Effect of Vinylene Carbonate Electrolyte Additive on the Surface Chemistry and Pseudocapacitive Sodium-Ion Storage of TiO2 Nanosheet Anodes
Abstract
1. Introduction
2. Experimental
2.1. Materials Synthesis
2.2. Materials Characterization
2.3. Electrochemical Measurements
3. Results and Discussion
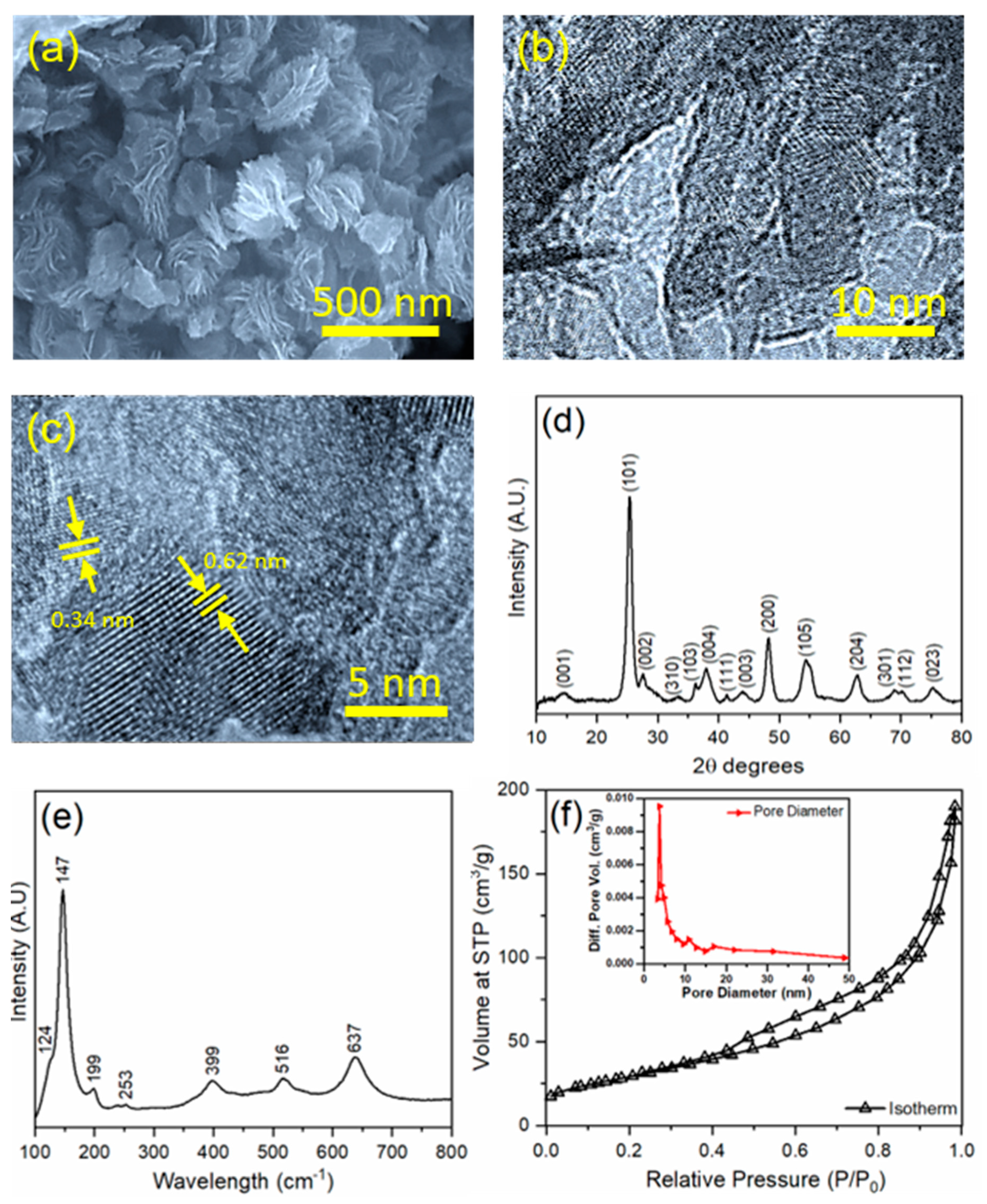
3.1. Synthesis and Characterization of TiO2 Nanosheets
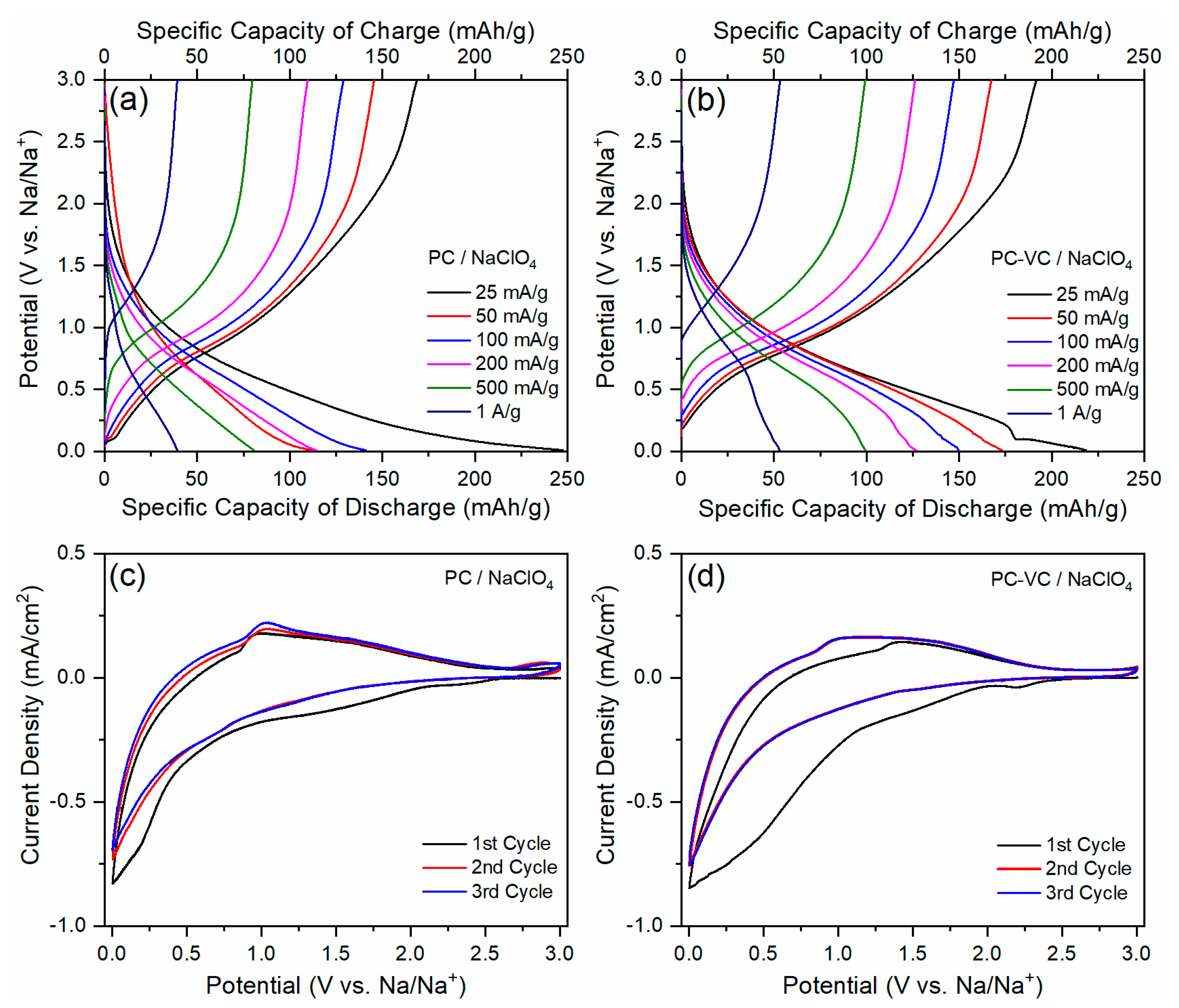
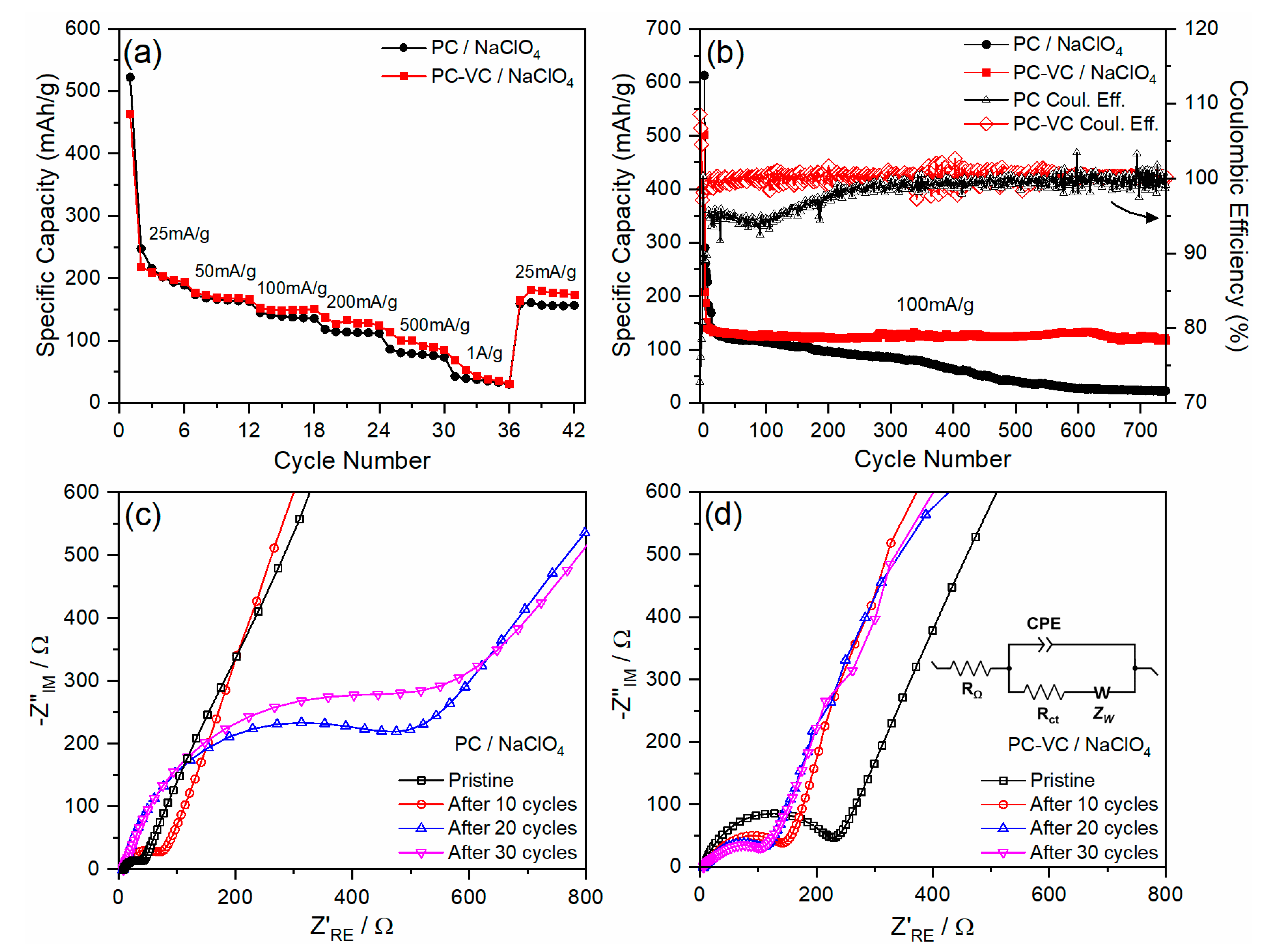
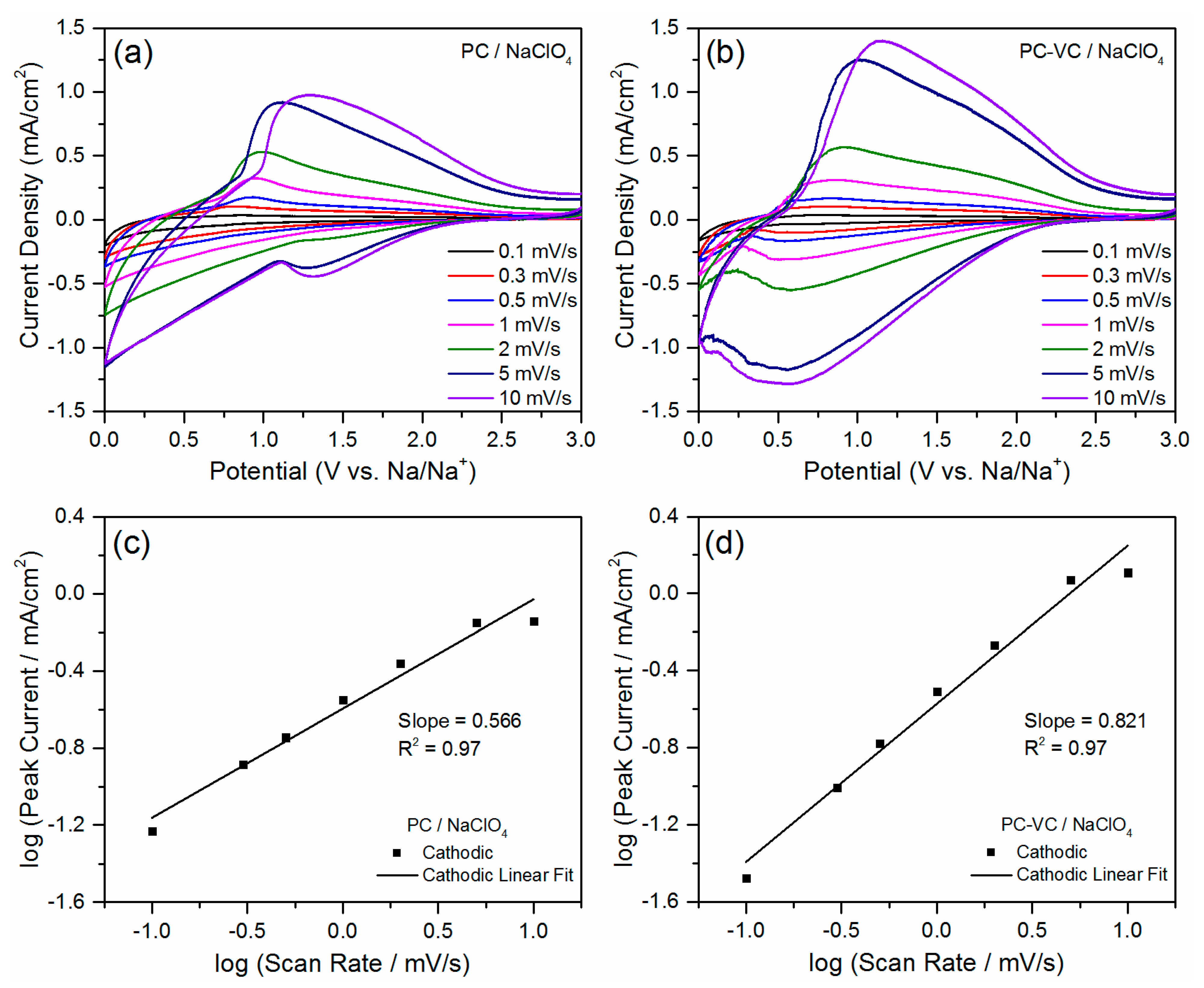
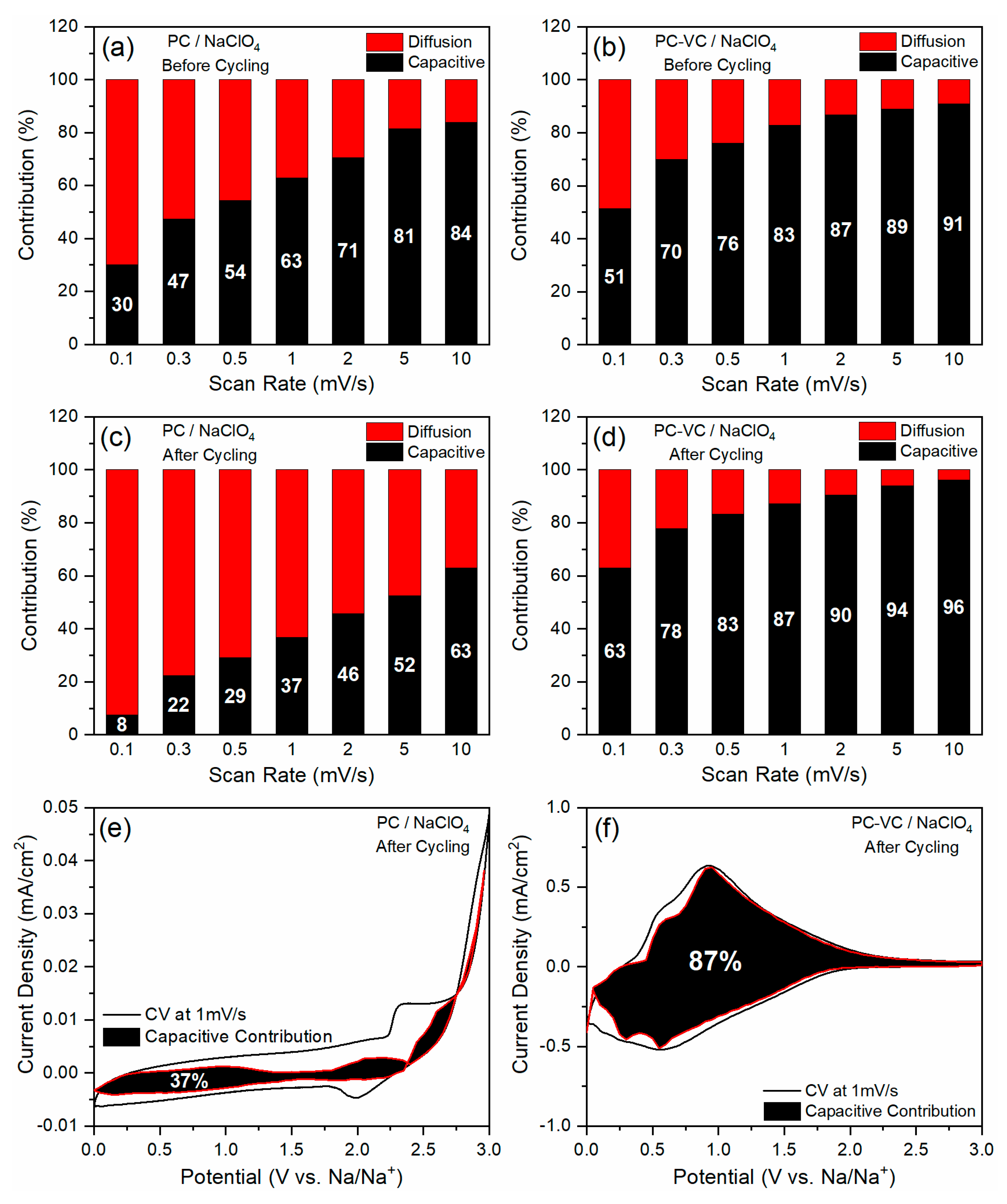
3.2. Electrochemical Performance of the TiO2 Nanosheets
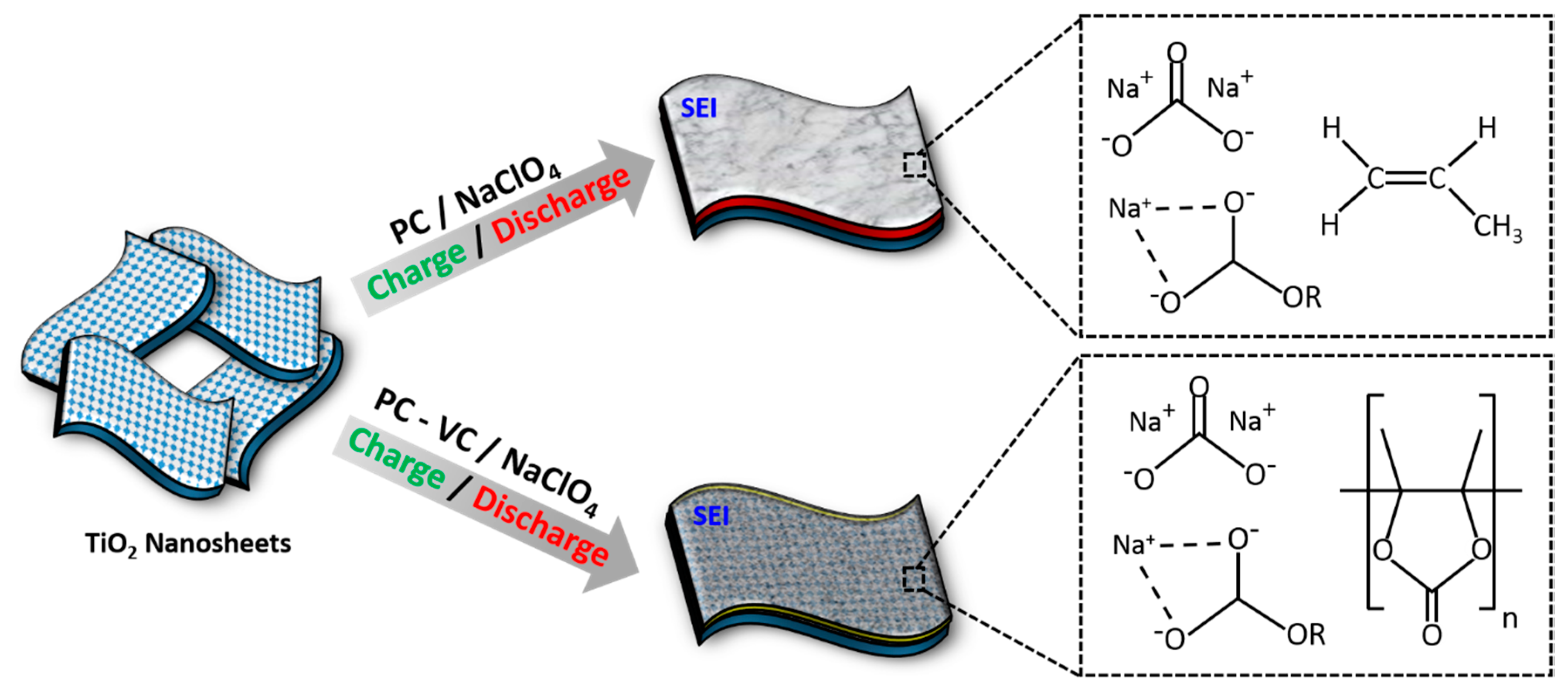
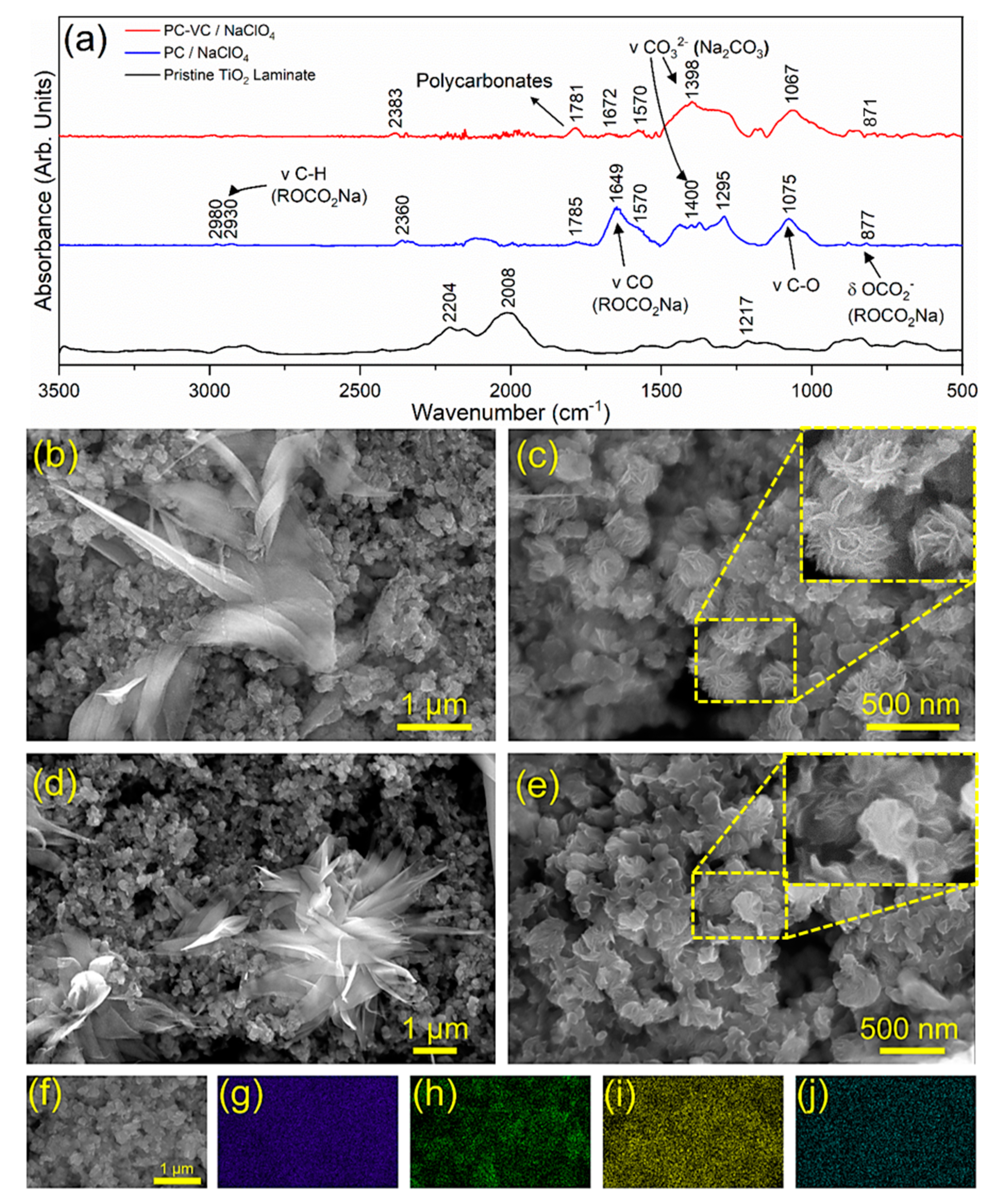
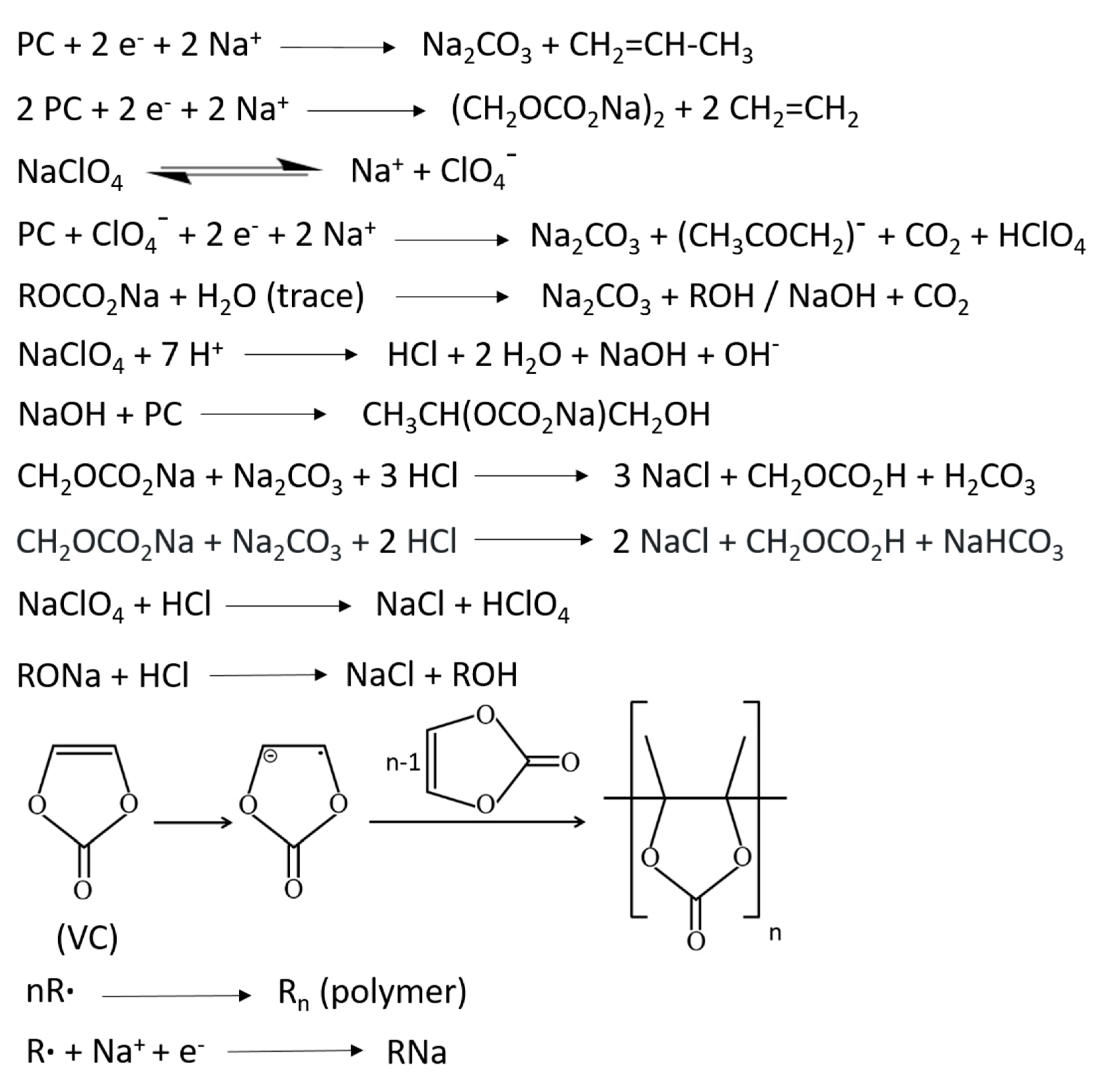
3.3. Surface Chemical Characterization of TiO2 Nanosheets
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef] [PubMed]
- Palomares, V.; Serras, P.; Villaluenga, I.; Hueso, K.B.; Carretero-González, J.; Rojo, T. Na-ion batteries, recent advances and present challenges to become low cost energy storage systems. Energy Environ. Sci. 2012, 5, 5884–5901. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Madian, M.; Eychmüller, A.; Giebeler, L. Current advances in TiO2-based nanostructure electrodes for high performance lithium ion batteries. Batteries 2018, 4, 7. [Google Scholar] [CrossRef]
- Dahbi, M.; Yabuuchi, N.; Fukunishi, M.; Kubota, K.; Chihara, K.; Tokiwa, K.; Yu, X.F.; Ushiyama, H.; Yamashita, K.; Son, J.Y.; et al. Black Phosphorus as a High-Capacity, High-Capability Negative Electrode for Sodium-Ion Batteries: Investigation of the Electrode/Electrolyte Interface. Chem. Mater. 2016, 28, 1625–1635. [Google Scholar] [CrossRef]
- Battistel, A.; Palagonia, M.S.; Brogioli, D.; La Mantia, F.; Trócoli, R. Electrochemical Methods for Lithium Recovery: A Comprehensive and Critical Review. Adv. Mater. 2020, 32, 1905440. [Google Scholar] [CrossRef] [PubMed]
- Rubio, S.; Maça, R.R.; Aragón, M.J.; Cabello, M.; Castillo-Rodríguez, M.; Lavela, P.; Tirado, J.L.; Etacheri, V.; Ortiz, G.F. Superior electrochemical performance of TiO2 sodium-ion battery anodes in diglyme-based electrolyte solution. J. Power Sources 2019, 432, 82–91. [Google Scholar] [CrossRef]
- Muñoz-Márquez, M.Á.; Saurel, D.; Gómez-Cámer, J.L.; Casas-Cabanas, M.; Castillo-Martínez, E.; Rojo, T. Na-Ion Batteries for Large Scale Applications: A Review on Anode Materials and Solid Electrolyte Interphase Formation. Adv. Energy Mater. 2017, 7, 1700463. [Google Scholar] [CrossRef]
