Evaluation of the Stability of Carbon Conductor in the Cathode of Aqueous Rechargeable Lithium Batteries against Overcharging
Abstract
1. Introduction
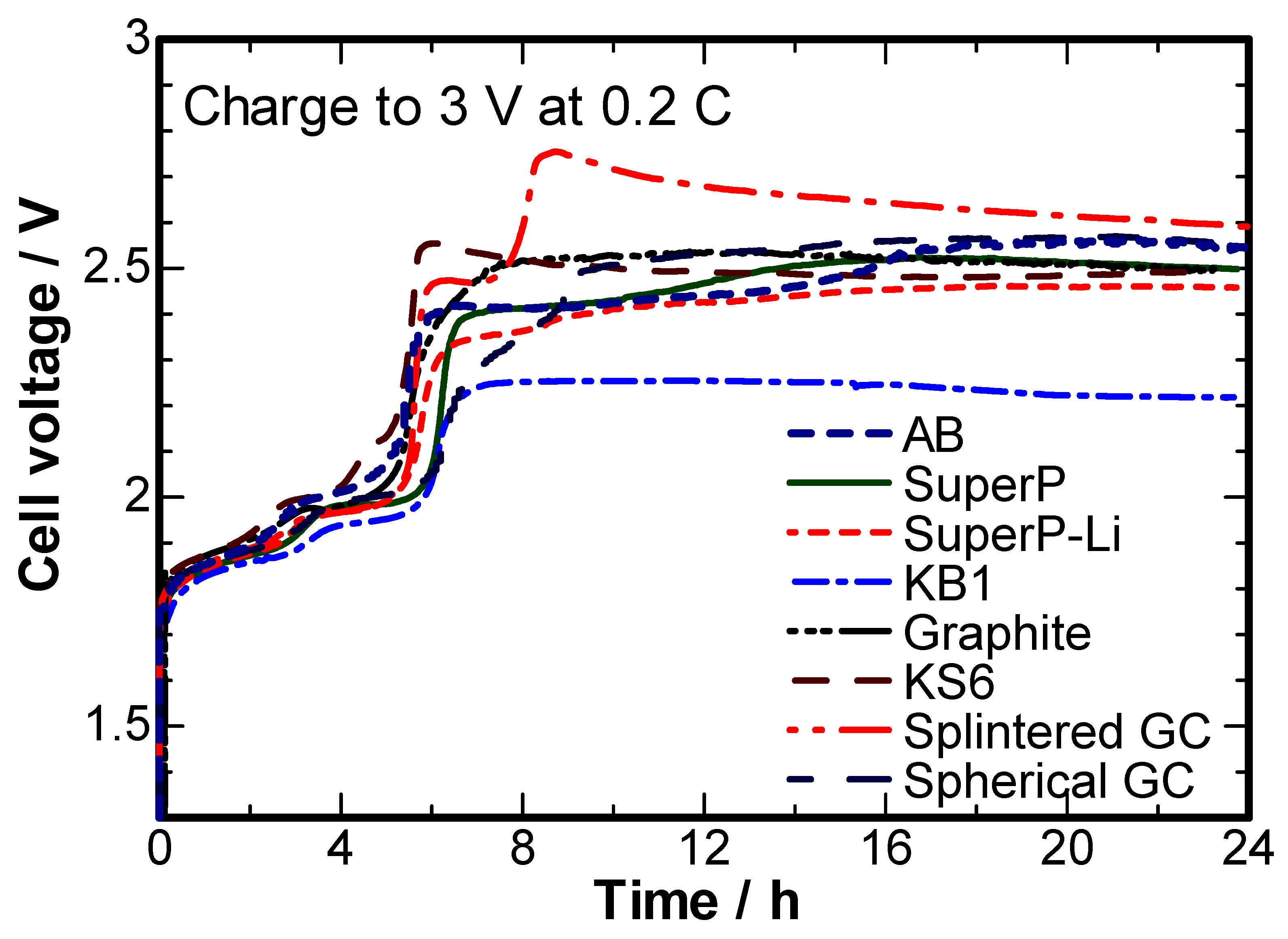
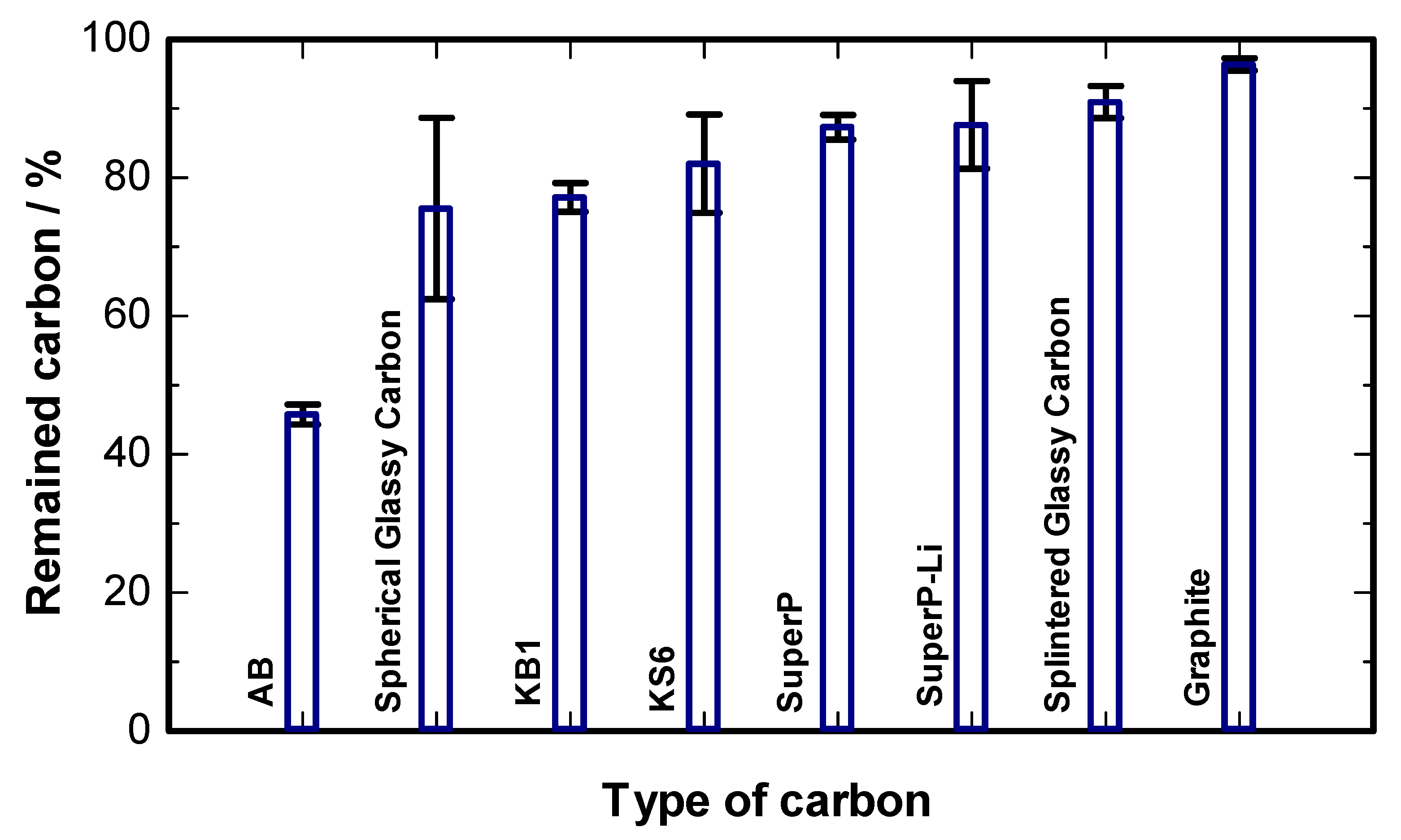
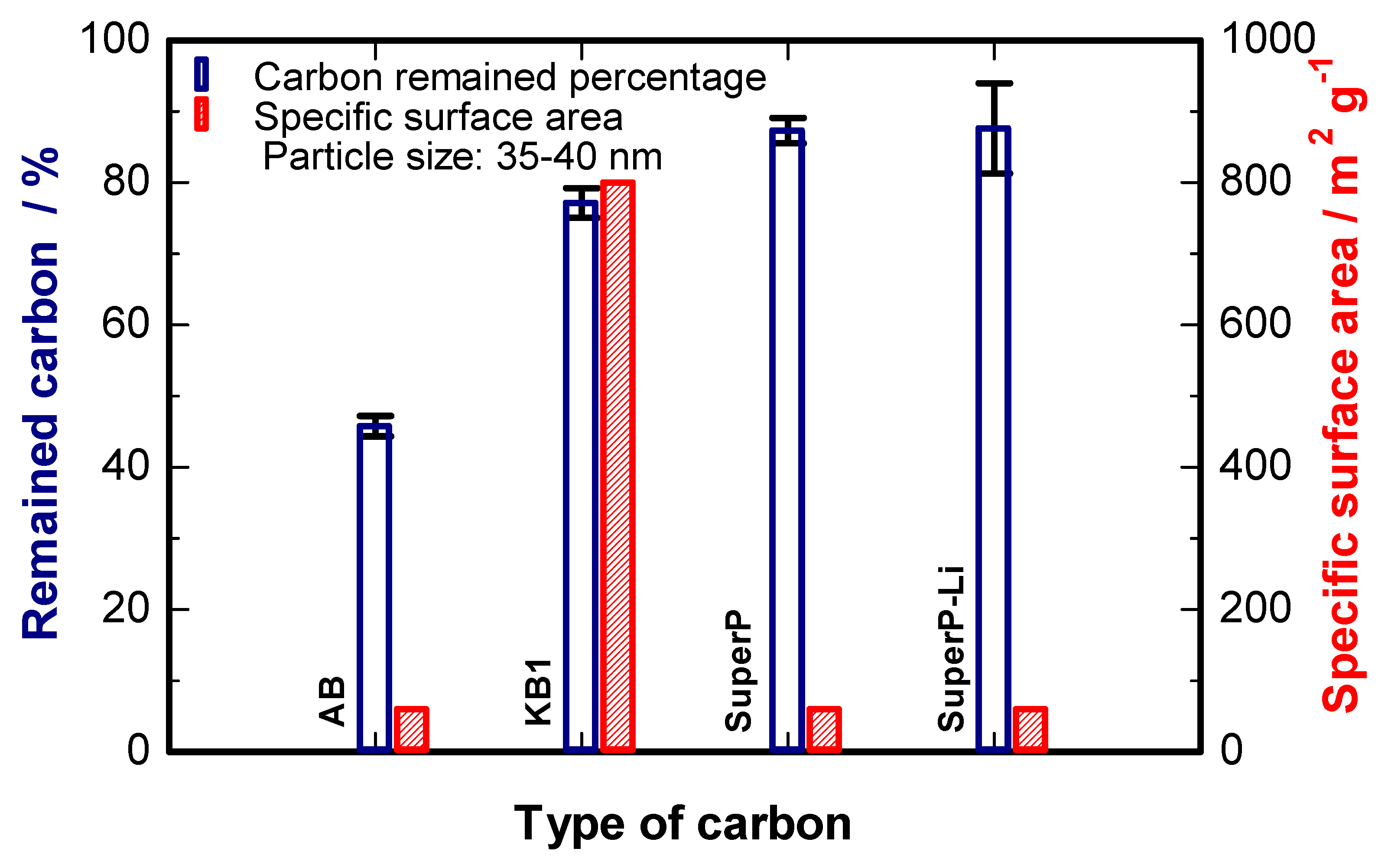
2. Results
3. Discussion
4. Materials and Methods
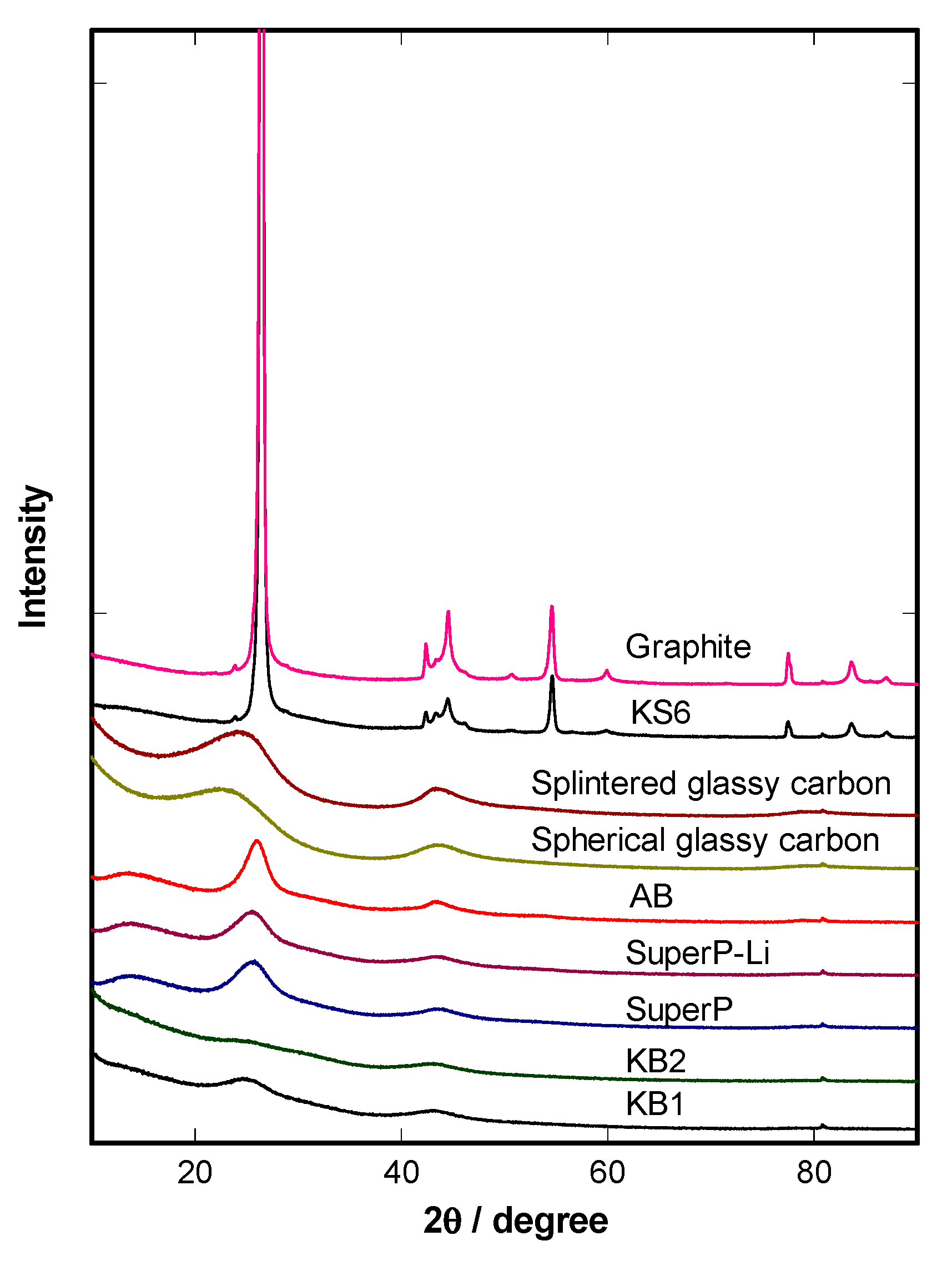
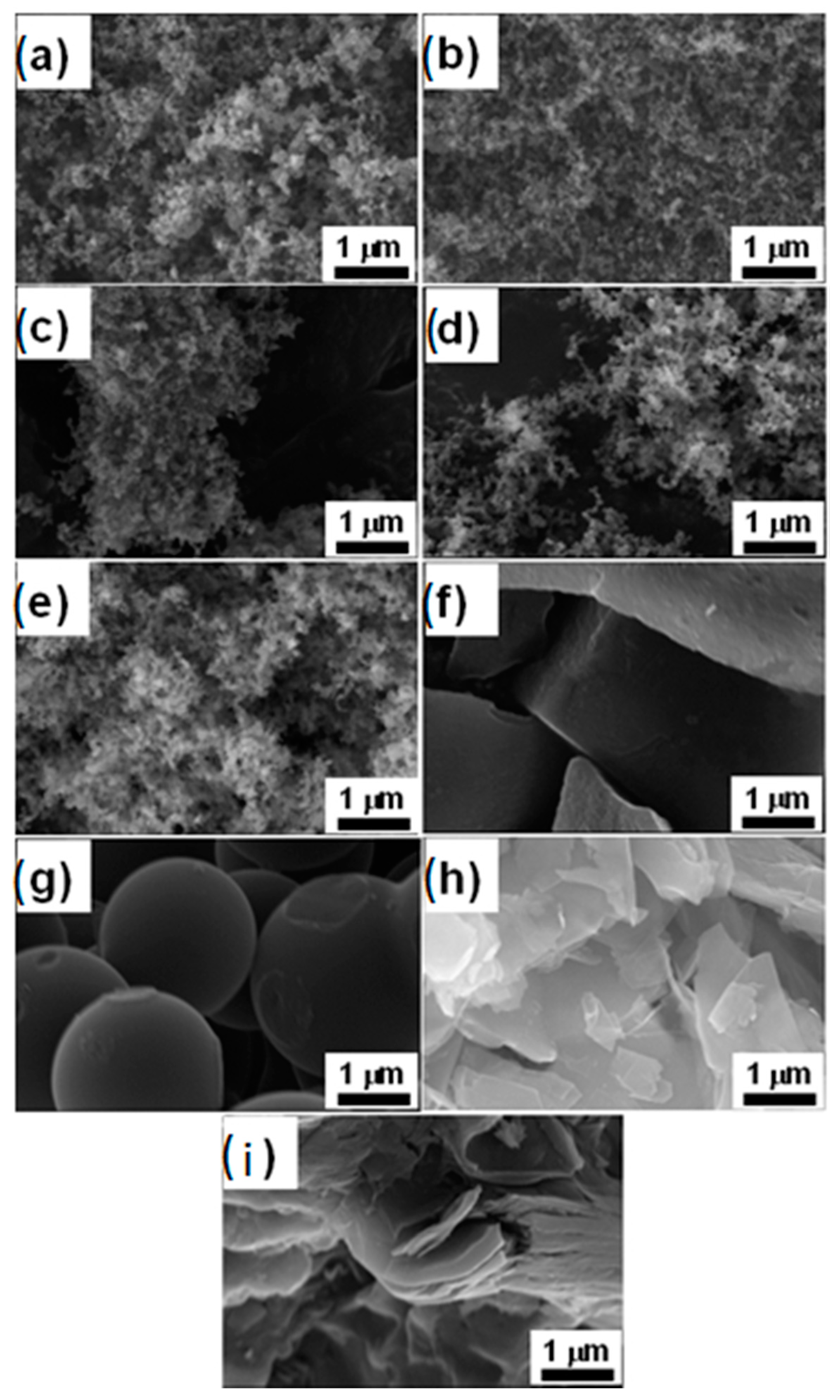
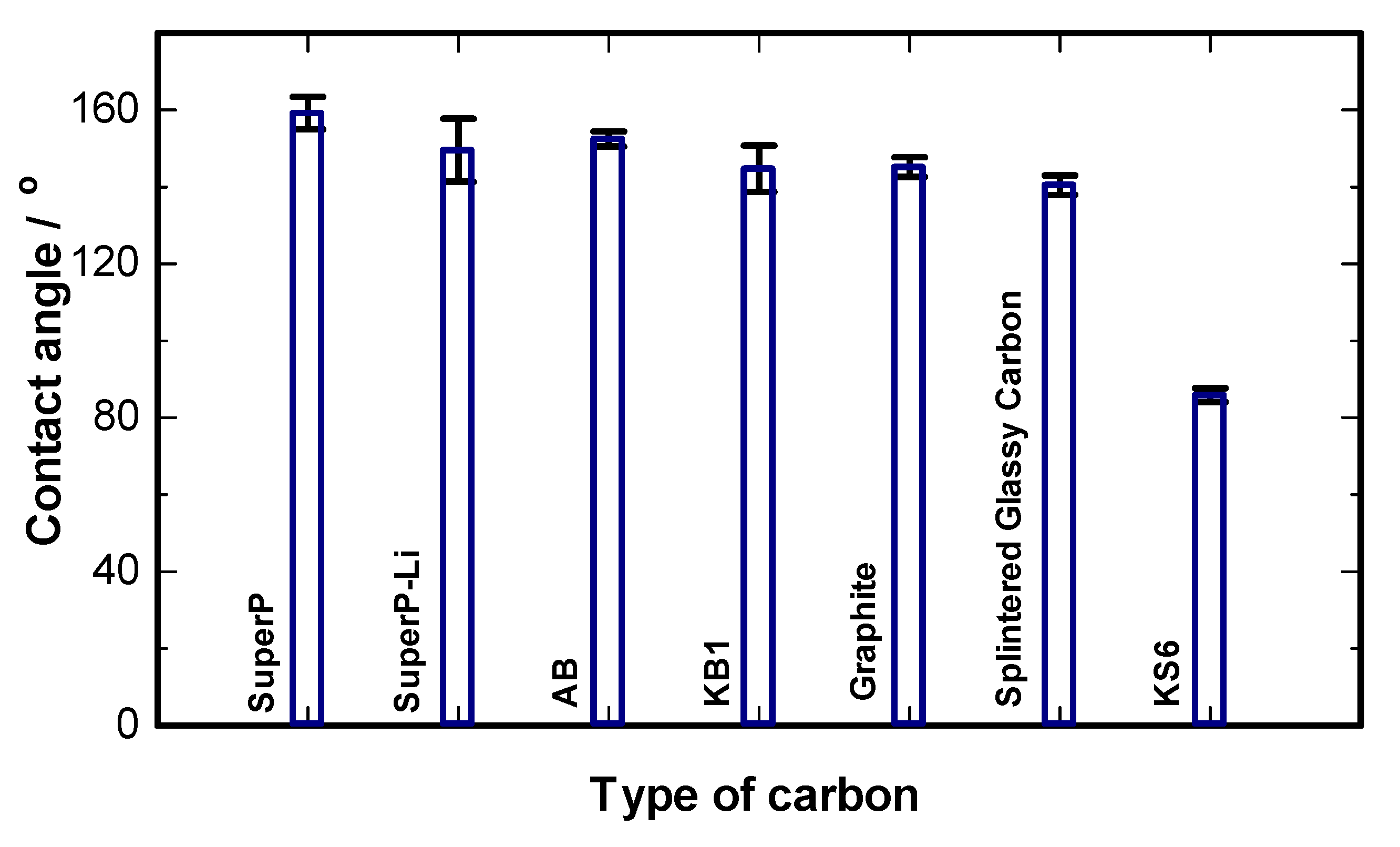
4.1. Physical Characterizations
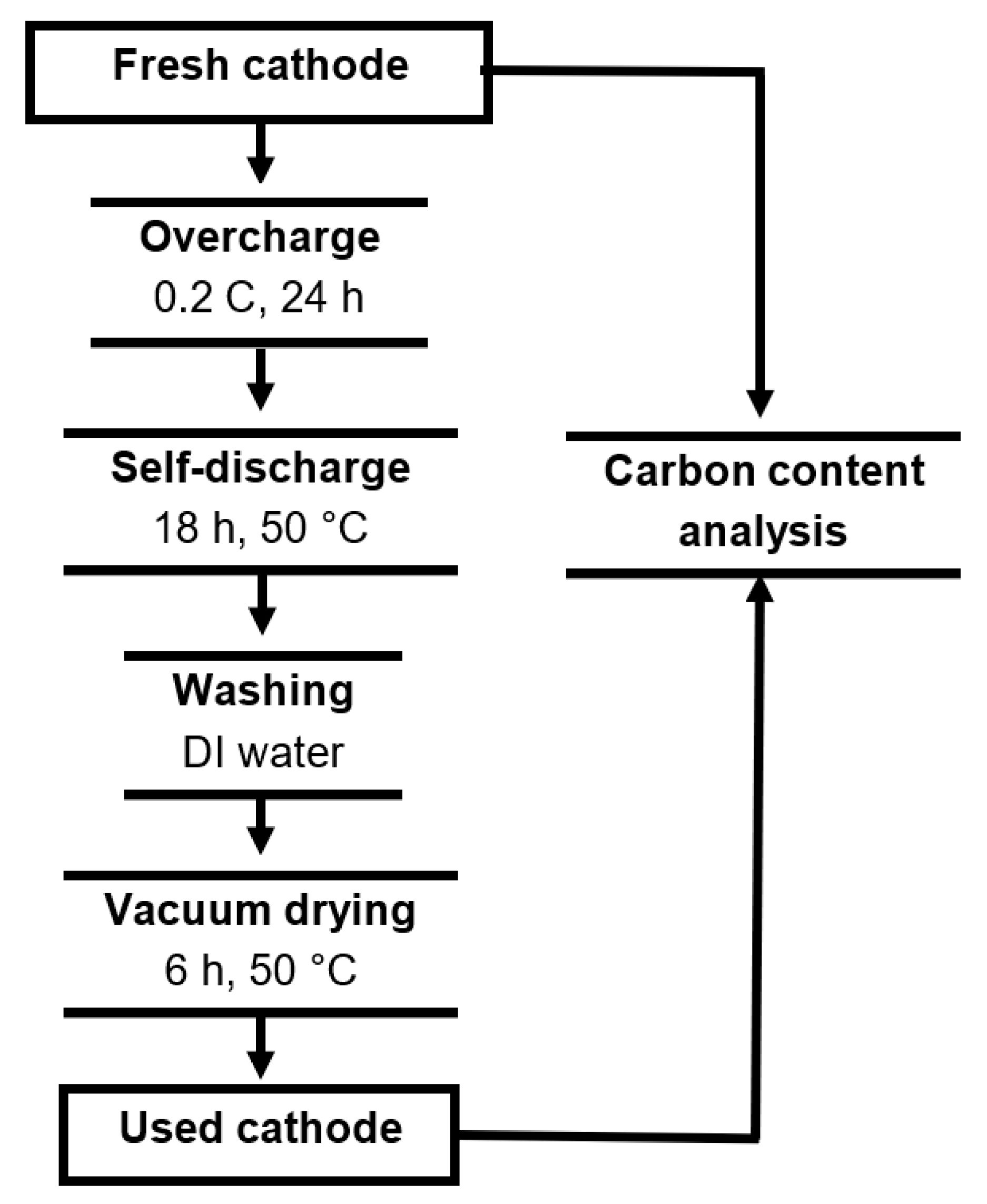
4.2. Carbon Stability Testing Procedure
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abou-Rjeily, J.; Bezza, I.; Laziz, N.A.; Autret-Lambert, C.; Sougrati, M.T.; Ghamouss, F. High-rate cyclability and stability of LiMn2O4 cathode materials for lithium-ion batteries from low-cost natural β−MnO2. Energy Storage Mater. 2020, 26, 423–432. [Google Scholar] [CrossRef]
- Yu, X.L.; Deng, J.J.; Yang, X.; Li, J.; Huang, Z.-H.; Li, B.H.; Kang, F.Y. A dual-carbon-anchoring strategy to fabricate flexible LiMn2O4 cathode for advanced lithium-ion batteries with high areal capacity. Nano Energy 2020, 67, 104256. [Google Scholar] [CrossRef]
- Cai, Z.F.; Ma, Y.Z.; Huang, X.N.; Yan, X.H.; Yu, Z.X.; Zhang, S.H.; Song, G.S.; Xu, Y.L.; Wen, C.; Yang, W.D. High electrochemical stability Al-doped spinel LiMn2O4 cathode material for Li-ion batteries. J. Energy Storage 2020, 27, 101036. [Google Scholar] [CrossRef]
