Mechanism of Ionic Impedance Growth for Palladium-Containing CNT Electrodes in Lithium-Oxygen Battery Electrodes and Its Contribution to Battery Failure
Abstract
:1. Introduction
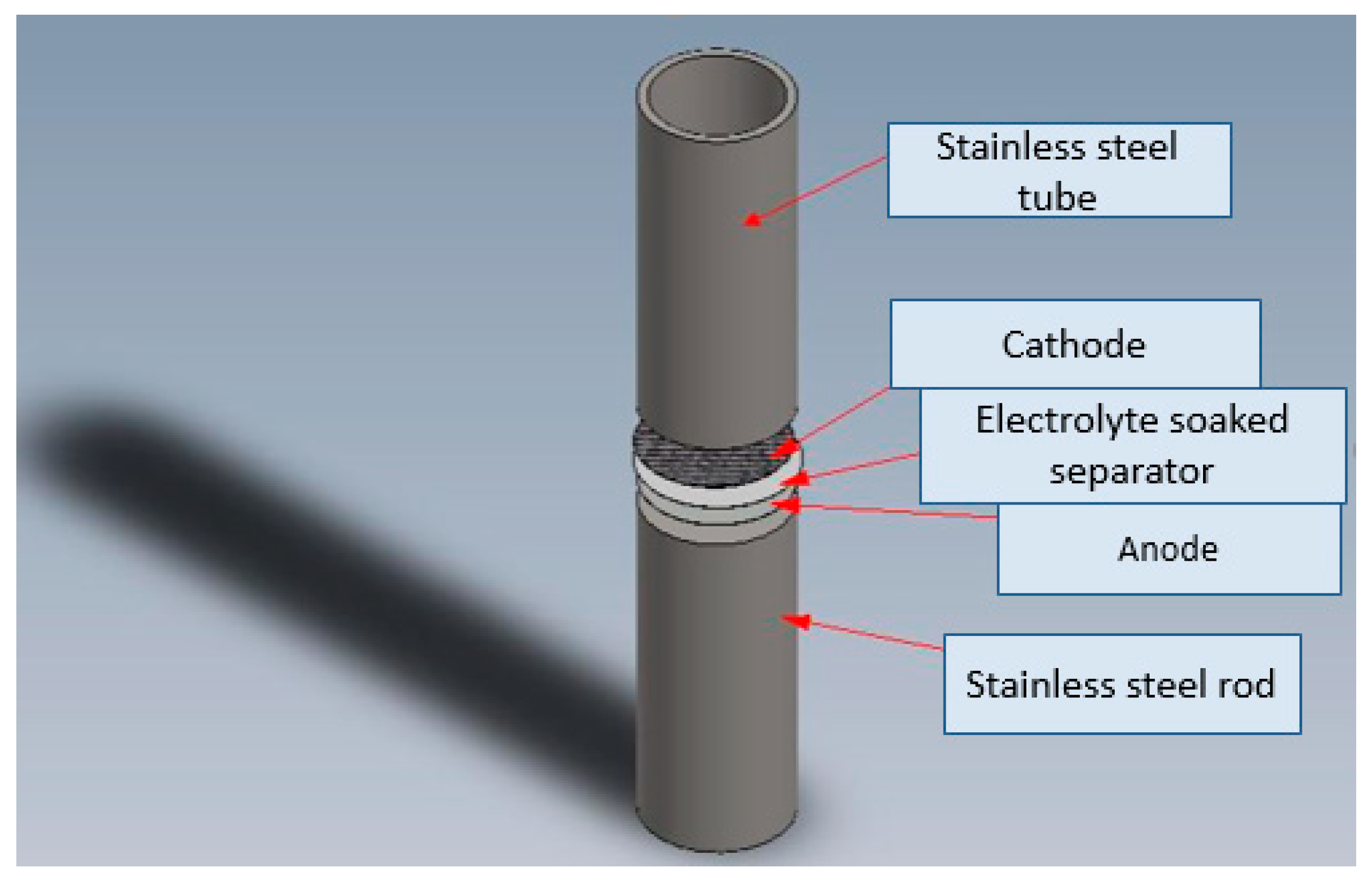
2. Experimental
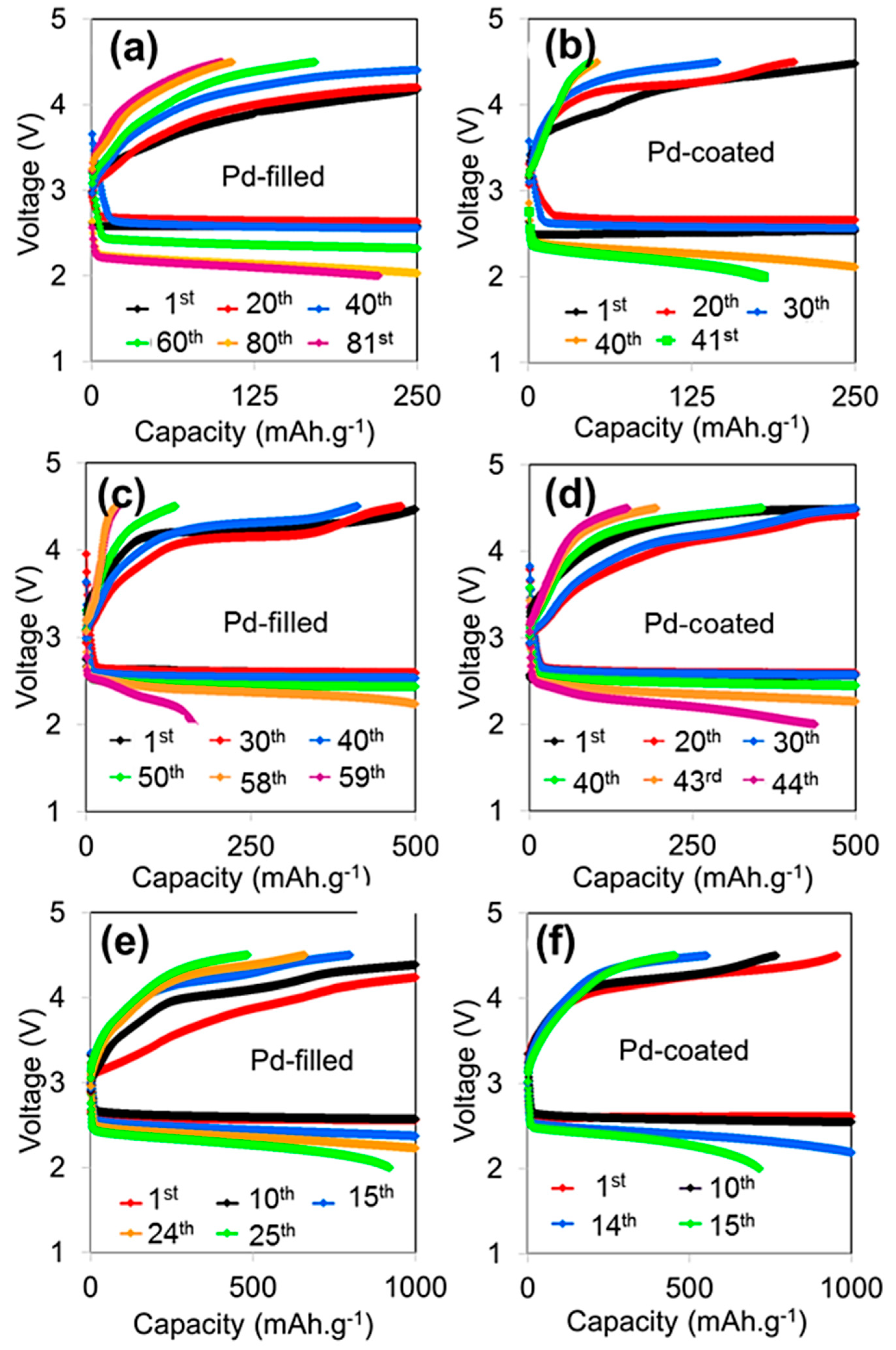
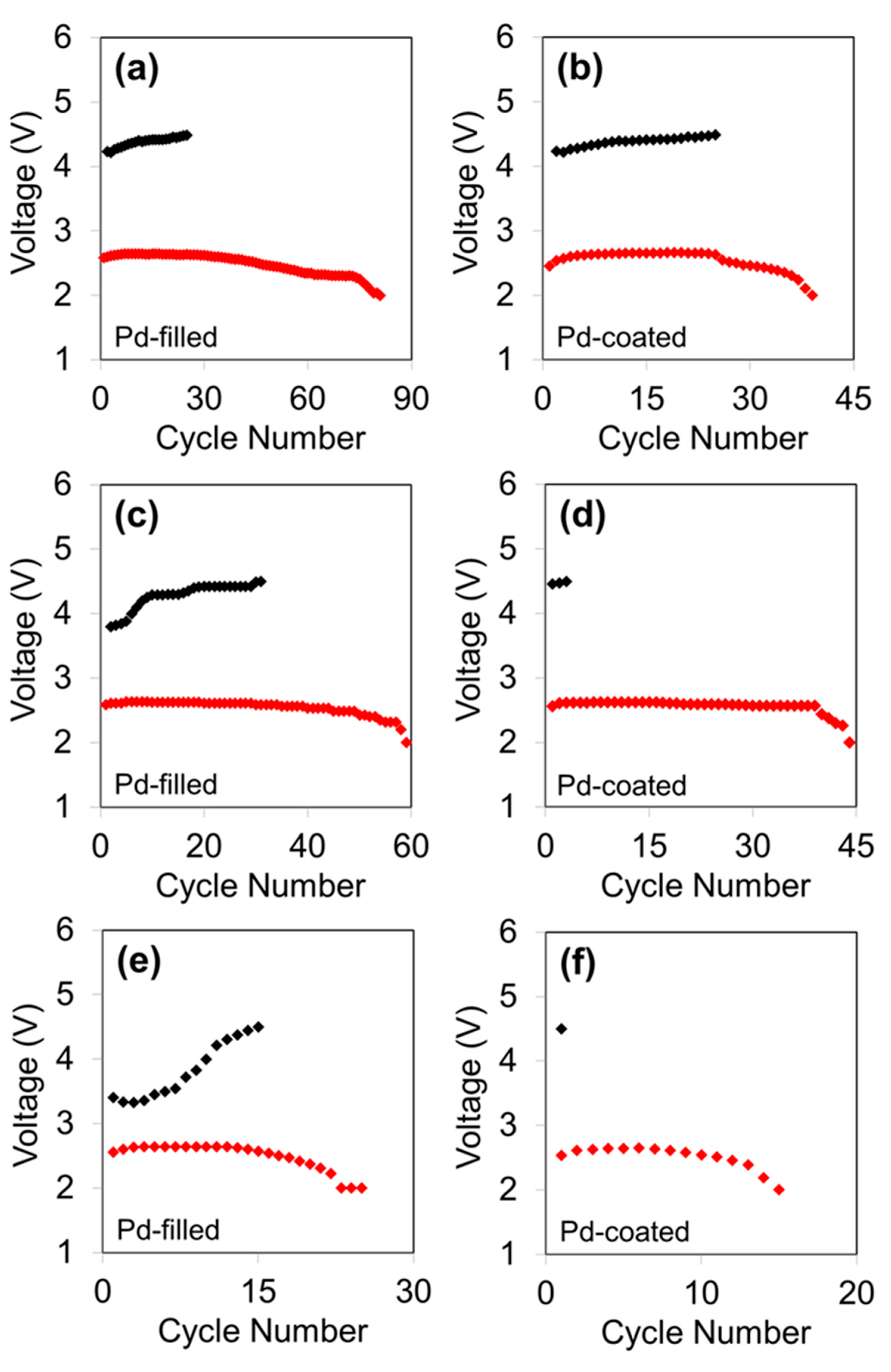
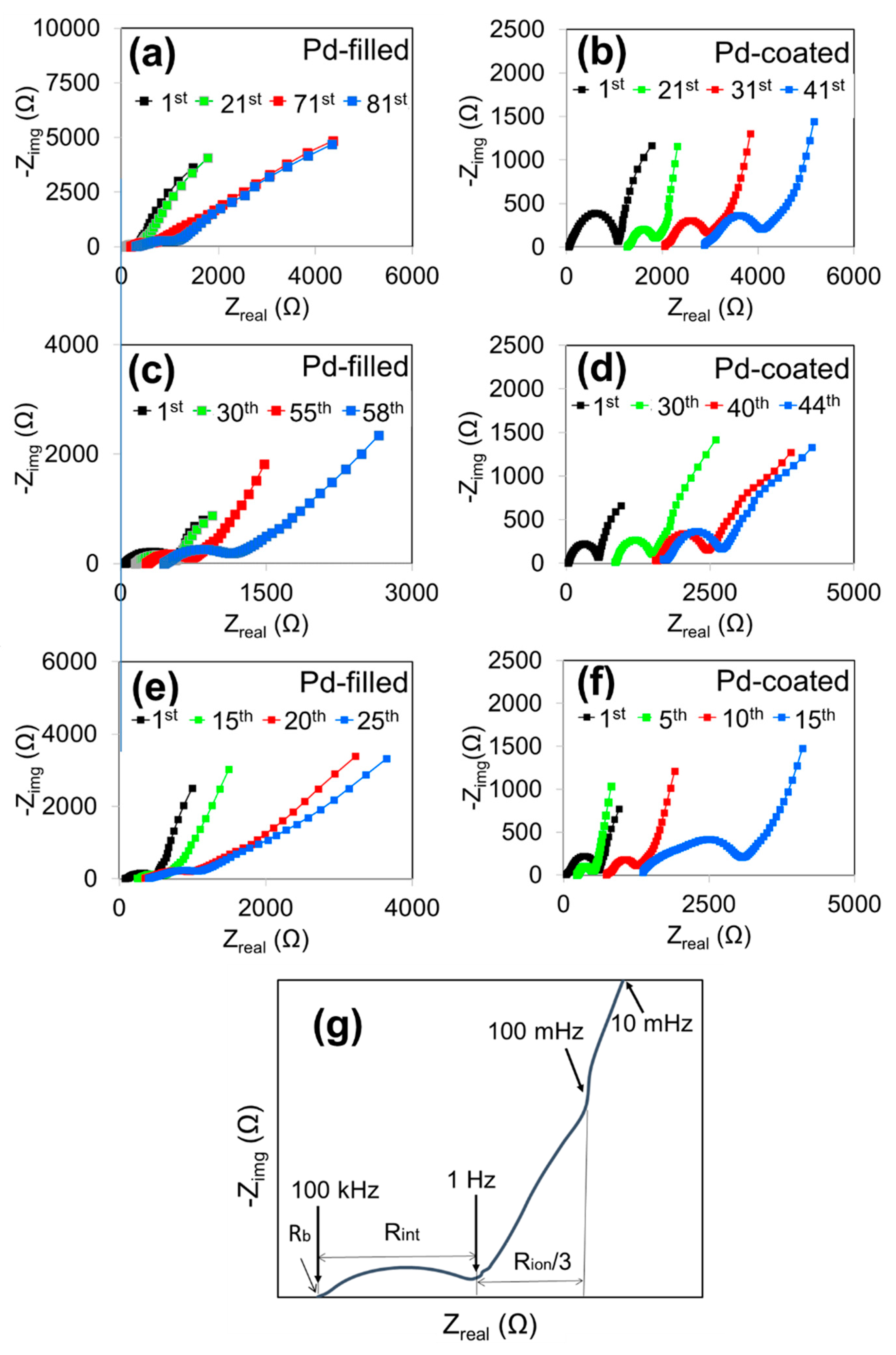
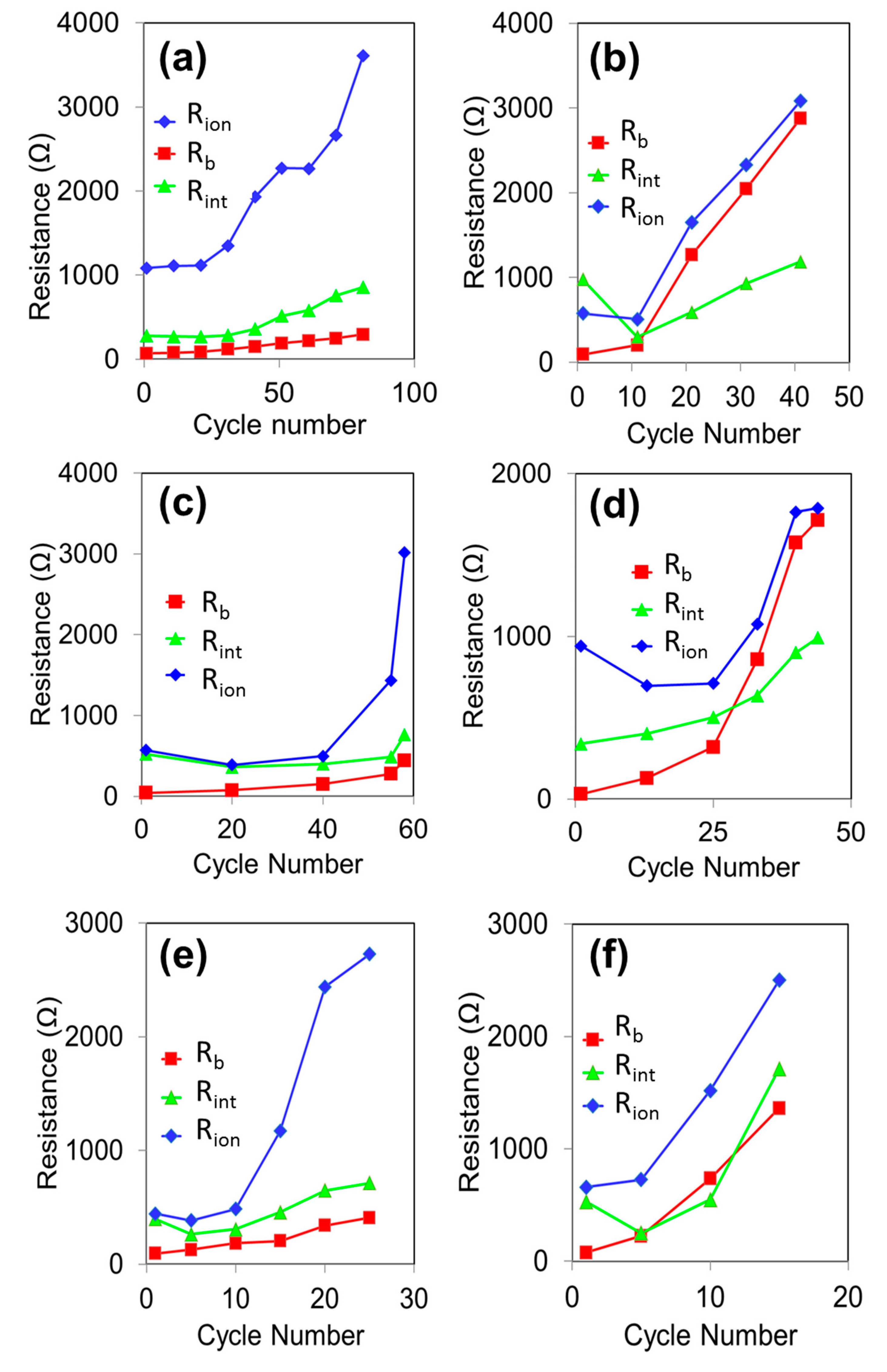
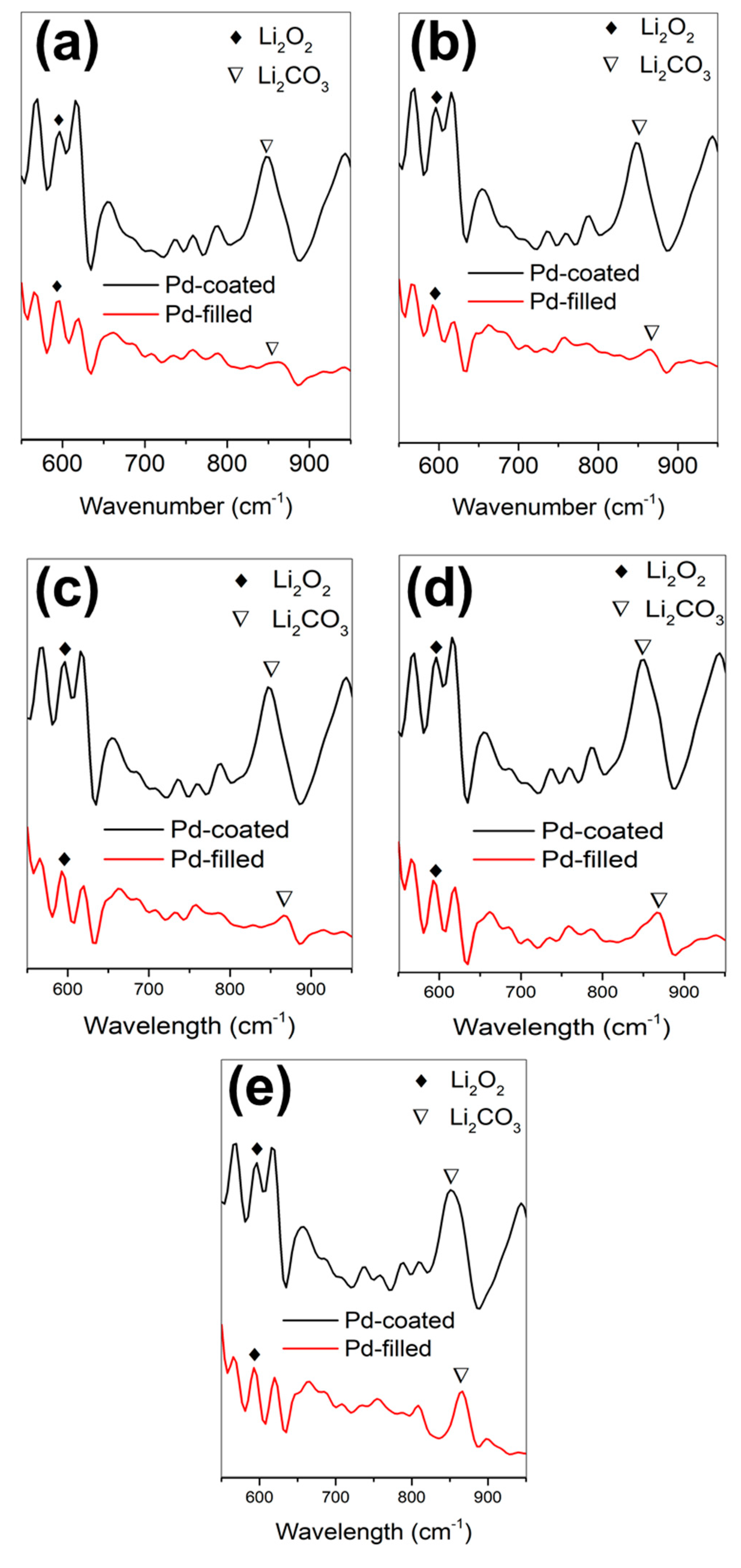
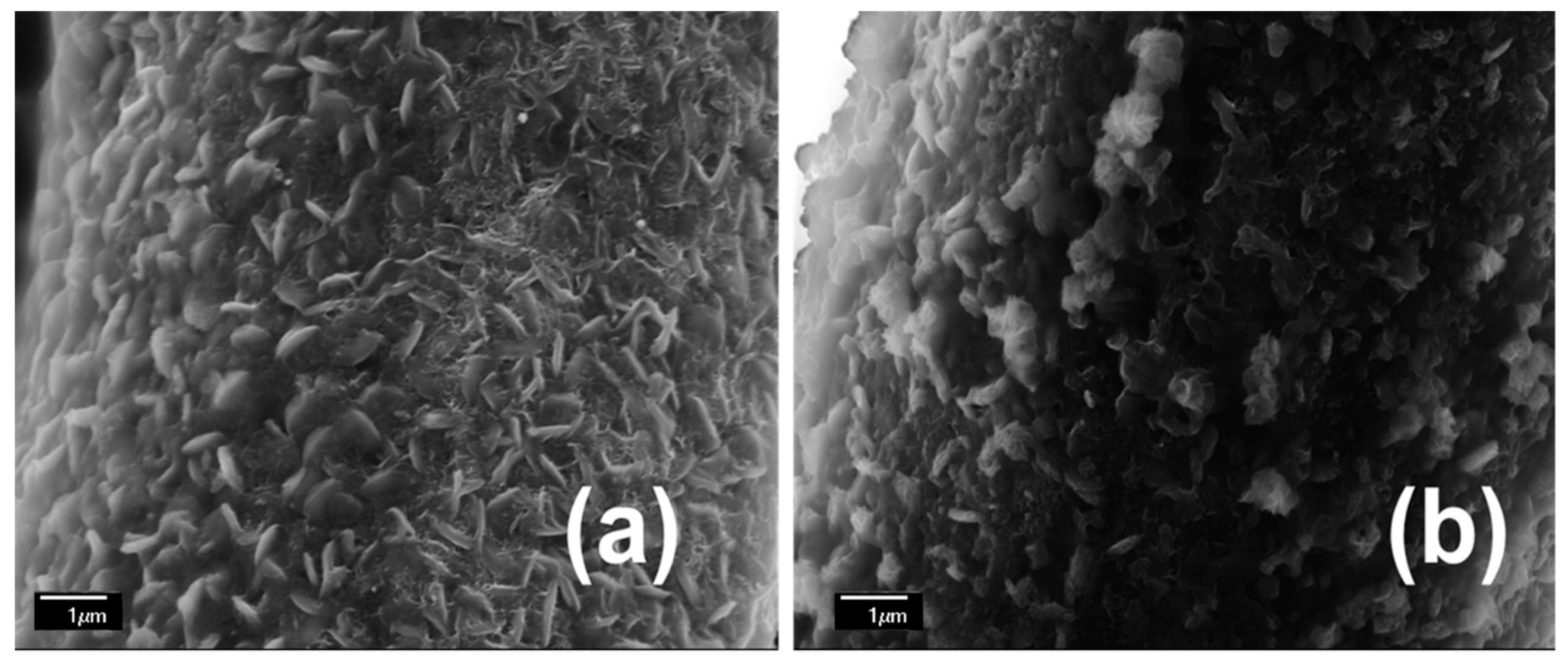
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Feng, N.; He, P.; Zhou, H. Critical Challenges in Rechargeable Aprotic Li-O2 Batteries. Adv. Energy Mater. 2016, 6, 1–24. [Google Scholar] [CrossRef]
- Panchal, S.; Mathewson, S.; Fraser, R.; Culham, R.; Fowler, M. Thermal Management of Lithium-Ion Pouch Cell with Indirect Liquid Cooling using Dual Cold Plates Approach. SAE Int. J. Altern. Powertrains 2015, 4, 293–307. [Google Scholar] [CrossRef]
- Safa, M.; Chamaani, A.; Chawla, N.; El-Zahab, B. Polymeric Ionic Liquid Gel Electrolyte for Room Temperature Lithium Battery Applications. Electrochim. Acta 2016, 213, 587–593. [Google Scholar] [CrossRef]
- Panchal, S.; Mathewson, S.; Fraser, R. Experimental Measurements of Thermal Characteristics of LiFePO4 Battery; SAE Tech. Paper; SAE: Warrendale, PA, USA, 2015. [Google Scholar]
- Geng, D.; Ding, N.; Hor, T.S.A.; Chien, S.W.; Liu, Z.; Wuu, D.; Sun, X.; Zong, Y. From Lithium-Oxygen to Lithium–air Batteries: Challenges and Opportunities. Adv. Energy Mater. 2016, 1–14. [Google Scholar] [CrossRef]
- Landa-Medrano, I.; Li, C.; Ortiz-Vitoriano, N.; de Larramendi, I.R.; Carrasco, J.; Rojo, T. Sodium-Oxygen Battery: Steps Toward Reality. J. Phys. Chem. Lett. 2016, 7, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Safa, M.; Hao, Y.; Chamaani, A.; Adelowo, E.; Chawla, N.; Wang, C.; El-Zahab, B. Capacity Fading Mechanism in Lithium-Sulfur Battery using Poly(ionic liquid) Gel Electrolyte. Electrochim. Acta 2017, 258, 1284–1292. [Google Scholar] [CrossRef]
