Abstract
Sodium-ion batteries (SIBs) are a recent development being promoted repeatedly as an economically promising alternative to lithium-ion batteries (LIBs). However, only one detailed study about material costs has yet been published for this battery type. This paper presents the first detailed economic assessment of 18,650-type SIB cells with a layered oxide cathode and a hard carbon anode, based on existing datasheets for pre-commercial battery cells. The results are compared with those of competing LIB cells, that is, with lithium-nickel-manganese-cobalt-oxide cathodes (NMC) and with lithium-iron-phosphate cathodes (LFP). A sensitivity analysis further evaluates the influence of varying raw material prices on the results. For the SIB, a cell price of 223 €/kWh is obtained, compared to 229 €/kWh for the LFP and 168 €/kWh for the NMC batteries. The main contributor to the price of the SIB cells are the material costs, above all the cathode and anode active materials. For this reason, the amount of cathode active material (e.g., coating thickness) in addition to potential fluctuations in the raw material prices have a considerable effect on the price per kWh of storage capacity. Regarding the anode, the precursor material costs have a significant influence on the hard carbon cost, and thus on the final price of the SIB cell. Organic wastes and fossil coke precursor materials have the potential of yielding hard carbon at very competitive costs. In addition, cost reductions in comparison with LIBs are achieved for the current collectors, since SIBs also allow the use of aluminum instead of copper on the anode side. For the electrolyte, the substitution of lithium with sodium leads to only a marginal cost decrease from 16.1 to 15.8 €/L, hardly noticeable in the final cell price. On the other hand, the achievable energy density is fundamental. While it seems difficult to achieve the same price per kWh as high energy density NMC LIBs, the SIB could be a promising substitute for LFP cells in stationary applications, if it also becomes competitive with LFP cells in terms of safety and cycle life.
1. Introduction
Currently, lithium-ion batteries (LIBs) are among the most relevant electrochemical energy storage technologies [1]. They are a relatively mature technology, show high gravimetric and volumetric energy densities, high charge–discharge efficiencies, and good power rates. These properties make them the technology of choice not only for mobile applications, but also to an increasing extent for stationary energy storage [2,3,4]. However, the still high cost of lithium-ion batteries is one of their principle shortcomings [5,6]. Additionally, the increasing demand raises concerns about the medium-term availability of certain raw materials like cobalt or lithium, where price peaks or supply shortages might further increase costs and economic risks for cell manufacturers [7,8]. For these reasons, alternative low-cost systems are being investigated, among them “beyond-lithium” chemistries like sodium-ion batteries (SIBs) [9,10]. SIBs are a recent development that is already in a pilot phase, being promoted as an economically promising alternative to LIBs [11,12,13]. Similar to LIBs, SIBs rely on the intercalation of sodium ions in the anode material (charging state) or the cathode materials (discharge). Promising cathode materials are (similar to LIBs) numerous types of layered oxides or polyanionic compounds, whereas for the anode, amorphous carbon material (mostly hard carbons) is usually applied. Graphite, the anode material of choice for current LIBs, is not suitable for SIBs since the larger sodium ions do not intercalate readily into the graphitic structure. Due to the very similar properties, SIBs are considered a drop-in technology that could be produced using the existing infrastructure for LIBs. Apart from the anode active material, another major difference of SIBs in comparison to existing LIBs is the possibility of also using aluminum for the anode current collectors, since sodium (unlike lithium) does not alloy with aluminum at the anode. A schematic visualization of the working principle of an SIB is shown in Figure 1, while Table 1 compares the key components of LIBs and SIBs. Due to the higher specific weight of sodium in comparison to lithium and a higher irreversible capacity of the hard carbon anodes, the theoretically achievable maximum energy densities of SIBs are lower than those of LIBs. Nevertheless, they are considered interesting especially for stationary applications, where energy density is less crucial [14]. Cost advantages are expected for SIBs above all due to the substitution of lithium with the more abundant (and thus cheaper) sodium, and the possibility of using aluminum instead of copper for the anode current collector. A recent life-cycle assessment found the SIBs to be promising under environmental aspects, and highlighted some still significant improvement potentials [15]. While cost aspects are repeatedly used as an argument for SIBs, only one tentative economic assessment of SIB cells has yet been published [8]. However, it focuses primarily on material costs and does not consider the differences in cell layout between SIBs and LIBs (driven by, for example, the higher irreversible capacity of hard carbon, requiring increased anode coating thicknesses). The present article therefore provides a study of the potential cost of a full 18,650 SIB cell in comparison with two existing types of LIBs, namely those based on lithium-nickel-manganese-cobalt-oxide (NMC) and lithium-iron-phosphate (LFP). For the first time, a cost comparison is done for 18,650-type round cells in a bottom-up approach based on the technical specifications of pre-commercial battery cells.
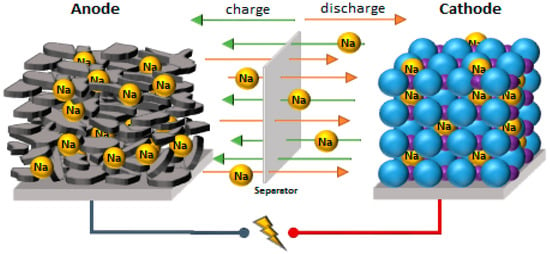
Figure 1.
Working principle of the sodium-ion cell [16].

Table 1.