- Abbas, S.M.; Hashem, A.M.; Abdel-Ghany, A.E.; Ismail, E.H.; Kotlár, M.; Winter, M.; Li, J.; Julien, C.M. Ag-Modified LiMn2O4 Cathode for Lithium-Ion Batteries: Coating Functionalization. Energies 2020, 13, 5194. [Google Scholar] [CrossRef]
- Palaniyandy, N.; Rambau, K.; Mysyoka, N.; Ren, J.W. A Facile Segregation Process and Restoration of LiMn2O4 Cathode Material From Spent Lithium-Ion Batteries. J. Electrochem. Soc. 2020, 167, 090510. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Li, H.; Hasa, D.; Passerini, S. Challenges and Strategies for High-Energy Aqueous Electrolyte Rechargeable Batteries. Angew. Chem. Int. Ed. 2020, 59, 2–21. [Google Scholar] [CrossRef]
- Luo, J.-Y.; Cui, W.-J.; He, P.; Xia, Y.Y. Raising the cycling stability of aqueous lithium-ion batteries by eliminating oxygen in the electrolyte. Nat. Chem. 2010, 2, 760–765. [Google Scholar] [CrossRef]
- Tang, W.; Zhu, Y.S.; Hou, Y.Y.; Liu, L.L.; Wu, Y.P.; Loh, K.P.; Zhang, H.P.; Zhu, K. Aqueous rechargeable lithium batteries as an energy storage system of superfast charging. Energy Environ. Sci. 2013, 6, 2093–2104. [Google Scholar] [CrossRef]