- Girishkumar, G.; McCloskey, B.; Luntz, A.C.; Swanson, S.; Wilcke, W. Lithium–air battery: Promise and challenges. J. Phys. Chem. Lett. 2010, 1, 2193–2203. [Google Scholar] [CrossRef]
- Abraham, K.M.; Jiang, Z. A Polymer Electrolyte—Based Rechargeable Lithium/Oxygen Battery technical papers electrochemical science and technology A Polymer Electrolyte-Based Rechargeable lithium/Oxygen Battery. J. Electrochem. Soc. 1996, 143, 1–5. [Google Scholar] [CrossRef]
- Chawla, N.; Chamaani, A.; Safa, M.; El-Zahab, B. PF- Carbon Nanotubes Cathode for Improved Electrolyte Stability and Cyclability Performance of Li-O2 Batteries. J. Electrochem. Soc. 2017, 164, A6303–A6307. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Li, J.; Wang, M.; Nie, H.; Zhang, F. The use of mixed carbon materials with improved oxygen transport in a Lithium–air battery. J. Power Sources 2013, 240, 390–396. [Google Scholar] [CrossRef]
- Fuentes, R.E.; Colón-Mercado, H.R.; Fox, E.B. Electrochemical evaluation of carbon nanotubes and carbon black for the cathode of Li-air batteries. J. Power Sources 2014, 255, 219–222. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Zheng, J.P.; Liang, R.; Zhang, C.; Wang, B.; Hendrickson, M.; Plichta, E.J. Lithium–Air Batteries Using SWNT/CNF Buckypapers as Air Electrodes. J. Electrochem. Soc. 2010, 157, A953. [Google Scholar] [CrossRef]
- Mitchell, R.R.; Gallant, B.M.; Thompson, C.V.; Shao-Horn, Y. All-carbon-nanofiber electrodes for high-energy rechargeable Li-O2 batteries. Energy Environ. Sci. 2011, 4, 2952. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Li, X.; Liu, J.; Geng, D.; Yang, J.; Li, R.; Sun, X. Nitrogen-doped carbon nanotubes as cathode for lithium–air batteries. Electrochem. Commun. 2011, 13, 668–672. [Google Scholar] [CrossRef]
- Chamaani, A.; Safa, M.; Chawla, N.; El-Zahab, B. Composite Gel polymer electrolyte for improved cyclability in lithium-oxygen batteries. ACS Appl. Mater. Interfaces 2017, 9, 33819–33826. [Google Scholar] [CrossRef]
