Recycling of Alkaline Batteries via a Carbothermal Reduction Process
Abstract
:1. Introduction
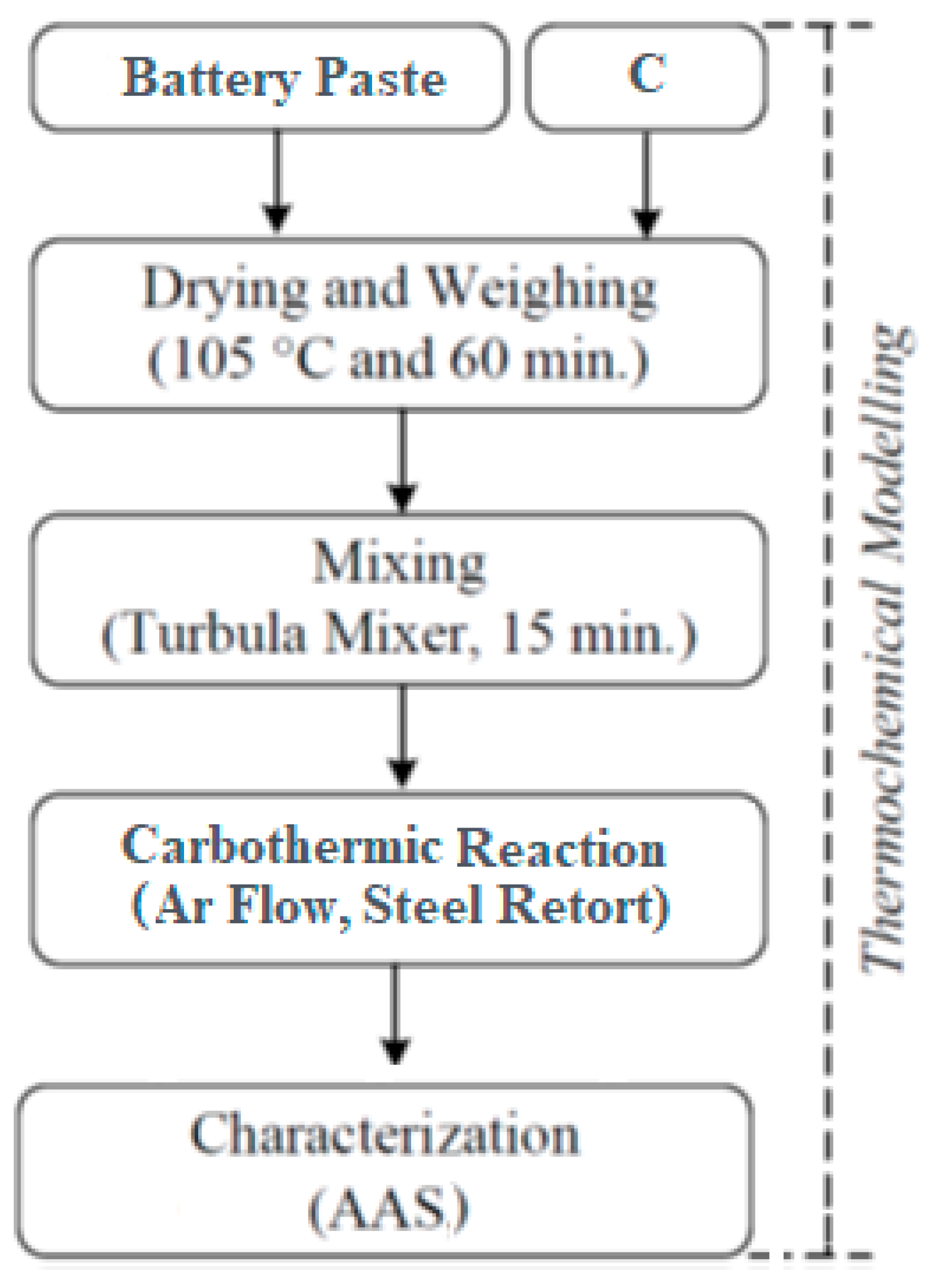
2. Experimental Studies
3. Results and Discussion
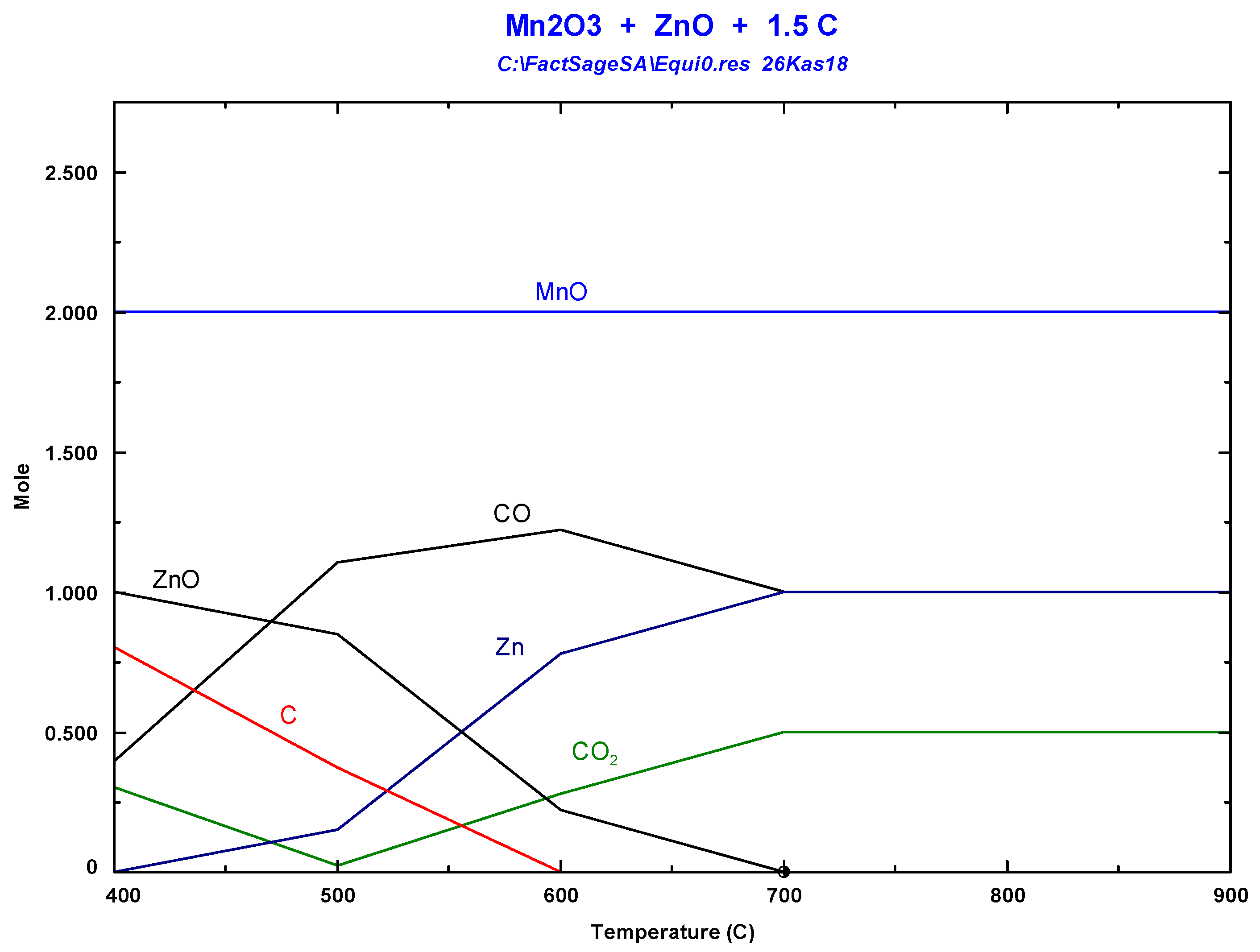
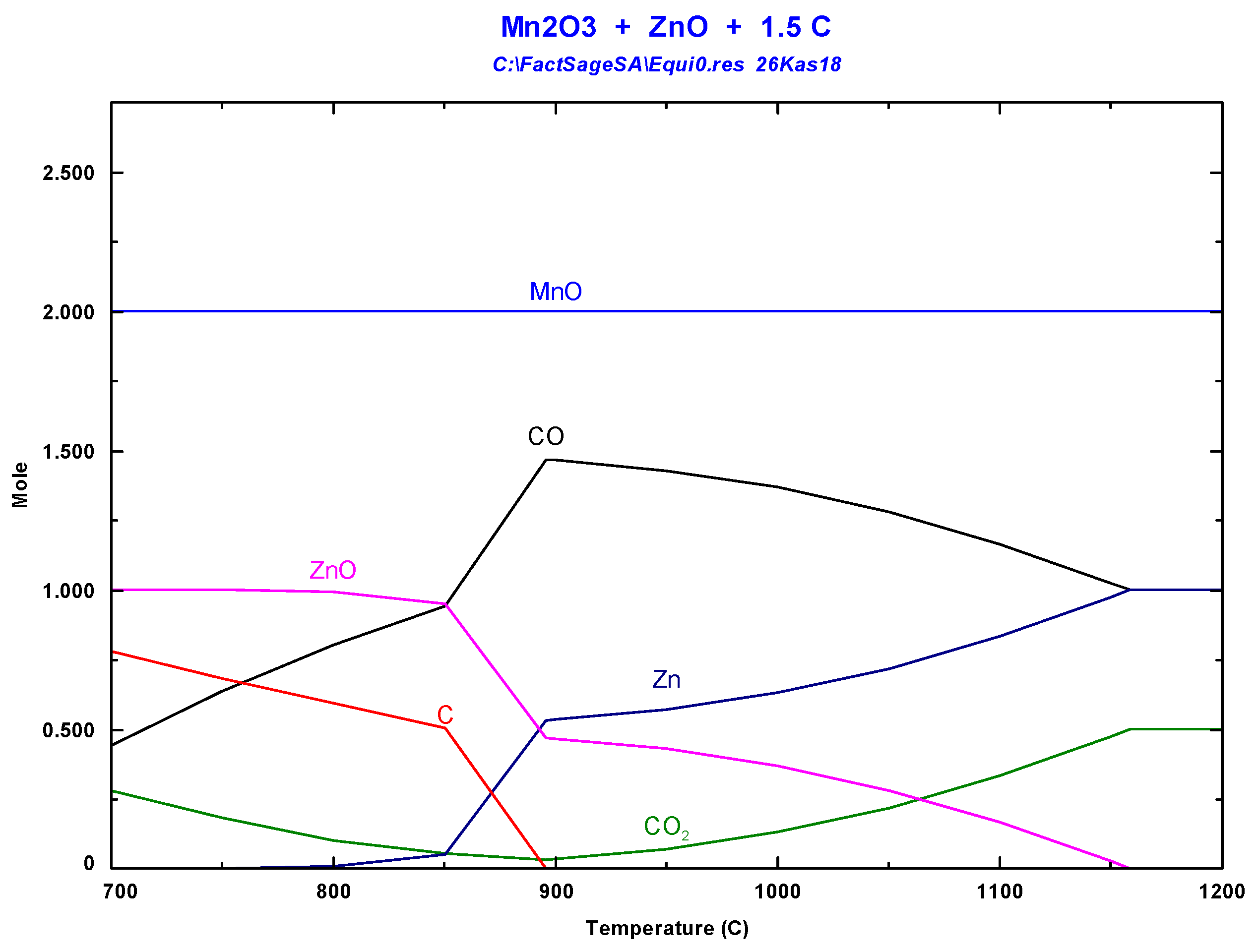
3.1. Thermodynamic Background
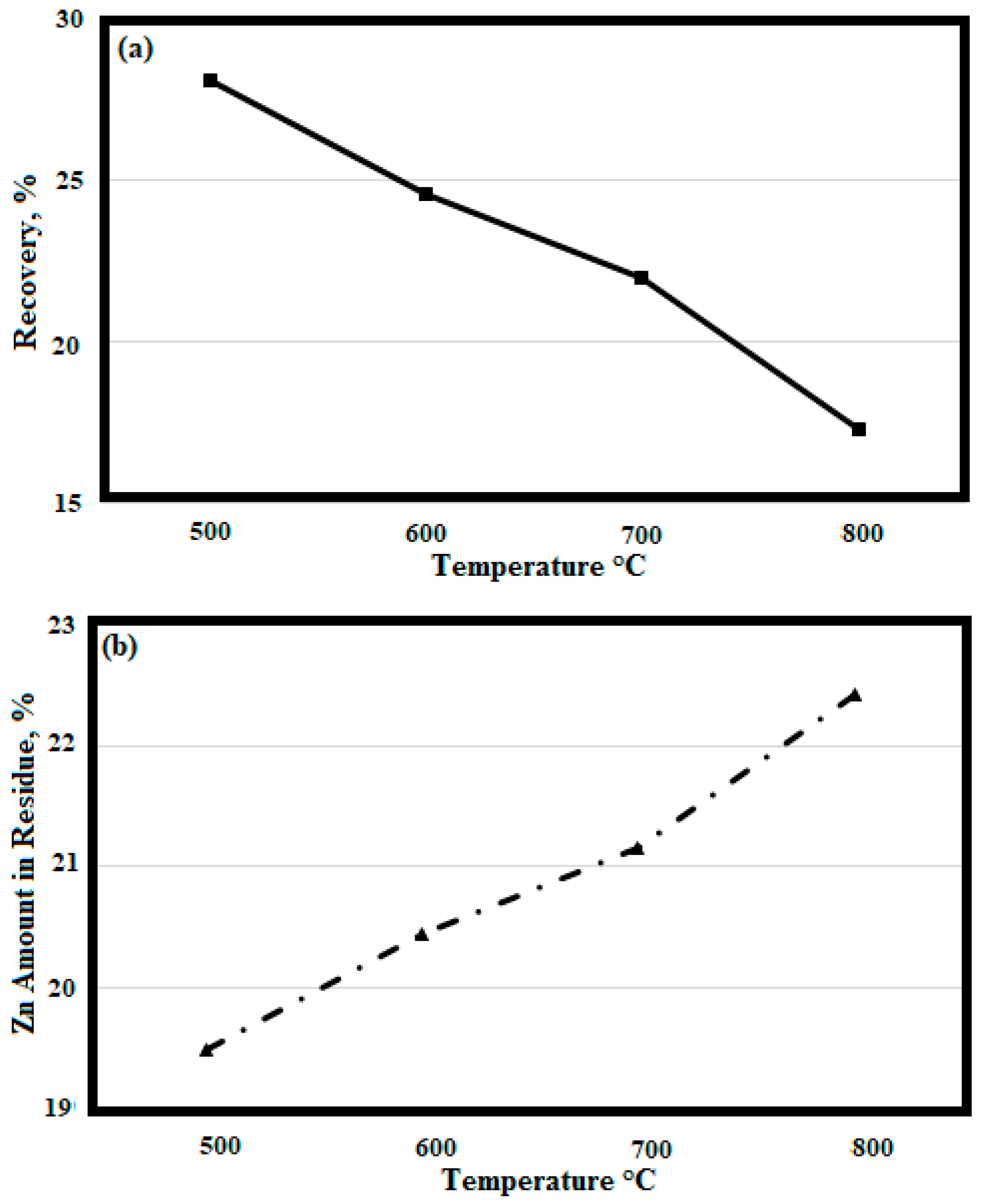
3.2. Carbothermal Reduction of Battery Wastes without Vacuum
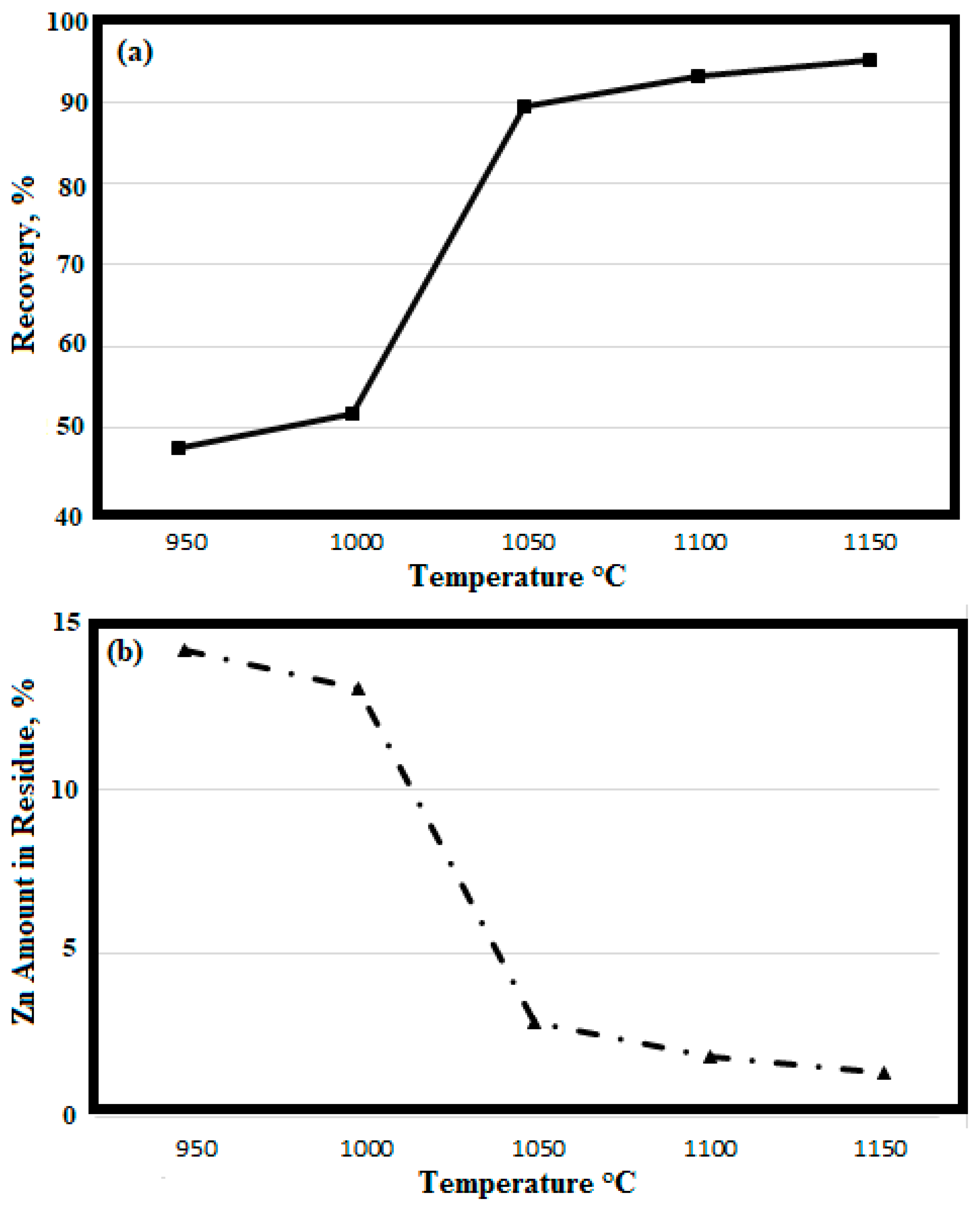
3.3. Vacuum Carbothermal Reduction of Battery Wastes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Espinosa, D.C.R.; Bernardes, A.M.; Tenorio, J.A.S. An overview on the current processes for the recycling of batteries. J. Power Sources 2004, 135, 311–319. [Google Scholar] [CrossRef]
- Ferella, F.; Michelis, I.D.; Beolchini, B.; Innocenzi, V.; Veglio, F. Extraction of zinc and manganese from alkaline and zinc-carbon spent batteries by citric-sulphuric acid solution. Int. J. Chem. Eng. 2010, 2010, 659434. [Google Scholar] [CrossRef]