- Miyazaki, K.; Shimada, T.; Ito, S.; Yokoyama, Y.; Fukutsuka, T.; Abe, T. Enhanced resistance to oxidative decomposition of aqueous electrolytes for aqueous lithium-ion batteries. Chem. Commun. 2016, 52, 4979–4982. [Google Scholar] [CrossRef]
- Nair, V.S.; Sreejith, S.; Joshi, H.; Zhao, Y.L.; West, A.; Madhavi, S. The fabrication of LiMn2O4 and Na1.16V3O8 based full cell aqueous rechargeable battery to power portable wearable electronics devices. Mater. Des. 2016, 93, 291–296. [Google Scholar] [CrossRef]
- Gordon, D.; Wu, M.Y.; Ramanujapuram, A.; Benson, J.; Lee, J.T.; Magasinski, A.; Nitta, N.; Huang, C.; Yushin, G. Enhancing Cycle Stability of Lithium Iron Phosphate in Aqueous Electrolytes by Increasing Electrolyte Molarity. Adv. Energy Mater. 2016, 6, 1501805. [Google Scholar] [CrossRef]
- Sun, D.; Tang, Y.; He, K.; Ren, Y.; Liu, S.; Wang, H. Long-lived Aqueous Rechargeable Lithium Batteries Using Mesoporous LiTi2(PO4)3@C Anode. Sci. Rep. 2015, 5, 17452. [Google Scholar] [CrossRef] [PubMed]
- Noerochim, L.; Yurwendra, A.O.; Susanti, D. Effect of carbon coating on the electrochemical performance of LiFePO4/C as cathode materials for aqueous electrolyte lithium-ion battery. Ionics 2016, 22, 341–346. [Google Scholar] [CrossRef]
