Enable High-Energy LiNi0.5Co0.2Mn0.3O2 by Ultra-Thin Coating through Wet Impregnation
Abstract
1. Introduction
2. Experimental Section
2.1. Surface Modification Process of NCM523
2.2. Characterization of Pristine and Surface-Modified NCM523
2.3. The Preparation of the Cathode Using Pristine and Modified NCM523 and the Corresponding Anode
2.4. Electrochemical Measurements of the Half and Full Cells Using Pristine and Modified NCM523
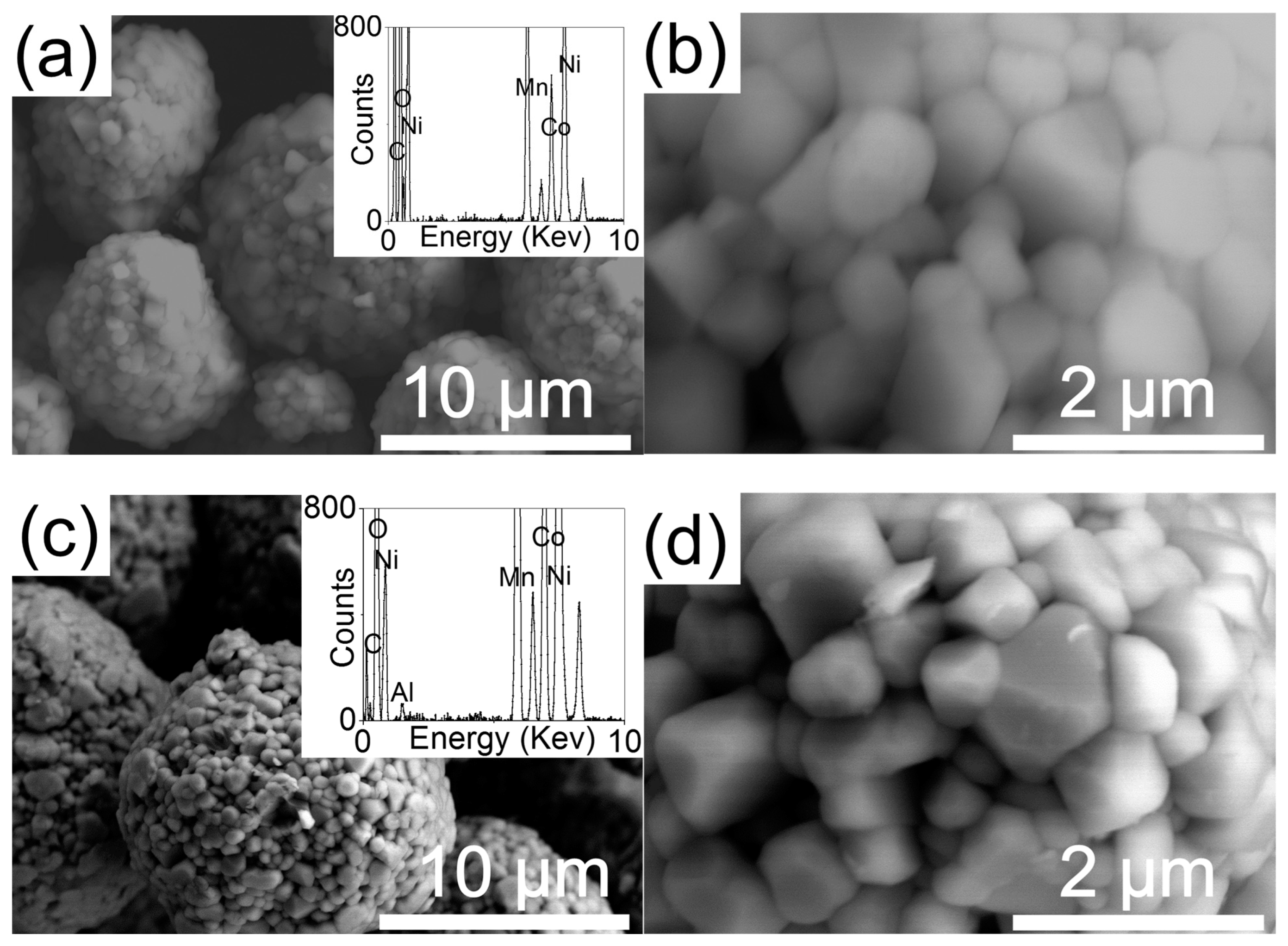
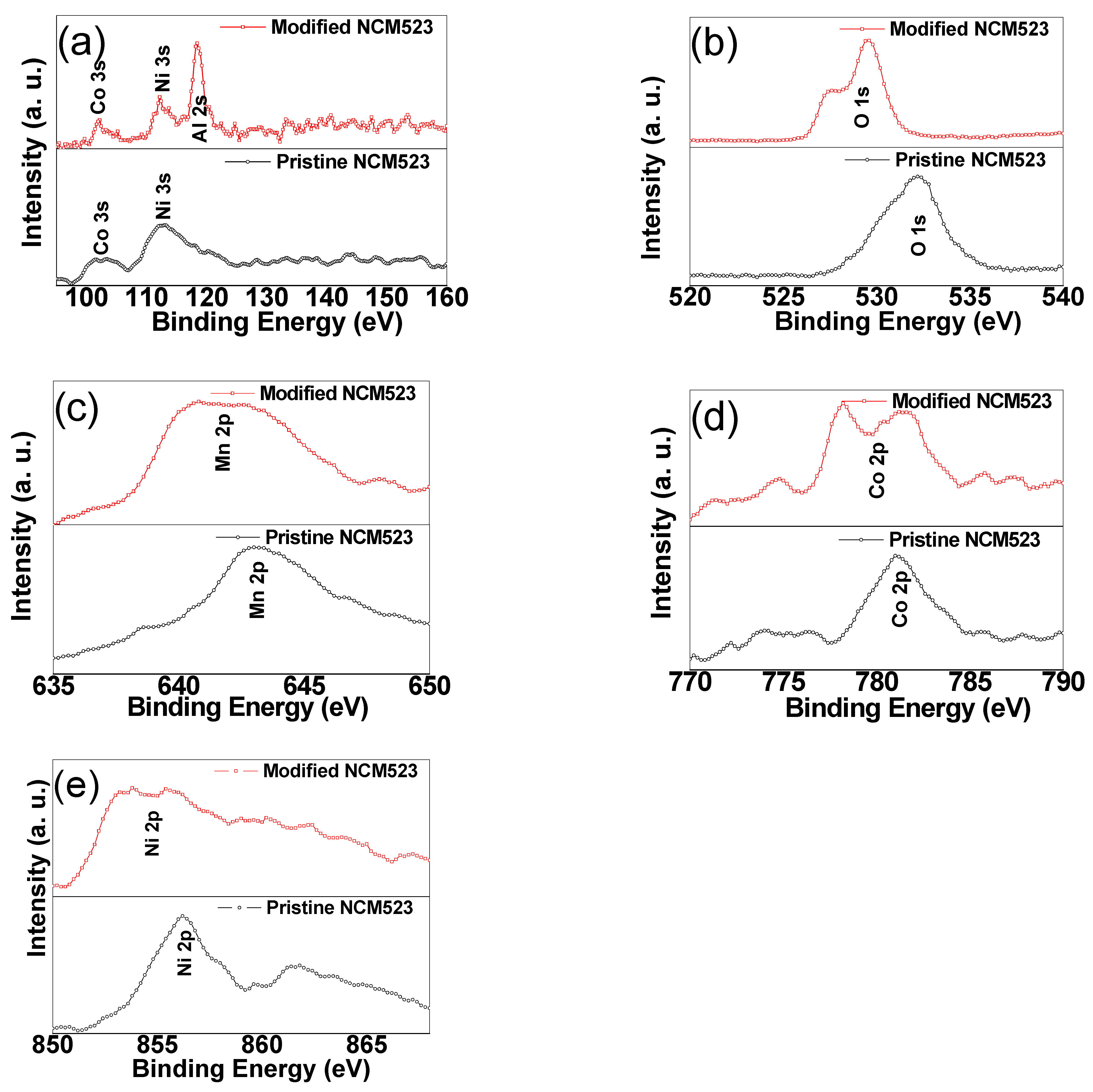
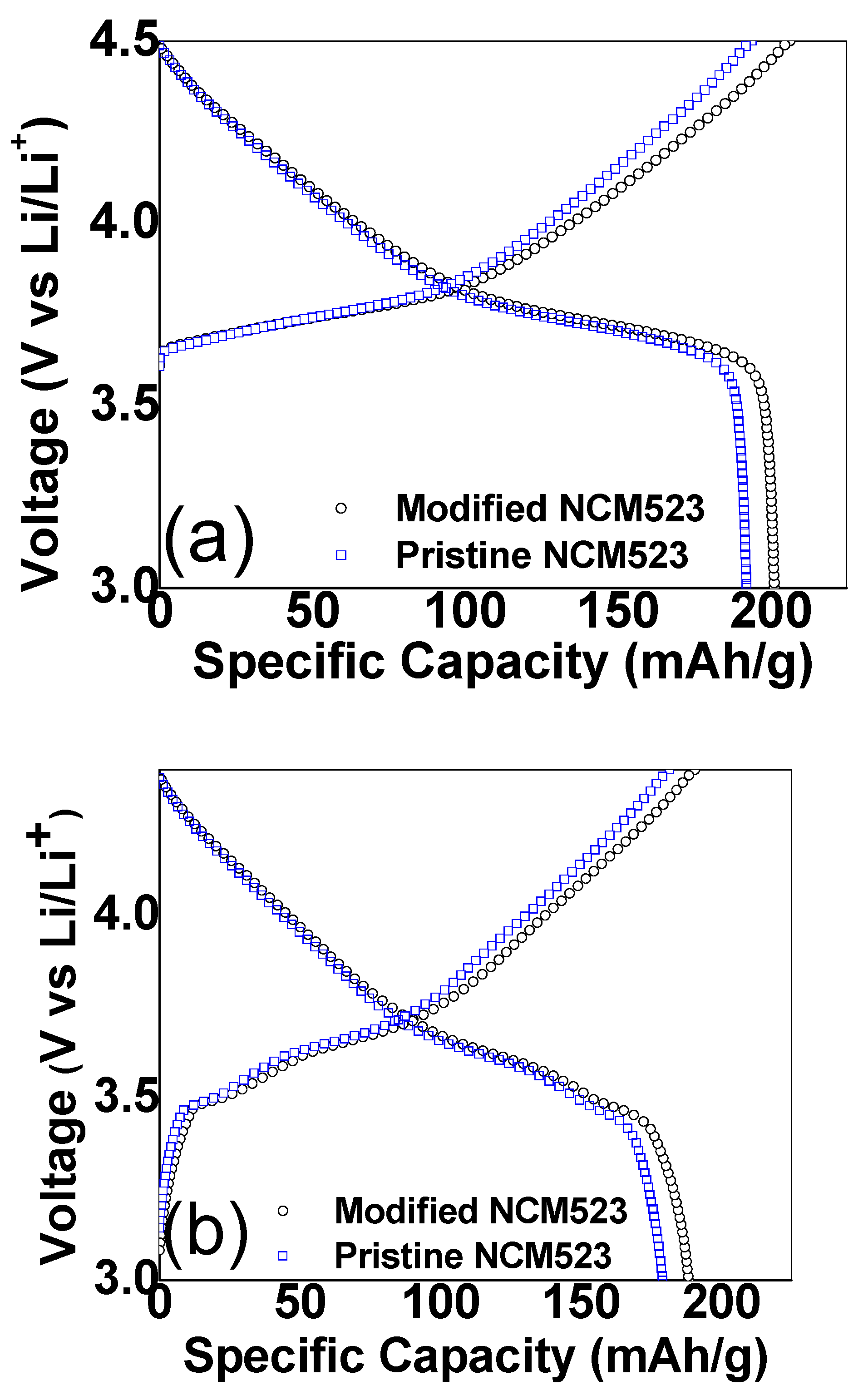
3. Result and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- de Meatza, I.; Landa-Medrano, I.; Sananes-Israel, S.; Eguia-Barrio, A.; Bondarchuk, O.; Lijó-Pando, S.; Boyano, I.; Palomares, V.; Rojo, T.; Grande, H.-J.; et al. Influence of the Ambient Storage of LiNi0.8Mn0.1Co0.1O2 Powder and Electrodes on the Electrochemical Performance in Li-ion Technology. Batteries 2022, 8, 79. [Google Scholar] [CrossRef]
- Coeler, M.; van Laack, V.; Langer, F.; Potthoff, A.; Höhn, S.; Reuber, S.; Koscheck, K.; Wolter, M. Infiltrated and Isostatic Laminated NCM and LTO Electrodes with Plastic Crystal Electrolyte Based on Succinonitrile for Lithium-Ion Solid State Batteries. Batteries 2021, 7, 11. [Google Scholar] [CrossRef]
- Li, W.; Erickson, E.M.; Manthiram, A. High-nickel layered oxide cathodes for lithium-based automotive batteries. Nat. Energy 2020, 5, 26–34. [Google Scholar] [CrossRef]
- Zappen, H.; Fuchs, G.; Gitis, A.; Sauer, D.U. In-Operando Impedance Spectroscopy and Ultrasonic Measurements during High-Temperature Abuse Experiments on Lithium-Ion Batteries. Batteries 2020, 6, 25. [Google Scholar] [CrossRef]
