Synthesis of a NiMoO4/3D-rGO Nanocomposite via Starch Medium Precipitation Method for Supercapacitor Performance
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of NiMoO4 Nanoparticles and NiMoO4 NPs/rGO Nanocomposite
2.3. Fabrication of NiMoO4 NPs and NiMoO4/3D-rGO Nanocomposite Electrodes
2.4. Material Characterization
2.5. Electrochemical Characterization
3. Results and Discussion
3.1. Investigation of Morphology and Structural Characterization
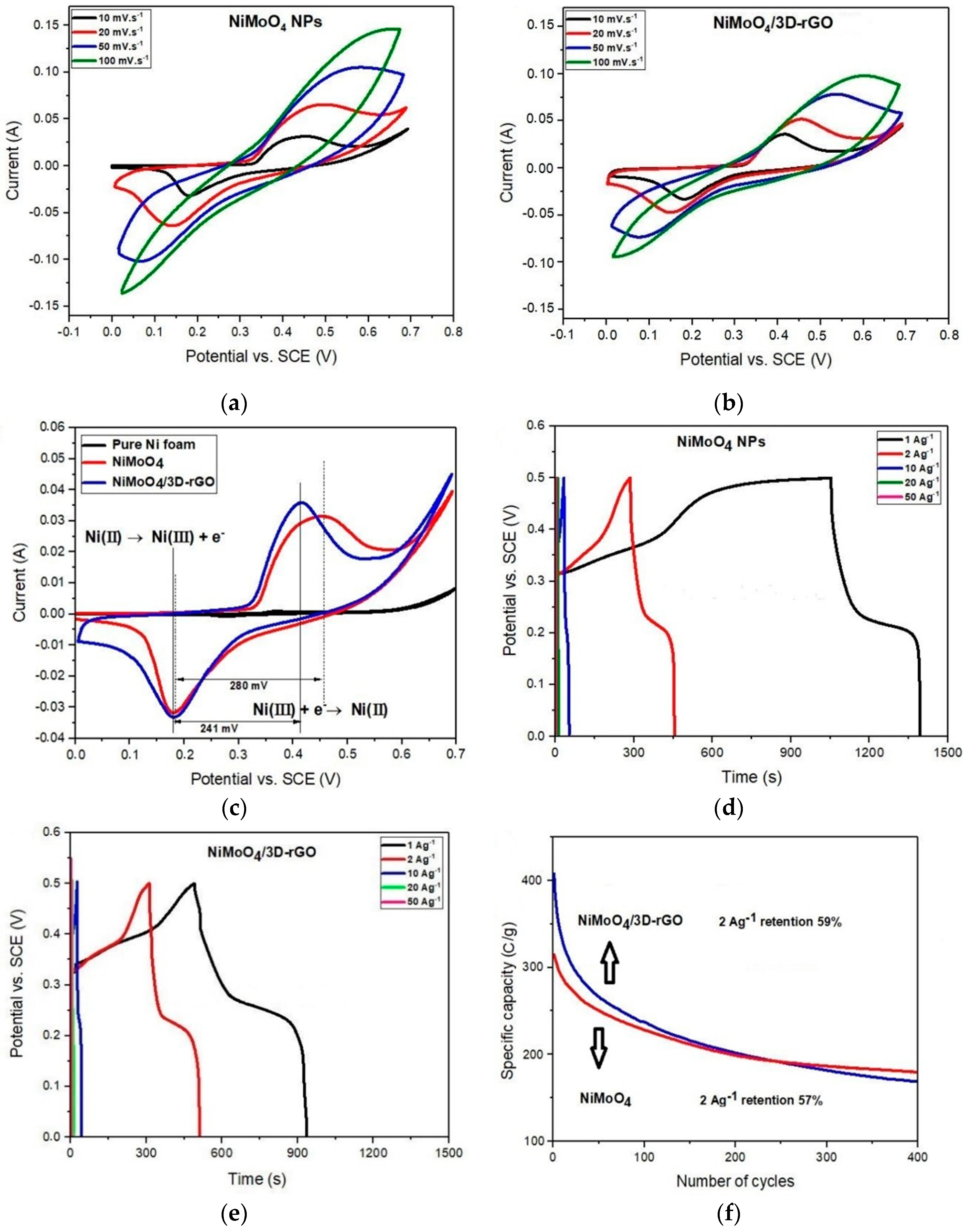
3.2. Electrochemical Measurement of the NiMoO4 NPs and NiMoO4/3D-rGO Electrodes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xia, X.; Zhang, Y.; Chao, D.; Guan, C.; Zhang, Y.; Li, L.; Fan, H.J. Solution synthesis of metal oxides for electrochemical energy storage applications. Nanoscale 2014, 6, 5008–5048. [Google Scholar] [CrossRef] [PubMed]
- Iro, Z.S.; Subramani, C.; Dash, S.S. A brief review on electrode materials for supercapacitor. Int. J. Electrochem. Sci. 2016, 11, 10628–10643. [Google Scholar] [CrossRef]
- Budhiraju, V.S.; Kumar, R.; Sharma, A.; Sivakumar, S. Structurally stable hollow mesoporous graphitized carbon nanofibers embedded with NiMoO4 nanoparticles for high performance asymmetric supercapacitors. Electrochim. Acta 2017, 238, 337–348. [Google Scholar] [CrossRef]
- Gund, G.S.; Lokhande, C.D.; Park, H.S. Controlled synthesis of hierarchical nanoflake structure of NiO thin film for supercapacitor application. Alloys Compd. 2018, 741, 549–556. [Google Scholar] [CrossRef]
- Wang, H.; Song, Y.; Liu, W.; Yan, L. Three dimensional Ni(OH)2/rGO hydrogel as binder-free electrode for asymmetric supercapacitor. Alloys Compd. 2018, 735, 2428–2435. [Google Scholar] [CrossRef]
- Liu, F.; Chu, X.; Zhang, H.; Zhang, B.; Su, H.; Jin, L.; Yang, W. Synthesis of self-assembly 3D porous Ni(OH)2 with high capacitance for hybrid supercapacitors. Electrochim. Acta 2018, 269, 102–110. [Google Scholar] [CrossRef]
- Brisse, A.L.; Stevens, P.; Toussaint, G.; Crosnier, O.; Brousse, T. Ni(OH)2 and NiO Based Composites: Battery Type Electrode Materials for Hybrid Supercapacitor Devices. Materials 2018, 11, 1178. [Google Scholar] [CrossRef]
- Chiu, H.Y.; Cho, C.P. Mixed-Phase MnO2/N-Containing Graphene Composites Applied as Electrode Active Materials for Flexible Asymmetric Solid-State Supercapacitors. Nanomaterials 2018, 8, 924. [Google Scholar] [CrossRef]
