Investigation of a Novel Ecofriendly Electrolyte-Solvent for Lithium-Ion Batteries with Increased Thermal Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrolyte Preparation
2.3. Cell Preparation
2.4. Electrochemical Characterization
3. Results
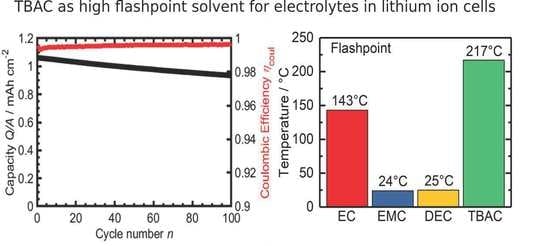
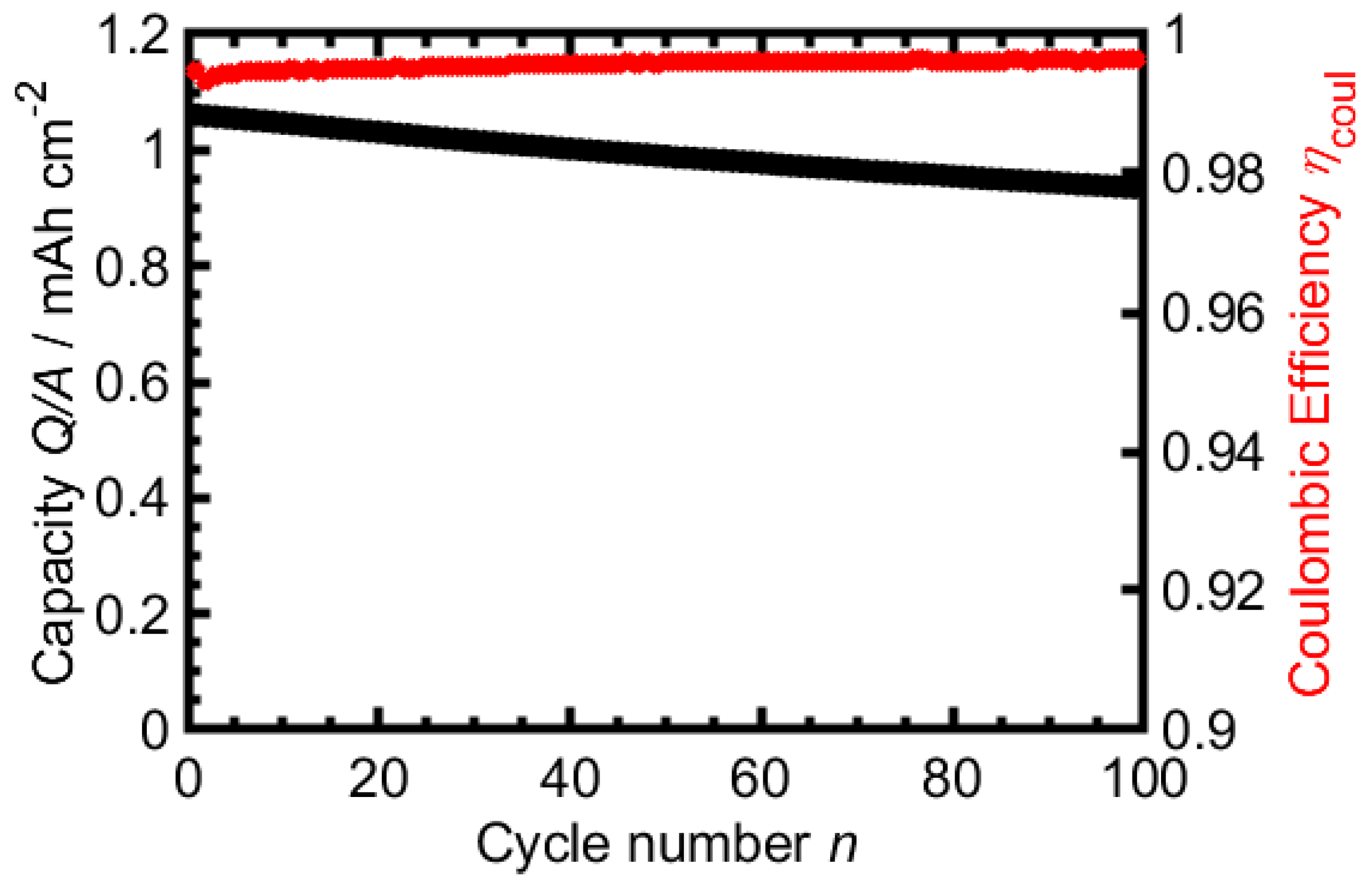
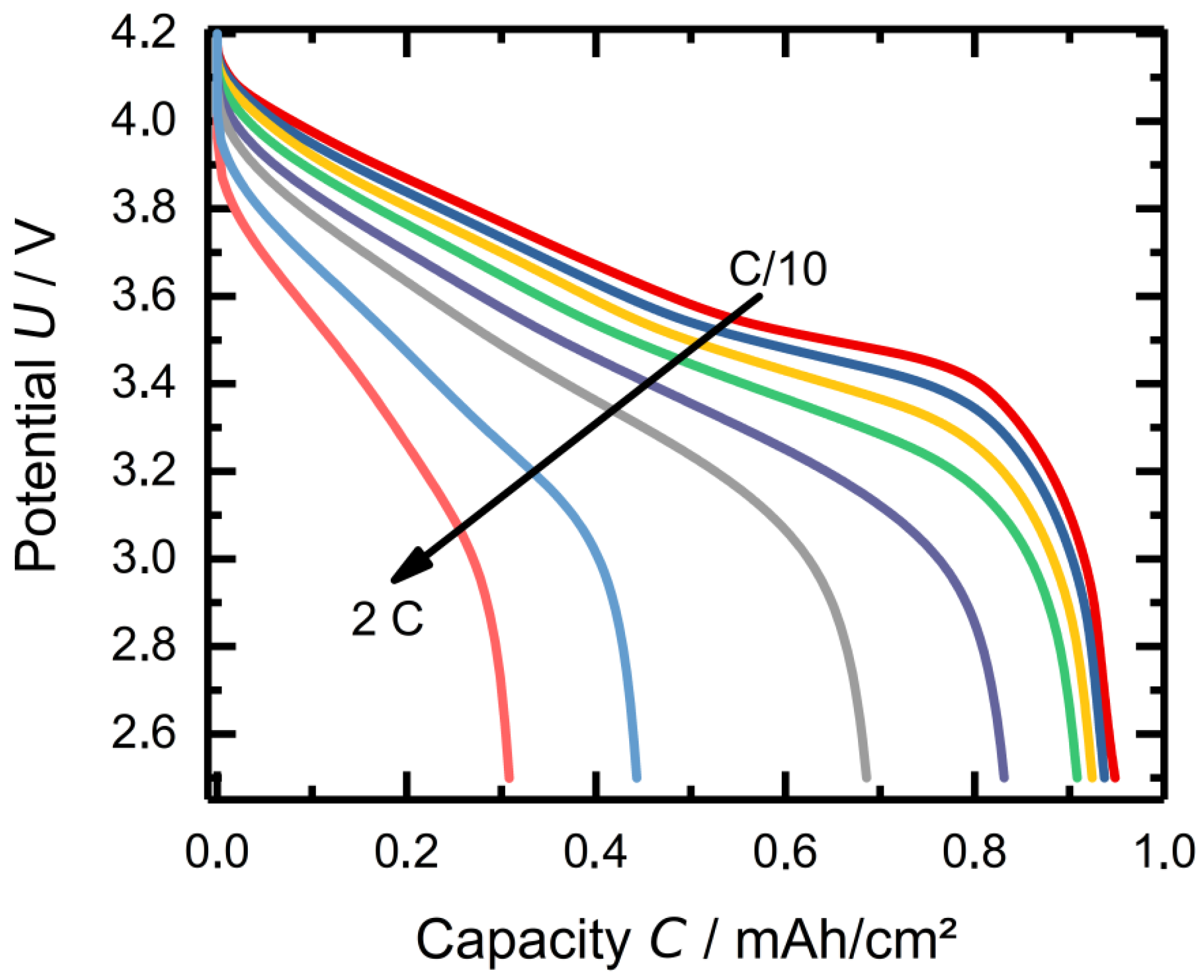
