Review of Achieved Purities after Li-ion Batteries Hydrometallurgical Treatment and Impurities Effects on the Cathode Performance
Abstract
:1. Introduction
2. Recycling
3. Battery Composition and Structure
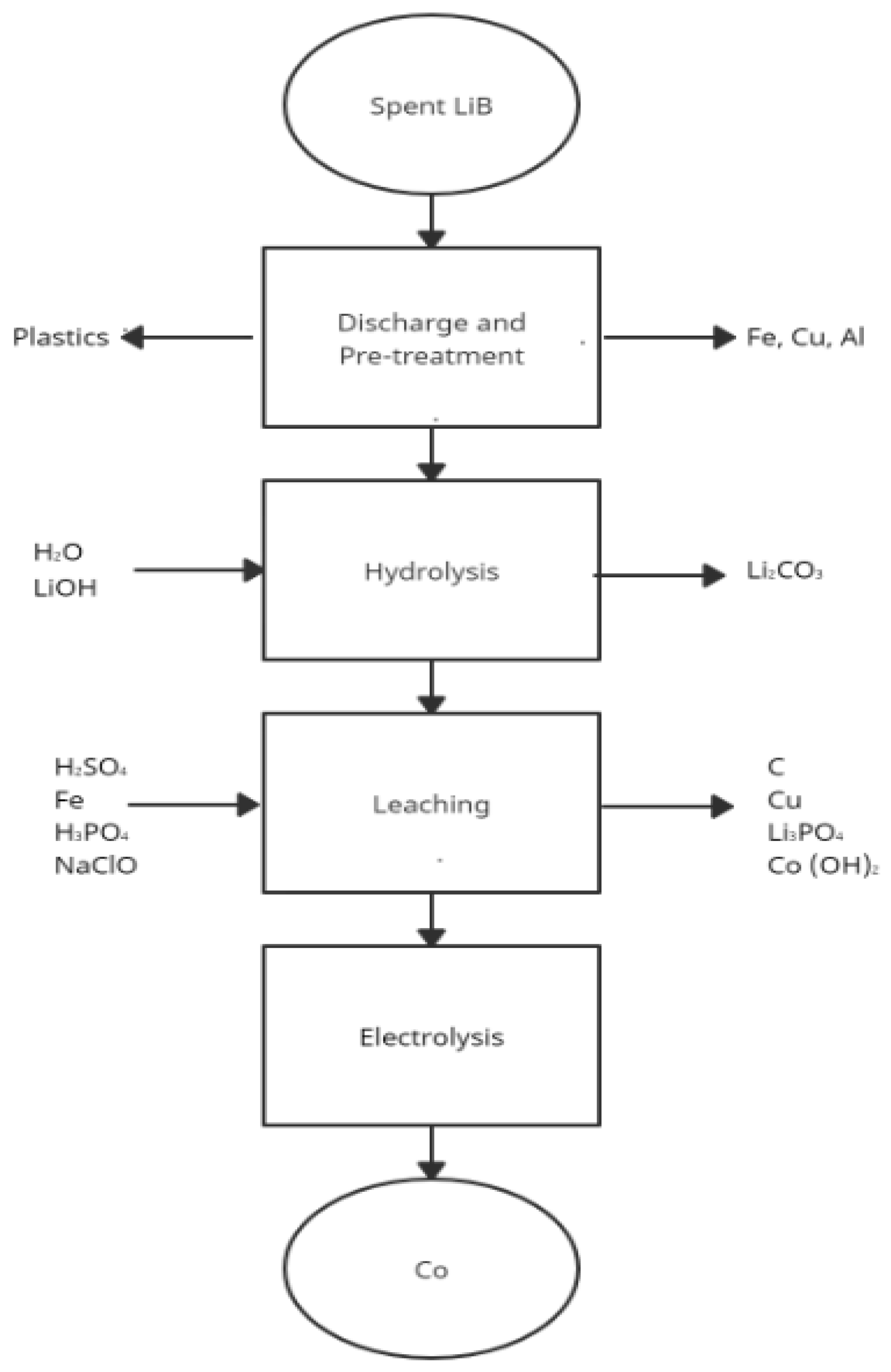
4. Hydrometallurgical Approach in Li-ion Batteries Recycling
5. Cu, Al and Fe as the Impurities in the Hydrometallurgical Process
6. Obtained Purity Degrees—Research
7. Obtained Purity Degrees—Hydrometallurgical Industrial Actors
8. The Effect of the Impurities on the Cathode Performance
8.1. Cu as the Impurity in Recycled Cathode Material
8.2. Fe as the Impurity in Recycled Cathode Material
8.3. Mg as the Impurity in Recycled Cathode Material
9. Producer Purity/Impurity Data of Battery Grade Chemical Compounds
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joulié, M.; Laucournet, R.; Billy, E. Hydrometallurgical process for the recovery of high value metals from spent lithium nickel cobalt aluminum oxide based lithium-ion batteries. J. Power Sources 2014, 247, 551–555. [Google Scholar] [CrossRef]
- Chen, X.; Yongbin, C.; Zhou, T.; Liu, D.; Hu, H.; Fan, S. Hydrometallurgical recovery of metal values from sulfuric acid leaching liquor of spent lithium-ion batteries. Waste Manag. 2015, 38, 349–356. [Google Scholar] [CrossRef]
- Peng, F.; Mu, D.; Li, R.; Liu, Y.; Ji, Y.; Dai, C.; Ding, F. Impurity removal with highly selective and efficient methods and the recycling of transition metals from spent lithium-ion batteries. RSC Adv. 2019, 35, 21922–21930. [Google Scholar] [CrossRef] [Green Version]
- Chagnes, A.; Pospiech, B. A brief review on hydrometallurgical technologies for recycling spent lithium-ion batteries. J. Chem. Technol. Biotechnol. 2013, 88, 1191–1199. [Google Scholar] [CrossRef]
- Liu, C.; Lin, J.; Cao, H.; Zhang, Y.; Sun, Z. Recycling of spent lithium-ion batteries in view of lithium recovery: A critical review. J. Clean. Prod. 2019, 228, 801–813. [Google Scholar] [CrossRef]
