Improvement of Cycle Stability for Graphite-Based Lithium-Ion Batteries via Usage of Phenyl Methanesulfonate as an Electrolyte Additive
Abstract
1. Introduction
2. Materials and Methods
2.1. Computational Section
2.2. Electrolyte Preparation
2.3. Electrode Preparation and Cell Assembling
2.4. Electrochemical Tests
2.5. Characterization of the Graphite Electrode Surface
3. Results and Discussion
3.1. DFT Calculations
3.2. Charge–Discharge Test
3.3. Electrochemical Measurements
3.3.1. EIS Analysis
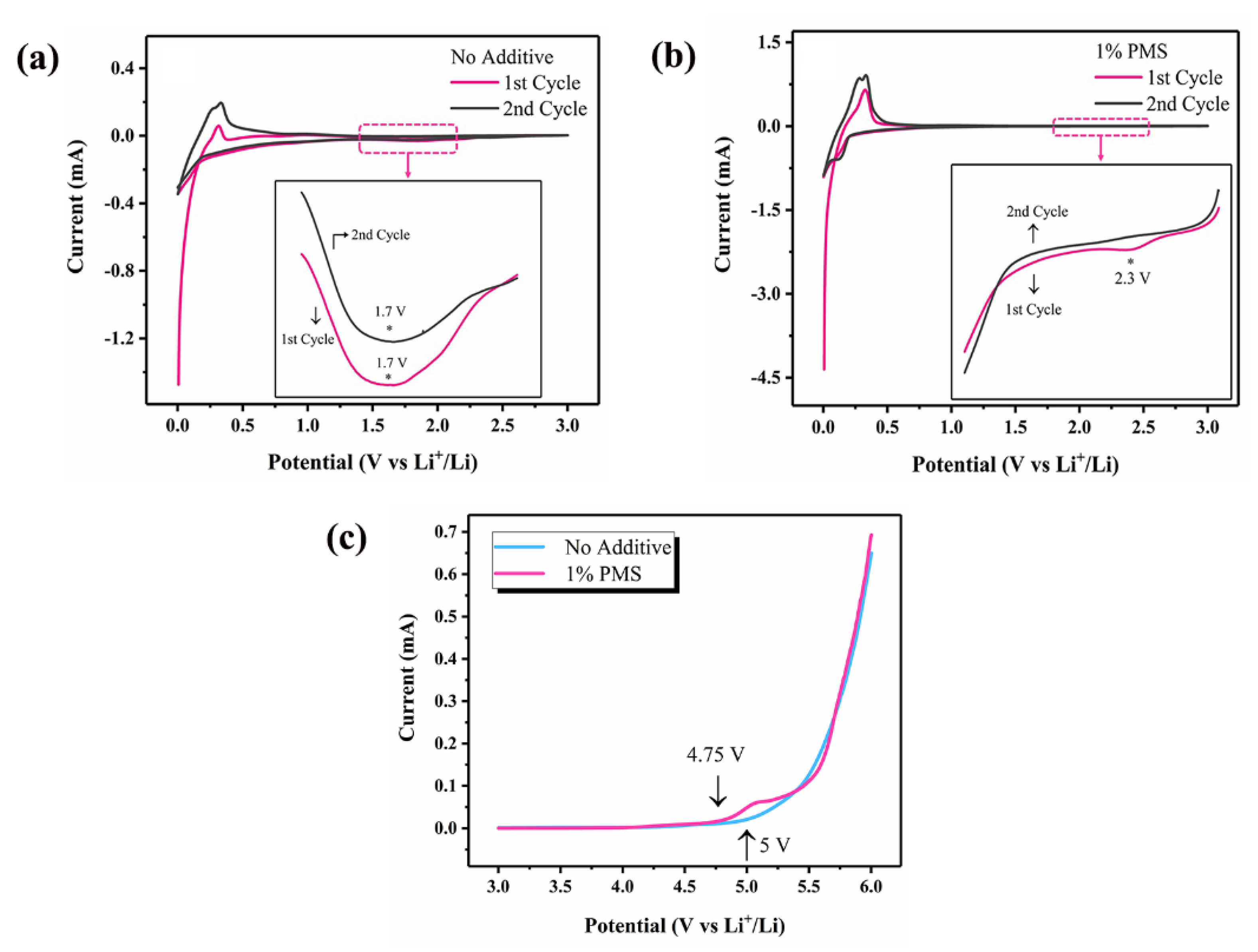
3.3.2. CV Measurements
3.3.3. Anodic Stability Evaluation
3.4. Surface Characterization Tests
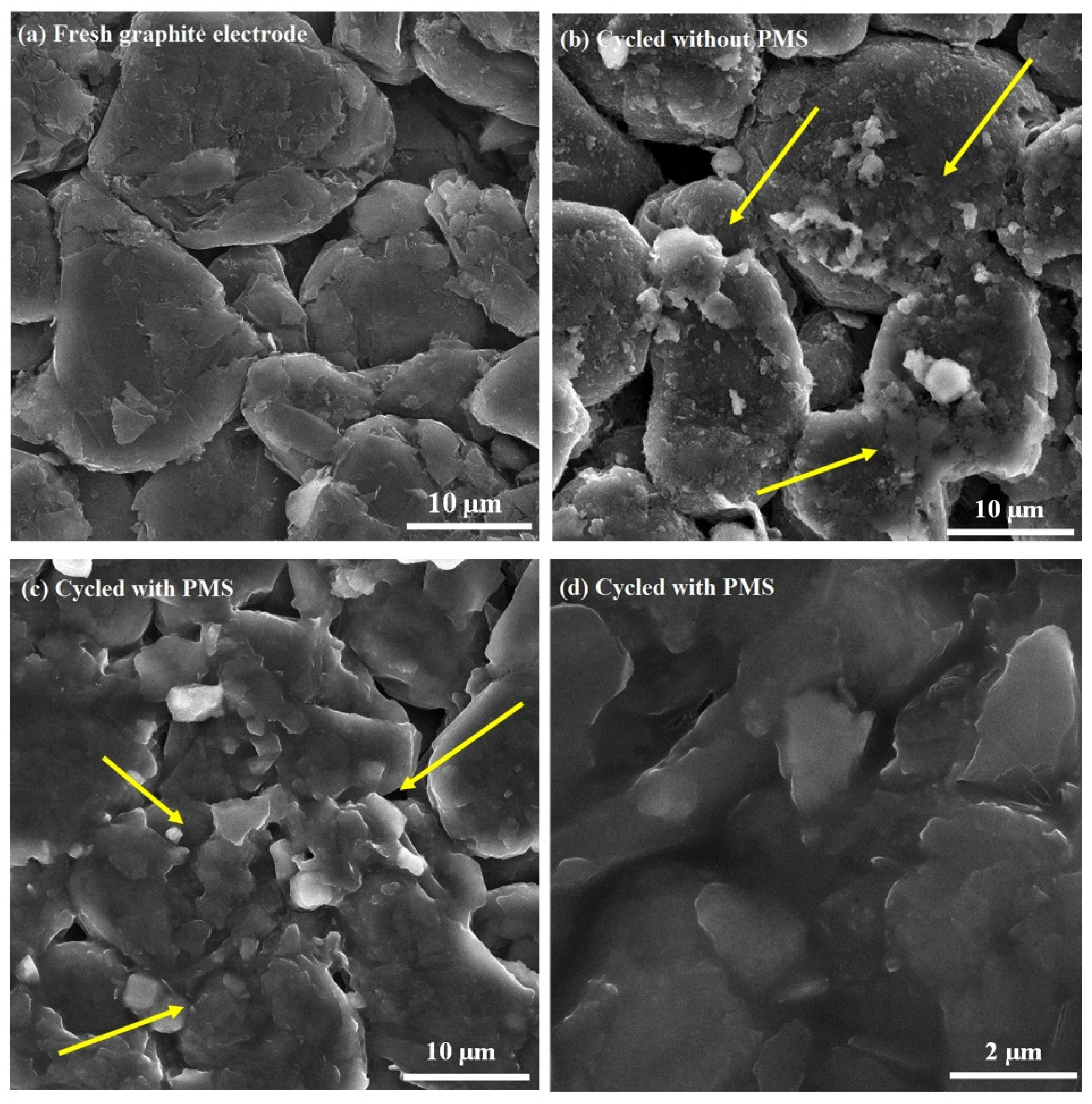
3.4.1. SEM and EDS Analyses
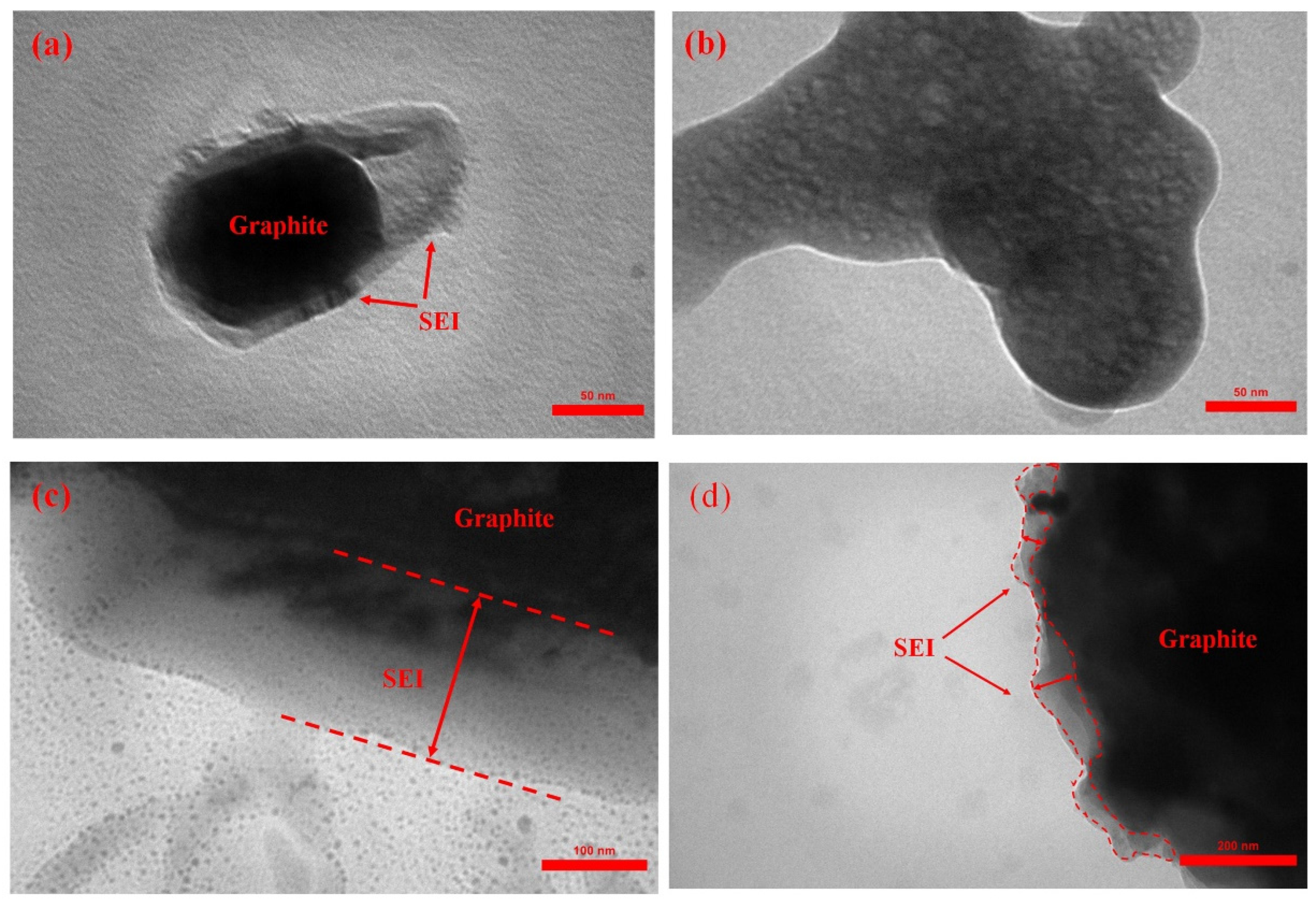
3.4.2. TEM Test
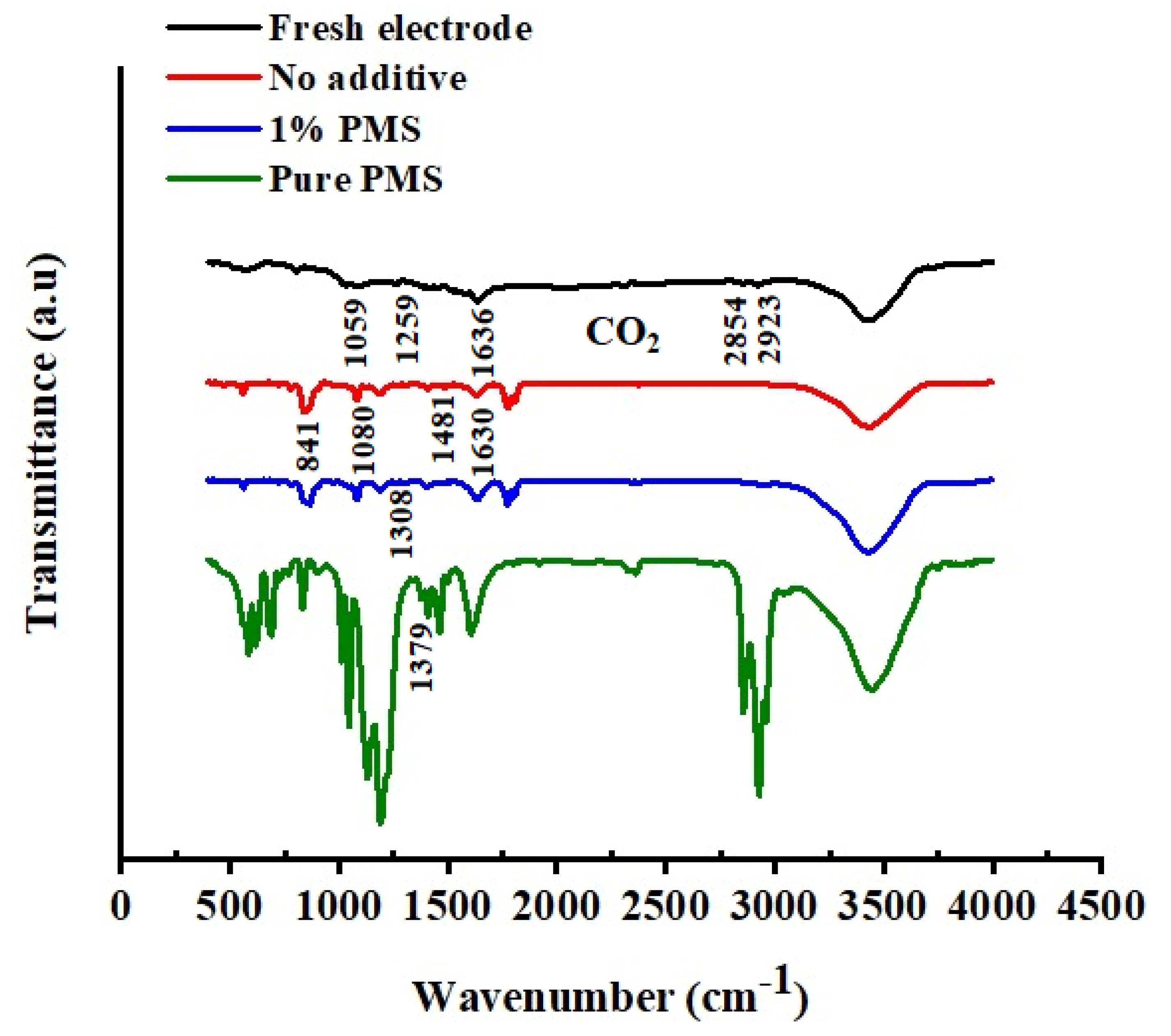
3.4.3. FT-IR Spectroscopy
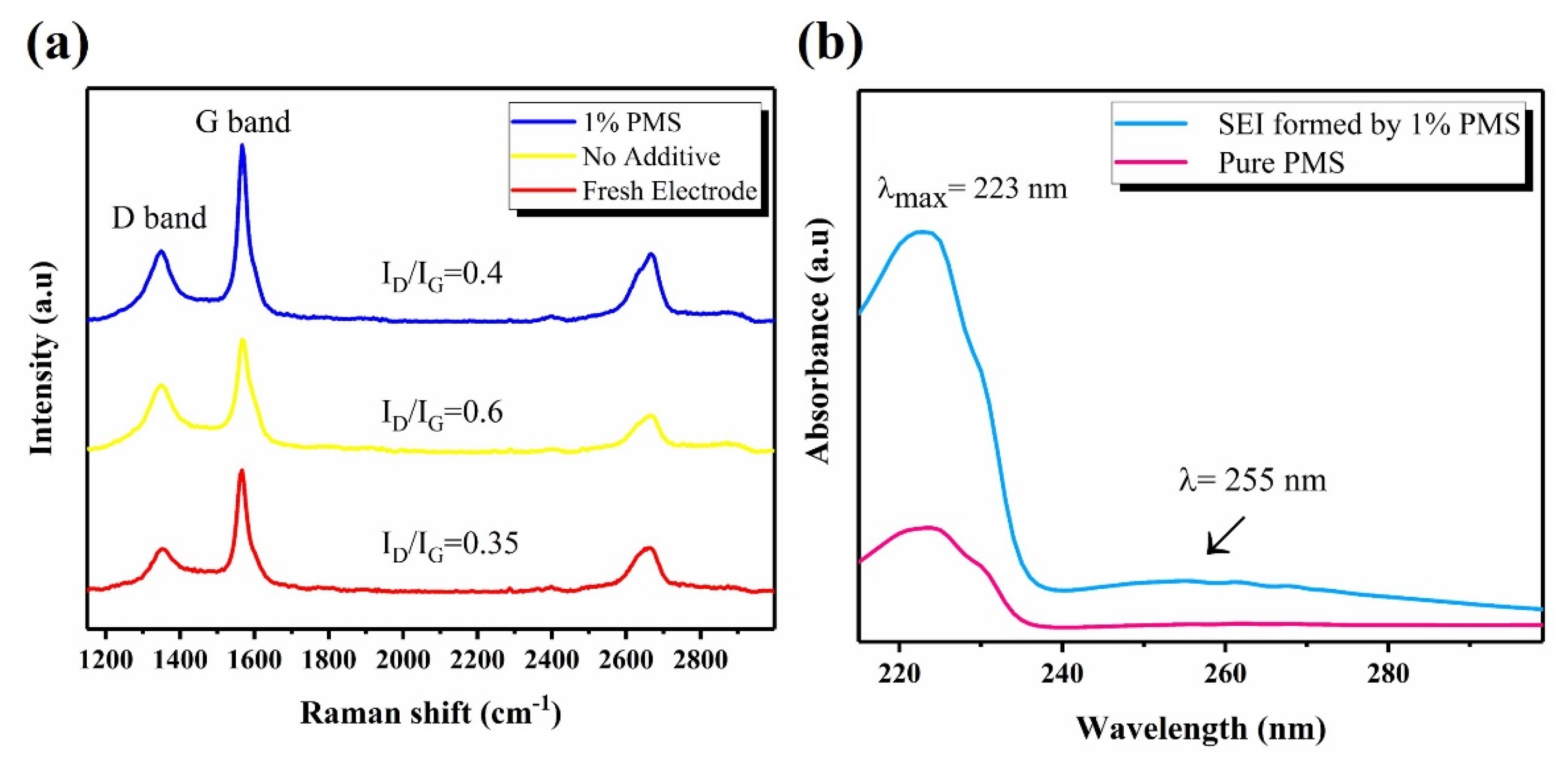
3.4.4. Raman Spectroscopy
3.4.5. UV-Visible Spectroscopy
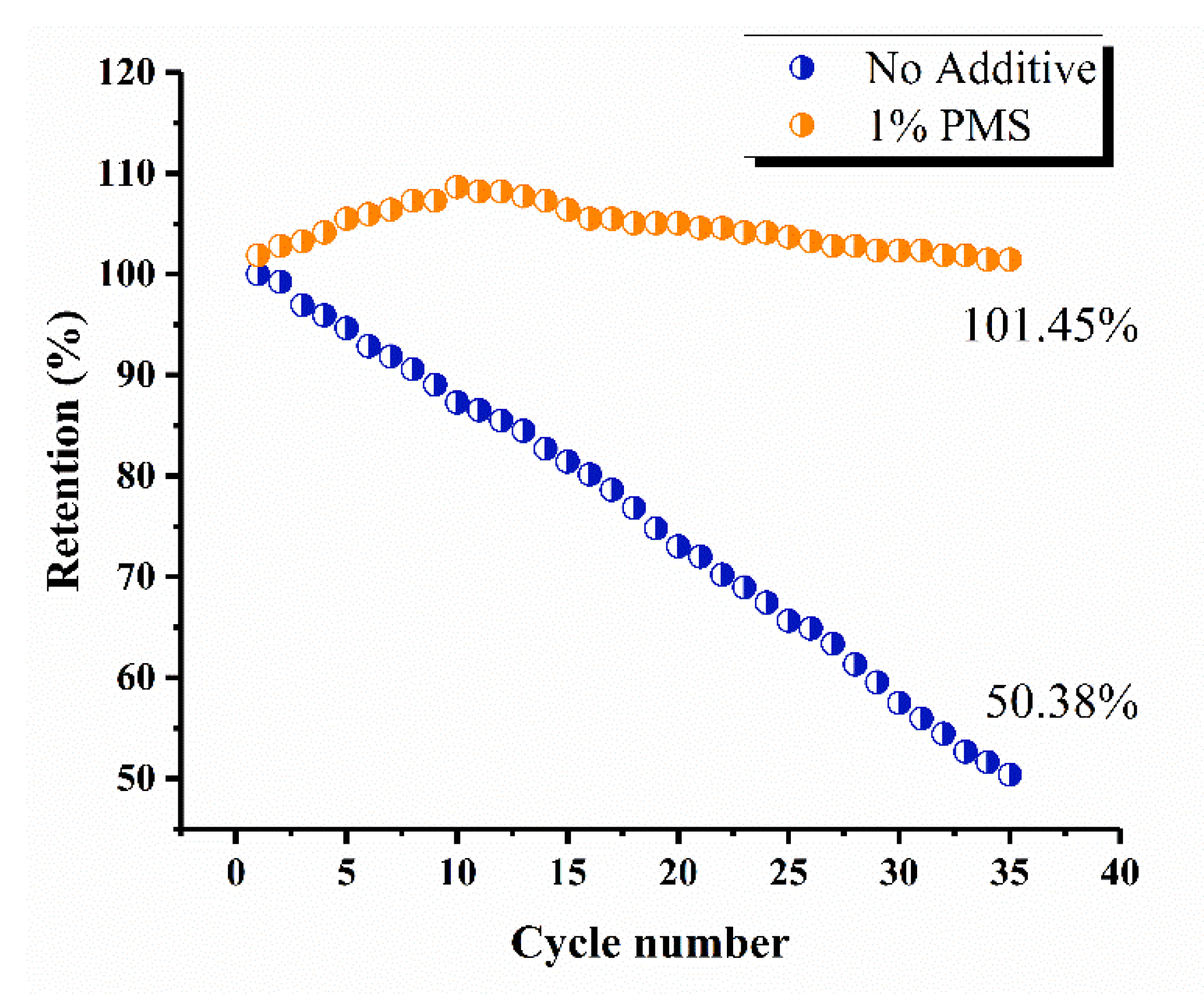
3.5. Cycling Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jin, R.; Ren, C.; Kang, H.; Gao, S.; Chen, S. Stacked Cu2−xSe nanoplates with 2D nanochannels as high performance anode for lithium batteries. J. Colloid Interface Sci. 2021, 590, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Jung, S. Thermal Stability and the Effect of Water on Hydrogen Fluoride Generation in Lithium-Ion Battery Electrolytes Containing LiPF6. Batteries 2022, 8, 61. [Google Scholar] [CrossRef]
- Madani, S.S.; Soghrati, R.; Ziebert, C. A Regression-Based Technique for Capacity Estimation of Lithium-Ion Batteries. Batteries 2022, 8, 31. [Google Scholar] [CrossRef]