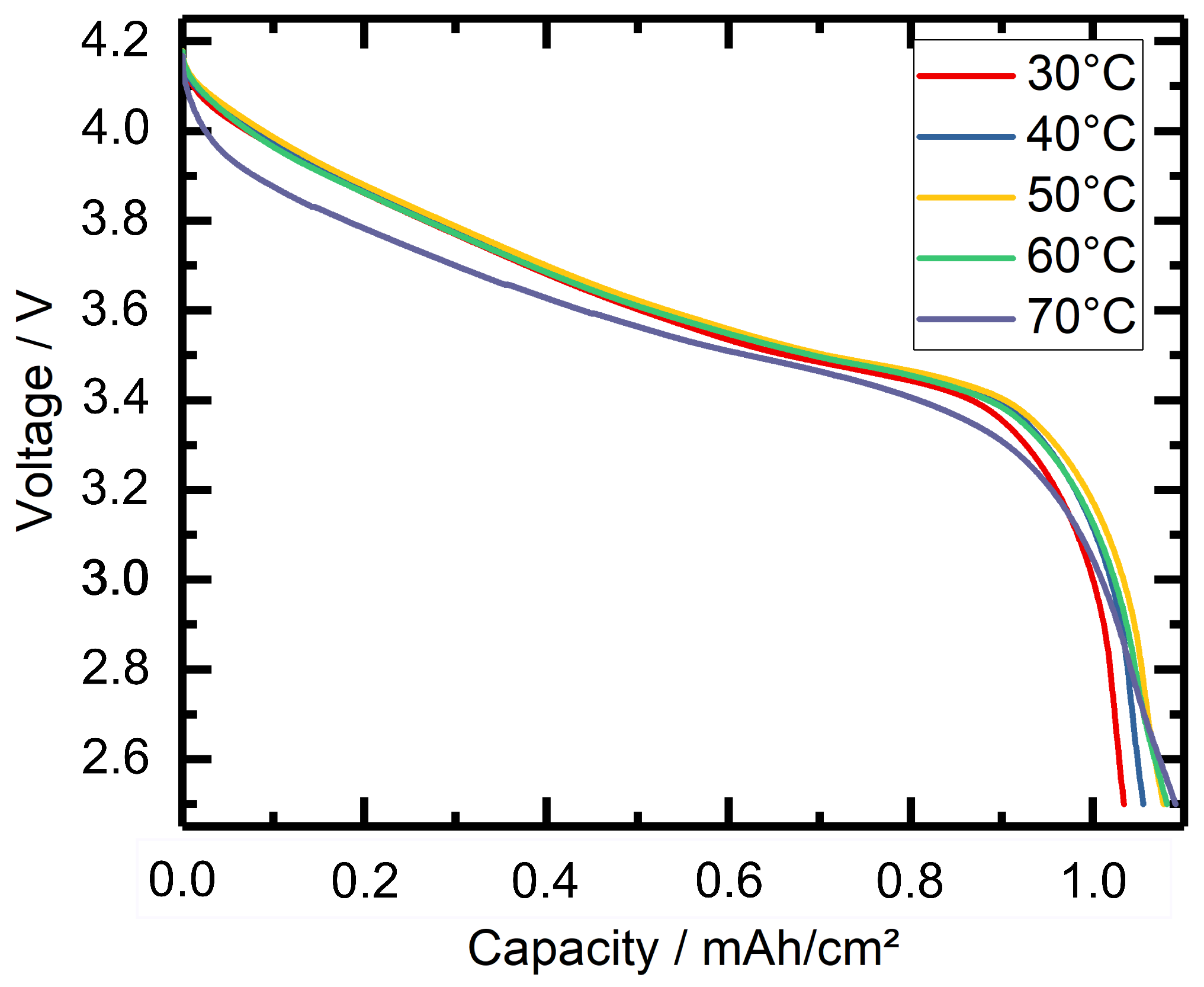
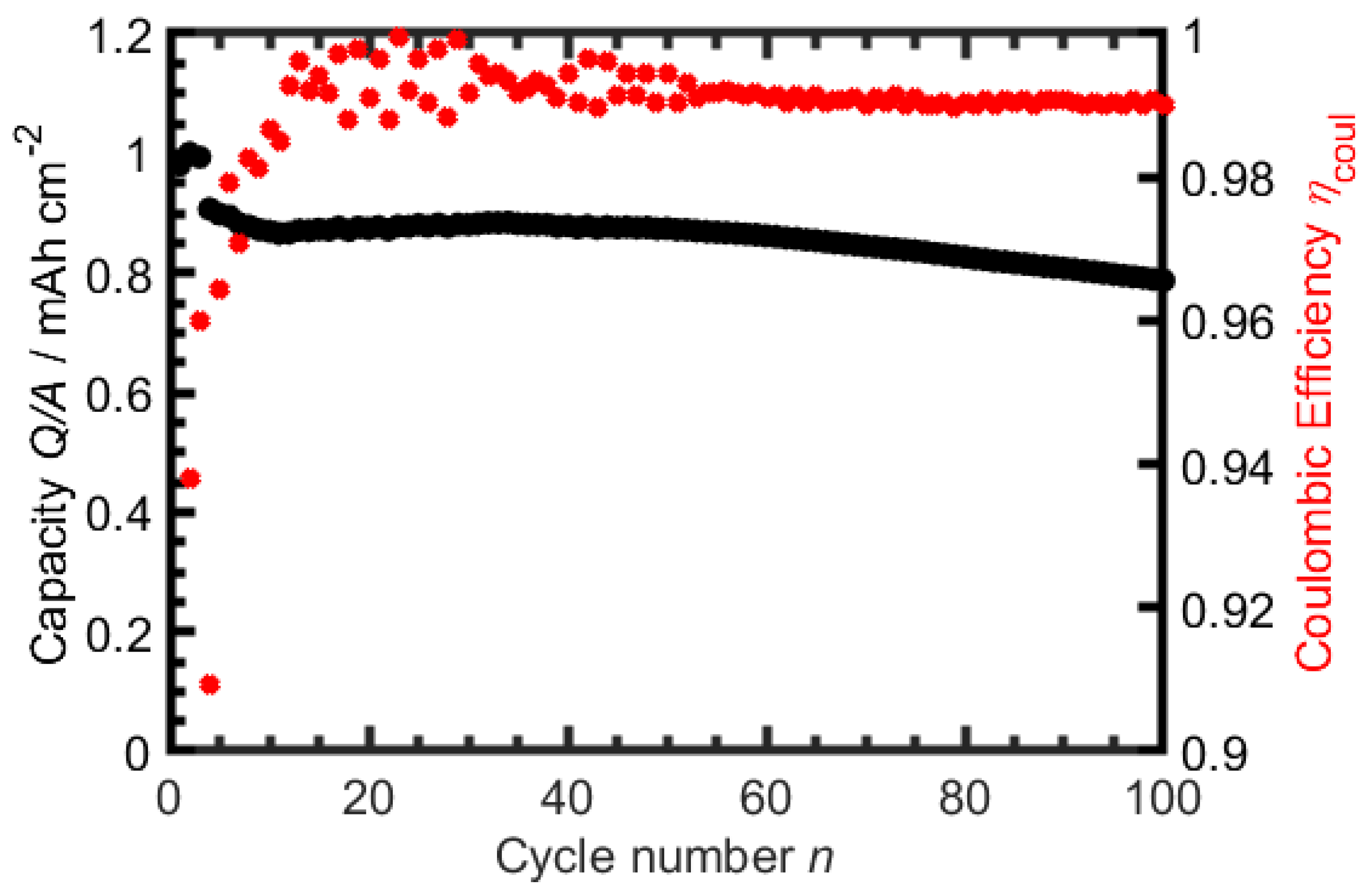
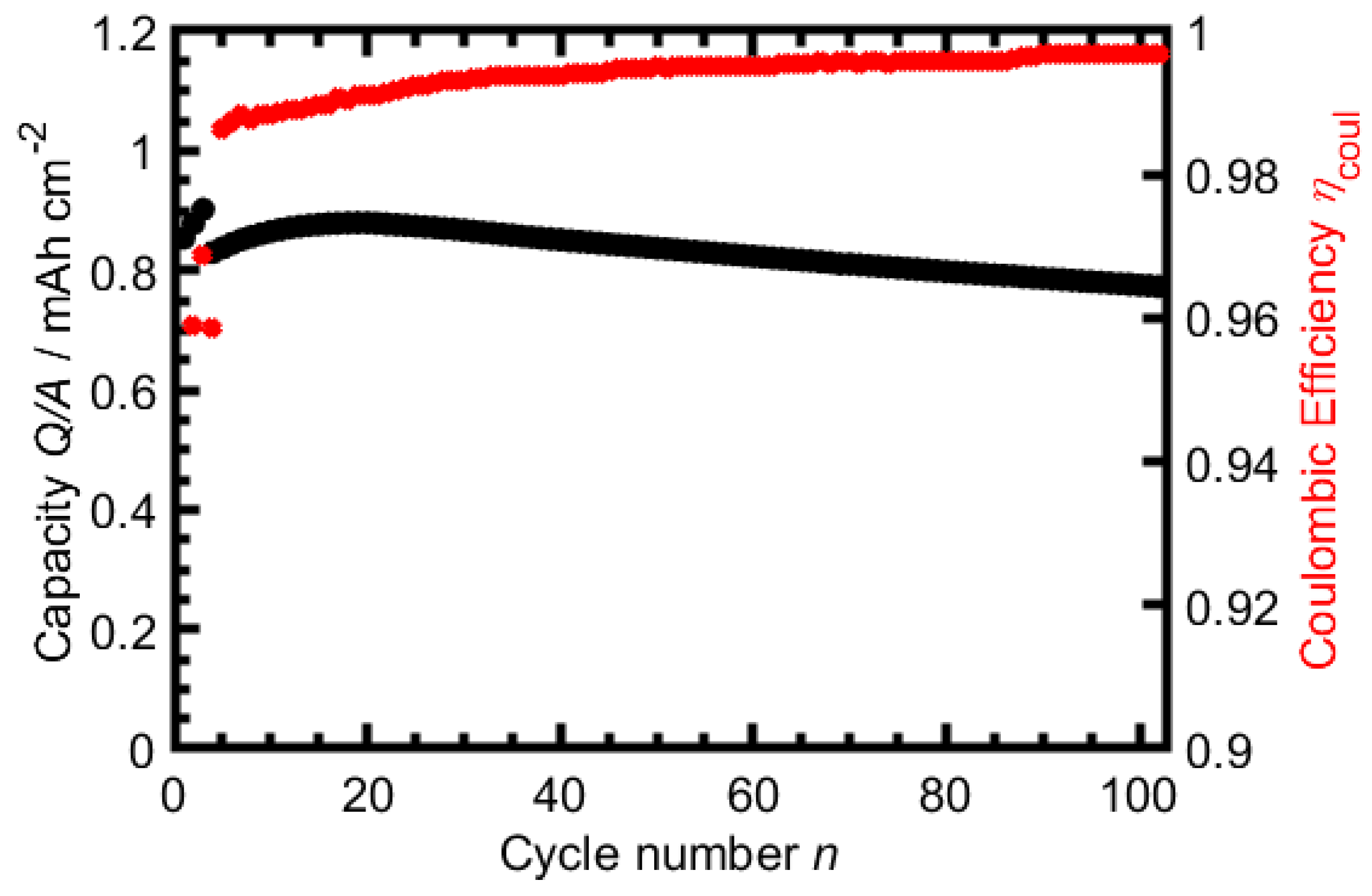
3.1. TBAC Solvent-Mixtures with LiTFSI
3.2. TBAC Solvent-Mixtures with LiBF