- Chamaani, A.; Chawla, N.; Safa, M.; El-Zahab, B. One-Dimensional Glass Micro-Fillers in Gel Polymer Electrolytes for Li-O2 Battery Applications. Electrochim. Acta 2017, 235, 56–63. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Li, X.; Geng, D.; Banis, M.N.; Li, R.; Sun, X. Nitrogen-doped graphene nanosheets as cathode materials with excellent electrocatalytic activity for high capacity lithium-oxygen batteries. Electrochem. Commun. 2012, 18, 12–15. [Google Scholar] [CrossRef]
- Zu, C.; Li, L.; Qie, L.; Manthiram, A. Expandable-graphite-derived graphene for next-generation battery chemistries. J. Power Sources 2015, 284, 60–67. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, H. A reversible long-life Lithium–air battery in ambient air. Nat. Commun. 2013, 4, 1817. [Google Scholar] [CrossRef]
- Lu, J.; Lei, Y.; Lau, K.C.; Luo, X.; Du, P.; Wen, J.; Assary, R.S.; Das, U.; Miller, D.J.; Elam, J.W.; et al. A nanostructured cathode architecture for low charge overpotential in lithium-oxygen batteries. Nat. Commun. 2013, 4, 2383. [Google Scholar] [CrossRef]
- Astinchap, B.; Moradian, R.; Ardu, A.; Cannas, C.; Varvaro, G.; Capobianchi, A. Bifunctional FePt @ MWCNTs/Ru Nanoarchitectures: Synthesis and Characterization. Chem. Mater. 2012, 24, 3393–3400. [Google Scholar] [CrossRef]
- Itkis, D.M.; Semenenko, D.A.; Kataev, E.Y.; Belova, A.I.; Neudachina, V.S.; Sirotina, A.P.; Hävecker, M.; Teschner, D.; Knop-Gericke, A.; Dudin, P.; et al. Reactivity of carbon in lithium-oxygen battery positive electrodes. Nano Lett. 2013, 13, 4697–4701. [Google Scholar] [CrossRef] [PubMed]
- Chamaani, A.; Safa, M.; Chawla, N.; Herndon, M.; El-zahab, B. Stabilizing e ff ect of ion complex formation in lithium—Oxygen battery electrolytes. J. Electroanal. Chem. 2018, 815, 143–150. [Google Scholar] [CrossRef]
- Rahman, M.A.; Wang, X.; Wen, C. A review of high energy density lithium–air battery technology. J. Appl. Electrochem. 2013, 44, 5–22. [Google Scholar] [CrossRef]
