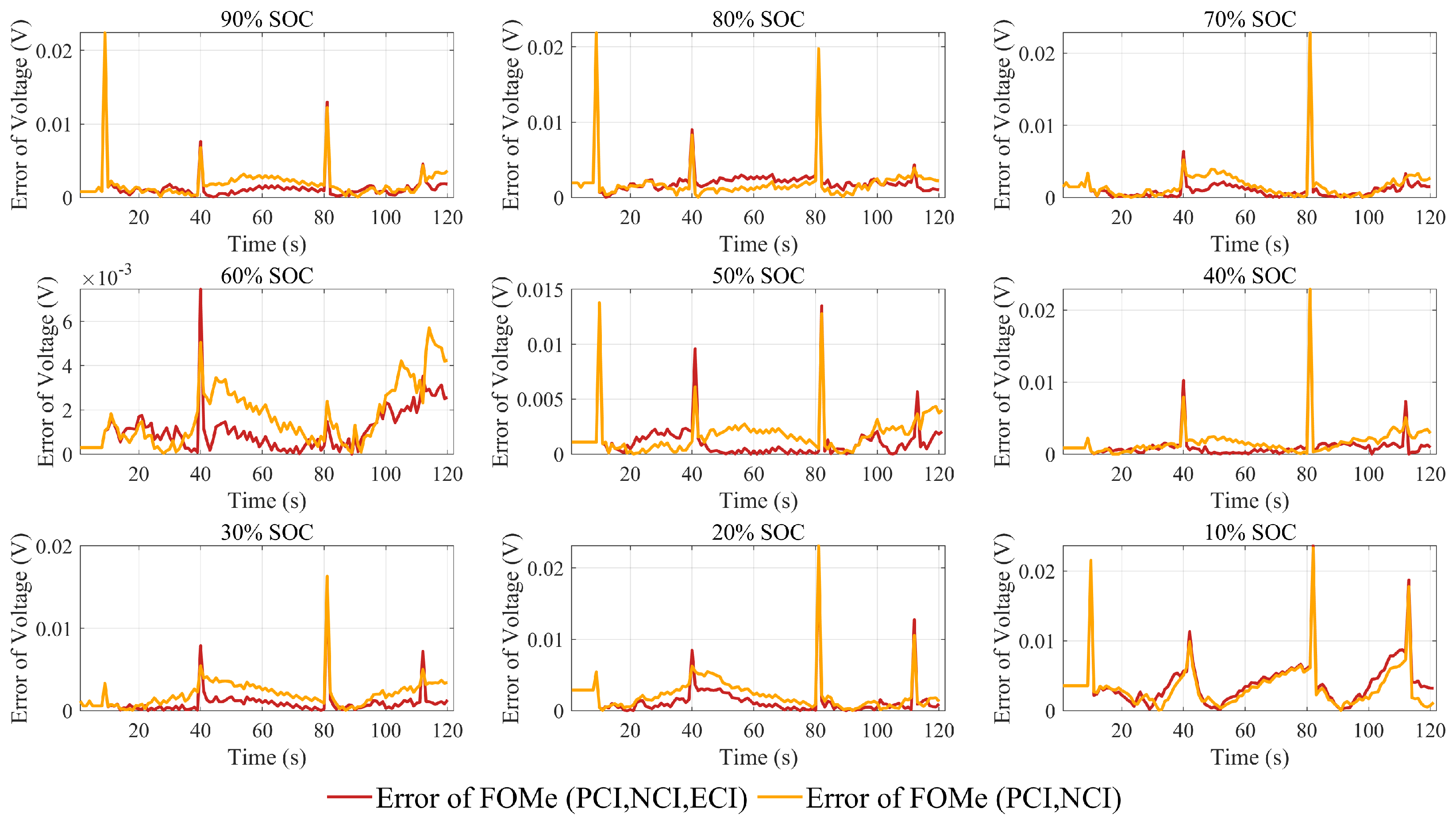
The results for the parameters identified by the above three methods are shown in
Table 6. The identification results using PCI and NCI data are substituted into FOMe, and the output voltages under HPPC profiles are shown in
Figure 10. The voltage errors compared with the experiments are shown in
Figure 12.
The error between the FOMe voltage and the experiment for the four profiles of HPPC, FUDS, DST, and NEDC under the above three sets of parameters are shown in
Table 5.
According to
Table 5, the identification results for the ACI allow FOMe to achieve the minimum error compared with the actual battery, followed by the identification results achieved using the PCI, NCI, and ECI. The identification results for the PCI and NCI allow FOMe to achieve the maximum error. In addition, the identification results for the ACI are similar to those of the PCI, NCI, and ECI, as shown in
Table 6. Considering the time consumed by the three identification methods, it can be concluded that the method that uses the PCI, NCI, and ECI can yield high identification efficiency while ensuring the accuracy of parameter identification.