Methodological Approaches to End-Of-Life Modelling in Life Cycle Assessments of Lithium-Ion Batteries
Abstract
1. Introduction
2. Technical Background
2.1. Waste Treatment of LIBs
2.2. Material Recovery from LIBs
3. EOL Modelling Approaches in LCA
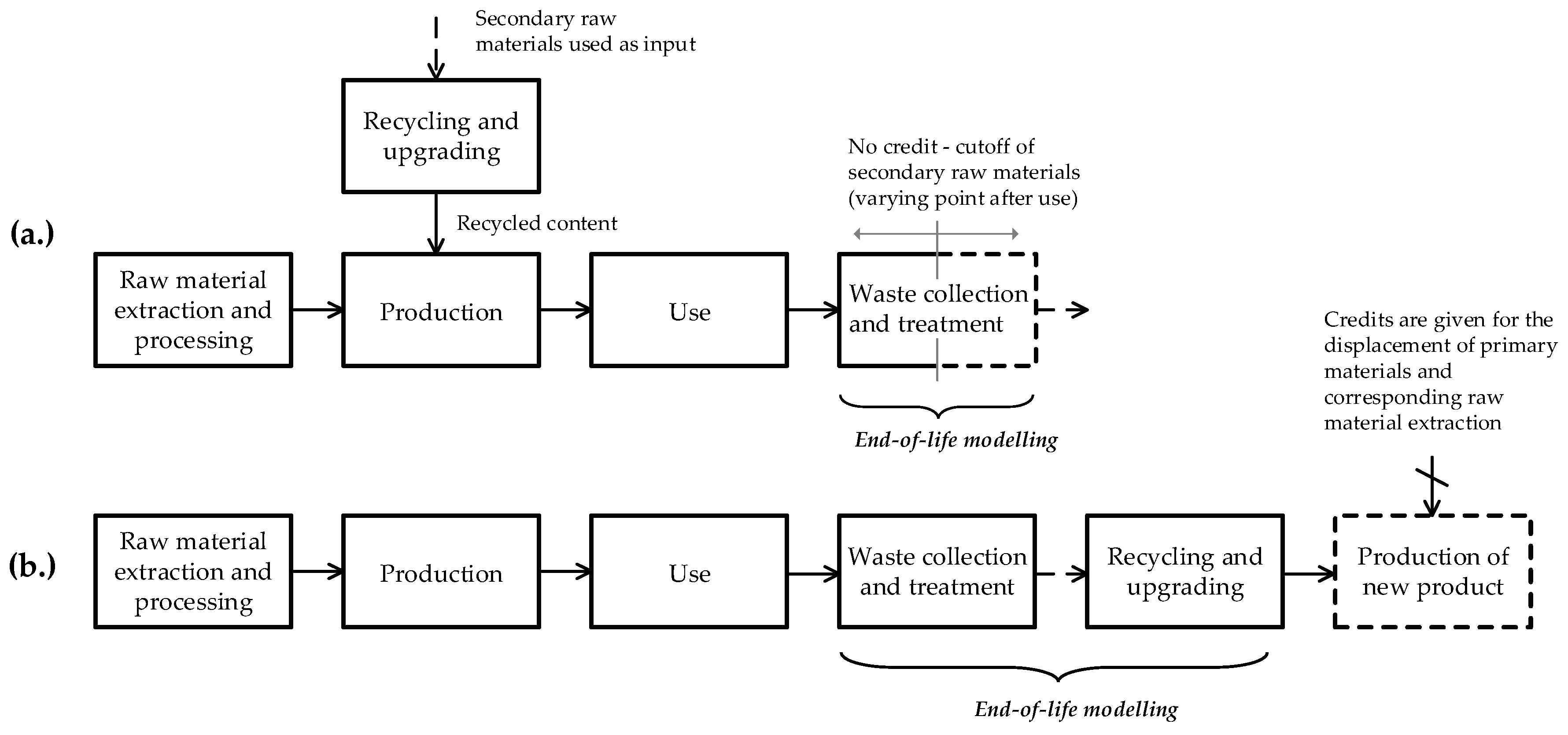
3.1. Cutoff and EOL Recycling
3.2. Closed-Loop, Open-Loop and Closed-Loop Approximation
4. Method
5. Results and Discussion
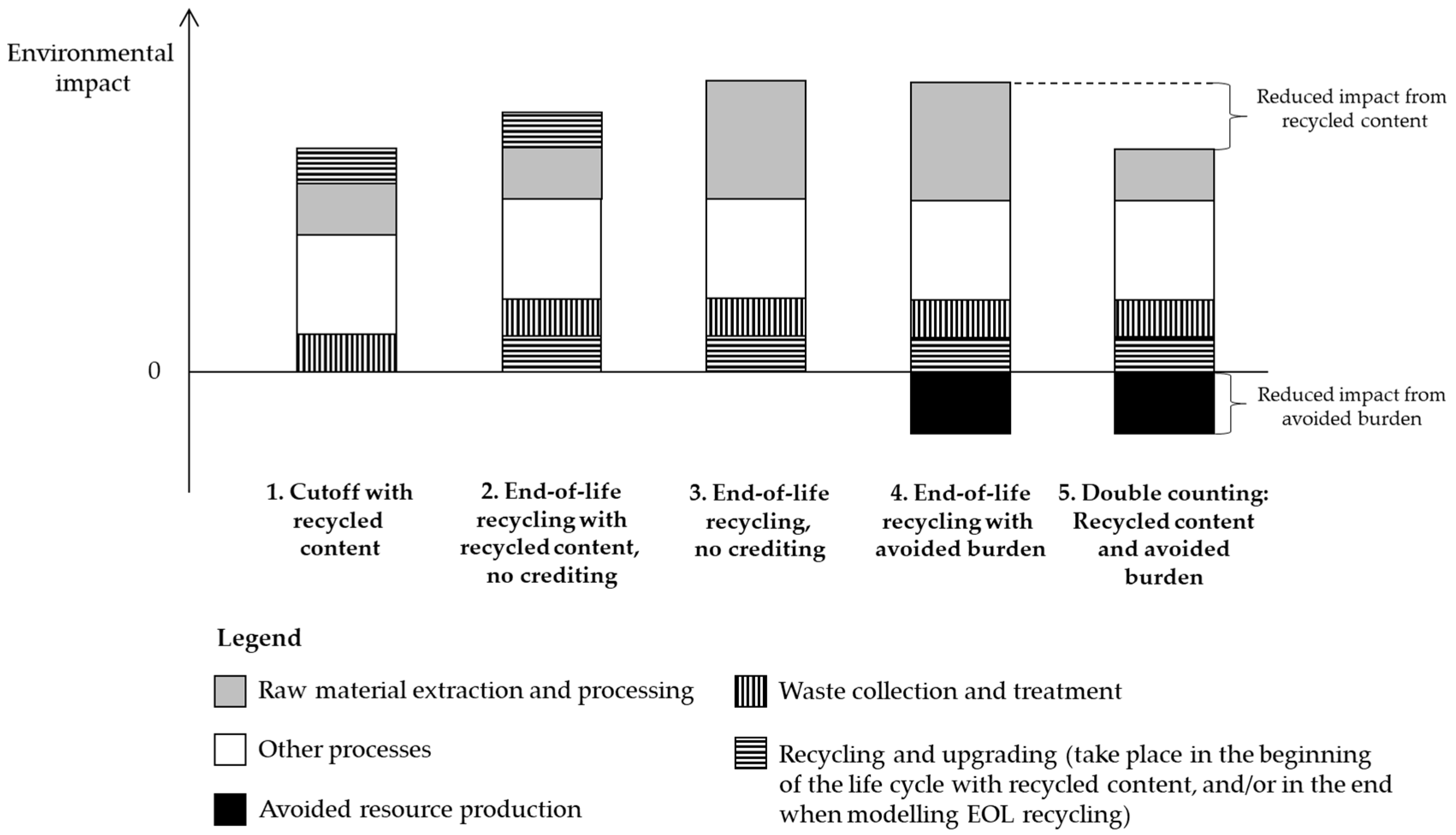
5.1. Cutoff, EOL Recycling and Hybrid Approaches
5.2. Recovery Procedures, Recycled Cell Materials and Waste Collection
6. Conclusions and Implications for Stakeholders
Author Contributions
Funding
Conflicts of Interest
References
- Whittingham, M.S. History, Evolution, and Future Status of Energy Storage. Proc. IEEE 2012, 100, 1518–1534. [Google Scholar] [CrossRef]
- Zubi, G.; Dufo-López, R.; Carvalho, M.; Pasaoglu, G. The lithium-ion battery: State of the art and future perspectives. Renew. Sustain. Energy Rev. 2018, 89, 292–308. [Google Scholar] [CrossRef]
- Pehlken, A.; Albach, S.; Vogt, T. Is there a resource constraint related to lithium ion batteries in cars? Int. J. Life Cycle Assess. 2017, 22, 40–53. [Google Scholar] [CrossRef]
- Oliveira, L.; Messagie, M.; Rangaraju, S.; Sanfelix, J.; Hernandez Rivas, M.; Van Mierlo, J. Key issues of lithium-ion batteries – from resource depletion to environmental performance indicators. J. Clean. Prod. 2015, 108, 354–362. [Google Scholar] [CrossRef]
- Miedema, J.H.; Moll, H.C. Lithium availability in the EU27 for battery-driven vehicles: The impact of recycling and substitution on the confrontation between supply and demand until 2050. Resour. Policy 2013, 38, 204–211. [Google Scholar] [CrossRef]
