Multi-Functional Potassium Ion Assists Ammonium Vanadium Oxide Cathode for High-Performance Aqueous Zinc-Ion Batteries
Abstract
:1. Introduction
2. Experimental
2.1. The Synthesis of KNVO
2.2. The Synthesis of NVO
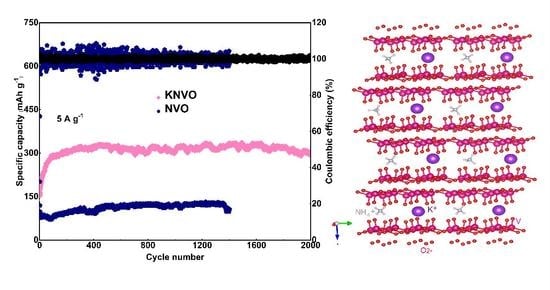
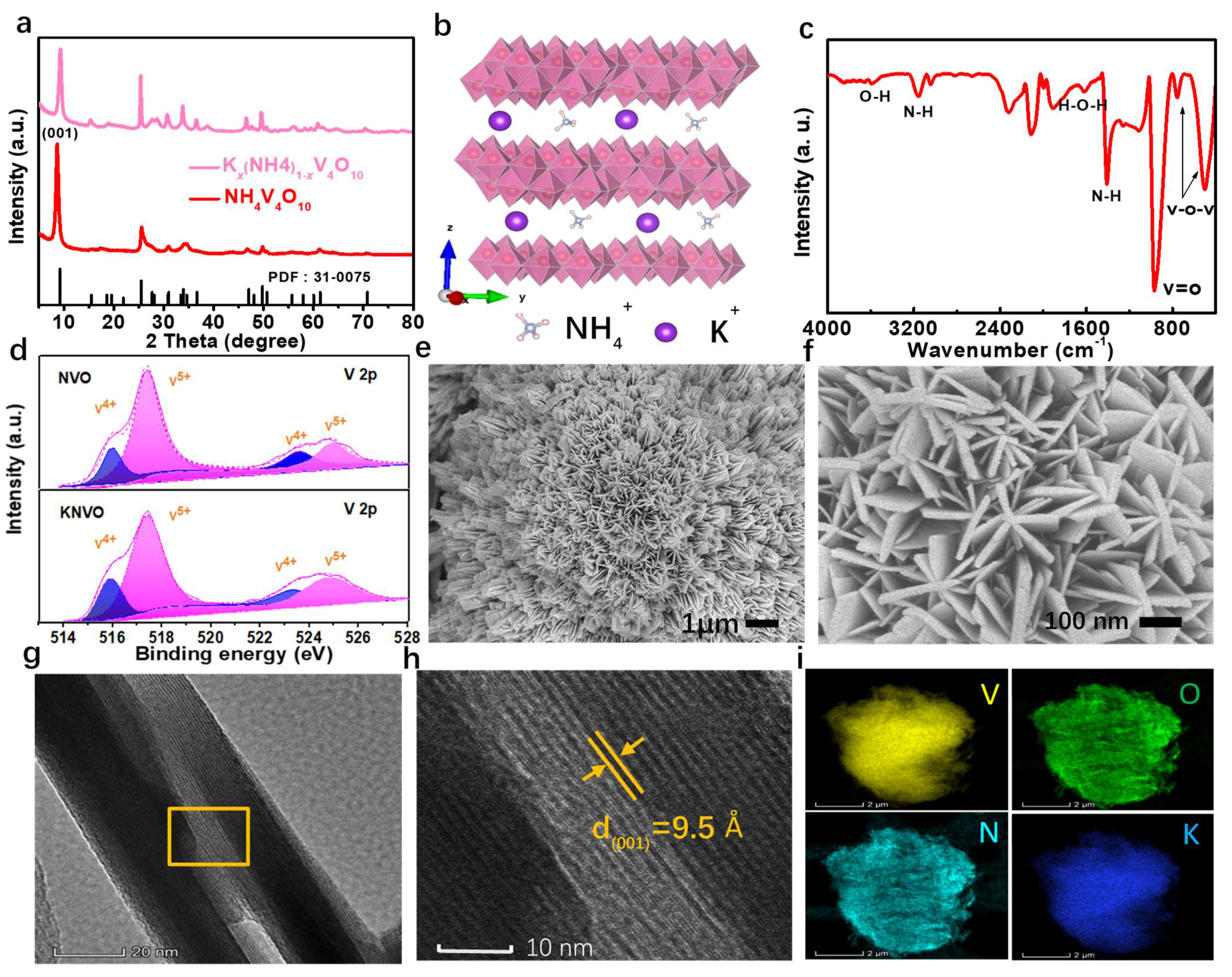
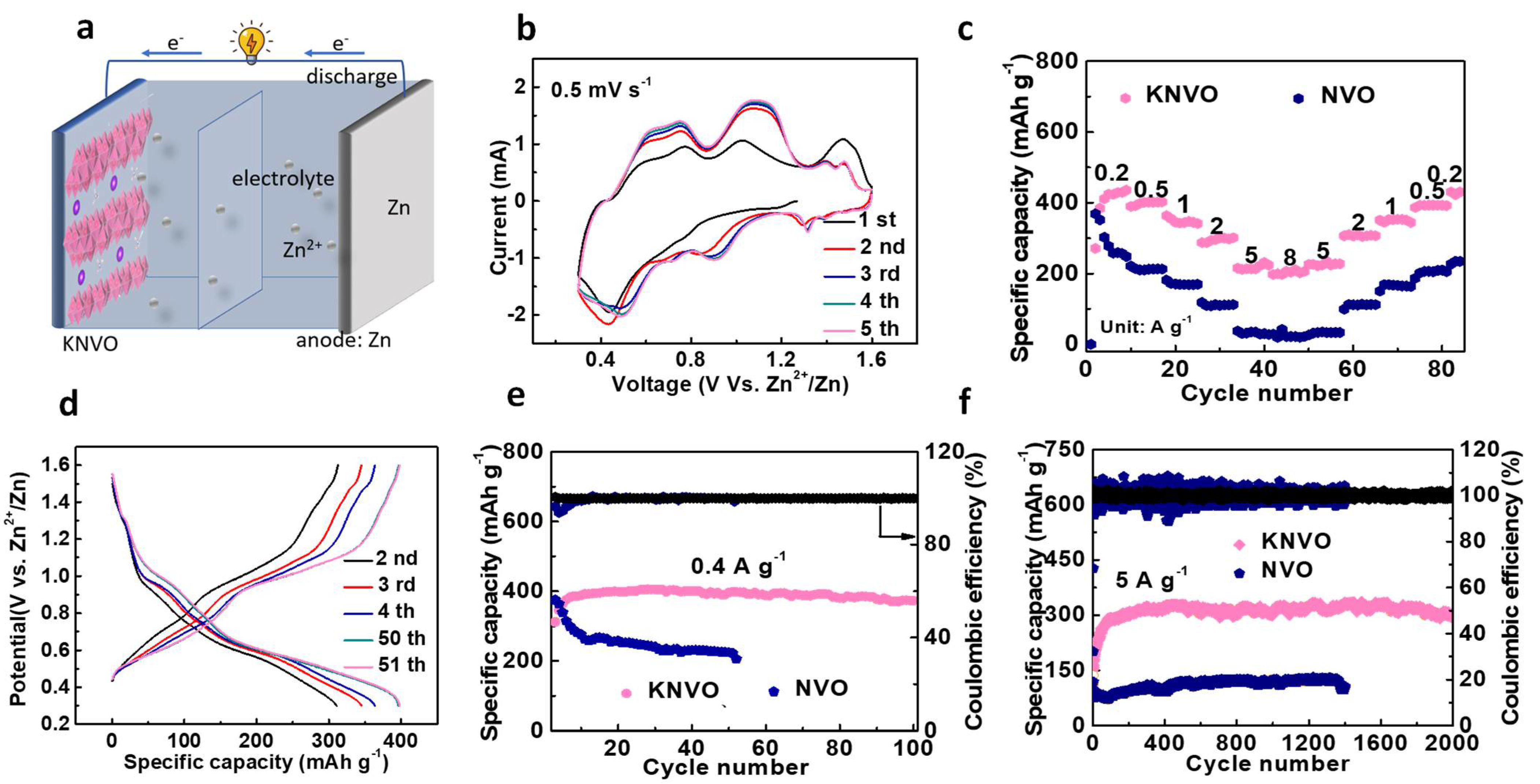
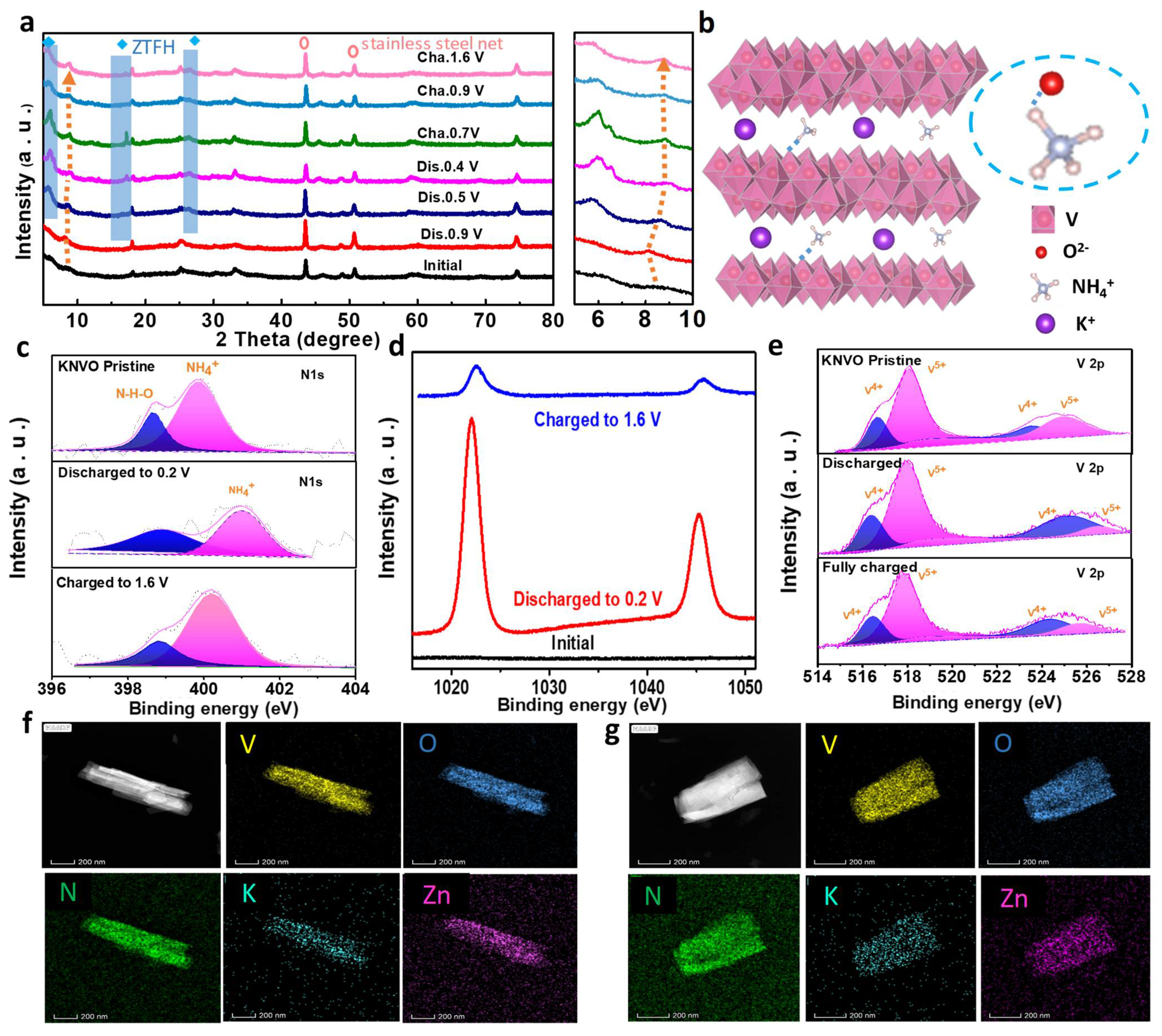
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, M.; Liu, C.; Zhang, F.; Dong, W.; Zhang, X.; Sang, Y.; Wang, J.J.; Guo, Y.G.; Liu, H.; Wang, S. Tunable Layered (Na, Mn)V8O20·nH2O Cathode Material for High-Performance Aqueous Zinc Ion Batteries. Adv. Sci. 2020, 7, 2000083. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, T.; Zheng, S.; Li, L.; Ma, T.; Liang, J. A New Tunnel-type V4O9 Cathode for High Power Density Aqueous Zinc ion Batteries. Inorg. Chem. Front. 2021, 8, 4497–4506. [Google Scholar] [CrossRef]
- Tang, B.; Shan, L.; Liang, S.; Zhou, J. Issues and Opportunities Facing Aqueous Zinc-Ion Batteries. Energy Environ. Sci. 2019, 12, 3288–3304. [Google Scholar] [CrossRef]
- Zhu, K.; Wu, T.; Huang, K. A High Capacity Bilayer Cathode for Aqueous Zn-Ion Batteries. ACS Nano 2019, 13, 14447–14458. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, H.; Zhang, B.; Li, G.; Zhu, H.; Ren, Y.; Geng, H.; Yang, Y.; Liu, Q.; Li, C.C. Tuning the Kinetics of Zinc Ion Insertion/Extraction in V2O5 by In Situ Polyaniline Intercalation Enables Improved Aqueous Zinc-Ion Storage Performance. Adv. Mater. 2020, 2001113. [Google Scholar] [CrossRef]
- Wang, B.; Yan, J.; Zhang, Y.; Ye, M.; Yang, Y.; Li, C.C. In Situ Carbon Insertion in Laminated Molybdenum Dioxide by Interlayer Engineering Toward Ultrastable “Rocking Chair” Zinc-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2102827. [Google Scholar] [CrossRef]