- Vogt, D.; Michalowski, P.; Kwade, A. Production and Characterisation of Fibre-Reinforced All-Solid-State Electrodes and Separator for the Application in Structural Batteries. Batteries 2022, 8, 55. [Google Scholar] [CrossRef]
- Li, Z.; Guo, A.; Liu, D. Water-Soluble Conductive Composite Binder for High-Performance Silicon Anode in Lithium-Ion Batteries. Batteries 2022, 8, 54. [Google Scholar] [CrossRef]
- Moradi Bilondi, A.; Kermani, M.J.; Heidary, H.; Abdollahzadehsangroudi, M. Investigation of the Effect of Anode Fuel Contaminants on the Performance of Polymer Electrolyte Membrane Fuel Cell. Amirkabir J. Mech. Eng. 2020, 52, 381–398. [Google Scholar] [CrossRef]
- Kosova, N.V.; Tsydypylov, D.Z. Effect of Mechanical Activation and Carbon Coating on Electrochemistry of TiNb2O7 Anodes for Lithium-Ion Batteries. Batteries 2022, 8, 52. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar] [CrossRef]
- Xu, K. Electrolytes and interphases in Li-ion batteries and beyond. Chem. Rev. 2014, 114, 11503–11618. [Google Scholar] [CrossRef]
- Xu, W.; Wang, J.; Ding, F.; Chen, X.; Nasybulin, E.; Zhang, Y.; Zhang, J.-G. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 2014, 7, 513–537. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Zhang, Q. Toward safe lithium metal anode in rechargeable batteries: A review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef]
- Zhang, S.S. Aromatic isocyanate as a new type of electrolyte additive for the improved performance of Li-ion batteries. J. Power Sources 2006, 163, 567–572. [Google Scholar] [CrossRef]
- Placke, T.; Siozios, V.; Schmitz, R.; Lux, S.F.; Bieker, P.; Colle, C.; Meyer, H.-W.; Passerini, S.; Winter, M. Influence of graphite surface modifications on the ratio of basal plane to ‘non-basal plane’ surface area and on the anode performance in lithium ion batteries. J. Power Sources 2012, 200, 83–91. [Google Scholar] [CrossRef]
