Nanostructured Lead Electrodes with Reduced Graphene Oxide for High-Performance Lead–Acid Batteries
Abstract
1. Introduction
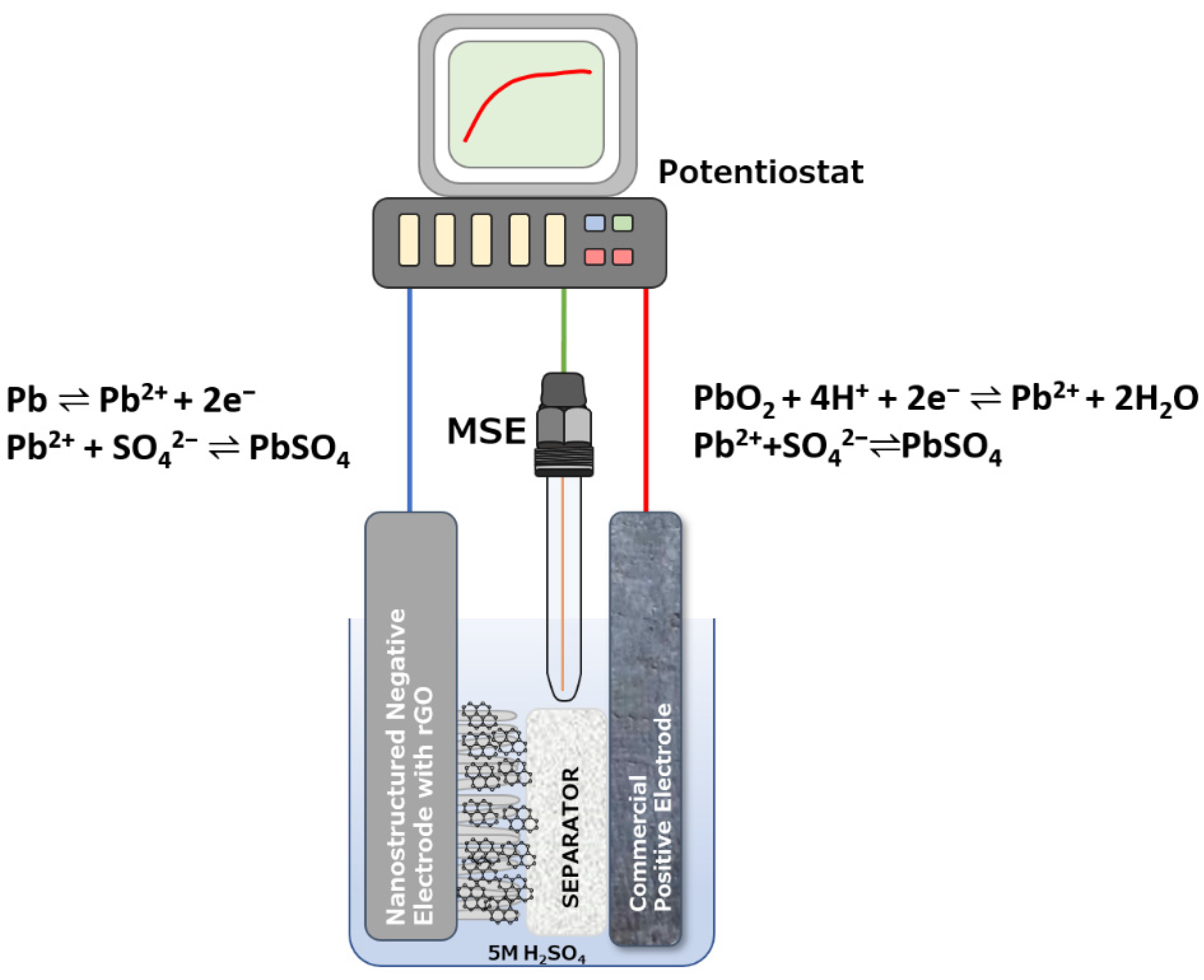
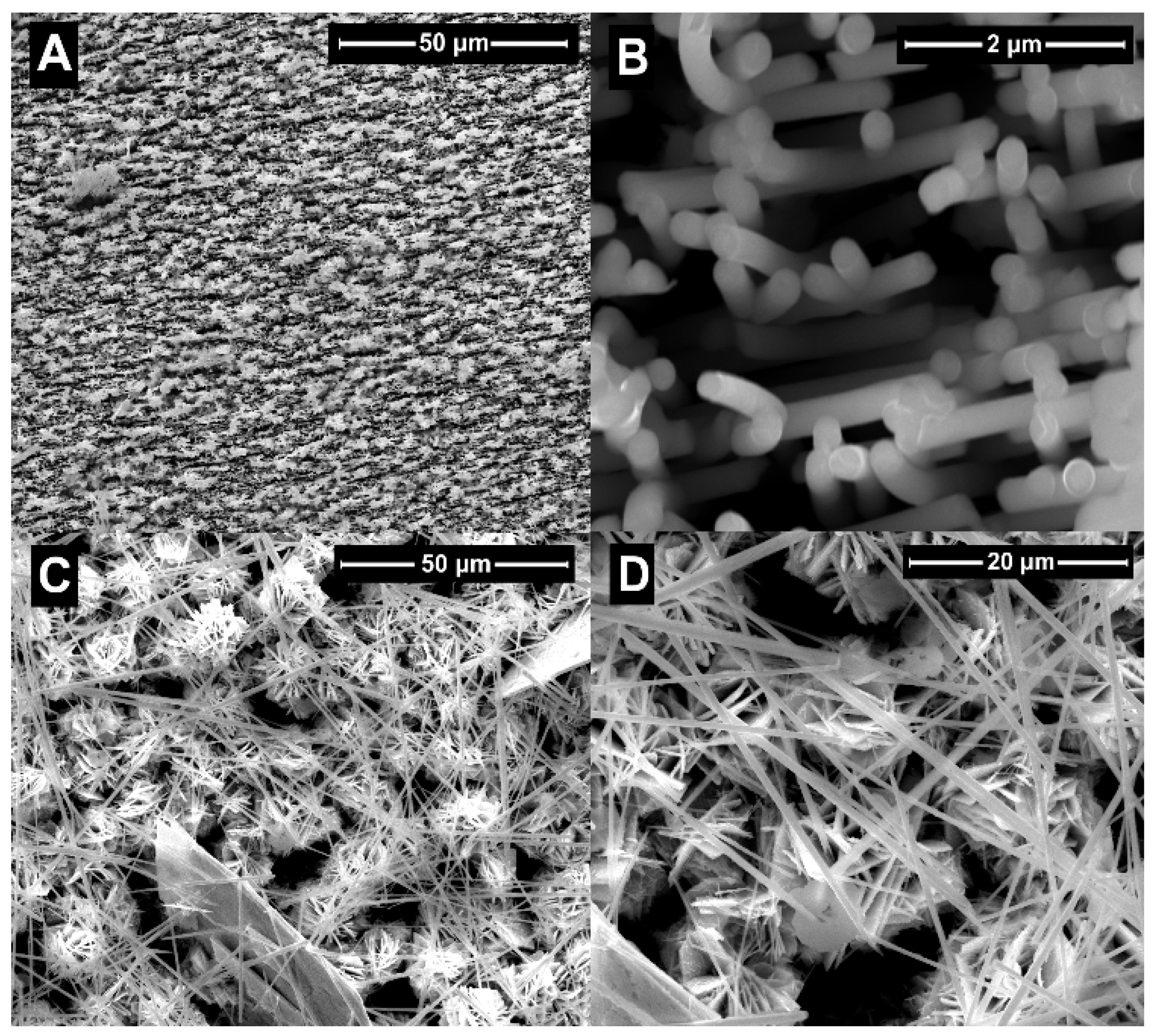
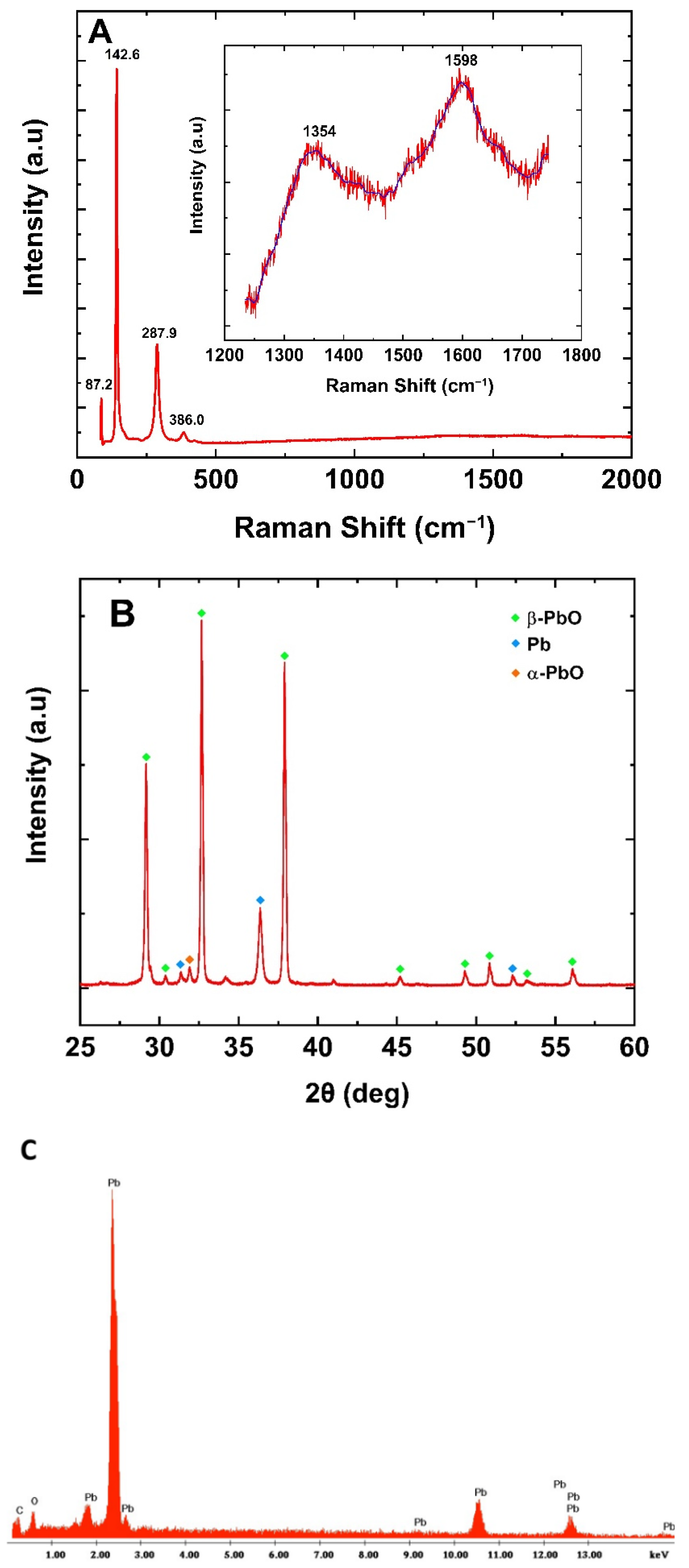
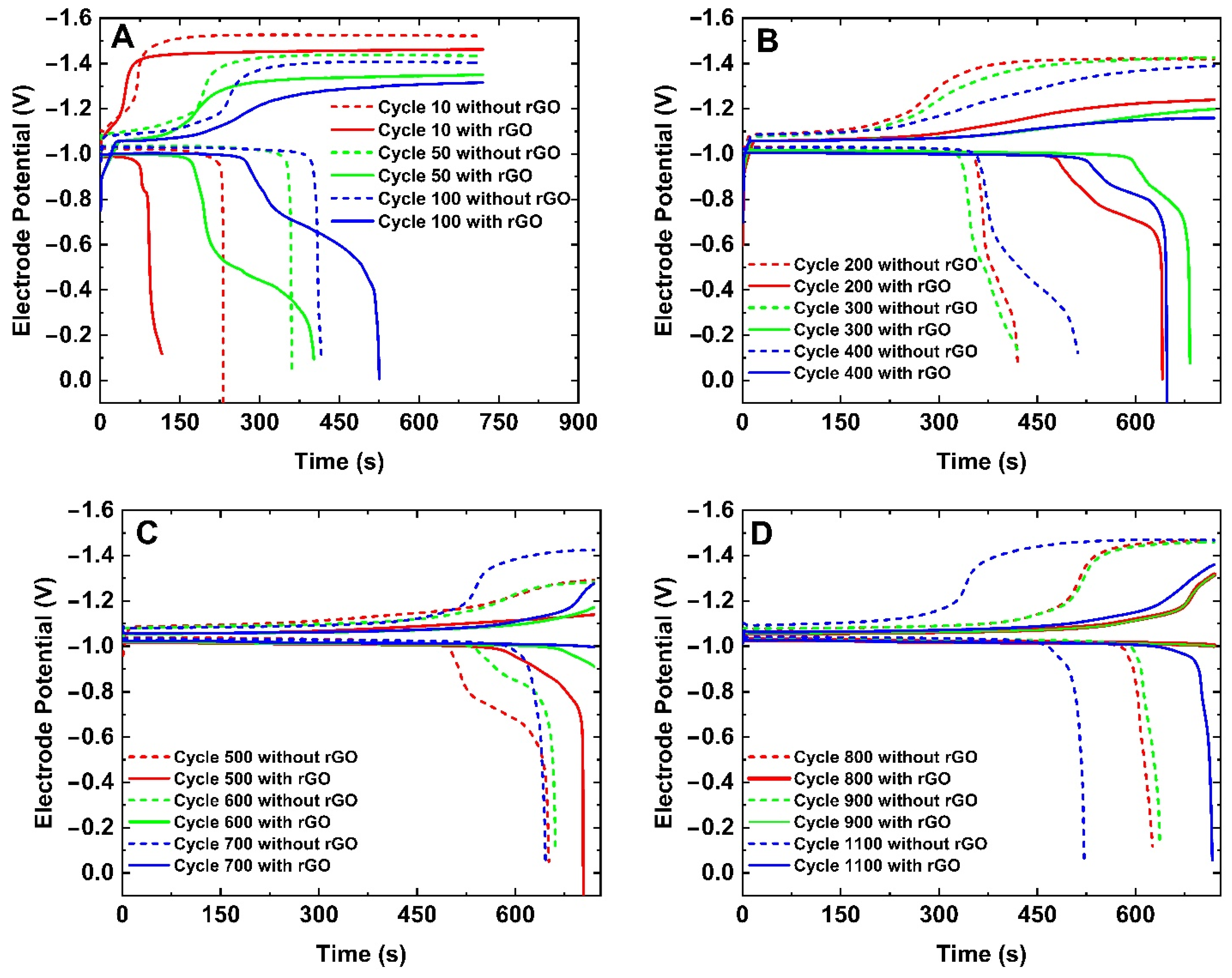
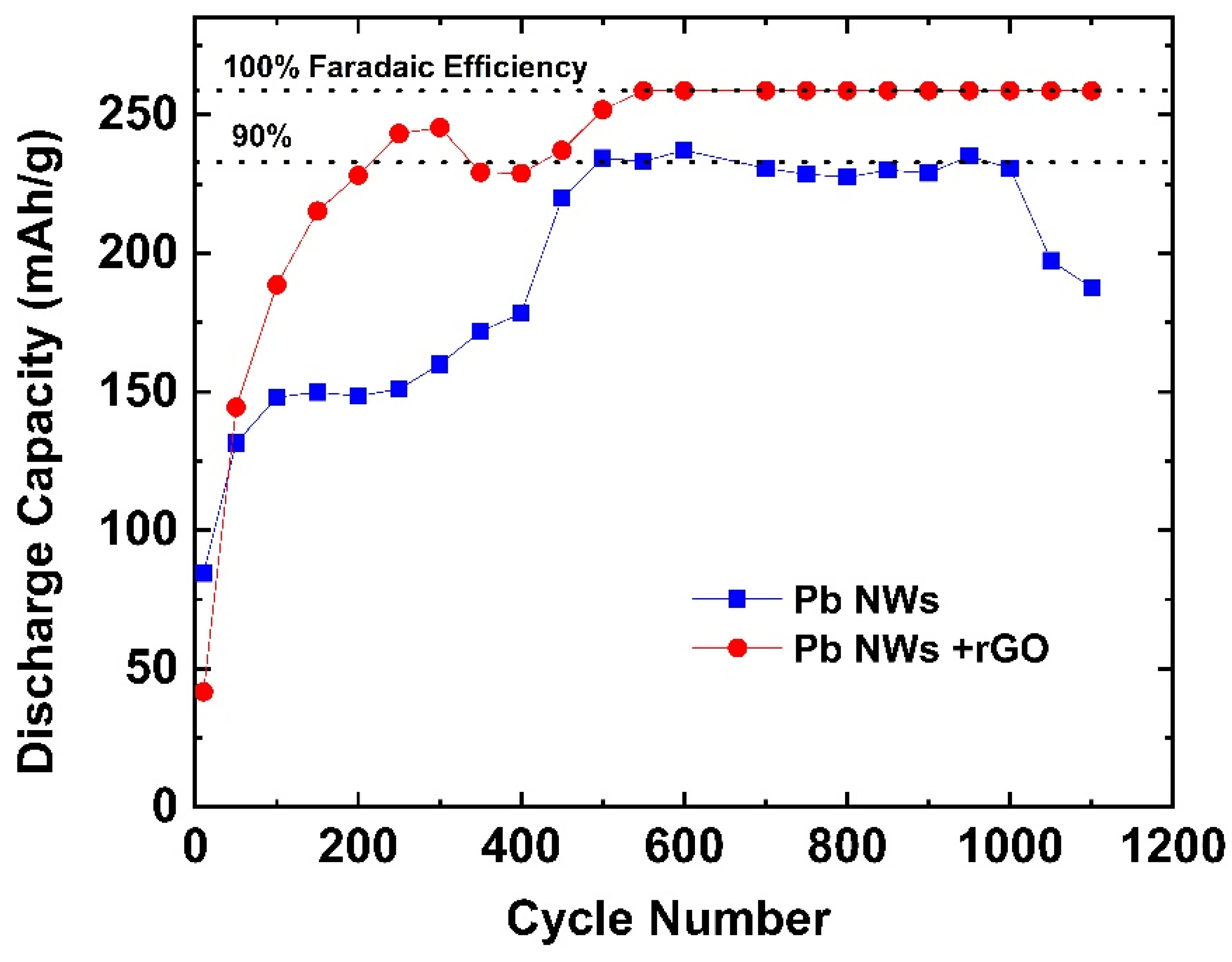
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pavlov, D. Invention and Development of the Lead–Acid Battery, Lead-Acid Batteries: Science and Technology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–32. [Google Scholar] [CrossRef]
- May, G.J.; Davidson, A.; Monahov, B. Lead batteries for utility energy storage: A review. J. Energy Storage 2018, 15, 145–157. [Google Scholar] [CrossRef]
- Vignarooban, K.; Chu, X.; Chimatapu, K.; Ganeshram, P.; Pollat, S.; Johnson, N.G.; Razdan, A.; Pelley, D.S.; Kannan, A.M. State of health determination of sealed lead acid batteries under various operating conditions. Sustain. Energy Technol. Assess. 2018, 18, 134–139. [Google Scholar] [CrossRef]
- Linden, D.; Beard, K.W.; Reddy, T.B. (Eds.) Linden’s Handbook of Batteries, 5th ed.; McGraw-Hill: New York, NY, USA, 2019. [Google Scholar]
- Castro, M.T.; del Rosario, J.A.D.; Chong, M.N.; Chuang, P.-Y.A.; Lee, J.; Ocon, J.D. Multiphysics modeling of lithium-ion, lead-acid, and vanadium redox flow batteries. J. Energy Storage 2021, 42, 102982. [Google Scholar] [CrossRef]
- Zubi, G.; Dufo-López, R.; Carvalho, M.; Pasaoglu, G. The lithium-ion battery: State of the art and future perspectives. Renew. Sustain. Energy Rev. 2018, 89, 292–308. [Google Scholar] [CrossRef]
- Garche, J. (Ed.) Encyclopedia of Electrochemical Power Sources; Elsevier: Amsterdam, The Netherlands; Academic Press: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Ibrahim, N.S.M.; Ponniran, A.; Rahman, R.A.; Martin, M.P.; Yassin, A.; Eahambram, A.; Aziz, M.H. Parameters observation of restoration capacity of industrial lead acid battery using high current pulses. Int. J. Power Electron. Drive Syst. 2020, 11, 1596. [Google Scholar] [CrossRef]
- Kim, S.J.; Co, L.S.E.; Seo, S.W.; An, S.Y.; Kim, B.-G.; Son, J.H.; Gil Jung, Y. Charging-Discharging Behavior and Performance of AGM Lead Acid Battery/EDLC Module for x-HEV. Korean J. Mater. Res. 2021, 31, 84–91. [Google Scholar] [CrossRef]
- Zhang, W.-L.; Yin, J.; Lin, Z.-Q.; Shi, J.; Wang, C.; Liu, D.-B.; Wang, Y.; Bao, J.-P.; Lin, H.-B. Lead-carbon electrode designed for renewable energy storage with superior performance in partial state of charge operation. J. Power Sources 2017, 342, 183–191. [Google Scholar] [CrossRef]