- Yilmaz, E.; Yogi, C.; Yamanaka, K.; Ohta, T.; Byon, H.R. Promoting Formation of Noncrystalline Li2O2 in the Li-O2 Battery with RuO2 Nanoparticles. Nano Lett. 2013, 13, 4679–4684. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Munroe, P.; Wang, G. Ruthenium nanocrystals as cathode catalysts for lithium-oxygen batteries with a superior performance. Sci. Rep. 2013, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Débart, A.; Paterson, A.J.; Bao, J.; Bruce, P.G. α-MnO2 Nanowires: A Catalyst for the O2 Electrode in Rechargeable Lithium Batteries. Angew. Chem. Int. Ed. 2008, 47, 4521–4524. [Google Scholar] [CrossRef]
- Chawla, N. Nanocatalysts in lithium oxygen batteries. Nano Sci. Nano Technol. Indian J. 2018, 12, 128. [Google Scholar]
- Lu, Y.C.; Gasteiger, H.a.; Shao-Horn, Y. Catalytic activity trends of oxygen reduction reaction for nonaqueous Li-air batteries. J. Am. Chem. Soc. 2011, 133, 19048–19051. [Google Scholar] [CrossRef]
- Xu, J.-J.; Wang, Z.-L.; Xu, D.; Zhang, L.-L.; Zhang, X.-B. Tailoring deposition and morphology of discharge products towards high-rate and long-life lithium-oxygen batteries. Nat. Commun. 2013, 4, 2438. [Google Scholar] [CrossRef]
- Zhao, G.; Niu, Y.; Zhang, L.; Sun, K. Ruthenium oxide modified titanium dioxide nanotube arrays as carbon and binder free lithium–air battery cathode catalyst. J. Power Sources 2014, 270, 386–390. [Google Scholar] [CrossRef]
- Wang, Y.; Rong, Z.; Wang, Y.; Qu, J. Ruthenium nanoparticles loaded on functionalized graphene for liquid-phase hydrogenation of fine chemicals: Comparison with carbon nanotube. J. Catal. 2016, 333, 8–16. [Google Scholar] [CrossRef]
- Guo, Z.; Zhou, D.; Liu, H.; Dong, X.; Yuan, S.; Yu, A.; Wang, Y.; Xia, Y. Synthesis of ruthenium oxide coated ordered mesoporous carbon nanofiber arrays as a catalyst for lithium oxygen battery. J. Power Sources 2015, 276, 181–188. [Google Scholar] [CrossRef]