- Xu, C.; Li, B.; Du, H.; Kang, F. Energetic Zinc Ion Chemistry: The Rechargeable Zinc Ion Battery. Angew. Chem. Int. Ed. 2012, 51, 933–935. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, D.; Tang, Z.; Liang, G.; Yang, Q.; Li, H.; Ma, L.; Mo, F.; Zhi, C. A Mechanically Durable and Device-Level tough Zn-MnO2 Battery with High Flexibility. Energy Storage Mater. 2019, 23, 636–645. [Google Scholar] [CrossRef]
- Sun, T.; Nian, Q.; Zheng, S.; Shi, J.; Tao, Z. Layered Ca0.28MnO2·0.5H2O as a High Performance Cathode for Aqueous Zinc-Ion Battery. Small 2020, 16, e2000597. [Google Scholar] [CrossRef]
- Wessells, C.D.; Huggins, R.A.; Cui, Y. Copper Hexacyanoferrate Battery Electrodes with Long Cycle Life and High Power. Nat. Commun. 2011, 2, 550. [Google Scholar] [CrossRef] [Green Version]
- Ge, J.; Fan, L.; Rao, A.; Zhou, J.; Lu, B. Surface-substituted Prussian blue analogue cathode for sustainable potassium-ion batteries. Nat. Sustain. 2022, 5, 225–234. [Google Scholar] [CrossRef]
- Chae, M.S.; Hong, S.-T. Prototype System of Rocking-Chair Zn-Ion Battery Adopting Zinc Chevrel Phase Anode and Rhombohedral Zinc Hexacyanoferrate Cathode. Batteries 2019, 5, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Chifotides, H.T.; Schottel, B.L.; Dunbar, K.R. The p-Accepting Arene HAT(CN)6 as a Halide Receptor through Charge Transfer: Multisite Anion Interactions and Self-Assembly in Solution and the Solid State. Angew. Chem. Int. Ed. 2010, 49, 7202–7207. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Wang, Q.; Hou, Y.; Li, L.; Tao, Z. Recent Progress and Strategies toward High Performance Zinc-Organic Batteries. J. Energy Chem. 2021, 63, 87–112. [Google Scholar] [CrossRef]
- Zheng, S.; Shi, D.; Yan, D.; Wang, Q.; Sun, T.; Ma, T.; Li, L.; He, D.; Tao, Z. Orthoquinone-Based Covalent Organic Frameworks with Ordered Channel Structures for Ultrahigh Performance Aqueous Zinc-Organic Batteries. Angew. Chem. Int. Ed. 2022, 61, e202117511. [Google Scholar] [CrossRef]
