Silicon/Mesoporous Carbon (Si/MC) Derived from Phenolic Resin for High Energy Anode Materials for Li-ion Batteries: Role of HF Etching and Vinylene Carbonate (VC) Additive
Abstract
1. Introduction
2. Results and Discussion
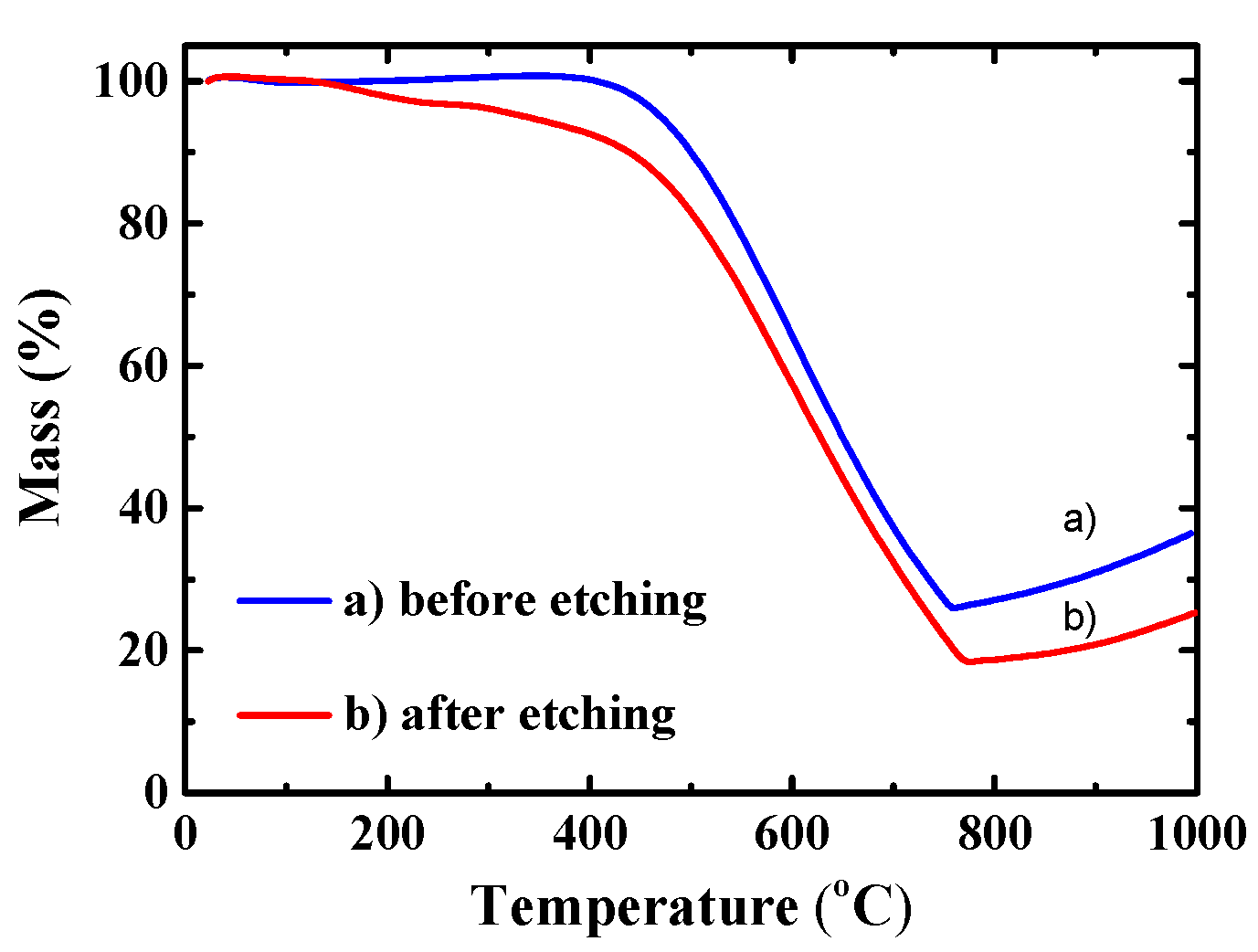
2.1. Properties of the Materials and the Effect of the HF Etching
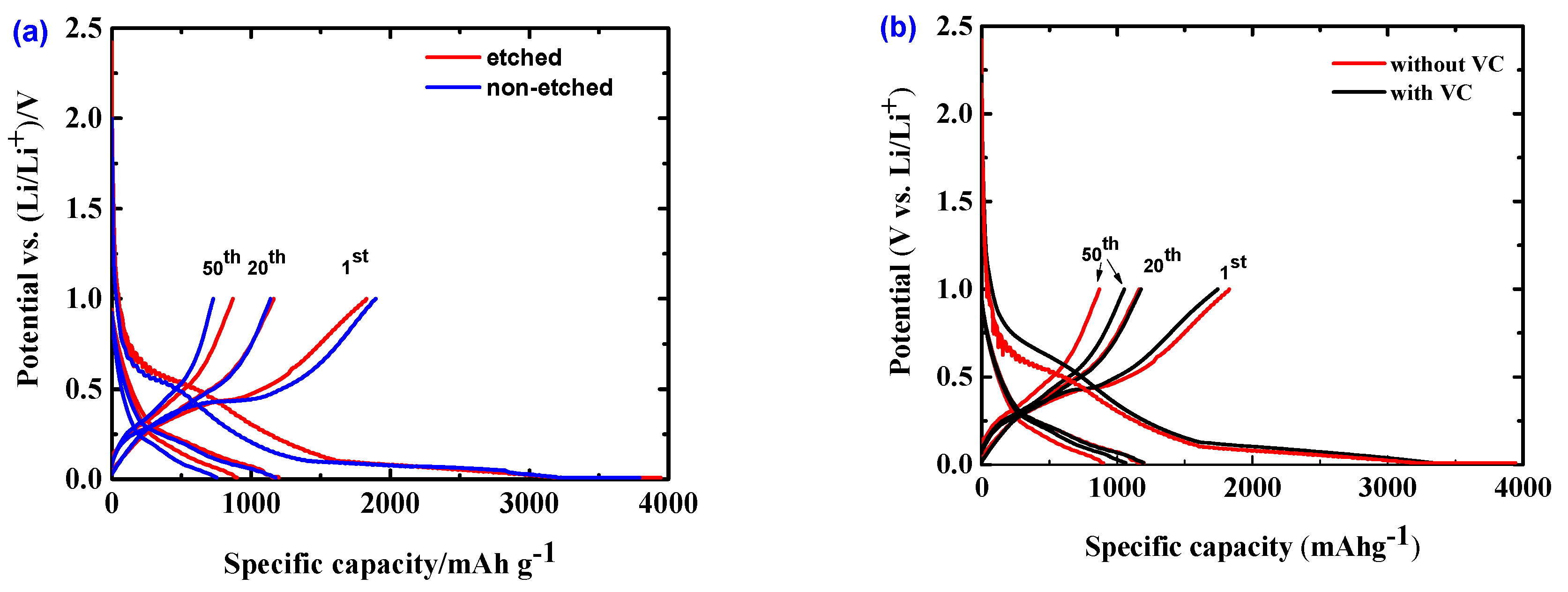
2.2. Initial Electrochemical Performance
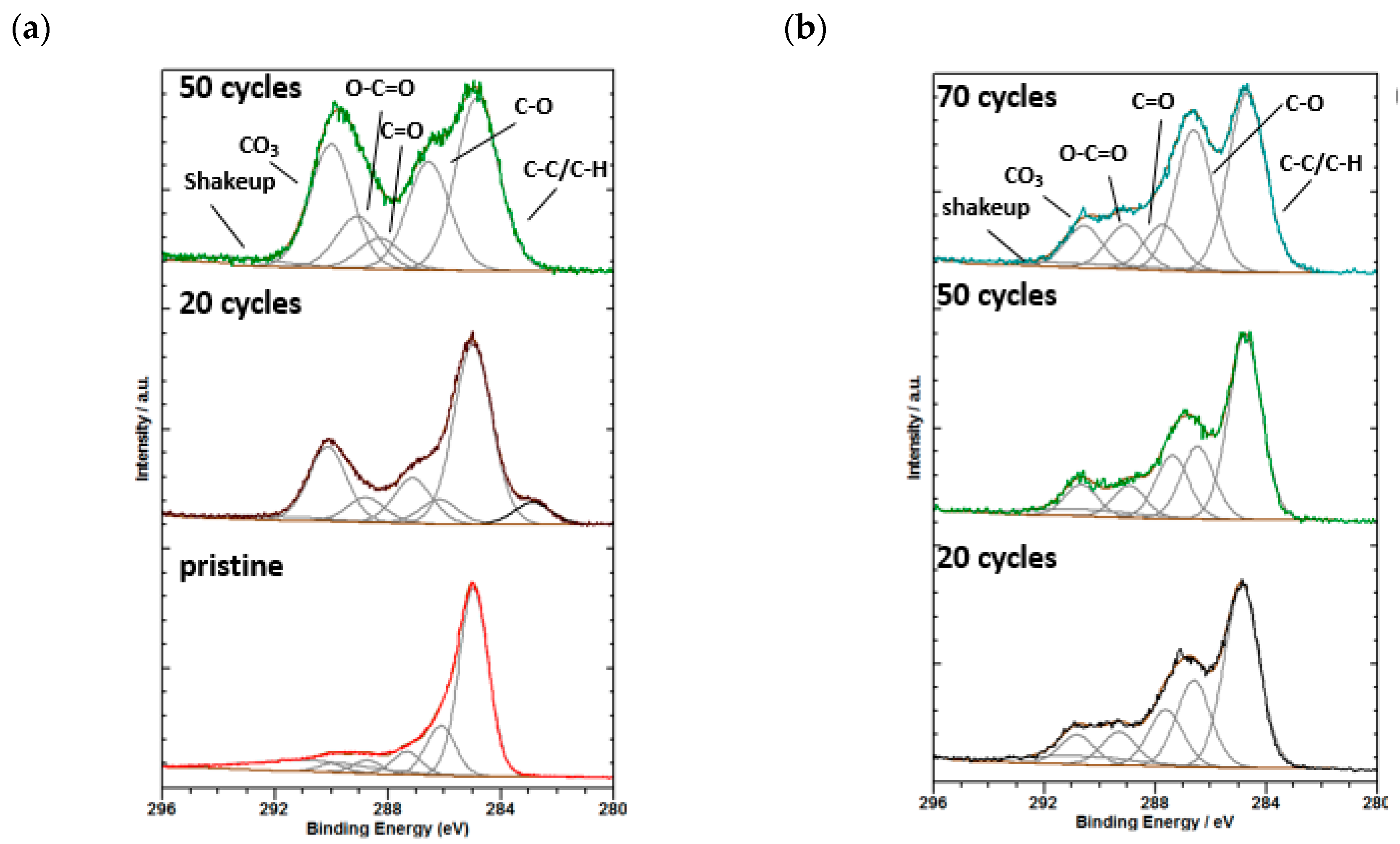
2.3. XPS Analysis of SEI Modification on Si/MC Electrodes
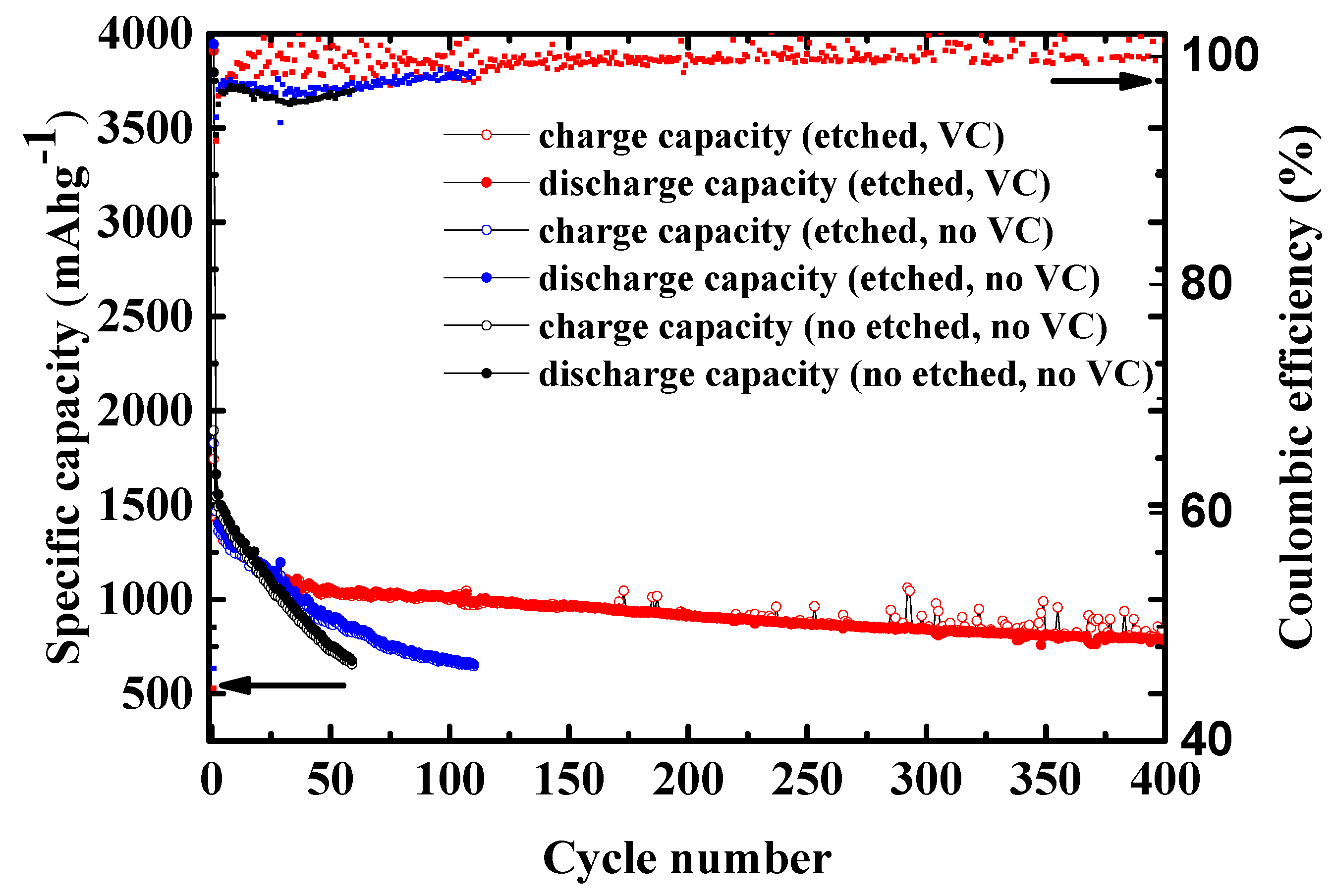
2.4. Long Cycling Performance
3. Materials and Methods
3.1. Si/MC Composites Preparation
3.2. Material Characterization
3.3. Electrochemical Characterization
3.4. XPS Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zuo, X.; Zhu, J.; Müller-Buschbaum, P.; Cheng, Y.-J. Silicon based lithium-ion battery anodes: A chronicle perspective review. Nano Energy 2017, 31, 113–143. [Google Scholar] [CrossRef]
- Yi, R.; Zai, J.; Dai, F.; Gordin, M.L.; Wang, D. Dual conductive network-enabled graphene/Si–C composite anode with high areal capacity for lithium-ion batteries. Nano Energy 2014, 6, 211–218. [Google Scholar] [CrossRef]
