A Performance and Cost Overview of Selected Solid-State Electrolytes: Race between Polymer Electrolytes and Inorganic Sulfide Electrolytes
Abstract
1. Introduction
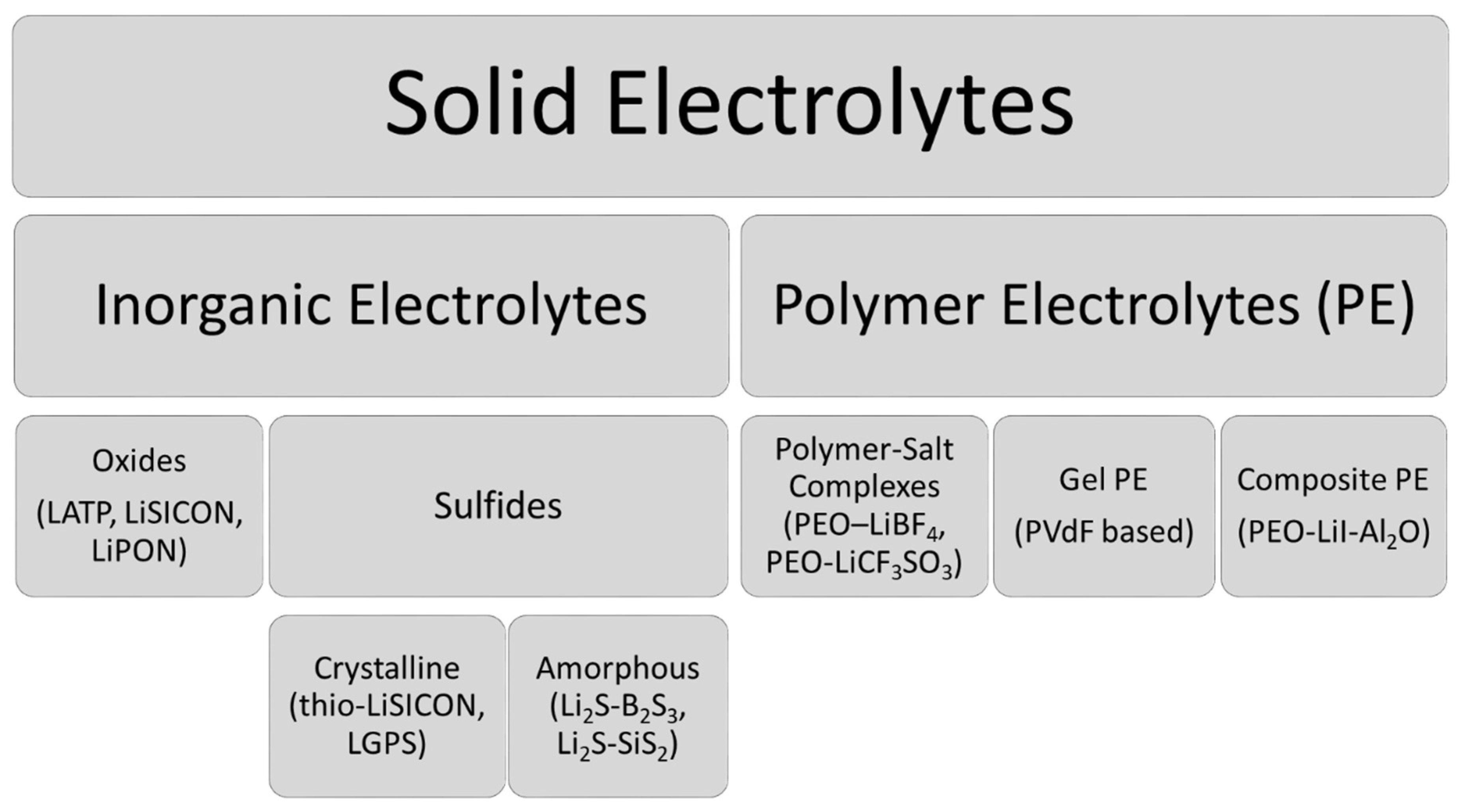
2. Solid Electrolytes
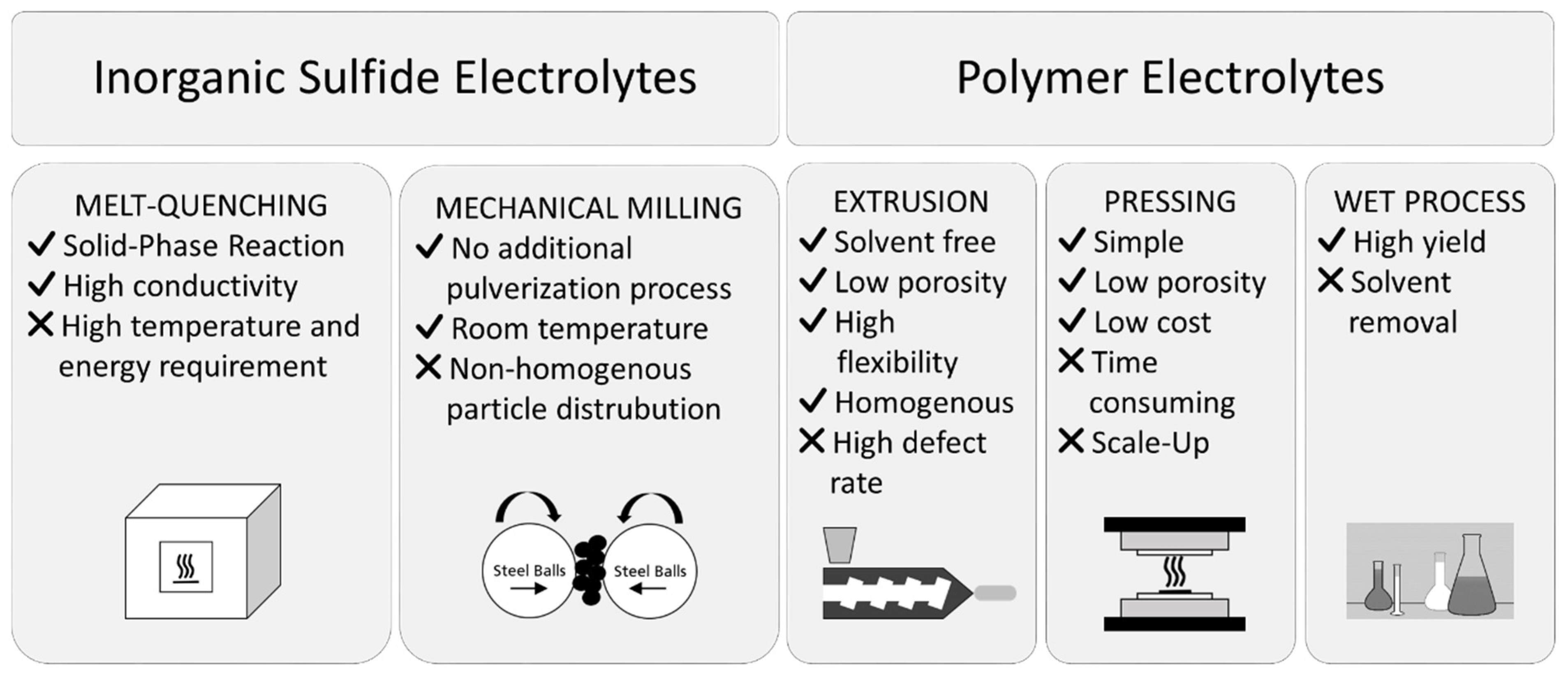
2.1. Inorganic Sulfide Electrolytes
2.2. Solid Polymer Electrolytes
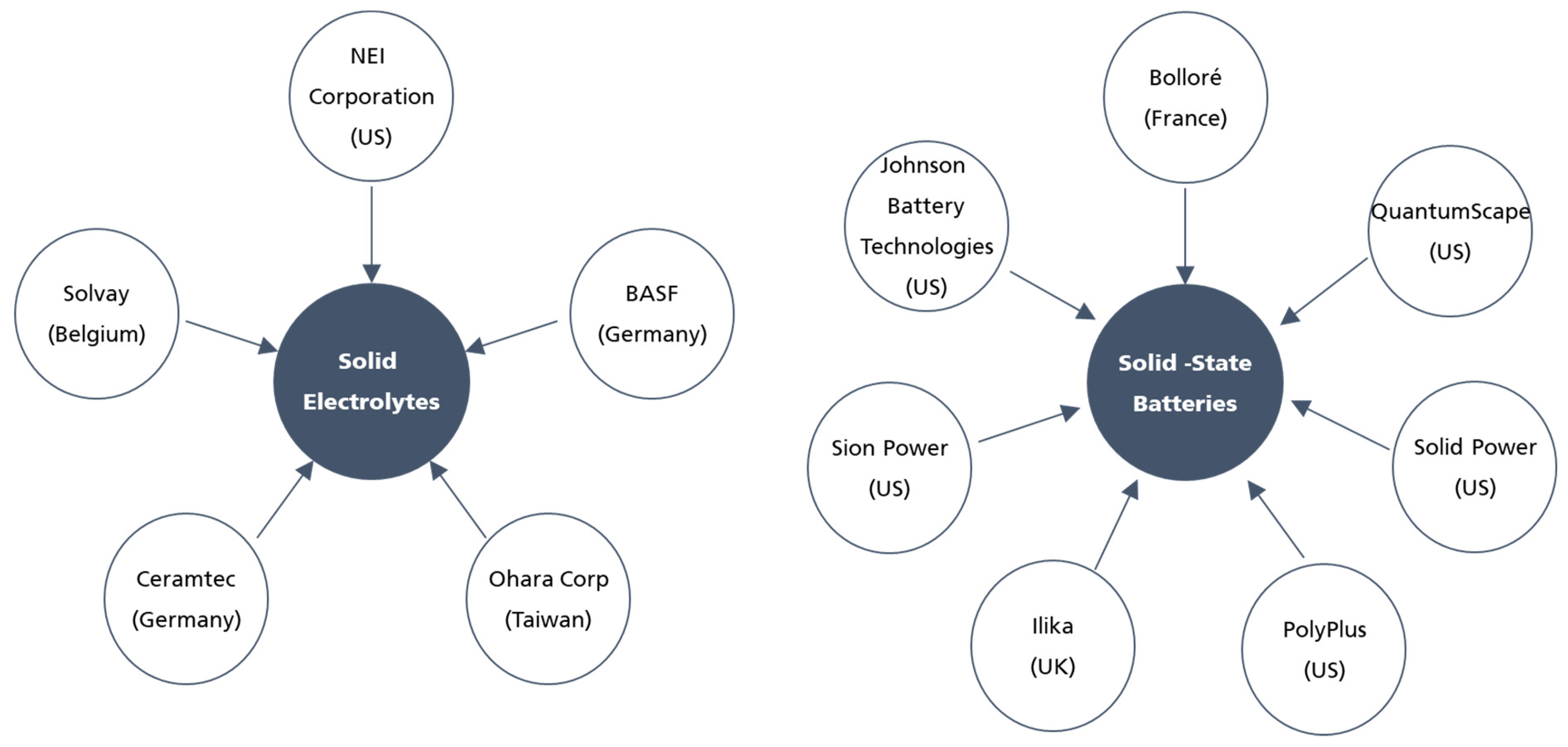
3. Choosing the Best Electrolyte: Commercialization and Mass Production Challenges
4. Summary and Outlook
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Whittingham, M.S. Electrical energy storage and intercalation chemistry. Science 1976, 192, 1126–1127. [Google Scholar] [CrossRef] [PubMed]
- Nishi, Y. Lithium ion secondary batteries; past 10 years and the future. J. Power Sources 2001, 100, 101–106. [Google Scholar] [CrossRef]
- Xiang, H.; Zhang, K.; Ji, G.; Lee, J.Y.; Zou, C.; Chen, X.; Wu, J. Graphene/nanosized silicon composites for Lithium battery anodes with improved cycling stability. Carbon 2011, 49, 1787–1796. [Google Scholar] [CrossRef]
- Chakrapani, V.; Rusli, F.; Filler, M.A.; Kohl, P.A. Silicon nanowire anode: Improved battery life with capacity-limited cycling. J. Power Sources 2012, 205, 433–438. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.-G.; Xu, W. Advancing Lithium Metal Batteries. Joule 2018, 2, 833–845. [Google Scholar] [CrossRef]
- Wanga, L.; Zhoua, Z.; Yan, X.; Hou, F.; Wen, L.; Luo, W.; Liang, J.; Dou, S.X. Engineering of Lithium-metal anodes towards a safe and stable battery. Energy Storage Mater. 2018, 14, 22–48. [Google Scholar] [CrossRef]
- Lau, J.; DeBlock, R.H.; Butts, D.M.; Ashby, D.S.; Choi, C.S.; Dunn, B.S. Sulfide Solid Electrolytes for Lithium Battery Applications. Adv. Energy Mater. 2018, 8, 1800933. [Google Scholar] [CrossRef]
- TechVision Group. Innovations in Solid State Batteries: Need for Safer Alternatives Drives Innovations in Solid State Batteries; Frost & Sullivan: Santa Clara, CA, USA, 2018. [Google Scholar]
- Cao, C.; Li, Z.-B.; Wang, X.-L.; Zhao, X.-B.; Han, W.-Q. Recent Advances in Inorganic Solid Electrolytes for Lithium Batteries. Front. Energy Res. 2014, 2, 25. [Google Scholar] [CrossRef]
- Aono, H.; Sugimoto, E.; Sadaoka, Y.; Imanaka, N.; Adachi, G.-Y. Ionic Conductivity of the Lithium Titanium Phosphate (Li1 + X M X Ti2 − X (PO4)3 M = Al Sc Y and La) Systems. J. Electrochem. Soc. 1989, 136, 590. [Google Scholar] [CrossRef]
- Xia, W.; Xu, B.; Duan, H.; Guo, Y.; Kang, H.; Li, H.; Liu, H. Ionic Conductivity and Air Stability of Al-Doped Li7La3Zr1O12 Sintered in Alumina and Pt Crucibles. ACS Appl. Mater. Interfaces 2016, 8, 5335–5342. [Google Scholar] [CrossRef]