- Liao, M.; Wang, J.; Ye, L.; Sun, H.; Wen, Y.; Wang, C.; Sun, X.; Wang, B.; Peng, H. A Deep-Cycle Aqueous Zinc-Ion Battery Containing an Oxygen-Deficient Vanadium Oxide Cathode. Angew. Chem. Int. Ed. 2020, 59, 2273–2278. [Google Scholar] [CrossRef]
- Yan, M.; He, P.; Chen, Y.; Wang, S.; Wei, Q.; Zhao, K.; Xu, X.; An, Q.; Shuang, Y.; Shao, Y.; et al. Water-Lubricated Intercalation in V2O5·nH2O for High-Capacity and High-Rate Aqueous Rechargeable Zinc Batteries. Adv. Mater. 2018, 30, 1703725. [Google Scholar] [CrossRef]
- Li, Q.; Rui, X.; Chen, D.; Feng, Y.; Xiao, N.; Gan, L.; Zhang, Q.; Yu, Y.; Huang, S. A High-Capacity Ammonium Vanadate Cathode for Zinc-Ion Battery. Nano Micro Lett. 2020, 12, 67. [Google Scholar] [CrossRef] [Green Version]
- Ding, J.; Du, Z.; Gu, L.; Li, B.; Wang, L.; Wang, S.; Gong, Y.; Yang, S. Ultrafast Zn2+ Intercalation and Deintercalation in Vanadium Dioxide. Adv. Mater. 2018, 30, 1800762. [Google Scholar] [CrossRef]
- Ke, L.; Dong, J.; Lin, B.; Yu, T.; Wang, H.; Zhang, S.; Deng, C. A NaV3(PO4)3@C hierarchical nanofiber in high alignment: Exploring a novel high-performance anode for aqueous rechargeable sodium batteries. Nanoscale 2017, 9, 12. [Google Scholar] [CrossRef]
- Cui, F.; Wang, D.; Hu, F.; Yu, X.; Guan, C.; Song, G.; Xu, F.; Zhu, K. Deficiency and Surface Engineering Boosting Electronic and Ionic Kinetics in NH4V4O10 for High-Performance Aqueous Zinc-Ion Battery. Energy Storage Mater. 2022, 44, 197–205. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Zheng, J.; Jiang, H.; Hu, T.; Meng, C. Ammonium Ion Intercalated Hydrated Vanadium Pentoxide for Advanced Aqueous Rechargeable Zn-Ion Batteries. Mater. Today Energy 2020, 18, 100509. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, Z.; Ye, F.; Pang, R.; Zhang, L.; Liu, Q.; Chang, X.; Sun, S.; Sun, Z.; Hu, L. Spontaneous Knitting Behavior of 6.7-nm Thin (NH4)0.38V2O5 Nano-Ribbons for Binder-Free Zinc-Ion Batteries. Energy Storage Mater. 2021, 42, 286–294. [Google Scholar] [CrossRef]