- Guo, X.; Liu, P.; Han, J.; Ito, Y.; Hirata, A.; Fujita, T.; Chen, M. 3D Nanoporous Nitrogen-Doped Graphene with Encapsulated RuO2 Nanoparticles for Li-O2 Batteries. Adv. Mater. 2015, 27, 6137–6143. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Dou, S.; Wang, G. Gold nanocrystals with variable index facets as highly effective cathode catalysts for lithium-oxygen batteries. NPG Asia Mater. 2015, 7, e155. [Google Scholar] [CrossRef]
- Li, F.; Tang, D.M.; Tao, Z.; Liao, K.; He, P.; Golberg, D.; Yamada, A.; Zhou, H. Superior performance of a Li-O2 battery with metallic RuO2 hollow spheres as the carbon-free cathode. Adv Energy Mater. 2015, 5. [Google Scholar] [CrossRef]
- Jian, Z.; Liu, P.; Li, F.; He, P.; Guo, X.; Chen, M.; Zhou, H. Core-shell-structured CNT@RuO2 composite as a high-performance cathode catalyst for rechargeable Li-O2 batteries. Angew. Chem. Int. Ed. Engl. 2014, 53, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Nazar, L.F. Oxide catalysts for rechargeable high-capacity Li-O2 batteries. Adv. Energy Mater. 2012, 2, 903–910. [Google Scholar] [CrossRef]
- Li, F.; Wu, S.; Li, D.; Zhang, T.; He, P.; Yamada, A.; Zhou, H. The water catalysis at oxygen cathodes of lithium–oxygen cells. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef]
- Yao, K.P.C.; Risch, M.; Sayed, S.Y.; Lee, Y.-L.; Harding, J.R.; Grimaud, A.; Pour, N.; Xu, Z.; Zhou, J.; Mansour, A.; et al. Solid-state activation of Li2O2 oxidation kinetics and implications for Li-O2 batteries. Energy Environ. Sci. 2015, 8, 2417–2426. [Google Scholar] [CrossRef]
- Yao, K.P.C.; Lu, Y.-C.; Amanchukwu, C.V.; Kwabi, D.G.; Risch, M.; Zhou, J.; Grimaud, A.; Hammond, P.T.; Bardé, F.; Shao-Horn, Y. The influence of transition metal oxides on the kinetics of Li2O2 oxidation in Li-O2 batteries: High activity of chromium oxides. Phys. Chem. Chem. Phys. 2014, 16, 2297–2304. [Google Scholar] [CrossRef] [PubMed]