- Speirs, J.; Contestabile, M.; Houari, Y.; Gross, R. The future of lithium availability for electric vehicle batteries. Renew. Sustain. Energy Rev. 2014, 35, 183–193. [Google Scholar] [CrossRef]
- Simon, B.; Ziemann, S.; Weil, M. Potential metal requirement of active materials in lithium-ion battery cells of electric vehicles and its impact on reserves: Focus on Europe. Resour. Conserv. Recycl. 2015, 104, 300–310. [Google Scholar] [CrossRef]
- Kushnir, D.; Sandén, B.A. The time dimension and lithium resource constraints for electric vehicles. Resour. Policy 2012, 37, 93–103. [Google Scholar] [CrossRef]
- Ellingsen, L.A.-W.; Hung, C.R.; Strømman, A.H. Identifying key assumptions and differences in life cycle assessment studies of lithium-ion traction batteries with focus on greenhouse gas emissions. Transp. Res. D Transp. Environ. 2017, 55, 82–90. [Google Scholar] [CrossRef]
- Peters, J.F.; Weil, M. Providing a common base for life cycle assessments of Li-Ion batteries. J. Clean. Prod. 2018, 171, 704–713. [Google Scholar] [CrossRef]
- Peters, J.F.; Baumann, M.; Zimmermann, B.; Braun, J.; Weil, M. The environmental impact of Li-Ion batteries and the role of key parameters—A review. Renew. Sustain. Energy Rev. 2017, 67, 491–506. [Google Scholar] [CrossRef]
- Gaines, L.; Sullivan, J.; Burnham, A.; Belharouak, I. Life-Cycle Analysis of Production and Recycling of Lithium Ion Batteries. Transp. Res. Rec.: J. Transp. Res. Board 2011, 2252, 57–65. [Google Scholar] [CrossRef]