- Park, K.-S.; Min, K.-M.; Seo, S.-D.; Lee, G.-H.; Shim, H.-W.; Kim, D.-W. Self-supported multi-walled carbon nanotube-embedded silicon nanoparticle films for anodes of Li-ion batteries. Mater. Res. Bull. 2013, 48, 1732–1736. [Google Scholar] [CrossRef]
- Usui, H.; Wasada, K.; Shimizu, M.; Sakaguchi, H. TiO2/Si composites synthesized by sol–gel method and their improved electrode performance as Li-ion battery anodes. Electrochim. Acta 2013, 111, 575–580. [Google Scholar] [CrossRef]
- Wang, M.S.; Fan, L.Z. Silicon/carbon nanocomposite pyrolyzed from phenolic resin as anode materials for lithium-ion batteries. J. Power Sources 2013, 244, 570–574. [Google Scholar] [CrossRef]
- Yue, L.; Zhang, W.; Yang, J.; Zhang, L. Designing Si/porous-C composite with buffering voids as high capacity anode for lithium-ion batteries. Electrochim. Acta 2014, 125, 206–217. [Google Scholar] [CrossRef]
- Deng, J.; Ji, H.; Yan, C.; Zhang, J.; Si, W.; Baunack, S.; Oswald, S.; Mei, Y.; Schmidt, O.G. Naturally Rolled-Up C/Si/C Trilayer Nanomembranes as Stable Anodes for Lithium-Ion Batteries with Remarkable Cycling Performance. Angew. Chem. 2013, 125, 2382–2386. [Google Scholar] [CrossRef]