- Zeng, X.Y.; Liu, Q.B.; Chen, M.F.; Leng, L.M.; Shu, T.; Du, L.; Song, H.Y.; Liao, S.J. Electrochemical Behavior of Spherical LiFePO4/C Nanomaterial in Aqueous Electrolyte, and Novel Aqueous Rechargeable Lithium Battery with LiFePO4/C anode. Electrochim. Acta 2015, 177, 277–282. [Google Scholar] [CrossRef]
- Sun, D.; Jiang, Y.F.; Wang, H.Y.; Yao, Y.; Xu, G.Q.; He, K.J.; Liu, S.Q.; Tang, Y.; Liu, Y.N.; Huang, X.B. Advanced aqueous rechargeable lithium battery using nanoparticulate LiTi2(PO4)3/C as a superior anode. Sci. Rep. 2015, 5, 10733. [Google Scholar] [CrossRef]
- Arun, N.; Aravindan, V.; Ling, W.C.; Madhavi, S. Importance of nanostructure for reversible Li-insertion into octahedral sites of LiNi0.5Mn1.5O4 and its application towards aqueous Li-ion chemistry. J. Power Sources 2015, 280, 240–245. [Google Scholar] [CrossRef]
- Yan, J.; Wang, J.; Liu, H.; Bakenov, Z.; Gosselink, D.; Chen, P. Rechargeable hybrid aqueous batteries. J. Power Sources 2012, 216, 222–226. [Google Scholar] [CrossRef]
- Yuan, G.H.; Bai, J.T.; Doan, T.N.L.; Chen, P. Synthesis and electrochemical investigation of nanosized LiMn2O4 as cathode material for rechargeable hybrid aqueous batteries. Mater. Lett. 2014, 137, 311–314. [Google Scholar] [CrossRef]
- Yuan, G.H.; Bai, J.T.; Doan, T.N.L.; Chen, P. Synthesis and electrochemical properties of LiFePO4/graphene composite as a novel cathode material for rechargeable hybrid aqueous battery. Mater. Lett. 2015, 158, 248–251. [Google Scholar] [CrossRef]