- Singh, M.; Kaiser, J.; Hahn, H. Effect of Porosity on the Thick Electrodes for High Energy Density Lithium Ion Batteries for Stationary Applications. Batteries 2016, 2, 35. [Google Scholar] [CrossRef]
- Baczyńska, A.; Niewiadomski, W.; Gonçalves, A.; Almeida, P.; Luís, R. Li-NMC Batteries Model Evaluation with Experimental Data for Electric Vehicle Application. Batteries 2018, 4, 11. [Google Scholar] [CrossRef]
- Liu, Q.; Su, X.; Lei, D.; Qin, Y.; Wen, J.; Guo, F.; Wu, Y.A.; Rong, Y.; Kou, R.; Xiao, X.; et al. Approaching the capacity limit of lithium cobalt oxide in lithium ion batteries via lanthanum and aluminium doping. Nat. Energy 2018, 3, 936–943. [Google Scholar] [CrossRef]
- Kosova, N.; Devyatkina, E.; Kaichev, V. Optimization of Ni2+/Ni3+ ratio in layered Li(Ni,Mn,Co)O2 cathodes for better electrochemistry. J. Power Sources 2007, 174, 965–969. [Google Scholar] [CrossRef]
- Cherkashinin, G.; Motzko, M.; Schulz, N.; Späth, T.; Jaegermann, W. Electron Spectroscopy Study of Li[Ni,Co,Mn]O2/Electrolyte Interface: Electronic Structure, Interface Composition, and Device Implications. Chem. Mater. 2015, 27, 2875–2887. [Google Scholar]
- Xiong, C.; Liu, F.; Gao, J.; Jiang, X. One-Spot Facile Synthesis of Single-Crystal LiNi0.5Co0.2Mn0.3O2 Cathode Materials for Li-ion Batteries. ACS Omega 2020, 5, 30356–30362. [Google Scholar] [CrossRef]
- Klein, S.; Bärmann, P.; Beuse, T.; Borzutzki, K.; Frerichs, J.E.; Kasnatscheew, J.; Winter, M.; Placke, T. Exploiting the Degradation Mechanism of NCM523 Graphite Lithium-Ion Full Cells Operated at High Voltage. ChemSusChem 2021, 14, 595–613. [Google Scholar] [CrossRef]