- Tang, B.; Zhou, J.; Fang, G.; Liu, F.; Zhu, C.; Wang, C.; Pan, A.; Liang, S. Engineering the Interplanar Spacing of Ammonium Vanadates as a High-performance Aqueous Zinc-Ion Battery Cathode. J. Mater. Chem. A 2019, 7, 940–945. [Google Scholar] [CrossRef]
- Jia, X.; Liu, C.; Neale, Z.G.; Yang, J.; Cao, G. Active Materials for Aqueous Zinc Ion Batteries: Synthesis, Crystal Structure, Morphology, and Electrochemistry. Chem. Rev. 2020, 120, 7795–7866. [Google Scholar] [CrossRef]
- Wang, H.; Jing, R.; Shi, J.; Zhang, M.; Jin, S.; Xiong, Z.; Guo, L.; Wang, Q. Mo-doped NH4V4O10 with Enhanced Electrochemical Performance in Aqueous Zn-Ion Batteries. J. Alloys Compd. 2021, 858, 158380. [Google Scholar] [CrossRef]
- Wu, T.; Lin, W. Boosting Proton Storage in Layered Vanadium Oxides for Aqueous Zinc−Ion Batteries. Electrochim. Acta 2021, 394, 139134. [Google Scholar] [CrossRef]
- Wu, T.; Li, Y.; Ni, K.; Li, T.; Lin, W. Vanadium Oxides Obtained by Chimie Douce Reactions: The Influences of Transition Metal Species on Crystal Structures and Electrochemical Behaviors in Zinc–Ion Batteries. J. Colloid. Interf. Sci. 2022, 608, 3122–3129. [Google Scholar] [CrossRef]
- Zhu, T.; Mai, B.; Hu, P.; Liu, Z.; Cai, C.; Wang, X.; Zhou, L. Ammonium Ion and Structural Water Co-Assisted Zn2+ Intercalation/De-Intercalation in NH4V4O10∙0.28H2O. Chin. J. Chem. 2021, 39, 1885–1890. [Google Scholar] [CrossRef]
- Sun, R.; Qin, Z.; Liu, X.; Wang, C.; Lu, S.; Zhang, Y.; Fan, H. Intercalation Mechanism of the Ammonium Vanadate (NH4V4O10) 3D Decussate Superstructure as the Cathode for High-Performance Aqueous Zinc-Ion Batteries. ACS Sustainable Chem. Eng. 2021, 9, 11769–11777. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, C.; Lan, B.; Chen, L.; Tang, W.; Zuo, C.; Dong, S.; An, Q.; Luo, P. K0.23V2O5 as A Promising Cathode Material for Rechargeable Aqueous Zinc Ion Batteries with Excellent Performance. J. Alloys Compd. 