- European Commission. Batteries and Accumulators. Available online: https://ec.europa.eu/environment/topics/waste-and-recycling/batteries-and-accumulators_en (accessed on 2 June 2021).
- Peng, C.; Liu, F.; Aji, A.T.; Wilson, B.P.; Lundström, M. Extraction of Li and Co from industrially produced Li-ion battery waste—Using the reductive power of waste itself. Waste Manag. 2019, 95, 604–611. [Google Scholar] [CrossRef]
- Zhang, R.; Meng, Z.; Ma, X.; Chen, M.; Chen, B.; Zheng, Y.; Yao, Z.; Vanaphuti, P.; Bong, S.; Yang, Z.; et al. Understanding fundamental effects of Cu impurity in different forms for recovered LiNi0.6Co0.2Mn0.2O2 cathode materials. Nano Energy 2020, 78, 105214. [Google Scholar] [CrossRef]
- Ardia, P.; Stallone, S.; Cericola, D. A quantification method for Fe based particle contaminants in high purity materials for lithium-ion batteries. Talanta 2021, 224, 121827. [Google Scholar] [CrossRef] [PubMed]
- Barik, S.P.; Prabaharan, G.; Kumar, B. An innovative approach to recover metal values from spent lithium-ion batteries. Waste Manag. 2016, 51, 222–226. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Recovery of valuable metals from cathodic active material of spent lithium ion batteries: Leaching and kinetic aspects. Waste Manag. 2015, 45, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhu, M.; Zhao, Z.; Tong, B.; Fan, Y.; Hua, Z. Hydrometallurgical Processes for Recycling Spent Lithium-Ion Batteries: A Critical Review. ASC Sustain. Chem. Eng. 2018, 6, 13611–13627. [Google Scholar] [CrossRef]
- Fortum. Lithium-ion Battery Recycling Solution. Available online: https://www.fortum.com/products-and-services/fortum-battery-solutions/recycling/lithium-ion-battery-recycling-solution?gclid=CjwKCAjw-e2EBhAhEiwAJI5jg3MY0vAhwcJdS3WfBH03GgrPqqerzcZFiY0sfjP8VXUaY75HdOJVKxoCAT8QAvD_BwE (accessed on 13 May 2021).
