Battery Scrap and Biochar Utilization for Improved Metal Recoveries in Nickel Slag Cleaning Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Reduction Experiments
2.3. Analytical Procedure
3. Results
3.1. Microstructure
3.2. Slag Composition
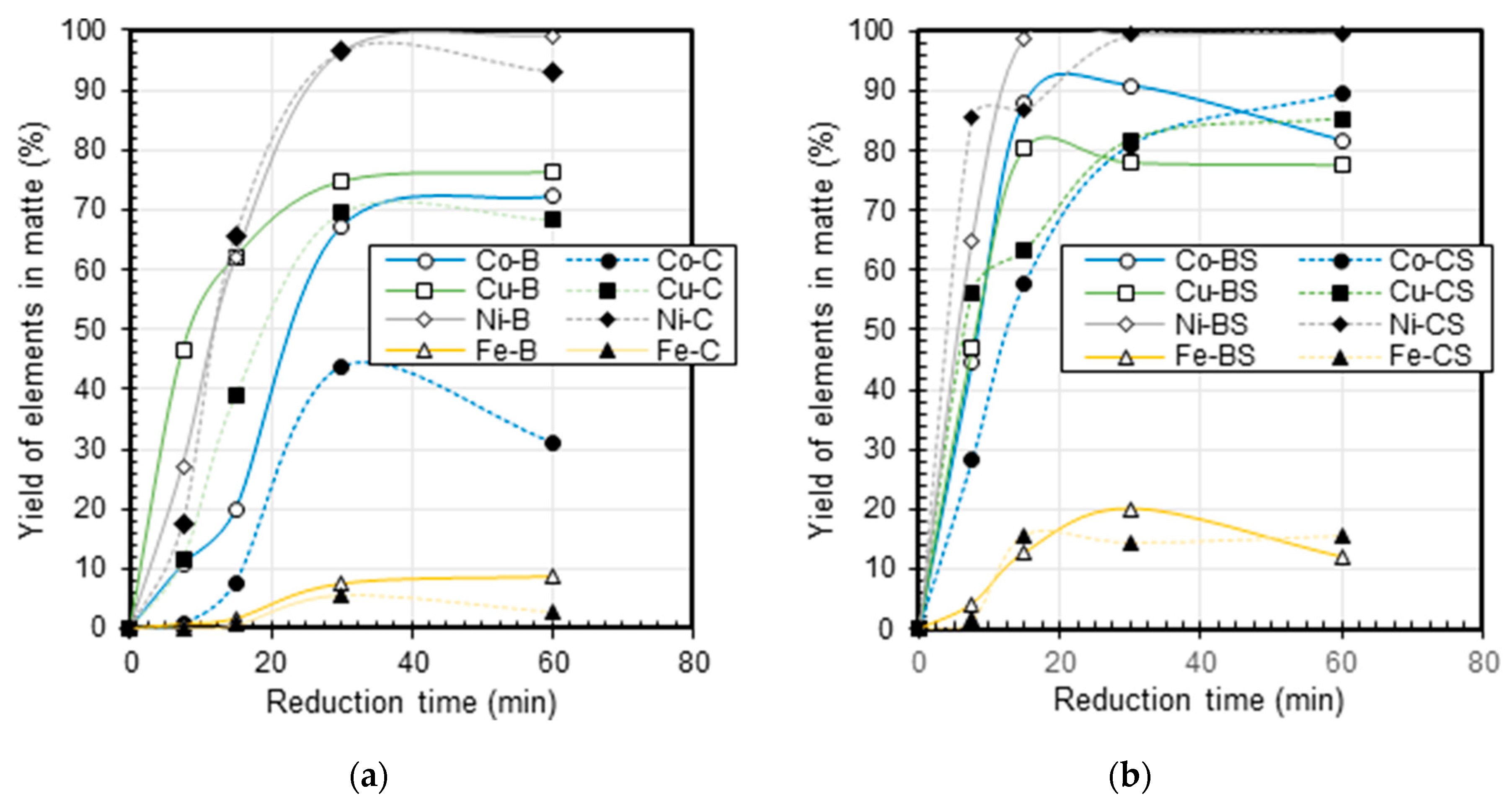
3.3. Matte Composition
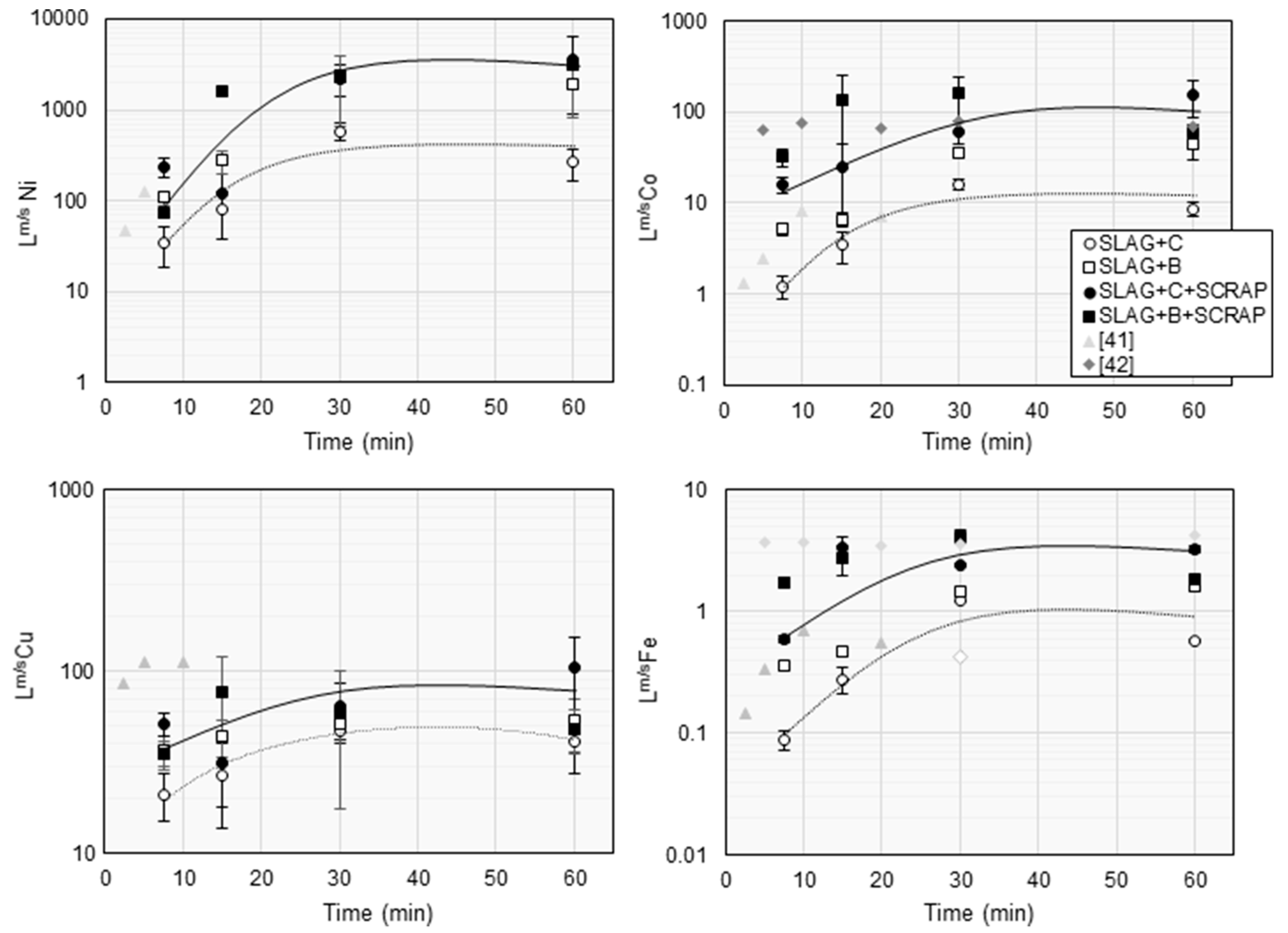
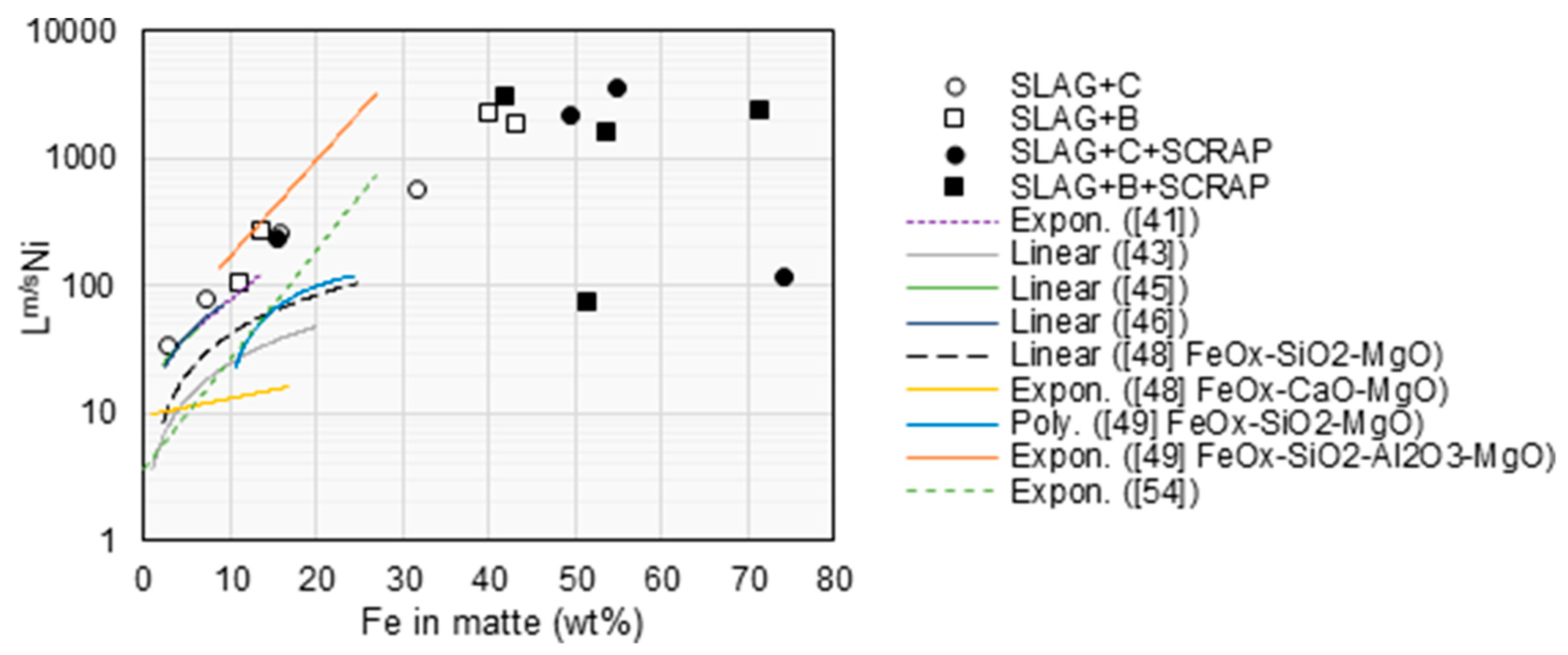
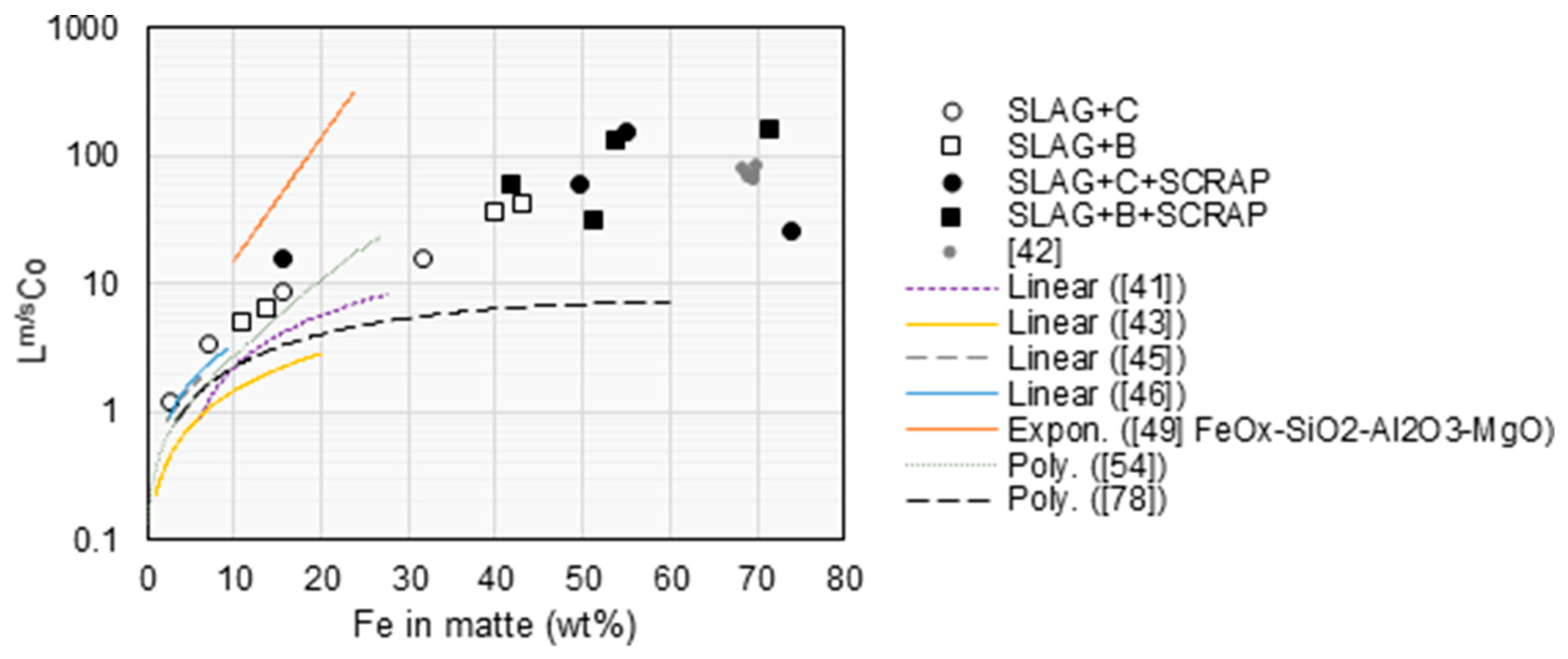
4. Discussion
Lm/sCu 70 >> Lm/sFe 4.
5. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alves Dias, P.; Blagoeva, D.; Pavel, C.; Arvanitidis, N. Cobalt Demand-Supply Balances in the Transition to Electric Mobility; Report EUR, 29381; Publications Office of the European Union: Luxembourg, 2018; p. 100. [Google Scholar]
- European Commission written by Deloitte Sustainability; British Geological Survey; Bureau de Recherches Géologiques et Minières; Netherlands Organisation for Applied Scientific Research. Study on the Review of the List of Critical Raw Materials. Critical Raw Materials Factsheet; Publications Office of the European Union: Luxembourg, 2017; p. 93. [Google Scholar]
- Barbera, R.J. Cobalt: Policy Options for a Strategic Mineral; Government Printing Office: Washington, DC, USA, 1982; p. 36.
- Blechman, B.M.; Sloss, D. National Security and Strategic Minerals: An Analysis of U.S. Dependence on Foreign Sources of Cobalt; Routledge: New York, NY, USA, 2018; p. 112. [Google Scholar]
- Scheele, F.; De Haan, E.; Kiezebrink, V. Cobalt Blues. Environmental Pollution and Human Rights Violations in Katanga’s Copper and Cobalt Mines; SOMO (Centre for Research on Multinational Corporations): Amsterdam, The Netherlands, 2016; p. 57. [Google Scholar]
- Crundwell, F.; Moats, M.S.; Ramachandran, V.; Robinson, T.G.; Davenport, W.G. Extractive Metallurgy of Nickel, Cobalt and Platinum-Group Metals; Elsevier: Oxford, UK; Amsterdam, The Netherlands, 2011; p. 610. [Google Scholar]
- Seetharaman, S.; McLean, A.; Guthrie, R.I.L.; Sridhar, S. Treatise on Process. Metallurgy; Elsevier: Oxford, UK; Waltham, MA, USA, 2014; Volume 3, p. 1810. [Google Scholar]
- Mäkinen, T.; Taskinen, P. State of the art in nickel smelting: Direct Outokumpu Nickel Technology. Miner. Process. Extr. Metall. 2008, 117, 86–94. [Google Scholar] [CrossRef]
- Taskinen, P.; Seppälä, K.; Laulumaa, J.; Poijärvi, J. Oxygen Pressure in the Outokumpu Flash Smelting Furnace—Part 2: The DON Process. Transactions of the Institutions of Mining and Metallurgy Section C: Mineral. Process. Extr. Metall. 2001, 110, C101–C108. [Google Scholar] [CrossRef]
- Toscano, P. Minimization of Dissolved Nickel and Cobalt Slag Losses at High Matte Grades. Master’s Thesis, Department of Material Sciences and Engineering, University of Toronto, Toronto, ON, Canada, 2001; p. 112. [Google Scholar]
- Jones, R.T.; Hayman, D.A.; Denton, G.M. Recovery of Cobalt, Nickel, and Copper from Slags, Using DC-Arc Furnace Technology. Pyrometallurgy Div. 1998, 2125, 1–17. [Google Scholar]
- Ye, L.; Peng, Z.; Wang, L.; Anzulevich, A.; Bychkov, I.; Kalganov, D.; Tang, H.; Rao, M.; Li, G.; Jiang, T. Use of Biochar for Sustainable Ferrous Metallurgy. JOM 2019, 71, 3931–3940. [Google Scholar] [CrossRef]
- Hakala, J.; Kangas, P.; Penttilä, K.; Alarotu, M.; Björnström, M.; Koukkari, P. Replacing Coal Used in Steelmaking with Biocarbon from Forest Industry Side Streams; VTT Technology Series 351; VTT Technical Research Centre of Finland: Espoo, Finland, 2019; p. 141. [Google Scholar]
- Suopajärvi, H.; Pongracz, E.; Fabritius, T. Bioreducer Use in Finnish Blast Furnace Ironmaking—Analysis of CO2 Emission Reduction Potential and Mitigation Cost. Appl. Energy 2014, 124, 82–93. [Google Scholar] [CrossRef]
- Zuo, Z.; Yu, Q.; Wei, M.; Xie, H.; Duan, W.; Wang, K.; Qin, Q. Thermogravimetric Study of the Reduction of Copper Slag by Biomass. J. Therm. Anal. Calorim. 2016, 126, 481–491. [Google Scholar] [CrossRef]
- Zhou, S.; Wei, Y.; Li, B.; Wang, H. Cleaner Recycling of Iron from Waste Copper Slag by Using Walnut Shell Char as Green Reductant. J. Clean. Prod. 2019, 217, 423–431. [Google Scholar] [CrossRef]
- Pillot, C. Lithium Ion Battery Raw Material Supply & Demand 2016–2025. In Proceedings of the Advanced Automotive Battery Conference, Mainz, Germany, 30 January–2 February 2017; Cambridge EnerTech: Needham, MA, USA, 2017; Volume 30. Available online: http://cii-resource.com/cet/AABE-03-17/Presentations/BRMT/Pillot_Christophe.pdf (accessed on 1 December 2020).
- Wang, S. Cobalt-Its Recovery, Recycling, and Application. JOM 2006, 58, 47–50. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J. On the Sustainability of Cobalt Utilization in China. Resour. Conserv. Recycl. 2015, 104, 12–18. [Google Scholar] [CrossRef]
- Sommer, P.; Rotter, V.S.; Ueberschaar, M. Battery Related Cobalt and REE Flows in WEEE Treatment. Waste Manag. 2015, 45, 298–305. [Google Scholar] [CrossRef]
- Georgi-Maschler, T.; Friedrich, B.; Weyhe, R.; Heegn, H.; Rutz, M. Development of a Recycling Process for Li-ion Batteries. J. Power Sources 2012, 207, 173–182. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J.; Singh, N. Recycling of Spent Lithium-Ion Battery: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1129–1165. [Google Scholar] [CrossRef]
- Pinegar, H.; Smith, Y. Recycling of End-of-Life Lithium Ion Batteries, Part I: Commercial Processes. J. Sustain. Metall. 2019, 5, 402–416. [Google Scholar] [CrossRef]
- Cobalt USGS Statistics, Annual and Monthly Publications. National Minerals Information Center. Available online: https://www.usgs.gov/centers/nmic/cobalt-statistics-and-information (accessed on 1 October 2020).
