Lightweight Polymer-Carbon Composite Current Collector for Lithium-Ion Batteries
Abstract
1. Introduction
2. Results and Discussion
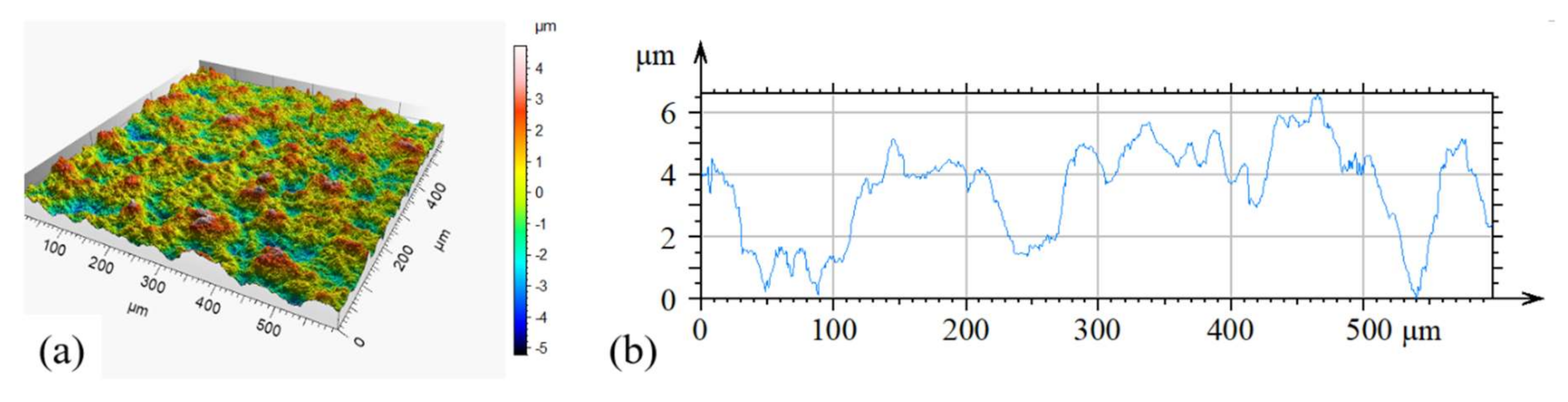
2.1. Characterization of the Polymer-Carbon Composite and Electrodes
2.2. Electrical and Electrochemical Characterization
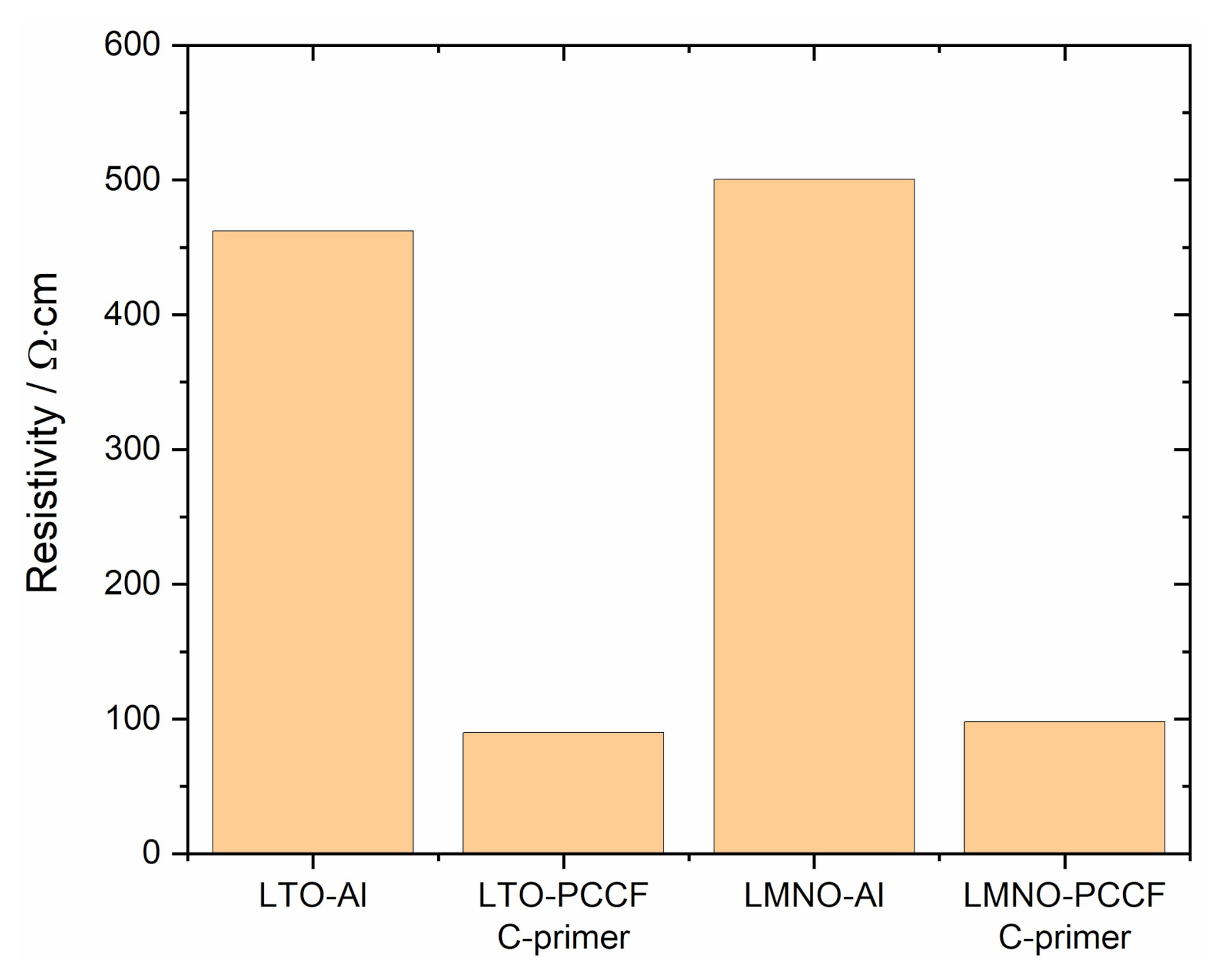
2.2.1. Electrical Properties and Advantage of Carbon Primer
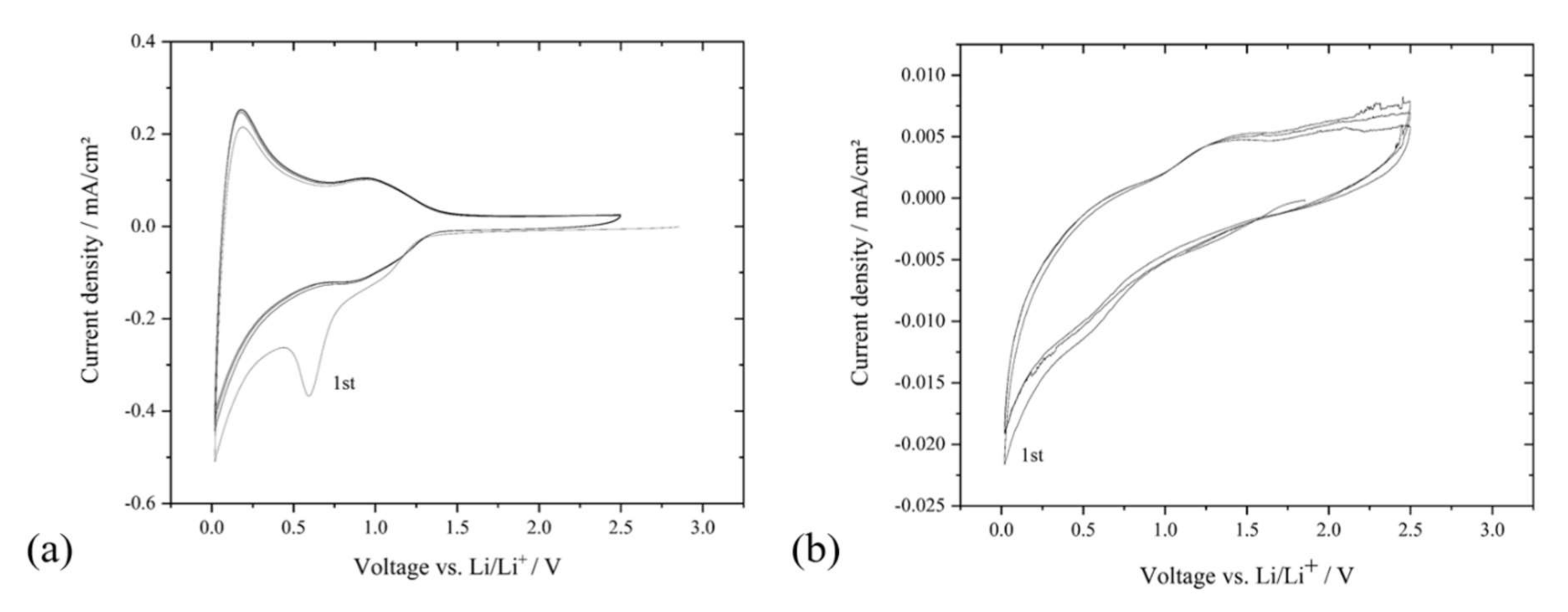
2.2.2. Electrochemical Stability of PCCF Collector between 0 V to 5 V
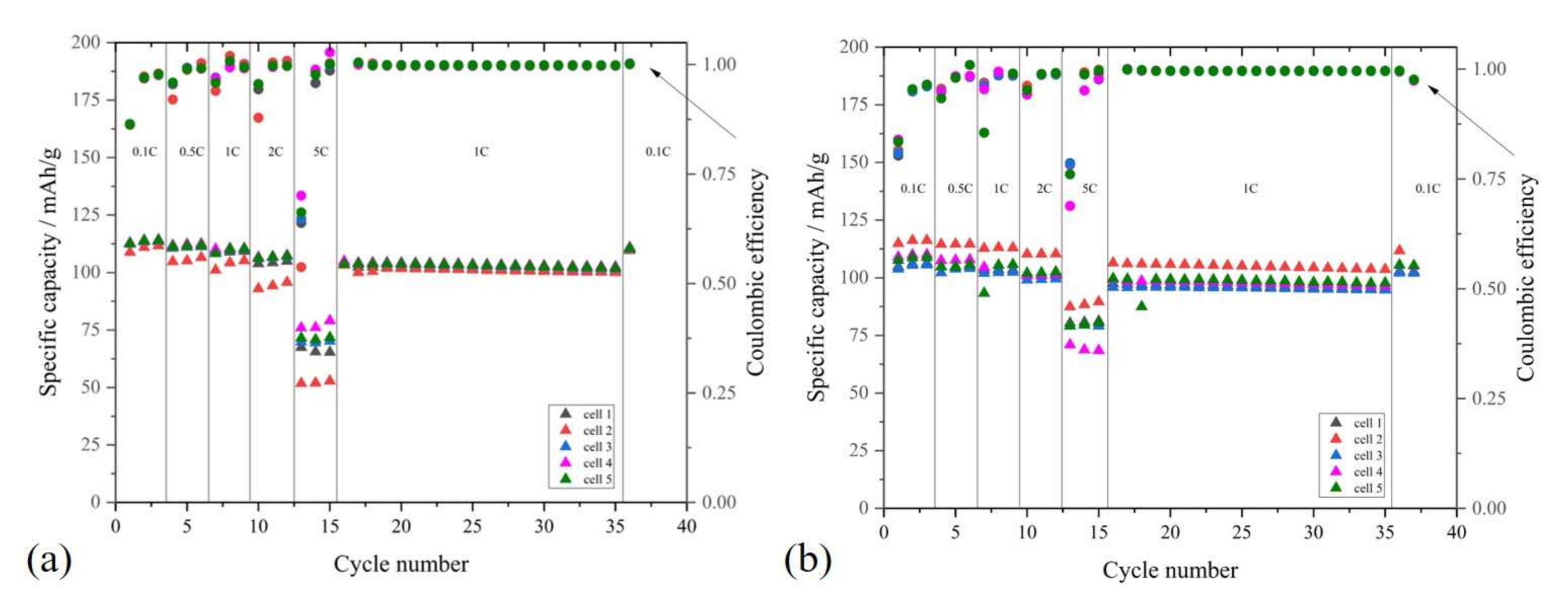
2.2.3. C-Rate Performance Test of LMNO and LTO on Al and PCCF Collector
2.3. Discussion of PCCF as an Alternative Current Collector for Li-Ion Batteries
3. Materials and Methods
3.1. Polymer-Carbon Collector Foil (PCCF)
3.2. LMNO, LTO and C-Primer Electrode Coatings
3.3. Material Characterization
3.3.1. Polymer-Carbon Collector Foil (PCCF)
3.3.2. Electrode Coatings
3.3.3. Electrical Measurements