- Northvolt. Northvolt: The Technologies Paving the Way for Li-ion Battery Recycling. Available online: https://northvolt.com/stories/RevoltTechnologies (accessed on 13 May 2020).
- Circular Energy Storage. State-of-the-Art in Reuse and Recycling of Lithium-Ion Batteries—A Research Review; The Swedish Energy Agency: Eskilstuna, Sweden, 2019. [Google Scholar]
- Po, D.H.; Kang, M.; Chen, O.; Ogunseitan, A. Potential environmental and human health impacts of rechargeable lithium batteries in electronic waste. Environ. Sci. Technol. 2013, 47, 5495–5503. [Google Scholar]
- Sun, L.; Qiu, K. Organic oxalate as leachant and precipitant for the recovery of valuable metals from spent lithium-ion batteries. Waste Manag. 2012, 32, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yokoyama, T.; Itabashi, O.; Wakui, Y.; Suzuki, T.M.; Inoue, K. Hydrometallurgical process for recovery of metal values from spent nickel-metal hydride secondary batteries. Hydrometallurgy 1998, 50, 61–75. [Google Scholar] [CrossRef]
- Park, S.; Kim, D.; Ku, H.; Jo, M.; Kim, S.; Song, J.; Yu, J.; Kwon, K. The effect of Fe as an impurity element for sustainable resynthesis of Li[Ni1/3Co1/3Mn1/3]O2 cathode material from spent lithium-ion batteries. Electrochim. Acta. 2019, 296, 814–822. [Google Scholar] [CrossRef]
- Fortum. Sustainability and Demand for Electric Cars to Improve with Fortum’s New Recycling Technology for Lithium Recovery. Available online: https://www.fortum.com/media/2020/11/sustainability-and-demand-electric-cars-improve-fortums-new-recycling-technology-lithium-recovery (accessed on 17 May 2021).
- Atia, T.A.; Elia, G.; Hahn, R.; Altimari, P.; Pagnanelli, F. Closed-loop hydrometallurgical treatment of end-of-life lithium ion batteries: Towards zero-waste process and metal recycling in advanced batteries. J. Energy Chem. 2019, 35, 220–227. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Peng, C.; Ma, Q.; Wang, J.; Zhou, S.; Chen, Z.; Wilson, B.P.; Lundström, M. Selective lithium recovery and integrated preparation of high-purity lithium hydroxide products from spent lithium-ion batteries. Sep. Purif. Technol. 2021, 259, 118181. [Google Scholar] [CrossRef]
- Peng, C.; Chang, C.; Wang, Z.; Wilson, B.P.; Liu, F.; Lundström, M. Recovery of high-purity MnO2 from the acid leaching solution of spent li-ion batteries. J. Miner. 2019, 72, 790–799. [Google Scholar]
- Jo, C.-H.; Myung, S.-T. Efficient recycling of valuable resources from discarded lithium-ion batteries. J. Power Sources 2019, 426, 259–265. [Google Scholar] [CrossRef]
- Granata, G.; Moscardini, E.; Pagnanelli, F.; Trabucco, F.; Toro, L. Product recovery from Li-ion battery wastes coming from an industrial pre-treatment plant: Lab scale tests and process simulations. J. Power Sources 2012, 206, 393–401. [Google Scholar] [CrossRef]
- Wang, W.Y.; Yen, C.H.; Lin, J.L.; Xu, R.B. Recovery of high-purity metallic cobalt from lithium nickel manganese cobalt oxide (NMC)-type Li-ion battery. J. Mater. Cycles Waste Manag. 2019, 21, 300–307. [Google Scholar] [CrossRef]
- AzoCleanTech, Pilkington, B.; Li-Cycle: Hydrometallurgical Recycling of Lithium-Ion Batteries. Available online: https://www.azocleantech.com/article.aspx?ArticleID=1261 (accessed on 1 September 2021).