- El-Rassi, K.P.; Utigard, T.A. Rate of Slag Reduction in a Laboratory Electric Furnace—Alternating vs. Direct Current. Metall. Mater. Trans. B 2000, 31, 1187–1194. [Google Scholar] [CrossRef]
- Ma, S.; Han, Y. Study of Extracting Valuable Metals from Nickel Smelting Slag by a Coal-based Reduction Method. J. China Univ. Min. Technol. 2014, 43, 305–308. [Google Scholar]
- Matusewicz, R.; Mounsey, E. Using Ausmelt Technology for the Recovery of Cobalt from Smelter Slags. J. Miner. Met. Mater. Soc. 1998, 50, 53–56. [Google Scholar] [CrossRef]
- Jones, R.; Deneys, A. Using a Direct-Current Arc Furnace to Recover Cobalt from Slags. JOM 1998, 50, 57–61. [Google Scholar] [CrossRef]
- Xu, S. Experimental and Modeling Study on Smelting Reduction Process. In Acta Polytechnica Scandinavica—Chemical Technology and Metallurgy Series; Finnish Academy Tech Sci: Espoo, Finland, 1994; Issue 217; p. 81. [Google Scholar]
- Hayes, P.C.; Okongwu, D.A.; Toguri, J.M. Some Observations of the Reactions between Molten Oxides and Solid Carbon. Can. Metall. Q. 1995, 34, 27–36. [Google Scholar] [CrossRef]
- Warczok, A.; Utigard, T.A. Fayalite Slag Reduction by Solid Graphite. Can. Metall. Q. 1998, 37, 27–39. [Google Scholar] [CrossRef]
- Heo, J.H.; Chung, Y.; Park, J.H. Recovery of Iron and Removal of Hazardous Elements from Waste Copper Slag via a Novel Aluminothermic Smelting Reduction (ASR) Process. J. Clean. Prod. 2016, 137, 777–787. [Google Scholar] [CrossRef]
- Zhou, S.; Wei, Y.; Li, B.; Wang, H. Effect of Iron Phase Evolution on Copper Separation from Slag Via Coal-Based Reduction. Metall. Mater. Trans. B 2018, 49, 3086–3096. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, R.; Chen, Q.; Wei, G.; Hu, S.; Guo, Y. Recovery of Fe, Ni, Co, and Cu from Nickel Converter Slag through Oxidation and Reduction. ISIJ Int. 2018, 58, 2191–2199. [Google Scholar] [CrossRef]
- Banks, C.; Harrison, D. The Recovery of Non-Ferrous Metals from Secondary Copper Smelter Discard Slags. Can. Metall. Q. 1975, 14, 183–190. [Google Scholar] [CrossRef]
- Sorokin, M.L.; Nikolaev, G.; Komkov, A. Slag Cleaning Operation in Nickel Production. In Co-Products and Minor Elements in Non-Ferrous Smelting, Proceedings of the TMS Annual Meeting, Las Vegas, NV, USA, 12–16 February 1995; Theo Lehner; Minerals, Metals & Materials Society: Warrendale, PA, USA, 1995; pp. 67–78. [Google Scholar]
- Pan, J.; Zheng, G.; Zhu, D.; Zhou, X. Utilization of Nickel Slag Using Selective Reduction Followed by Magnetic Separation. Trans. Nonferr. Met. Soc. China 2013, 23, 3421–3427. [Google Scholar] [CrossRef]
- Banda, W.; Morgan, N.; Eksteen, J. The Role of Slag Modifiers on the Selective Recovery of Cobalt and Copper from Waste Smelter Slag. Miner. Eng. 2002, 15, 899–907. [Google Scholar] [CrossRef]