- Fei, H.; Saha, N.; Kazantseva, N.; Moucka, R.; Cheng, Q.; Saha, P. A highly flexible supercapacitor based on MnO2/RGO nanosheets and bacterial cellulose-filled gel electrolyte. Materials 2017, 10, 1251. [Google Scholar] [CrossRef]
- Prakash, N.G.; Dhananjaya, M.; Narayana, A.L.; Shaik, D.P.; Rosaiah, P.; Hussain, O.M. High Performance One Dimensional α-MoO3 Nanorods for Supercapacitor Applications. Ceram. Int. 2018, 44, 9967–9975. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, Y.; Ma, Y.; Yao, J.; Li, X.; Sun, Y.; Li, D. RF magnetron sputtering synthesis of three-dimensional graphene@Co3O4 nanowire array grown on Ni foam for application in supercapacitors. Alloys Compd. 2018, 740, 174–179. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Z.; Yuan, X.; Yang, Z.; Zhang, M.; Meng, A.; Li, Q. A High-Energy Density Asymmetric Supercapacitor Based on Fe2O3 Nanoneedle Arrays and NiCo2O4/Ni(OH)2 Hybrid Nanosheet Arrays Grown on SiC Nanowire Networks as Free-Standing Advanced Electrodes. Adv. Energy Mater. 2018, 8, 1702787–1702791. [Google Scholar] [CrossRef]
- Zhang, C.; Lei, C.; Cen, C.; Tang, S.; Deng, M.; Li, Y.; Du, Y. Interface polarization matters: Enhancing supercapacitor performance of spinel NiCo2O4 nanowires by reduced graphene oxide coating. Electrochim. Acta 2018, 260, 814–822. [Google Scholar] [CrossRef]
- Umapathy, V.; Neeraja, P.; Manikandan, A.; Ramu, P. Synthesis of NiMoO4 nanoparticles by sol–gel method and their structural, morphological, optical, magnetic and photocatlytic properties. Trans. Nonferrous Met. Soc. China 2017, 27, 1785–1793. [Google Scholar] [CrossRef]
- Xuan, H.; Xu, Y.; Zhang, Y.; Li, H.; Han, P.; Du, Y. One-step combustion synthesis of porous CNTs/C/NiMoO4 composites for high-performance asymmetric supercapacitors. Alloys Compd. 2018, 745, 135–146. [Google Scholar] [CrossRef]
- Nti, F.; Anang, D.A.; Han, J.I. Facilely synthesized NiMoO4/CoMoO4 nanorods as electrode material for high performance supercapacitor. Alloys Compd. 2018, 742, 342–350. [Google Scholar] [CrossRef]
- Yesuraj, J.; Elumalai, V.; Bhagavathiachari, M.; Samuel, A.S.; Elaiyappillai, E.; Johnson, P.M. A facile sonochemical assisted synthesis of α-MnMoO4/PANI nanocomposite electrode for supercapacitor applications. Electroanal. Chem. 2017, 797, 78–88. [Google Scholar] [CrossRef]
- Xiao, K.; Xia, L.; Liu, G.; Wang, S.; Ding, L.X.; Wang, H. Honeycomb-like NiMoO4 ultrathin nanosheet arrays for high-performance electrochemical energy storage. J. Mater. Chem. A 2015, 3, 6128–6135. [Google Scholar] [CrossRef]
