Detection of Lithium Plating in Li-Ion Cell Anodes Using Realistic Automotive Fast-Charge Profiles
Abstract
:1. Introduction
- consumption of active Lithium;
- clogging of electrode porosity, with consequent loss of Li+ ions mobility;
- risk of short circuits associated with the formation of Li dendrites.
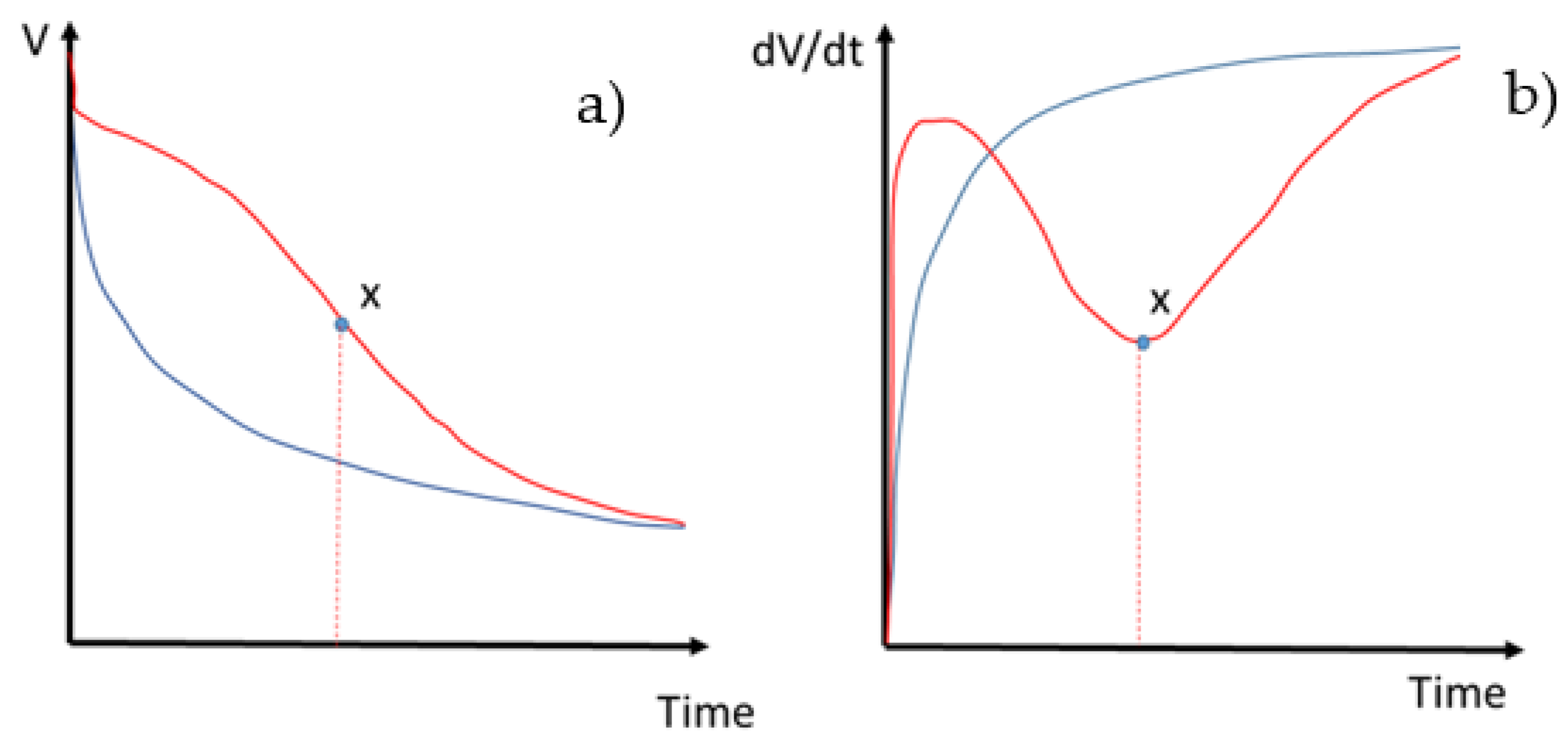
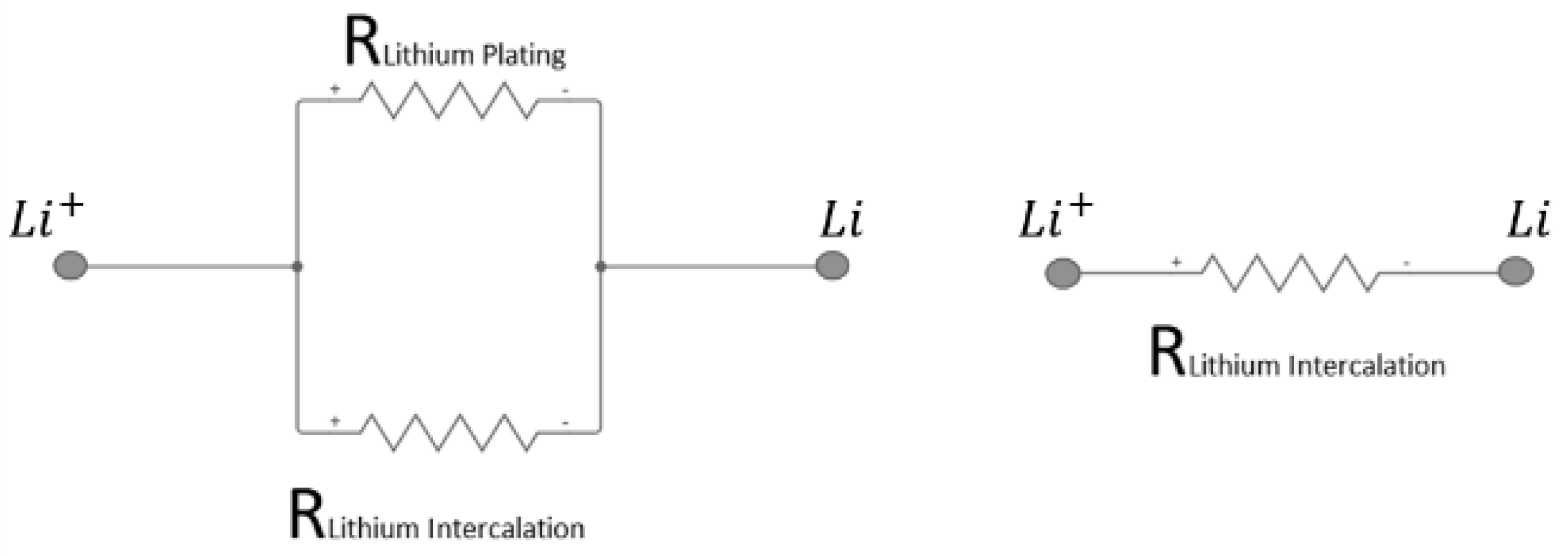
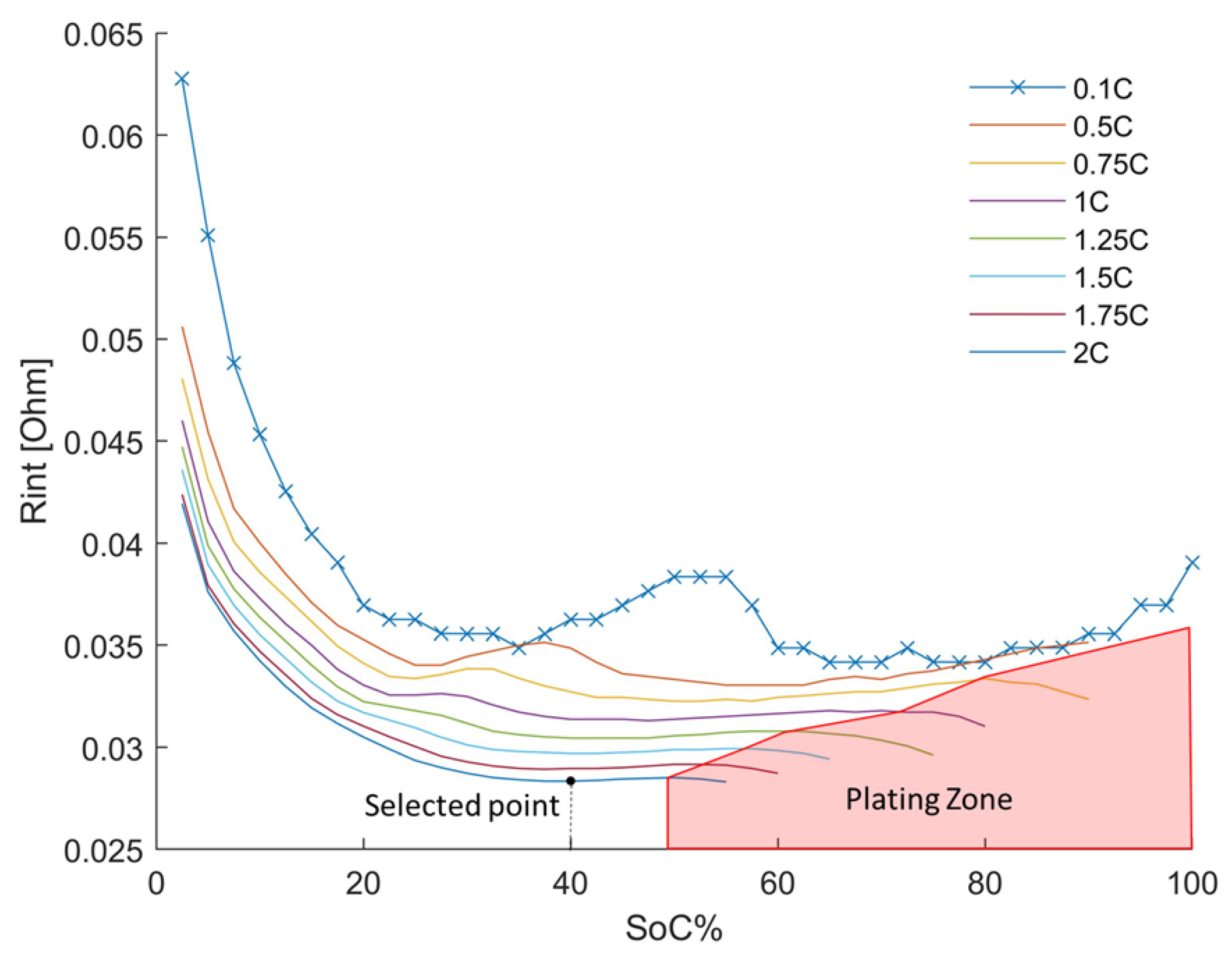
- evaluation of the evolution as a function of time of the internal resistance during the charge process [28].