Key components of lithium-ion and sodium-ion batteries (LIBs and SIBs). Major differences in bold. EC = ethylene carbonate, DMC = dimethyl carbonate, PE = polyethylene.
2. Methodology
The battery cost calculation was based on the BatPaC cost calculation model presented by Argonne National Laboratories [17]. This model was modified and adapted for estimating the manufacturing costs of individual 18650 round cells (BatPaC originally only considers prismatic cells mounted in automotive battery packs). The individual battery components and the dimensioning of the battery cells required as input for the cost estimation were modelled based on technical datasheets and scientific literature and are described in detail in the following sections. All prices, unless indicated otherwise, are provided in €2017. The battery production was assumed to be situated in Germany. Therefore, prices from other years and currencies were converted into euros according to the average annual currency exchange rate of the corresponding year and then adjusted to the reference year 2017 according to the Industrial Producer Price Index (IPP; see Table S1) [18,19]. An alternative low-labor-cost location is evaluated in the sensitivity analysis.
The assessed SIB was based on a layered oxide cathode (NMMT: Sodium Nickel Manganese Magnesium Titanate Oxide; Na1.1Ni0.3Mn0.5Mg0.05Ti0.05O2 [20]) in combination with a hard carbon anode and an electrolyte made of sodium hexafluorophosphate (NaPF6) salt in an organic solvent [13,15]. The layout of the battery cell was taken from technical datasheets of a battery manufacturer that produces pre-commercial SIB cells in a pilot stage [13]. Other battery components like collector foils, electrode binders, separator, and housing were identical between the SIBs and LIBs. Prices for these standard components were taken from published literature and are provided, together with the corresponding data source, in Table 2. The batteries were assembled as 18650-type round cells and the final battery cell price was calculated on a cell level, giving a price estimation independent of the later application.

Table 2.
Inputs required for the production of 1 kg of hard carbon and final hard carbon costs.
2.1. Cost of Cathode Active Material
Since the studied NMMT cathode material was not available on the market, its price had to be estimated based on the cost of the raw materials plus the manufacturing costs. This was done according to Equation (1) [17]. For validation purposes, the cost of the lithium-ion cathode material (NMC) was also calculated in the same way. This allowed the comparison of the obtained results with market prices for an established active material and thus for validation of the cost estimation model.
where:
C = Final cost (€/kg),
Co = Baseline cost (€),
Ci = Price of the raw materials (€/kg),
xi = Molar stoichiometry (-),
MWi = Molecular weight of the raw material (g/mol), and
MW = Molecular weight of the final product (g/mol).
The baseline cost is the cost associated with the installation and operation of the manufacturing line/plant and thus depends on the material properties, but not the cost of the precursor materials. A baseline cost of 9.15 €/kg for co-precipitated metal oxides such as NMC is given in the literature [17]. The same value is also used as the baseline cost for NMMT production, assuming that the synthesis processes of both layered oxides are very similar. According to Equation (1) and using the raw material prices provided in the supplementary information (SI), a price of 23.42 €/kg is obtained for the NMC active material (more specifically NMC333, that is, containing stoichiometrically equivalent shares of nickel, manganese, and cobalt; hereafter referred to as NMC), and of 12.46 €/kg for NMMT. The NMC price obtained in this way is well between the values published in the literature (19–28 €/kg) [17,21,22,23,24,25,26]. Since no information about the baseline cost of phosphate compounds such as LFP is publicly available, Equation (1) is not applicable directly to this material. Calculating with the same baseline costs as for layered oxides, an LFP price of 13.9 € can nevertheless be obtained, which is significantly lower than the values that can be found in the literature [17,21,22,24,25]. For this reason, the price of the LFP active material is not calculated explicitly via Equation (1), but the average value from the literature is taken instead (16.4 €/kg) [17,21,24,25]. More information about the calculation of the cathode active material costs (amounts and costs of the required precursor materials of the cathode active materials) are provided in Tables S2 and S3 in the Supplementary Material (SM).
2.2. Cost of Anode Active Material
Graphite is the most widely used anode active material in lithium-ion cells. However, it is not applicable for sodium-ion cells, since sodium does not intercalate readily with graphite, giving very low capacities. Therefore, amorphous carbon materials are usually used for SIB anodes, mostly hard carbon [1,27]. Due to the lack of an established market, no market price data was available for hard carbon, and the price for synthetic graphite (13.73 €/kg [17,28]) was used as a first approximation. Keeping the anode material price identical allowed us to focus the comparison on the cathode materials and the differences in battery layout. For the sensitivity analysis, a possible cost range was determined in a simplified way based on the required raw materials and auxiliary inputs required for its production. The required inputs were obtained from the literature, and the corresponding data sources are indicated together with the mass balances in Table S2 of the SM. With the purpose of analyzing the influence of the anode active material’s price on the final price of the cell, three options were considered: hard carbon (i) from sugar [13]; (ii) from coconut shell [29,30]; and (iii) from petroleum coke [15]. The energy and auxiliary inputs required per kg of raw material fed into the hard carbon production process were taken from a previous publication [15], where a carbonization temperature of 1100 °C was assumed. The yield of hard carbon per kg of raw material was estimated based on its fixed carbon content, assuming that 100% of the fixed carbon was converted into hard carbon. Correspondingly, sugar with a hard carbon content of 9% [31] requires the processing of 11 kg of feedstock to obtain 1 kg of hard carbon; coconut shells, with 20% hard carbon content [31], requires 4.5 kg; and petroleum coke, with 87% hard carbon content [32], requires 1.14 kg. This a very simple modelling approach; for a more detailed assessment of this aspect, the whole carbonization process would have to be modelled in detail, achieving potentially different electrochemical performances of the obtained hard carbons. However, this sensitivity analysis aims at pointing out tendencies rather than providing a detailed assessment of the performance of different hard carbons, which would require a series of experiments, and is out of this study’s scope.