- Fatullah, M.A.; Rahardjo, A.; Husnayain, F. Analysis of Discharge Rate and Ambient Temperature Effects on Lead Acid Battery Capacity. In Proceedings of the 2019 IEEE International Conference on Innovative Research and Development (ICIRD), Jakarta, Indonesia, 28–30 June 2019; pp. 1–5. [Google Scholar] [CrossRef]
- Blecua, M.; Fatas, E.; Ocon, P.; Valenciano, J.; de la Fuente, F.; Trinidad, F. Influences of carbon materials and lignosulfonates in the negative active material of lead-acid batteries for microhybrid vehicles. J. Energy Storage 2017, 11, 55–63. [Google Scholar] [CrossRef]
- Deveau, J.; White, C.; Swan, L.G. Lead-acid battery response to various formation levels—Part A: Recommended formation levels for off-grid solar and conventional applications. Sustain. Energy Technol. Assess. 2015, 11, 1–10. [Google Scholar] [CrossRef]
- Yin, J.; Lin, N.; Lin, Z.; Wang, Y.; Chen, C.; Shi, J.; Bao, J.; Lin, H.; Feng, S.; Zhang, W. Hierarchical porous carbon@PbO1-x composite for high-performance lead-carbon battery towards renewable energy storage. Energy 2019, 193, 116675. [Google Scholar] [CrossRef]
- Fernández, M.; Valenciano, J.; Trinidad, F.; Muñoz, N. The Use of Activated Carbon and Graphite for the Development of Lead-Acid Batteries for Hybrid Vehicle Applications. J. Power Sources 2010, 195, 4458–4469. [Google Scholar] [CrossRef]
- Pavlov, D. Lead-Acid Batteries—Science and Technology—A Handbook of Lead-Acid Battery Technology and Its Influence on the Product, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Yang, F.; Zhou, H.; Hu, J.; Ji, S.; Lai, C.; Wang, H.; Sun, J.; Lei, L. Thorn-like and dendrite lead sulfate as negative electrode materials for enhancing the cycle performance of lead-acid batteries. J. Energy Storage 2022, 49, 104112. [Google Scholar] [CrossRef]
- Tao, S.; Fan, H.; Lei, Y.; Xu, X.; Sun, Y.; You, B.; Gao, Y. The proactive maintenance for the irreversible sulfation in lead-based energy storage systems with a novel resonance method. J. Energy Storage 2021, 42, 103093. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.; Yu, J.; Wu, L.; Zhou, S.; Rao, Y.; Cao, J. The critical role of aluminum sulfate as electrolyte additive on the electrochemical performance of lead-acid battery. Electrochim. Acta 2022, 407, 139877. [Google Scholar] [CrossRef]
