3.1. Effect of Current Rate
The battery cells were cyclically discharged and charged at different current rates and between a lower and upper voltage limit.
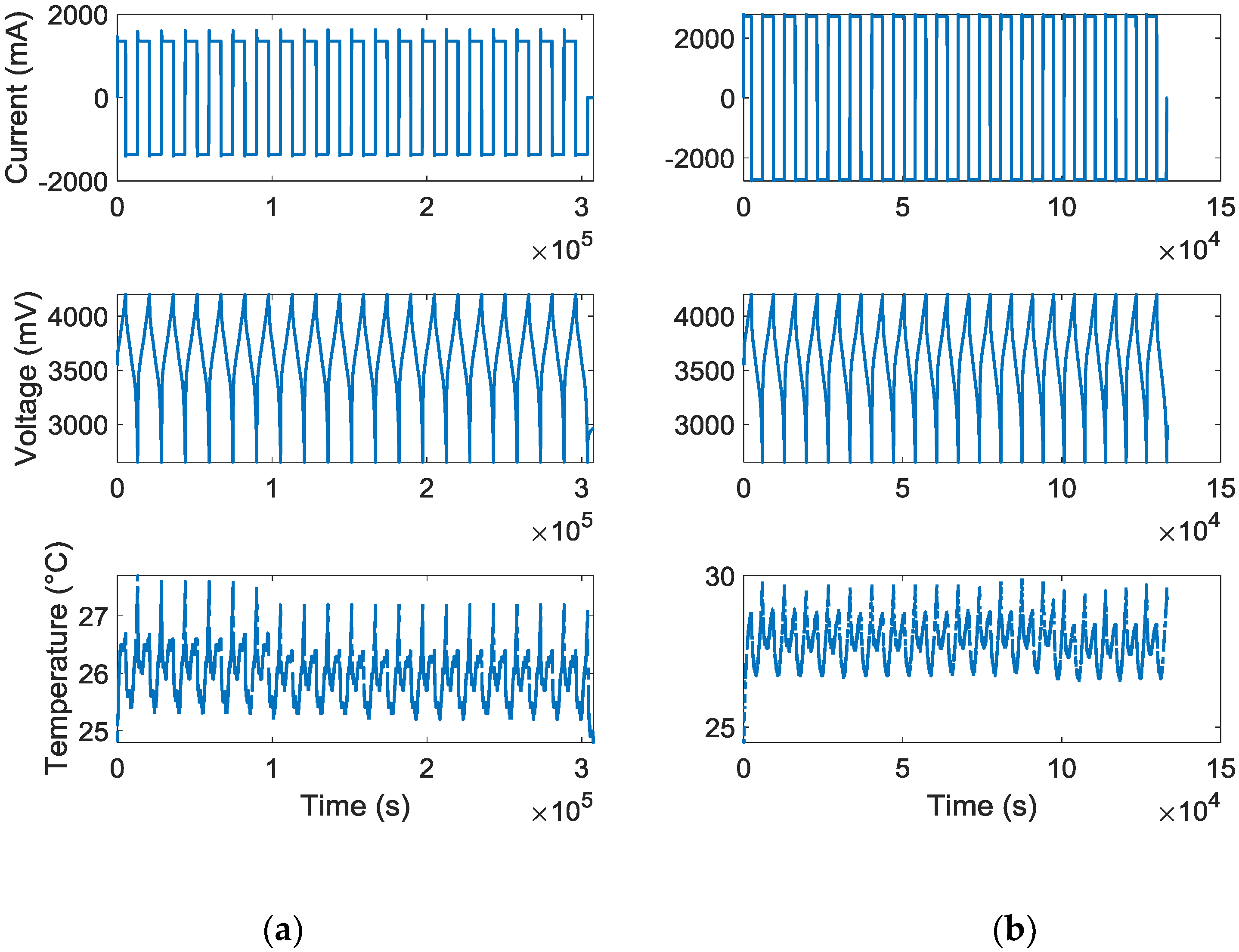
Figure 1 illustrates the current, voltage, and temperature profiles from the applied experiment for 0.4 C and 0.8 C.
When performing the test, each of the battery cells were initially rested for 24 h, and then being charged at a constant current rate equal to 0.4 C and 0.8 C. Following each of the charging processes, immediately, the battery cells were being discharged at a constant current rate equal to 0.4 C and 0.8 C, correspondingly. Lower and upper voltage limits were assigned as 2.65 V and 4.2 V to fulfill the lesser and uppermost voltage limit, correspondingly.
To automate the experiments, safety procedures were applied in the battery cycler to stop the experiment in the case special events are triggered. Each part of the experiment is finished if the measured voltage attains some limits, for example, 2.65 V and 4.2 V during discharging and charging, correspondingly.
In addition, another constraint was implemented, which was restricting the charging and discharging time. For example, in case the current rate is C/5, the battery cell needs maximum 5 h to attain each voltage limitation. This time was selected in such a way that the battery cells reach the lower and upper voltage thresholds. Because, stopping each discharge and charge cycle before reaching the threshold voltage leads to forcing the battery cells to settle to a dissimilar relaxed voltage. Two different loading profiles were applied to the batteries. One of them (number 1) was charged and then discharged with 0.4 C for 20 times and another cell (number 2) was charged and discharged with 0.8 C with the same amount of cycle number.