2.3. Cost of Electrolyte
Lithium or sodium hexafluorophosphate (LiPF6 or NaPF6) salt in an organic solvent like dimethyl carbonate (DMC), ethylene carbonate (EC), or a combination of those two is the electrolyte most commonly used in LIB or SIB cells [40,41,42]. In this work, a 1 M solution of LiPF6 or NaPF6 in an 80 wt %–20 wt % mixture of EC and DMC was considered as the electrolyte for the LIB and the SIB, respectively [13,15,43,44]. Since the synthesis route of LiPF6 and NaPF6 is identical, the cost estimation for the SIB electrolyte was based on available data for LIBs. In the synthesis process, the same precursor materials were used, except for the alkali metal precursors (Li2CO3/Na2CO3). However, since it was very difficult to find information about the contribution of the different precursors to the final electrolyte price, this was estimated based on stoichiometric calculations and available market prices for the precursors. For the LIB electrolyte, an average price of 16.06 €/l was used [17], and the corresponding price of the SIB electrolyte was then calculated by substituting the Li2CO3 with Na2CO3, obtaining 15.84 €/l. More details about the electrolyte composition and the stoichiometric calculations can be found in the SM (Table S4).
2.4. Cost of Other Materials
Apart from the electrodes and electrolyte, an LIB cell contains other components such as separators, collector foils, binders, and the cell container. These are usually not dependent on the LIB chemistry, and it is therefore assumed that they do not differ significantly between the lithium- and the sodium-ion battery. In fact, one of the advantages of SIBs is that they are considered a “drop-in” technology, allowing the same manufacturing lines to be used for cell production and having a large proportion of identical parts [10,15]. For the battery model, it was assumed that the separators were uniformly made of polyethylene foil. The common conductive additive for the electrodes is carbon black. For the positive electrode, a binder based on polyvinylidene fluoride (PVdF; the most frequently used organic binder for LIBs) is used in combination with N-Methyl-2-pyrrolidone (NMP) as organic solvent, whereas the anode uses carboxymethyl cellulose (CMC) as an organic water-based binder [15]. Other types of binders are also frequently used, but for the cost comparison, which focuses on the different active materials, these were assumed to be identical between the compared battery types. The price for both types of binder was taken from the literature [17]. Compared to the lithium-ion cells (NMC- and LFP-type) under study that use aluminum as the positive collector foil and copper as the negative collector foil, aluminum can be used for both collector foils in SIBs [14]. Cost data for 18650-type cell casings were hard to find, and the only publication that provided information in this regard contained unrealistically high values of 0.2 € per cell container plus another 0.2 € for cell terminals [23]. Thus, retail prices from Internet suppliers were used instead as an approximation, with an average value of 0.1 € per cell container including cap and insulation [45,46]. An overview of the assumed costs for all materials and battery cell components is provided in Table 3. The detailed layout and mass balances for the battery cells under study are provided in detail in Tables S5 and S6 of the SM.

Table 3.
Cost data for other battery materials.
2.5. 18650 Cell Composition and Performance
A detailed mass balance is required to determine the exact amounts of materials required for manufacturing a single battery cell. In that respect, significant deviations existed not only between different scientific publications, but also between technical datasheets from manufacturers. The principal data source for the present assessment were technical datasheets published by Faradion, an SIB manufacturer that provides information about the layout and electrochemical performance of pre-commercial SIB cells, in addition to a competing comparable LFP cell [13]. The NMC cell was dimensioned correspondingly based on literature data [48,49]. Data about electrode dimensions and coating thicknesses allowed for a detailed determination of the required mass and corresponding volume of active materials, collector foils, and separator per single round cell. An estimation of the active material void fraction (porosity) was done based on the difference between the density of the unprocessed active material and the actual coating density according to the datasheets [11]. The amount of electrolyte required for cell filling was then calculated by the difference between the inner volume of an 18650 can and the volume of all battery components (cathode, anode, and separator, including the void due to porosity of the active material). Table 4 provides the key performance parameters of the compared battery cells, whereas more details about the battery layout and dimensioning are provided in Table S5 of the SM. The battery model was considered to represent the battery as specified in the underlying datasheets. Batteries can be designed for different purposes (high power/high energy), what affects the electrode thicknesses and thus material balances. However, such a detailed battery model is out of the scope of the present study.

Table 4.
Performance parameters per single 18650 battery cell.