- Gaines, L. To recycle, or not to recycle, that is the question: Insights from life-cycle analysis. MRS Bull. 2012, 37, 333–338. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J.; Singh, N. Recycling of Spent Lithium-Ion Battery: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1129–1165. [Google Scholar] [CrossRef]
- Dewulf, J.; Van der Vorst, G.; Denturck, K.; Van Langenhove, H.; Ghyoot, W.; Tytgat, J.; Vandeputte, K. Recycling rechargeable lithium ion batteries: Critical analysis of natural resource savings. Resour. Conserv. Recycl. 2010, 54, 229–234. [Google Scholar] [CrossRef]
- Nordelöf, A.; Messagie, M.; Tillman, A.-M.; Ljunggren Söderman, M.; Van Mierlo, J. Environmental impacts of hybrid, plug-in hybrid, and battery electric vehicles—what can we learn from life cycle assessment? Int. J. Life Cycle Assess. 2014, 19, 1866–1890. [Google Scholar] [CrossRef]
- Kim, H.C.; Wallington, T.J.; Arsenault, R.; Bae, C.; Ahn, S.; Lee, J. Cradle-to-Gate Emissions from a Commercial Electric Vehicle Li-Ion Battery: A Comparative Analysis. Environ. Sci. Technol. 2016, 50, 7715–7722. [Google Scholar] [CrossRef]
- Li, B.; Gao, X.; Li, J.; Yuan, C. Life Cycle Environmental Impact of High-Capacity Lithium Ion Battery with Silicon Nanowires Anode for Electric Vehicles. Environ. Sci. Technol. 2014, 48, 3047–3055. [Google Scholar] [CrossRef]
- Sanfélix, J.; Messagie, M.; Omar, N.; Van Mierlo, J.; Hennige, V. Environmental performance of advanced hybrid energy storage systems for electric vehicle applications. Appl. Energy 2015, 137, 925–930. [Google Scholar] [CrossRef]