- Han, Z.X.; Askhatova, D.; Doan, T.N.L.; Hoang, T.K.A.; Chen, P. Experimental and mathematical studies on cycle life of rechargeable hybrid aqueous batteries. J. Power Sources 2015, 279, 238–245. [Google Scholar] [CrossRef]
- Zhu, X.; Doan, T.N.L.; Yu, Y.; Tian, Y.; Sun, K.E.K.; Zhao, H.B.; Chen, P. Enhancing rate performance of LiMn2O4 cathode in rechargeable hybrid aqueous battery by hierarchical carbon nanotube/acetylene black conductive pathways. Ionics 2016, 22, 71–76. [Google Scholar] [CrossRef]
- Lu, C.Y.; Hoang, T.K.A.; Doan, T.N.L.; Zhao, H.B.; Pan, R.; Yang, L.; Guan, W.S.; Chen, P. Rechargeable hybrid aqueous batteries using silica nanoparticle doped aqueous electrolytes. Appl. Energy 2016, 170, 58–64. [Google Scholar] [CrossRef]
- Gallagher, K.G.; Fuller, T.F. Kinetic model of the electrochemical oxidation of graphitic carbon in acidic environments. Phys. Chem. Chem. Phys. 2009, 11, 11557–11567. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.J.; Xu, Q.; Yan, C.W.; Qiao, Y.L. Corrosion behavior of a positive graphite electrode in vanadium redox flow battery. Electrochim. Acta 2011, 56, 8783–8790. [Google Scholar] [CrossRef]