2.6. Price of Final Battery Cell
The estimation of the full cell price was based on BatPaC Version 3.0 by Argonne National Laboratories, one of the standard battery cost assessment tools that allows dimensioning and cost estimation of electric vehicle battery modules [17]. However, major modifications were made for adapting the model to the calculation of single cell costs independent of their final application instead of configuring automotive battery packs. The parts of the manufacturing plant cost estimations were modified where necessary and adapted to the fabrication of 18650 round cells, mainly based on the data provided by Ciez and Whitacre [23]. The plant throughput was assumed to be 200 Mio battery cells per year. Since the different cell chemistries showed different storage capacities (see Table 4 and Table S7), this was equivalent to 1.2 GWh/y for the SIB and 1.7 GWh/y for the NMC (due to its higher energy density). Compared to the original BatPaC model (0.8 GWh/year), this was a significantly higher plant output in terms of manufactured storage capacity (GWh/y). Additionally, since the 18,650 round cells showed a significantly lower capacity per single cell than the prismatic cells assumed by BatPaC, the number of cells produced annually was significantly higher, thereby increasing the costs associated with cell filling, sealing, and cycling. Since the production was assumed to be in Germany, the electricity and labor costs were adjusted accordingly (0.12 €/kWh and 25 €/h) [51].
3. Results
The final cell prices obtained for the three different battery chemistries are displayed in Figure 2. The LFP battery shows the highest price per kWh of storage capacity (229.3 €/kWh), followed by the SIB at 223.4 €/kWh. The NMC-type LIB is the cheapest (168.5 €/kWh), basically due to its high energy density. The material costs make up between 37% and 42% of the final cell price, and another 18–19% represent the investment costs (depreciation). Compared with Vaalma et al. [8], who did a comparison of the material costs of a 11.5 kWh SIB and LIB, these values are significantly lower, but Vaalma et al. did not assess the battery on the cell level, but rather for a whole battery pack. The additional peripheral components and corresponding lower energy density increased the price per kWh of storage capacity. When looking at the material costs without the cell hardware, values in the same order of magnitude are obtained, with the different energy densities of the modelled batteries and different cathode compositions being the principal reason for the discrepancies. The operation of the manufacturing plant (incl. R&D) contributes between 32 and 35%. Naturally, these (and thus also the final cell price) depend strongly on the plant size and the annual throughput. This is one of the reasons for the high variation in prices published in the literature, where values of between 145 and 289 €/kWh for NMC and between 225 and 303 €/kWh for LFP are given [17,21,22,23,24,25,26,52,53,54], with the lower values originating from the most recent publications. The price estimations from this work are situated at the lower end of this range, corresponding with this trend towards decreasing battery prices. In addition, scale effects influence battery prices significantly, but are not investigated further in this work since they would affect all battery types identically, and since this study’s main scope is the comparison of different battery types under comparable conditions. For all battery chemistries, the materials make up the highest share, although with varying contributions from the different cell components. For comparison, Figure 3 displays the contribution of the different cell components to the total material costs per single 18650 cell (i.e., comparing individual cells without considering their different energy densities). This provides a better picture of the influences from the different materials, since on a single cell basis, identical components also show the same contributions (the mass of the final cells varies only slightly between the different battery types). When comparing the obtained prices for LFP and NMC LIB with those available in the literature, a good fit can be observed.
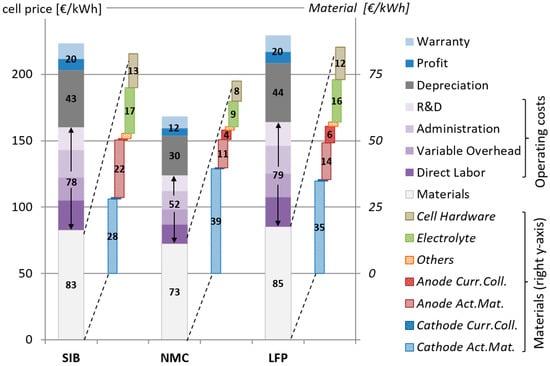
Figure 2.
Cell costs per kWh of storage capacity broken down to battery components and battery materials.
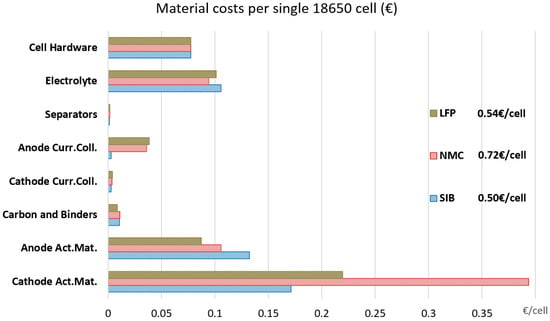
Figure 3.
Contribution of battery components to total material costs per single 18650 round cell (€/cell; disregarding different energy densities/storage capacities of the individual cells).
When looking at the contribution of the battery materials to the final cell costs (per single 18,650 cell; see Figure 3), the benefits of the SIB on a material level become clearer. Here, the SIB shows the lowest costs per single cell, whereas the materials for the NMC-type cells are the most expensive. However, these are costs per single cell and do not consider the storage capacity. Comparing these numbers with the results from Figure 2 gives an idea of the relevance of the cell energy density. Per single cell, significant cost benefits are obtained for the SIB cathode active material due to the use of less expensive raw materials, and for the anode current collectors due to the avoidance of copper (aluminum is significantly cheaper than copper). For the electrolyte, the differences are comparably small: the amount of lithium in the electrolyte is very low (0.5% for a 1 M LiPF6 solution in organic solvent), and correspondingly low is the potential for cost reductions by substituting it with an electrolyte using a less expensive sodium salt. On the other hand, a significant increase in anode costs can be observed, although for the base case assessment, the hard carbon is assumed to have the same price as battery grade graphite (different hard carbon prices are evaluated later on in the sensitivity analysis). This is a result of the lower specific density of hard carbon, requiring thicker anodes and thus leaving less space for the cathode active material, reducing the overall energy density. According to the underlying datasheets [13], the anode coating thickness of the SIB is almost double in comparison with the LIB. The higher irreversible capacity of hard carbon might also contribute to this effect, increasing the required amount of anode active material for compensating these losses. These effects are not explicitly modelled in this study, but contained implicitly in the anode thickness values provided in the datasheets of the battery manufacturer [13]. However, hard carbon can be produced from numerous types of precursors including organic waste materials [55,56], what might be a promising approach to reducing hard carbon costs. This aspect is evaluated further in the subsequent sensitivity analysis. A high share of the material costs is also caused by the cell hardware (cell container and sealing), and a different cell container might therefore offer further potentials for cost reduction. However, this would again affect all battery types identically and is therefore not targeted within this work.