- Xiong, X.; Ding, D.; Chen, D.; Waller, G.; Bu, Y.; Wang, Z.; Liu, M. Three-dimensional ultrathin Ni(OH)2 nanosheets grown on nickel foam for high-performance supercapacitors. Nano. Energy 2015, 11, 154–161. [Google Scholar] [CrossRef]
- Mohapatra, D.; Parida, S.; Singh, B.K.; Sutar, D.S. Importance of microstructure and interface in designing metal oxide nanocomposites for supercapacitor electrodes. Electroanal. Chem. 2017, 803, 30–39. [Google Scholar] [CrossRef]
- Liu, M.C.; Kong, L.B.; Lu, C.; Ma, X.J.; Li, X.M.; Luo, Y.C.; Kang, L. Design and synthesis of CoMoO4–NiMoO4·xH2O bundles with improved electrochemical properties for supercapacitors. J. Mater. Chem. A 2013, 1, 1380–1387. [Google Scholar] [CrossRef]
- Liu, T.; Chai, H.; Jia, D.; Su, Y.; Wang, T.; Zhou, W. Rapid microwave-assisted synthesis of mesoporous NiMoO4 nanorod/reduced graphene oxide composites for high-performance supercapacitors. Electrochim. Acta 2015, 180, 998–1006. [Google Scholar] [CrossRef]
- Kianpour, G.; Salavati-Niasari, M.; Emadi, H. Sonochemical synthesis and characterization of NiMoO4 nanorods. Ultrason. Sonochem. 2013, 20, 418–424. [Google Scholar] [CrossRef] [PubMed]
- De Moura, A.P.; de Oliveira, L.H.; Rosa, I.L.; Xavier, C.S.; Lisboa-Filho, P.N.; Li, M.S.; Varela, J.A. Structural, optical, and magnetic properties of NiMoO4 nanorods prepared by microwave sintering. Sci. World J. 2015, 1, 1–9. [Google Scholar] [CrossRef]
- Jinlong, L.; Miura, H.; Meng, Y. A novel mesoporous NiMoO4@rGO nanostructure for supercapacitor applications. Mater. Lett. 2017, 194, 94–97. [Google Scholar] [CrossRef]
- Arshadi Rastabi, S.; Sarraf Mamoory, R.; Dabir, F.; Blomquist, N.; Phadatare, M.; Olin, H. Synthesis of NiMoO4/3D-rGO Nanocomposite in Alkaline Environments for Supercapacitor Electrodes. Crystals 2019, 9, 31. [Google Scholar] [CrossRef]
- Kumar, Y.; Kim, H.J. Effect of Time on a Hierarchical Corn Skeleton-Like Composite of CoO@ZnO as Capacitive Electrode Material for High Specific Performance Supercapacitors. Energies 2018, 11, 3285. [Google Scholar] [CrossRef]
- Ossonon, B.D.; Bélanger, D. Synthesis and characterization of sulfophenyl-functionalized reduced graphene oxide sheets. RSC Adv. 2017, 7, 27224–27234. [Google Scholar] [CrossRef]
- Srivastava, M.; Uddin, M.E.; Singh, J.; Kim, N.H.; Lee, J.H. Preparation and characterization of self-assembled layer by layer NiCo2O4–reduced graphene oxide nanocomposite with improved electrocatalytic properties. Alloys Compd. 2014, 590, 266–276. [Google Scholar] [CrossRef]