3.4. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marsha, R.A.; Russell, P.G.; Reddy, T.B. Bipolar lithium-ion battery development. J. Power Sources 1997, 65, 133–141. [Google Scholar] [CrossRef]
- Jung, K.; Shin, H.; Park, M. Solid-State Lithium Batteries: Bipolar Design, Fabrication and Electrochemistry. ChemElectroChem 2019, 6, 3842–3859. [Google Scholar] [CrossRef]
- Iwakura, C.; Fukumoto, Y.; Inoue, H.; Ohashi, S.; Kobayashi, S.; Tada, H.; Abe, M. Electrochemical characterization of various metal collectors as a current collector of positive electrode for rechargeable lithium batteries. J. Power Sources 1997, 68, 301–303. [Google Scholar] [CrossRef]
- Whitehead, A.H.; Schreiber, M. Current Collectors for Positive Electrodes of Lithium-Based Batteries. J. Electrochem. Soc. 2005, 152, A2105–A2113. [Google Scholar] [CrossRef]
- Myung, S.-T.; Hitoshi, Y.; Sun, Y.-K. Electrochemical behavior and passivation of current collectors in lithium-ion batteries. J. Mater. Chem. 2011, 21, 9891–9911. [Google Scholar] [CrossRef]
- Stich, M.; Fritz, M.; Roscher, M.; Peipmann, R. Korrosionsverhalten von bipolaren Stromableitern für Lithium-Ionen-Batterien (Teil 1). Galvanotechnik 2018, 12, 2330–2336. [Google Scholar]
- Stich, M.; Fritz, M.; Roscher, M.; Peipmann, R. Korrosionsverhalten von bipolaren Stromableitern für Lithium-Ionen-Batterien (Teil 2). Galvanotechnik 2019, 1, 67–72. [Google Scholar]
- Beikai, Z. The Height that a Kind of Bipolar Template Transitionality Unit Lithium Battery of Cu-Al Bimetal and Its Series Connection Are Formed Forces down Changeable Internal Damp Battery Heap and Method for Packing. China Patent 104916864B, 16 January 2018. [Google Scholar]
- Culver, D.; Dyer, C.K.; Epstein, M.L. Modular Battery with Battery Cell Having Bimetallic End. U.S. Patent 20110200867, 18 August 2011. [Google Scholar]
- Hossain, S. Bipolar Lithium-Ion Rechargeable Battery. U.S. Patent 5595839, 21 January 1997. [Google Scholar]