- Maça, R.R.; Cíntora Juárez, D.; Castillo Rodríguez, M.; Etacheri, V. Nanointerface-Driven Pseudocapacitance Tuning of TiO2 Anode for High-Rate, Ultralong-Life and Enhanced Capacity Sodium-Ion Batteries. Chem. Eng. J. 2020, 391, 123598. [Google Scholar] [CrossRef]
- Feng, W.; Maça, R.R.; Etacheri, V. High-Energy-Density Sodium-Ion Hybrid Capacitors Enabled by Interface-Engineered Hierarchical TiO2 Nanosheet Anodes. ACS Appl. Mater. Interfaces 2020, 12, 4443–4453. [Google Scholar] [CrossRef]
- Sanchez, J.S.; Maça, R.R.; Pendashteh, A.; Etacheri, V.; de la Peña O’Shea, V.A.; Castillo-Rodríguez, M.; Palma, J.; Marcilla, R. Hierarchical Co3O4 nanorods anchored on nitrogen doped reduced graphene oxide: A highly efficient bifunctional electrocatalyst for rechargeable Zn–air batteries. Catal. Sci. Technol. 2020, 10, 1444–1457. [Google Scholar] [CrossRef]
- Cao, Y.; Xiao, L.; Sushko, M.L.; Wang, W.; Schwenzer, B.; Xiao, J.; Nie, Z.; Saraf, L.V.; Yang, Z.; Liu, J. Sodium ion insertion in hollow carbon nanowires for battery applications. Nano Lett. 2012, 12, 3783–3787. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zou, G.; Hou, H.; Zhang, Y.; Huang, Z.; Ji, X. Pinecone-like hierarchical anatase TiO2 bonded with carbon enabling ultrahigh cycling rates for sodium storage. J. Mater. Chem. A 2016, 4, 12591–12601. [Google Scholar] [CrossRef]
- Chao, D.; Zhu, C.; Yang, P.; Xia, X.; Liu, J.; Wang, J.; Fan, X.; Savilov, S.V.; Lin, J.; Fan, H.J.; et al. Array of nanosheets render ultrafast and high-capacity Na-ion storage by tunable pseudocapacitance. Nat. Commun. 2016, 7, 12122. [Google Scholar] [CrossRef]
- Rubio, S.; Maça, R.R.; Ortiz, G.F.; Vicente, C.P.; Lavela, P.; Etacheri, V.; Tirado, J.L. Iron Oxide-Iron Sulfide Hybrid Nanosheets as High-Performance Conversion-Type Anodes for Sodium-Ion Batteries. Appl. Energy Mater. 2020, 3, 10765–10775. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef]
- Dahbi, M.; Nakano, T.; Yabuuchi, N.; Fujimura, S.; Chihara, K.; Kubota, K.; Son, J.Y.; Cui, Y.T.; Oji, H.; Komaba, S. Effect of Hexafluorophosphate and Fluoroethylene Carbonate on Electrochemical Performance and the Surface Layer of Hard Carbon for Sodium-Ion Batteries. ChemElectroChem 2016, 3, 1856–1867. [Google Scholar] [CrossRef]