4. Discussion and Sensitivity Analysis
4.1. Active Material Prices
The cost assessment of the chosen cell chemistries, namely, NMC, LFP (LIB), and NMMT (SIB), shows that the main contributors to the final cell cost are the electrode active materials. Although the electrolyte also contributes considerably to the final cell price, the difference between the lithium and sodium-ion cells is small. This is because the electrolyte salts (LiPF6/NaPF6) make up only about 10% of the total electrolyte mass and, within the salts, the amount of lithium and sodium metal is comparably small.
One of the principal differences of the SIBs in comparison to the LIBs is the anode active material, which shows the second highest contribution to the final cell material costs. No commercial hard carbon prices are available, and a wide price range is possible for this material, depending on the assumed precursor material (Table 2). While the influence of different precursors on the hard carbon’s electrochemical properties cannot be assessed in this work, it seems possible to produce hard carbons at prices below that of battery grade graphite. This would lead to significant price reductions for the SIB cell, as discussed in the following section (for the base case, the hard carbon price is assumed to be identical to the graphite price).
Regarding the cathode active materials, the SIB shows a significant cost advantage compared to the NMC battery, although more due to the avoidance of cobalt than due to the substitution of lithium (see Figure 4). While the NMC and NMMT cathode material costs calculated according to Equation (1) correlate well with published values, BatPaC does not provide information about the underlying calculation or how the baseline cost is determined. Since this hinders the calculation of a reliable price for the LFP material, literature values (avg. 16.4 €/kg) are used for the battery cell price calculations. When also calculating the LFP price with Equation (1) using the same baseline costs as for layered oxide cathode materials, a final LFP price of 13.9 €/kg is obtained, similar to that of the NMMT material. This reduces the final LFP cell price to 221 €/kWh, slightly below that of the SIB. Thus, more data about the actual baseline costs for the different active material production processes or the contribution of the precursor materials to the final active material price are needed for increasing the robustness of the results.
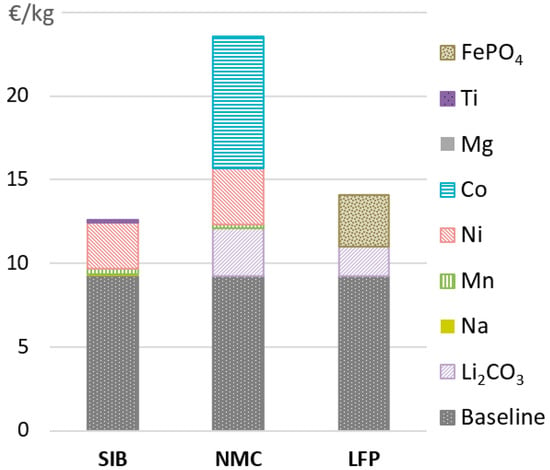
Figure 4.
Breakdown of the cathode active material cost [€/kg].
The main cost reduction potential for the NMMT cathode material is the reduction of the nickel content. All remaining cathode precursors are comparably abundant and thus inexpensive materials, causing the material costs (e.g., for LFP) to contribute only a minor share to the cathode material costs. For the LIBs, the cathode active material cost is more decisive for the final battery cell price. Above all, the prices of cobalt and nickel, which are highly fluctuating, and also the price of lithium are relevant factors.
4.2. Variations in Raw Material Prices
Fluctuations in raw material prices are a major factor of concern for battery manufacturers [24]. For the assessment, the 10-year average raw material prices were used [28]. In order to determine the influence of potential variations in material prices on the final battery price, Figure 5 displays the variation in the final cell price with changes in the price for selected raw materials (while all the remaining material costs are maintained constant). For this purpose, the 10-year maximum and 10-year minimum prices were used. For lithium, where a significant increase in price has been recently observed, a very high price (50% above the 10-year maximum) was also considered. As can be seen, a high sensitivity was given especially to fluctuations in the graphite/hard carbon prices. This is more severe for the SIB, where the share of anode active material is higher. Thus, the highest cost reduction potential is identified in the purchase cost of the anode carbon material. For the SIB, the hard carbon price was assumed to be identical to that of graphite in the base case, whereas in reality there would most probably be differences. No market prices were available for hard carbon, thereby providing no fluctuations. Thus, the possible price range for hard carbon is taken from Table 2, where the different potential hard carbon prices are determined. High costs can be expected for carbohydrate-based raw materials, whereas for carbons based on organic waste materials or petroleum coke, a very favorable price of significantly below 10 €/kg is possible due to the high potential yields and thus low process costs for this raw material (Table 2). Such inexpensive hard carbon materials could reduce the final battery price to 204 €/kWh, significantly below that of the LFP cells. However, with the given battery configuration and assuming identical performance of the different hard carbons, it still seems very difficult to achieve the same price per kWh as the NMC battery. In this regard, a more detailed study considering the varying electrochemical properties of different hard carbons would be a promising future work. Identifying hard carbons with superior performance would also be highly beneficial, reducing the required amount of anode material and increasing thus the energy density.