- Xia, S.; Wu, X.; Zhang, Z.; Cui, Y.; Liu, W. Practical Challenges and Future Perspectives of All-Solid-State Lithium-Metal Batteries. Chem 2019, 5, 753–785. [Google Scholar] [CrossRef]
- Minafra, N.; Culver, S.P.; Li, C.; Senyshyn, A.; Zeier, W.G. Influence of the Lithium Substructure on the Diffusion Pathways and Transport Properties of the Thio-LISICON Li 4 Ge 1– x Sn x S 4. Chem. Mater. 2019, 31, 3794–3802. [Google Scholar] [CrossRef]
- Rao, R.P.; Adams, S. Studies of Lithium argyrodite solid electrolytes for all-solid-state batteries. Phys. Status Solidi A 2011, 208, 1804–1807. [Google Scholar] [CrossRef]
- Tatsumisago, M.; Nagao, M.; Hayashi, A. Recent development of sulfide solid electrolytes and interfacial modification for all-solid-state rechargeable Lithium batteries. J. Asian Ceram. Soc. 2013, 1, 17–25. [Google Scholar] [CrossRef]
- Sakuda, A.; Hayashi, A.; Tatsumisago, M. Sulfide Solid Electrolyte with Favorable Mechanical Property for All-Solid-State Lithium Battery. Sci. Rep. 2013, 3, 1–5. [Google Scholar] [CrossRef]
- Zhao, Q.; Stalin, S.; Zhao, C.-Z.; Archer, L.A. Designing solid-state electrolytes for safe, energy-dense batteries. Nat. Rev. Mater. 2020, 5, 229–252. [Google Scholar] [CrossRef]
- Barai, P.; Higa, K.; Srinivasan, V. Lithium dendrite growth mechanisms in polymer electrolytes and prevention strategies. Phys. Chem. Chem. Phys. 2017, 19, 20493–20505. [Google Scholar] [CrossRef] [PubMed]
- Fergus, J.W. Ceramic and polymeric solid electrolytes for Lithium-ion batteries. J. Power Sources 2010, 195, 4554–4569. [Google Scholar] [CrossRef]
- Lagadec, M.F.; Zahn, R.; Wood, V. Characterization and performance evaluation of Lithium-ion battery separators. Nat. Energy 2019, 4, 16–25. [Google Scholar] [CrossRef]
- Kanno, R.; Murayama, M. Lithium Ionic Conductor Thio-LISICON: The Li2 S-GeS2-P 2 S 5 System. J. Electrochem. Soc. 2001, 148, A742. [Google Scholar] [CrossRef]
- Kamaya, N.; Homma, K.; Yamakawa, Y.; Hirayama, M.; Kanno, R.; Yonemura, M.; Kamiyama, T.; Kato, Y.; Hama, S.; Kawamoto, K.; et al. A Lithium superionic conductor. Nat. Mater. 2011, 10, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hu, J.; Chu, Y.; Wan, W.; Zhao, L.; Zhu, Y. Electrochemical performance of sulfide solid electrolyte Li10GeP2S12 synthesized by a new method. Mater. Lett. 2019, 248, 153–156. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, S.; Yang, J.; Wang, J.; Yao, L.; Yao, X.; Cui, P.; Xu, X. Interface Re-Engineering of Li10GeP2S12 Electrolyte and Lithium anode for All-Solid-State Lithium Batteries with Ultralong Cycle Life. ACS Appl. Mater. Interfaces 2018, 10, 2556–2565. [Google Scholar] [CrossRef]