- Dekanski, A.; Stevanović, J.; Stevanović, R.; Nikolić, B.Z.; Jovanović, V.M. Glassy carbon electrodes I. Characterization and electrochemical activation. Carbon 2001, 39, 1195–1205. [Google Scholar] [CrossRef]
- Hassel, O. Ueber die Kristallstruktur des Graphits. Z. Phys. 1924, 25, 317–337. [Google Scholar] [CrossRef]
- Lipson, H.; Stokes, A.R. The Structure of Graphite. Proc. R. Soc. Math. Phys. Eng. Sci. 1942, 181, 101–105. [Google Scholar]
- Biscoe, J.; Warren, B.E. An X-ray Study of Carbon Black. J. Appl. Phys. 1942, 13, 364. [Google Scholar] [CrossRef]
- Harris, P.J.F. Fullerene-related structure of commercial glassy carbons. Phisolophical Mag. 2004, 84, 3159–3167. [Google Scholar] [CrossRef]
- Marinho, B.; Ghislandi, M.; Tkalya, E.; Koning, C.E.; de With, G. Electrical conductivity of compacts of graphene, multi-wall carbon nanotubes, carbon black, and graphite powder. Powder Technol. 2012, 221, 351–358. [Google Scholar] [CrossRef]
- Giet, C. Acetylene Black with High Electrical Conductivity and High Absorptive Power. US Patent US4279880A, 21 July 1981. [Google Scholar]
- Rimbu, G.A.; Stamatin, I.; Jackson, C.L.; Scott, K. The Morphology Control of Poloyaniline as Conducting Polymer in Fuel Cell Technology. J. Optoelectron. Adv. Mater. 2006, 8, 670–674. [Google Scholar]
