Effects of Excessive Prelithiation on Full-Cell Performance of Li-Ion Batteries with a Hard-Carbon/Nanosized-Si Composite Anode
Abstract
1. Introduction
2. Results and Discussion
2.1. Half-Cell Evaluation
2.2. Physical Characterization of Materials and Electrodes
2.3. Specific Capacity of HC8/N-Si2 Anode
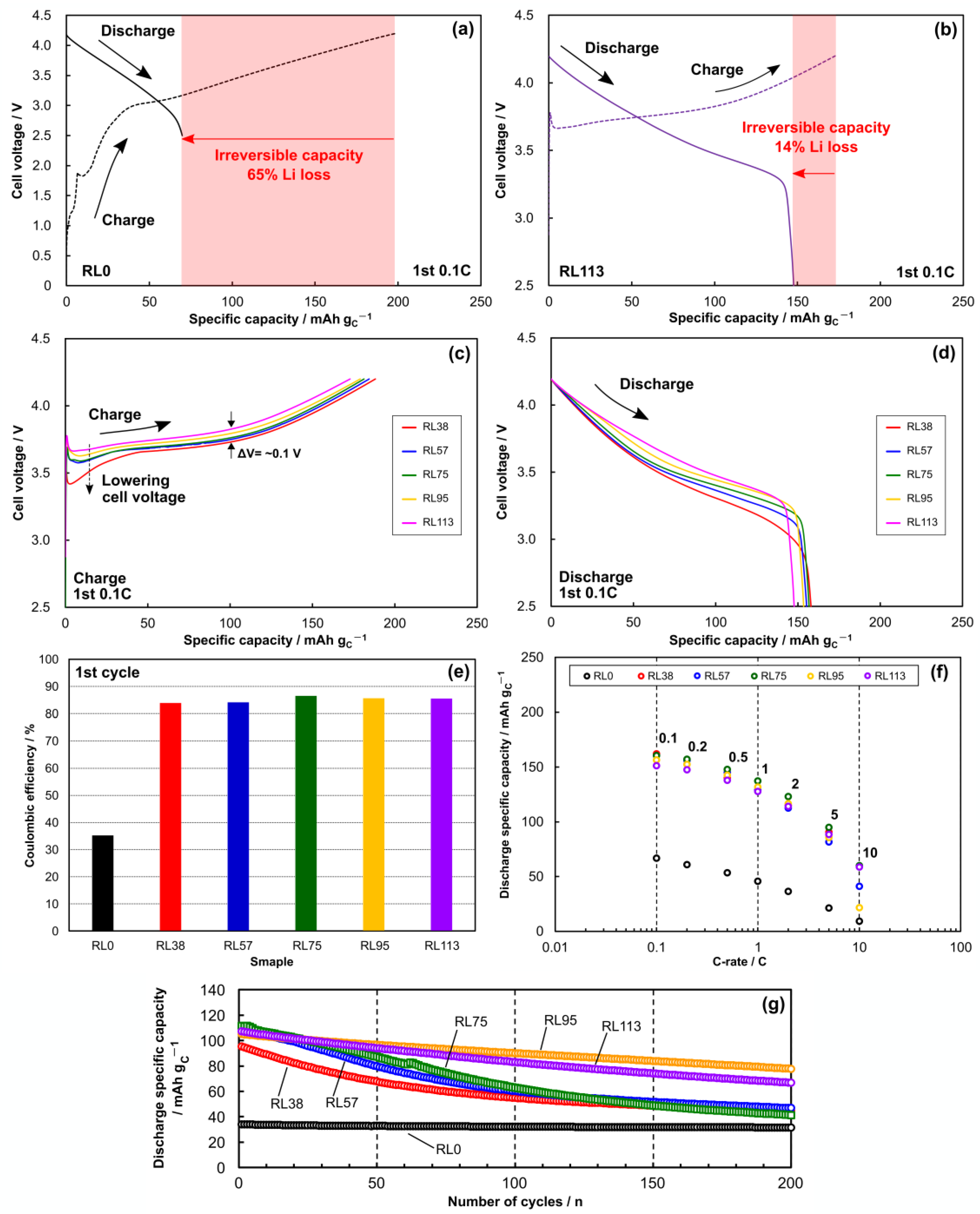
2.4. Anode Prelithiation and Full-Cell Assembly
2.5. Full-Cell Evaluation
2.6. Effects of Blend Ratio and Excessive Prelithiation on Performance of HC/N-Si Composite Anode
3. Materials and Methods
3.1. Electrode Fabrication
3.2. Physical Characterization
3.3. Electrochemical Measurements
3.4. Design, Assembly, and Performance Evaluation of Full Cell
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miao, Y.; Hynan, P.; von Jouanne, A.; Yokochi, A. Current Li-ion battery technologies in electric vehicles and opportunities for advancements. Energies 2019, 12, 1074. [Google Scholar] [CrossRef]
- Li, H. Practical evaluation of Li-ion batteries. Joule 2019, 3, 908–919. [Google Scholar] [CrossRef]
- McCloskey, B.D. Expanding the Ragone plot: Pushing the limits of energy storage. J. Phys. Chem. Lett. 2015, 6, 3592–3593. [Google Scholar] [CrossRef] [PubMed]
- Buiel, E.; Dahn, J.R. Li-insertion in hard carbon anode materials for Li-ion batteries. Electrochim. Acta 1999, 45, 121–130. [Google Scholar] [CrossRef]
- Shobukawa, H.; Alvarado, J.; Yang, Y.; Meng, Y.S. Electrochemical performance and interfacial investigation on Si composite anode for lithium ion batteries in full cell. J. Power Sources 2017, 359, 173–181. [Google Scholar] [CrossRef]
- Kim, J.-H.; Jung, M.-J.; Kim, M.-J.; Lee, Y.-S. Electrochemical performances of lithium and sodium ion batteries based on carbon materials. J. Ind. Eng. Chem. 2018, 61, 368–380. [Google Scholar] [CrossRef]
- Khomenko, V.G.; Barsukov, V.Z. Characterization of silicon- and carbon-based composite anodes for lithium-ion batteries. Electrochim. Acta 2007, 52, 2829–2840. [Google Scholar] [CrossRef]
- Fergus, J.W. Recent developments in cathode materials for lithium ion batteries. J. Power Sources 2010, 195, 939–954. [Google Scholar] [CrossRef]
- Terranova, M.L.; Orlanducci, S.; Tamburri, E.; Guglielmotti, V.; Rossi, M. Si/C hybrid nanostructures for Li-ion anodes: An overview. J. Power Sources 2014, 246, 167–177. [Google Scholar] [CrossRef]
- Eom, K.; Joshi, T.; Bordes, A.; Do, I.; Fuller, T.F. The design of a Li-ion full cell battery using a nano silicon and nano multi-layer graphene composite anode. J. Power Sources 2014, 249, 118–124. [Google Scholar] [CrossRef]
- Kobayashi, N.; Inden, Y.; Endo, M. Silicon/soft-carbon nanohybrid material with low expansion for high capacity and long cycle life lithium-ion battery. J. Power Sources 2016, 326, 235–241. [Google Scholar] [CrossRef]
- Kierzek, K.; Machnikowski, J. Factors influencing cycle-life of full Li-ion cell built from Si/C composite as anode and conventional cathodic material. Electrochim. Acta 2016, 192, 475–481. [Google Scholar] [CrossRef]