- Veloso, L.R.S.; Rodrigues, L.E.O.C.; Ferreira, D.A.; Magalhaes, F.S.; Mansur, M.B. Development of a hydrometallurgical route for the recovery of zinc and manganese from spent alkaline batteries. J. Power Sources 2005, 152, 295–302. [Google Scholar] [CrossRef]
- Xiong, L.Z.; Xiang, Y.H.; Wu, X.W.; He, Z.Q.; Yin, Z.L. Preparation of high purity zinc from zinc oxide ore by vacuum carbothermic reduction. Vacuum 2017, 146, 200–205. [Google Scholar] [CrossRef]
- Habashi, F. Handbook of Extractive Metallurgy, Zinc; Wiley-VCH: Weinheim, Germany, 1997; Volume 2, pp. 641–682. [Google Scholar]
- Mathewson, H.C. Zinc: The Metal, Its Alloys and Compounds in Physical Metallurgy of Zinc; Reinhold Publishing Corporation: New York, NY, USA, 1959; pp. 400–422. [Google Scholar]
- Onem, K. Searching of Manufacturing Parameters Effecting Ductility of Zinc Wires for Thermal Spraying Application. Master’s Thesis, Istanbul Technical University, Istanbul, Turkey, 2002. [Google Scholar]
- Yun, T.H.; Park, J.H.; Kim, J.-S.; Park, J.M. Effect of the surface modification of zinc powders with organo silanes on the corrosion resistance of a zinc pigmented organic coating. Prog. Org. Coat. 2014, 77, 1780–1788. [Google Scholar] [CrossRef]