- Wenzel, S.; Randau, S.; Leichtweiß, T.; Weber, D.A.; Sann, J.; Zeier, W.G.; Janek, J. Direct Observation of the Interfacial Instability of the Fast Ionic Conductor Li10GeP2S12 at the Lithium Metal Anode. Chem. Mater. 2016, 28, 2400–2407. [Google Scholar] [CrossRef]
- Woo, J.H.; Trevey, J.E.; Cavanagh, A.S.; Choi, Y.S.; Kim, S.C.; George, S.M.; Oh, K.H.; Lee, S.H. Nanoscale Interface Modification of LiCoO2 by Al2O3 Atomic Layer Deposition for Solid-State Li Batteries. J. Electrochem. Soc. 2012, 159, A1120. [Google Scholar] [CrossRef]
- Xie, D.; Chen, S.; Zhang, Z.; Ren, J.; Yao, L.; Wu, L.; Yao, X.; Xu, X. High ion conductive Sb2O5-doped β-Li3PS4 with excellent stability against Li for all-solid-state Lithium batteries. J. Power Sources 2018, 389, 140–147. [Google Scholar] [CrossRef]
- Yao, X.; Liu, D.; Wang, C.; Long, P.; Peng, G.; Hu, Y.S.; Li, H.; Chen, L.; Xu, X. High-Energy All-Solid-State Lithium Batteries with Ultralong Cycle Life. Nano Lett. 2016, 16, 7148–7154. [Google Scholar] [CrossRef]
- Zhang, Q.; Peng, G.; Mwizerwa, J.P.; Wan, H.; Cai, L.; Xu, X.; Yao, X. Nickel sulfide anchored carbon nanotubes for all-solid-state Lithium batteries with enhanced rate capability and cycling stability. J. Mater. Chem. A 2018, 6, 12098–12105. [Google Scholar] [CrossRef]
- Kato, Y.; Hori, S.; Saito, T.; Suzuki, K.; Hirayama, M.; Mitsui, A.; Yonemura, M.; Iba, H.; Kanno, R. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 2016, 1, 1–7. [Google Scholar] [CrossRef]
- Kato, Y.; Hori, S.; Kanno, R. Li10GeP2S12—Type Superionic Conductors: Synthesis, Structure, and Ionic Transportation. Adv. Energy Mater. 2020, 10. [Google Scholar] [CrossRef]
- Ruiz, A.G.; Sola, P.C.; Palmerola, N.M. Advanced Material and Device Applications with Germanium: Germanium: Current and Novel Recovery Processes; InTech Open: London, UK, 2018. [Google Scholar]
- Kato, Y.; Saito, R.; Sakano, M.; Mitsui, A.; Hirayama, M.; Kanno, R. Synthesis, structure and Lithium ionic conductivity of solid solutions of Li10(Ge1−xMx)P2S12 (M = Si, Sn). J. Power Sources 2014, 271, 60–64. [Google Scholar] [CrossRef]
- Deiseroth, H.J.; Kong, S.T.; Eckert, H.; Vannahme, J.; Reiner, C.; Zaiß, T.; Schlosser, M. Li6PS5X: A Class of Crystalline Li-Rich Solids With an Unusually High Li+ Mobility. Angew. Chem. 2008, 120, 767–770. [Google Scholar] [CrossRef]
- Hanghofer, I.; Brinek, M.; Eisbacher, S.L.; Bitschnau, B.; Volck, M.; Hennige, V.; Hanzu, I.; Rettenwander, D.; Wilkening, H.M.R. Substitutional disorder: Structure and ion dynamics of the argyrodites Li6PS5Cl, Li6PS5Br and Li6PS5I. Phys. Chem. Chem. Phys. 2019, 21, 8489–8507. [Google Scholar] [CrossRef]