- Ge, C.; Fan, Z.; Zhang, J.; Qiao, Y.; Wang, J.; Lingd, L. Novel hard carbon/graphite composites synthesized by a facile in situ anchoring method as high-performance anodes for lithium-ion batteries. RSC Adv. 2018, 8, 34682–34689. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Liang, R.; Zheng, J.P. Freestanding flexible Si nanoparticles–multiwalled carbon nanotubes composite anodes for Li-ion batteries and their prelithiation by stabilized Li metal powder. J. Electrochem. Energy Convers. Storage 2016, 13, 011004. [Google Scholar] [CrossRef]
- Hu, B.; Jiang, S.; Shkrob, I.A.; Zhang, J.; Trask, S.E.; Polzin, B.J.; Jansen, A.; Chen, W.; Liao, C.; Zhang, Z.; et al. Understanding of pre-lithiation of poly(acrylic acid) binder: Striking the balances between the cycling performance and slurry stability for silicon-graphite composite electrodes in Li-ion batteries. J. Power Sources 2019, 416, 125–131. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, J.-S.; Song, S.-W. Uniform distribution of siloxane-grafted SiOx nanoparticles in micron hard-carbon matrix for high-rate composite anode in Li-ion batteries. J. Solid State Chem. 2019, 270, 479–486. [Google Scholar] [CrossRef]
- Zhong, X.; Wei, Y.; Guo, Y.; Cui, C.; Zhai, T.; Li, H. Double the energy storage of hard carbon anode for Li-ion batteries via a simple blending strategy. Electrochim. Acta 2020, 336, 135729. [Google Scholar] [CrossRef]
- Kim, K.H.; Shon, J.; Jeong, H.; Park, H.; Lim, S.-J.; Heo, J.S. Improving the cyclability of silicon anodes for lithium-ion batteries using a simple pre-lithiation method. J. Power Sources 2020, 459, 228066. [Google Scholar] [CrossRef]
- Park, H.; Yoon, N.; Kang, D.H.; Young, C.; Lee, J.K. Electrochemical characteristics and energy densities of lithium-ion batteries using mesoporous silicon and graphite as anodes. Electrochim. Acta 2020, 357, 136870. [Google Scholar] [CrossRef]
- Li, H.; Ji, W.; He, Z.; Zhang, Y.; Zhao, J. Distinct capacity fade modes of nickel-rich/graphite-SiOx power lithium ion battery. J. Energy Storage 2022, 47, 103830. [Google Scholar] [CrossRef]
- Huang, W.-J.; Zheng, J.-Y.; Liu, J.-J.; Yang, R.M.; Cheng, F.X.; Suo, H.B.; Guo, H.; Xia, S.B. Boosting rate performance of LiNi0.8Co0.15Al0.05O2 cathode by simply mixing lithium iron phosphate. J. Alloys Compd. 2020, 827, 154296. [Google Scholar] [CrossRef]
- Chen, K.H.; Goel, V.; Namkoong, M.J.; Wied, M.; Müller, S.; Wood, V.; Sakamoto, J.; Thornton, K.; Dasgupta, N.P. Enabling 6C fast charging of Li-ion batteries with graphite/hard carbon hybrid anodes. Adv. Energy Mater. 2020, 11, 2003336. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.L.; Wang, Y.G.; Wang, C.X.; Xia, Y.Y. Pitch modified hard carbons as negative materials for lithium-ion batteries. Electrochim. Acta 2012, 74, 1–7. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, B.; Dong, X.; Wang, Y.; Xia, Y. A simple prelithiation strategy to build a high-rate and long-life lithium-ion battery with improved low-temperature performance. Angew. Chem. Int. Ed. 2017, 56, 16606–16610. [Google Scholar] [CrossRef] [PubMed]