- Stamatiou, A.; Loutzenhiser, P.G.; Steinfeld, A. Syngas production from H2O and CO2 over Zn particles in a packed-bed reactor. AIChE J. 2012, 58, 625–631. [Google Scholar] [CrossRef]
- Ebin, B.; Petranikova, M.; Steenari, B.-M.; Ekberg, C. Effects of gas flow rate on zinc recovery rate and particle properties by pyrolysis of alkaline and zinc carbon battery waste. J. Anal. Appl. Pyrolysis 2016, 121, 333–341. [Google Scholar] [CrossRef]
- Ito, Y.; Nyce, M.; Plivelich, R.; Klein, M.; Steingart, D.; Banerjee, S. Zinc morphology in zinc-nickel flow assisted batteries and impact on performance. J. Power Sources 2011, 196, 2340–2345. [Google Scholar] [CrossRef]
- Schaefer, K.; Miszczyk, A. Improvement of electrochemical action of zinc-rich paints by addition of nano particulate zinc. Corros. Sci. 2013, 66, 380–391. [Google Scholar] [CrossRef]
- Gallegos, M.V.; Falco, L.R.; Peluso, M.A.; Sambeth, J.E.; Thomas, H.J. Recovery of manganese oxides from spent alkaline and zinc-carbon batteries: An application as catalysts for VOCs elimination. Waste Manag. 2013, 33, 1483–1490. [Google Scholar] [CrossRef]