- Binh, P.T.; van Anh, N.T.; Thuy, M.T.T.; Xuan, M.T.; Duyen, N.T. Electrochemical study on the structure of PbO2 in mixed gel electrolytes during cycling by cyclic voltammetry. Vietnam J. Chem. 2021, 59, 767–774. [Google Scholar] [CrossRef]
- Yin, J.; Lin, N.; Zhang, W.; Lin, Z.; Zhang, Z.; Wang, Y.; Shi, J.; Bao, J.; Lin, H. Highly reversible lead-carbon battery anode with lead grafting on the carbon surface. J. Energy Chem. 2018, 27, 1674–1683. [Google Scholar] [CrossRef]
- Van Anh, N.T.; Thuy, M.T.T.; Xuan, M.T.; Binh, P.T. Study on electrochemical properties of gelled electrolytes using nano fumed silica in the presence of some organic additives. Int. J. Nanotechnol. 2020, 17, 648. [Google Scholar] [CrossRef]
- Lim, T.S.; Kim, S.J.; Kim, S.D.; Yang, S.C.; Jung, Y.-G. Performance Characteristics of Lead Acid Battery with the Contents of Sodium Perborate Tetrahydrate (SPT) in Positive Plate Active Material. Korean J. Mater. Res. 2020, 30, 426–434. [Google Scholar] [CrossRef]
- Zhang, W.; Yin, J.; Lin, H.; Lu, K.; Feng, F.; Qiu, X. Design principles of lead-carbon additives toward better lead-carbon batteries. Curr. Opin. Electrochem. 2021, 30, 426–434. [Google Scholar] [CrossRef]
- Blecua, M.; Fatas, E.; Ocon, P.; Gonzalo, B.; Merino, C.; de la Fuente, F.; Valenciano, J.; Trinidad, F. Graphitized Carbon Nanofibers: New additive for the Negative Active Material of Lead Acid Batteries. Electrochim. Acta 2017, 257, 109–117. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, W.; Sun, G.; Xiao, S.; Lin, H. Oxygen-functionalized defect engineering of carbon additives enable lead-carbon batteries with high cycling stability. J. Energy Storage 2021, 43, 103205. [Google Scholar] [CrossRef]
- Sawai, K.; Funato, T.; Watanabe, M.; Wada, H.; Nakamura, K.; Shiomi, M.; Osumi, S. Development of additives in negative active-material to suppress sulfation during high-rate partial-state-of-charge operation of lead–acid batteries. J. Power Sources 2006, 158, 1084–1090. [Google Scholar] [CrossRef]
- Garche, J.; Karden, E.; Moseley, P.T.; Rand, D.A.J. (Eds.) Lead-Acid Batteries for Future Automobiles; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Yin, J.; Lin, H.; Shi, J.; Lin, Z.; Bao, J.; Wang, Y.; Lin, X.; Qin, Y.; Qiu, X.; Zhang, W. Lead-Carbon Batteries toward Future Energy Storage: From Mechanism and Materials to Applications. Electrochem. Energy Rev. 2022, 5, 1–32. [Google Scholar] [CrossRef]
- Morales, J.; Petkova, G.; Cruz, M.; Caballero, A. Nanostructured Lead Dioxide Thin Electrode. Electrochem. Solid-State Lett. 2004, 7, A75–A77. [Google Scholar] [CrossRef]