- Kasavajjula, U.; Wang, C.; Appleby, A.J. Nano- and bulk-silicon-based insertion anodes for lithium-ion secondary cells. J. Power Sources 2007, 163, 1003–1039. [Google Scholar] [CrossRef]
- Azam, M.A.; Safie, N.E.; Ahmad, A.S.; Yuza, N.A.; Zulkifli, N.S.A. Recent advances of silicon, carbon composites and tin oxide as new anode materials for lithium-ion battery: A comprehensive review. J. Energy Storage 2021, 33, 102096. [Google Scholar] [CrossRef]
- Wu, H.; Cui, Y. Designing nanostructured Si anodes for high energy lithium ion batteries. Nano Today 2012, 7, 414–429. [Google Scholar] [CrossRef]
- Bärmann, P.; Diehl, M.; Göbel, L.; Ruttert, M.; Nowak, S.; Winter, M.; Placke, T. Impact of the silicon particle size on the pre-lithiation behavior of silicon/carbon composite materials for lithium ion batteries. J. Power Sources 2020, 464, 228224. [Google Scholar] [CrossRef]
- Song, B.F.; Dhanabalan, A.; Biswal, S.L. Evaluating the capacity ratio and prelithiation strategies for extending cyclability in porous silicon composite anodes and lithium iron phosphate cathodes for high capacity lithium-ion batteries. J. Energy Storage 2020, 28, 101268. [Google Scholar] [CrossRef]
- Wu, M.-S.; Chiang, P.-C.J.; Lin, J.C. Electrochemical investigations on advanced lithium-ion batteries by three-electrode measurements. J. Electrochem. Soc. 2005, 152, A47–A52. [Google Scholar] [CrossRef]
- Zhang, S.S.; Xu, K.; Jow, T.R. Study of the charging process of a LiCoO2-based Li-ion battery. J. Power Sources 2006, 160, 1349–1354. [Google Scholar] [CrossRef]
- Loeffler, N.; von Zamory, J.; Laszczynski, N.; Doberdo, I.; Kim, G.-T.; Passerini, S. Performance of LiNi1/3Mn1/3Co1/3O2/graphite batteries based on aqueous binder. J. Power Sources 2014, 248, 915–922. [Google Scholar] [CrossRef]
- Kim, C.-S.; Jeong, K.M.; Kim, K.; Yi, C.W. Effects of capacity ratios between anode and cathode on electrochemical properties for lithium polymer batteries. Electrochim. Acta 2015, 155, 431–436. [Google Scholar] [CrossRef]
- Liu, S.; Xiong, L.; He, C. Long cycle life lithium ion battery with lithium nickel cobalt manganese oxide (NCM) cathode. J. Power Sources 2014, 261, 285–291. [Google Scholar] [CrossRef]
- Abe, Y.; Kumagai, S. Effect of negative/positive capacity ratio on the rate and cycling performances of LiFePO4/graphite lithium-ion batteries. J. Energy Storage 2018, 19, 96–102. [Google Scholar] [CrossRef]
- Sun, H.; He, X.; Ren, J.; Li, J.; Jiang, C.; Wan, C. Hard carbon/lithium composite anode materials for Li-ion batteries. Electrochim. Acta 2007, 52, 4312–4316. [Google Scholar] [CrossRef]
- Wang, H.; Lai, C.; Xiao, Y.; Ai, X. A new lithium-ion battery with LiNi0.80Co0.15Al0.05O2 cathode and lithium pre-doping hard carbon anode. Mater. Lett. 2015, 160, 250–254. [Google Scholar] [CrossRef]