- Zhou, L.; Park, K.H.; Sun, X.; Lalѐre, F.; Adermann, T.; Hartmann, P.; Nazar, L.F. Solvent-engineered design of argyrodite Li6PS5X (X = Cl, Br, I) solid electrolytes with high ionic conductivity. ACS Energy Lett. 2018, 4, 265–270. [Google Scholar] [CrossRef]
- Boulineau, S.; Courty, M.; Tarascon, J.; Viallet, V. Mechanochemical synthesis of Li-argyrodite Li6PS5X (X=Cl, Br, I) as sulfur-based solid electrolytes for all solid state batteries application. Solid State Ion. 2012, 221, 1–5. [Google Scholar] [CrossRef]
- Kasemchainan, J.; Zekoll, S.; Jolly, D.S.; Ning, Z.; Hartley, G.O.; Marrow, J.; Bruce, P.G. Critical stripping current leads to dendrite formation on plating in Lithium anode solid electrolyte cells. Nat. Mater 2019, 18, 1105–1111. [Google Scholar] [CrossRef]
- Zhou, Y.; Doerrer, C.; Kasemchainan, J.; Bruce, P.G.; Pasta, M.; Hardwick, L.J. Observation of Interfacial Degradation of Li6PS5Cl against Lithium Metal and LiCoO2 via In Situ Electrochemical Raman Microscopy. Batter Supercaps 2020, 3, 647–652. [Google Scholar] [CrossRef]
- Viallet, V.; Tarascon, J.-M.; Boulineau, S. Improvement of Electrochemical Performances of All-Solid-State Argyrodite-Based Lithium Batteries. In Proceedings of the 17th International Meeting on Lithium Batteries, Como, Italy, 10–14 June 2014; p. 757. [Google Scholar] [CrossRef]
- Lee, Y.G.; Fujiki, S.; Jung, C.; Suzuki, N.; Yashiro, N.; Omoda, R.; Ko, D.S.; Shiratsuchi, T.; Sugimoto, T.; Ryu, S.; et al. High-energy long-cycling all-solid-state Lithium metal batteries enabled by silver–carbon composite anodes. Nat. Energy 2020, 5, 299–308. [Google Scholar] [CrossRef]
- Wu, Z.; Xie, Z.; Yoshida, A.; Wang, Z.; Hao, X.; Abudula, A.; Guan, G. Utmost limits of various solid electrolytes in all-solid-state Lithium batteries: A critical review. Renew. Sustain. Energy Rev. 2019, 109, 367–385. [Google Scholar] [CrossRef]
- Cheng, S.H.S.; He, K.Q.; Liu, Y.; Zha, J.W.; Kamruzzaman, M.; Ma, R.L.W.; Dang, Z.M.; Li, R.K.; Chung, C.Y. Electrochemical performance of all-solid-state Lithium batteries using inorganic Lithium garnets particulate reinforced PEO/LiClO4 electrolyte. Electrochim. Acta 2017, 253, 430–438. [Google Scholar] [CrossRef]
- Gorecki, W.; Jeannin, M.; Belorizky, E.; Roux, C.; Armand, M. Physical properties of solid polymer electrolyte PEO(LiTFSI) complexes. J. Phys. Condens. Matter 1995, 7, 6823. [Google Scholar] [CrossRef]
- Rey, I.; Lassègues, J.C.; Grondin, J.; Servant, L. Infrared and Raman study of the PEO-LiTFSI polymer electrolyte. Electrochim. Acta 1998, 43, 1505–1510. [Google Scholar] [CrossRef]