- Cao, G.; Jin, Z.; Zhu, J.; Li, Y.; Xu, B.; Xiong, Y.; Yang, J. A green Al2O3 metal oxide coating method for LiNi0.5Co0.2Mn0.3O2 cathode material to improve the high voltage performance. J. Alloys Compd. 2020, 832, 153788. [Google Scholar] [CrossRef]
- Neudeck, S.; Mazilkin, A.; Reitz, C.; Hartmann, P.; Janek, J.; Brezesinski, T. Effect of Low-Temperature Al2O3 ALD Coating on Ni-Rich Layered Oxide Composite Cathode on the Long-Term Cycling Performance of Lithium-Ion Batteries. Sci. Rep. 2019, 9, 5328. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, M.; Qian, D.; Meng, Y.S. Ultrathin Al2O3 Coatings for Improved Cycling Performance and Thermal Stability of LiNi0.5Co0.2Mn0.3O2 Cathode Material. Electrochim. Acta 2016, 203, 154–161. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, G.; Weng, Y.; Yan, T.; Shi, L.; An, Z.; Zhang, D. Precise Al2O3 Coating on LiNi0.5Co0.2Mn0.3O2 by Atomic Layer Deposition Restrains the Shuttle Effect of Transition Metals in Li-Ion Capacitors. Chem. Eng. J. 2020, 401, 126138. [Google Scholar] [CrossRef]
- Guan, P.; Zhou, L.; Yu, Z.; Sun, Y.; Liu, Y.; Wu, F.; Jiang, Y.; Chu, D. Recent progress of surface coating on cathode materials for high-performance lithium-ion batteries. J. Energy Chem. 2020, 43, 220–235. [Google Scholar] [CrossRef]
- Hou, Q.; Cao, G.; Wang, P.; Zhao, D.; Cui, X.; Li, S.; Li, C. Carbon coating nanostructured-LiNi1/3Co1/3Mn1/3O2 cathode material synthesized by chemical vapor deposition method for high performance lithium-ion batteries. J. Alloys Compd. 2018, 747, 796–802. [Google Scholar] [CrossRef]
- Son, H.I.; Park, K.; Park, J.H. Improvement in high-voltage and high rate cycling performance of nickel-rich layered cathode materials via facile chemical vapor deposition with methane. Electrochim. Acta 2017, 230, 308–315. [Google Scholar]