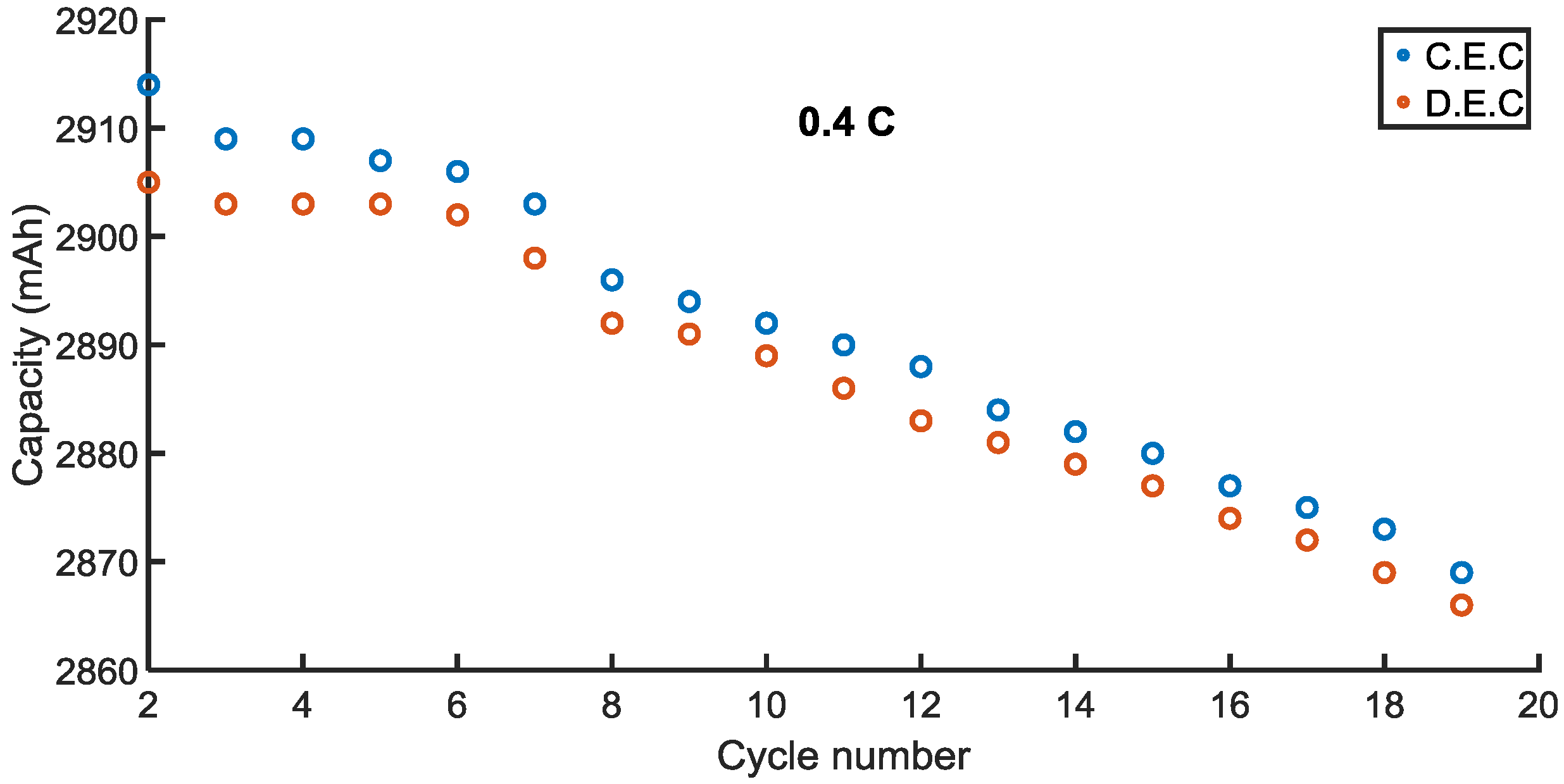
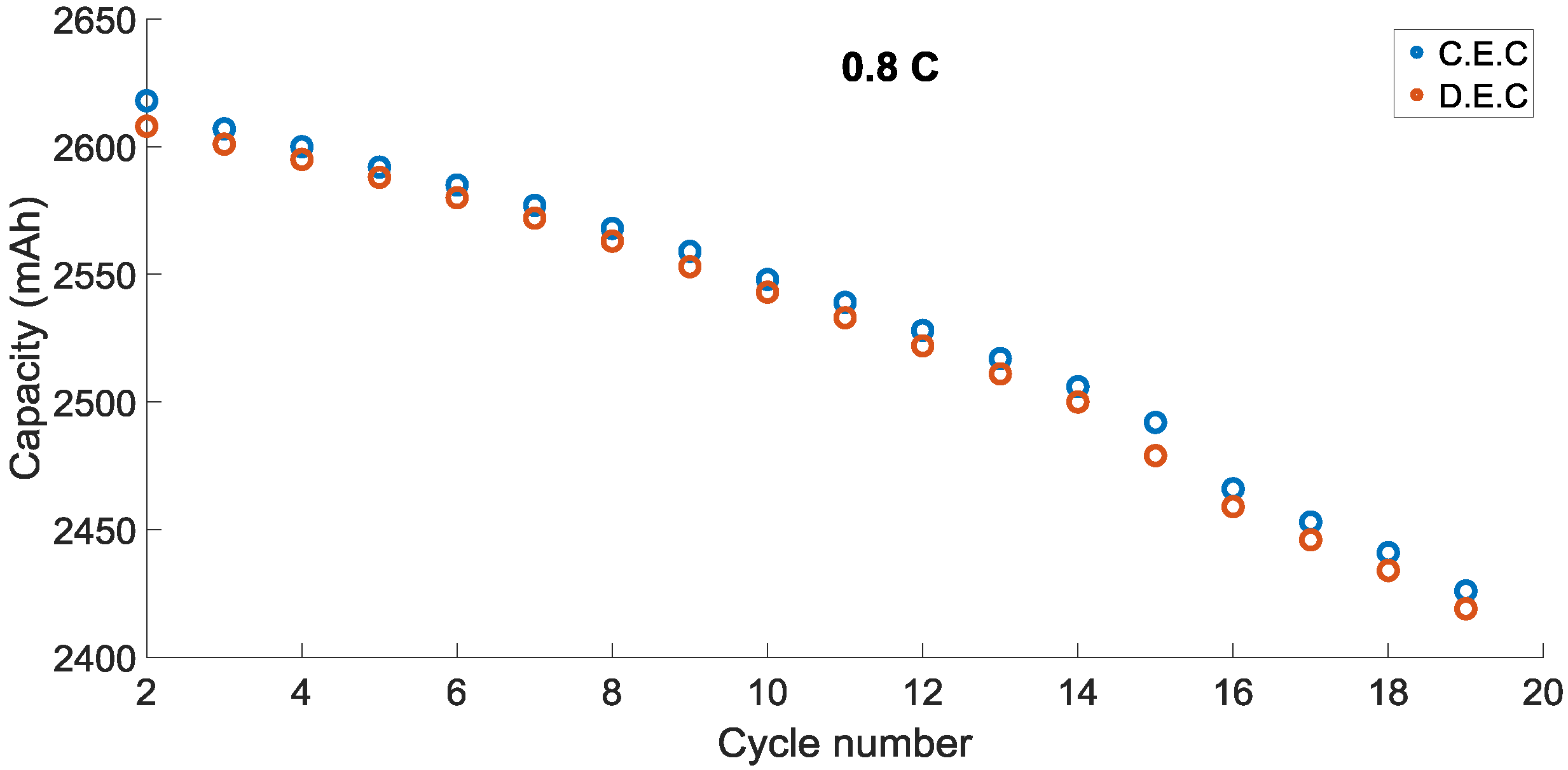
Charge End Capacity (CEC) and Discharge End Capacity (DEC) of the battery cells are illustrated in
Figure 2 and
Figure 3, correspondingly. All of the end points of charge and discharge capacities continually decrease as lithium-ion cells are cycled and this could be a conventional feature of all lithium-ion battery cells.
The battery cell which was cycled at bigger C rates lose capacity quicker than another battery which was cycled at lower C rates. Discharge end capacity is less than charge end capacity for 0.4 C in all cycles. Notwithstanding, the cycling type less affects 0.8 C. In other words, the charge end capacity and discharge end capacity are almost the same for 0.8 C. The average capacity loss rates for discharge and charge during 0.4 C were approximately 0.076% and 0.09% per cycle, correspondingly. This was calculated over the 19 cycles.
The life cycle of a lithium-ion battery cell is not unlimited, because of smart parts of battery cell ingredients that are utilized by parasitic reactions throughout the time of each cycle likely constructing electrolyte oxidation and capacity fade [
10]. The quantity of these parasitic reactions could be displayed by accurate measurements of coulombic efficiency [
10]:
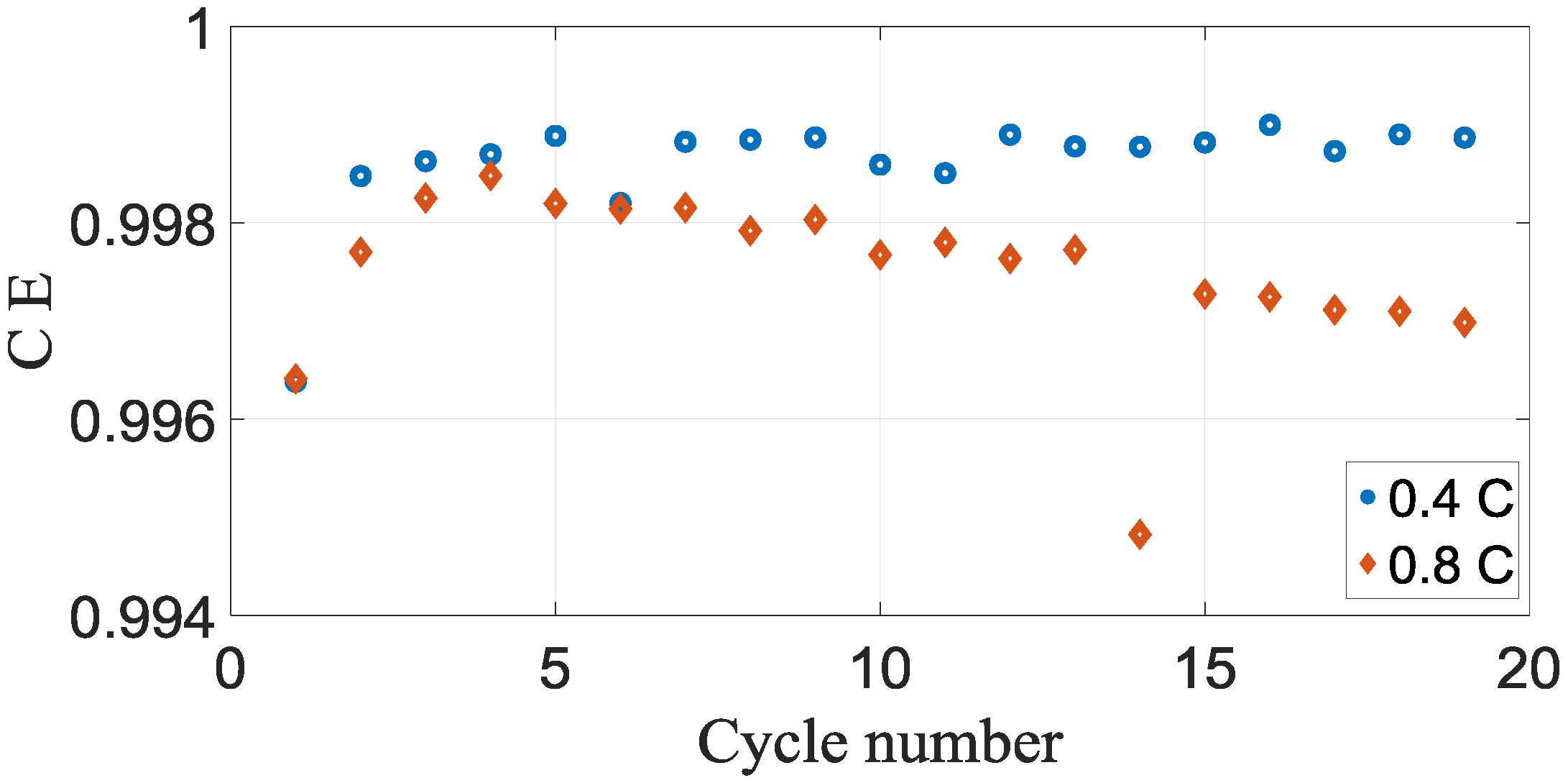
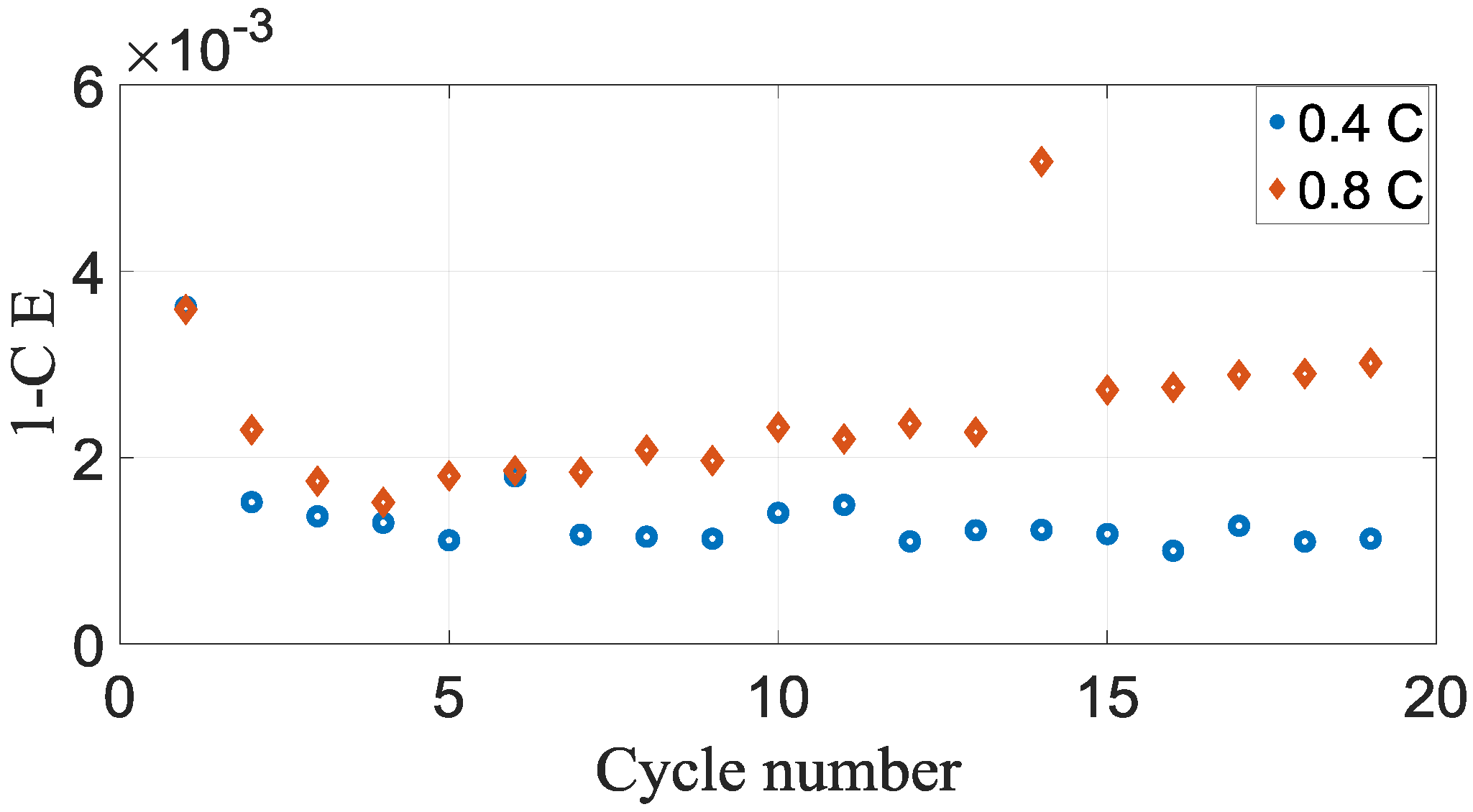