- Baggetto, L.; Keum, J.K.; Browning, J.F.; Veith, G.M. Germanium as negative electrode material for sodium-ion batteries. Electrochem. Commun. 2013, 34, 41–44. [Google Scholar] [CrossRef]
- Kalubarme, R.S.; Lee, J.Y.; Park, C.J. Carbon Encapsulated Tin Oxide Nanocomposites: An Efficient Anode for High Performance Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 17226–17237. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Chen, Y.; Wu, L.; Cao, Y.; Ai, X.; Yang, H. High capacity Na-storage and superior cyclability of nanocomposite Sb/C anode for Na-ion batteries. Chem. Commun. 2012, 48, 7070–7072. [Google Scholar] [CrossRef]
- Augustyn, V.; Come, J.; Lowe, M.A.; Kim, J.W.; Taberna, P.-L.; Tolbert, S.H.; Abruña, H.D.; Simon, P.; Dunn, B. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 2013, 12, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Longoni, G.; Pena Cabrera, R.L.; Polizzi, S.; D’Arienzo, M.; Mari, C.M.; Cui, Y.; Ruffo, R. Shape-Controlled TiO2 Nanocrystals for Na-Ion Battery Electrodes: The Role of Different Exposed Crystal Facets on the Electrochemical Properties. Nano Lett. 2017, 17, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Cargnello, M.; Gordon, T.R.; Murray, C.B. Solution-phase synthesis of titanium dioxide nanoparticles and nanocrystals. Chem. Rev. 2014, 114, 9319–9345. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Hwang, J.; Yoon, C.S.; Lu, J.; Amine, K.; Belharouak, I.; Sun, Y. High Electrochemical Performances of Microsphere C-TiO2 Anode for Sodium-Ion Battery. ACS Appl. Mater. Interfaces 2014, 6, 11295–11301. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Fukunishi, M.; Morgan, B.J.; Borkiewicz, O.J.; Chapman, K.W.; Pralong, V.V.; Maignan, A.; Lebedev, O.I.; Ma, J.; Groult, H.; et al. A Reversible Phase Transition for Sodium Insertion in Anatase TiO2. Chem. Mater. 2017, 29, 1836–1844. [Google Scholar] [CrossRef]
- Ge, Y.; Jiang, H.; Zhu, J.; Lu, Y.; Chen, C.; Hu, Y.; Qiu, Y.; Zhang, X. High cyclability of carbon-coated TiO2 nanoparticles as anode for sodium-ion batteries. Electrochim. Acta 2015, 157, 142–148. [Google Scholar] [CrossRef]
- Chen, J.; Ding, Z.; Wang, C.; Hou, H.; Zhang, Y.; Wang, C.; Zou, G.; Ji, X. Black Anatase Titania with Ultrafast Sodium-Storage Performances Stimulated by Oxygen Vacancies. ACS Appl. Mater. Interfaces 2016, 8, 9142–9151. [Google Scholar] [CrossRef] [PubMed]