- Bogart, T.D.; Oka, D.; Lu, X.; Gu, M.; Wang, C.; Korgel, B.A.; Al, B.E.T. Lithium Ion Battery Peformance of Silicon Nanowires with Carbon Skin. ACS Nano 2014, 8, 915–922. [Google Scholar] [CrossRef]
- Zhu, S.; Zhu, C.; Ma, J.; Meng, Q.; Guo, Z.; Yu, Z.; Lu, T.; Li, Y.; Zhang, D.; Lau, W.M. Controlled fabrication of Si nanoparticles on graphene sheets for Li-ion batteries. RSC Adv. 2013, 3, 6141. [Google Scholar] [CrossRef]
- Ren, J.-G.; Wu, Q.-H.; Hong, G.; Zhang, W.-J.; Wu, H.; Amine, K.; Yang, J.; Lee, S.-T. Silicon-Graphene Composite Anodes for High-Energy Lithium Batteries. Energy Technol. 2013, 1, 77–84. [Google Scholar] [CrossRef]
- Park, J.; Kim, G.-P.; Nam, I.; Park, S.; Yi, J. One-pot synthesis of silicon nanoparticles trapped in ordered mesoporous carbon for use as an anode material in lithium-ion batteries. Nanotechnology 2013, 24, 025602. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhu, Y.; Wang, C. Mesoporous carbon/silicon composite anodes with enhanced performance for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 9751–9757. [Google Scholar] [CrossRef]