- Qiu, B.; Wang, J.; Xia, Y.; Wei, Z.; Han, S.; Liu, Z. Enhanced Electrochemical Performance with Surface Coating by Reactive Magnetron Sputtering on Lithium-Rich Layered Oxide Electrodes. ACS Appl. Mater. Interfaces 2014, 6, 9185–9193. [Google Scholar] [CrossRef]
- Yu, H.; Wang, S.; Hu, Y.; He, G.; Bao, L.Q.; Parkin, I.P.; Jiang, H. Lithium-conductive LiNbO3 coated high-voltage LiNi0.5Co0.2Mn0.3O2 cathode with enhanced rate and cyclability. Green Energy Environ. 2020, 7, 266–274. [Google Scholar] [CrossRef]
- Yao, C.; Mo, Y.; Jia, X.; Chen, X.; Xia, J.; Chen, Y. LiMnPO4 surface coating on LiNi0.5Co0.2Mn0.3O2 by a simple sol-gel method and improving electrochemical properties. Solid State Ion. 2018, 317, 156–163. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, S.; Liu, H.; Liu, P. Protective coatings for lithium metal anodes: Recent progress and future perspectives. J. Power Sources 2020, 450, 227632. [Google Scholar] [CrossRef]
- Xu, Q.; Li, X.; Kheimeh Sari, H.M.; Li, W.; Liu, W.; Hao, Y.; Qin, J.; Cao, B.; Xiao, W.; Xu, Y.; et al. Surface engineering of LiNi0.8Mn0.1Co0.1O2 towards boosting lithium storage: Bimetallic oxides versus monometallic oxides. Nano Energy 2020, 77, 105034. [Google Scholar] [CrossRef]
- Ahn, J.; Jang, E.K.; Yoon, S.; Lee, S.-J.; Sung, S.-J.; Kim, D.-H.; Cho, K.Y. Ultrathin ZrO2 on LiNi0.5Mn0.3Co0.2O2 electrode surface via atomic layer deposition for high-voltage operation in lithium-ion batteries. Appl. Surf. Sci. 2019, 484, 701–709. [Google Scholar] [CrossRef]