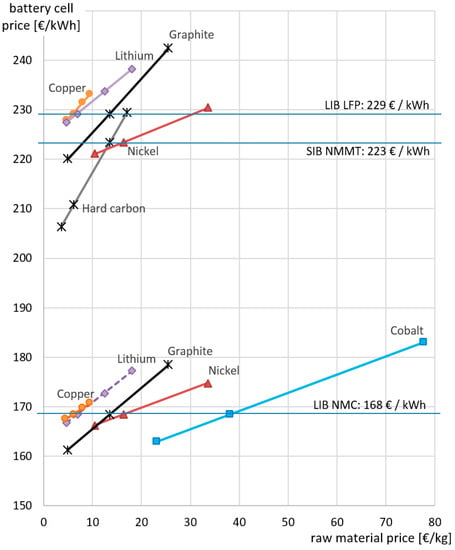
Figure 5.
Sensitivity of the final battery price on variations in raw material prices (10-year minimum, average, and maximum; for lithium additionally 10y max + 50%).
Regarding the cathode materials, the highest fluctuations can be observed for cobalt and nickel. Lithium and copper, despite the variations in their price which have a stronger impact on the final cell price, are comparably stable metals that did not fluctuate heavily over the past 10 years. However, recent increases in the price of lithium have been significant and might be triggered by an increasing demand for batteries, leading to potentially stronger impacts in LIB prices than previously noted. Thus, the high dependency of the actual NMC price on current nickel market prices, and cobalt market prices to a great degree, produces significant uncertainty for future price predictions. Since this situation also affects the SIB due to the high nickel content in the cathode, alternative nickel-free SIB cathode chemistries could be an interesting option in this regard.
4.3. Variation of Region
No commercial production of SIB yet exists. The SIBs in the present assessment are assumed to be manufactured in Germany, and (for providing a fair basis of comparison) the same is also assumed for the LIBs. However, the major share of LIBs is currently produced in Asia, especially China, and current activities for installing LIB production facilities in Europe are aimed at east European countries, with different wages and electricity costs. Battery manufacturing in these countries would affect the cell prices accordingly. However, the BatPaC model does not provide sufficient details to allow modifying these parameters explicitly, since, for example, electricity prices are not considered and R&D expenses are calculated as a fixed share of depreciation costs. When modifying the labor costs assuming 5 €/h, final cell prices would drop to 147.2 €/kWh for the NMC batteries, 196.2€/kWh for the LFP, and 190.1 €/kWh for the SIBs. Larger plant sizes would allow for further cost reductions. However, since this affects all battery chemistries identically, assessing this aspect further is of only minor interest for the present comparison of cell chemistries.
5. Conclusions
The present work provides an in-depth assessment of the potential prices for 18650-type sodium-ion battery cells relative to comparable lithium-ion cells. The cells are modelled on existing datasheets for pre-commercial cells, allowing for a detailed breakdown of costs for components having individual layout requirements with different cell chemistries. Per single battery cell, the sodium-ion battery (SIB) cells show advantages compared to the lithium-ion battery (LIB) cells due to cheaper cathode active materials and the avoidance of copper for the anode current collector. An additional potential for further cost reduction is identified especially for the hard carbon anode material. However, when the different energy density of the cells is considered, NMC-type LIB cells turn out to be the most competitive, simply by requiring less battery cells for providing a certain amount of storage capacity. Even considering the all-time high raw material prices, the final price per Wh of storage capacity of the NMC-type LIB is below those of LFP-type LIB and SIB, highlighting the importance of energy density even under cost aspects. On the other hand, LFP-type LIBs are also widely used in spite of their higher price, what can be explained in large part by their high stability and safety compared to the NMC. This shows the relevance of other parameters such as cycle life, which are not covered by the present assessment. With an estimated price in the same order of magnitude as LFP-type LIBs, the SIB shows a high potential of becoming cost-competitive in comparison to these, as long as SIBs also achieve a similar performance in these disciplines. Apart from that point, one must consider that LIBs are an established technology, whereas the sodium-ion cell is in the early phase of development and further improvements regarding materials and achievable energy densities as well as cycle stability can be expected. Additionally, the weak database available for the SIB (the modelling is based on one single datasheet) reduces the robustness of the results for this battery significantly, which also must be taken into account when comparing the SIB with established commercial batteries like the LIB.
Supplementary Materials
The following are available online at http://www.mdpi.com/2313-0105/5/1/10/s1, Table S1: IPP and exchange rate 2010–2017; Table S2: Prices of metals and other components according to USGS; Table S3: Precursor materials for the cathode active material synthesis; Table S4: Composition of the electrolytes used for the SIB and LIB; Table S5: Cell dimensioning parameters used for the base case assessment; Table S6: Mass balance by cell components for the battery cells based on different literature sources; Table S7: Parameters used for the calculation of the battery cell energy density.