- Kucharski, M.; Sak, T.; Madej, P.; Wedrychowicz, M.; Mroz, W. A Study on the Copper Recovery from the Slag of the Outokumpu Direct-to-Copper Process. Metall. Mater. Trans. B 2014, 45, 590–602. [Google Scholar] [CrossRef]
- González, C.; Parra, R.; Klenovcanova, A.; Imris, I.; Sánchez, M. Reduction of Chilean Copper Slags: A Case of Waste Management Project. Scand. J. Metall. 2005, 34, 143–149. [Google Scholar] [CrossRef]
- Ruismäki, R.; Dańczak, A.; Klemettinen, L.; Taskinen, P.; Lindberg, D.; Jokilaakso, A. Integrated Battery Scrap Recycling and Nickel Slag Cleaning with Methane Reduction. Minerals 2020, 10, 435. [Google Scholar] [CrossRef]
- Ruismäki, R.; Rinne, T.; Dańczak, A.; Taskinen, P.; Serna Guerrero, R.; Jokilaakso, A. Integrating Flotation and Pyrometallurgy for Recovering Graphite and Valuable Metals from Battery Scrap. Metals 2020, 10, 680. [Google Scholar] [CrossRef]
- Toscano, P.; Utigard, T. Nickel, Copper, and Cobalt Slag Losses During Converting. Metall. Mater. Trans. B 2003, 34, 121–125. [Google Scholar] [CrossRef]
- Choi, N.; Cho, W.D. Distribution Behavior of Cobalt, Selenium, and Tellurium between Nickel-Copper-Iron Matte and Silica-Saturated Iron Silicate Slag. Metall. Mater. Trans. B 1997, 28, 429–438. [Google Scholar] [CrossRef]
- Sukhomlinov, D.; Klemettinen, L.; Virtanen, O.; Lahaye, Y.; Latostenmaa, P.; Jokilaakso, A.; Taskinen, P. Trace Element Distributions between Matte and Slag in Direct Nickel Matte Smelting. Can. Metall. Q. 2020, 59, 67–77. [Google Scholar] [CrossRef]
- Piskunen, P.; Avarmaa, K.; Klemettinen, L.; Johto, H.; Taskinen, P. Precious Metal Distributions in Direct Nickel Matte Smelting with Low-Cu Mattes. Metall. Mater. Trans. B 2018, 49, 98–112. [Google Scholar] [CrossRef]
- Henao, H.; Itagaki, K. Phase Equilibrium and Distribution of Minor Elements between Ni-S Melt and Al2O3-CaO-MgO Slag at 1873 K. Metall. Mater. Trans. 2004, 35, 1041–1049. [Google Scholar] [CrossRef][Green Version]
- Henao, H.; Hino, M.; Itagaki, K. Phase Equilibrium between Ni-S Melt and FeOx-SiO2 or FeOx-CaO Based Slag under Controlled Partial Pressures. Mater. Trans. 2002, 43, 2219–2227. [Google Scholar] [CrossRef][Green Version]
- Henao, H.; Hino, M.; Itagaki, K. Distribution of Ni, Cr, Mn, Co and Cu between Fe-Ni alloy and FeOx-MgO-SiO2 Base Slags. Mater. Trans. 2001, 42, 1959–1966. [Google Scholar] [CrossRef][Green Version]
- Font, J.; Hino, M.; Itagaki, K. Phase Equilibrium and Minor Elements Distribution between Iron-Silicate Base Slag and Nickel-Copper-Iron Matte at 1573 K under High Partial Pressures of SO2. Mater. Trans. JIM 1999, 40, 20–26. [Google Scholar] [CrossRef][Green Version]
- Font, J.; Hino, M.; Itagaki, K. Minor Elements Distribution between Iron-Silicate Base Slag and Ni3S2-FeS Matte under High Partial Pressures of SO2. Mater. Trans. JIM 1998, 39, 834–840. [Google Scholar] [CrossRef]