- Fayer, M.D. Ultrafast Infrared Vibrational Spectroscopy; CRC Press: New York, NY, USA, 2013; Volume 4, p. 128. [Google Scholar]
- Li, Y.; Jian, J.; Fan, Y.; Wang, H.; Yu, L.; Cheng, G.; Sun, M. Facile one-pot synthesis of a NiMoO4/reduced graphene oxide composite as a pseudocapacitor with superior performance. RSC Adv. 2016, 6, 69627–69633. [Google Scholar] [CrossRef]
- Bankar, P.K.; Ratha, S.; More, M.A.; Late, D.J.; Rout, C.S. Enhanced field emission performance of NiMoO4 nanosheets by tuning the phase. Appl. Surf. Sci. 2017, 418, 270–274. [Google Scholar] [CrossRef]
- Ezeigwe, E.R.; Khiew, P.S.; Siong, C.W.; Tan, M.T. Synthesis of NiMoO4 nanorods on graphene and superior electrochemical performance of the resulting ternary based composites. Ceram. Int. 2017, 43, 13772–13780. [Google Scholar] [CrossRef]
- Azarang, M.; Shuhaimi, A.; Yousefi, R.; Sookhakian, M. Effects of graphene oxide concentration on optical properties of ZnO/RGO nanocomposites and their application to photocurrent generation. Appl. Phys. 2014, 116, 84307–84313. [Google Scholar] [CrossRef]
- Jamali-Sheini, F.; Azarang, M. Effect of annealing temperature and graphene concentrations on photovoltaic and NIR-detector applications of PbS/rGO nanocomposites. Ceram. Int. 2016, 42, 15209–15216. [Google Scholar] [CrossRef]
- Uddin, A.S.M.I.; Phan, D.T.; Chung, G.S. Synthesis of ZnO nanoparticles-reduced graphene oxide composites and their intrinsic gas sensing properties. Surf. Rev. Lett. 2014, 21, 1450086–1450097. [Google Scholar] [CrossRef]
- Guan, X.H.; Lan, X.; Lv, X.; Yang, L.; Wang, G.S. Synthesis of NiMoSO/rGO Composites Based on NiMoO4 and Reduced Graphene with High-Performance Electrochemical Electrodes. ChemistrySelect 2018, 3, 6719–6728. [Google Scholar] [CrossRef]
- Jothi, P.R.; Kannan, S.; Velayutham, G. Enhanced methanol electro-oxidation over in-situ carbon and graphene supported one dimensional NiMoO4 nanorods. J. Power Sources 2015, 277, 350–359. [Google Scholar] [CrossRef]
- Ghosh, D.; Giri, S.; Das, C.K. Synthesis, characterization and electrochemical performance of graphene decorated with 1D NiMoO4·nH2O nanorods. Nanoscale 2013, 5, 10428–10437. [Google Scholar] [CrossRef]