2. Materials and Methods
2.1. Evaluation of Electrode Potential Measured in a Three-Electrode Cell against a Li/Li+ Pseudo-Reference Electrode
- CC–CV charge C/2 up to 4.2 V (CV step with an exit condition I < C/40);
- CC discharge at expected 1 C down to 2.75 V;
2.2. Evaluation of the Evolution of the Internal Resistance as a Function of Time during the Charge Process
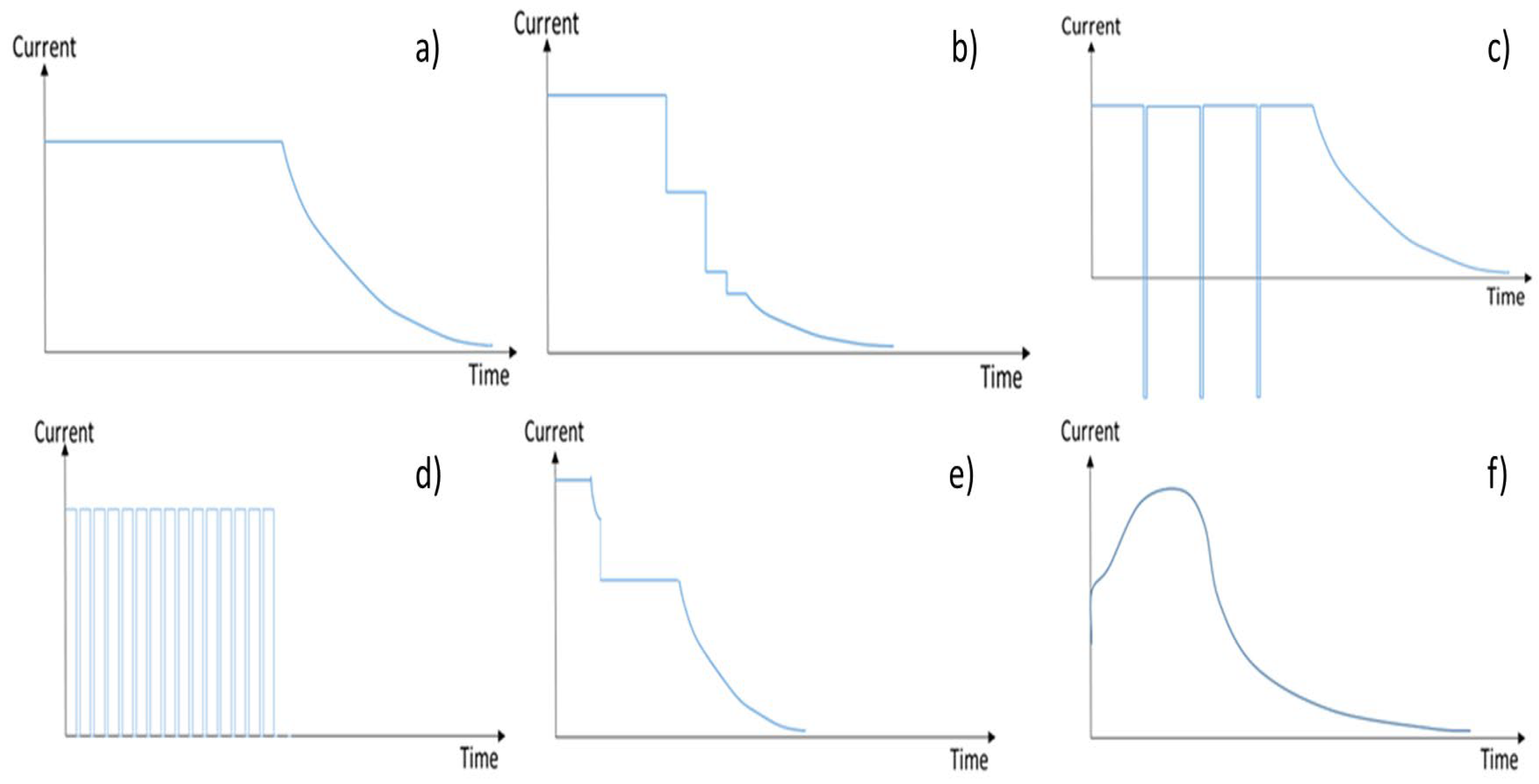
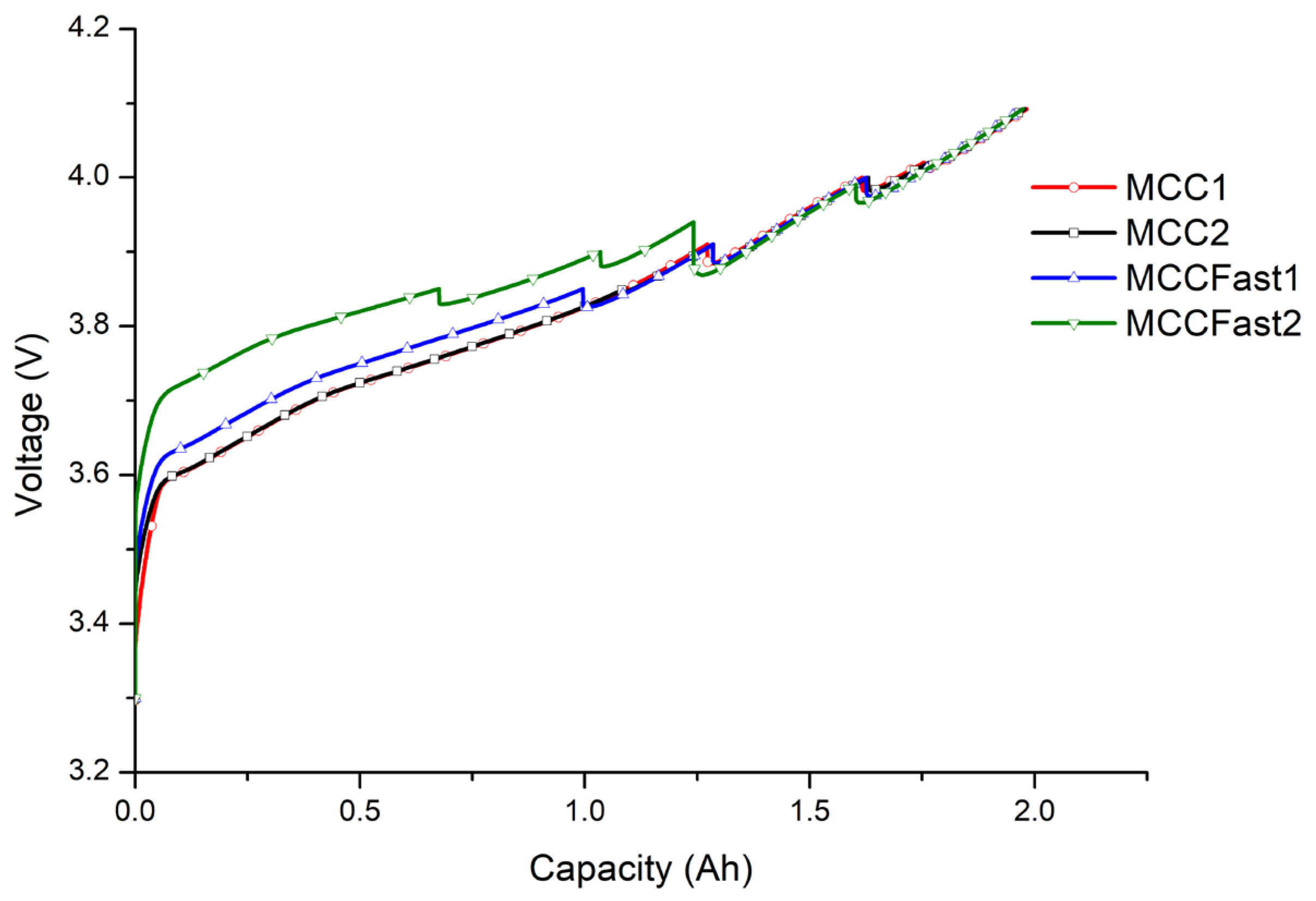
2.3. Definition of Multi-Stage Constant-Current Profiles
- MCC1
- MCC2
- MCCFast1
- MCCFast2
2.3.1. MCC1