- Su, L.; Weaver, J.L.; Groenenboom, M.; Nakamura, N.; Rus, E.; Anand, P.; Jha, S.K.; Okasinski, J.S.; Dura, J.A.; Reeja-Jayan, B. Tailoring Electrode–Electrolyte Interfaces in Lithium-Ion Batteries Using Molecularly Engineered Functional Polymers. ACS Appl. Mater. Interfaces 2021, 13, 9919–9931. [Google Scholar] [CrossRef]
- Xu, G.-L.; Liu, Q.; Lau, K.K.S.; Liu, Y.; Liu, X.; Gao, H.; Zhou, X.; Zhuang, M.; Ren, Y.; Li, J.; et al. Building ultraconformal protective layers on both secondary and primary particles of layered lithium transition metal oxide cathodes. Nat. Energy 2019, 4, 484–494. [Google Scholar] [CrossRef]
- Su, L.; Smith, P.M.; Anand, P.; Reeja-Jayan, B. Surface Engineering of a LiMn2O4 Electrode Using Nanoscale Polymer Thin Films via Chemical Vapor Deposition Polymerization. ACS Appl. Mater. Interfaces 2018, 10, 27063–27073. [Google Scholar] [CrossRef]
- Zhang, Y.; Kim, C.S.; Song, H.W.; Chang, S.-J.; Kim, H.; Park, J.; Hu, S.; Zhao, K.; Lee, S. Ultrahigh active material content and highly stable Ni-rich cathode leveraged by oxidative chemical vapor deposition. Energy Storage Mater. 2022, 48, 1–11. [Google Scholar] [CrossRef]
- Tago, T.; Kataoka, N.; Tanaka, H.; Kinoshita, K.; Kishida, S. XPS study from a clean surface of Al2O3 single crystals. Procedia Eng. 2017, 216, 175–181. [Google Scholar] [CrossRef]
- Singh, A.N.; Kim, M.-H.; Meena, A.; Wi, T.-U.; Lee, H.-W.; Kim, K.S. Na/Al Codoped Layered Cathode with Defects as Bifunctional Electrocatalyst for High-Performance Li-Ion Battery and Oxygen Evolution Reaction. Small 2021, 17, 2005605. [Google Scholar] [CrossRef]