- Jothi, P.R.; Shanthi, K.; Salunkhe, R.R.; Pramanik, M.; Malgras, V.; Alshehri, S.M.; Yamauchi, Y. Synthesis and Characterization of α-NiMoO4 Nanorods for Supercapacitor Application. Eur. J. Inorg. Chem. 2015, 22, 3694–3699. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, G.; Zhou, Y.; Gao, M.; Yang, F. One-step potentiodynamic synthesis of poly (1,5-diaminoanthraquinone)/reduced graphene oxide nanohybrid with improved electrocatalytic activity. J. Mater. Chem. A 2013, 1, 13902–13913. [Google Scholar] [CrossRef]
- Li, Y.; Jian, J.; Xiao, L.; Wang, H.; Yu, L.; Cheng, G.; Sun, M. Synthesis of NiMoO4 nanosheets on graphene sheets as advanced supercapacitor electrode materials. Mater. Lett. 2016, 184, 21–24. [Google Scholar] [CrossRef]
- Trafela, Š.; Zavašnik, J.; Šturm, S.; Rožman, K.Ž. Formation of a Ni(OH)2/NiOOH active redox couple on nickel nanowires for formaldehyde detection in alkaline media. Electrochim. Acta 2019, 309, 346–353. [Google Scholar] [CrossRef]
- Jiang, G.; Zhang, M.; Li, X.; Gao, H. NiMoO4@Ni(OH)2 core/shell nanorods supported on Ni foam for high-performance supercapacitors. RSC Adv. 2015, 85, 69365–69370. [Google Scholar] [CrossRef]
- Yedluri, A.K.; Anitha, T.; Kim, H.J. Fabrication of Hierarchical NiMoO4/NiMoO4 Nanoflowers on Highly Conductive Flexible Nickel Foam Substrate as a Capacitive Electrode Material for Supercapacitors with Enhanced Electrochemical Performance. Energies 2019, 12, 1143. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arshadi Rastabi, S.; Sarraf Mamoory, R.; Blomquist, N.; Phadatare, M.; Olin, H. Synthesis of a NiMoO4/3D-rGO Nanocomposite via Starch Medium Precipitation Method for Supercapacitor Performance. Batteries 2020, 6, 5. https://doi.org/10.3390/batteries6010005
Arshadi Rastabi S, Sarraf Mamoory R, Blomquist N, Phadatare M, Olin H. Synthesis of a NiMoO4/3D-rGO Nanocomposite via Starch Medium Precipitation Method for Supercapacitor Performance. Batteries. 2020; 6(1):5. https://doi.org/10.3390/batteries6010005
Chicago/Turabian StyleArshadi Rastabi, Shahrzad, Rasoul Sarraf Mamoory, Nicklas Blomquist, Manisha Phadatare, and Håkan Olin. 2020. "Synthesis of a NiMoO4/3D-rGO Nanocomposite via Starch Medium Precipitation Method for Supercapacitor Performance" Batteries 6, no. 1: 5. https://doi.org/10.3390/batteries6010005
APA StyleArshadi Rastabi, S., Sarraf Mamoory, R., Blomquist, N., Phadatare, M., & Olin, H. (2020). Synthesis of a NiMoO4/3D-rGO Nanocomposite via Starch Medium Precipitation Method for Supercapacitor Performance. Batteries, 6(1), 5. https://doi.org/10.3390/batteries6010005