- Bouchet, R.; Lascaud, S.; Rosso, M. An EIS Study of the Anode Li/PEO-LiTFSI of a Li Polymer Battery. J. Electrochem. Soc. 2003, 150, A1385. [Google Scholar] [CrossRef]
- Stolwijk, N.A.; Wiencierz, M.; Heddier, C.; Kösters, J. What can we learn from ionic conductivity measurements in polymer electrolytes? A case study on poly(ethylene oxide) (PEO)-NaI and PEO-LiTFSI. J. Phys. Chem. B 2012, 116, 3065–3074. [Google Scholar] [CrossRef] [PubMed]
- Marzantowicz, M.; Dygas, J.R.; Krok, F. Impedance of interface between PEO:LiTFSI polymer electrolyte and blocking electrodes. Electrochim. Acta 2008, 53, 7417–7425. [Google Scholar] [CrossRef]
- Molinari, N.; Mailoa, J.P.; Kozinsky, B. Effect of Salt Concentration on Ion Clustering and Transport in Polymer Solid Electrolytes: A Molecular Dynamics Study of PEO–LiTFSI. Chem. Mater. 2018, 30, 6298–6306. [Google Scholar] [CrossRef]
- Utpalla, P.; Sharma, S.K.; Sudarshan, K.; Sahu, M.; Pujari, P.K. Investigation of the free volume characteristics of PEO based solid state polymer electrolyte by means of positron annihilation spectroscopy. Solid State Ion. 2019, 339, 114990. [Google Scholar] [CrossRef]
- Wan, J.; Xie, J.; Kong, X.; Liu, Z.; Liu, K.; Shi, F.; Pei, A.; Chen, H.; Chen, W.; Chen, J. Ultrathin, flexible, solid polymer composite electrolyte enabled with aligned nanoporous host for Lithium batteries. Nat. Nanotechnol. 2019, 14, 705–711. [Google Scholar] [CrossRef]
- Qiu, J.; Yang, L.; Sun, G.; Yu, X.; Li, H.; Chen, L. A stabilized PEO-based solid electrolyte via a facile interfacial engineering method for a high voltage solid-state Lithium metal battery. Chem. Commun. 2020, 56, 5633–5636. [Google Scholar] [CrossRef] [PubMed]
- Wurster, V.; Engel, C.; Graebe, H.; Ferber, T.; Jaegermann, W.; Hausbrand, R. Characterization of the Interfaces in LiFePO4/PEO-LiTFSI Composite Cathodes and to the Adjacent Layers. J. Electrochem. Soc. 2019, 166, A5410. [Google Scholar] [CrossRef]
- Chen, H.; Adekoya, D.; Hencz, L.; Ma, J.; Chen, S.; Yan, C.; Zhao, H.; Cui, G.; Zhang, S. Stable Seamless Interfaces and Rapid Ionic Conductivity of Ca—CeO2/LiTFSI/PEO Composite Electrolyte for High—Rate and High—Voltage All—Solid—State Battery. Adv. Energy Mater. 2020, 10. [Google Scholar] [CrossRef]
- Chen, R.J.; Zhang, Y.B.; Liu, T.; Xu, B.Q.; Lin, Y.H.; Nan, C.W.; Shen, Y. Addressing the Interface Issues in All-Solid-State Bulk-Type Lithium Ion Battery via an All-Composite Approach. ACS Appl. Mater. Interfaces 2017, 9, 9654–9661. [Google Scholar] [CrossRef]