- El-Genk, M.S.; Tournier, J.-M.P. Development and validation of a model for the chemical kinetics of graphite oxidation. J. Nucl. Mater. 2011, 411, 193–207. [Google Scholar] [CrossRef]
- Winter, M.; Novák, P.; Monnier, A. Graphites for lithium-ion cells: The correlation of the first-cycle charge loss with the Brunauer-Emmett-Teller surface area. J. Electrochem. Soc. 1998, 145, 428–436. [Google Scholar] [CrossRef]
- Smith, T.E.; McCrory, S.; Lou Dunzik-Gougar, M. Limited oxidation of irradiated graphite waste to remove surface carbon-14. Nucl. Eng. Technol. 2013, 45, 211–218. [Google Scholar] [CrossRef]
- Zhao, H.; Yu, X.; Li, J.; Li, B.; Shao, H.; Li, L.; Deng, Y. Film-forming electrolyte additives for rechargeable lithium-ion batteries: Progress and outlook. J. Mater. Chem. A 2019, 7, 8700–8722. [Google Scholar] [CrossRef]
- Zhang, S.S. A review on electrolyte additives for lithium-ion batteries. J. Power Sources 2006, 162, 1379–1394. [Google Scholar] [CrossRef]
- Haregewoin, A.M.; Wotango, A.S.; Hwang, B.-J. Electrolyte additives for lithium ion battery electrodes: Progress and perspectives. Energy Environ. Sci. 2016, 9, 1955–1988. [Google Scholar] [CrossRef]
- Zhao, W.; Ji, Y.; Zhang, Z.; Lin, M.; Wu, Z.; Zheng, X.; Li, Q.; Yang, Y. Recent advances in the research of functional electrolyte additives for lithium-ion batteries. Curr. Opin. Electrochem. 2017, 6, 84–91. [Google Scholar] [CrossRef]
- Park, G.; Nakamura, H.; Lee, Y.; Yoshio, M. The important role of additives for improved lithium ion battery safety. J. Power Sources 2009, 189, 602–606. [Google Scholar] [CrossRef]