- Heelan, J.; Gratz, E.; Zheng, Z.; Wang, Q.; Chen, M.; Apelian, D.; Wang, Y. Current and prospective li-ion battery recycling and recovery processes. J. Miner. 2016, 68, 2632–2638. [Google Scholar] [CrossRef] [Green Version]
- Velázquez-Martínez, O.; Valio, J.; Santasalo-Aarnio, A.; Reuter, M.; Serna-Guerrero, R. A critical review of lithium-ion battery recycling processes from a circular economy perspective. Batteries 2019, 5, 68. [Google Scholar] [CrossRef] [Green Version]
- Linneen, N.; Bhave, R.; Woerner, D. Purification of industrial grade lithium chloride for the recovery of high purity battery grade lithium carbonate. Sep. Purif. Technol. 2019, 214, 168–173. [Google Scholar] [CrossRef]
- Wu, Z.J.; Wang, D.; Gao, Z.F.; Yue, H.F.; Liu, W.M. Effect of Cu substitution on structures and electrochemical properties of Li[NiCo1−xCuxMn]1/3O2 as cathode materials for lithium ion batteries. Dalton Trans. 2015, 42, 18624–18631. [Google Scholar] [CrossRef]
- Yang, L.; Ren, F.; Feng, Q.; Xu, G.; Li, X.; Li, Y.; Zhao, E.; Ma, J.; Fan, S. Effect of Cu doping on the structural and electrochemical performance of LiNi1/3Co1/3Mn1/3O2 cathode materials. J. Electron. Mater. 2018, 47, 3996–4002. [Google Scholar] [CrossRef]
- Fear, C.; Juarez-Robles, D.; Jeevarajan, J.A.; Mukherjee, P.P. Elucidating copper dissolution phenomenon in Li-Ion cells under overdischarge extremes. J. Electrochem. Soc. 2018, 165, A1639–A1647. [Google Scholar] [CrossRef]
- Zhao, M.; Dewald, H.; Doch, R.; Staniewicz, J. Quantitation of the dissolution of battery-grade copper foils in lithium-ion battery electrolytes by flame atomic absorption spectroscopy. Electrochim. Acta. 2004, 49, 683–689. [Google Scholar] [CrossRef]
- Contestabile, M.; Panero, S.; Scrosati, B. A laboratory-scale lithium-ion battery recycling process. J. Power Sources. 2001, 92, 65–69. [Google Scholar] [CrossRef]
- Nan, J.; Han, D.; Zuo, X. Recovery of metal values from spent lithium-ion batteries with chemical deposition and solvent extraction. J. Power Sources. 2005, 152, 278–284. [Google Scholar] [CrossRef]
- Weng, Y.; Xu, S.; Huang, G.; Jiang, C. Synthesis and performance of Li[(Ni1/3Co1/3Mn1/3)1−xMgx]O2 prepared from spent lithium ion batteries. J. Hazard. Mater. 2013, 1, 2246–2247. [Google Scholar] [CrossRef] [PubMed]
- Via, T.T. Northvolt och Volvo i Nytt Batteriprojekt. Available online: https://via.tt.se/pressmeddelande/northvolt-och-volvo-i-nytt-batteriprojekt?publisherId=3235770&releaseId=3297508 (accessed on 17 May 2021).