- Braunovic, M.; Aleksandrov, N. Effect of electrical current on the morphology and kinetics of formation of intermetallic phases in bimetallic aluminum-copper joints. In Proceedings of the IEEE Holm Conference on Electrical Contacts, Pittsburgh, PA, USA, 27–29 September 1993; pp. 261–268. [Google Scholar] [CrossRef]
- Silveria, V.L.A.; Mury, A.G. Analysis of the behavior of bimetallic joints (Al /Cu). J. Microstruct. Sci. 1987, 14, 277–287. [Google Scholar]
- Abbasi, M.; Taheri, A.K.; Salehi, M.T. Growth rate of intermetallic compounds in Al/Cu bimetal produced by cold roll welding process. J. Alloy. Compd. 2001, 319, 233–241. [Google Scholar] [CrossRef]
- Fritsch, M.; Standke, G.; Heubner, C.; Langklotz, U.; Michaelis, A. 3D-cathode design with foam-like aluminum current collector for high energy density lithium-ion batteries. J. Energy Storage 2018, 16, 125–132. [Google Scholar] [CrossRef]
- Li, S. Corrosion of Aluminum Current Collector in Cost Effective Rechargeable Lithium-Ion Batteries. Dissertation, No.1384, University of Wisconsin-Milwaukee, USA. 2016. Available online: https://dc.uwm.edu/etd/1384 (accessed on 4 December 2020).
