Cell Replacement Strategies for Lithium Ion Battery Packs
Abstract
1. Introduction
- treating the battery pack as a single cell of high voltage and capacity;
- applying single-cell state-of-charge (SOC) estimation methods to every cell in a pack, but this approach is computationally intensive and cumbersome for practical application;
- quantifying individual cell SOC by analyzing variations in open circuit voltage and internal resistance.
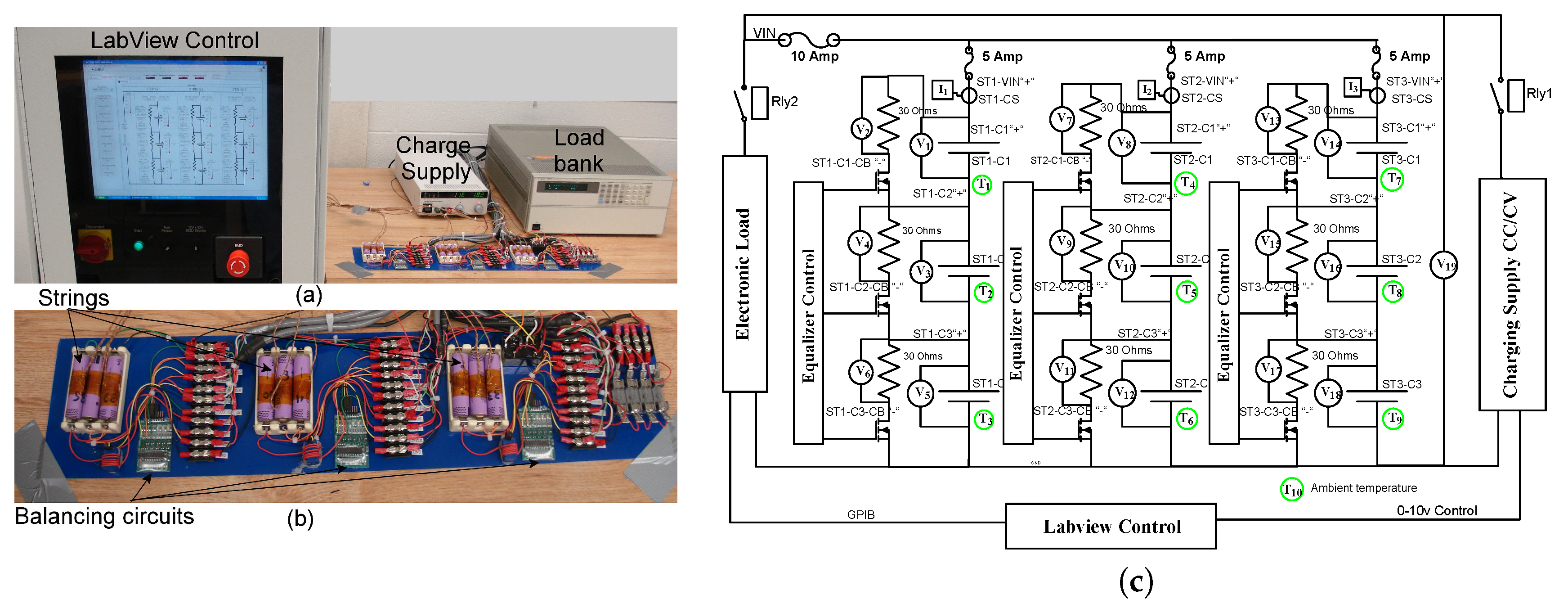
2. Methodology
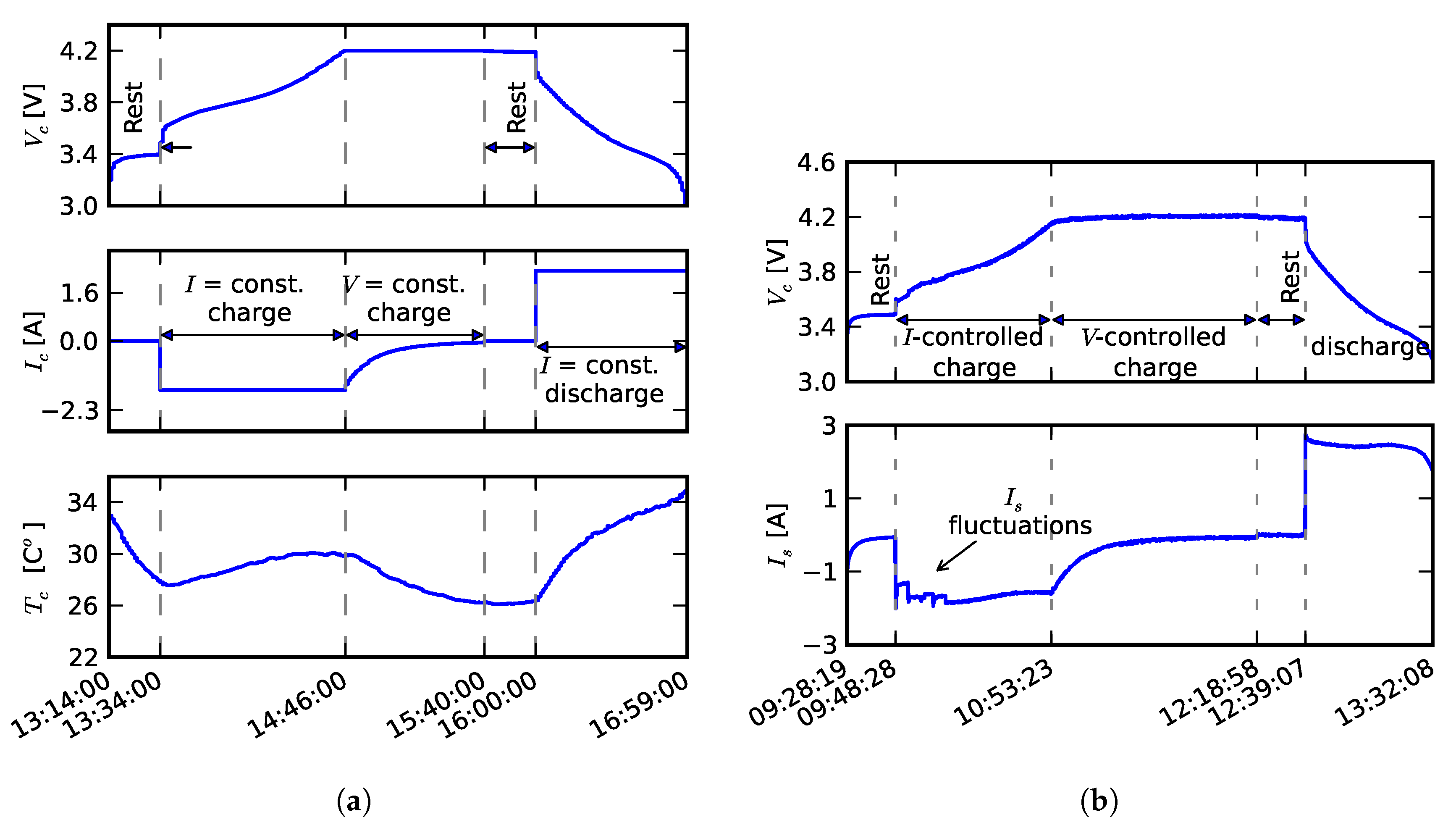
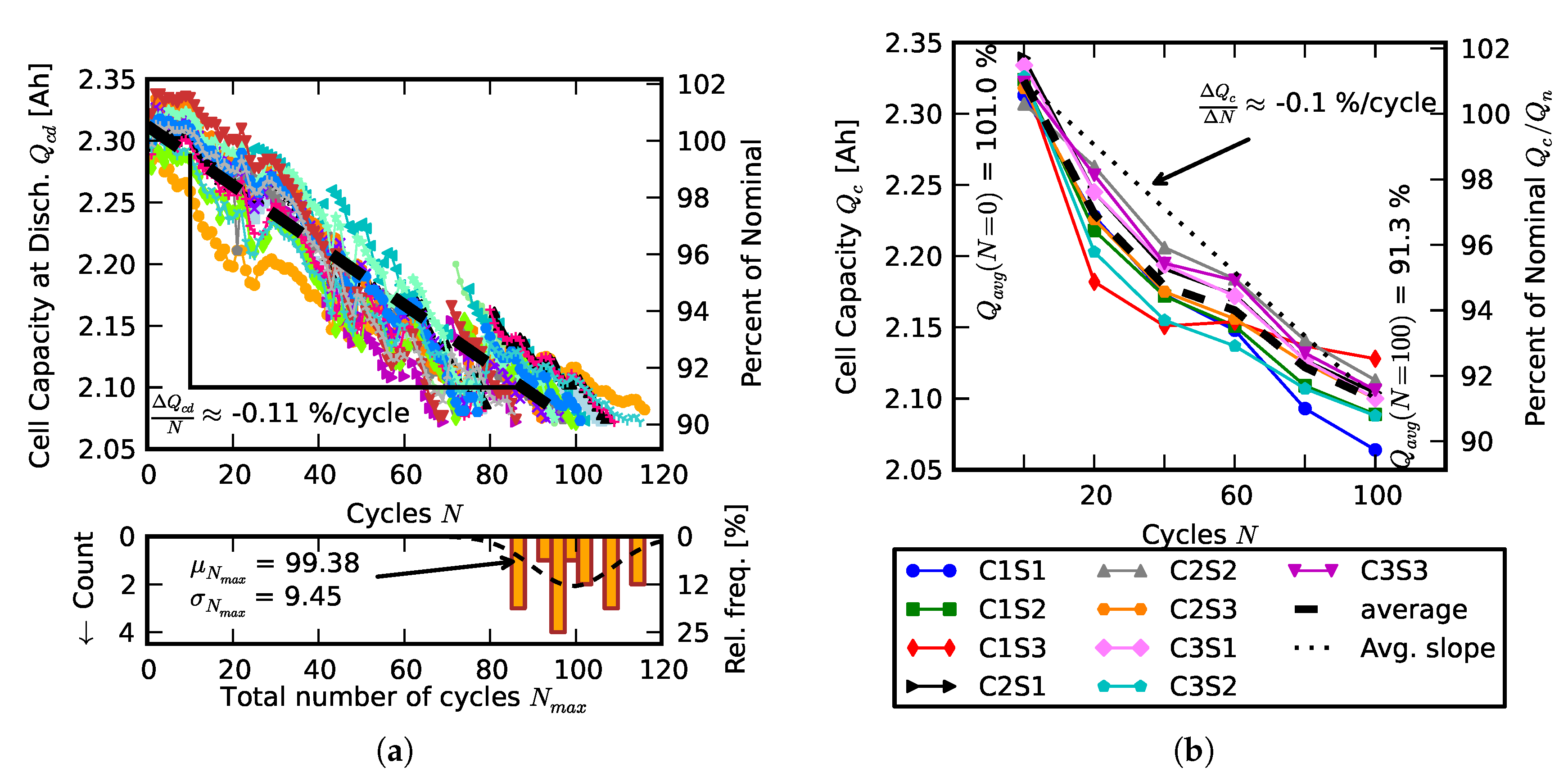
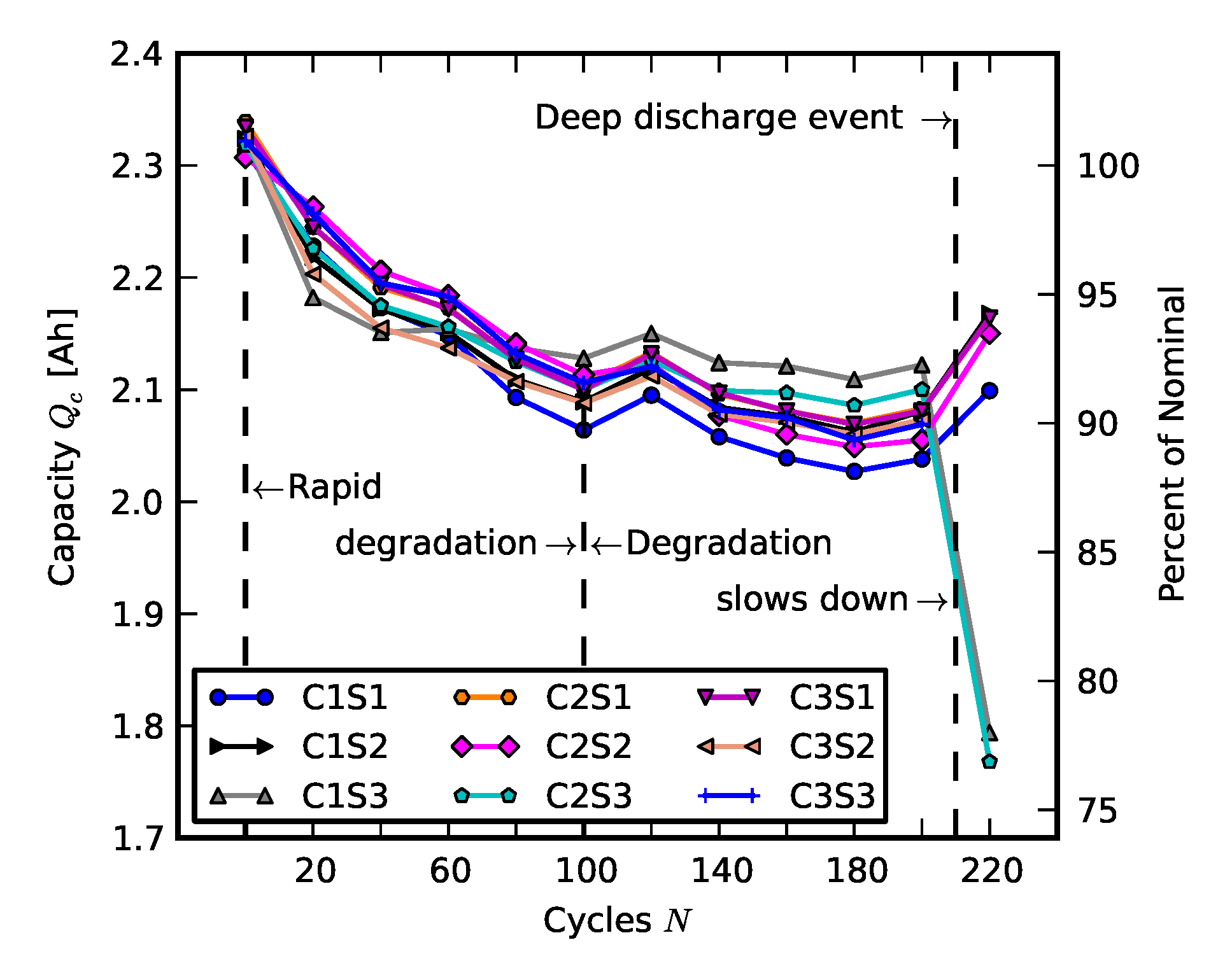
2.1. Individual and Pack Aging of 18650 LiCo Cells
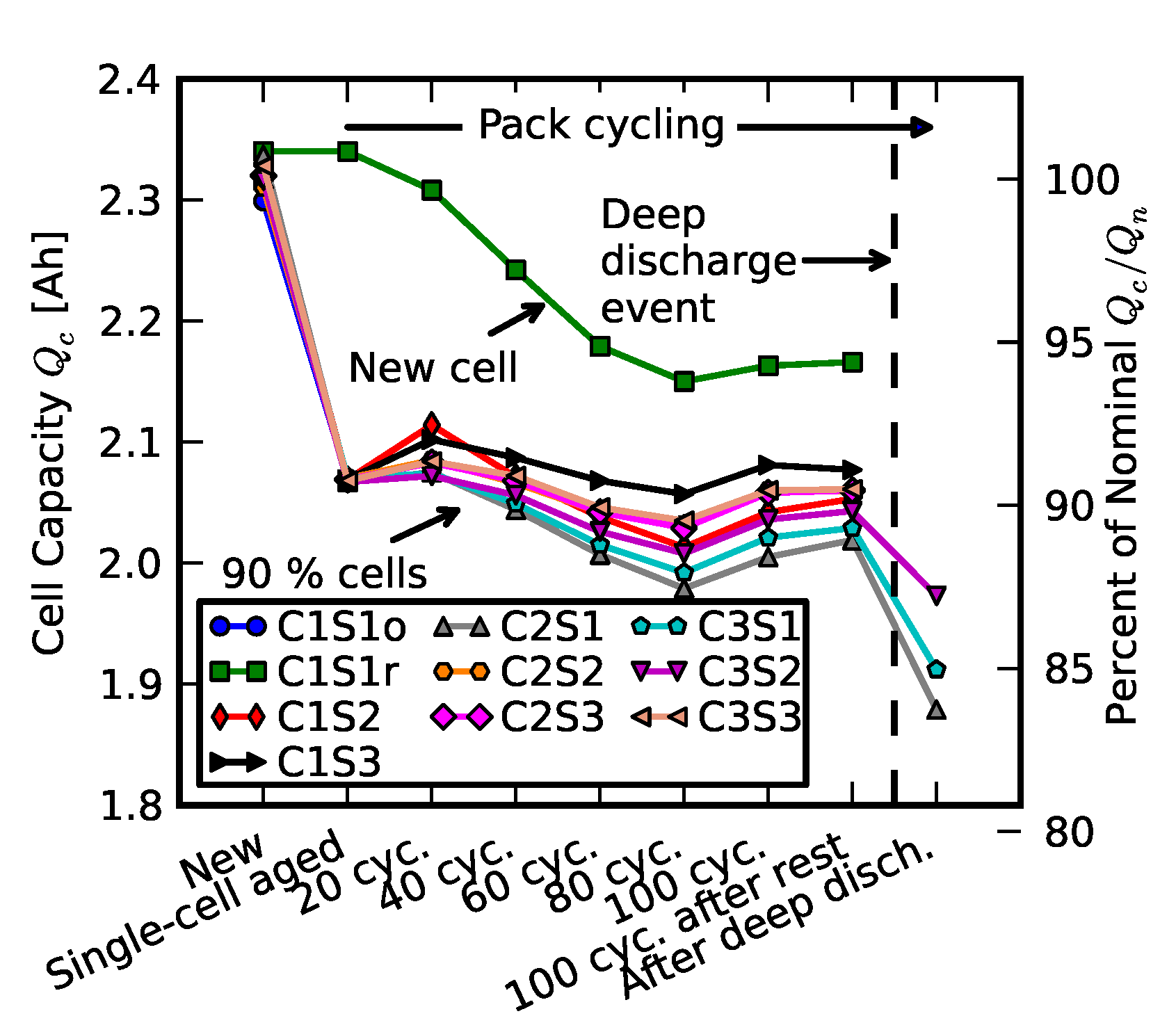
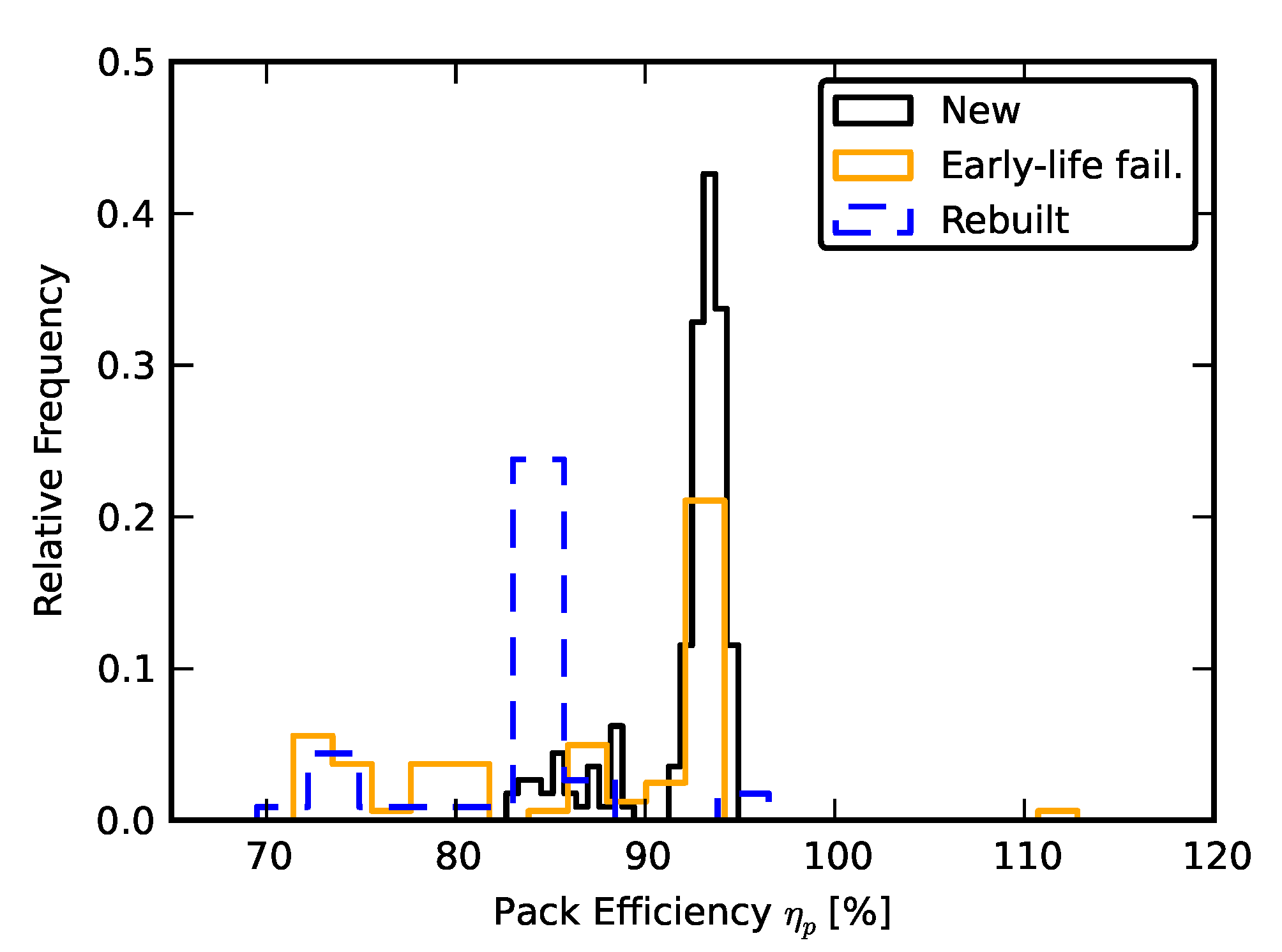