- Livent. Lithium Carbonate. Battery Grade. Available online: https://livent.com/product/lithium-carbonate-battery-grade/ (accessed on 7 June 2021).
- Livent. Lithium Hydroxide Monohydrate. High Purity. Available online: https://livent.com/product/lithium-hydroxide-monohydrate-high-purity/ (accessed on 7 June 2021).
- CoreMax. Battery Materials. Available online: https://www.coremaxcorp.com/uploads/product/Battery_Materials/Cobalt_Sulfate_V4.pdf (accessed on 7 June 2021).
- CoreMax. Battery Materials. Available online: https://www.coremaxcorp.com/uploads/product/Battery_Materials/Nickel_Sulfate_Battery_V1.pdf (accessed on 7 June 2021).
- iSkyChem. Battery Grade Manganese Sulfate Monohydrate. Available online: http://www.iskychem.com/wap_product_detail_en/id/17.html (accessed on 7 June 2021).
- Sigma-Aldrich. 746762 Lithium Hexafluorophosphate Solution in Ethyl Methyl Carbonate. 1.0 M LiPF6 in EMC. Battery Grade. Available online: https://www.sigmaaldrich.com/SE/en/product/aldrich/746762 (accessed on 7 June 2021).
- Sigma-Aldrich. 746789 Lithium Hexafluorophosphate Solution in Propylene Carbonate. 1.0 M LiPF6 in PC. Battery Grade. Available online: https://www.sigmaaldrich.com/SE/en/product/aldrich/746789 (accessed on 7 June 2021).
- Sigma-Aldrich. 746754 Lithium Hexafluorophosphate Solution in Dimethyl Carbonate. 1.0 M LiPF6 in DMC. Battery Grade. Available online: https://www.sigmaaldrich.com/SE/en/product/aldrich/746754 (accessed on 7 June 2021).
- MSE Supplies. Lithium Nickel Manganese Cobalt Oxide NMC 622 Cathode Powder 500g. LiNi0.6Mn0.2Co0.2O2. Available online: https://www.msesupplies.com/products/lithium-nickel-manganese-cobalt-oxide-nmc622-cathode-powder?variant=7141237060 (accessed on 7 June 2021).
- MSE Supplies. Single Crystal NMC 532 Cathode Powder 500g. Lithium Nickel Manganese Cobalt Oxide. LiNi0.5Mn0.3Co0.2O2. Available online: https://www.msesupplies.com/products/single-crystal-nmc532-cathode-powder-lithium-nickel-manganese-cobalt-oxide-lini0-5mn0-3co0-2o2?ref=isp_rel_prd&isp_ref_pos=1&variant=31800717082682 (accessed on 6 June 2021).
- MSE Supplies. Lithium Nickel Manganese Cobalt Oxide. LiNi0.8Co0.1Mn0.1O2 NMC 811 Cathode Powder 500 g. Available online: https://www.msesupplies.com/collections/cathode-materials/products/nmc-811-cathode-powder-500g-per-bag-lithium-nickel-manganese-cobalt-oxide-powder?variant=16116292714554 (accessed on 7 June 2021).
- MSE Supplies. Lithium Nickel Manganese Cobalt Oxide NMC 111 Cathode Powder 500g. Li1.05Ni0.33Mn0.33Co0.33O2. Available online: https://www.msesupplies.com/collections/cathode-materials/products/lithium-nickel-manganese-cobalt-oxide-cathode-powder-nmc111-500g?variant=7141062212 (accessed on 7 June 2021).
- MSE Supplies. Single Crystal High Nickle NMC Ni83 Cathode Powder 500 g Lithium Nickel Manganese Cobalt Oxide. LiNi0.83Mn0.06Co0.11O2. Available online: https://www.msesupplies.com/collections/cathode-materials/products/single-crystal-high-nickle-nmc-cathode-powder-lithium-nickel-manganese-cobalt-oxide-lini-sub-0-83-sub-mn-sub-0-06-sub-co-sub-0-11-sub-o-sub-2-sub?variant=31800758403130 (accessed on 7 June 2021).