- Gittleson, F.S.; Sekol, R.C.; Doubek, G.; Linardi, M.; Taylor, A.D. Catalyst and electrolyte synergy in Li-O2 batteries. Phys. Chem. Chem. Phys. 2014, 16, 3230–3237. [Google Scholar] [CrossRef] [PubMed]
- Gittleson, F.S.; Ryu, W.-H.; Schwab, M.; Tong, X.; Taylor, A.D. Pt and Pd catalyzed oxidation of Li2O2 and DMSO during Li-O2 battery charging. Chem. Commun. 2016, 52, 6605–6608. [Google Scholar] [CrossRef] [PubMed]
- Ryu, W.H.; Gittleson, F.S.; Schwab, M.; Goh, T.; Taylor, A.D. A mesoporous catalytic membrane architecture for lithium-oxygen battery systems. Nano Lett. 2015, 15, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, M.A.; Pearse, A.J.; Kozen, A.C.; Chen, X.; Gregorczyk, K.; Han, X.; Cao, A.; Hu, L.; Lee, S.B.; Rubloff, G.W.; et al. Investigation of the Cathode-Catalyst-Electrolyte Interface in Aprotic Li-O2 Batteries. Chem. Mater. 2015, 27, 5305–5313. [Google Scholar] [CrossRef]
- Huang, X.; Yu, H.; Tan, H.; Zhu, J.; Zhang, W.; Wang, C.; Zhang, J.; Wang, Y.; Lv, Y.; Zeng, Z.; et al. Carbon nanotube-encapsulated noble metal nanoparticle hybrid as a cathode material for Li-oxygen batteries. Adv. Funct. Mater. 2014, 24, 6516–6523. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, B.; Xie, Y.Y.; Lye, W.W.K.; Xu, Z.L.; Abouali, S.; Garakani, M.A.; Huang, J.Q.; Zhang, T.Y.; Huang, B.; et al. Electrospun graphitic carbon nanofibers with in-situ encapsulated Co-Ni nanoparticles as freestanding electrodes for Li-O2 batteries. Carbon N. Y. 2016, 100, 329–336. [Google Scholar] [CrossRef]
- Hevia, S.; Homm, P.; Cortes, A.; Núñez, V.; Contreras, C.; Vera, J.; Segura, R. Selective growth of palladium and titanium dioxide nanostructures inside carbon nanotube membranes. Nanoscale Res. Lett. 2012, 7, 342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, R.; Huang, T.; Yu, A. Novel composite polymer electrolyte for lithium air batteries. J. Power Sources 2010, 195, 1202–1206. [Google Scholar] [CrossRef]