- Porcarelli, L.; Aboudzadeh, M.A.; Rubatat, L.; Nair, J.R.; Shaplov, A.S.; Gerbaldi, C.; Mecerreyes, D. Single-ion triblock copolymer electrolytes based on poly(ethylene oxide) and methacrylic sulfonamide blocks for Lithium metal batteries. J. Power Sources 2017, 364, 191–199. [Google Scholar] [CrossRef]
- Wakayama, H.; Yonekura, H.; Kawai, Y. Three-Dimensional Bicontinuous Nanocomposite from a Self-Assembled Block Copolymer for a High-Capacity All-Solid-State Lithium Battery Cathode. Chem. Mater. 2016, 28, 4453–4459. [Google Scholar] [CrossRef]
- Hovington, P.; Lagacé, M.; Guerfi, A.; Bouchard, P.; Mauger, A.; Julien, C.M.; Armand, M.; Zaghib, K. New Lithium metal polymer solid state battery for an ultrahigh energy: Nano C-LiFePO4 versus nano Li1.2V3O8. Nano Lett. 2015, 15, 2671–2678. [Google Scholar] [CrossRef] [PubMed]
- Bouchet, R.; Maria, S.; Meziane, R.; Aboulaich, A.; Lienafa, L.; Bonnet, J.P.; Phan, T.N.; Bertin, D.; Gigmes, D.; Devaux, D.; et al. Single-ion BAB triblock copolymers as highly efficient electrolytes for Lithium-metal batteries. Nat. Mater. 2013, 12, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Randau, S.; Weber, D.A.; Kötz, O.; Koerver, R.; Braun, P.; Weber, A.; Ivers-Tiffée, E.; Adermann, T.; Kulisch, J.; Zeier, W.G.; et al. Benchmarking the performance of all-solid-state Lithium batteries. Nat. Energy 2020, 5, 259–270. [Google Scholar] [CrossRef]
- Homann, G.; Stolz, L.; Nair, J.; Laskovic, I.C.; Winter, M.; Kasnatscheew, J. Poly(Ethylene Oxide)-based Electrolyte for Solid-State-Lithium-Batteries with High Voltage Positive Electrodes: Evaluating the Role of Electrolyte Oxidation in Rapid Cell Failure. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, N.; Zhang, M.; Li, Y.; Chu, P.K.; Guo, X.; Di, Z.; Wang, X.; Li, H. Flexible and ion-conducting membrane electrolytes for solid-state Lithium batteries: Dispersion of garnet nanoparticles in insulating polyethylene oxide. Nano Energy 2016, 28, 447–454. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Zhang, N.; Liu, J.; Cong, L.; Liu, J.; Sun, L.; Mauger, A.; Julien, C.M.; Xie, H.; et al. Synthesis and interface stability of polystyrene-poly(ethylene glycol)-polystyrene triblock copolymer as solid-state electrolyte for Lithium-metal batteries. J. Power Sources 2019, 428, 93–104. [Google Scholar] [CrossRef]
- Mauger, A.; Armand, M.; Julien, C.M.; Zaghib, K. Challenges and issues facing Lithium metal for solid-state rechargeable batteries. J. Power Sources 2017, 353, 333–342. [Google Scholar] [CrossRef]