- Ellingsen, L.A.-W.; Majeau-Bettez, G.; Singh, B.; Srivastava, A.K.; Valøen, L.O.; Strømman, A.H. Life Cycle Assessment of a Lithium-Ion Battery Vehicle Pack. J. Ind. Ecol. 2014, 18, 113–124. [Google Scholar] [CrossRef]
- Dunn, J.B.; Gaines, L.; Sullivan, J.; Wang, M.Q. Impact of Recycling on Cradle-to-Gate Energy Consumption and Greenhouse Gas Emissions of Automotive Lithium-Ion Batteries. Environ. Sci. Technol. 2012, 46, 12704–12710. [Google Scholar] [CrossRef] [PubMed]
- Saw, L.H.; Ye, Y.; Tay, A.A.O. Integration issues of lithium-ion battery into electric vehicles battery pack. J. Clean. Prod. 2016, 113, 1032–1045. [Google Scholar] [CrossRef]
- Kushnir, D. Lithium Ion Battery Recycling Technology 2015. In Current State and Future Prospects; ESAReport # 2015:18; Chalmers University of Tehcnology: Gothenburg, Sweden, 2015. [Google Scholar]
- Huisman, J.; Leroy, P.; Tertre, F.; Söderman, M.L.; Chancerel, P.; Cassard, D.; Løvik, A.N.; Wäge, P.; Kushnir, D.; Rotter, V.S. Prospecting Secondary Raw Materials in the Urban Mine and Mining Wastes (ProSUM)—Final Report. 21 December 2017: Brussels, Belgium. Available online: http://www.prosumproject.eu/sites/default/files/DIGITAL_Final_Report.pdf. (accessed on 28 March 2019).
- Melin, H.E. Forskningsöversikt om återvinning och återbruk av litiumjonbatterier; Circular Energy Storage, Swedish Energy Agency: Eskilstuna, Sweden; Available online: http://www.energimyndigheten.se/globalassets/forskning-innovation/overgripande/forskningsoversikt-om-atervinning-och-aterbruk-av-litiumjonbatterier-2019.pdf. (accessed on 29 March 2019).