- Li, Y.J.; Yang, D.J. Study of leaching zinc from difficult dealt zinc oxide ore with high silicon in the alkaline. Adv. Mater. Res. 2015, 1120–1121, 105–109. [Google Scholar] [CrossRef]
- El-Nadi, Y.A.; Daoud, J.A.; Aly, H.F. Leaching and separation of zinc from the black paste of spent MnO2-Zn dry cell batteries. J. Hazard. Mater. 2007, 143, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Sayilgan, E.; Kukrer, T.; Civelekoglu, G.; Ferella, F.; Akcil, A.; Veglio, F.; Kitis, M. A review of technologies for the recovery of metals from spent alkaline andzinc-carbon batteries. Hydrometallurgy 2009, 97, 158–166. [Google Scholar] [CrossRef]
- Bernardes, A.M.; Espinosa, D.C.R.; Tenório, J.A.S. Recycling of batteries: A review of current processes and technologies. J. Power Sources 2004, 130, 291–298. [Google Scholar] [CrossRef]
- Sun, Y.; Shen, X.; Zhai, Y. Thermodynamics and kinetics of extracting zinc from zinc oxide ore by the ammonium sulfate roasting method. Int. J. Miner. Metall. Mater. 2015, 22, 467–475. [Google Scholar] [CrossRef]