- Oh, J.; Kim, J.; Lee, Y.M.; Shin, D.O.; Kim, J.Y.; Lee, Y.-G.; Kim, K.M. High-rate cycling performance and surface analysis of LiNi1−xCox/2Mnx/2O2 (x = 2/3, 0.4, 0.2) cathode materials. Mater. Chem. Phys. 2019, 222, 1–10. [Google Scholar] [CrossRef]
- Kim, U.-H.; Kuo, L.-Y.; Kaghazchi, P.; Yoon, C.; Sun, Y. Quaternary Layered Ni-Rich NCMA Cathode for Lithium-Ion Batteries. ACS Energy Lett. 2019, 4, 576–582. [Google Scholar] [CrossRef]
- Song, X.; Liu, G.; Yue, H.; Luo, L.; Yang, S.; Huang, Y.; Wang, C. A novel low-cobalt long-life LiNi0.88Co0.06Mn0.03Al0.03O2 cathode material for lithium ion batteries. Chem. Eng. J. 2021, 407, 126301. [Google Scholar] [CrossRef]
- Fly, A.; Chen, R. Rate dependency of incremental capacity analysis (dQ/dV) as a diagnostic tool for lithium-ion batteries. J. Energy Storage 2020, 29, 101329. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, X.; Wang, X.; Bareno, J.; Qin, Y.; Aguesse, F.; Lu, W. Enable High-Energy LiNi0.5Co0.2Mn0.3O2 by Ultra-Thin Coating through Wet Impregnation. Batteries 2022, 8, 136. https://doi.org/10.3390/batteries8100136
Su X, Wang X, Bareno J, Qin Y, Aguesse F, Lu W. Enable High-Energy LiNi0.5Co0.2Mn0.3O2 by Ultra-Thin Coating through Wet Impregnation. Batteries. 2022; 8(10):136. https://doi.org/10.3390/batteries8100136
Chicago/Turabian StyleSu, Xin, Xiaoping Wang, Javier Bareno, Yan Qin, Frederic Aguesse, and Wenquan Lu. 2022. "Enable High-Energy LiNi0.5Co0.2Mn0.3O2 by Ultra-Thin Coating through Wet Impregnation" Batteries 8, no. 10: 136. https://doi.org/10.3390/batteries8100136
APA StyleSu, X., Wang, X., Bareno, J., Qin, Y., Aguesse, F., & Lu, W. (2022). Enable High-Energy LiNi0.5Co0.2Mn0.3O2 by Ultra-Thin Coating through Wet Impregnation. Batteries, 8(10), 136. https://doi.org/10.3390/batteries8100136