- Zheng, X.; Zhu, Z.; Lin, X.; Zhang, Y.; Hec, Y.; Cao, H.; Sun, Z. A Mini-Review on Metal Recycling from Spent Lithium Ion Batteries. Engineering 2018, 4, 361–370. [Google Scholar] [CrossRef]
- Lv, W.; Wang, Z.; Cao, H.; Sun, Y.; Zhang, Y.; Sun, Z. A Critical Review and Analysis on the Recycling of Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 1504–1521. [Google Scholar] [CrossRef]
- Andersson, M.; Ljunggren Söderman, M.; Sandén, B.A. Are scarce metals in cars functionally recycled? Waste Manag. 2017, 60, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Gaines, L. The future of automotive lithium-ion battery recycling: Charting a sustainable course. Sustain. Mater. Technol. 2014, 1–2, 2–7. [Google Scholar] [CrossRef]
- Ekberg, C.; Petranikova, M. Chapter 7—Lithium Batteries Recycling. In Lithium Process Chemistry; Chagnes, A., Światowska, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 233–267. [Google Scholar]
- Dunn, J.B.; Gaines, L.; Barnes, M.; Sullivan, J.; Wang, M. Material and Energy Flows in the Materials Production, Assembly, and End-of-Life Stages of the Automotive Lithium-Ion Battery Life Cycle. In Energy Systems Division, Argonne National Laboratory; ANL/ESD/12-3 Rev; U.S. Department of Energy: Argonne, IL, USA, 2014. [Google Scholar]
- Ekvall, T.; Tillman, A.-M. Open-loop recycling: Criteria for allocation procedures. Int. J. Life Cycle Assess. 1997, 2, 155. [Google Scholar] [CrossRef]
- Guérin, B.L.E. Modelling of Recycling in Life Cycle Assessment. Master’s Thesis, Department of Environmental Engineering, Technical University of Denmark, Copenhagen, Denmark, 2017. [Google Scholar]
- Atherton, J. Declaration by the Metals Industry on Recycling Principles. Int. J. Life Cycle Assess. 2007, 12, 59–60. [Google Scholar] [CrossRef]
- Frischknecht, R. LCI modelling approaches applied on recycling of materials in view of environmental sustainability, risk perception and eco-efficiency. Int. J. Life Cycle Assess. 2010, 15, 666–671. [Google Scholar] [CrossRef]
- Zackrisson, M.; Avellán, L.; Orlenius, J. Life cycle assessment of lithium-ion batteries for plug-in hybrid electric vehicles – Critical issues. J. Clean. Prod. 2010, 18, 1519–1529. [Google Scholar] [CrossRef]
- Wernet, G.; Bauer, C.; Steubing, B.; Reinhard, J.; Moreno-Ruiz, E.; Weidema, B. The ecoinvent database version 3 (part I): overview and methodology. Int. J. Life Cycle Assess. 2016, 21, 1218–1230. [Google Scholar] [CrossRef]
- ISO. Environmental Management—Life Cycle Assessment—Requirements and Guidelines; ISO 14044:2006; International Organization for Standardization: Geneva, Switzerland, 2016; p. 46. [Google Scholar]
- Classen, M.; Althaus, H.-J.; Blaser, S.; Scharnhorst, W.; Tuchschmid, M.; Jungbluth, N.; Emmenegger, M.F. Life Cycle Inventories of Metals; Ecoinvent report No. 10. (Data v2.1); Swiss Centre for Life Cycle Inventories: Dübendorf, Switzerland, 2009. [Google Scholar]
- Baumann, H.; Tillman, A.-M. The Hitch Hiker’s Guide to LCA—An Orientation in Life Cycle Assessment Methodology and Application; Studentlitteratur: Lund, Sweden, 2004. [Google Scholar]
- Finnveden, G.; Hauschild, M.Z.; Ekvall, T.; Guinée, J.; Heijungs, R.; Hellweg, S.; Koehler, A.; Pennington, D.; Suh, S. Recent developments in Life Cycle Assessment. J. Environ. Manag. 2009, 91, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Schrijvers, D.L.; Loubet, P.; Sonnemann, G. Critical review of guidelines against a systematic framework with regard to consistency on allocation procedures for recycling in LCA. Int. J. Life Cycle Assess. 2016, 21, 994–1008. [Google Scholar] [CrossRef]
- Vadenbo, C.; Hellweg, S.; Astrup, T.F. Let’s Be Clear(er) about Substitution: A Reporting Framework to Account for Product Displacement in Life Cycle Assessment. J. Ind. Ecol. 2017, 21, 1078–1089. [Google Scholar] [CrossRef]
- Geyer, R.; Kuczenski, B.; Zink, T.; Henderson, A. Common Misconceptions about Recycling. J. Ind. Ecol. 2016, 20, 1010–1017. [Google Scholar] [CrossRef]
- Scopus. Online Subscription-Based Scientific Multi-Disciplinary Database 2019. Available online: https://www.scopus.com (accessed on 27 February 2019).