2.2. Scenario 1: Early Life Failure
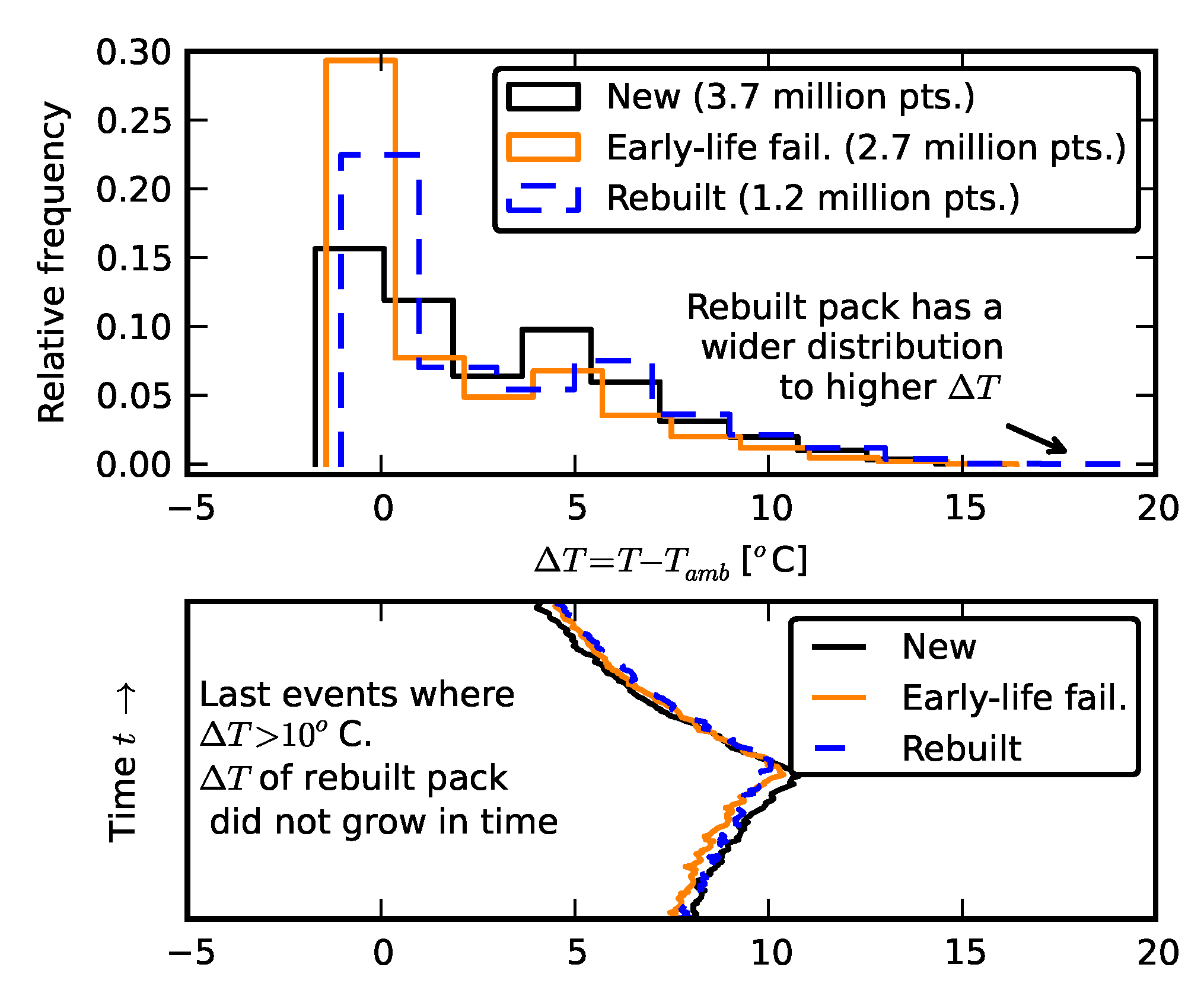
2.3. Scenario 2: Rebuilding a Pack from Two Failed Packs
3. Results and Analysis
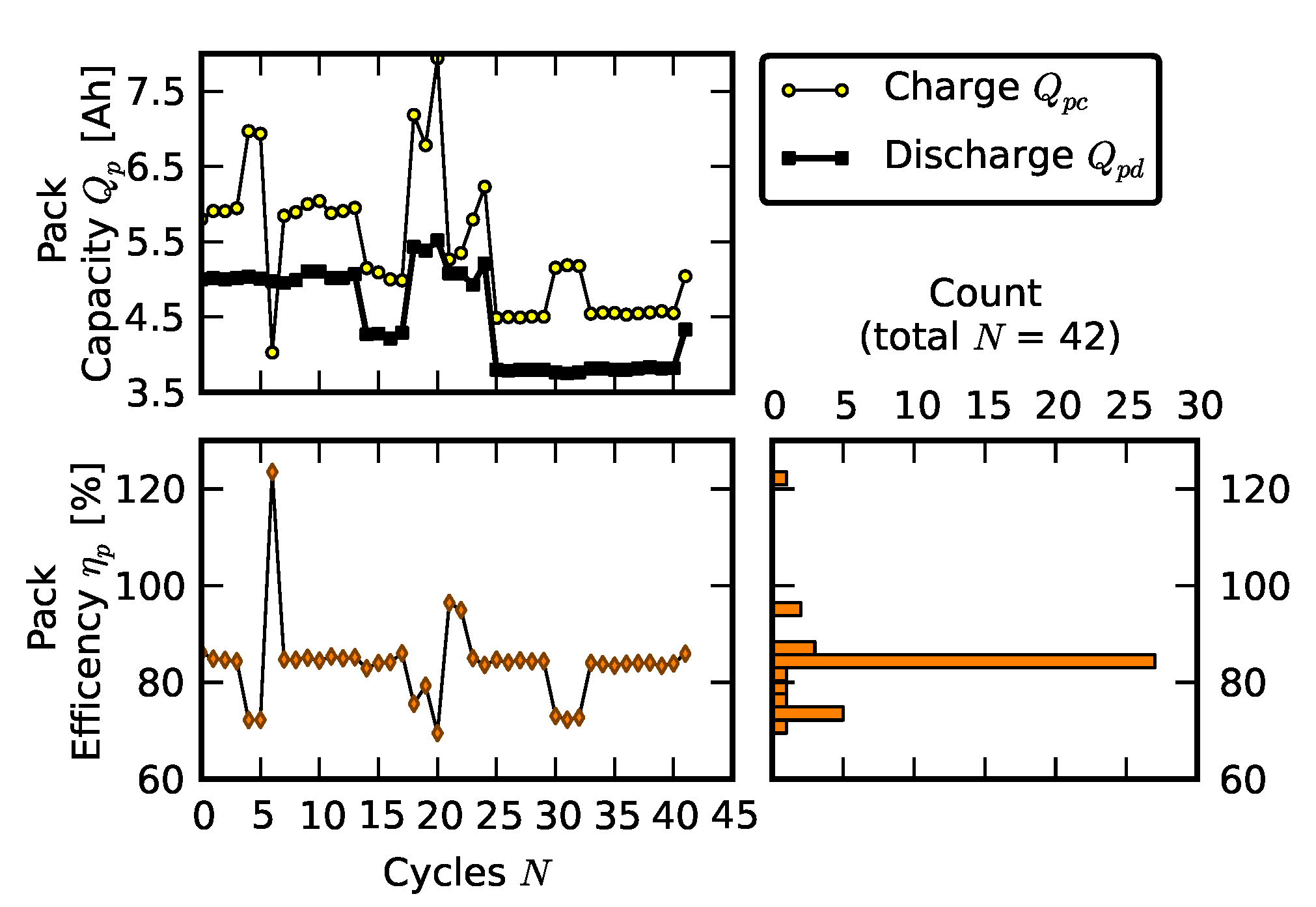
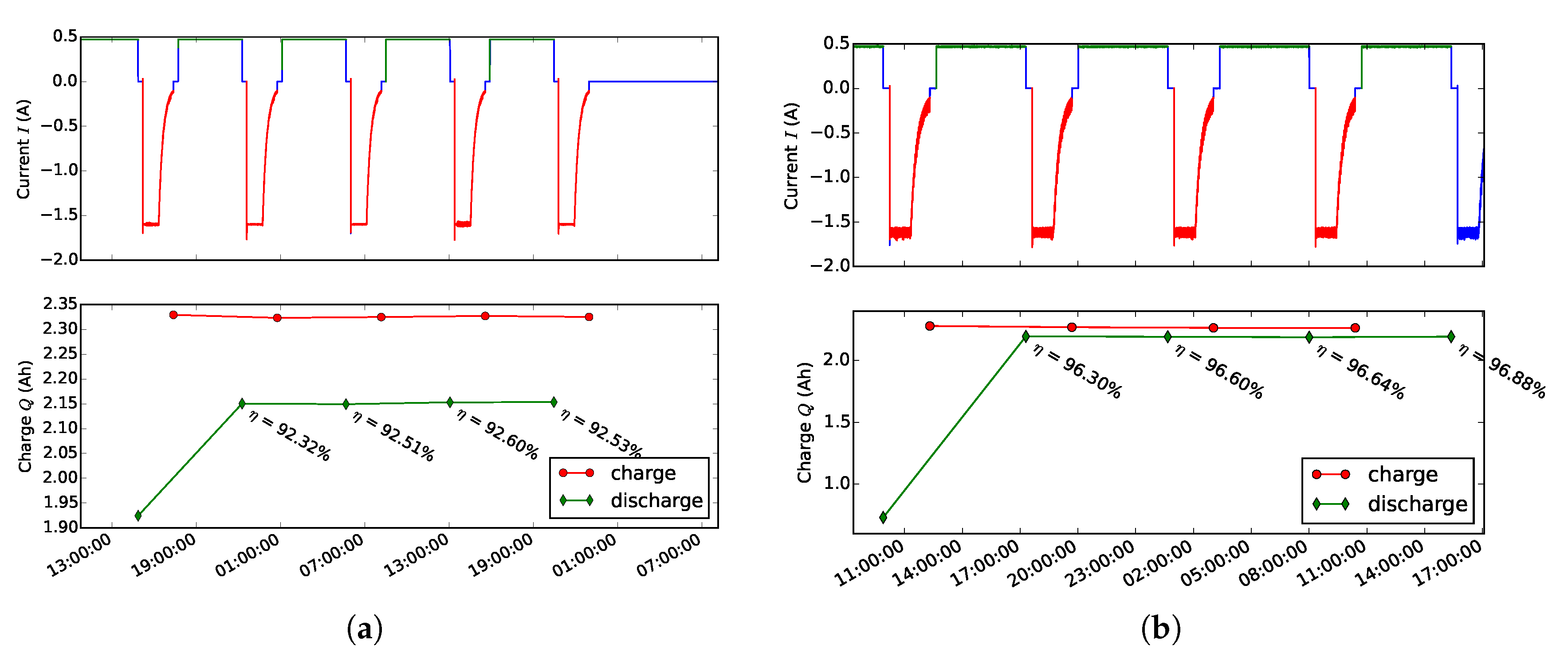
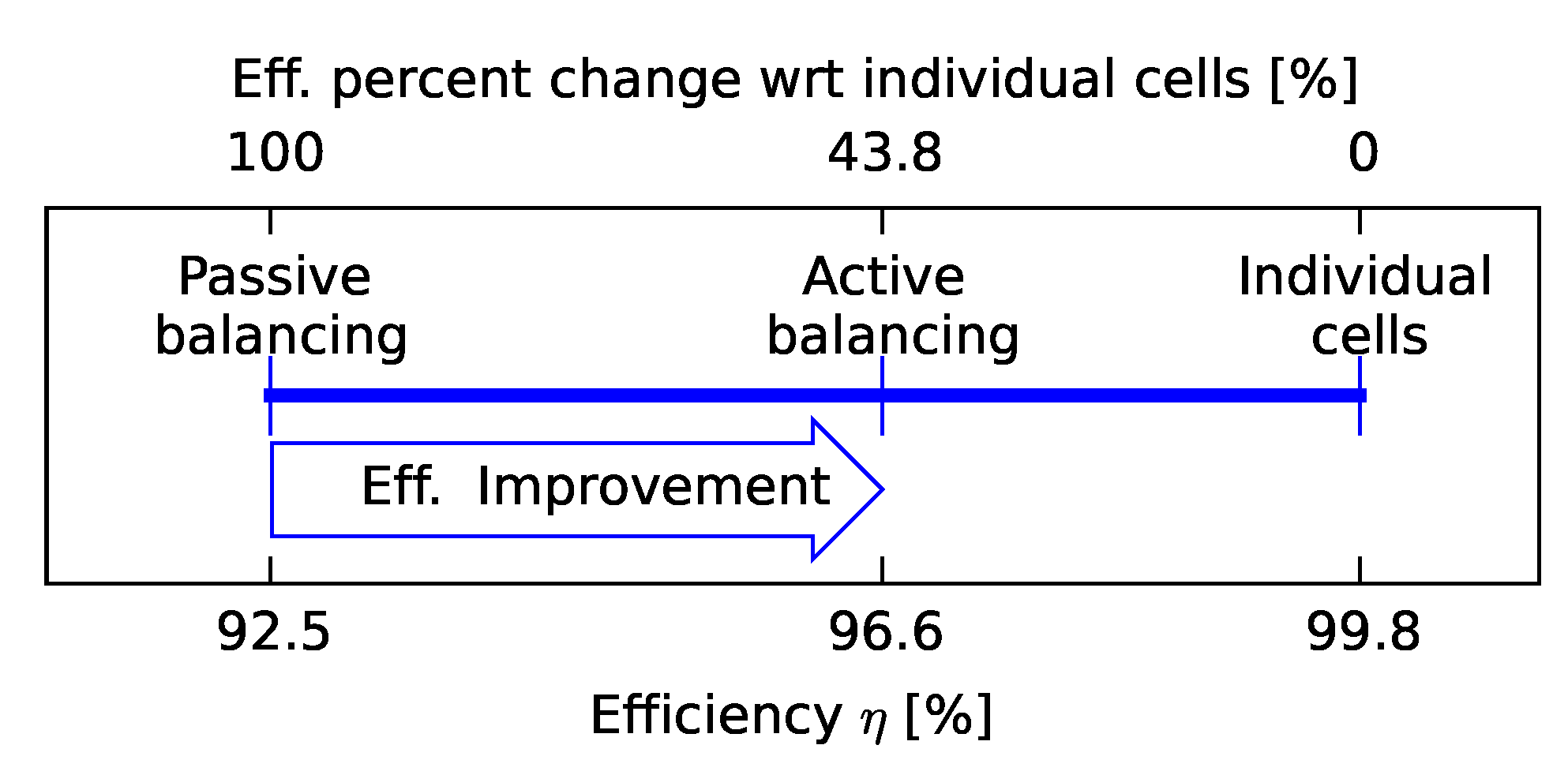
3.1. Efficiency Comparison
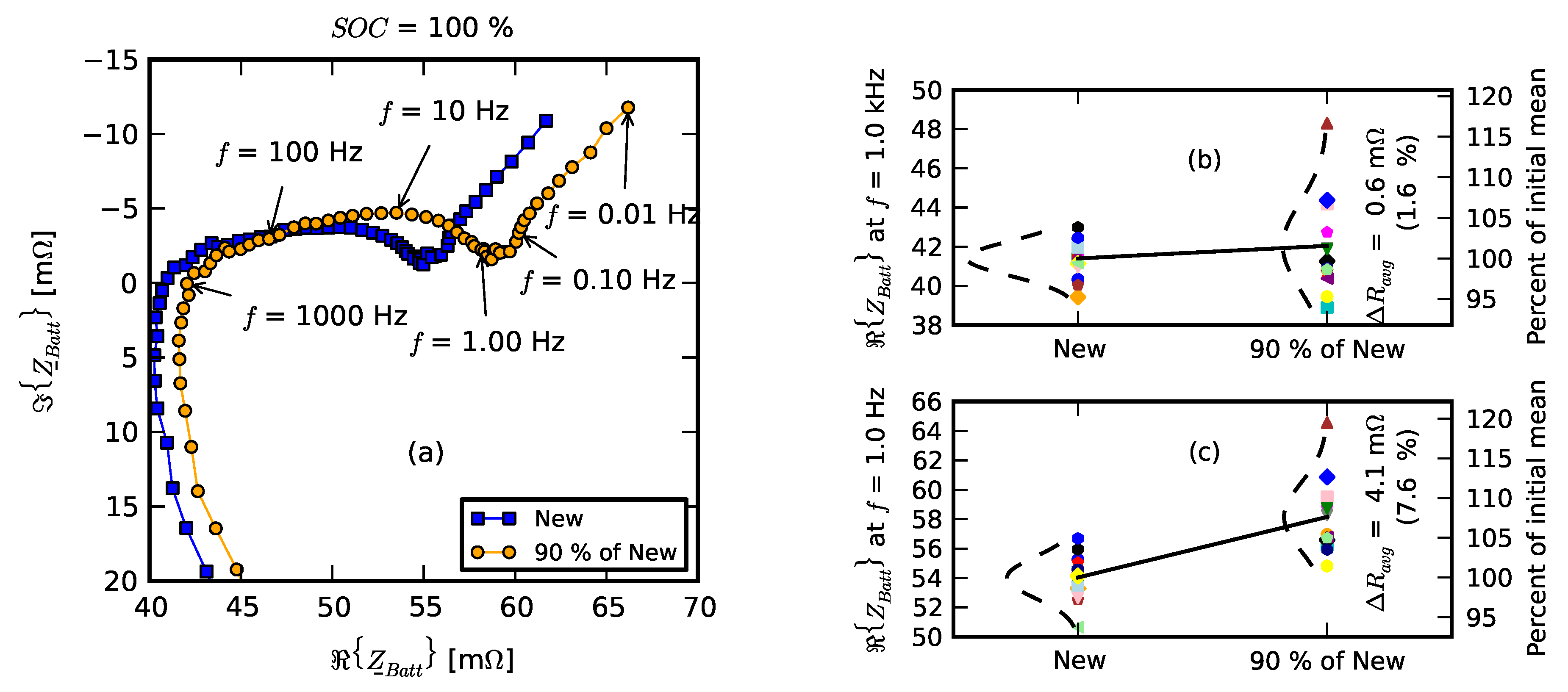