- Dash, R.; Pannala, S. Theoretical Limits of Energy Density in Silicon-Carbon Composite Anode Based Lithium Ion Batteries. Sci. Rep. 2016, 6, 27449. [Google Scholar] [CrossRef] [PubMed]
- Peled, E.; Menkin, S. Review—SEI: Past, Present and Future. J. Electrochem. Soc. 2017, 164, A1703–A1719. [Google Scholar] [CrossRef]
- Martin, L.; Martinez, H.; Ulldemolins, M.; Pecquenard, B.; Le Cras, F. Evolution of the Si electrode/electrolyte interface in lithium batteries characterized by XPS and AFM techniques: The influence of vinylene carbonate additive. Solid State Ionics 2012, 215, 36–44. [Google Scholar] [CrossRef]
- Nguyen, C.C.; Lucht, B.L. Comparative Study of Fluoroethylene Carbonate and Vinylene Carbonate for Silicon Anodes in Lithium Ion Batteries. J. Electrochem. Soc. 2014, 161, A1933–A1938. [Google Scholar] [CrossRef]
- Soto, F.A.; Ma, Y.; Martinez De La Hoz, J.M.; Seminario, J.M.; Balbuena, P.B. Formation and Growth Mechanisms of Solid-Electrolyte Interphase Layers in Rechargeable Batteries. Chem. Mater. 2015, 27, 7990–8000. [Google Scholar] [CrossRef]