- Font, J.; Takeda, Y.; Itagaki, K. Phase Equilibrium between Iron-Silicate Base Slag and Nickel-Iron Matte at 1573 K under High Partial Pressures of SO2. Mater. Trans. JIM 1998, 39, 652–657. [Google Scholar] [CrossRef][Green Version]
- Andrews, L.; Pistorius, P.C. Nickel, Copper and Cobalt Distribution and Equilibria in Anglo Platinum Furnace Slags. Miner. Process. Extr. Metall. 2010, 119, 52–59. [Google Scholar] [CrossRef]
- Li, G.; Tsukihashi, F. Distribution Equilibria of Fe, Co and Ni between MgO-Saturated FeOx-MgO-SiO2 Slag and Ni Alloy. ISIJ Int. 2001, 41, 1303–1308. [Google Scholar] [CrossRef][Green Version]
- Pagador, R.U.; Hino, M.; Itagaki, K. Phase Equilibrium between FeOx-MgO-SiO2 or FeOx-CaO-MgO-SiO2 Slag and Nickel Alloy. Shigen-to-Sozai 1998, 114, 127–132. [Google Scholar] [CrossRef][Green Version]
- Pagador, R.; Hino, M.; Itagaki, K. Distribution of Minor Elements between MgO Saturated FeOx-MgO-SiO2 or FeOx-CaO-MgO-SiO2 Slag and Nickel Alloy. Mater. Trans. JIM 1999, 40, 225–232. [Google Scholar] [CrossRef][Green Version]
- Lu, X.; Miki, T.; Nagasaka, T. Activity Coefficients of NiO and CoO in CaO–Al2O3–SiO2 Slag and Their Application to the Recycling of Ni–Co–Fe-Based End-Of-Life Superalloys via Remelting. Int. J. Miner. Metall. Mater. 2017, 24, 25–36. [Google Scholar] [CrossRef]
- Derin, B.; Yucel, O. The Distribution of Cobalt between Co-Cu Alloys and Al2O3-FeO-Fe2O3-SiO2 slags. Scand. J. Metall. 2002, 31, 12–19. [Google Scholar] [CrossRef]
- Katyal, A.; Jeffes, J. Activities of Cobalt and Copper Oxides in Silicate and Ferrite Slags. In Proceedings of the 3rd International Conference on Molten Slags and Fluxes, Glasgow, UK, 27–29 June 1988; Institute of Metals: London, UK; Institute of Metals, Nort American Publications Center: Brookfield, VT, USA, 1989; pp. 46–55. [Google Scholar]
- Wang, S.; Kurtis, A.; Toguri, J. Distribution of Copper-Nickel and Copper-Cobalt between Copper-Nickel and Copper-Cobalt Alloys and Silica Saturated Fayalite Slags. Can. Metall. Q. 1973, 12, 383–390. [Google Scholar] [CrossRef]
- Grimsey, E.J.; Toguri, J.M. Cobalt in Silica Saturated Fayalite Slags. Can. Metall. Q. 1988, 27, 331–333. [Google Scholar] [CrossRef]
- Wang, S.; Santander, N.; Toguri, J. The Solubility of Nickel and Cobalt in Iron Silicate Slags. Metall. Trans. 1974, 5, 261–265. [Google Scholar]
- Kitamura, S.; Kuriyama, H.; Maruoka, N.; Yamaguchi, K.; Hasegawa, A. Distribution of Cobalt between MgO-Saturated FeOx-MgO-CaO-SiO2 Slag and Fe-Cu-Co Molten Alloy. Mater. Trans. 2008, 49, 2636–2641. [Google Scholar] [CrossRef]
- Grimsey, E.J.; Liu, X. The Activity Coefficient of Cobalt Oxide in Silica-Saturated Iron Silicate Slags. Metall. Mater. Trans. B 1995, 26, 229–233. [Google Scholar] [CrossRef]
- Teague, K.; Swinbourne, D.; Jahanshahi, S. A Thermodynamic Study on Cobalt Containing Calcium Ferrite and Calcium Iron Silicate Slags at 1573 K. Metall. Mater. Trans. B 2001, 32, 47–54. [Google Scholar] [CrossRef]