- Zuo, X.; Xu, M.; Li, W.; Su, D.; Liu, J. Electrochemical reduction of 1,3-propane sultone on graphite electrodes and its application in Li-ion batteries. Electrochem. Solid State Lett. 2006, 9, A196. [Google Scholar] [CrossRef]
- Xu, M.; Li, W.; Lucht, B.L. Effect of propane sultone on elevated temperature performance of anode and cathode materials in lithium-ion batteries. J. Power Sources 2009, 193, 804–809. [Google Scholar] [CrossRef]
- Jung, H.M.; Park, S.-H.; Jeon, J.; Choi, Y.; Yoon, S.; Cho, J.-J.; Oh, S.; Kang, S.; Han, Y.-K.; Lee, H. Fluoropropane sultone as an SEI-forming additive that outperforms vinylene carbonate. J. Mater. Chem. A 2013, 1, 11975–11981. [Google Scholar] [CrossRef]
- Lin, Y.; Yue, X.; Zhang, H.; Yu, L.; Fan, W.; Xie, T. Using phenyl methanesulfonate as an electrolyte additive to improve performance of LiNi0.5Co0.2Mn0.3O2/graphite cells at low temperature. Electrochim. Acta 2019, 300, 202–207. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Barone, V.; Cossi, M.; Tomasi, J. Geometry optimization of molecular structures in solution by the polarizable continuum model. J. Comput. Chem. 1998, 19, 404–417. [Google Scholar] [CrossRef]
- Han, Y.-K.; Yoo, J.; Yim, T. Why is tris (trimethylsilyl) phosphite effective as an additive for high-voltage lithium-ion batteries? J. Mater. Chem. A 2015, 3, 10900–10909. [Google Scholar] [CrossRef]
- Mosallanejad, B. Phthalimide Derivatives: New Promising Additives for Functional Electrolyte in Lithium-ion Batteries. Chem. Methodol. 2019, 3, 261–275. [Google Scholar] [CrossRef]
- Borodin, O.; Behl, W.; Jow, T.R. Oxidative stability and initial decomposition reactions of carbonate, sulfone, and alkyl phosphate-based electrolytes. J. Phys. Chem. C 2013, 117, 8661–8682. [Google Scholar] [CrossRef]