- Ghasemi, S.; Mousavi, M.F.; Karami, H.; Shamsipur, M.; Kazemi, S. Energy storage capacity investigation of pulsed current formed nano-structured lead dioxide. Electrochim. Acta 2006, 52, 1596–1602. [Google Scholar] [CrossRef]
- Bervas, M.; Perrin, M.; Geniès, S.; Mattera, F. Low-cost synthesis and utilization in mini-tubular electrodes of nano PbO2. J. Power Sources 2007, 173, 570–577. [Google Scholar] [CrossRef]
- Perret, P.; Brousse, T.; Bélanger, D.; Guay, D. Electrochemical Template Synthesis of Ordered Lead Dioxide Nanowires. J. Electrochem. Soc. 2009, 156, A645–A651. [Google Scholar] [CrossRef]
- Egan, D.; Low, C.; Walsh, F. Electrodeposited nanostructured lead dioxide as a thin film electrode for a lightweight lead-acid battery. J. Power Sources 2011, 196, 5725–5730. [Google Scholar] [CrossRef]
- Chen, T.; Huang, H.; Ma, H.; Kong, D. Effects of surface morphology of nanostructured PbO2 thin films on their electrochemical properties. Electrochim. Acta 2012, 88, 79–85. [Google Scholar] [CrossRef]
- Fan, N.; Sun, C.; Kong, D.; Qian, Y. Chemical synthesis of PbO2 particles with multiple morphologies and phases and their electrochemical performance as the positive active material. J. Power Sources 2014, 254, 323–328. [Google Scholar] [CrossRef]
- Zhao, X. Electrodeposited PbO2 Thin Films with Different Surface Structure as Positive Plate in Lead Acid Batteries. Int. J. Electrochem. Sci. 2018, 3745–3756. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Ma, X.; Tang, K.; Wang, Z.; Hao, X.; Abudula, A.; Guan, G. Template-free electro-synthesis of PbO2 nanorod with chrysanthemum-like array. Mater. Lett. 2018, 238, 85–88. [Google Scholar] [CrossRef]
- Yu, N.; Gao, L.; Zhao, S.; Wang, Z. Electrodeposited PbO2 thin film as positive electrode in PbO2/AC hybrid capacitor. Electrochim. Acta 2009, 54, 3835–3841. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, H.; Kong, H.; Lu, H.; Yang, Z.; Liu, T. Preparation and characterization of lead dioxide electrode with three-dimensional porous titanium substrate for electrochemical energy storage. Electrochim. Acta 2014, 139, 209–216. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, X.; Yu, N.; Wei, F.; Feng, H. Preparation and Supercapacitive Performance of Lead Dioxide Electrodes with Three-Dimensional Porous Structure. Russ. J. Electrochem. 2018, 54, 585–591. [Google Scholar] [CrossRef]
- Yao, Y.; Li, M.; Zhang, X.; Lu, Z.; Wei, F. Influence of manganese content on the supercapacitive performance of PbO2-MnO2 electrodes. Turk. J. Chem. 2018, 42, 472–481. [Google Scholar] [CrossRef]