- Son, I.H.; Park, J.H.; Park, S.; Park, K.; Han, S.; Shin, J.; Doo, S.-G.; Hwang, Y.; Chang, H.; Choi, J.W. Graphene balls for lithium rechargeable batteries with fast charging and high volumetric energy densities. Nat. Commun. 2017, 8, 1561. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, C.; Han, S. Improving the initial Coulombic efficiency of hard carbon-based anode for rechargeable batteries with high energy density. J. Mater. Sci. 2017, 52, 10418–10430. [Google Scholar] [CrossRef]
- Kulova, T.L.; Skundin, A.M. Irreversible capacity elimination via immediate contact of carbon with lithium metal. J. Solid State Electrochem. 2003, 8, 59–65. [Google Scholar] [CrossRef]
- Sigma-Aldrich. High-Performance Silicon Anode. Available online: https://www.sigmaaldrich.com/JP/ja/product/aldrich/918334 (accessed on 19 February 2022).
- Agubra, V.; Fergus, J. Lithium ion battery anode aging mechanisms. Materials 2013, 6, 1310–1325. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Li, X.; Zheng, J.; Yan, P.; Cao, R.; Jung, H.J.; Wang, C.; Liu, J.; Zhang, J.-G. Hard carbon coated nano-Si/graphite composite as a high performance anode for Li-ion batteries. J. Power Sources 2016, 329, 323–329. [Google Scholar] [CrossRef]
- Ni, J.; Huang, Y.; Gao, L. A high-performance hard carbon for Li-ion batteries and supercapacitors application. J. Power Sources 2013, 223, 306–311. [Google Scholar] [CrossRef]
- Parekh, M.H.; Parikh, V.P.; Kim, P.J.; Misra, S.; Qi, Z.; Wang, H.; Pol, V.G. Encapsulation and networking of silicon nanoparticles using amorphous carbon and graphite for high performance Li-ion batteries. Carbon 2019, 148, 36–43. [Google Scholar] [CrossRef]
- Mu, T.; Zhang, Z.; Li, Q.; Lou, S.; Zuo, P.; Du, C.; Yin, G. Scalable submicron/micron silicon particles stabilized in a robust graphite-carbon architecture for enhanced lithium storage. J. Colloid Interface Sci. 2019, 555, 783–790. [Google Scholar] [CrossRef]
- Jiang, S.; Ji, Q.; Yun, S.; Zhang, Z.; Jiang, Q.; Chen, H.C. Tuning the microstructures of uniform carbon spheres by controlling the annealing conditions for high-performance lithium-ion full batteries and lithium-ion capacitors. J. Energy Storage 2021, 39, 102625. [Google Scholar] [CrossRef]
- Yu, H.; Dong, X.; Pang, Y.; Wang, Y.; Xia, Y. High power lithium-ion battery based on spinel cathode and hard carbon anode. Electrochim. Acta 2017, 228, 251–258. [Google Scholar] [CrossRef]
- Simonetti, W.; Maresca, G.; Appetecchi, G.B.; Kim, G.-T.; Loeffler, N.; Passerini, S. Towards Li(Ni0.33Mn0.33Co0.33)O2/graphite batteries with ionic liquid-based electrolytes. I. Electrodes behavior in lithium half-cells. J. Power Sources 2016, 331, 426–434. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, R.; Wang, L.; Cheng, K.; Zhao, Z.; Mu, D.; Wu, B. A short review on layered LiNi0.8Co0.1Mn0.1O2 positive electrode material for lithium-ion batteries. Energy Procedia 2017, 105, 2941–2952. [Google Scholar] [CrossRef]