- Trasatti, S. The absolute electrode potential: An explanatory note (Recommendations 1986). Pure Appl. Chem. 1986, 58, 955–966. [Google Scholar] [CrossRef]
- Leggesse, E.G.; Jiang, J.-C. Theoretical study of the reductive decomposition of ethylene sulfite: A film-forming electrolyte additive in lithium ion batteries. J. Phys. Chem. A 2012, 116, 11025–11033. [Google Scholar] [CrossRef]
- Li, X.; Yin, Z.; Li, X.; Wang, C. Ethylene sulfate as film formation additive to improve the compatibility of graphite electrode for lithium-ion battery. Ionics 2014, 20, 795–801. [Google Scholar] [CrossRef]
- Li, B.; Xu, M.; Li, B.; Liu, Y.; Yang, L.; Li, W.; Hu, S. Properties of solid electrolyte interphase formed by prop-1-ene-1,3-sultone on graphite anode of Li-ion batteries. Electrochim. Acta 2013, 105, 1–6. [Google Scholar] [CrossRef]
- Wang, R.; Li, X.; Wang, Z.; Zhang, H. Electrochemical analysis graphite/electrolyte interface in lithium-ion batteries: P-Toluenesulfonyl isocyanate as electrolyte additive. Nano Energy 2017, 34, 131–140. [Google Scholar] [CrossRef]
- Wang, R.-H.; Li, X.-H.; Wang, Z.-X.; Guo, H.-J.; He, Z.-J. Electrochemical analysis for enhancing interface layer of spinel Li4Ti5O12: P-toluenesulfonyl isocyanate as electrolyte additive. ACS Appl. Mater. Interfaces 2015, 7, 23605–23614. [Google Scholar] [CrossRef]
- Yan, G.; Li, X.; Wang, Z.; Guo, H.; Wang, J. Compatibility of graphite with 1,3-(1,1,2,2-Tetrafluoroethoxy) propane and fluoroethylene carbonate as cosolvents for nonaqueous electrolyte in lithium-ion batteries. J. Phys. Chem. C 2014, 118, 6586–6593. [Google Scholar] [CrossRef]
- Zhao, L.; Jing, D.; Shi, Y.; Zhuang, Q.; Cui, Y.; Ju, Z.; Cui, Y. TriMethylene sulfite as a novel additive for SEI film formation in lithium-ion batteries. Ionics 2020, 26, 4813–4824. [Google Scholar] [CrossRef]