2020, 819, 152971. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, T.; Zhou, H.; Jin, H.; Liu, K.; Luo, Y.; Jiang, H.; Huang, K.; Huang, L.; Zhou, J. Rich Alkali Ions Preintercalated Vanadium Oxides for Durable and Fast Zinc-Ion Storage. ACS Energy Lett. 2021, 6, 2111–2120. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, Y.; Sun, J.; Liu, Y.; Jiang, H.; Cui, M.; Hu, T.; Meng, C. Dual Ions Enable Vanadium Oxide Hydration with Superior Zn2+ Storage for Aqueous Zinc-Ion Batteries. Chem. Eng. J. 2022, 433, 133795. [Google Scholar] [CrossRef]
- Zong, Q.; Wang, Q.; Liu, C.; Tao, D.; Wang, J.; Zhang, J.; Du, H.; Chen, J.; Zhang, Q.; Cao, G. Potassium Ammonium Vanadate with Rich Oxygen Vacancies for Fast and Highly Stable Zn-Ion Storage. ACS Nano 2022, 16, 4588–4598. [Google Scholar] [CrossRef]
- Chine, M.K.; Sediri, F.; Gharbi, N. Solvothermal Synthesis of V4O9 Flake-Like Morphology and its Photocatalytic Application in the Degradation of Methylene Blue. Mater. Res. Bull. 2012, 47, 3422–3426. [Google Scholar] [CrossRef]
- Subba Reddy, C.V.; Jin, A.-P.; Han, X.; Zhu, Q.-Y.; Mai, L.-Q.; Chen, W. Preparation and Characterization of (PVP + V2O5) Cathode for Battery Applications. Electrochem. Commun. 2006, 8, 279–283. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, M.; Zeng, M.; Li, X.; Chen, L.; Yang, Z.; Chen, J.; Guo, B.; Ma, Z.; Li, X. Sulfite Modified and Ammonium Ion Intercalated Vanadium Hydrate with Enhanced Redox Kinetics for Aqueous Zinc Ion Batteries. J. Power Sources 2021, 496, 229832. [Google Scholar] [CrossRef]
- Dai, X.; Wan, F.; Zhang, L.; Cao, H.; Niu, Z. Freestanding Graphene/VO2 Composite Films for Highly Stable Aqueous Zn-ion Batteries with Superior Rate Performance. Energy Storage Mater. 2019, 17, 143–150. [Google Scholar] [CrossRef]
- Yu, X.; Hu, F.; Cui, F.; Zhao, J.; Guan, C.; Zhu, K. The Displacement Reaction Mechanism of the CuV2O6 Nanowire Cathode for Rechargeable Aqueous Zinc Ion Batteries. Dalton Trans. 2020, 49, 1048–1055. [Google Scholar] [CrossRef]