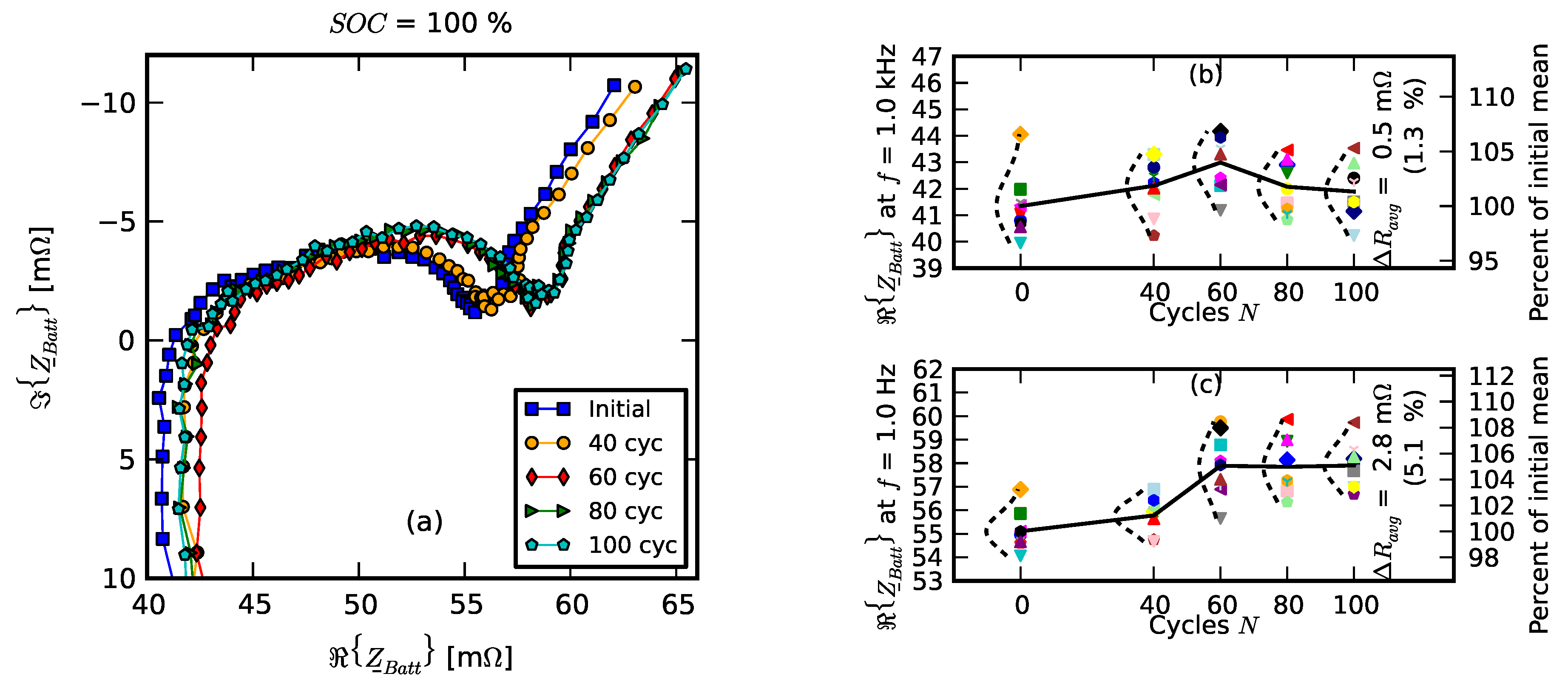
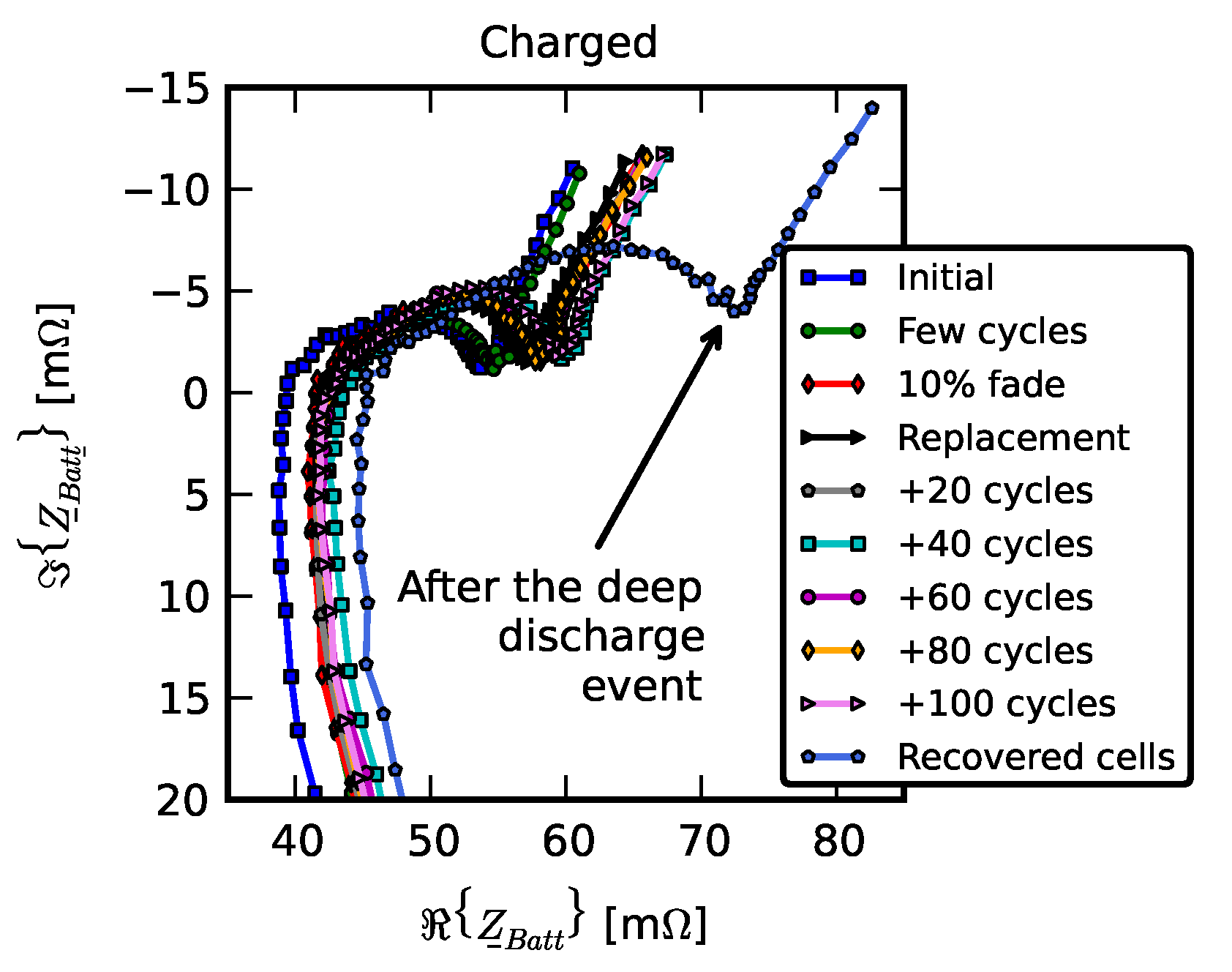
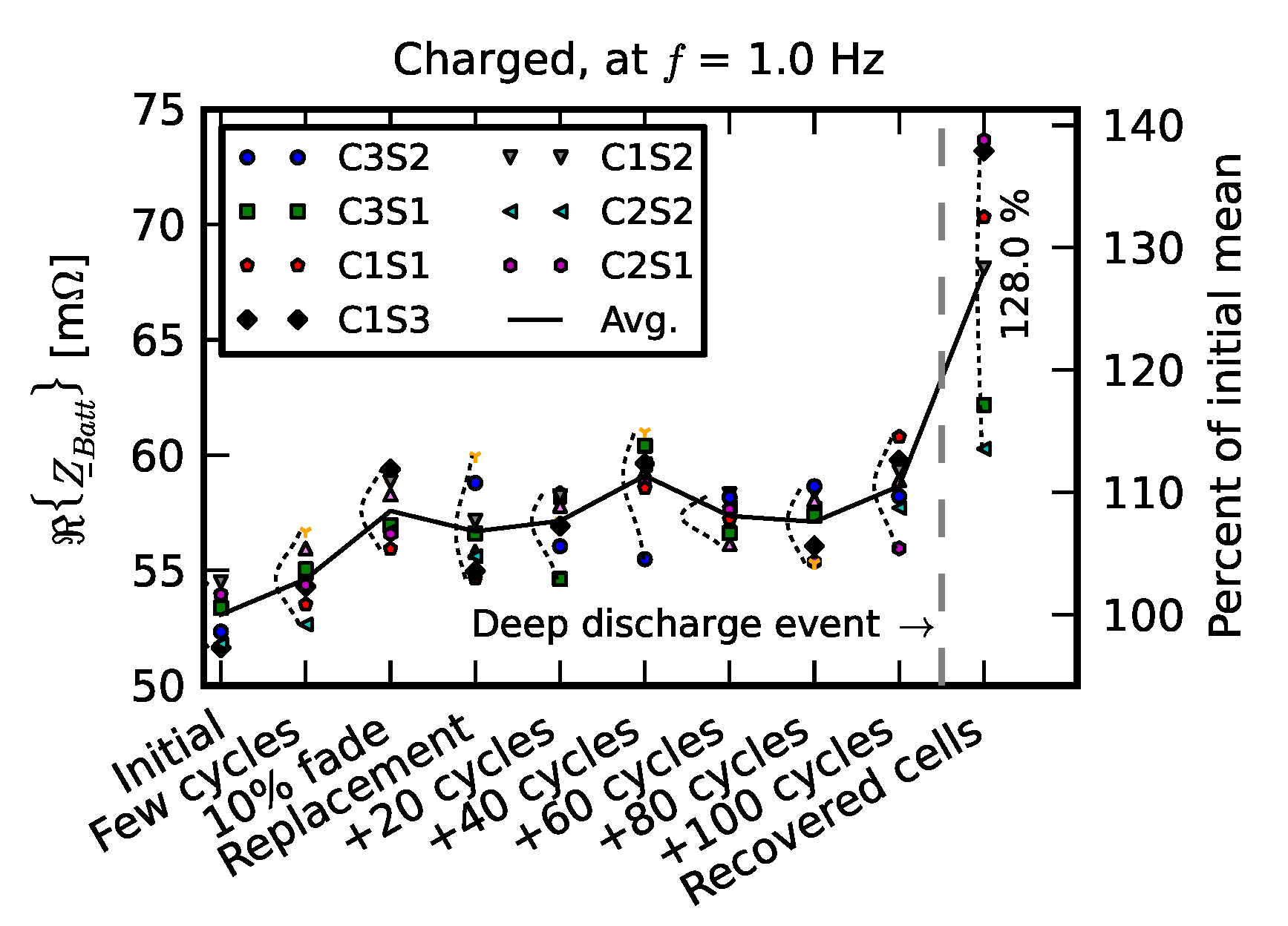
- Initial signifies the measurements on new cells.
- Few cycles signifies the impedance of nine cells after they were initially run in the pack for three cycles.
- 10% fade signifies the measurements on the cell after they were individually aged on the single-cell tester to 90% of their nominal capacity.
- Replacement signifies resistance measurements after one cell was replaced with a new cell; +20 cycles, +40 cycles, +60 cycles, +80 cycles, and +100 cycles signify measurements as the “repaired” pack was operated for 100 cycles.
- Recovered cells signify the impedance of a few cells that survived the deep discharge event.