- Goriparti, S.; Miele, E.; Prato, M.; Scarpellini, A.; Marras, S.; Monaco, S.; Toma, A.; Messina, G.C.; Alabastri, A.; De Angelis, F.; et al. Direct Synthesis of Carbon-Doped TiO2-Bronze Nanowires as Anode Materials for High Performance Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 25139–25146. [Google Scholar] [CrossRef]
- Etacheri, V.; Kuo, Y.; Van Der Ven, A.; Bartlett, B.M. Mesoporous TiO2-B microflowers composed of (1-1 0) facet-exposed nanosheets for fast reversible lithium-ion storage. J. Mater. Chem. A 2013, 1, 12028–12032. [Google Scholar] [CrossRef]
- Mehraeen, S.; Taşdemir, A.; Gürsel, S.A.; Yürüm, A. Homogeneous growth of TiO2-based nanotubes on nitrogen-doped reduced graphene oxide and its enhanced performance as a Li-ion battery anode. Nanotechnology 2018, 29, 255402. [Google Scholar] [CrossRef]
- Su, D.; Dou, S.; Wang, G.; Su, D.; Dou, S.; Wang, G. Anatase TiO2: Better Anode Material than Amorphous and Rutile Phases of TiO2 for Na-ion Batteries. Chem. Mater. 2015, 27, 6022–6029. [Google Scholar] [CrossRef]
- Xu, Y.; Lotfabad, M.; Wang, H.; Farbod, B.; Xu, Z.; Mitlin, D. Nanocrystalline anatase TiO2: A new anode material for rechargeable sodium ion batteries. Chem. Commun. 2013, 49, 8973–8975. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Wu, X.; Wang, J.; Fu, L.; Zhu, Y.; Wu, Y.; Liu, X. Advances of TiO2 as Negative Electrode Materials for Sodium-Ion Batteries. Adv. Mater. Technol. 2018, 3, 1800004. [Google Scholar] [CrossRef]
- Usui, H.; Domi, Y.; Yoshioka, S.; Kojima, K.; Sakaguchi, H. Electrochemical Lithiation and Sodiation of Nb-Doped Rutile TiO2. ACS Sustain. Chem. Eng. 2016, 4, 6695–6702. [Google Scholar] [CrossRef]
- Tanaka, Y.; Usui, H.; Domi, Y.; Ohtani, M.; Kobiro, K.; Sakaguchi, H. Mesoporous spherical aggregates consisted of Nb-doped anatase TiO2 nanoparticles for Li and Na storage materials. ACS Appl. Energy Mater. 2019, 2, 636–643. [Google Scholar] [CrossRef]
- Chen, C.; Wen, Y.; Ji, X.; Yan, M.; Mai, L.; Hu, P.; Shan, B.; Hu, X.; Huang, Y. Na+ intercalation pseudocapacitance in graphene-coupled titanium oxide enabling ultra-fast sodium storage and long-term cycling. Nat. Commun. 2015, 6, 6929–6936. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Yang, J.; Zhang, Q.; Wang, N.; Niu, F.; Xu, X.; Yang, J.; Fan, W.; Qian, Y. Biphase-Interface Enhanced Sodium Storage and Accelerated Charge Transfer: Flower-Like Anatase/Bronze TiO2/C as an Advanced Anode Material for Na-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 43648–43656. [Google Scholar] [CrossRef]