| Cathode Material | Charging and Discharging Capacity | Initial Coulomb Efficiencies | Substantial Polarization of the Electrode | Capacity through Cycle | Specific Capacity Retention at 10 C | Discharg Capacity Recovery at 0.1 C |
|---|---|---|---|---|---|---|
| LiNi0.8Co0.1Mn0.1O2 synthesized with commercial Li source | Higher | 90.5% | - | Worse | 75% | Close to initial value |
| LiNi0.8Co0.1Mn0.1O2 synthesized with recovered Li source | Lower | 90.7% | Occurs | Better due to stabilizing Al-O bonds | 63% | Close to initial value |
| Study Reference | Product | Purity Grade | Battery Grade | Recovery Method | Year |
|---|---|---|---|---|---|
| (Atia et al., 2019) | Co3O4 CoC2O4 NiO Li2CO3 | 83% 96% 89% 99.8% | − − − + | 1. Fe-precipitation 2. liquor-liquor extraction 3. Li-Na precipitation | 2019 |
| (Liu et al., 2021) | LiOH⋅H2O | 99.9% | + | 1. Reductive hydrogen roasting 2. Water leaching 3. Crystallization | 2021 |
| (Peng et al., 2019) | MnO2 | >99.5% | ? | 1. Solvent extraction (D2EHPA) 2. Scrubbing (MnSO4) 3. Stripping 4. Oxidative precipitation (KMnO4 and MnO2) | 2019 |
| (Jo and Myung, 2019) | Li2CO3 | 99.5% | - | Based on different material solubilities depending on solvent and temperature | 2019 |
| (Chen et al., 2015) | CoC2O4⋅H2O Li2CO3 MnSO4 Ni+ | 97.9% 99.2% rel.pure 97.8% | ? − − ? | 1. Ni—precipitation (CH3C(NOH)C(NOH)CH3) 2. Mn, Co—solvent extraction (D2EHPA) 3. Co-precipitation (NH₄OH) and Li-precipitation (Na₂CO₃) | 2015 |
| (Wang et al., 2019) | Metallic Co | 98.8% | ? | 1. Extraction with (I)P204 (II)P507 2. Electrowinning | 2019 |
| (Granata et al., 2012) | Li2CO3 CoCO3 | >98% 36–37% | − − | 1. Precipitation in pH 5.5 2. Carbonatation | 2012 |
| (Granata et al., 2012) | Li2CO3 CoCO3 | >98% 47% | − − | 1. Precipitation in pH 5,5 2. Solvent extraction 3. Carbonatation | 2012 |
| Company | Process Type | Product | Method | Purity Degree | Product Application |
|---|---|---|---|---|---|
| Northvolt | hydrometallurgy | Precipitation, SX | Battery grade | ||
| Fortum | hydrometallurgy | Precipitaiton | |||
| Li-Cycle | hydrometallurgy | CoSO4, NiSO4, Li2CO3, MnCO3 | Precipitation, SX | Battery grade | |
| Recupyl | hydrometallurgy | Li2CO3 Li3PO4 | precipitation with CO2 | no data | cathode production |
| OnTo | hydrometallurgy | Li2CO3 | anode purification: low and high pH cathode purification: basic solution | 99% | cathode production |
| Precursor | Purity Level | H2O | Na | Ca | SO4 | Fe | Al | Cu | Ni | Mg | Co | Zn | Cl | Mn | Pb | Cr | Cd | Si | K | LiOH | CO2 | HF | LiPF4 | Insolubles |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Li2CO3 [39] | ≥ 99.5 wt% | 0.5 wt% | 0.05 wt% | 0.04 wt% | 0.1 wt% | 5 | 10 | 5 | 6 | 5 | 0.01 wt% | 0.02 wt% | ||||||||||||