- Xu, M.Q.; Li, W.S.; Zuo, X.X.; Liu, J.S.; Xu, X. Performance improvement of lithium ion battery using PC as a solvent component and BS as an SEI forming additive. J. Power Sources 2007, 174, 705–710. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Orfao, J.J.M. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Soliman, A.B.; Abdel-Samad, H.S.; Rehim, S.S.A.; Hassan, H.H. Surface functionality and electrochemical investigations of a graphitic electrode as a candidate for alkaline energy conversion and storage devices. Sci. Rep. 2016, 6, 256. [Google Scholar] [CrossRef]
- Xing, M.L.; Li, W. Interphases between electrolytes and anodes in Li-ion battery. In Electrolytes for Lithium and Lithium-Ion Batteries; Jow, T.R., Xu, K., Borodin, O., Ue, M., Eds.; Springer: New York, NY, USA, 2014; pp. 227–277. [Google Scholar]
- Wang, L.; Menakath, A.; Han, F.; Wang, Y.; Zavalij, P.Y.; Gaskell, K.J.; Borodin, O.; Iuga, D.; Brown, S.P.; Wang, C.; et al. Identifying the components of the solid–electrolyte interphase in Li-ion batteries. Nat. Chem. 2019, 11, 789–796. [Google Scholar] [CrossRef]
- Choi, N.-S.; Profatilova, I.A.; Kim, S.-S.; Song, E.-H. Thermal reactions of lithiated graphite anode in LiPF6-based electrolyte. Thermochim. Acta 2008, 480, 10–14. [Google Scholar] [CrossRef]
- Ota, H.; Kominato, A.; Chun, W.-J.; Yasukawa, E.; Kasuya, S. Effect of cyclic phosphate additive in non-flammable electrolyte. J. Power Sources 2003, 119, 393–398. [Google Scholar] [CrossRef]
- Hu, Y.; Kong, W.; Li, H.; Huang, X.; Chen, L. Experimental and theoretical studies on reduction mechanism of vinyl ethylene carbonate on graphite anode for lithium ion batteries. Electrochem. Commun. 2004, 6, 126–131. [Google Scholar] [CrossRef]