Figure 4 illustrates the coulombic efficiency vs cycle number. The presence of these reactions could be distinguished by a coulombic efficiency fewer than 1.000. The coulombic inefficiency vs cycle number is shown in
Figure 5. Coulombic inefficiency divided by time of each discharge and charge cycle vs time is illustrated in
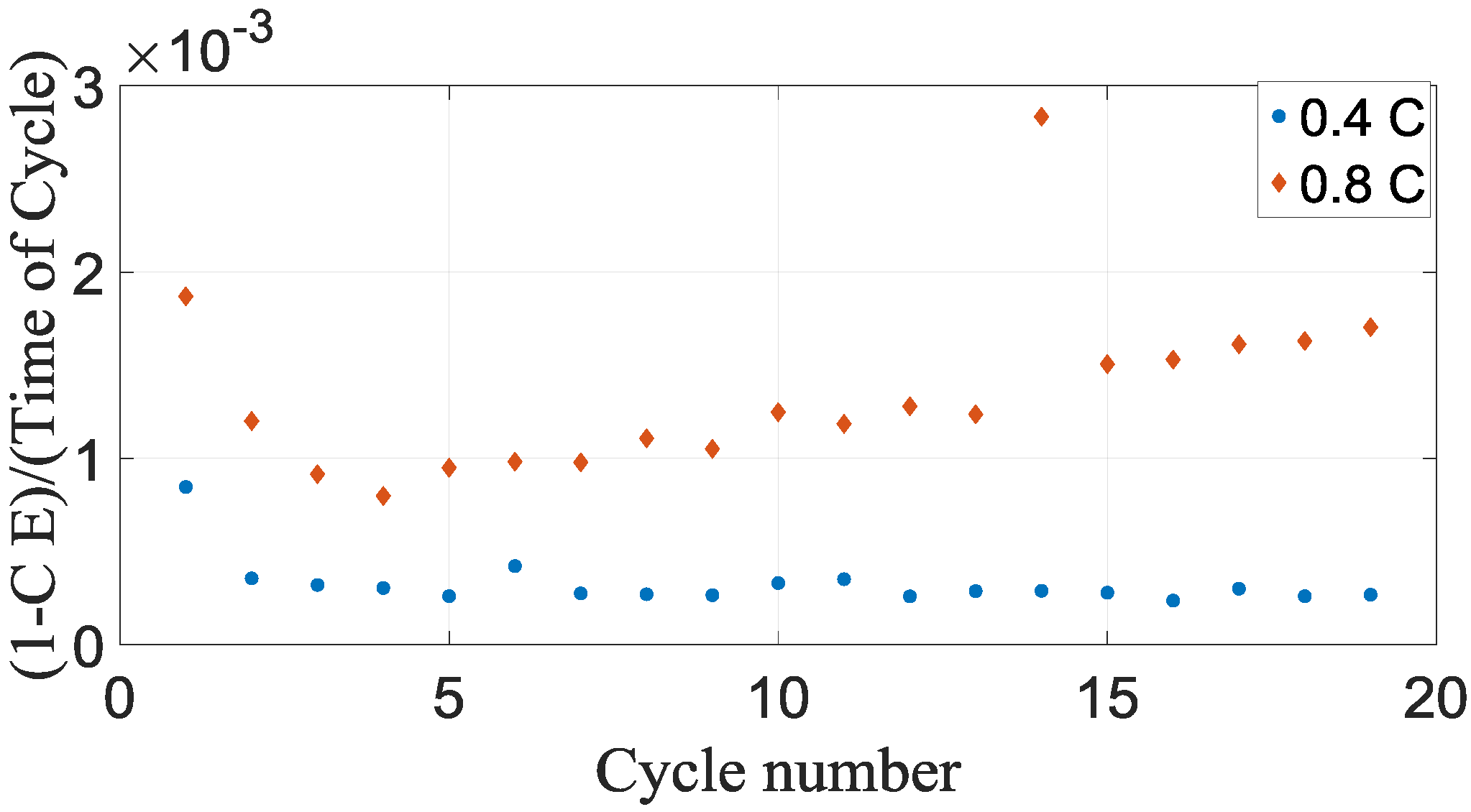
Figure 3.
The quantity of parasitic reactions that happen for a specified cycle is straightforwardly the time of each cycle multiplied by the parasitic reaction rate. This causes a conventional expression for the coulombic inefficiency for any particular cycle [
10]:
where
t: Calendar time;
k(
T,
t): The parasitic reaction;
T: The cell temperature.
Reaction processes that use electrolyte ingredients or active lithium in lithium-ion battery cells are frequently considered as parasitic reactions [
11]. As was mentioned before, parasitic reactions that occur in the battery cell and
k(
T,
t) are related to this parasitic reaction rate, which is as a function of the battery cell calendar time and temperature. As could be seen from
Figure 6, these parasitic reactions are higher for 0.8 C when compared to 0.4 C.
The intensity of these parasitic reactions decreases as the battery cells age for the reason that coulombic inefficiency comes within reach of unification [