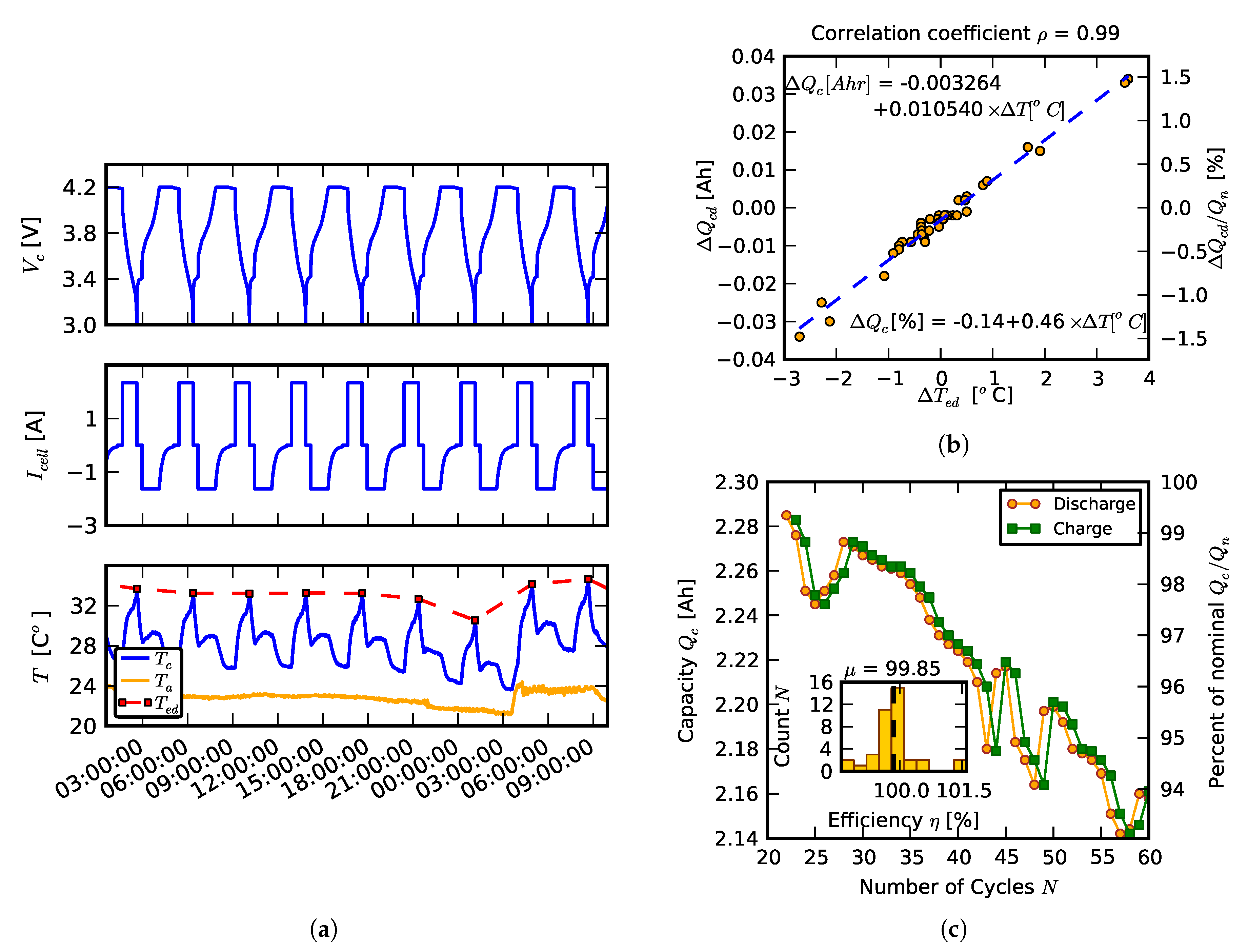
3.2. Temperature Effects
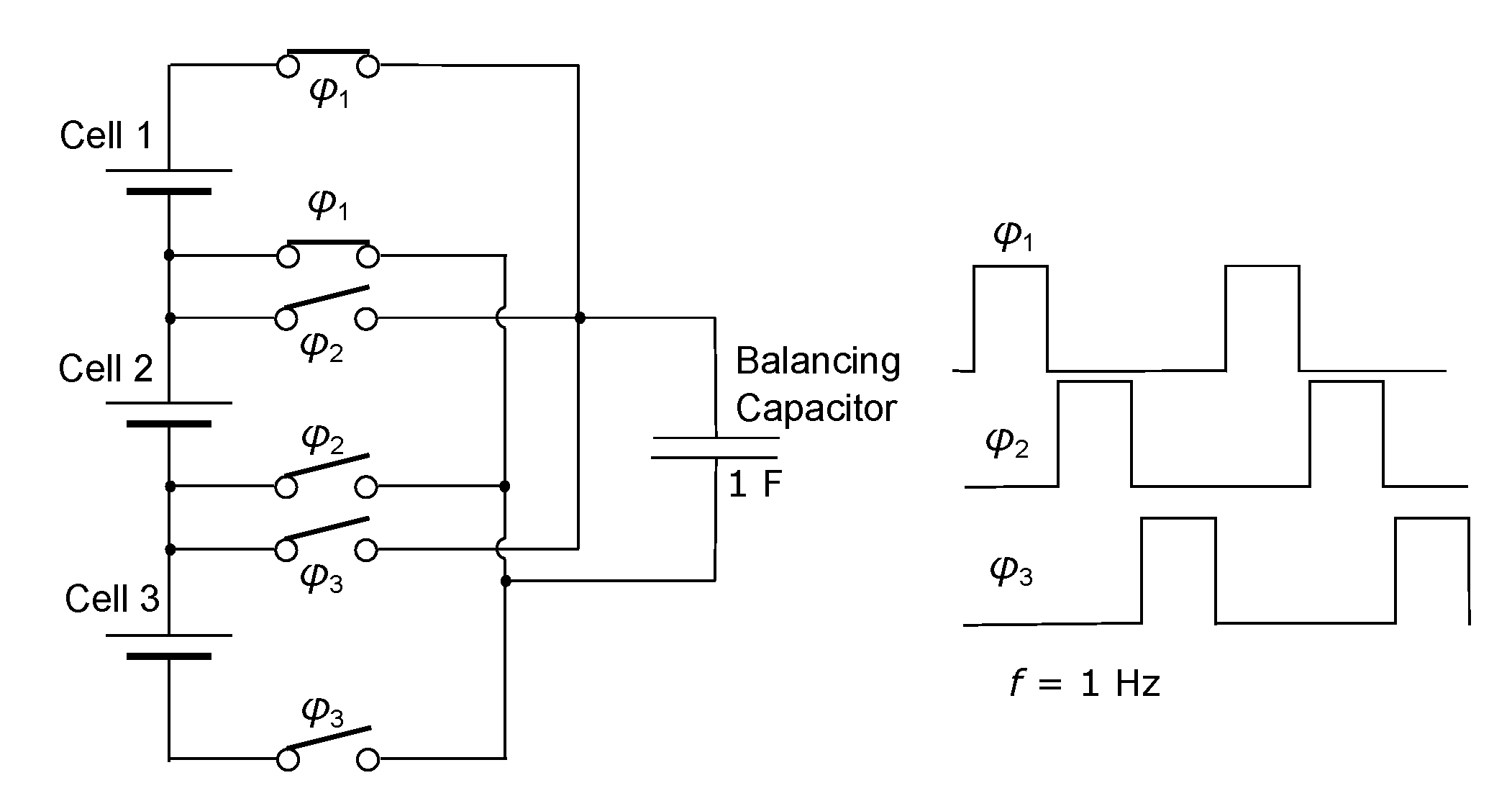
3.3. Effects of Balancing Scheme
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Disclaimer
Acknowledgments
Conflicts of Interest
References
- Wagner, F.T.; Lakshmanan, B.; Mathias, M.F. Electrochemistry and the future of the automobile. J. Phys. Chem. Lett. 2010, 1, 2204–2219. [Google Scholar] [CrossRef]
- Williams, B.D.; Lipman, T.E. Strategy for Overcoming Cost Hurdles of Plug-In-Hybrid Battery in California. Transp. Res. Rec. J. Transp. Res. Board 2010, 2191, 59–66. [Google Scholar] [CrossRef]
- Ralston, M.; Nigro, N. Plug-in Electric Vehicles: Literature Review; Pew Center on Global Climate Change: Arlington, VA, USA, 2011; Available online: https://www.c2es.org/site/assets/uploads/2011/07/plug-in-electric-vehicles-literature-review.pdf (accessed on 22 July 2020).
- Witkin, J. A Second Life for the Electric Car Battery. 2011. Available online: https://green.blogs.nytimes.com/2011/04/27/a-second-life-for-the-electric-car-battery/ (accessed on 22 July 2020).
- Marano, V.; Onori, S.; Guezennec, Y.; Rizzoni, G.; Madella, N. Lithium-ion batteries life estimation for plug-in hybrid electric vehicles. In Proceedings of the 2009 IEEE Vehicle Power and Propulsion Conference, Dearborn, MI, USA, 7–10 September 2009; pp. 536–543. [Google Scholar] [CrossRef]
- Waag, W.; Fleischer, C.; Sauer, D.U. Critical review of the methods for monitoring of lithium-ion batteries in electric and hybrid vehicles. J. Power Sources 2014, 258, 321–339. [Google Scholar] [CrossRef]
- Rezvanizaniani, S.M.; Liu, Z.; Chen, Y.; Lee, J. Review and recent advances in battery health monitoring and prognostics technologies for electric vehicle (EV) safety and mobility. J. Power Sources 2014, 256, 110–124. [Google Scholar] [CrossRef]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Roscher, M.A.; Bohlen, O.S.; Sauer, D.U. Reliable state estimation of multicell lithium-ion battery systems. IEEE Trans. Energy Convers. 2011, 26, 737–743. [Google Scholar] [CrossRef]
- Kim, G.H.; Smith, K.; Ireland, J.; Pesaran, A. Fail-safe design for large capacity lithium-ion battery systems. J. Power Sources 2012, 210, 243–253. [Google Scholar] [CrossRef]
- Offer, G.J.; Yufit, V.; Howey, D.A.; Wu, B.; Brandon, N.P. Module design and fault diagnosis in electric vehicle batteries. J. Power Sources 2012, 206, 383–392. [Google Scholar] [CrossRef]
- Paul, S.; Diegelmann, C.; Kabza, H.; Tillmetz, W. Analysis of ageing inhomogeneities in lithium-ion battery systems. J. Power Sources 2013, 239, 642–650. [Google Scholar] [CrossRef]
- Zheng, Y.; Han, X.; Lu, L.; Li, J.; Ouyang, M. Lithium ion battery pack power fade fault identification based on Shannon entropy in electric vehicles. J. Power Sources 2013, 223, 136–146. [Google Scholar] [CrossRef]
- Li, J.; Barillas, J.K.; Guenther, C.; Danzer, M.A. Multicell state estimation using variation based sequential Monte Carlo filter for automotive battery packs. J. Power Sources 2015, 277, 95–103. [Google Scholar] [CrossRef]
- Dubarry, M.; Truchot, C.; Cugnet, M.; Liaw, B.Y.; Gering, K.; Sazhin, S.; Jamison, D.; Michelbacher, C. Evaluation of commercial lithium-ion cells based on composite positive electrode for plug-in hybrid electric vehicle applications. Part I: Initial characterizations. J. Power Sources 2011, 196, 10328–10335. [Google Scholar] [CrossRef]
- Dubarry, M.; Truchot, C.; Liaw, B.Y.; Gering, K.; Sazhin, S.; Jamison, D.; Michelbacher, C. Evaluation of commercial lithium-ion cells based on composite positive electrode for plug-in hybrid electric vehicle applications. Part II: Degradation mechanism under 2C cycle aging. J. Power Sources 2011, 196, 10336–10343. [Google Scholar] [CrossRef]
- Dubarry, M.; Vuillaume, N.; Liaw, B.Y. Origins and accommodation of cell variations in Li-ion battery pack modeling. Int. J. Energy Res. 2009, 34, 216–231. [Google Scholar] [CrossRef]
- Gering, K.L.; Sazhin, S.V.; Jamison, D.K.; Michelbacher, C.J.; Liaw, B.Y.; Dubarry, M.; Cugnet, M. Investigation of path dependence in commercial lithium-ion cells chosen for plug-in hybrid vehicle duty cycle protocols. J. Power Sources 2011, 196, 3395–3403. [Google Scholar] [CrossRef]
- Rao, Z.; Wang, S. A review of power battery thermal energy management. Renew. Sustain. Energy Rev. 2011, 15, 4554–4571. [Google Scholar] [CrossRef]
- Sarre, G.; Blanchard, P.; Broussely, M. Aging of lithium-ion batteries. J. Power Sources 2004, 127, 65–71. [Google Scholar] [CrossRef]
- Nenadic, N.G.; Bussey, H.E.; Ardis, P.A.; Thurston, M.G. Estimation of State-of-Charge and Capacity of Used Lithium-Ion Cells. Int. J. Progn. Health Manag. 2014, 5, 12. [Google Scholar]
- Dudek, K.; Lane, R. Synergy between electrified vehicle and community energy storage batteries and markets: Super session 4. Energy storage. In Proceedings of the Power and Energy Society General Meeting, Detroit, MI, USA, 24–28 July 2011; p. 1. [Google Scholar]
- Williams, D.M.; Gole, A.M.; Wachal, R.W. Repurposed battery for energy storage in applications of renewable energy for grid applications. In Proceedings of the 2011 24th Canadian Conference on Electrical and Computer Engineering (CCECE), Niagara Falls, ON, Canada, 8–11 May 2011; pp. 1446–1450. [Google Scholar]
- Schneider, E.L.; Kindlein, W., Jr.; Souza, S.; Malfatti, C.F. Assessment and reuse of secondary batteries cells. J. Power Sources 2009, 189, 1264–1269. [Google Scholar] [CrossRef]
- Lih, W.C.; Jieh-Hwang, Y.; Fa-Hwa, S.; Yu-Min, L. Second Use of Retired Lithium-ion Battery Packs from Electric Vehicles: Technological Challenges, Cost Analysis and Optimal Business Model. In Proceedings of the 2012 International Symposium on Computer, Consumer and Control (IS3C), Taichung, Taiwan, 4–6 June 2012; pp. 381–384. [Google Scholar] [CrossRef]
- Kamath, H. Lithium Ion Batteries in Utility Applications. In Proceedings of the 27th International Battery Seminar & Exhibit, Fort Lauderdale, FL, USA, 15–18 March 2010. [Google Scholar]
- Neubauer, J.; Pesaran, A.; Howell, D. Secondary Use of PHEV and EV Batteries: Opportunities & Challenges (Presentation); No. NREL/PR-540-48872; National Renewable Energy Lab (NREL): Golden, CO, USA, 2010. Available online: https://www.nrel.gov/docs/fy10osti/48872.pdf (accessed on 22 July 2020).