- Jak, E.; Zhao, B.; Hayes, P.C.; Liu, N. Experimental Study of Phase Equilibria in the System PbO-ZnO-SiO2. Metall. Mater. Trans. B 1999, 30, 21–27. [Google Scholar] [CrossRef]
- Hamuyuni, J.; Hellsten, N.; Akdogan, G.; Taskinen, P. The Liquidus in Cu-O-CaO System at Metallic Copper Saturation up to 1698 K. J. Am. Ceram. Soc. 2015, 98, 320–323. [Google Scholar] [CrossRef]
- Shishin, D.; Hidayat, T.; Chen, J.; Hayes, P.; Jak, E. Experimental Investigation and Thermodynamic Modeling of the Distributions of Ag and Au Between Slag, Matte, and Metal in the Cu–Fe–O–S–Si System. J. Sustain. Metall. 2019, 5, 240–249. [Google Scholar] [CrossRef]
- Avarmaa, K.; O’Brien, H.; Johto, H.; Taskinen, P. Equilibrium Distribution of Precious Metals Between Slag and Copper Matte at 1250–1350 °C. J. Sustain. Metall. 2015, 1, 216–228. [Google Scholar] [CrossRef]
- Klemettinen, L.; Aromaa, R.; Dańczak, A.; O’Brien, H.; Taskinen, P.; Jokilaakso, A. Distribution Kinetics of Rare Earth Elements in Copper Smelting. Sustainability 2020, 12, 208. [Google Scholar] [CrossRef]
- Wan, X.; Shen, L.; Jokilaakso, A.; Eriç, H.; Taskinen, P. Experimental Approach to Matte-Slag Reactions in the Flash Smelting Process. Miner. Process. Extr. Metall. Rev. 2020, 1–11. [Google Scholar] [CrossRef]
- Pouchou, J.; Pichoir, L. Basic expression of “PAP” computation for quantitative EPMA. In Proceedings of the 11th International Congress on X-ray Optics and Microanalysis (ICXOM), London, ON, Canada, 4–8 August 1986; Brown, J.D., Packwood, R.H., Eds.; University of Western Ontario: London, ON, Canada, 1986; pp. 249–256. [Google Scholar]
- Hidayat, T.; Mehrjardi, A.F.; Hayes, P.C.; Jak, E. Experimental Study of Gas/Slag/Matte/Spinel Equilibria and Minor Elements Partitioning in the Cu-Fe-O-S-Si System. Advances in Molten Slags, Fluxes, and Salts. In Proceedings of the 10th International Conference on Molten Slags, Fluxes and Salts, Seattle, WA, USA, 22–25 May 2016; Reddy, R., Chaubal, P., Pistorius, P., Pal, U., Eds.; Springer International Publishing AG: Cham, Switzerland, 2016; pp. 1207–1220. [Google Scholar]
- Warner, A.; Díaz, C.; Dalvi, A.; Mackey, P. JOM World Nonferrous Smelter Survey Part IV: Nickel: Sulfide. JOM 2007, 59, 58–72. [Google Scholar] [CrossRef]
- Waanders, F.B.; Nell, J. Phase Chemical Composition of Slag from a Direct Nickel Flash Furnace and Associated Slag Cleaning Furnace. Hyperfine Interact 2013, 218, 101–105. [Google Scholar] [CrossRef]
- Whyte, R.M.; Orjans, J.R.; Harris, G.B.; Thomas, J.A. Development of a Process for the Recovery of Electrolytic Copper and Cobalt from Rokana Converter Slag. Adv. Extr. Metall. 1997, 77, 57–68. [Google Scholar]
- Strengell, D.; Avarmaa, K.; Johto, H.; Taskinen, P. Distribution Equilibria and Slag Chemistry of DON Smelting. Can. Metall. Q. 2016, 55, 234–242. [Google Scholar] [CrossRef]