- Rezqita, A.; Sauer, M.; Foelske, A.; Kronberger, H.; Trifonova, A. The effect of electrolyte additives on electrochemical performance of silicon/mesoporous carbon (Si/MC) for anode materials for lithium-ion batteries. Electrochim. Acta 2017, 247, 600–609. [Google Scholar] [CrossRef]
- Hassan, F.M.; Chabot, V.; Elsayed, A.R.; Xiao, X.; Chen, Z. Engineered Si electrode nanoarchitecture: A scalable postfabrication treatment for the production of next-generation Li-ion batteries. Nano Lett. 2014, 14, 277–283. [Google Scholar] [CrossRef]
- Fang, Y.; Lv, Y.Y.; Che, R.C.; Wu, H.Y.; Zhang, X.H.; Gu, D.; Zheng, G.F.; Zhao, D.Y. Two-Dimensional Mesoporous Carbon Nanosheets and Their Derived Graphene Nanosheets: Synthesis and Efficient Lithium Ion Storage. J. Am. Chem. Soc. 2013, 135, 1524–1530. [Google Scholar] [CrossRef]
- Journal, F. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database. Cell 2016, 164, 591–592. [Google Scholar]
- Naumkin, A.V.; Kraut-Vass, A.; Gaarenstroom, S.W.; Powell, C.J. NIST X-ray Photoelectron Spectroscopy Database; Version 4.1; National Institute of Standards and Technology (NIST): Gaithersburg, MD, USA, 2012. [Google Scholar]