- Li, J.; Sattayasamitsathit, S.; Dong, R.; Gao, W.; Tam, R.; Feng, X.; Ai, S.; Wang, J. Template electrosynthesis of tailored-made helical nanoswimmers. Nanoscale 2013, 6, 9415–9420. [Google Scholar] [CrossRef]
- Patella, B.; Piazza, S.; Sunseri, C.; Inguanta, R. Anodic Alumina Membranes: From Electrochemical Growth to Use as Template for Fabrication of Nanostructured Electrodes. Appl. Sci. 2022, 12, 869. [Google Scholar] [CrossRef]
- Lai, M.; Riley, J. Templated electrosynthesis of nanomaterials and porous structures. J. Colloid Interface Sci. 2008, 323, 203–212. [Google Scholar] [CrossRef]
- Inguanta, R.; Vergottini, F.; Ferrara, G.; Piazza, S.; Sunseri, C. Effect of temperature on the growth of α-PbO2 nanostructures. Electrochim. Acta 2010, 55, 8556–8562. [Google Scholar] [CrossRef]
- Insinga, M.G.; Oliveri, R.L.; Sunseri, C.; Inguanta, R. Template electrodeposition and characterization of nanostructured Pb as a negative electrode for lead-acid battery. J. Power Sources 2018, 413, 107–116. [Google Scholar] [CrossRef]
- Inguanta, R.; Rinaldo, E.; Piazza, S.; Sunseri, C. Lead Nanowires for Microaccumulators Obtained Through Indirect Electrochemical Template Deposition. Electrochem. Solid-State Lett. 2010, 13, K1–K4. [Google Scholar] [CrossRef]
- Oliveri, R.; Insinga, M.; Pisana, S.; Patella, B.; Aiello, G.; Inguanta, R. High-Performance Lead-Acid Batteries Enabled by Pb and PbO2 Nanostructured Electrodes: Effect of Operating Temperature. Appl. Sci. 2021, 11, 6357. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Gu, Z. A comprehensive review of template-synthesized multi-component nanowires: From interfacial design to sensing and actuation applications. Sens. Actuators Rep. 2021, 3, 100029. [Google Scholar] [CrossRef]
- Inguanta, R.; Piazza, S.; Sunseri, C.; Cino, A.; di Dio, V.; Cascia, D.L.; Miceli, R.; Rando, C.; Zizzo, G. An Electrochemical Route Towards the Fabrication of Nanostructured Semiconductor Solar Cells, SPEEDAM 2010; IEEE: Pisa, Italy, 2010; pp. 1166–1171. [Google Scholar] [CrossRef]
- Kelaidis, N.; Zervos, M.; Lathiotakis, N.N.; Chroneos, A.; Tanasă, E.; Vasile, E. Vapor–liquid–solid growth and properties of one dimensional PbO and PbO/SnO2 nanowires. Mater. Adv. 2021, 3, 1695–1702. [Google Scholar] [CrossRef]
- Battaglia, M.; Inguanta, R.; Piazza, S.; Sunseri, C. Fabrication and characterization of nanostructured Ni–IrO2 electrodes for water electrolysis. Int. J. Hydrog. Energy 2014, 39, 16797–16805. [Google Scholar] [CrossRef]