3.3. TBAC Solvent-Mixtures with LiFSI
3.4. TBAC Solvent-Mixtures with LiPF
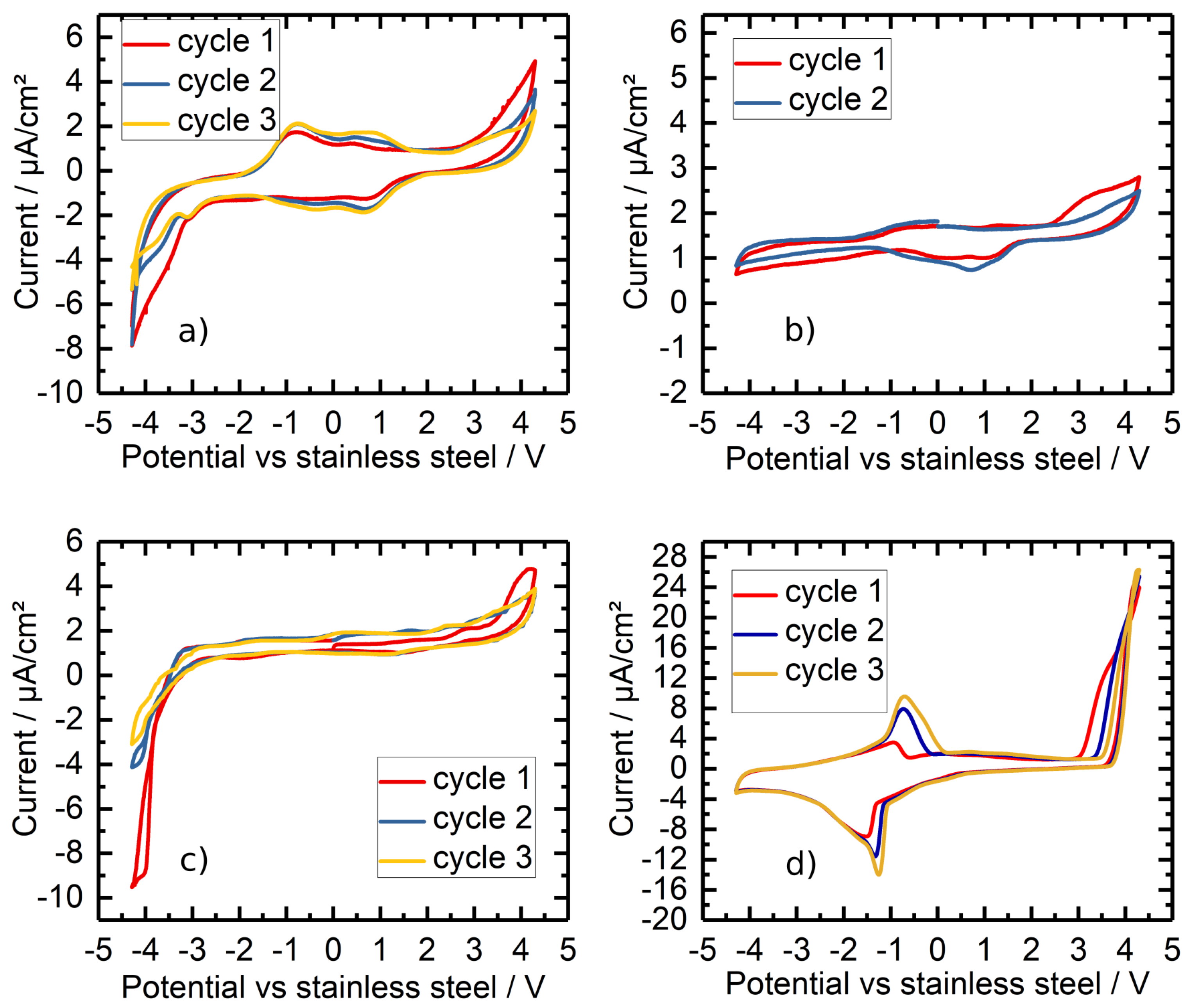
3.5. Cyclic Voltammetry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar] [CrossRef]
- Xu, K. Electrolytes and interphases in Li-ion batteries and beyond. Chem. Rev. 2014, 114, 11503–11618. [Google Scholar] [CrossRef] [PubMed]
- Dimethyl Carbonate, CAS Number 616-38-6; MERCK KGaA: Darmstadt, Germany, 2020.
- Ethyl Methyl Carbonate, CAS Number 623-53-0; MERCK KGaA: Darmstadt, Germany, 2020.
- Diethyl Carbonate, CAS Number 105-58-8; MERCK KGaA: Darmstadt, Germany, 2020.