10]. This is unquestionably attributable to the growing of interface layers between the electrolyte and electrodes [
10].
As could be seen from the
Figure 4, the battery cells demonstrated well capacity retention throughout the beginning twenty cycles. However, there are clear differences in the coulombic efficiency for both cases, which would cause differences in the capacity retentions. The coulombic efficiency outcomes demonstrated that all the battery cells that were discharged and charged have coulombic efficiency less than 1000.
The rate of battery capacity loss is proximate to the divergence of the coulombic efficiency from 1000. This correspondence is to be assumed in the application which Li absent at the negative electrode by becoming more concentrated of solid electrolyte interphase that the derivation of the coulombic inefficiency is [
12]. It is essential to contemplate the experimental factors that should be controlled to measure the coulombic efficiency accurately during a constant current discharge and charge among fixed voltage limits. There are several factors that need to be contemplated, such as accuracy of voltage, currents, battery cell temperature, and time among voltage measurements [
12].
3.2. Effect of Prior Cycling
The investigation of rechargeable batteries, particularly lithium-ion battery cells, in the present circumstances, is of great technological and scientific attentiveness. Additionally, experimental investigations targeting at engineer more accomplished batteries. A considerable quantity of modeling has been attempted to comprehend the electrochemical processes that happen throughout battery application.
To satisfy the demands for some applications, it is needed to prolong the lifetime of Li-ion batteries. For instance, solar and wind energy storage systems have more demanding lifetime requirements. The capacity of the Li-ion batteries decreases during cycling. In an automotive application, this lessening in Li-ion battery capacity demonstrates a lessening in the uttermost driving scope of an electric vehicle. Li-ion battery cell capacity, accordingly, is an appropriate metric for characterizing the state of health of a Li-ion battery cell [
13,
14,
15].
The lithium-ion batteries are distinguished rechargeable batteries. In these batteries, lithium ions are commuted internally between two electrodes, during which electrons are carried by the external circuit and perform the electrical function. The electrodes are generally inserting porous electrodes that, in a perfect instance, reversibly keep lithium in their construction. The electrode that is at the greater electrochemical potential is considered the positive electrode and another that at the lesser potential is considered the negative electrode.