- Pretsch, E.; Bühlmann, P.; Badertscher, M. Structure Determination of Organic Compounds, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Mistry, B.D. A Handbook of Spectroscopic Data: Chemistry; Oxford Book Co.: Jaipur, India, 2009. [Google Scholar]
- Sethuraman, V.A.; Hardwick, L.J.; Srinivasan, V.; Kostecki, R. Surface structural disordering in graphite upon lithium intercalation/deintercalation. J. Power Sources 2010, 195, 3655–3660. [Google Scholar] [CrossRef]
- Markervich, E.; Salitra, G.; Levi, M.D.; Aurbach, D. Capacity fading of lithiated graphite electrodes studied by a combination of electroanalytical methods, Raman spectroscopy and SEM. J. Power Sources 2005, 146, 146–150. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, W.; Peng, H.; Qian, J.; Ou, E.; Xu, W. Water-soluble graphene dispersion functionalized by Diels–Alder cycloaddition reaction. J. Iran. Chem. Soc. 2017, 14, 89–93. [Google Scholar] [CrossRef]
- Komaba, S.; Itabashi, T.; Tatsuya Ohtsuka, H.; Groult, N.; Kumagai, N.; Kaplan, B.; Yashiro, H. Impact of 2-vinylpyridine as electrolyte additive on surface and electrochemistry of graphite for C/LiMn2O4 Li-ion Cells. J. Electrochem. Soc. 2005, 152, A937. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y.; Lin, H.; Wang, X.; Xu, M.; Wang, Y.; Xing, L.; Li, W. Performance improvement of phenyl acetate as propylene carbonate-based electrolyte additive for lithium ion battery by fluorine-substituting. J. Power Sources 2014, 267, 182–187. [Google Scholar] [CrossRef]