- Shirley, D.A. High-resolution x-ray photoemission spectrum of the valence bands of gold. Phys. Rev. B 1972, 5, 4709–4714. [Google Scholar] [CrossRef]
- Scofield, J.H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
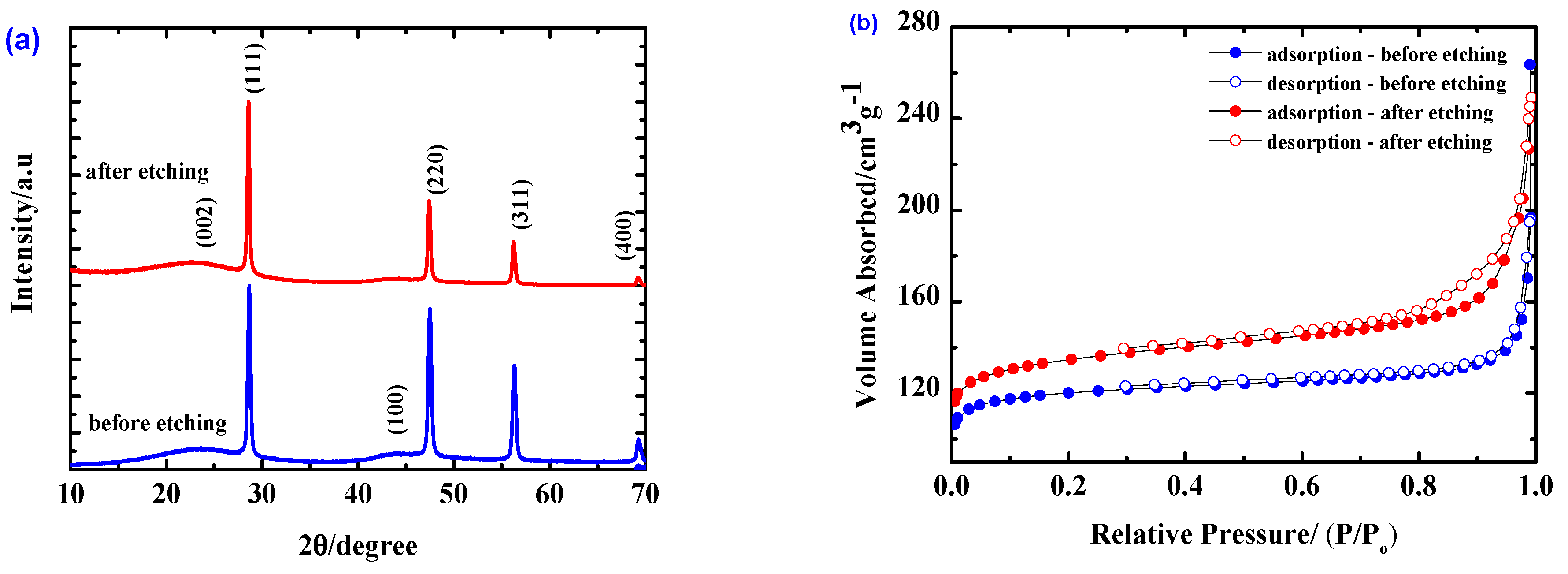
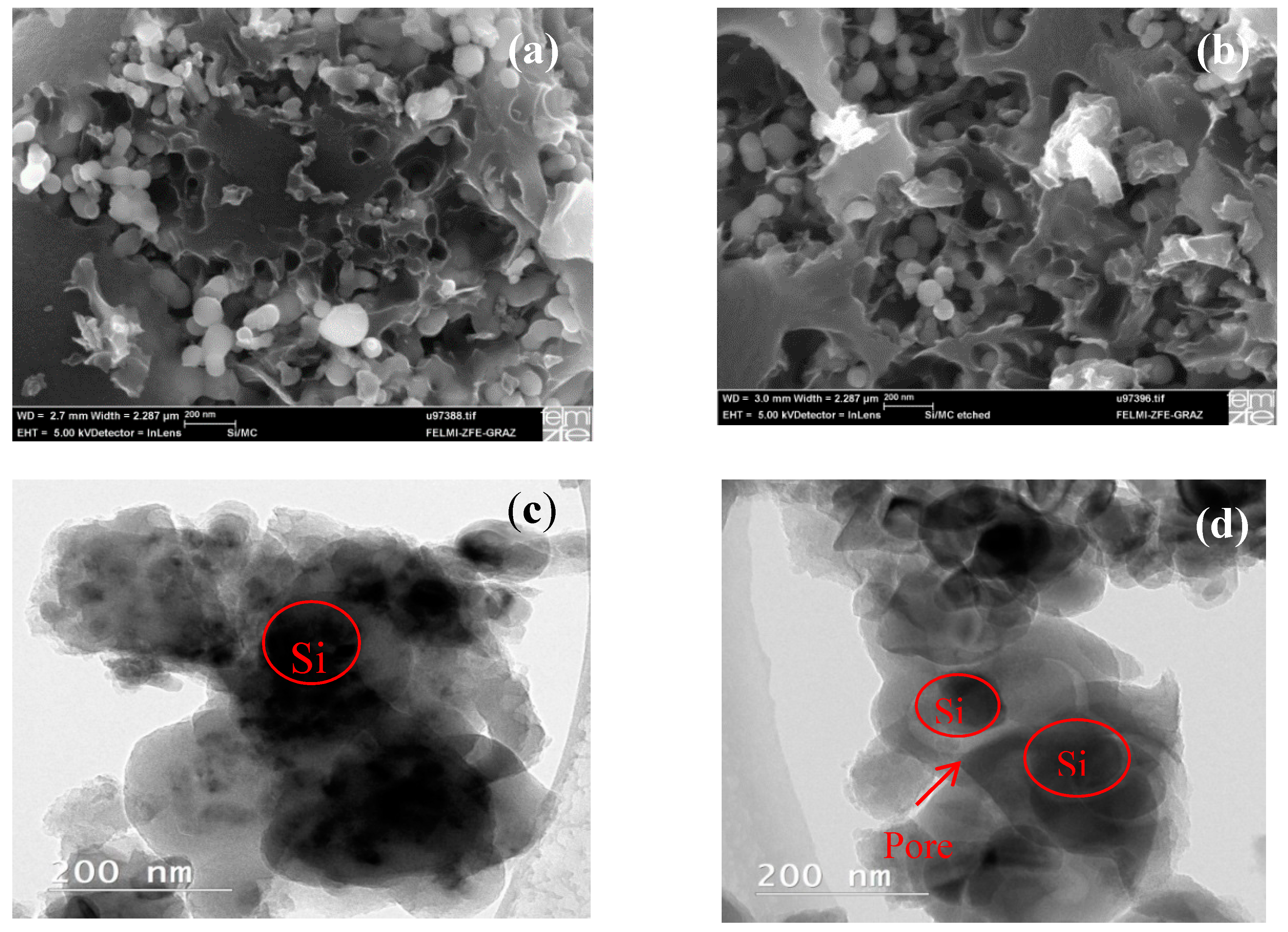
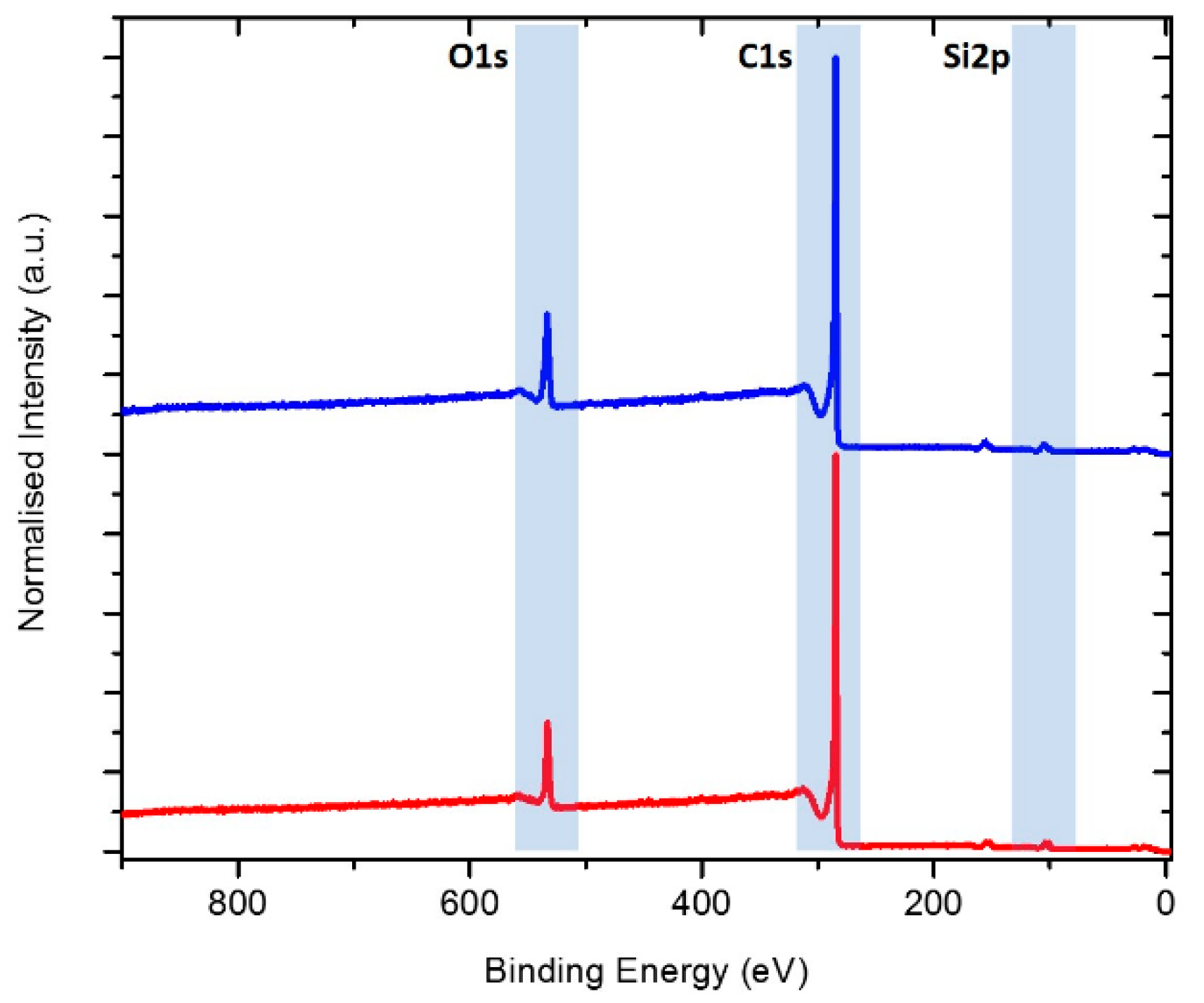
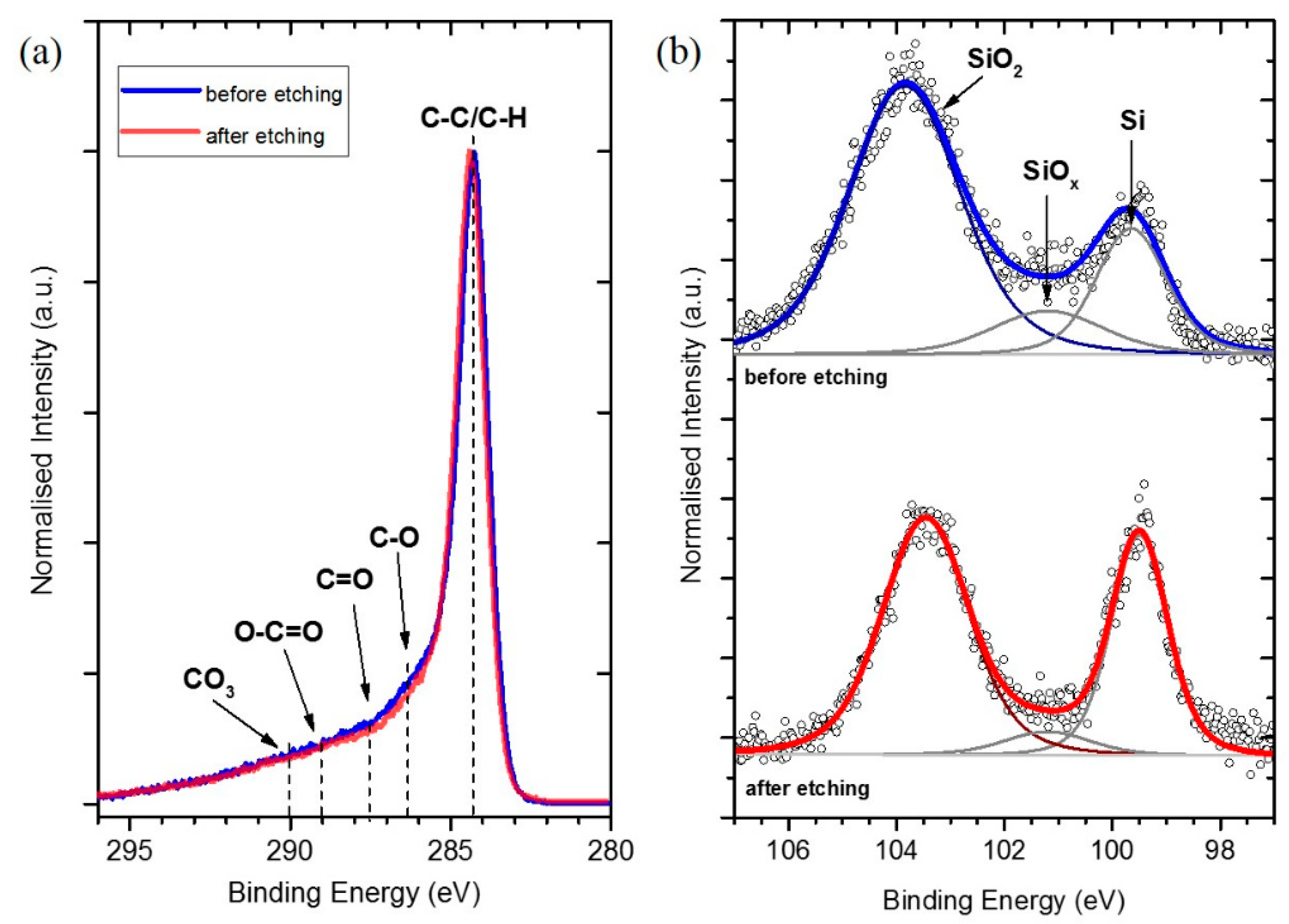
| Electrodes | Si Content | HF Etching | Electrolyte | Electrochemical Performance |
|---|---|---|---|---|
| Si/C nanocomposites | 42 wt% | No | 1M LiPF6 in EC: DEC: DMC (1:1:1 by volume) | initial reversible capacity of 904 mAhg−1 and a high capacity retention of 75% after 50 cycles at a current density of 100 mA g−1 [5] |
| Si/porous-C | 36.2 wt% | Yes | 1 M in LiPF6 EC/DEC/DMC (1:1:1 by volume) | a reversible capacity of 980 mAh g−1 over 80 cycles at a current density of 100 mA g−1 [6] |
| Si/OMC | 82 wt% | Yes | 1 M LiPF6 in EC:DEC (1:1 by weight) | reversible capacity above 700 mAh g−1 during 50 cycles at 2 A g−1 [11] |
| Mesoporous C/Si composite | 76 wt% | No | 1 M LiPF6 in a mixture of FEC:DMC (1:1 by volume) | specific capacity of 1018 mAh g−1 after 100 cycles at a current density of 500 mA g−1 [12] |