- Cho, I.; Choi, J.; Kim, K.; Ryou, M.-H.; Lee, Y.M. A comparative investigation of carbon black (Super-P) and vapor-grown carbon fibers (VGCFs) as conductive additives for lithium-ion battery cathodes. RSC Adv. 2015, 5, 95073–95078. [Google Scholar] [CrossRef]
- Jurkiewicz, K.; Pawlyta, M.; Burian, A. Structure of Carbon Materials Explored by Local Transmission Electron Microscopy and Global Powder Diffraction Probes. J. Carbon Res. 2018, 4, 68. [Google Scholar] [CrossRef]
- Parobek, D.; Liu, H.T. Wettability of graphene. 2D Mater. 2015, 2, 032001. [Google Scholar] [CrossRef]
- Kwok, D.Y.; Neumann, A.W. Contact Angle Measurement and Contact Angle Interpretation. Adv. Colloid Interface Sci. 1999, 81, 167–249. [Google Scholar] [CrossRef]
- Tavana, H.; Lam, C.N.C.; Friedel, P.; Grundke, K.; Kwok, D.Y.; Hair, M.L.; Neumann, A.W. Contact angle measurements with liquids consisting of bulky molecules. J. Colloid Interface Sci. 2004, 279, 493–502. [Google Scholar] [CrossRef]
- Tavana, H.; Neumann, A.W. Recent progress in the determination of solid surface tensions from contact angles. Adv. Colloid Interface Sci. 2007, 132, 1–32. [Google Scholar] [CrossRef]
- Del Río, O.I.; Neumann, A.W. Axisymmetric drop shape analysis: Computational methods for the measurement of interfacial properties from the shape and dimensions of pendant and sessile drops. J. Colloid Interface Sci. 1997, 196, 136–147. [Google Scholar]