- Hofmann, A.; Hanemann, T. Novel electrolyte mixtures based on dimethyl sulfone, ethylene carbonate and LiPF6 for lithium-ion batteries. J. Power Sources 2015, 298, 322–330. [Google Scholar] [CrossRef]
- Hess, S.; Wohlfahrt-Mehrens, M.; Wachtler, M. Flammability of Li-ion battery electrolytes: Flash point and self-extinguishing time measurements. J. Electrochem. Soc. 2015, 162, A3084. [Google Scholar] [CrossRef]
- Lisbona, D.; Snee, T. A review of hazards associated with primary lithium and lithium-ion batteries. Process. Saf. Environ. Prot. 2011, 89, 434–442. [Google Scholar] [CrossRef]
- Lei, B.; Zhao, W.; Ziebert, C.; Uhlmann, N.; Rohde, M.; Seifert, H.J. Experimental analysis of thermal runaway in 18650 cylindrical Li-ion cells using an accelerating rate calorimeter. Batteries 2017, 3, 14. [Google Scholar] [CrossRef] [Green Version]
- Hammami, A.; Raymond, N.; Armand, M. Runaway risk of forming toxic compounds. Nature 2003, 424, 635–636. [Google Scholar] [CrossRef]
- Larsson, F. Assessment of Safety Characteristics for Li-Ion Battery Cells by Abuse Testing; Dempartment of Applied Physics, Chalmers University of Technology: Gothenborg, Sweden, 2014; p. 94. [Google Scholar]
- Larsson, F.; Andersson, P.; Blomqvist, P.; Lorén, A.; Mellander, B.E. Characteristics of lithium-ion batteries during fire tests. J. Power Sources 2014, 271, 414–420. [Google Scholar] [CrossRef]
- Xu, K.; Ding, M.S.; Zhang, S.; Allen, J.L.; Jow, T.R. Evaluation of fluorinated alkyl phosphates as flame retardants in electrolytes for Li-ion batteries: I. Physical and electrochemical properties. J. Electrochem. Soc. 2003, 150, A161. [Google Scholar] [CrossRef]
- Dalavi, S.; Xu, M.; Ravdel, B.; Zhou, L.; Lucht, B.L. Nonflammable electrolytes for lithium-ion batteries containing dimethyl methylphosphonate. J. Electrochem. Soc. 2010, 157, A1113. [Google Scholar] [CrossRef]
- Kalhoff, J.; Eshetu, G.G.; Bresser, D.; Passerini, S. Safer electrolytes for lithium-ion batteries: State of the art and perspectives. ChemSusChem 2015, 8, 2154–2175. [Google Scholar] [CrossRef]
- Kim, G.; Jeong, S.; Joost, M.; Rocca, E.; Winter, M.; Passerini, S.; Balducci, A. Use of natural binders and ionic liquid electrolytes for greener and safer lithium-ion batteries. J. Power Sources 2011, 196, 2187–2194. [Google Scholar] [CrossRef]
- Lux, S.F.; Schmuck, M.; Jeong, S.; Passerini, S.; Winter, M.; Balducci, A. Li-ion anodes in air-stable and hydrophobic ionic liquid-based electrolyte for safer and greener batteries. Int. J. Energy Res. 2010, 34, 97–106. [Google Scholar] [CrossRef]
- Tsurumaki, A.; Agostini, M.; Poiana, R.; Lombardo, L.; Lufrano, E.; Simari, C.; Matic, A.; Nicotera, I.; Panero, S.; Navarra, M.A. Enhanced safety and galvanostatic performance of high voltage lithium batteries by using ionic liquids. Electrochim. Acta 2019, 316, 1–7. [Google Scholar] [CrossRef]
- Navarra, M.A. Ionic liquids as safe electrolyte components for Li-metal and Li-ion batteries. MRS Bull. 2013, 38, 548. [Google Scholar] [CrossRef]
- Adiponitrile, CAS Number 111-69-3; MERCK KGaA: Darmstadt, Germany, 2020.
- Isken, P.; Dippel, C.; Schmitz, R.; Schmitz, R.; Kunze, M.; Passerini, S.; Winter, M.; Lex-Balducci, A. High flash point electrolyte for use in lithium-ion batteries. Electrochim. Acta 2011, 56, 7530–7535. [Google Scholar] [CrossRef]
- Sun, X.G.; Angell, C.A. New sulfone electrolytes for rechargeable lithium batteries.: Part I. Oligoether-containing sulfones. Electrochem. Commun. 2005, 7, 261–266. [Google Scholar] [CrossRef]
- Xu, K.; Angell, C.A. Sulfone-based electrolytes for lithium-ion batteries. J. Electrochem. Soc. 2002, 149, A920. [Google Scholar] [CrossRef]
- Tributyl Acetylcitrat, CAS Number 77-90-7; MERCK KGaA: Darmstadt, Germany, 2020.