| LiOH⋅H2O [40] | 20 | 15 | 0.01 wt% | 5 | 10 | 5 | 10 | 10 | 0.002 wt% | 10 | 5 | 30 | 10 | ≥56.5 wt% | ≤0.35 wt% | 0.01 wt% | ||||||||
| NiSO4 [41] | 15 | 4 | 3 | 5 | 1 | 22 wt% | 10 | 100 | 3 | 10 | 2 | 5 | 5 | 1 | 10 | 10 | 50 | |||||||
| CoSO4 [42] | 12 | 12 | 5 | 12 | 3 | 100 | 10 | ≥21 wt% | 5 | 12 | 5 | 5 | 5 | 5 | 12 | 5 | 50 | |||||||
| MnSO4·H2O [43] | 50 | 50 | 10 | 10 | 50 | 10 | ≥32% | 10 | 10 | |||||||||||||||
| LiPF6 Solution in EMC [44] | 15 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 50 | 0.95–1.10 M | ||||||||||||||
| LiPF6 solution in PC [45] | 15 | 1 | 1 | 2 | 2 | 1 | 1 | 1 | 50 | 0.95–1.10 M | ||||||||||||||
| LiPF6 solution in DMC [46] | 15 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 50 | 0.95–1.10 M |
| Cathode Material [47,48,49,50,51] | Li+Mn+Co (wt%) | Li (wt%) | Ni (wt%) | Co (wt%) | Mn (%) | Fe (wt%) | Cu (wt%) | Zn | Ca (wt%) | Na (wt%) | S | Moisture | Li2CO3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMC 622 | 58.0 | 7.0~8.0 | 0.01 | 0.005 | 0.03 | 0.05 wt% | 0.2 wt% | ||||||
| NMC 532 | 57.0~62.0 | 7.0~7.6 | 50.0 ± 1.0 | 20.0 ± 1.0 | 30.0 ± 1.0 | ≤0.005 | ≤0.002 | ≤0.01 | ≤0.03 | ||||
| NMC 811 | 58.0–60.5 | 7.1–7.6 | <0.005 | <0.002 | <0.02 wt% | <0.01 | <0.03 | ||||||
| NMC 111 | 7.60 ± 1.0 | 20.0 ± 1.5 | 20.0 ± 1.5 | 16.0 ± 1.5 | ≤100 ppm | ≤20 ppm | ≤20 ppm | ≤300 ppm | ≤100 ppm | ≤4000 ppm | ≤1500 ppm | ||
| NMC Ni83 | 58.5~60.5 | 7.0~7.6 | 83.0 ± 0.7 | 11.0 ± 0.5 | 6.0 ± 0.5 | ≤0.005 | ≤0.002 | ≤0.01 | ≤0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasser, O.A.; Petranikova, M. Review of Achieved Purities after Li-ion Batteries Hydrometallurgical Treatment and Impurities Effects on the Cathode Performance. Batteries 2021, 7, 60. https://doi.org/10.3390/batteries7030060
Nasser OA, Petranikova M. Review of Achieved Purities after Li-ion Batteries Hydrometallurgical Treatment and Impurities Effects on the Cathode Performance. Batteries. 2021; 7(3):60. https://doi.org/10.3390/batteries7030060
Chicago/Turabian StyleNasser, Olimpia A., and Martina Petranikova. 2021. "Review of Achieved Purities after Li-ion Batteries Hydrometallurgical Treatment and Impurities Effects on the Cathode Performance" Batteries 7, no. 3: 60. https://doi.org/10.3390/batteries7030060
APA StyleNasser, O. A., & Petranikova, M. (2021). Review of Achieved Purities after Li-ion Batteries Hydrometallurgical Treatment and Impurities Effects on the Cathode Performance. Batteries, 7(3), 60. https://doi.org/10.3390/batteries7030060