| Silicon/mesoporous carbon (Si/MC) | 12.98 wt% | Yes | 1 M LiPF6 in EC and DMC (1:1 by volume) with 5 wt% VC | reversible capacity of ~793 mAhg−1 with an average coulombic efficiency of ~99 % after more than 400 cycles [this study] |
| Sample | SBET (m2/g) | Micropore Surface Area (m2/g) | Mesopore Surface Area (m2/g) | Vtotal (cm3/g) | Vmicro (cm3/g) | Vmeso (cm3/g) |
|---|---|---|---|---|---|---|
| before etching | 481.88 | 461.09 | 20.79 | 0.305 | 0.179 | 0.126 |
| after etching | 530.23 | 486.59 | 43.64 | 0.386 | 0.192 | 0.194 |
| Sample | Element (at%) | ||
|---|---|---|---|
| C | O | Si | |
| before etching | 85.3 | 11.6 | 3.2 |
| after etching | 87.2 | 10.2 | 2.6 |
| Sample | Component (at%) | ||
|---|---|---|---|
| Si | SiOx/Si3N4 | SiO2 | |
| before etching | 20.3 | 11.0 | 68.6 |
| after etching | 35.6 | 5.8 | 58.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezqita, A.; Vasilchina, H.; Hamid, R.; Sauer, M.; Foelske, A.; Täubert, C.; Kronberger, H. Silicon/Mesoporous Carbon (Si/MC) Derived from Phenolic Resin for High Energy Anode Materials for Li-ion Batteries: Role of HF Etching and Vinylene Carbonate (VC) Additive. Batteries 2019, 5, 11. https://doi.org/10.3390/batteries5010011