- Peled, E.; Menkin, S. Review-SEI: Past, Present and Future. J. Electrochem. Soc. 2017, 164, A1703–A1719. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, L.; Li, L.; Xie, M.; Wu, F.; Chen, R. Electrolytes and Electrolyte/Electrode Interfaces in Sodium-Ion Batteries: From Scientific Research to Practical Application. Adv. Mater. 2019, 31, 1808393. [Google Scholar] [CrossRef] [PubMed]
- Ponrouch, A.; Marchante, E.; Courty, M.; Tarascon, J.-M.; Palacín, M.R. In search of an optimized electrolyte for Na-ion batteries. Energy Environ. Sci. 2012, 5, 8572–8583. [Google Scholar] [CrossRef]
- Etacheri, V.; Haik, O.; Goffer, Y.; Roberts, G.A.; Stefan, I.C.; Fasching, R.; Aurbach, D. Effect of Fluoroethylene Carbonate (FEC) on the Performance and Surface Chemistry of Si-Nanowire Li-Ion Battery Anodes. Langmuir 2012, 28, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Xiao, B.; Lin, Y.; Xu, K.; Li, X. Interphases in Sodium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1703082. [Google Scholar] [CrossRef]
- Peled, E.; Patolsky, F.; Golodnitsky, D.; Freedman, K.; Davidi, G.; Schneier, D. Tissue-like Silicon Nanowires-Based Three-Dimensional Anodes for High-Capacity Lithium Ion Batteries. Nano Lett. 2015, 15, 3907–3916. [Google Scholar] [CrossRef] [PubMed]
- Ushirogata, K.; Sodeyama, K.; Tateyama, Y.; Okuno, Y.; Tateyama, Y. Additive Effect on Reductive Decomposition and Binding of Carbonate-Based Solvent toward Solid Electrolyte Interphase Formation in Lithium-Ion Battery. J. Am. Chem. Soc. 2013, 135, 11967–11974. [Google Scholar] [CrossRef] [PubMed]
- Jaumann, T.; Balach, J.; Langklotz, U.; Sauchuk, V.; Fritsch, M.; Michaelis, A.; Teltevskij, V.; Mikhailova, D.; Oswald, S.; Klose, M.; et al. Lifetime vs. rate capability: Understanding the role of FEC and VC in high-energy Li-ion batteries with nano-silicon anodes. Energy Storage Mater. 2017, 6, 26–35. [Google Scholar] [CrossRef]
- Wang, L.; Światowska, J.; Dai, S.; Cao, M.; Zhong, Z.; Shen, Y.; Wang, M. Promises and challenges of alloy-type and conversion-type anode materials for sodium–ion batteries. Mater. Today Energy 2019, 11, 46–60. [Google Scholar] [CrossRef]
- Liu, Q.; Mu, D.; Wu, B.; Wang, L.; Gai, L.; Wu, F. Density Functional Theory Research into the Reduction Mechanism for the Solvent/Additive in a Sodium-Ion Battery. ChemSusChem 2017, 10, 786–796. [Google Scholar] [CrossRef]