An electrolyte is employed as surroundings of transmission for the lithium ions among the electrodes. A separator that permits ion transportation is employed to stop physical contact among the electrodes.
From beginning to end of charging, lithium ions are transferred from the positive electrode to the negative electrode by the separator and electrolyte. Electrons relocate in the corresponding direction by the exterior circuit. The opposite process happens throughout discharging.
The effectiveness of lithium-ion batteries worsens over time, even if they are used or not. Ageing without and with use are called calendar ageing and cycle ageing, correspondingly.
The two principal outcomes of ageing are power and energy fade. In an electric vehicle utilization, for example, the power specifies the utmost acceleration the vehicle could gain, and the energy specifies the utmost distance that the vehicle could travel through a single charge.
Energy declining could be induced through a diminution of battery capacity or in the increase of the impedance. Diminution of counterbalancing of the active electrode material or the cyclable lithium is the principal origins of capacity fade.
The increase in impedance is attributable to the physical or chemical conversion of the diverse interfaces and materials. An increase in impedance consequently results in a power fade moreover to an energy fade. Typically, both power and energy fades happen contemporary and their comparative importance relies on the specific application. For example, power fade is less critical than energy fade in an electric vehicle.
Lithium-ion batteries have different classifications of ageing mechanism. They could be either mechanical or chemical in character. The mechanisms are dissimilar on the negative electrode side and on the positive electrode side. The most essential ageing mechanism on the negative electrode side is the development of a solid electrolyte interphase, which utilizes the cyclable lithium.
This interphase layer between the electrolyte and graphite is produced due to the fact that the functioning graphite potential on the surface is greater than the stability range of the mostly utilized carbonate electrolytes.
Generally, configuration cycling is accomplished in a lithium-ion battery after battery cell structure where the commencing solid electrolyte interphase is made. Notwithstanding, continuing cycling induces the graphite particles to thicken and construct cracks in the solid electrolyte interphase layer. This revealing novel surface is responsible for supplementary solid electrolyte interphase expansion.
The solid electrolyte interphase enlargement declines at rate that is accompanied by time. Nevertheless, it proceeds over the length and breadth of the lifetime of the battery cell and it uses the cyclable lithium [
16].
As was mentioned before, there are different types of ageing mechanism. There is ageing mechanisms within the confines of the graphite that comprise gas development, lithium plating, graphite depilation, and current collector erosion. A considerably slim impervious layer of the electrolyte oxidation production establishes on the electrode surface that brings about the increase in the battery cell impedance.
Life cycle is essentially necessary in implementations of rechargeable batteries. Nevertheless, lifetime prognostication is predominantly based upon empirical trends, instead of mathematical models. In practicable lithium-ion batteries, capacity fade happens over a large amount of cycles, which is restricted by sluggish electrochemical processes, for instance, the creation of a solid-electrolyte interphase in the negative electrode.
Throughout the discharge and charge of a lithium-ion battery cell, the active lithium-ion in the battery cell is inserted out of and into the negative electrode, correspondingly. For the duration of each cycle a tiny quantity of that active lithium-ion reacts with the intention of thickening a passive layer on the surface of the electrode. This is identified under the name of solid electrolyte interphase.
The life cycle of a lithium-ion battery cell is not boundless because little fractions of battery cell ingredients are used up by parasitic reactions throughout each cycle. These undesirable reactions could appear by several different processes, such as solid electrolyte interphase repair and growth, electrolyte oxidation, progression metal ions from out of the positive electrode, and destruction of the positive electrode. Each of these processes could have different reasons for instance solid electrolyte interphase growth and repair is because of lithium-ion loss at the negative electrode [
17].
The significance of the coulombic efficiency was acknowledged in a thoughtful research paper on factors that influence capacity retention of lithium ion cells. In the mentioned research paper, it was declared that matched coulombic efficiencies for the negative and positive electrodes, notwithstanding could result in outstanding life cycle for full Li-ion battery cells [
4].
It was shown that accuracy measurements of coulombic efficiency are achievable and could lead to bigger comprehension of the degradation processes to be accomplished at the electrodes of Li-ion battery cells [
4].
As mentioned before, coulombic efficiencies for the Li-ion battery cells were calculated as the ratio of the capacity of the discharge instantaneously following the previous charge capacity. Consequently, for the Li-ion battery cells:
where:
Qd: Discharge capacity;
Qc: Charge capacity
Three new Li-ion battery cells were selected for the experiments. Two of the Li-ion battery cells were discharged and then charged at 25 °C by using currents corresponding to 0.4 C and 0.8 C. Another Li-ion battery cell was discharged and charged at the same temperature, but with the currents corresponding to 0.2 C, 0.4 C, 0.6 C, and 0.8 C.