- Sun, Y.; Ning, G.; Qi, C.; Li, J.; Ma, X.; Xu, C.; Li, Y.; Zhang, X.; Gao, J. An advanced lithium ion battery based on a sulfur-doped porous carbon anode and a lithium iron phosphate cathode. Electrochim. Acta 2016, 190, 141–149. [Google Scholar] [CrossRef]
- Zheng, J.-S.; Zhang, L.; Shellikeri, A.; Cao, W.; Wu, Q.; Zheng, J.P. A hybrid electrochemical device based on a synergetic inner combination of Li ion battery and Li ion capacitor for energy storage. Sci. Rep. 2017, 7, 41910. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Sung, J.; Lee, Y.; Son, Y.; Chae, S.; Kim, N.; Choi, S.-H.; Cho, J. Strategic pore architecture for accommodating volume change from high Si content in lithium-ion battery anodes. Adv. Energy Mater. 2020, 10, 1903400. [Google Scholar] [CrossRef]
- Abe, Y.; Saito, T.; Kumagai, S. Effect of prelithiation process for hard carbon negative electrode on the rate and cycling behaviors of lithium-ion batteries. Batteries 2018, 4, 71. [Google Scholar] [CrossRef]





| Graphite | HC | N-Si | |
|---|---|---|---|
| Dm * (μm) | 16 | 10 | 0.1 [41] |
| SBET ** (m2 g−1) | 8.0 | 8.2 | 35.2 |
| Cell Parameter | NCM523|HC8/N-Si2 Cell | |||||
|---|---|---|---|---|---|---|
| RL0 | RL38 | RL57 | RL75 | RL95 | RL113 | |
| CC (mAh) | 1.03 | 1.05 | 1.03 | 1.04 | 1.04 | 1.04 |
| CA (mAh) | 2.13 | 2.09 | 2.08 | 2.11 | 2.17 | 2.10 |
| RA/C | 2.07 | 1.99 | 2.02 | 2.03 | 2.09 | 2.02 |
| LC (mg cm−2) | 3.90 | 3.95 | 3.90 | 3.90 | 3.91 | 3.92 |
| LA (mg cm−2) | 2.27 | 2.24 | 2.22 | 2.25 | 2.32 | 2.25 |
| tC (μm) | 40 | 38 | 41 | 40 | 38 | 60 |
| tA (μm) | 75 | 70 | 70 | 72 | 72 | 88 |
| CLi (mAh) | - | 0.79 | 1.18 | 1.59 | 2.05 | 2.38 |
| RL | 0 | 0.38 | 0.57 | 0.75 | 0.95 | 1.13 |
| Max_CLi (mAh) | 2.91 | 2.11 | 1.71 | 1.34 | 0.96 | 0.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abe, Y.; Saito, I.; Tomioka, M.; Kabir, M.; Kumagai, S. Effects of Excessive Prelithiation on Full-Cell Performance of Li-Ion Batteries with a Hard-Carbon/Nanosized-Si Composite Anode. Batteries 2022, 8, 210. https://doi.org/10.3390/batteries8110210
Abe Y, Saito I, Tomioka M, Kabir M, Kumagai S. Effects of Excessive Prelithiation on Full-Cell Performance of Li-Ion Batteries with a Hard-Carbon/Nanosized-Si Composite Anode. Batteries. 2022; 8(11):210. https://doi.org/10.3390/batteries8110210
Chicago/Turabian StyleAbe, Yusuke, Ippei Saito, Masahiro Tomioka, Mahmudul Kabir, and Seiji Kumagai. 2022. "Effects of Excessive Prelithiation on Full-Cell Performance of Li-Ion Batteries with a Hard-Carbon/Nanosized-Si Composite Anode" Batteries 8, no. 11: 210. https://doi.org/10.3390/batteries8110210
APA StyleAbe, Y., Saito, I., Tomioka, M., Kabir, M., & Kumagai, S. (2022). Effects of Excessive Prelithiation on Full-Cell Performance of Li-Ion Batteries with a Hard-Carbon/Nanosized-Si Composite Anode. Batteries, 8(11), 210. https://doi.org/10.3390/batteries8110210