- Font, J.M.; Hino, M.; Itagaki, K. Phase Equilibrium between FeOx-SiO2 Base Slag and Cu2S-Ni3S2-FeS Mattes with Different Cu and Ni Contents at 1573 K. Shigen-to-Sozai 1999, 115, 460–465. [Google Scholar] [CrossRef]
- Yucel, O.; Addemir, O.; Tekin, A.; Nizamoglu, S. Recovery of Cobalt from Copper Slags. Miner. Process. Extr. Metall. Rev. 1992, 10, 99–107. [Google Scholar] [CrossRef]
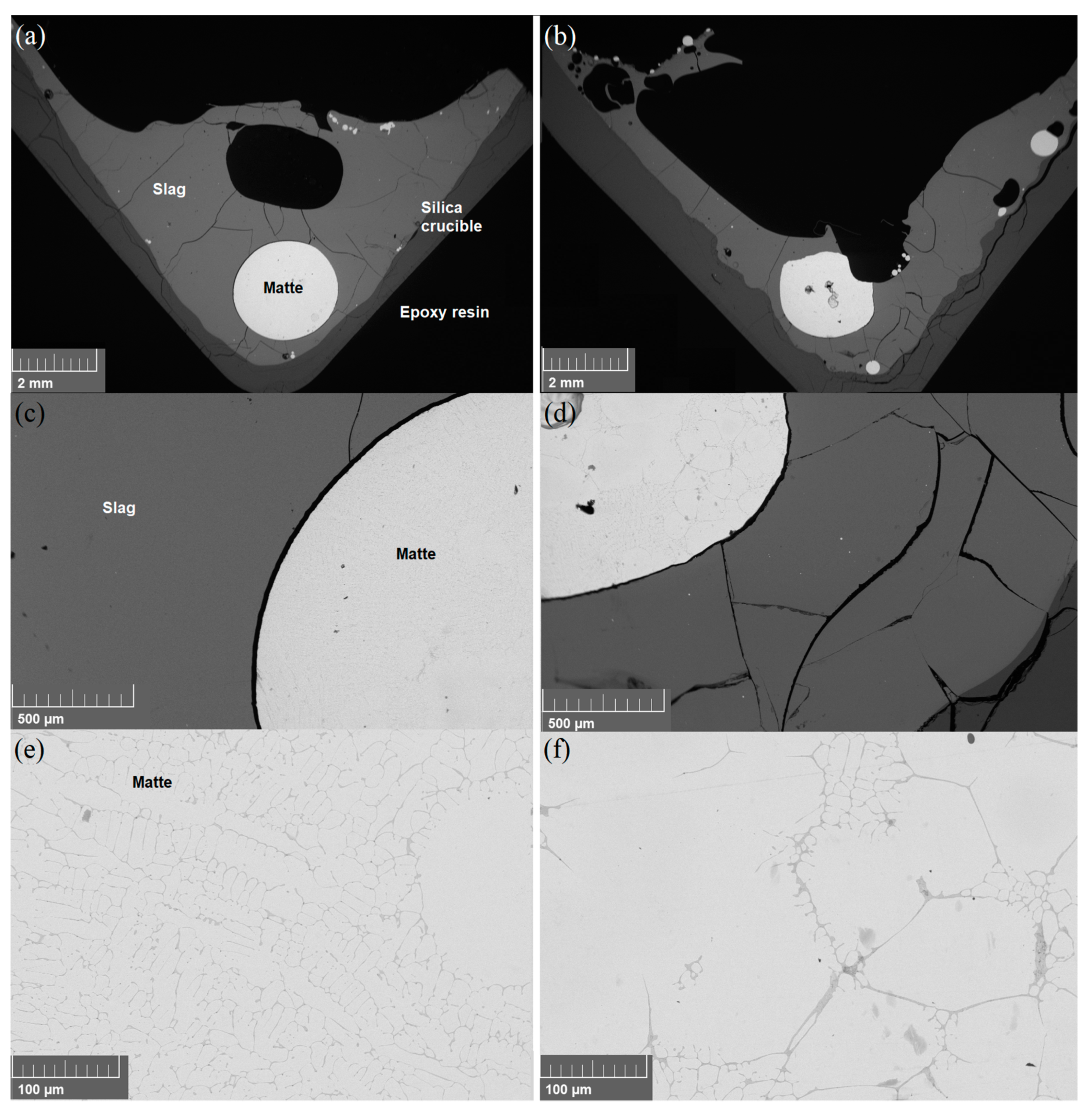
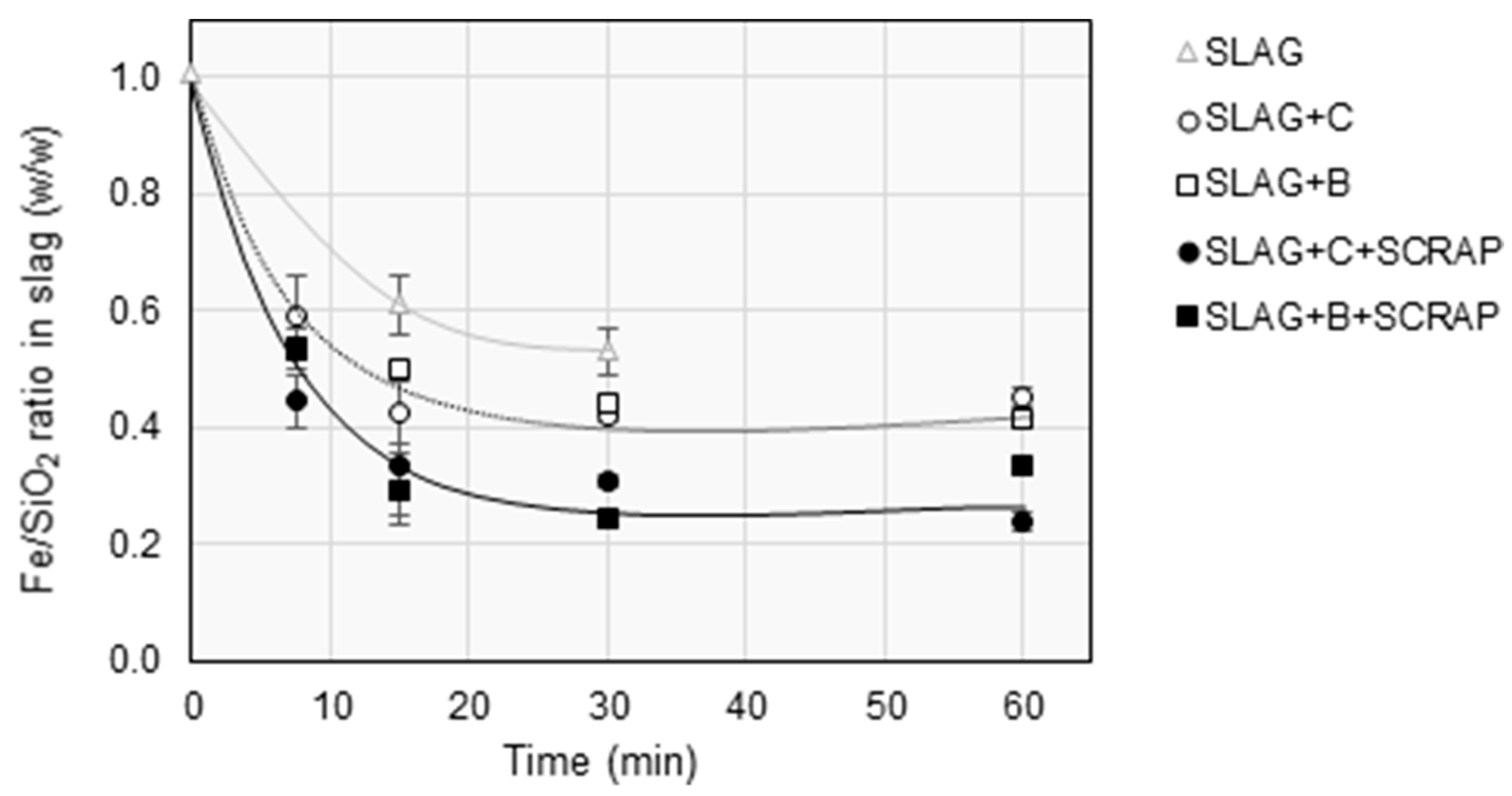
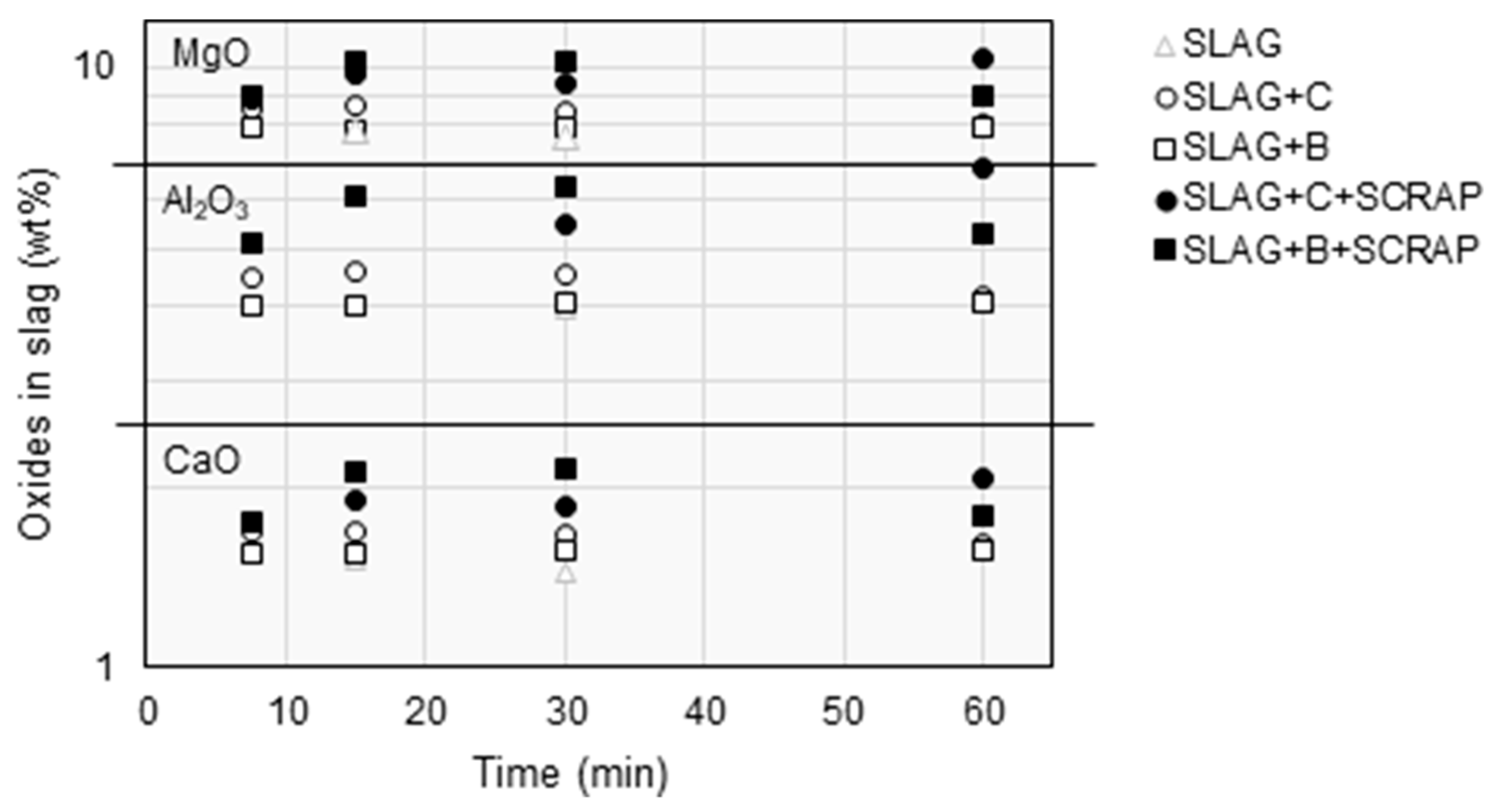

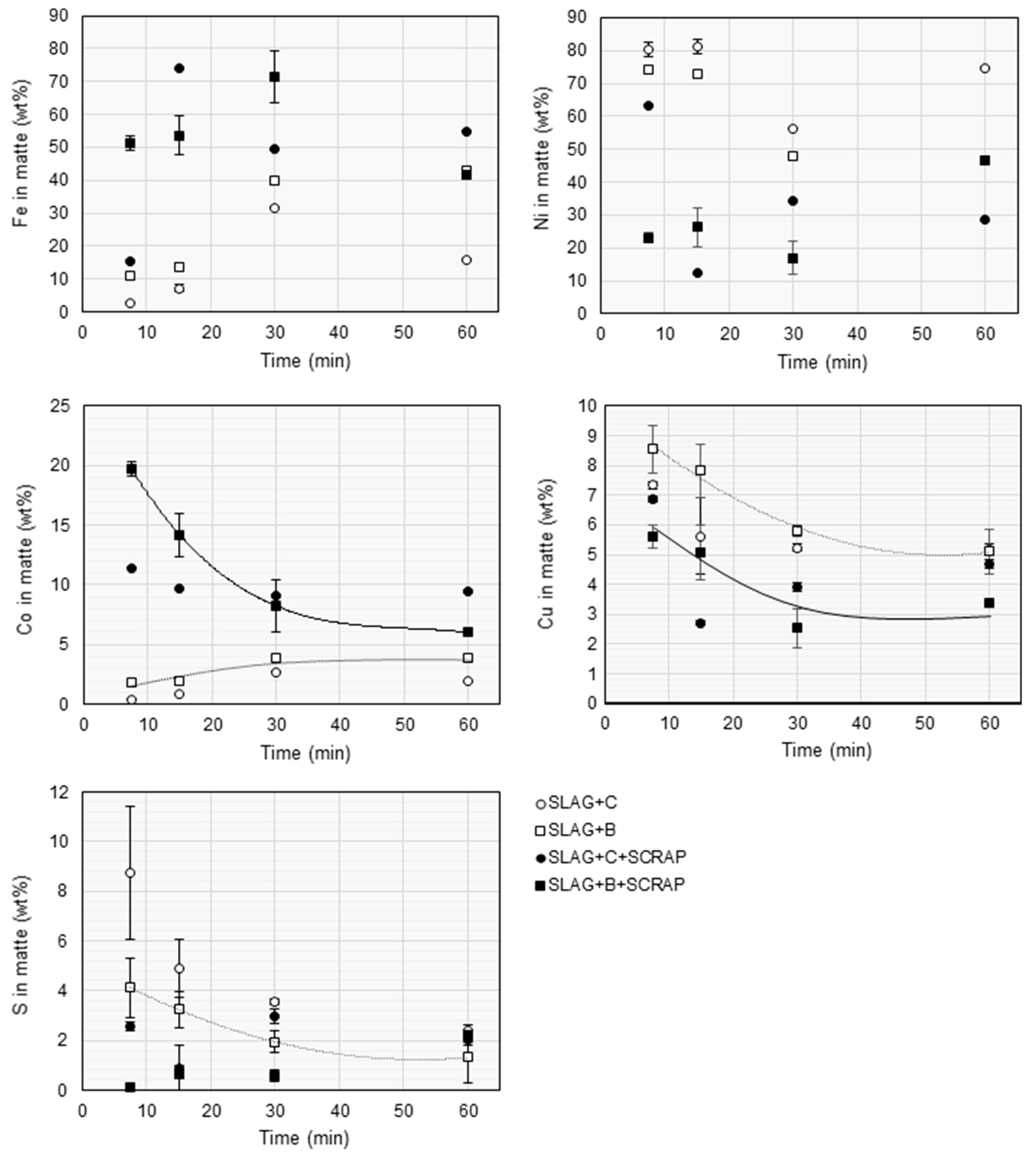

| Fe | SiO2 | MgO | Ni | Al2O3 | CaO | Cu | Co | Cr | S | Zn | SUM |
| 34.3 | 34.1 | 8.7 | 4.23 | 2.7 | 1.73 | 0.65 | 0.47 | 0.097 | 0.08 | 0.05 | 87.107 |
| Ag | Au | Mg | Al | Ca | Mn | Fe | Co |
| 9.5 ppm | 2 ppm | 0.09 | 2.16 | 0.09 | 1.65 | 0.57 | 25.5 |
| Ni | Cu | Zn | C | F− | SiO2 | Volatiles | |
| 2.6 | 2.92 | 0.06 | 32.7 | 2.09 | 0.38 | 32.9 |
| # | S0 | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 | S12 | S13 | S14 | S15 | S16 | S17 | S18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SLAG | SLAG + C | SLAG + B | SLAG + C + SCRAP | SLAG + B + SCRAP | |||||||||||||||
| Reductant | - | - | - | ||||||||||||||||
| Scrap | - | - | - | - | - | - | - | - | - | - | - | ||||||||
| Time (min) | 0 | 15 | 30 | 15 | 30 | 75 | 60 | 75 | 15 | 30 | 60 | 7.5 | 15 | 30 | 60 | 75 | 15 | 30 | 60 |
| =Coke | =Biochar | =Scrap (Co 25.5 wt%) | |||||||||||||||||
| Phase | O | Si | Mg | S | K | Ni | Co | Fe | Cu | Al | Ca | Cr | Zn | Ti |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slag | 1687 | 339 | 349 | 182 | 163 | 355 | 395 | 344 | 446 | 279 | 152 | 264 | 509 | 267 |
| Matte | 1053 | 483 | 744 | 252 | 190 | 478 | 449 | 318 | 611 | 466 | 212 | 283 | 671 | 319 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avarmaa, K.; Järvenpää, M.; Klemettinen, L.; Marjakoski, M.; Taskinen, P.; Lindberg, D.; Jokilaakso, A. Battery Scrap and Biochar Utilization for Improved Metal Recoveries in Nickel Slag Cleaning Conditions. Batteries 2020, 6, 58. https://doi.org/10.3390/batteries6040058
Avarmaa K, Järvenpää M, Klemettinen L, Marjakoski M, Taskinen P, Lindberg D, Jokilaakso A. Battery Scrap and Biochar Utilization for Improved Metal Recoveries in Nickel Slag Cleaning Conditions. Batteries. 2020; 6(4):58. https://doi.org/10.3390/batteries6040058
Chicago/Turabian StyleAvarmaa, Katri, Marko Järvenpää, Lassi Klemettinen, Miikka Marjakoski, Pekka Taskinen, Daniel Lindberg, and Ari Jokilaakso. 2020. "Battery Scrap and Biochar Utilization for Improved Metal Recoveries in Nickel Slag Cleaning Conditions" Batteries 6, no. 4: 58. https://doi.org/10.3390/batteries6040058
APA StyleAvarmaa, K., Järvenpää, M., Klemettinen, L., Marjakoski, M., Taskinen, P., Lindberg, D., & Jokilaakso, A. (2020). Battery Scrap and Biochar Utilization for Improved Metal Recoveries in Nickel Slag Cleaning Conditions. Batteries, 6(4), 58. https://doi.org/10.3390/batteries6040058