- Neubauer, J.; Pesaran, A. PHEV/EV Li-Ion Battery Second-Use Project; National Renewable Energy Laboratory: Golden, CO, USA, 2010. Available online: https://www.nrel.gov/docs/fy10osti/48018.pdf (accessed on 22 July 2020).
- Neubauer, J.; Pesaran, A.; Williams, B.; Ferry, M.; Eyer, J. Techno-Economic Analysis of PEV Battery Second Use: Repurposed-Battery Selling Price and Commercial and Industrial End-User Value; Report; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2012.
- Hawkins, J.M. Some field experience with battery impedance measurement as a useful maintenance tool. In Proceedings of the 16th International Telecommunications Energy Conference, INTELEC ’94, Vancouver, BC, Canada, 30 October–3 November 1994; pp. 263–269. [Google Scholar] [CrossRef]
- Lasia, A. Electrochemical impedance spectroscopy and its applications. Mod. Asp. Electrochem. 1999, 32, 143–248. [Google Scholar]
- Amine, K.; Chen, C.H.; Liu, J.; Hammond, M.; Jansen, A.; Dees, D.; Bloom, I.; Vissers, D.; Henriksen, G. Factors responsible for impedance rise in high power lithium ion batteries. J. Power Sources 2001, 97–98, 684–687. [Google Scholar] [CrossRef]
- Broussely, M.; Biensan, P.; Bonhomme, F.; Blanchard, P.; Herreyre, S.; Nechev, K.; Staniewicz, R.J. Main aging mechanisms in Li ion batteries. J. Power Sources 2005, 146, 90–96. [Google Scholar] [CrossRef]
- Dees, D.; Gunen, E.; Abraham, D.; Jansen, A.; Prakash, J. Alternating Current Impedance Electrochemical Modeling of Lithium-Ion Positive Electrodes. J. Electrochem. Soc. 2005, 152, A1409–A1417. [Google Scholar] [CrossRef]
- Tröltzsch, U.; Kanoun, O.; Tränkler, H.R. Characterizing aging effects of lithium ion batteries by impedance spectroscopy. Electrochim. Acta 2006, 51, 1664–1672. [Google Scholar] [CrossRef]
- Li, J.; Murphy, E.; Winnick, J.; Kohl, P.A. The effects of pulse charging on cycling characteristics of commercial lithium-ion batteries. J. Power Sources 2001, 102, 302–309. [Google Scholar] [CrossRef]
- Chen, M.; Rincon-Mora, G.A. Accurate electrical battery model capable of predicting runtime and IV performance. IEEE Trans. Energy Convers. 2006, 21, 504–511. [Google Scholar] [CrossRef]
- Saha, B.; Goebel, K. Uncertainty Management for Diagnostics and Prognostics of Batteries using Bayesian Techniques. In Proceedings of the 2008 IEEE Aerospace Conference, Big Sky, MT, USA, 1–8 March 2008; pp. 1–8. [Google Scholar]