- Morcali, M.H. Reductive atmospheric acid leaching of spent alkaline batteries in H2SO4/Na2SO3 solutions. Int. J. Miner. Metall. Mater. 2015, 22, 674–681. [Google Scholar] [CrossRef]
- Ebin, B.; Petranikova, M.; Steenari, B.-M.; Ekberg, C. Investigation of zinc recovery by hydrogen reduction assisted pyrolysis of alkaline and zinc-carbon battery waste. Waste Manag. 2017, 68, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Yang, B.; Dai, Y.-N.; Liu, D.-C.; Yang, B.-Z. Applications of vacuum metallurgical technology in recovery of secondary zinc-based resources. Vacuum 2008, 45, 20–22. [Google Scholar]
- Xiong, L.; Chen, Q.; Yin, Z.; Zhang, P.; Ding, Z.; Liu, Z. Preparation of metal zinc from hemimorphite by vacuum carbothermic reduction with CaF2 as catalyst. Trans. Nonferrous Met. Soc. China 2011, 22, 694–699. [Google Scholar] [CrossRef]
- Xiong, L.; He, Z.; Liu, Z.; Yin, Z. Study on vacuum carbothermic reduction kinetics of hemimorphite catalyzed by calcium fluoride. Vacuum 2015, 119, 163–167. [Google Scholar] [CrossRef]