Author Contributions
J.F.P.: Conceptual idea; supervision; verification, updating and visualization of results; writing of manuscript. A.P.C.: literature research; data collection; creation of modelling framework; BatPaC adaptation; calculation of results. M.W.: funding, supervision, proofreading.
Funding
The research received no other external funding.
Acknowledgments
The authors acknowledge the basic funding of the Helmholtz Society. The authors gratefully thank also Daniel Buchholz for his valuable input and fruitful discussions on earlier versions of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CMC | Carboxymethyl cellulose (aqueous binder for electrode active material) |
| DMC | Dimethyl carbonate (electrolyte solvent) |
| EC | Ethylene carbonate (electrolyte solvent) |
| IPP | Industrial Producer Price Index |
| LFP | Lithium-iron-phosphate (cathode active material) |
| LIB | Lithium-ion battery |
| NMC | Lithium-nickel-manganese-cobalt-oxide (cathode active material) |
| NMMT | Sodium-nickel-manganese-magnesium-titanium-oxide (cathode active material) |
| NMP | N-Methyl-2-pyrrolidone (organic solvent for active material processing) |
| PVdF | Polyvinylidene fluoride (organic binder for electrode active material) |
| SIB | Sodium-ion battery |
References
- Kim, H.; Kim, H.; Ding, Z.; Lee, M.H.; Lim, K.; Yoon, G.; Kang, K. Recent Progress in Electrode Materials for Sodium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1600943. [Google Scholar] [CrossRef]
- Baumann, M.; Peters, J.F.; Weil, M.; Grunwald, A. CO2 Footprint and Life-Cycle Costs of Electrochemical Energy Storage for Stationary Grid Applications. Energy Technol. 2017, 5, 1071–1083. [Google Scholar] [CrossRef]
- Battke, B.; Schmidt, T.S.; Grosspietsch, D.; Hoffmann, V.H. A review and probabilistic model of lifecycle costs of stationary batteries in multiple applications. Renew. Sustain. Energy Rev. 2013, 25, 240–250. [Google Scholar] [CrossRef]
- CARMEN eV. Marktübersicht Batteriespeicher; Centrales Agrar-Rohstoff Marketing- und Energie-Netzwerk: Straubing, Germany, 2017. [Google Scholar]
- Weil, M.; Peters, J.F.; Baumann, M.J.; Dura, H.; Zimmermann, B.M. Elektrochemische Energiespeicher für mobile Anwendungen im Fokus der Systemanalyse. Tech. Theor. Prax. 2015, 24, 20–29. [Google Scholar]
- Weil, M.; Tübke, J. Energiespeicher für Energiewende und Elektromobilität. Entwicklungen, Herausforderungen und systemische Analysen. Tech. Theor. Prax. 2015, 24, 4–9. [Google Scholar]
- Peters, J.F.; Weil, M. A Critical Assessment of the Resource Depletion Potential of Current and Future Lithium-Ion Batteries. Resources 2016, 5, 46. [Google Scholar] [CrossRef]
- Vaalma, C.; Buchholz, D.; Weil, M.; Passerini, S. A cost and resource analysis of sodium-ion batteries. Nat. Rev. Mater. 2018, 3, 18013. [Google Scholar] [CrossRef]
- Pan, H.; Hu, Y.-S.; Chen, L. Room-temperature stationary sodium-ion batteries for large-scale electric energy storage. Energy Environ. Sci. 2013, 6, 2338–2360. [Google Scholar] [CrossRef]
- Palomares, V.; Serras, P.; Villaluenga, I.; Hueso, K.B.; Carretero-González, J.; Rojo, T. Na-ion batteries, recent advances and present challenges to become low cost energy storage systems. Energy Environ. Sci. 2012, 5, 5884–5901. [Google Scholar] [CrossRef]
- Barker, J.; Heap, R.; Roche, N.; Tan, C.; Sayers, R.; Liu, Y. Low Cost Na-ion Battery Technology. In Proceedings of the 224th ECS Meeting, San Francisco, CA, USA, 27 October–1 November 2013. [Google Scholar]
- BCC Research. Global Market for Sodium-Ion Batteries to Nearly Triple in Value by 2022; BCC Market Research Reports; BCC Research LLC: Wellesley, MA, USA, 18 January 2018. [Google Scholar]
- Barker, J.; Heap, R.; Roche, N.; Tan, C.; Sayers, R.; Liu, Y. Low Cost Na-Ion Battery Technology; Faradion Limited: Sheffield, UK, 2014. [Google Scholar]
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef]
- Peters, J.; Buchholz, D.; Passerini, S.; Weil, M. Life cycle assessment of sodium-ion batteries. Energy Environ. Sci. 2016, 9, 1744–1751. [Google Scholar] [CrossRef]
- Daniel, C.; Besenhard, J.O. Handbook of Battery Materials; John Wiley & Sons: Weinheim, Germany, 2012. [Google Scholar]
- Nelson, P.A.; Gallagher, K.G.; Bloom, I.; Dees, D.W. Modeling the Performance and Cost of Lithium-Ion Batteries for Electric-Drive Vehicles; Argonne National Laboratories (ANL), Chemical Sciences and Engineering Division: Lemont, IL, USA, 2012. [Google Scholar]