- Web of Science. Online Subscription-Based Aggregated Scientific Multi-Disciplinary Database. 2019. Available online: http://webofknowledge.com/WOS (accessed on 27 February 2019).
- Wang, Y.; Yu, Y.; Huang, K.; Chen, B.; Deng, W.; Yao, Y. Quantifying the environmental impact of a Li-rich high-capacity cathode material in electric vehicles via life cycle assessment. Environ. Sci. Pollut. Res. 2017, 24, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, B.; Huang, K.; Wang, X.; Wang, D. Environmental Impact Assessment and End-of-Life Treatment Policy Analysis for Li-Ion Batteries and Ni-MH Batteries. Int. J. Environ. Res. Public Health 2014, 11, 3185–3198. [Google Scholar] [CrossRef] [PubMed]
- Casals, L.C.; García, B.A.; Aguesse, F.; Iturrondobeitia, A. Second life of electric vehicle batteries: relation between materials degradation and environmental impact. Int. J. Life Cycle Assess. 2017, 22, 82–93. [Google Scholar] [CrossRef]
- Bobba, S.; Mathieux, F.; Ardente, F.; Blengini, G.A.; Cusenza, M.A.; Podias, A.; Pfrang, A. Life Cycle Assessment of repurposed electric vehicle batteries: An adapted method based on modelling energy flows. J. Energy Storage 2018, 19, 213–225. [Google Scholar] [CrossRef]
- Argonne National Laboratory. GREET® Model—The Greenhouse Gases, Regulated Emissions, and Energy Use in Transportation Model. 2019. Available online: https://greet.es.anl.gov (accessed on 12 March 2019).
- Sanfélix, J.; de la Rúa, C.; Schmidt, J.H.; Messagie, M.; Van Mierlo, J. Environmental and economic performance of an li-ion battery pack: A multiregional input-output approach. Energies 2016, 9, 584. [Google Scholar] [CrossRef]
- Vandepaer, L.; Cloutier, J.; Bauer, C.; Amor, B. Integrating Batteries in the Future Swiss Electricity Supply System: A Consequential Environmental Assessment. J. Ind. Ecol. 2018, 23, 709–725. [Google Scholar] [CrossRef]
- Ahmadi, L.; Young, S.B.; Fowler, M.; Fraser, R.A.; Achachlouei, M.A. A cascaded life cycle: reuse of electric vehicle lithium-ion battery packs in energy storage systems. Int. J. Life Cycle Assess. 2017, 22, 111–124. [Google Scholar] [CrossRef]
- Ciez, R.E.; Whitacre, J.F. Examining different recycling processes for lithium-ion batteries. Nat. Sustain. 2019, 2, 148–156. [Google Scholar] [CrossRef]
- Cusenza, M.A.; Bobba, S.; Ardente, F.; Cellura, M.; Di Persio, F. Energy and environmental assessment of a traction lithium-ion battery pack for plug-in hybrid electric vehicles. J. Clean. Prod. 2019, 215, 634–649. [Google Scholar] [CrossRef] [PubMed]
- de Souza, L.L.P.; Lora, E.E.S.; Palacio, J.C.E.; Rocha, M.H.; Renó, M.L.G.; Venturini, O.J. Comparative environmental life cycle assessment of conventional vehicles with different fuel options, plug-in hybrid and electric vehicles for a sustainable transportation system in Brazil. J. Clean. Prod. 2018, 203, 444–468. [Google Scholar] [CrossRef]
- Dunn, J.B.; Gaines, L.; Kelly, J.C.; James, C.; Gallagher, K.G. The significance of Li-ion batteries in electric vehicle life-cycle energy and emissions and recycling’s role in its reduction. Energy Environ. Sci. 2015, 8, 158–168. [Google Scholar] [CrossRef]