- Ma, T.; Xu, G.-L.; Li, Y.; Wang, L.; He, X.; Zheng, J.; Liu, J.; Engelhard, M.H.; Zapol, P.; Curtiss, L.A.; et al. Revisiting the Corrosion of the Aluminum Current Collector in Lithium-Ion Batteries. J. Phys. Chem. Lett. 2017, 8, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Cho, K.Y. Current Collectors for Flexible Lithium Ion Batteries: A Review of Materials. J. Electrochem. Sci. Technol. 2015, 6, 1–6. [Google Scholar] [CrossRef]
- Foreman, E.; Zakri, W.; Sanatimoghaddam, M.H.; Modjtahedi, A.; Pathak, S.; Kashkooli, A.G.; Garafolo, N.G.; Farhad, S. A Review of Inactive Materials and Components of Flexible Lithium-Ion Batteries. Adv. Sustain. Syst. 2017, 1, 1700061. [Google Scholar] [CrossRef]
- Wang, K.; Wu, Y.; Wu, H.; Luo, Y.; Wang, D.; Jiang, K.; Li, Q.; Li, Y.; Fan, S.; Wang, J. Super-aligned carbon nanotube films with a thin metal coating as highly conductive and ultralight current collectors for lithium-ion batteries. J. Power Sources 2017, 351, 160–168. [Google Scholar] [CrossRef]
- Evanko, B.; Yoo, S.J.; Lipton, J.; Chun, S.-E.; Moskovits, M.; Ji, X.; Boettcher, S.W.; Stucky, G.D. Stackable bipolar pouch cells with corrosion resistant current collectors enable high-power aqueous electrochemical energy storage. Energy Environ. Sci. 2018, 11, 2865–2875. [Google Scholar] [CrossRef]
- Krause, B.; Barbier, C.; Kunz, K.; Pötschke, P. Comparative study of singlewalled, multiwalled, and branched carbon nanotubes melt mixed in different thermoplastic matrices. Polymer 2018, 159, 75–85. [Google Scholar] [CrossRef]
- Kunz, K.; Krause, B.; Kretzschmar, B.; Juhasz, L.; Kobsch, O.; Jenschke, W.; Ulrich, M.; Pötschke, P. Direction dependent electrical conductivity of polymer/carbon filler composites. Polymers 2019, 11, 591. [Google Scholar] [CrossRef]
- Krause, B.; Kunz, K.; Kretzschmar, B.; Kühnert, I.; Pötschke, P. Effect of filler synergy and cast film extrusion parameters on extrudability and direction-dependent conductivity of PVDF/carbon nanotube/carbon black composites. Polymers 2020. Submitted for publication. [Google Scholar]
- Hampel, U.; Denkmann, V.; Siemen, A.; Eckhard, K.; Schenkel, W.; Eberhard, S.; Bögershausen, D. Chemically Treated Current Collector Foil Made of Aluminium or an Aluminium Alloy. U.S. Patent 9160006B2, 13 October 2015. [Google Scholar]
- Kunz, K.; de Limé, A.d.B.; Seeba, J.; Reuber, S.; Wolter, M.; Michaelis, A. Possibilities for Processing of All Solid State Batteries/Components as Pilot Plant Scale. Poster, Dresden Battery Days, 23–25 September 2019. Available online: https://www.energy-saxony.net/veranstaltungen/dresden-battery-days-23-25092019.html (accessed on 4 December 2020).
- Sacher, E. (Ed.) Metallization of Polymers 2; Springer: Boston, MA, USA, 2002; ISBN 978-1-4615-0563-1. [Google Scholar] [CrossRef]
- Nakanishi, S.; Suzuki, T.; Cui, Q.; Akikusa, J.; Nakamura, K. Effect of surface treatment for aluminum foils on discharge properties of lithium-ion battery. Trans. Nonferr. Met. Soc. China 2014, 24, 2314–2319. [Google Scholar] [CrossRef]
- Nara, H.; Mukoyama, D.; Shimizu, R.; Momma, T.; Osaka, T. Systematic analysis of interfacial resistance between the cathode layer and the current collector in lithium-ion batteries by electrochemical impedance spectroscopy. J. Power Sources 2019, 409, 139–147. [Google Scholar] [CrossRef]