- Chen, R.; Li, Q.; Yu, X.; Chen, L.; Li, H. Approaching Practically Accessible Solid-State Batteries: Stability Issues Related to Solid Electrolytes and Interfaces. Chem. Rev. 2020, 120, 6820–6877. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Z.; Zou, J.; Gao, P.; Niu, X.; Li, H.; Chen, L. Li-free Cathode Materials for High Energy Density Lithium Batteries. Joule 2019, 3, 2086–2102. [Google Scholar] [CrossRef]
- Takeda, Y.; Yamamoto, O.; Imanishi, N. Lithium Dendrite Formation on a Lithium Metal Anode from Liquid, Polymer and Solid Electrolytes. Electrochemistry 2016, 84, 210–218. [Google Scholar] [CrossRef]
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and cost of materials for Lithium-based rechargeable automotive batteries. Nat. Energy 2018, 3, 267–278. [Google Scholar] [CrossRef]
- Schnell, J.; Günther, T.; Knoche, T.; Vieider, C.; Köhler, L.; Just, A.; Keller, M.; Passerini, S.; Reinhart, G. All-solid-state Lithium-ion and Lithium metal batteries—Paving the way to large-scale production. J. Power Sources 2018, 382, 160–175. [Google Scholar] [CrossRef]
- Zhang, T.; He, W.; Zhang, W.; Wang, T.; Li, P.; Sun, Z.; Yu, X. Designing composite solid-state electrolytes for high performance Lithium ion or Lithium metal batteries. Chem. Sci. 2020, 11, 8686–8707. [Google Scholar] [CrossRef]
- Zhu, Y.; Mo, Y. Materials Design Principles for Air—Stable Lithium/Sodium Solid Electrolytes. Angew. Chem. Int. Ed. 2020, 59, 17472–17476. [Google Scholar] [CrossRef]
- Bernhardt, W.; Gabaldon, D.; Zheng, R.; Schmitt, P.; Hotz, T.; Braun, S.; Kampker, A.; Offermanns, C.; Krämer, S. Rising Opportunities for Battery Equipment Manufacturers: Boom in Electric Cars and Li-Ion Batteries Shifts Manufacturing Equipment into Drive; Roland Berger GmbH: Munich, Germany, 2020. [Google Scholar]
- Schnell, J.; Knörzer, H.; Imbsweiler, A.J.; Reinhart, G. Solid versus Liquid—A Bottom—Up Calculation Model to Analyze the Manufacturing Cost of Future High—Energy Batteries. Energy Technol. 2020, 8, 1901237. [Google Scholar] [CrossRef]
- TechVision Analysis. Technological Advancements in Solid State Batteries for Electric Vehicles: Game-Changing Solid-state Batteries Will Push the Future Electric Vehicles to the Next Level. 2020. Available online: https://www.globenewswire.com/news-release/2021/01/27/2164871/0/en/Global-Solid-State-Batteries-for-Electric-Vehicles-Market-Report-2020-Game-Changing-Solid-state-Batteries-Will-Push-the-Future-Electric-Vehicles-to-the-Next-Level.html (accessed on 1 December 2020).
- Frost Perspectives. Solid-State Electrolytes—Next-Generation Safer Alternative in Li-Ion Batteries. 2017. Available online: https://ww2.frost.com/frost-perspectives/solid-state-electrolytes-next-generation-safer-alternative-li-ion-batteries/ (accessed on 1 December 2020).