2.3.2. MCC2
2.3.3. MCC Fast1
2.3.4. MCC Fast2
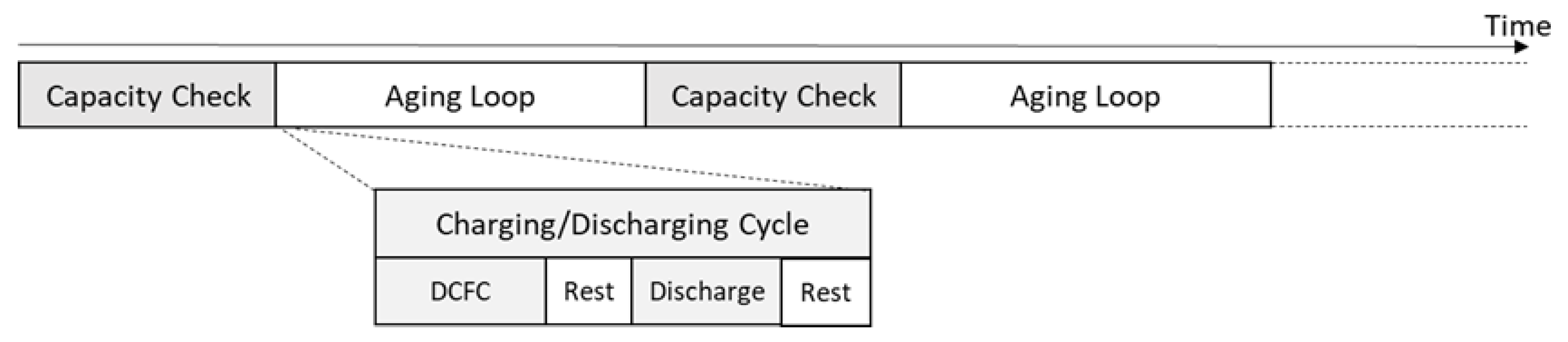
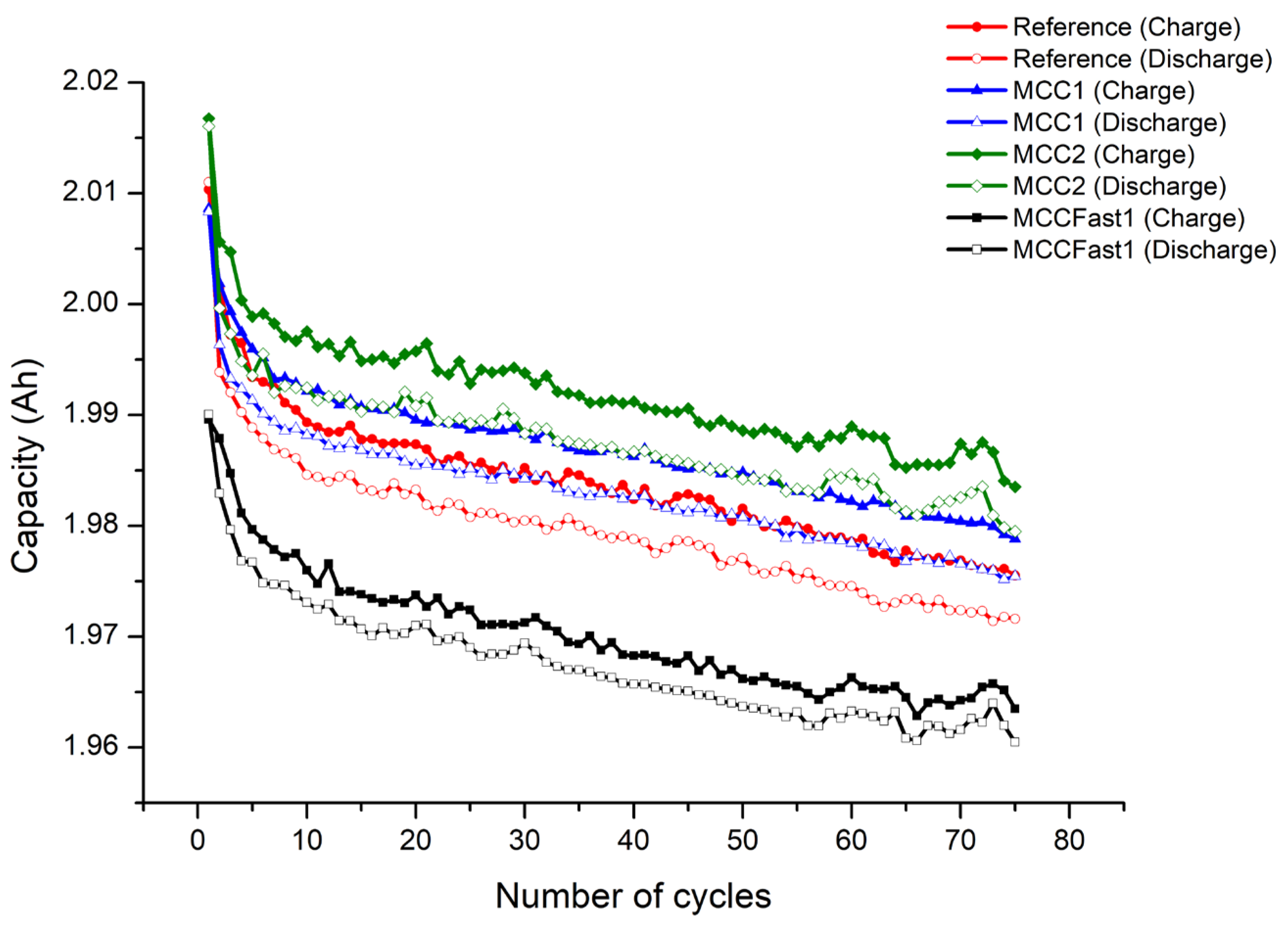
2.4. Cycle Aging Evaluation Test
- charging: performed by applying for each cell, one of the profiles described above;
- rest: each charging phase was followed by a resting time (30 min);
- discharging: at the end of the resting time, the cell discharge started at 1 C up to a voltage value equal to 2.98 V;
- rest: the discharging phase was followed by a resting time (30 min);
- CC–CV charge C/2 up to 4.2 V (CV step with an exit condition I < C/100);