- Saad, S.M.I.; Policova, Z.; Neumann, A.W. Design and accuracy of pendant drop methods for surface tension measurement. Colloids Surf. Phys. Eng. Asp. 2011, 384, 442–452. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doan, T.N.L.; Hoang, T.K.A.; Saad, S.M.I.; Chen, P. Evaluation of the Stability of Carbon Conductor in the Cathode of Aqueous Rechargeable Lithium Batteries against Overcharging. Batteries 2020, 6, 59. https://doi.org/10.3390/batteries6040059
Doan TNL, Hoang TKA, Saad SMI, Chen P. Evaluation of the Stability of Carbon Conductor in the Cathode of Aqueous Rechargeable Lithium Batteries against Overcharging. Batteries. 2020; 6(4):59. https://doi.org/10.3390/batteries6040059
Chicago/Turabian StyleDoan, The Nam Long, Tuan K. A. Hoang, Sameh M. I. Saad, and P. Chen. 2020. "Evaluation of the Stability of Carbon Conductor in the Cathode of Aqueous Rechargeable Lithium Batteries against Overcharging" Batteries 6, no. 4: 59. https://doi.org/10.3390/batteries6040059
APA StyleDoan, T. N. L., Hoang, T. K. A., Saad, S. M. I., & Chen, P. (2020). Evaluation of the Stability of Carbon Conductor in the Cathode of Aqueous Rechargeable Lithium Batteries against Overcharging. Batteries, 6(4), 59. https://doi.org/10.3390/batteries6040059