- Heubner, C.; Nickol, A.; Seeba, J.; Reuber, S.; Junker, N.; Wolter, M.; Schneider, M.; Michaelis, A. Understanding thickness and porosity effects on the electrochemical performance of LiNi0.6Co0.2Mn0.2O2-based cathodes for high energy Li-ion batteries. J. Power Sources 2019, 419, 119–126. [Google Scholar] [CrossRef]
- Heubner, C.; Schneider, M.; Michaelis, A. Diffusion-Limited C-Rate: A Fundamental Principle Quantifying the Intrinsic Limits of Li-Ion Batteries. Adv. Energy Mater. 2020, 10, 1902523. [Google Scholar] [CrossRef]
- Seidel, M.; Kugaraj, M.; Nikolowski, K.; Wolter, M.; Kinski, I.; Jähnert, T.; Michaelis, A. Comparison of Electrochemical Degradation for Spray Dried and Pulse Gas Dried LiNi0.5Mn1.5O4. J. Electrochem. Soc. 2019, 166, A2860–A2869. [Google Scholar] [CrossRef]
- Kovachev, G.; Schröttner, H.; Gstrein, G.; Aiello, L.; Hanzu, I.; Martin, H.; Wilkening, R.; Foitzik, A.; Wellm, M.; Sinz, W.; et al. Analytical Dissection of an Automotive Li-Ion Pouch Cell. Batteries 2019, 5, 67. [Google Scholar] [CrossRef]
- Wang, M.; Tan, Q.; Liu, L.; Li, J.; Facile, A. Environmentally Friendly, and Low-Temperature Approach for Decomposition of Polyvinylidene Fluoride from the Cathode Electrode of Spent Lithium-ion Batteries. ACS Sustain. Chem. Eng. 2019, 7, 12799–12806. [Google Scholar] [CrossRef]
- Berga, H.; Zackrisson, M. Perspectives on environmental and cost assessment of lithium metal negative electrodes in electric vehicle traction batteries. J. Power Sources 2019, 415, 83–90. [Google Scholar] [CrossRef]
- Emilsson, E.; Dahllöf, L. Lithium-Ion Vehicle Battery Production—Status 2019 on Energy Use, CO2 Emissions, Use of Metals, Products Environmental Footprint, and Recycling; Report number C 444; IVL Swedish Environmental Research Institute Ltd.: Stockholm, Sweden, 2019; ISBN 978-91-7883-112-8. [Google Scholar]
- Nanomanufacturing—Key Control Characteristics—Part 4-3: Nano-Enabled Electrical Energy Storage—Contact and Coating Resistivity Measurements for Nanomaterials, DIN IEC/TS 62607-4-3:2018-07. 2018. Available online: https://dx.doi.org/10.31030/2534286 (accessed on 4 December 2020).






| Collector Type | Et. (MPa) | σB (MPa) | εB (%) |
|---|---|---|---|
| PCCF collector ‖ | 2105 ± 158 | 39 ± 1 | 3.9 ± 0.3 |
| PCCF collector ⊥ | 1557 ± 518 | 31 ± 3 | 4.1 ± 0.9 |
| Al-foil *1 | 45205 ± 2760 | 146 ± 3 | 1.2 ± 0.1 |
| Sample | Material | Size (cm2) | Thickness (µm) | Area Weight (mg/cm2) |
|---|---|---|---|---|
| PCCF | PVDF-carbon composite | roll | 55 ± 5 | 8.7 ± 0.8 |
| PCCF + C-primer | batch of 9 sheets | 12 × 16 | 70 ± 2 | 9.8 ± 0.1 |
| Al collector | Al-alloy EN AW 1085-L H18 | roll | 19 ± 1 | 4.8 ± 0.3 |
| Sample | Thickness (µm) | Area Weight (mg/cm2) | Electrode Film | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
densification
| Sample | Electrode film | Sample | Electrode film | Density (g/cm3) | Porosity *3 (vol.-%) | |||||
| (1) | (2) | (1) | (2) | (1,2) | (1,2) | theor. | (1) | (2) | (1) | (2) | |
| LTO on Al *1 | 127 | 88 | 108 | 69 | 15.94 | 11.13 | 2.77 | 1.03 | 1.61 | 63 | 42 |
| LTO on PCCF *2 | 210 | 149 | 140 | 79 | 23.11 | 13.31 | 2.77 | 0.95 | 1.68 | 66 | 39 |