- CC discharge at the expected 1 C down to 2.5 V;
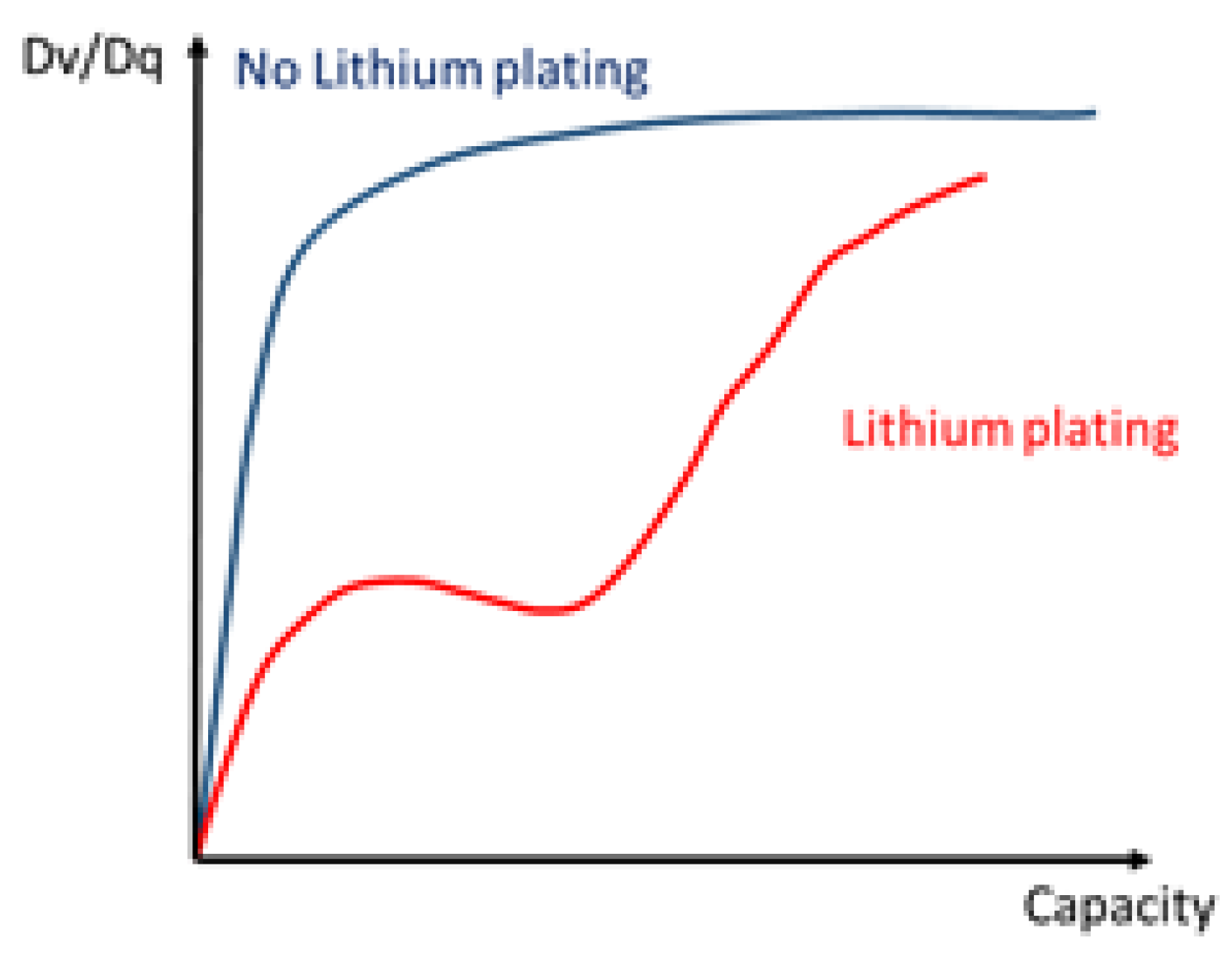
3. Results
4. Conclusions
- Evaluation of electrode potential as a function of time, measured in a three-electrode cell (EL-Cell) against a Li/Li+ pseudo-reference electrode (described in Section 2.1)
- -
- Pros: consolidated method based on well-known electrochemical principles; precise check on electrode potential (i.e., anode vs. Li).
- -
- Cons: complex preparative procedure and need of specific facilities (i.e., Glove box with argon flux); risk of degradation for the electrodes during the dismantling of the cell.
- Evaluation of the evolution as a function of time of the internal resistance during the charge process (described in Section 2.2)
- -
- Pros: no complex preparative procedure and no need of specific facilities; no risk of degradation for the electrodes, since no dismantling operation is necessary; fast check on plating evolution; easy to be applied to a high number of samples.
- -
- Cons: further investigation required, above all, on cells with higher capacities and different anode chemistry.