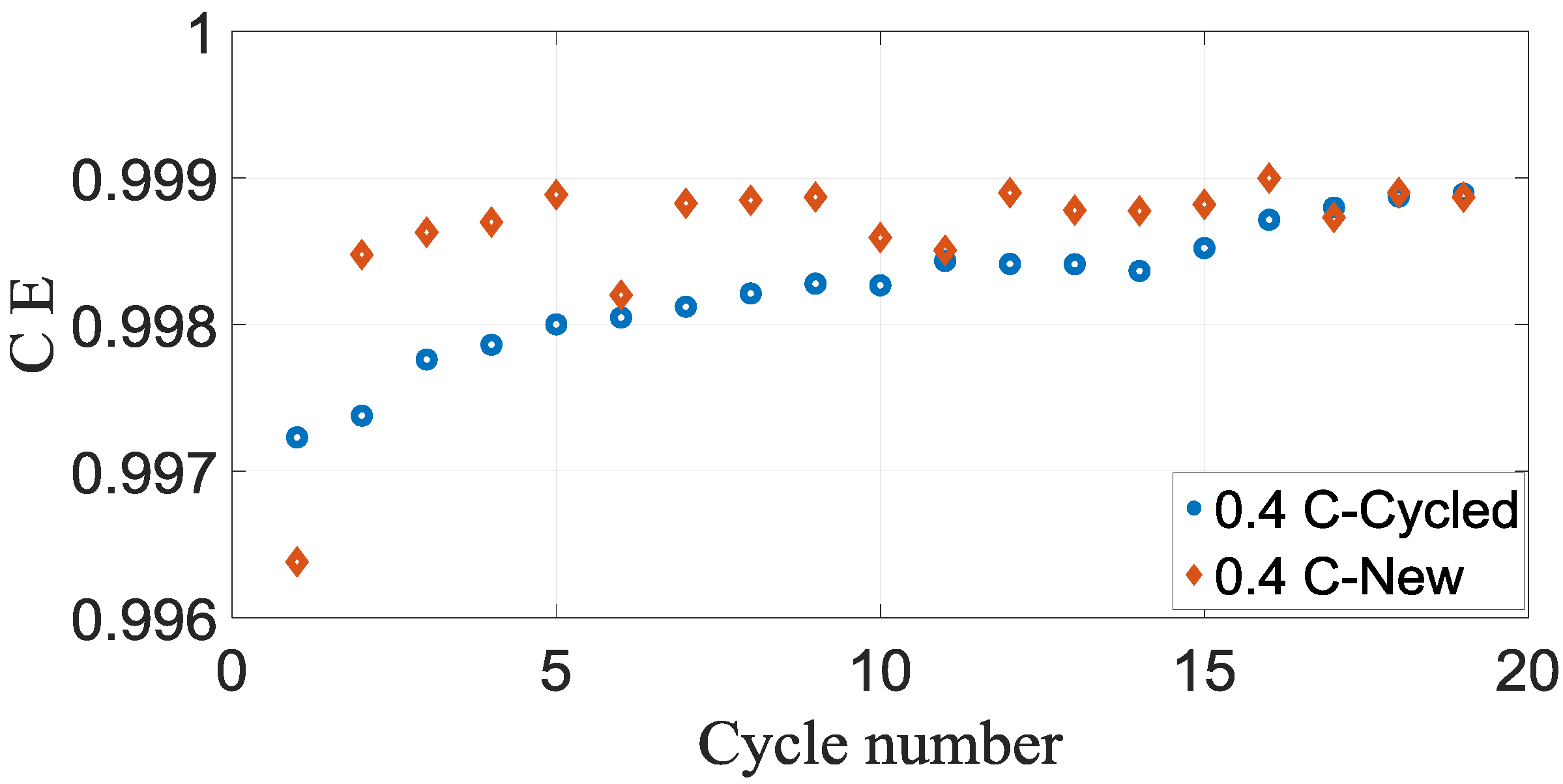
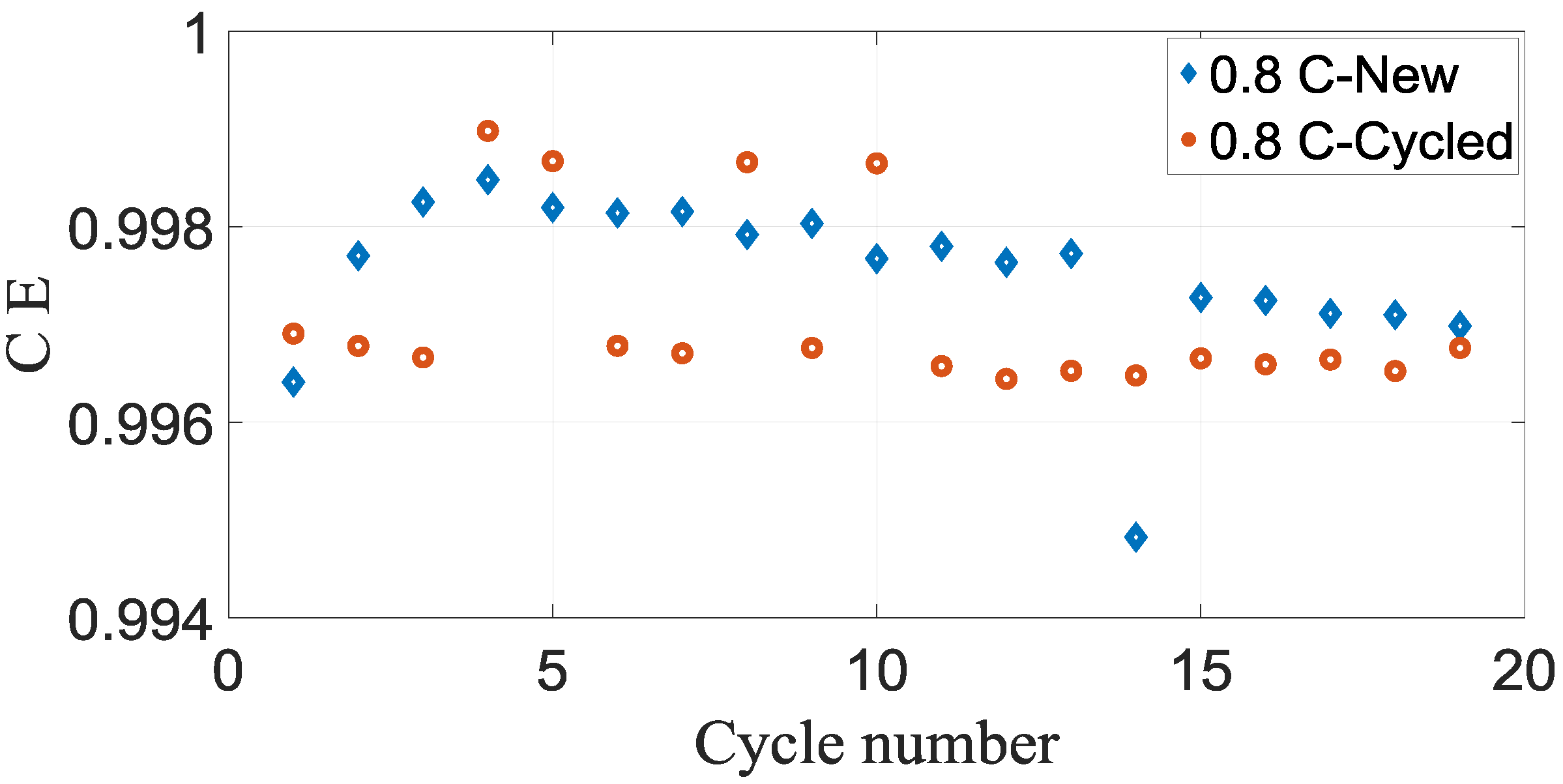
Figure 7 and
Figure 8 demonstrate the coulombic efficiency of the new and cycled commercial battery cells plotted vs cycle number.