| LMNO on Al *1 | 140 | 83 | 121 | 64 | 16.98 | 12.17 | 3.29 | 1.01 | 1.90 | 69 | 42 |
| LMNO on PCCF *2 | 194 | 133 | 124 | 63 | 21.88 | 12.03 | 3.29 | 0.97 | 1.91 | 71 | 42 |
| Component | Direction | Resistivity (Ω·cm) |
|---|---|---|
| PCCF *1 | in-plane parallel and | 0.7 |
| perpendicular to extrusion direction | 2.7 | |
| through-thickness | 26 | |
| C-primer *2 | in-plane | 0.3 |
| Al-foil *3 | in-plane and through-thickness | 5.7 × 10−6 |
| LTO on Al | through-thickness | 460 |
| LTO on PCCF *4 | 90 | |
| LMNO on Al | 500 | |
| LMNO on PCCF *4 | 100 |
| Filler Content | σx/σz (-) * | σy/σz (-) * | σx/σy (-) * |
|---|---|---|---|
| 1 wt% b-MWCNT [22] | 166 | 42 | 4 |
| 4 wt% CB [22] | 5 | 4 | 1 |
| PCCF (1 wt% b-MWCNT + 3 wt% CB) [23] | 26 | 8 | 3 |
| Sample | Average Capacity at 0.1 C Begin of Cycling (mAh/g) | Average Capacity at 0.1 C End of Cycling (mAh/g) | Average Cell Resistance Begin of Cycling (Ω) | Average Cell Resistance End of Cycling (Ω) |
|---|---|---|---|---|
| LTO on Al | 167 ± 0.6 | 166 ± 0.5 | 39 ± 7.1 | 18 ± 1.0 |
| LTO on PCCF | 165 ± 3.8 | 163 ± 3.9 | 40 ± 10.6 | 18 ± 1.9 |
| LMNO on Al | 114 ± 0.4 | 102 ± 1.0 | 24 ± 6.1 | 15 ± 2.2 |
| LMNO on PCCF | 108 ± 2.4 | 104 ± 2.0 | 28 ± 2.4 | 20 ± 2.8 |
| Collector Material | Density (g/cm3) | Typical Thickness (µm) | Mass Loading (mg/cm2) | Mass Loading Relative to Al *3 (%) |
|---|---|---|---|---|
| Al-foil | 2.5–2.7 | 20–30 *1 | 5.0–8.1 | 100 |
| Cu-foil | 8.9 | 9–18 *1 | 8.0–16.0 | 160 |
| PCCF-C-primer (this study) | 1.4 | 70 *2 | 9.8 | 196 |
| PCCF-C-primer (potential) | 1.4 to 1.5 *4 | 25 to 40 | 3.8 to 6.0 | 76 |
| PCCF composite (potential) *5 | 1.58 | 20 to 30 | 3.2 to 4.7 | 64 |
| Component | Price (EUR/kg) | Thickness (µm) | Density (g/cm3) | Volume (cm3 @ 1 m2) | Mass (g @ 1 m2) | Price (EURct for 1 m2) | Price Factor Rel. to Al |
|---|---|---|---|---|---|---|---|
| Al collector | 26.85 | 20 | 2.5 | 20 | 50 | 1.34 | 1.0 |
| Cu-collector | 66.74 | 10 | 8.9 | 10 | 89 | 5.94 | 4.4 |
| PCCF-C-primer (this study) | 122.20 | 70 | 1.4 | 70 | 98 | 11.98 | 8.9 |
| PCCF-C-primer (potential) | 38.0 | 25 | 1.4 | 25 | 35 | 1.33 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fritsch, M.; Coeler, M.; Kunz, K.; Krause, B.; Marcinkowski, P.; Pötschke, P.; Wolter, M.; Michaelis, A. Lightweight Polymer-Carbon Composite Current Collector for Lithium-Ion Batteries. Batteries 2020, 6, 60. https://doi.org/10.3390/batteries6040060
Fritsch M, Coeler M, Kunz K, Krause B, Marcinkowski P, Pötschke P, Wolter M, Michaelis A. Lightweight Polymer-Carbon Composite Current Collector for Lithium-Ion Batteries. Batteries. 2020; 6(4):60. https://doi.org/10.3390/batteries6040060
Chicago/Turabian StyleFritsch, Marco, Matthias Coeler, Karina Kunz, Beate Krause, Peter Marcinkowski, Petra Pötschke, Mareike Wolter, and Alexander Michaelis. 2020. "Lightweight Polymer-Carbon Composite Current Collector for Lithium-Ion Batteries" Batteries 6, no. 4: 60. https://doi.org/10.3390/batteries6040060
APA StyleFritsch, M., Coeler, M., Kunz, K., Krause, B., Marcinkowski, P., Pötschke, P., Wolter, M., & Michaelis, A. (2020). Lightweight Polymer-Carbon Composite Current Collector for Lithium-Ion Batteries. Batteries, 6(4), 60. https://doi.org/10.3390/batteries6040060