- reduction of charging time,
- improvement of capacity retention for MCC1, MCC2, MCC Fast1 (best result obtained with MCC2).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Pollet, B.G.; Staffell, I.; Shang, J.L. Current status of hybrid, battery and fuel cell electric vehicles: From electrochemistry to market prospects. Electrochim. Acta 2012, 84, 235–249. [Google Scholar] [CrossRef]
- Pelegov, D.V.; Pontes, J. Main Drivers of Battery Industry Changes: Electric Vehicles—A Market Overview. Batteries 2018, 4, 65. [Google Scholar] [CrossRef] [Green Version]
- Pevec, D.; Babic, J.; Carvalho, A.; Ghiassi-Farrokhfal, Y.; Ketter, W.; Podobnik, V. A survey-based assessment of how existing and potential electric vehicle owners perceive range anxiety. J. Clean. Prod. 2020, 276, 122779. [Google Scholar] [CrossRef]
- Manthiram, A. Materials for High-energy Density Batteries. In Energy Harvesting Technologies; Springer: Boston, MA, USA, 2009; pp. 365–385. [Google Scholar]
- Cui, Z.; Xie, Q.; Manthiram, A. Zinc-Doped High-Nickel, Low-Cobalt Layered Oxide Cathodes for High-Energy-Density Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 9, 15324–15332. [Google Scholar] [CrossRef] [PubMed]
- Namsar, O. Improved electrochemical performance of anode materials for high energy density lithium-ion batteries through Sn(SnO2)–SiO2/graphene-based nanocomposites prepared by a facile and low-cost approach. Sustain. Energy Fuels 2020, 12, 4625–4636. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Maitra, A.; Halliwell, J.; Davis, M.; Duvall, M. Fast Charging: An In-Depth Look at Market Penetration, Charging Characteristics, and Advanced Technologies. In Proceedings of the 2013 World Electric Vehicle Symposium and Exhibition (EVS27), Barcelona, Spain, 17–20 November 2013; pp. 1–11. [Google Scholar]
- Tomaszewska, A.; Chu, Z.; Feng, X.; O’Kane, S.; Liu, X.; Chen, J.; Ji, C.; Endler, E.; Li, R.; Liu, L.; et al. Lithium-ion battery fast charging: A review. eTransportation 2019, 1, 100011. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Luo, Y.-F. Search for an Optimal Rapid-Charging Pattern for Li-Ion Batteries Using the Taguchi Approach. IEEE Trans. Ind. Electron. 2010, 57, 3963–3971. [Google Scholar] [CrossRef]
- Waldmann, T.; Kasper, M.; Wohlfahrt-Mehrens, M. Optimization of Charging Strategy by Prevention of Lithium Deposition on Anodes in high-energy Lithium-ion Batteries–Electrochemical Experiments. Electrochim. Acta 2015, 178, 525–532. [Google Scholar] [CrossRef]
- Abdel Monem, M.; Trad, K.; Omar, N.; Hegazy, O.; Mantels, B.; Mulder, G.; Van den Bossche, P.; Van Mierlo, J. Lithium-ion batteries: Evaluation study of different charging methodologies based on aging process. Appl. Energy 2015, 152, 143–155. [Google Scholar] [CrossRef]
- Monem, M.A.; Trad, K.; Omar, N.; Hegazy, O.; Mantels, B. A Comparative Study of Different Fast Charging methodologies for Lithium-Ion Batteries Based on Aging Process. Available online: https://www.researchgate.net/publication/276057968_A_comparative_study_of_different_fast_charging_methodologies_for_lithium-ion_batteries_based_on_aging_process (accessed on 18 May 2021).
- Keil, P. Charging protocols for lithium-ion batteries and their impact on cycle life—An experimental study with different 18650 high-power cells. J. Energy Storage 2016, 17, 125–141. [Google Scholar] [CrossRef]
- Li, J.; Murphy, E.; Winnick, J.; Kohl, P.A. The effects of pulse charging on cycling characteristics of commercial lithium-ion batteries. J. Power Sources 2001, 102, 302–309. [Google Scholar] [CrossRef]
- Cho, I.-H.; Lee, P.-Y.; Kim, J.-H. Analysis of the Effect of the Variable Charging Current Control Method on Cycle Life of Li-ion Batteries. Energies 2019, 12, 3023. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W. Effects of external mechanical loading on stress generation during lithiation in Li-ion battery electrodes. Electrochim. Acta 2015, 185, 28–33. [Google Scholar] [CrossRef] [Green Version]
- Christensen, J.; Newman, J. A Mathematical Model of Stress Generation and Fracture in Lithium Manganese Oxide. J. Electrochem. Soc. 2006, 153, A1019. [Google Scholar] [CrossRef]
- Zhou, W.; Hao, F.; Fang, D. The effects of elastic stiffening on the evolution of the stress field within a spherical electrode particle of lithium-ion batteries. Int. J. Appl. Mech. 2013, 5, 1350040. [Google Scholar] [CrossRef]