- Cecchetto, L.; Salomon, M.; Scrosati, B.; Croce, F. Study of a Li-air battery having an electrolyte solution formed by a mixture of an ether-based aprotic solvent and an ionic liquid. J. Power Sources 2012, 213, 233–238. [Google Scholar] [CrossRef]
- Laoire, C.O.; Mukerjee, S.; Plichta, E.J.; Hendrickson, M.a.; Abraham, K.M. Rechargeable Lithium/TEGDME-LiPF-6/O2 Battery. J. Electrochem. Soc. 2011, 158, A302. [Google Scholar] [CrossRef]
- Kichambare, P.; Kumar, J.; Rodrigues, S.; Kumar, B. Electrochemical performance of highly mesoporous nitrogen doped carbon cathode in lithium-oxygen batteries. J. Power Sources 2011, 196, 3310–3316. [Google Scholar] [CrossRef]
- Knudsen, K.B.; Nichols, J.E.; Vegge, T.; Luntz, A.C.; McCloskey, B.D.; Hjelm, J. An Electrochemical Impedance Study of the Capacity Limitations in Na-O2Cells. J. Phys. Chem. C 2016, 120, 10799–10805. [Google Scholar] [CrossRef]
- Højberg, J.; McCloskey, B.D.; Hjelm, J.; Vegge, T.; Johansen, K.; Norby, P.; Luntz, A.C. An electrochemical impedance spectroscopy investigation of the overpotentials in Li-O2 batteries. ACS Appl. Mater. Interfaces. 2015, 7, 4039–4047. [Google Scholar] [CrossRef] [PubMed]
- Eswaran, M.; Munichandraiah, N.; Scanlon, L.G. High Capacity Li-O2 Cell and Electrochemical Impedance. Electrochem. Solid-State Lett. 2010, 13, A121–A124. [Google Scholar] [CrossRef]
- Bardenhagen, I.; Yezerska, O.; Augustin, M.; Fenske, D.; Wittstock, A.; Bäumer, M. In situ investigation of pore clogging during discharge of a Li/O2battery by electrochemical impedance spectroscopy. J. Power Sources 2015, 278, 255–264. [Google Scholar] [CrossRef]
- Yi, J.; Wu, S.; Bai, S.; Liu, Y.; Li, N.; Zhou, H. Interfacial Construction of Li2O2 for a Performance-Improved Polymer Li-O2 Battery. J. Mater. Chem. A 2016, 4, 2403–2407. [Google Scholar] [CrossRef]
- Yi, J.; Zhou, H. A Unique Hybrid Quasi-Solid-State Electrolyte for Li-O2 Batteries with Improved Cycle Life and Safety. ChemSusChem 2016, 9, 2391–2396. [Google Scholar] [CrossRef]