- Shim, J.; Kostecki, R.; Richardson, T.; Song, X.; Striebel, K.A. Electrochemical analysis for cycle performance and capacity fading of a lithium-ion battery cycled at elevated temperature. J. Power Sources 2002, 112, 222–230. [Google Scholar] [CrossRef]
- Waag, W.; Käbitz, S.; Sauer, D.U. Experimental investigation of the lithium-ion battery impedance characteristic at various conditions and aging states and its influence on the application. Appl. Energy 2013, 102, 885–897. [Google Scholar] [CrossRef]
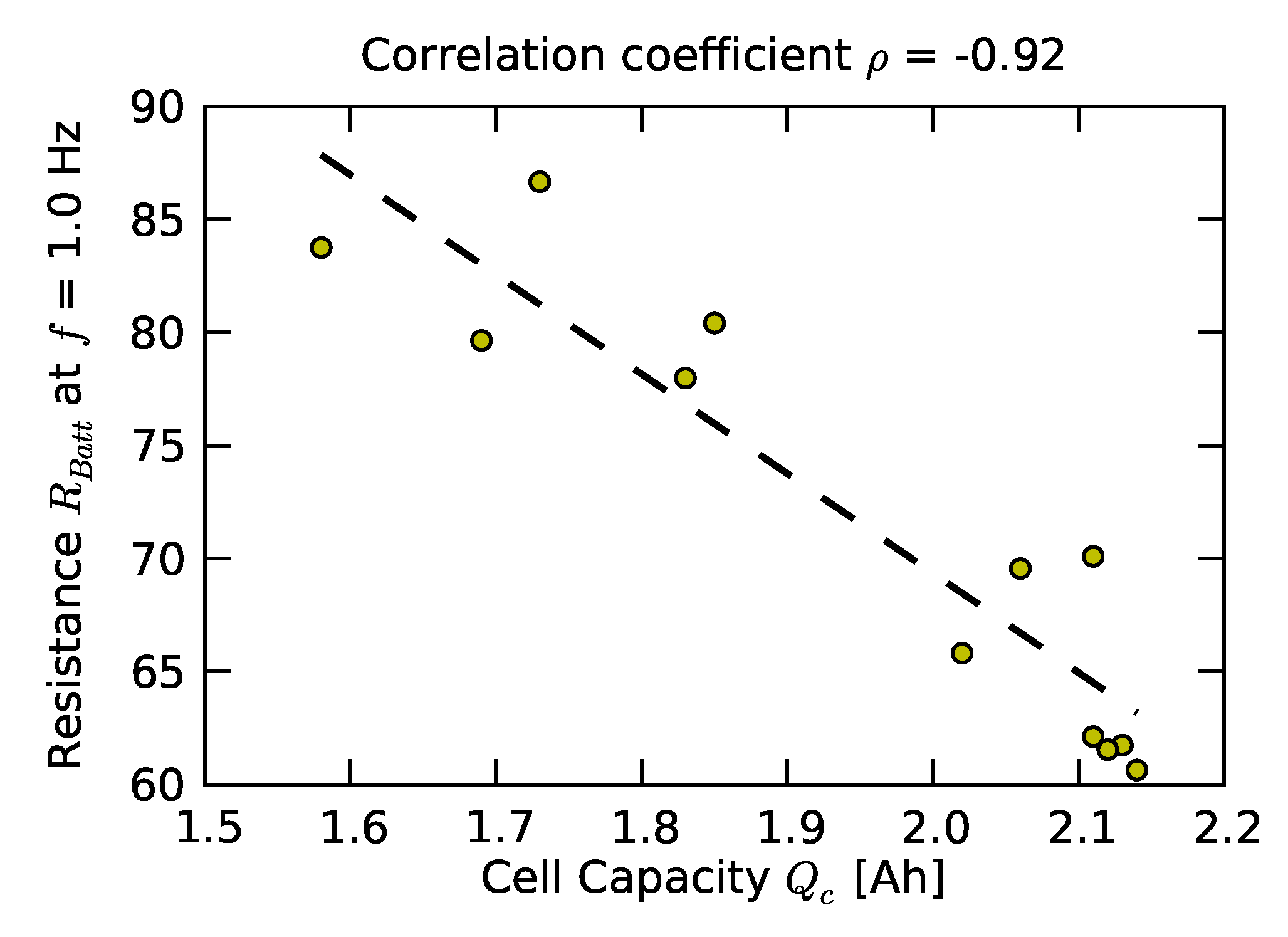
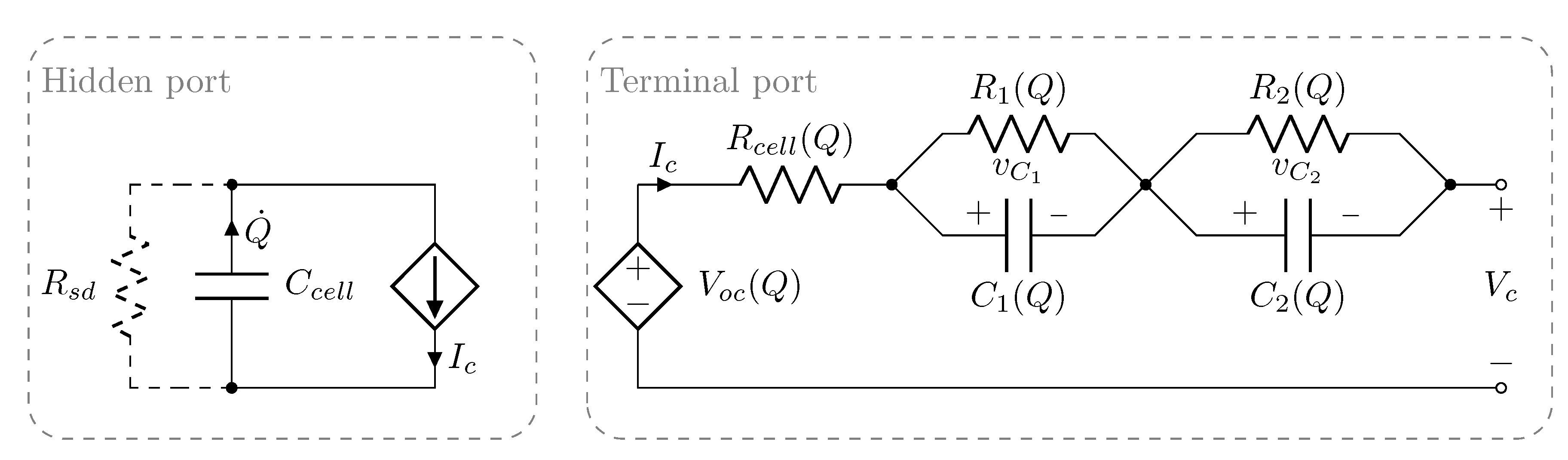
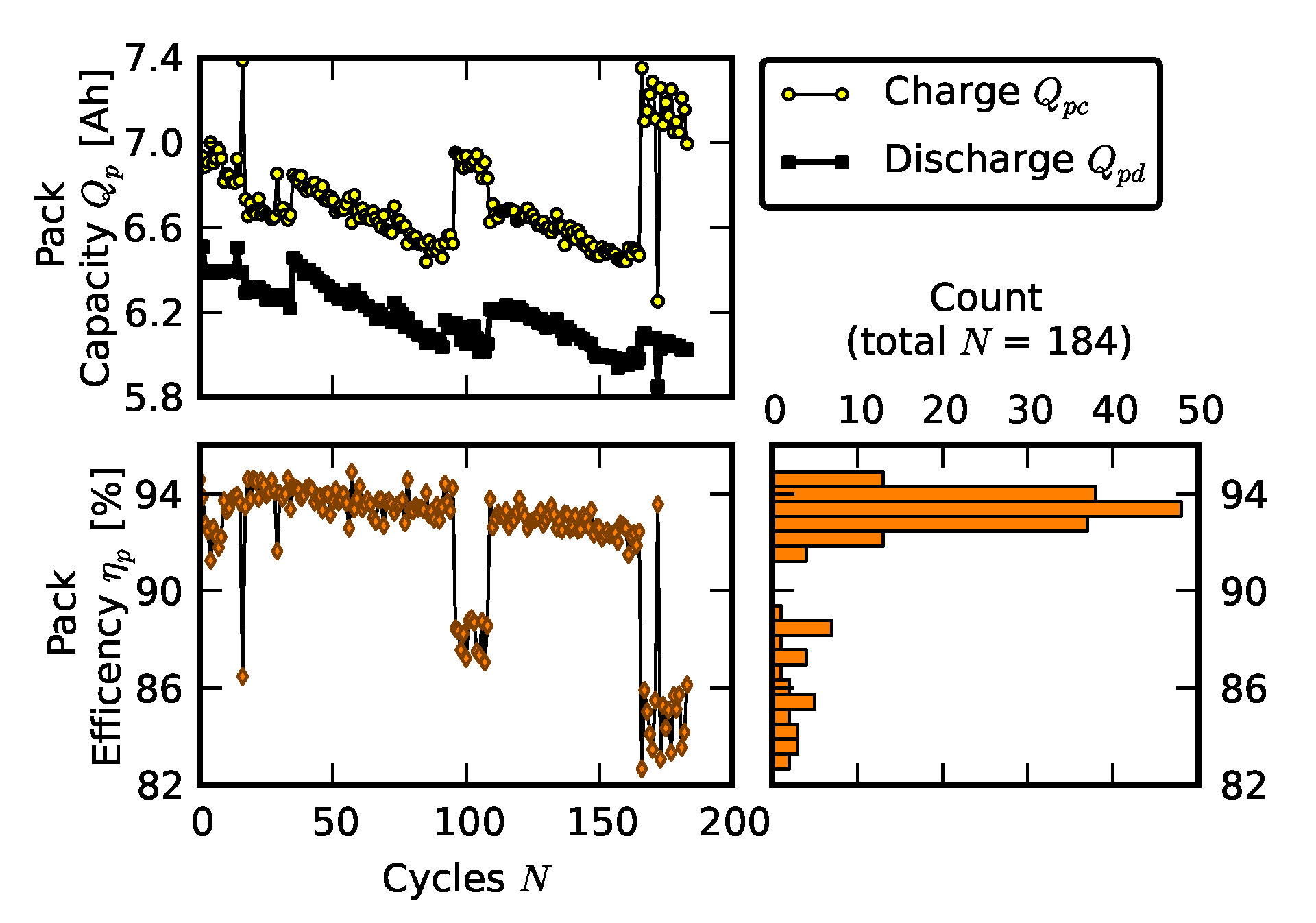
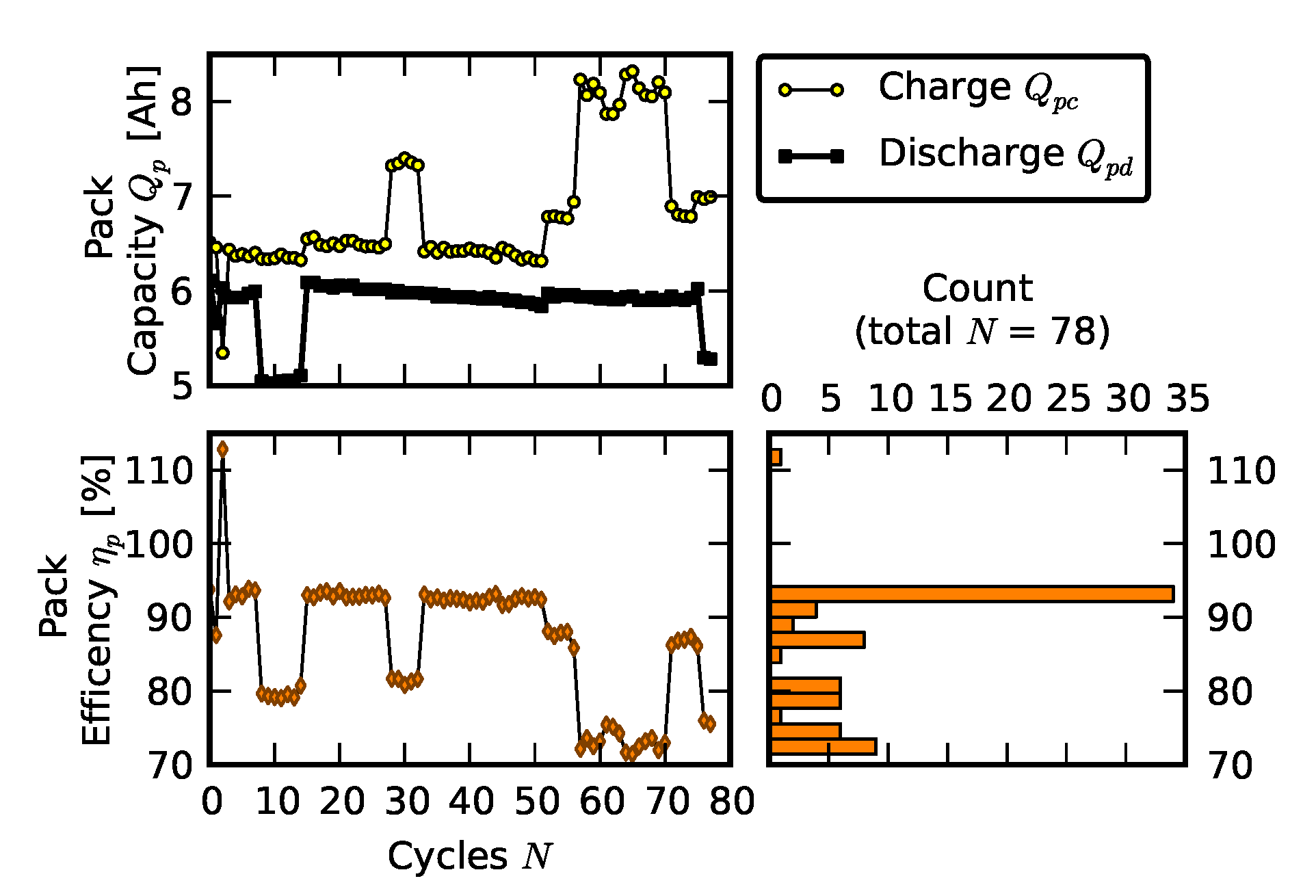
| Cell ID | Cell Capacity (Ah) |
|---|---|
| 6 | 1.83 |
| 8 | 1.85 |
| 14 | 2.11 |
| 16 | 2.11 |
| 17 | 2.06 |
| 19 | 2.13 |
| 20 | max→ 2.14 |
| 21 | 2.12 |
| 23 | 1.73 |
| 24 | 1.69 |
| 25 | min → 1.58 |
| 27 | 2.02 |
| 1.95 | |
| 0.20 |
| Cell ID | (m) | (m) | (m) |
|---|---|---|---|
| 6 | 88.18 | 77.98 | 49.01 |
| 8 | 90.24 | 80.41 | max→52.23 |
| 14 | 64.85 | 62.12 | 44.00 |
| 16 | 72.07 | 70.09 | 51.55 |
| 17 | 72.42 | 69.55 | 50.27 |
| 19 | 64.47 | 61.74 | 43.56 |
| 20 | min→62.61 | min→60.64 | 44.74 |
| 21 | 64.15 | 61.54 | 43.56 |
| 23 | max → 113.70 | max→86.66 | min→43.41 |
| 24 | 99.37 | 79.64 | 49.14 |
| 25 | 105.02 | 83.75 | 49.15 |
| 27 | 69.98 | 65.81 | 45.65 |
| 80.59 | 71.66 | 47.19 | |
| 17.96 | 9.56 | 3.36 |
| Configuration | String 1 | String 2 | String 3 |
|---|---|---|---|
| 2.14 | 2.13 | 2.12 | |
| 2.06 | 2.11 | 2.11 | |
| 2.02 | 1.85 | 1.83 | |
| Total | 6.22 | 6.09 | 6.06 |
| Configuration | String 1 | String 2 | String 3 |
|---|---|---|---|
| 1.83 | 2.11 | 2.06 | |
| 1.85 | 2.12 | 1.73 | |
| 1.85 | 1.58 | 2.02 | |
| Total | 5.81 | 5.81 | 5.81 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nenadic, N.G.; Trabold, T.A.; Thurston, M.G. Cell Replacement Strategies for Lithium Ion Battery Packs. Batteries 2020, 6, 39. https://doi.org/10.3390/batteries6030039
Nenadic NG, Trabold TA, Thurston MG. Cell Replacement Strategies for Lithium Ion Battery Packs. Batteries. 2020; 6(3):39. https://doi.org/10.3390/batteries6030039
Chicago/Turabian StyleNenadic, Nenad G., Thomas A. Trabold, and Michael G. Thurston. 2020. "Cell Replacement Strategies for Lithium Ion Battery Packs" Batteries 6, no. 3: 39. https://doi.org/10.3390/batteries6030039
APA StyleNenadic, N. G., Trabold, T. A., & Thurston, M. G. (2020). Cell Replacement Strategies for Lithium Ion Battery Packs. Batteries, 6(3), 39. https://doi.org/10.3390/batteries6030039