- Yucel, O.; Demirci, F.; Turan, A.; Alkan, M. Determination of Direct Reduction Conditions of Mill Scale. High Temp. Mater. Process. 2013, 32, 405–412. [Google Scholar] [CrossRef]
- Shuqing, Z.; Jie, Y.; Xu, Z.; Xingbin, S. One-step Pyrolysis Process for Recover Zinc and Mercury from Spent Zinc-manganese Batteries. Adv. Mater. Res. 2010, 113–116, 2224–2227. [Google Scholar]
- Belardi, G.; Medici, F.; Piga, L. Influence of gaseous atmosphere during a thermal process for recovery of manganese and zinc from spent batteries. J. Power Sources 2014, 248, 1290–1298. [Google Scholar] [CrossRef]
- Bale, C.W.; Chartran, P.; Degterov, S.A.; Eriksson, G.; Hack, K.; Mahfoud, R.B.; Melançon, J.; Pelton, A.D.; Petersen, S. FactSage “Thermochemical Software and Databases”. Calphad 2002, 26, 189–228. [Google Scholar] [CrossRef]




| Element | Weight, % |
|---|---|
| Zn | 12–21 |
| Mn | 26–33 |
| K | 5.5–7.3 |
| Fe | 0.17 |
| Pb | 0.005 |
| Cd | - |
| Hg | - |
| Element | Weight, % |