- Madusanka, S.A.U.A.; Mahadiulwewa, D.M.O.R.; Samarakoon, S.P.A.A.J.; Sandeepanie, K.A.H.; Damayanthi, R.M.T. Improving the Performance of Lead Acid Batteries using Nano-Technology. In Proceedings of the Moratuwa Engineering Research Conference (MERCon), Moratuwa, Sri Lanka, 27–30 July 2019; pp. 589–593. [Google Scholar] [CrossRef]
- Logeshkumar, S.; Manoharan, R. Influence of some nanostructured materials additives on the performance of lead acid battery negative electrodes. Electrochim. Acta 2014, 144, 147–153. [Google Scholar] [CrossRef]
- Lach, J.; Wróbel, K.; Wróbel, J.; Podsadni, P.; Czerwiński, A. Applications of carbon in lead-acid batteries: A review. J. Solid State Electrochem. 2019, 23, 693–705. [Google Scholar] [CrossRef]
- Blecua, M.; Romero, A.; Ocon, P.; Fatas, E.; Valenciano, J.; Trinidad, F. Improvement of the lead acid battery performance by the addition of graphitized carbon nanofibers together with a mix of organic expanders in the negative active material. J. Energy Storage 2019, 23, 106–115. [Google Scholar] [CrossRef]
- Arun, S.; Kiran, K.U.V.; Mayavan, S. Effects of carbon surface area and morphology on performance of stationary lead acid battery. J. Energy Storage 2020, 32, 101763. [Google Scholar] [CrossRef]
- Tian, Y.; Yu, Z.; Cao, L.; Zhang, X.L.; Sun, C.; Wang, D.-W. Graphene oxide: An emerging electromaterial for energy storage and conversion. J. Energy Chem. 2020, 55, 323–344. [Google Scholar] [CrossRef]
- Berbeć, S.; Żołądek, S.; Jabłońska, A.; Palys, B. Electrochemically reduced graphene oxide on gold nanoparticles modified with a polyoxomolybdate film. Highly sensitive non-enzymatic electrochemical detection of H2O2. Sens. Actuators B Chem. 2018, 258, 745–756. [Google Scholar] [CrossRef]
- Nong, J.; Lan, G.; Jin, W.; Luo, P.; Guo, C.; Tang, X.; Zang, Z.; Wei, W. Eco-friendly and high-performance photoelectrochemical anode based on AgInS2 quantum dots embedded in 3D graphene nanowalls. J. Mater. Chem. C 2019, 7, 9830–9839. [Google Scholar] [CrossRef]
- Liu, X.; Xu, T.; Li, Y.; Zang, Z.; Peng, X.; Wei, H.; Zha, W.; Wang, F. Enhanced X-ray photon response in solution-synthesized CsPbBr3 nanoparticles wrapped by reduced graphene oxide. Sol. Energy Mater. Sol. Cells 2018, 187, 249–254. [Google Scholar] [CrossRef]