- Bae, J.W.; Yeo, M.; Shin, E.J.; Park, W.H.; Lee, J.E.; Nam, B.U.; Kim, S.Y. Eco-friendly plasticized poly (vinyl chloride)–acetyl tributyl citrate gels for varifocal lens. RSC Adv. 2015, 5, 94919–94925. [Google Scholar] [CrossRef]
- Johnson, W., Jr. Final report on the safety assessment of acetyl triethyl citrate, acetyl tributyl citrate, acetyl trihexyl citrate, and acetyl trioctyl citrate. Int. J. Toxicol. 2002, 21, 1–17. [Google Scholar]
- Renard, C. Tributyl Acetylcitrat as Component of Nail Polish. U.S. Patent 20040170584A1, 2 September 2004. [Google Scholar]
- Ethylcarbonate, CAS Number 96-49-1; MERCK KGaA: Darmstadt, Germany, 2021.
- Propylencarbonate, CAS Number 108-32-7; MERCK KGaA: Darmstadt, Germany, 2021.
- Dahbi, M.; Ghamouss, F.; Tran-Van, F.; Lemordant, D.; Anouti, M. Comparative study of EC/DMC LiTFSI and LiPF6 electrolytes for electrochemical storage. J. Power Sources 2011, 196, 9743–9750. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, G.; Lucht, B.L. Cycling performance and surface analysis of Lithium bis (trifluoromethanesulfonyl) imide in propylene carbonate with graphite. Electrochim. Acta 2016, 217, 269–273. [Google Scholar] [CrossRef] [Green Version]
- Krämer, E.; Schedlbauer, T.; Hoffmann, B.; Terborg, L.; Nowak, S.; Gores, H.J.; Passerini, S.; Winter, M. Mechanism of anodic dissolution of the aluminum current collector in 1 M LiTFSI EC: DEC 3: 7 in rechargeable lithium batteries. J. Electrochem. Soc. 2012, 160, A356. [Google Scholar] [CrossRef]
- Morita, M.; Shibata, T.; Yoshimoto, N.; Ishikawa, M. Anodic behavior of aluminum current collector in LiTFSI solutions with different solvent compositions. J. Power Sources 2003, 119, 784–788. [Google Scholar] [CrossRef]
- Abouimrane, A.; Ding, J.; Davidson, I. Liquid electrolyte based on lithium bis-fluorosulfonyl imide salt: Aluminum corrosion studies and lithium ion battery investigations. J. Power Sources 2009, 189, 693–696. [Google Scholar] [CrossRef] [Green Version]

| (a) | |||||
|---|---|---|---|---|---|
| Mixture | TBAC | EC | EMC | PC | DEC |
| F1 | 85% | 15% | 0% | 0% | 0% |
| F2 | 80% | 15% | 5% | 0% | 0% |
| F3 | 60% | 15% | 5% | 20% | 0% |
| F4 | 60% | 15% | 5% | 0% | 20% |
| (b) | |||||
| Salt | Solvent-Mixtures | ||||
| LiBF | F2 | ||||
| LiTFSI | F1 | F2 | F3 | F4 | |
| LiPF | F2 | F3 | F4 | ||
| LiFSI | F2 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ströbel, M.; Kiefer, L.; Birke, K.P. Investigation of a Novel Ecofriendly Electrolyte-Solvent for Lithium-Ion Batteries with Increased Thermal Stability. Batteries 2021, 7, 72. https://doi.org/10.3390/batteries7040072
Ströbel M, Kiefer L, Birke KP. Investigation of a Novel Ecofriendly Electrolyte-Solvent for Lithium-Ion Batteries with Increased Thermal Stability. Batteries. 2021; 7(4):72. https://doi.org/10.3390/batteries7040072
Chicago/Turabian StyleStröbel, Marco, Larissa Kiefer, and Kai Peter Birke. 2021. "Investigation of a Novel Ecofriendly Electrolyte-Solvent for Lithium-Ion Batteries with Increased Thermal Stability" Batteries 7, no. 4: 72. https://doi.org/10.3390/batteries7040072
APA StyleStröbel, M., Kiefer, L., & Birke, K. P. (2021). Investigation of a Novel Ecofriendly Electrolyte-Solvent for Lithium-Ion Batteries with Increased Thermal Stability. Batteries, 7(4), 72. https://doi.org/10.3390/batteries7040072