|---|---|
| Zn | 27.1 |
| Mn | 38.12 |
| K | 6.47 |
| Fe | 1.35 |
| Pb | 0.0018 |
| Loss of Ignition | 20.21 |
| Moisture | 6.75 |
| Element | mg/L |
|---|---|
| K | 6400 |
| Zn | 0.0147 |
| Mn | 0.0007 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeşiltepe, S.; Buğdaycı, M.; Yücel, O.; Şeşen, M.K. Recycling of Alkaline Batteries via a Carbothermal Reduction Process. Batteries 2019, 5, 35. https://doi.org/10.3390/batteries5010035
Yeşiltepe S, Buğdaycı M, Yücel O, Şeşen MK. Recycling of Alkaline Batteries via a Carbothermal Reduction Process. Batteries. 2019; 5(1):35. https://doi.org/10.3390/batteries5010035
Chicago/Turabian StyleYeşiltepe, Selçuk, Mehmet Buğdaycı, Onuralp Yücel, and Mustafa Kelami Şeşen. 2019. "Recycling of Alkaline Batteries via a Carbothermal Reduction Process" Batteries 5, no. 1: 35. https://doi.org/10.3390/batteries5010035
APA StyleYeşiltepe, S., Buğdaycı, M., Yücel, O., & Şeşen, M. K. (2019). Recycling of Alkaline Batteries via a Carbothermal Reduction Process. Batteries, 5(1), 35. https://doi.org/10.3390/batteries5010035