- Tang, B.; Fang, G.; Zhou, J.; Wang, L.; Lei, Y.; Wang, C.; Lin, T.; Tang, Y.; Liang, S. Potassium Vanadates with Stable Structure and Fast Ion Diffusion Channel as Cathode for Rechargeable Aqueous Zinc-Ion Batteries. Nano Energy 2018, 51, 579–587. [Google Scholar] [CrossRef]
- He, T.; Ye, Y.; Li, H.; Weng, S.; Zhang, Q.; Li, M.; Liu, T.; Cheng, J.; Wang, X.; Lu, J.; et al. Oxygen-Deficient Ammonium Vanadate for Flexible Aqueous Zinc Batteries with High Energy Density and Rate Capability at −30 °C. Mater. Today 2021, 43, 53–61. [Google Scholar] [CrossRef]
- Mannix, A.J.; Zhou, X.-F.; Kiraly, B.; Wood, J.D.; Alducin, D.; Myers, B.D.; Liu, X.; Fisher, B.L.; Santiago, U.; Guest, J.R.; et al. Synthesis of Borophenes: Anisotropic, Two Dimensional Boron Polymorphs. Science 2015, 350, 1513–1516. [Google Scholar] [CrossRef] [Green Version]
- Chao, D.; Liang, P.; Chen, Z.; Bai, L.; Shen, H.; Liu, X.; Xia, X.; Zhao, Y.; Savilov, S.V.; Lin, J.; et al. Pseudocapacitive Na-Ion Storage Boosts High Rate and Areal Capacity of Self-Branched 2D Layered Metal Chalcogenide Nanoarrays. ACS Nano 2016, 10, 10211–10219. [Google Scholar] [CrossRef]
- Augustyn, V.; Come, J.; Lowe, M.A.; Kim, J.W.; Taberna, P.L.; Tolbert, S.H.; Abruna, H.D.; Simon, P.; Dunn, B. High-rate Electrochemical Energy Storage through Li+ Intercalation Pseudocapacitance. Nat. Mater. 2013, 12, 518–522. [Google Scholar] [CrossRef]
- Weppner, W.; Huggins, R. Determination of the Kinetic Parameters of Mixed-Conducting Electrodes and Application to the System Li3Sb. J. Electrochem. Soc. 1977, 124, 1569–1578. [Google Scholar] [CrossRef]
- Liu, X.; Xu, G.; Zhang, Q.; Huang, S.; Li, L.; Wei, X.; Cao, J.; Yang, L.; Chu, P.K. Ultrathin Hybrid Nanobelts of Single-Crystalline VO2 and Poly(3,4-ethylenedioxythiophene) as Cathode Materials for Aqueous Zinc Ion Batteries with Large Capacity and High-Rate Capability, Layered Vanadium Oxides with Proton and Zinc Ion Insertion for Zinc Ion Batteries. J. Power Sources 2020, 463, 228223. [Google Scholar]