- Faria, R.; Marques, P.; Garcia, R.; Moura, P.; Freire, F.; Delgado, J.; de Almeida, A.T. Primary and secondary use of electric mobility batteries from a life cycle perspective. J. Power Sources 2014, 262, 169–177. [Google Scholar] [CrossRef]
- Hendrickson, T.P.; Kavvada, O.; Shah, N.; Sathre, R.; Scown, C.D. Life-cycle implications and supply chain logistics of electric vehicle battery recycling in California. Environ. Res. Lett. 2015, 10, 014011. [Google Scholar] [CrossRef]
- Notter, D.A.; Gauch, M.; Widmer, R.; Wäger, P.; Stamp, A.; Zah, R.; Althaus, H.-J. Contribution of Li-Ion Batteries to the Environmental Impact of Electric Vehicles. Environ. Sci. Technol. 2010, 44, 6550–6556. [Google Scholar] [CrossRef]
- Oliveira, L.; Messagie, M.; Mertens, J.; Laget, H.; Coosemans, T.; Van Mierlo, J. Environmental performance of electricity storage systems for grid applications, a life cycle approach. Energy Convers. Manag. 2015, 101, 326–335. [Google Scholar] [CrossRef]
- Raugei, M.; Winfield, P. Prospective LCA of the production and EoL recycling of a novel type of Li-ion battery for electric vehicles. J. Clean. Prod. 2019, 213, 926–932. [Google Scholar] [CrossRef]
- Richa, K.; Babbitt, C.W.; Nenadic, N.G.; Gaustad, G. Environmental trade-offs across cascading lithium-ion battery life cycles. Int. J. Life Cycle Assess. 2017, 22, 66–81. [Google Scholar] [CrossRef]
- Ryan, N.A.; Lin, Y.; Mitchell-Ward, N.; Mathieu, J.L.; Johnson, J.X. Use-Phase Drives Lithium-Ion Battery Life Cycle Environmental Impacts When Used for Frequency Regulation. Environ. Sci. Technol. 2018, 52, 10163–10174. [Google Scholar] [CrossRef] [PubMed]
- Swart, P.; Dewulf, J.; Biernaux, A. Resource demand for the production of different cathode materials for lithium ion batteries. J. Clean. Prod. 2014, 84, 391–399. [Google Scholar] [CrossRef]
- Tagliaferri, C.; Evangelisti, S.; Acconcia, F.; Domenech, T.; Ekins, P.; Barletta, D.; Lettieri, P. Life cycle assessment of future electric and hybrid vehicles: A cradle-to-grave systems engineering approach. Chem. Eng. Res. Des. 2016, 112, 298–309. [Google Scholar] [CrossRef]
- Unterreiner, L.; Jülch, V.; Reith, S. Recycling of Battery Technologies – Ecological Impact Analysis Using Life Cycle Assessment (LCA). Energy Procedia 2016, 99, 229–234. [Google Scholar] [CrossRef]
- Vandepaer, L.; Cloutier, J.; Amor, B. Environmental impacts of Lithium Metal Polymer and Lithium-ion stationary batteries. Renew. Sustain. Energy Rev. 2017, 78, 46–60. [Google Scholar] [CrossRef]
- Zhao, S.; You, F. Comparative Life-Cycle Assessment of Li-Ion Batteries through Process-Based and Integrated Hybrid Approaches. ACS Sustain. Chem. Eng. 2019, 7, 5082–5094. [Google Scholar] [CrossRef]
- Richa, K.; Babbitt, C.W.; Nenadic, N.G.; Gaustad, G. Supplementary Material for Environmental Trade-Offs Across Cascading Lithium-Ion Battery Life Cycles; Golisano Institute for Sustainability, Rochester Institute of Technology: Rochester, NY, USA, 1 January 2017; p. 35. Available online: https://doi.org/10.1007/s11367-015-0942-3 (accessed on 12 March 2019).