- Shui, J.-L.; Okasinski, J.S.; Kenesei, P.; Dobbs, H.A.; Zhao, D.; Almer, J.D.; Liu, D.-J. Reversibility of anodic lithium in rechargeable lithium–oxygen batteries. Nat. Commun. 2013, 4, 1–7. [Google Scholar] [CrossRef]
- Qiao, Y.; Ye, S. Spectroscopic Investigation for Oxygen Reduction and Evolution Reactions on Carbon Electrodes in Li-O2 Battery. J. Phys. Chem. C 2016, 120, 8033–8047. [Google Scholar] [CrossRef]
- Thotiyl, M.M.O.; Freunberger, S.a.; Peng, Z.; Bruce, P.G. The carbon electrode in nonaqueous Li-O2 cells. J. Am. Chem. Soc. 2013, 135, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.B.; Pham, T.V.; Guo, H.P.; Liu, H.K.; Dou, S.X. Three-Dimensional Array of TiN@Pt3Cu Nanowires as an Efficient Porous Electrode for the Lithium-Oxygen Battery. ACS Nano 2017, 11, 1747–1754. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chawla, N.; Chamaani, A.; Safa, M.; Herndon, M.; El-Zahab, B. Mechanism of Ionic Impedance Growth for Palladium-Containing CNT Electrodes in Lithium-Oxygen Battery Electrodes and Its Contribution to Battery Failure. Batteries 2019, 5, 15. https://doi.org/10.3390/batteries5010015
Chawla N, Chamaani A, Safa M, Herndon M, El-Zahab B. Mechanism of Ionic Impedance Growth for Palladium-Containing CNT Electrodes in Lithium-Oxygen Battery Electrodes and Its Contribution to Battery Failure. Batteries. 2019; 5(1):15. https://doi.org/10.3390/batteries5010015
Chicago/Turabian StyleChawla, Neha, Amir Chamaani, Meer Safa, Marcus Herndon, and Bilal El-Zahab. 2019. "Mechanism of Ionic Impedance Growth for Palladium-Containing CNT Electrodes in Lithium-Oxygen Battery Electrodes and Its Contribution to Battery Failure" Batteries 5, no. 1: 15. https://doi.org/10.3390/batteries5010015
APA StyleChawla, N., Chamaani, A., Safa, M., Herndon, M., & El-Zahab, B. (2019). Mechanism of Ionic Impedance Growth for Palladium-Containing CNT Electrodes in Lithium-Oxygen Battery Electrodes and Its Contribution to Battery Failure. Batteries, 5(1), 15. https://doi.org/10.3390/batteries5010015