Rezqita A, Vasilchina H, Hamid R, Sauer M, Foelske A, Täubert C, Kronberger H. Silicon/Mesoporous Carbon (Si/MC) Derived from Phenolic Resin for High Energy Anode Materials for Li-ion Batteries: Role of HF Etching and Vinylene Carbonate (VC) Additive. Batteries. 2019; 5(1):11. https://doi.org/10.3390/batteries5010011
Chicago/Turabian StyleRezqita, Arlavinda, Hristina Vasilchina, Raad Hamid, Markus Sauer, Annette Foelske, Corina Täubert, and Hermann Kronberger. 2019. "Silicon/Mesoporous Carbon (Si/MC) Derived from Phenolic Resin for High Energy Anode Materials for Li-ion Batteries: Role of HF Etching and Vinylene Carbonate (VC) Additive" Batteries 5, no. 1: 11. https://doi.org/10.3390/batteries5010011
APA StyleRezqita, A., Vasilchina, H., Hamid, R., Sauer, M., Foelske, A., Täubert, C., & Kronberger, H. (2019). Silicon/Mesoporous Carbon (Si/MC) Derived from Phenolic Resin for High Energy Anode Materials for Li-ion Batteries: Role of HF Etching and Vinylene Carbonate (VC) Additive. Batteries, 5(1), 11. https://doi.org/10.3390/batteries5010011