- Tao, H.; Zhou, M.; Wang, K.; Cheng, S.; Jiang, K. Glycol Derived Carbon-TiO2 as Low Cost and High Performance Anode Material for Sodium-Ion Batteries. Sci. Rep. 2017, 7, 43895–43901. [Google Scholar] [CrossRef]
- Song, W.; Jiang, Q.; Xie, X.; Brookfield, A.; McInnes, E.J.L.; Shearing, P.R.; Brett, D.J.L.; Xie, F.; Riley, D.J. Synergistic storage of lithium ions in defective anatase/rutile TiO2 for high-rate batteries. Energy Storage Mater. 2019, 22, 441–449. [Google Scholar] [CrossRef]
- Yang, P.D.; Zhao, D.Y.; Margolese, D.I.; Chmelka, B.F.; Stucky, G.D. Generalized syntheses of large-pore mesoporous metal oxides with semicrystalline frameworks. Lett. to Nat. 1998, 396, 152–155. [Google Scholar] [CrossRef]
- Yu, X.; Kim, B.; Kim, Y.K. Highly enhanced photoactivity of anatase TiO2 nanocrystals by controlled hydrogenation-induced surface defects. ACS Catal. 2013, 3, 2479–2486. [Google Scholar] [CrossRef]
- Beuvier, T.; Richard-plouet, M.; Brohan, L. Accurate Methods for Quantifying the Relative Ratio of Anatase and TiO2(B) Nanoparticles. J. Phys. Chem. C 2009, 113, 13703–13706. [Google Scholar] [CrossRef]
- Wu, L.; Bresser, D.; Buchholz, D.; Giffi, G.; Castro, C.R.; Ochel, A.; Passerini, S.; Giffin, G.A.; Castro, C.R.; Ochel, A.; et al. Unfolding the Mechanism of Sodium Insertion in Anatase TiO2 Nanoparticles. Adv. Energy Mater. 2014, 5, 1401142. [Google Scholar] [CrossRef]
- Zhang, Y.; Pu, X.; Yang, Y.; Zhu, Y.; Hou, H.; Jing, M.; Yang, X.; Chen, J.; Ji, X. An Electrochemical Investigation of Rutile TiO2 Microspheres Anchored by Nanoneedle Clusters for its Sodium Storage. Phys. Chem. Chem. Phys. 2015, 17, 15764–15770. [Google Scholar] [CrossRef] [PubMed]
- Randles, J.E.B. Kinetics of Rapid Electrode Reactions. Discuss. Faraday Soc. 1947, 1, 11–19. [Google Scholar] [CrossRef]
- Mogi, R.; Inaba, M.; Jeong, S.-K.; Iriyama, Y.; Abe, T.; Ogumi, Z. Effects of Some Organic Additives on Lithium Deposition in Propylene Carbonate. J. Electrochem. Soc. 2002, 149, A1578–A1583. [Google Scholar] [CrossRef]
- Aurbach, D.; Gamolsky, K.; Markovsky, B.; Gofer, Y.; Schmidt, M.; Heider, U. On the use of vinylene carbonate (VC) as an additive to electrolyte solutions for Li-ion batteries. Electrochim. Acta 2002, 47, 1423–1439. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef]