- European Union. Directive 2006/66/EC of the European Parliament and of the Council; Official Journal of the European Union: Luxembourg, 2006. [Google Scholar]
| Study | EOL Modelling Approach | Production of Raw Materials | EOL Crediting |
|---|---|---|---|
| Ahmadi et al. [54] | EOL recycling | Recycled content (Al) | No crediting |
| Ciez and Whitacre [55] 1,2 | EOL recycling | Recycled content (Fe, Ni) | Avoided burden |
| Cusenza et al. [56] | EOL recycling | Primary only | Avoided burden |
| de Souza et al. [57] | EOL recycling | Primary only | Avoided burden |
| Dunn et al. [21] 1 | EOL recycling | Recycled content (Al) | Avoided burden |
| Dunn et al. [58] 1 | EOL recycling | Not reported | Avoided burden |
| Faria et al. [59] | EOL recycling | Not reported | Not reported |
| Gaines et al. [12] | Cutoff | Recycled content | No crediting |
| Hendrickson et al. [60] 1 | EOL recycling | Not reported | Avoided burden |
| Li et al. [18] 2 | EOL recycling | Recycled content (Al) | No crediting |
| Notter et al. [61] 2 | Cutoff | Recycled content (Al) | No crediting |
| Oliveira et al. [62] | Cutoff | Recycled content | No crediting |
| Oliveira et al. [4] | EOL recycling | Not reported | Avoided burden |
| Raugei and Winfield [63] 2 | EOL recycling | Recycled content (Al, Cu) | Avoided burden |
| Richa et al. [64] | EOL recycling | Recycled content (Al, Cu, Fe) | Avoided burden |
| Ryan et al. [65] 1,3 | Cutoff | Recycled content (Al, Fe, Li) | No crediting |
| Sanfélix et al. [19] | EOL recycling | Not reported | Avoided burden |
| Sanfélix et al. [52] | EOL recycling | Not reported | Avoided burden |
| Swart et al. [66] 4 | EOL recycling | Primary only | Avoided burden |
| Tagliaferri et al. [67] 2 | EOL recycling | Not reported | Avoided burden |
| Unterreiner et al. [68] 5 | EOL recycling | Primary only | Avoided burden |
| Vandepaer et al. [69] | Cutoff | Not reported | No crediting |
| Vandepaer et al. [53] 2,6 | EOL recycling | Not reported | Avoided burden |
| Zackrisson et al. [36] 2 | Cutoff | Recycled content | No crediting |
| Zhao and You [70] | EOL recycling | Recycled content | No crediting |
| EOL recycling approach | EOL recycling | Primary only | Avoided burden |
| Cutoff approach | Cutoff | Recycled content | No crediting |
| No. of Studies Accounting for Waste Collection | |
| Include or discuss collection rate | 2 |
| Model the transportation required for collection | 6 |
| Report no aspects of waste collection | 17 |
| No. of Studies Modelling Specific Cell Material Recovery Procedures in the EOL Recycling Approach | |
| Direct recycling | 4 |
| Hydrometallurgy | 16 |
| Pyrometallurgy | 12 |
| Other procedures 1 | 2 |
| No. of Studies Modelling Recovery of Specific Cell Materials in the EOL Recycling Approach | |
| Cobalt | 11 |
| Lithium | 12 |
| Manganese | 6 |
| Nickel | 7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nordelöf, A.; Poulikidou, S.; Chordia, M.; Bitencourt de Oliveira, F.; Tivander, J.; Arvidsson, R. Methodological Approaches to End-Of-Life Modelling in Life Cycle Assessments of Lithium-Ion Batteries. Batteries 2019, 5, 51. https://doi.org/10.3390/batteries5030051
Nordelöf A, Poulikidou S, Chordia M, Bitencourt de Oliveira F, Tivander J, Arvidsson R. Methodological Approaches to End-Of-Life Modelling in Life Cycle Assessments of Lithium-Ion Batteries. Batteries. 2019; 5(3):51. https://doi.org/10.3390/batteries5030051
Chicago/Turabian StyleNordelöf, Anders, Sofia Poulikidou, Mudit Chordia, Felipe Bitencourt de Oliveira, Johan Tivander, and Rickard Arvidsson. 2019. "Methodological Approaches to End-Of-Life Modelling in Life Cycle Assessments of Lithium-Ion Batteries" Batteries 5, no. 3: 51. https://doi.org/10.3390/batteries5030051
APA StyleNordelöf, A., Poulikidou, S., Chordia, M., Bitencourt de Oliveira, F., Tivander, J., & Arvidsson, R. (2019). Methodological Approaches to End-Of-Life Modelling in Life Cycle Assessments of Lithium-Ion Batteries. Batteries, 5(3), 51. https://doi.org/10.3390/batteries5030051