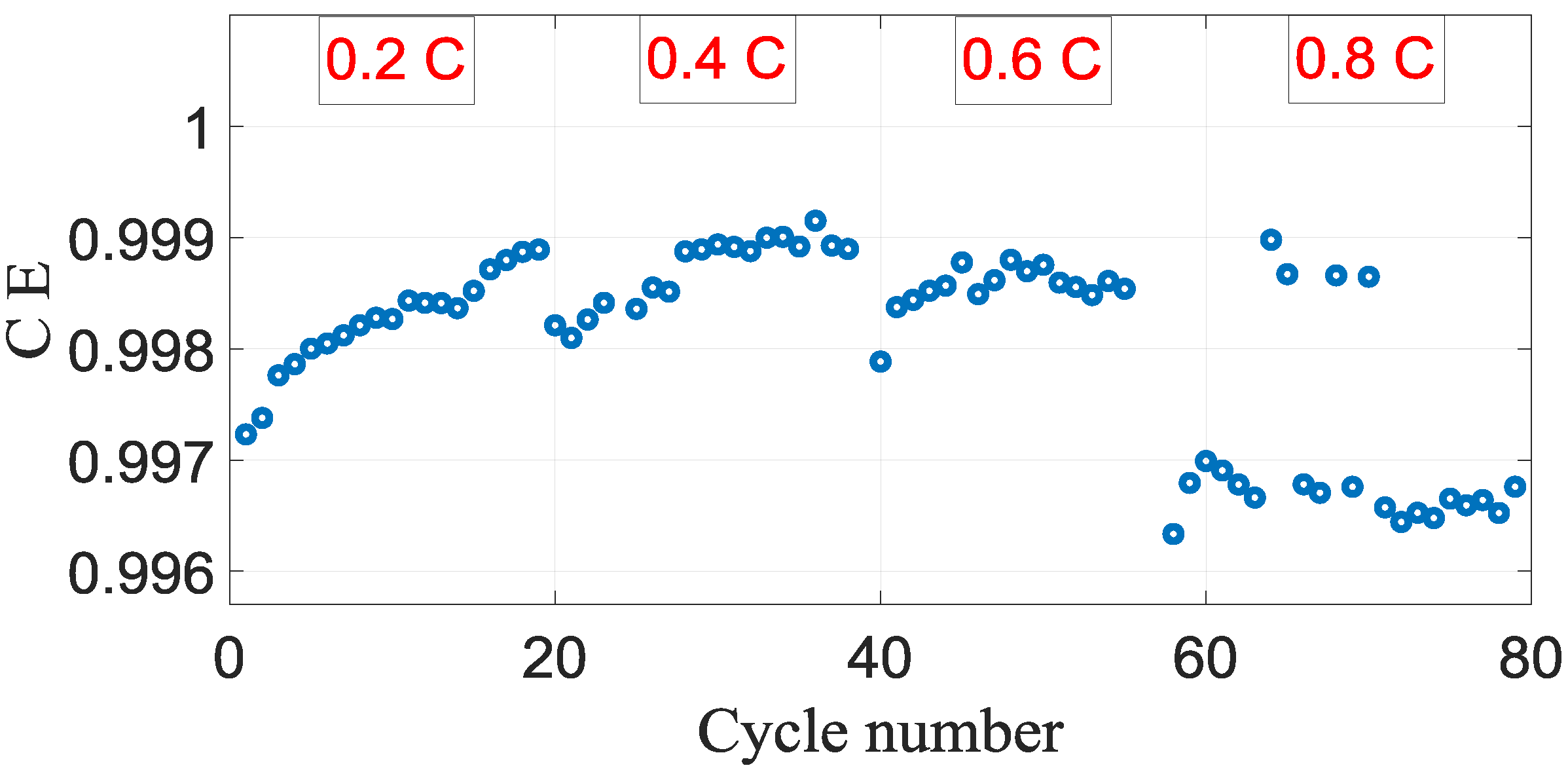
Figure 9 demonstrates the result of coulombic efficiency measurements of Li-ion battery cells for different current rates. It can be seen from
Figure 7 and
Figure 8 that cases coulombic efficiencies of the new cell is approximately bigger than the cycled battery cell for both 0.4 C and 0.8 C. Another observation from the figures is an almost similar pattern of coulombic efficiencies for both 0.4 C and 0.8 C cases.
Figure 9 shows a comparison between coulombic efficiencies for different current rates from 0.2 C and 0.8 C. It is clear from the figure that the coulombic efficiency for 0.8 C is lesser than other cases and, in addition, it follows an almost different pattern as compared to other C rates.