- Ciosek Högström, K.; Malmgren, S.; Hahlin, M.; Rensmo, H.; Thébault, F.; Johansson, P.; Edstrom, K. The influence of PMS-additive on the electrode/electrolyte interfaces in LiFePO4/graphite Li-ion batteries. J. Phys. Chem. C 2013, 117, 23476–23486. [Google Scholar] [CrossRef]


| Material | Redox Potentials (eV) | |
|---|---|---|
| RP | OP | |
| EC | −0.33 | 6.94 |
| PMS | 1.99 | 5.32 |
| Sample | After 5 Cycles | After 50 Cycles | ||||
|---|---|---|---|---|---|---|
| Rs (Ω) | Rf (Ω) | Rct (Ω) | Rs (Ω) | Rf (Ω) | Rct (Ω) | |
| No additive | 5.88 | 52.87 | 68.09 | 12.24 | 35.15 | 34.6 |
| 1% PMS | 12.49 | 27.07 | 31.58 | 12.92 | 32.14 | 23.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosallanejad, B.; Javanbakht, M.; Shariatinia, Z.; Akrami, M. Improvement of Cycle Stability for Graphite-Based Lithium-Ion Batteries via Usage of Phenyl Methanesulfonate as an Electrolyte Additive. Batteries 2022, 8, 152. https://doi.org/10.3390/batteries8100152
Mosallanejad B, Javanbakht M, Shariatinia Z, Akrami M. Improvement of Cycle Stability for Graphite-Based Lithium-Ion Batteries via Usage of Phenyl Methanesulfonate as an Electrolyte Additive. Batteries. 2022; 8(10):152. https://doi.org/10.3390/batteries8100152
Chicago/Turabian StyleMosallanejad, Behrooz, Mehran Javanbakht, Zahra Shariatinia, and Mohammad Akrami. 2022. "Improvement of Cycle Stability for Graphite-Based Lithium-Ion Batteries via Usage of Phenyl Methanesulfonate as an Electrolyte Additive" Batteries 8, no. 10: 152. https://doi.org/10.3390/batteries8100152
APA StyleMosallanejad, B., Javanbakht, M., Shariatinia, Z., & Akrami, M. (2022). Improvement of Cycle Stability for Graphite-Based Lithium-Ion Batteries via Usage of Phenyl Methanesulfonate as an Electrolyte Additive. Batteries, 8(10), 152. https://doi.org/10.3390/batteries8100152