- Guo, Z.; Zhu, J.; Feng, J.; Du, S. Direct in situ observation and explanation of lithium dendrite of commercial graphite electrodes. RSC Adv. 2015, 5, 69514–69521. [Google Scholar] [CrossRef]
- Petzl, M.; Kasper, M.; Danzer, M.A. Lithium plating in a commercial lithium-ion battery–A low-temperature aging study. J. Power Sources 2015, 275, 799–807. [Google Scholar] [CrossRef]
- von Lüders, C.; Zinth, V.; Erhard, S.V.; Osswald, P.J.; Hofmann, M.; Gilles, R.; Jossen, A. Lithium plating in lithium-ion batteries investigated by voltage relaxation and in situ neutron diffraction. J. Power Sources 2017, 342, 17–23. [Google Scholar] [CrossRef]
- Campbell, I.D.; Marzook, M.; Marinescu, M.; Offer, G.J. How Observable Is Lithium Plating? Differential Voltage Analysis to Identify and Quantify Lithium Plating Following Fast Charging of Cold Lithium-Ion Batteries. J. Electrochem. Soc. 2019, 166, A725–A739. [Google Scholar] [CrossRef]
- Bloom, I.; Christophersen, J.; Gering, K. Differential voltage analyses of high-power lithium-ion cells. J. Power Sources 2005, 139, 304–313. [Google Scholar] [CrossRef]
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.-C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in lithium-ion batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Rangarajan, S.P.; Barsukov, Y.; Mukherjee, P.P. Anode potential controlled charging prevents lithium plating. J. Mater. Chem. A 2020, 8, 13077–13085. [Google Scholar] [CrossRef]
- Ahmed, S.; Bloom, I.; Jansen, A.N.; Tanim, T.; Dufek, E.J.; Pesaran, A.; Burnham, A.; Carlson, R.B.; Dias, F.; Hardy, K.; et al. Enabling fast charging—A battery technology gap assessment. J. Power Sources 2017, 367, 250–262. [Google Scholar] [CrossRef]
- Koleti, U.R.; Dinh, T.Q.; Marco, J. A new on-line method for lithium plating detection in lithium-ion batteries. J. Power Sources 2020, 451, 227798. [Google Scholar] [CrossRef]
- Bitzer, B.; Gruhle, A. A new method for detecting lithium plating by measuring the cell thickness. J. Power Sources 2014, 262, 297–302. [Google Scholar] [CrossRef]




| Electric Vehicle | Pack Energy [KWh] | Peak Power [kW] | Charging Time Up to SoC Equal to 80% [min] |
|---|---|---|---|
| Peugeot 208 | 50 | 100 | 30 |
| Fiat 500 | 42 | 85 | 35 |
| Renault Zoe 2 | 50 | 50 | 65 |
| Honda E | 35.5 | 50 | 31 |
| Kia Niro | 64 | 100 | 60 |
| Volkswagen ID 3 | 45 | 100 | 27 |
| 58 | 35 |
| Property | Cathode (NMC 811) | Anode (Si-C) |
|---|---|---|
| Particle size distribution (µm) * | 5–15 | 10–20 |
| Mass Loading (mg/cm2) | 43 | 24 |
| Porosity (%) ** | 29–31 | 21–24 |
| Specific Capacity (mAh/g) | ~200 | ~408 |
| C-Rate | Cutoff Voltage [V] |
|---|---|
| MCC1 | |
| From 0.500 to 1.090 | Slope = 6.6 × 10−3 |
| 1.090 | 3.910 |
| 0.800 | 4.000 |
| 0.600 | 4.020 |
| 0.500 | 4.092 |
| MCC2 | |
| 1.090 | 3.850 |
| 1.038 | 3.910 |
| 0.750 | 4.000 |
| 0.560 | 4.020 |
| 0.500 | 4.092 |
| MCC Fast1 | |
| 1.320 | 3.850 |
| 1.038 | 3.910 |
| 0.756 | 4.000 |
| 0.500 | 4.092 |
| MCC Fast2 | |
| 2.000 | 3.850 |
| 1.750 | 3.900 |
| 1.500 | 3.940 |
| 0.750 | 3.990 |
| 0.500 | 4.092 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dotoli, M.; Milo, E.; Giuliano, M.; Rocca, R.; Nervi, C.; Baricco, M.; Ercole, M.; Sgroi, M.F. Detection of Lithium Plating in Li-Ion Cell Anodes Using Realistic Automotive Fast-Charge Profiles. Batteries 2021, 7, 46. https://doi.org/10.3390/batteries7030046
Dotoli M, Milo E, Giuliano M, Rocca R, Nervi C, Baricco M, Ercole M, Sgroi MF. Detection of Lithium Plating in Li-Ion Cell Anodes Using Realistic Automotive Fast-Charge Profiles. Batteries. 2021; 7(3):46. https://doi.org/10.3390/batteries7030046
Chicago/Turabian StyleDotoli, Matteo, Emanuele Milo, Mattia Giuliano, Riccardo Rocca, Carlo Nervi, Marcello Baricco, Massimiliano Ercole, and Mauro Francesco Sgroi. 2021. "Detection of Lithium Plating in Li-Ion Cell Anodes Using Realistic Automotive Fast-Charge Profiles" Batteries 7, no. 3: 46. https://doi.org/10.3390/batteries7030046
APA StyleDotoli, M., Milo, E., Giuliano, M., Rocca, R., Nervi, C., Baricco, M., Ercole, M., & Sgroi, M. F. (2021). Detection of Lithium Plating in Li-Ion Cell Anodes Using Realistic Automotive Fast-Charge Profiles. Batteries, 7(3), 46. https://doi.org/10.3390/batteries7030046