- Liu, W.; Dong, L.; Jiang, B.; Huang, Y.; Wang, X.; Xu, C.; Kang, Z.; Mou, J.; Kang, F. Layered Vanadium Oxides with Proton and Zinc Ion Insertion for Zinc Ion Batteries. Electrochim. Acta 2019, 320, 134565. [Google Scholar] [CrossRef]
- Wan, F.; Zhang, L.; Dai, X.; Wang, X.; Niu, Z.; Chen, J. Aqueous Rechargeable Zinc/Sodium Vanadate Batteries with Enhanced Performance from Simultaneous Insertion of Dual Carriers. Nat. Commun. 2018, 9, 1656. [Google Scholar] [CrossRef] [Green Version]
- Ge, J.; Wang, B.; Wang, J.; Zhang, Q.; Lu, B. Nature of FeSe2/N-C Anode for High Performance Potassium Ion Hybrid Capacitor. Adv. Energy Mater. 2020, 10, 1903277. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, D.; Sun, T.; Wang, Q.; Ma, T.; Zheng, S.; Tao, Z.; Liang, J. Multi-Functional Potassium Ion Assists Ammonium Vanadium Oxide Cathode for High-Performance Aqueous Zinc-Ion Batteries. Batteries 2022, 8, 84. https://doi.org/10.3390/batteries8080084
He D, Sun T, Wang Q, Ma T, Zheng S, Tao Z, Liang J. Multi-Functional Potassium Ion Assists Ammonium Vanadium Oxide Cathode for High-Performance Aqueous Zinc-Ion Batteries. Batteries. 2022; 8(8):84. https://doi.org/10.3390/batteries8080084
Chicago/Turabian StyleHe, Dan, Tianjiang Sun, Qiaoran Wang, Tao Ma, Shibing Zheng, Zhanliang Tao, and Jing Liang. 2022. "Multi-Functional Potassium Ion Assists Ammonium Vanadium Oxide Cathode for High-Performance Aqueous Zinc-Ion Batteries" Batteries 8, no. 8: 84. https://doi.org/10.3390/batteries8080084
APA StyleHe, D., Sun, T., Wang, Q., Ma, T., Zheng, S., Tao, Z., & Liang, J. (2022). Multi-Functional Potassium Ion Assists Ammonium Vanadium Oxide Cathode for High-Performance Aqueous Zinc-Ion Batteries. Batteries, 8(8), 84. https://doi.org/10.3390/batteries8080084