- Wei, J.; Zang, Z.; Zhang, Y.; Wang, M.; Du, J.; Tang, X. Enhanced performance of light-controlled conductive switching in hybrid cuprous oxide/reduced graphene oxide (Cu2O/rGO) nanocomposites. Opt. Lett. 2017, 42, 911–914. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Klinowski, J.; Forster, M.; Lerf, A. A new structural model for graphite oxide. Chem. Phys. Lett. 1998, 287, 53–56. [Google Scholar] [CrossRef]
- Alam, S.N.; Sharma, N.; Kumar, L. Synthesis of Graphene Oxide (GO) by Modified Hummers Method and Its Thermal Reduction to Obtain Reduced Graphene Oxide (rGO)*. Graphene 2017, 6, 1–18. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Toh, S.Y.; Loh, K.S.; Kamarudin, S.K.; Daud, W.R.W. Graphene production via electrochemical reduction of graphene oxide: Synthesis and characterization. Chem. Eng. J. 2014, 251, 422–434. [Google Scholar] [CrossRef]
- Yeung, K.K.; Zhang, X.; Kwok, S.C.T.; Ciucci, F.; Yuen, M.M.F. Enhanced cycle life of lead-acid battery using graphene as a sulfation suppression additive in negative active material. RSC Adv. 2015, 5, 71314–71321. [Google Scholar] [CrossRef]
- Chang, S.-H.; Kung, K.-C.; Huang, W.-C.; Liu, W.-R. Few-layer graphene as an additive in negative electrodes for lead-acid batteries. Thin Solid Film. 2022, 753, 139273. [Google Scholar] [CrossRef]
- Li, J.; Cao, J.; Chen, Z.; Yu, J.; Zhang, J.; Chen, B.; Wu, L.; Zhou, S.; Rao, Y. Improving the performance of recovered lead oxide powder from waste lead paste as active material for lead-acid battery. Int. J. Energy Res. 2022, 46, 14268–14282. [Google Scholar] [CrossRef]
- Yang, H.; Qiu, Y.; Guo, X. Effects of PPy, GO and PPy/GO composites on the negative plate and on the high-rate partial-state-of-charge performance of lead-acid batteries. Electrochim. Acta 2016, 215, 346–356. [Google Scholar] [CrossRef]
- Yang, H.; Qi, K.; Gong, L.; Liu, W.; Zaman, S.; Guo, X.; Qiu, Y.; Xia, B.Y. Lead Oxide Enveloped in N-Doped Graphene Oxide Composites for Enhanced High-Rate Partial-State-of-Charge Performance of Lead-Acid Battery. ACS Sustain. Chem. Eng. 2018, 6, 11408–11413. [Google Scholar] [CrossRef]
- Wang, X.-R.; Zhong, J.; Zhu, K.-D.; Wang, S.-L. Nitrogen-doped redox graphene as a negative electrode additive for lead-acid batteries. J. Energy Storage 2021, 44, 103454. [Google Scholar] [CrossRef]
- Long, Q.; Ma, G.; Xu, Q.; Ma, C.; Nan, J.; Li, A.; Chen, H. Improving the cycle life of lead-acid batteries using three-dimensional reduced graphene oxide under the high-rate partial-state-of-charge condition. J. Power Sources 2017, 343, 188–196. [Google Scholar] [CrossRef]