- Babu, B.; Shaijumon, M.M. High performance sodium-ion hybrid capacitor based on Na2Ti2O4(OH)2 nanostructures. J. Power Sources 2017, 353, 85–94. [Google Scholar] [CrossRef]
- Lesel, B.K.; Cook, J.B.; Yan, Y.; Lin, T.C.; Tolbert, S.H. Using Nanoscale Domain Size to Control Charge Storage Kinetics in Pseudocapacitive Nanoporous LiMn2O4 Powders. ACS Energy Lett. 2017, 2, 2293–2298. [Google Scholar] [CrossRef]
- De La Llave, E.; Borgel, V.; Park, K.J.; Hwang, J.Y.; Sun, Y.K.; Hartmann, P.; Chesneau, F.F.; Aurbach, D. Comparison between Na-Ion and Li-Ion Cells: Understanding the Critical Role of the Cathodes Stability and the Anodes Pretreatment on the Cells Behavior. ACS Appl. Mater. Interfaces 2016, 8, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Costentin, C.; Porter, T.R.; Save, J. How Do Pseudocapacitors Store Energy? Theoretical Analysis and Experimental Illustration. ACS Appl. Mater. Interfaces 2017, 9, 8649–8658. [Google Scholar] [CrossRef] [PubMed]
- El Ouatani, L.; Dedryvère, R.; Siret, C.; Biensan, P.; Gonbeau, D. Effect of Vinylene Carbonate Additive in Li-Ion Batteries: Comparison of LiCoO2/C, LiFePO4/C, and LiCoO2/Li4Ti5O12 Systems. J. Electrochem. Soc. 2009, 156, A468–A477. [Google Scholar] [CrossRef]
- Kumar, H.; Detsi, E.; Abraham, D.P.; Shenoy, V.B. Fundamental Mechanisms of Solvent Decomposition Involved in Solid-Electrolyte Interphase Formation in Sodium Ion Batteries. Chem. Mater. 2016, 28, 8930–8941. [Google Scholar] [CrossRef]
- Leggesse, E.G.; Lin, R.T.; Teng, T.F.; Chen, C.L.; Jiang, J.C. Oxidative decomposition of propylene carbonate in lithium ion batteries: A DFT study. J. Phys. Chem. A 2013, 117, 7959–7969. [Google Scholar] [CrossRef]
- Fondard, J.; Irisarri, E.; Courrèges, C.; Palacin, M.R.; Ponrouch, A.; Dedryvère, R. SEI Composition on Hard Carbon in Na-Ion Batteries After Long Cycling: Influence of Salts (NaPF6, NaTFSI) and Additives (FEC, DMCF). J. Electrochem. Soc. 2020, 167, 070526. [Google Scholar] [CrossRef]
- Moshkovich, M.; Gofer, Y.; Aurbach, D. Investigation of the Electrochemical Windows of Aprotic Alkali Metal (Li, Na, K) Salt Solutions. J. Electrochem. Soc. 2001, 148, E155–E167. [Google Scholar] [CrossRef]
- Aurbach, D.; Weissman, I.; Schechter, A.; Cohen, H. X-ray Photoelectron Spectroscopy Studies of Lithium Surfaces Prepared in Several Important Electrolyte Solutions. A Comparison with Previous Studies by Fourier Transform Infrared Spectroscopy. Langmuir 1996, 12, 3991–4007. [Google Scholar] [CrossRef]