- Chen, A.; Qu, C.; Shi, Y.; Shi, F. Manufacturing Strategies for Solid Electrolyte in Batteries. Front. Energy Res. 2020, 8, 226. [Google Scholar] [CrossRef]
- Hayashi, A.; Sakuda, A.; Tatsumisago, M. Development of Sulfide Solid Electrolytes and Interface Formation Processes for Bulk-Type All-Solid-State Li and Na Batteries. Front. Energy Res. 2016, 4, 25. [Google Scholar] [CrossRef]
| Solid Inorganic Electrolyte | Cathode | Energy Density (Wh kg−1) | Full Cell Capacity (mAh g−1) | Cycle | Reference |
|---|---|---|---|---|---|
| Li10GeP2S12 | LCO-LiNbO3 | - | 114 (at 0.12 C) | 8 | [22] |
| Li10GeP2S12//Li9.6P3S12 | LCO-LiNbO3 | 33 | 114 (at 0.12 C) | 30 | [30] |
| Li10GeP2S12//Li2S-P2S5 | NiS-CNT | - | 170 | 150 | [29] |
| Li10GeP2S12//Li2S-P2O5 | LCO-Li7P3S11 | 17 | 647 (at 0.06C) | 1000 | [28] |
| Li10GeP2S12//Li3P0.98Sb0.02S3.95O0.05 | LCO-LiNbO3 | 14 | 134 (at 0.09C) | 50 | [27] |
| Li3.15Ge0.15P0.85S4//Li2S-P2S5 | LCO-Al2O3 | 7 | 121 (at 0.13C) | 25 | [26] |
| Solid Polymer Electrolyte | Cathode | Energy Density (Wh kg−1) | Full Cell Capacity (mAh g−1) | Cycle | Ref. |
|---|---|---|---|---|---|
| Ca–CeO2/LiTFSI/PEO | LFP | - | 157 (at 0.2C) | 40 | [54] |
| PEO/LiTFSI/ Al-Li6.75La3Zr1.75Ta0.25 O12 | LFP-In2O5Sn | 334 (Wh l−1) | 155 (at 0.01C) | 10 | [55] |
| poly(-LiMTFSI)-b-PEO-b-poly(LiMTFSI) | LFP | 168 | 158 (at 0.1C) | 300 | [56] |
| PEO-LiTFSI | LCO-Li7La3Zr2O12 | 141 | 136 (at 0.05C) | 20 | [57] |
| poly(LiTFSI)-b-PEO-b-poly(LiTFSI) | LFP | 282 | 158 (at 0.36C) | 1400 | [58] |
| poly(STFSILi)-b-PEO-b-poly(STFSILi) | LFP | 120 | 162 (at 0.07C) | - | [59] |
| Solid Electrolyte (SE) | Conductivity (S cm−1) | Cost of SE from Producers ($/10 g) | Cost of Raw Materials ($) | Amount (g) | Ref. | |
|---|---|---|---|---|---|---|
| Ca–CeO2/LiTFSI/PEO | 1.3 × 10−4 (60 °C) | Not commercial | Ce(NO3)3 6H2O | 356 | 100 | [54] |
| Ca(NO3)2·4H2O | 15 | 500 | ||||
| PVP | 54 | 100 | ||||
| LiTFSI | 198 | 100 | ||||
| PEO | 70 | 100 | ||||
| PEO/LiTFSI/ Al-Li6.75La3Zr1.75Ta0.25 O12 | 2.4 × 10−4 (30 °C) | Not commercial | LiOH · H2O | 150 | 25 | [55] |
| La2O3 | 75 | 100 | ||||
| ZrO2 | 45 | 100 | ||||
| Ta2O5 | 260 | 50 | ||||
| Al2O3 | 30 | 100 | ||||
| LiTFSI | 198 | 100 | ||||
| PEO | 70 | 100 | ||||
| Li10GeP2S12 | 1.2 × 10−2 (at RT) | 695 | L2S | 670 | 50 | [67] |
| P2S5 | 34 | 100 | ||||
| GeS2 | 500 | 1 | ||||
| Li6PS5Cl | 10−4 (at RT) | 360 | L2S | 670 | 50 | [7] |
| P2S5 | 34 | 100 | ||||
| LiCl | 70 | 100 | ||||
| Liquid electrolyte | 10−2 (at RT) | $12/kg | - | [68] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karabelli, D.; Birke, K.P.; Weeber, M. A Performance and Cost Overview of Selected Solid-State Electrolytes: Race between Polymer Electrolytes and Inorganic Sulfide Electrolytes. Batteries 2021, 7, 18. https://doi.org/10.3390/batteries7010018
Karabelli D, Birke KP, Weeber M. A Performance and Cost Overview of Selected Solid-State Electrolytes: Race between Polymer Electrolytes and Inorganic Sulfide Electrolytes. Batteries. 2021; 7(1):18. https://doi.org/10.3390/batteries7010018
Chicago/Turabian StyleKarabelli, Duygu, Kai Peter Birke, and Max Weeber. 2021. "A Performance and Cost Overview of Selected Solid-State Electrolytes: Race between Polymer Electrolytes and Inorganic Sulfide Electrolytes" Batteries 7, no. 1: 18. https://doi.org/10.3390/batteries7010018
APA StyleKarabelli, D., Birke, K. P., & Weeber, M. (2021). A Performance and Cost Overview of Selected Solid-State Electrolytes: Race between Polymer Electrolytes and Inorganic Sulfide Electrolytes. Batteries, 7(1), 18. https://doi.org/10.3390/batteries7010018