- Vangapally, N.; Jindal, S.; Gaffoor, S.; Martha, S.K. Titanium dioxide-reduced graphene oxide hybrid as negative electrode additive for high performance lead-acid batteries. J. Energy Storage 2018, 20, 204–212. [Google Scholar] [CrossRef]
- Tao, D.; Liu, X.; Li, Z.; Yang, H.; Wang, J.; Zhang, Q. PbO nanoparticles anchored on reduced graphene oxide for enhanced cycle life of lead-carbon battery. Electrochim. Acta 2022, 432, 141228. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, Y.; Zhai, Y.; Zhai, J.; Ren, W.; Wang, F.; Dong, S. Controlled Synthesis of Large-Area and Patterned Electrochemically Reduced Graphene Oxide Films. Chem. A Eur. J. 2009, 15, 6116–6120. [Google Scholar] [CrossRef]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. The power of databases: The RRUFF project. In Highlights in Mineralogical Crystallography; Armbruster, T., Danisi, R.M., Eds.; De Gruyter: Berlin, Germany, 2015; pp. 1–30. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, X.; Zhang, H.; Wen, T.; Huang, F.; Li, G.; Wang, Y.; Liao, F.; Lin, J. Selected-control hydrothermal growths of α- and β-PbO crystals and orientated pressure-induced phase transition. CrystEngComm 2012, 15, 3513–3516. [Google Scholar] [CrossRef]
- Güngör, A.; Genç, R.; Özdemir, T. Facile Synthesis of Semiconducting Nanosized 0d and 2d Lead Oxides Using a Modified Co-Precipitation Method. J. Turk. Chem. Soc. Sect. A Chem. 2017, 4, 1017–1030. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossini, M.; Ganci, F.; Zanca, C.; Patella, B.; Aiello, G.; Inguanta, R. Nanostructured Lead Electrodes with Reduced Graphene Oxide for High-Performance Lead–Acid Batteries. Batteries 2022, 8, 211. https://doi.org/10.3390/batteries8110211
Rossini M, Ganci F, Zanca C, Patella B, Aiello G, Inguanta R. Nanostructured Lead Electrodes with Reduced Graphene Oxide for High-Performance Lead–Acid Batteries. Batteries. 2022; 8(11):211. https://doi.org/10.3390/batteries8110211
Chicago/Turabian StyleRossini, Matteo, Fabrizio Ganci, Claudio Zanca, Bernardo Patella, Giuseppe Aiello, and Rosalinda Inguanta. 2022. "Nanostructured Lead Electrodes with Reduced Graphene Oxide for High-Performance Lead–Acid Batteries" Batteries 8, no. 11: 211. https://doi.org/10.3390/batteries8110211
APA StyleRossini, M., Ganci, F., Zanca, C., Patella, B., Aiello, G., & Inguanta, R. (2022). Nanostructured Lead Electrodes with Reduced Graphene Oxide for High-Performance Lead–Acid Batteries. Batteries, 8(11), 211. https://doi.org/10.3390/batteries8110211