- Herstedt, M.; Abraham, D.P.; Kerr, J.B.; Edström, K. X-Ray Photoelectron Spectroscopy of Negative Electrodes from High-Power Lithium-Ion Cells Showing Various Levels of Power Fade. Electrochim. Acta 2004, 49, 5097–5110. [Google Scholar] [CrossRef]
- Muñoz-Márquez, M.A.; Zarrabeitia, M.; Castillo-Martínez, E.; Eguía-Barrio, A.; Rojo, T.; Casas-Cabanas, M. Composition and evolution of the solid-electrolyte interphase in Na2Ti3O7 electrodes for Na-Ion batteries: XPS and Auger parameter analysis. ACS Appl. Mater. Interfaces 2015, 7, 7801–7808. [Google Scholar] [CrossRef]
- Matsuta, S.; Asada, T.; Kitaura, K. Vibrational Assignments of Lithium Alkyl Carbonate and Lithium Alkoxide in the Infrared Spectra. J. Electrochem. Soc. 2000, 147, 1695–1702. [Google Scholar] [CrossRef]
- Tsubouchi, S.; Domi, Y.; Doi, T.; Ochida, M.; Nakagawa, H.; Yamanaka, T.; Abe, T.; Ogumi, Z. Spectroscopic Characterization of Surface Films Formed on Edge Plane Graphite in Ethylene Carbonate-Based Electrolytes Containing Film-Forming Additives. J. Electrochem. Soc. 2012, 159, A1786–A1790. [Google Scholar] [CrossRef]


| Composition | C Content (at.%) | O Content (at.%) | Cl Content (at.%) | Na Content (at.%) | Ti Content (at.%) |
|---|---|---|---|---|---|
| PC/NaClO4 | 21.101 | 33.412 | 0.706 | 44.590 | 0.191 |
| PC-VC/NaClO4 | 27.711 | 37.928 | 0.496 | 33.614 | 0.251 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maça, R.R.; Etacheri, V. Effect of Vinylene Carbonate Electrolyte Additive on the Surface Chemistry and Pseudocapacitive Sodium-Ion Storage of TiO2 Nanosheet Anodes. Batteries 2021, 7, 1. https://doi.org/10.3390/batteries7010001
Maça RR, Etacheri V. Effect of Vinylene Carbonate Electrolyte Additive on the Surface Chemistry and Pseudocapacitive Sodium-Ion Storage of TiO2 Nanosheet Anodes. Batteries. 2021; 7(1):1. https://doi.org/10.3390/batteries7010001
Chicago/Turabian StyleMaça, Rudi Ruben, and Vinodkumar Etacheri. 2021. "Effect of Vinylene Carbonate Electrolyte Additive on the Surface Chemistry and Pseudocapacitive Sodium-Ion Storage of TiO2 Nanosheet Anodes" Batteries 7, no. 1: 1. https://doi.org/10.3390/batteries7010001
APA StyleMaça, R. R., & Etacheri, V. (2021). Effect of Vinylene Carbonate Electrolyte Additive on the Surface Chemistry and Pseudocapacitive Sodium-Ion Storage of TiO2 Nanosheet Anodes. Batteries, 7(1), 1. https://doi.org/10.3390/batteries7010001