- Eurostat. Producer Prices in Industry, Non Domestic Market-Annual Data; Statistical Office of the European Union, European Commission: Brussels, Belgium, 2017. [Google Scholar]
- Eurostat. EUR Exchange Rates Versus National Currencies; Statistical Office of the European Union, European Commission: Brussels, Belgium, 2017. [Google Scholar]
- Barker, J.; Heap, R. Doped nickelate compounds. International Patent Application No. WO2014/009710 A1, 16 January 2014. [Google Scholar]
- Patry, G.; Romagny, A.; Martinet, S.; Froelich, D. Cost modeling of lithium-ion battery cells for automotive applications. Energy Sci. Eng. 2015, 3, 71–82. [Google Scholar] [CrossRef]
- Petri, R.; Giebel, T.; Zhang, B.; Schünemann, J.-H.; Herrmann, C. Material cost model for innovative li-ion battery cells in electric vehicle applications. Int. J. Precis. Eng. Manuf.-Green Technol. 2015, 2, 263–268. [Google Scholar] [CrossRef]
- Ciez, R.E.; Whitacre, J.F. Comparison between cylindrical and prismatic lithium-ion cell costs using a process based cost model. J. Power Sources 2017, 340, 273–281. [Google Scholar] [CrossRef]
- Renard, F. 2020 cathode materials cost competition for large scale applications and promising LFP best-in-class performer in term of price per kWh. In Proceedings of the International Conference on Olivines for Rechargeable Batteries, Montreal, QC, Canada, 25–28 May 2014. [Google Scholar]
- Rempel, J.; Barnett, B.; Hyung, Y. PHEV Battery Cost Assessment. In Proceedings of the TIAX LLC, Lexington, KY, USA, 14 May 2013. [Google Scholar]
- Wood, D.L., III; Li, J.; Daniel, C. Prospects for reducing the processing cost of lithium ion batteries. J. Power Sources 2015, 275, 234–242. [Google Scholar] [CrossRef]
- Slater, M.D.; Kim, D.; Lee, E.; Johnson, C.S. Sodium-ion batteries. Adv. Funct. Mater. 2013, 23, 947–958. [Google Scholar] [CrossRef]
- US Geological Survey. Mineral Commodity Summaries 2016; US Geological Survey: Reston, VA, USA, 2017; ISBN 978-1-4113-4011-4.
- Irisarri, E.; Ponrouch, A.; Palacin, M.R. Review—Hard Carbon Negative Electrode Materials for Sodium-Ion Batteries. J. Electrochem. Soc. 2015, 162, A2476–A2482. [Google Scholar] [CrossRef]
- Olontsev, V.F.; Borisova, I.A.; Sazonova, E.A. Pyrolysis of coconut shells for the manufacture of carbon sorbents. Solid Fuel Chem. 2011, 45, 44–49. [Google Scholar] [CrossRef]
- ECN-Biomass Phyllis 2, Database for Biomass and Waste. 2017. Available online: http://www.ecn.nl/phyllis2/ (accessed on 5 October 2017).
- Jungbluth, N. Ecoinvent report No. 4—Erdöl. In Sachbilanzen von Energiesystemen: Grundlagen für den ökologischen Vergleich von Energiesystemen und den Einbezug von Energiesystemen in Ökobilanzen für die Schweiz; Dones, R., Ed.; Swiss Centre for Life Cycle Inventories: Dübendorf, Switzerland, 2007. [Google Scholar]
- Index Mundi. Commodity Price Indices. 2017. Available online: http://www.indexmundi.com/commodities/ (accessed on 30 November 2018).
- CDA. Coconut Development Authority—Info Portal. Ministry of Plantation Industries from Sri Lanka. 2017. Available online: http://www.cda.lk/web/ (accessed on 15 October 2018).
- SCI. Sublime China Information. Chinese Commodity Market Statistics. 2017. Available online: http://intl.sci99.com/# (accessed on 10 May 2018).
- Eurostat. Energy Statistics; Statistical Office of the European Union, European Commission: Brussels, Belgium, 2017. [Google Scholar]
- Ross, D. Cryocoolers 11; Springer: Berlin, Germany, 2001. [Google Scholar]
- Fan, K. The Physics Factbook—Price of Liquid Nitrogen. 2007. Available online: http://hypertextbook.com/facts/2007/KarenFan.shtml (accessed on 10 April 2017).
- O’Brien, C.; Heravi, B. Irish water charges cheapest in Europe under revised package. The Irish Time, 19 November 2014. [Google Scholar]
- Ellingsen, L.A.-W.; Majeau-Bettez, G.; Singh, B.; Srivastava, A.K.; Valøen, L.O.; Strømman, A.H. Life Cycle Assessment of a Lithium-Ion Battery Vehicle Pack: LCA of a Li-Ion Battery Vehicle Pack. J. Ind. Ecol. 2014, 18, 113–124. [Google Scholar] [CrossRef]
- Warner, J.T. The Handbook of Lithium-Ion Battery Pack Design: Chemistry, Components, Types and Terminology; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 978-0-12-801456-1. [Google Scholar]
- Pistoia, G. (Ed.) Lithium-Ion Batteries. Advances and Applications; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Ponrouch, A.; Marchante, E.; Courty, M.; Tarascon, J.-M.; Palacín, M.R. In search of an optimized electrolyte for Na-ion batteries. Energy Environ. Sci. 2012, 5, 8572. [Google Scholar] [CrossRef]
- Ponrouch, A.; Monti, D.; Boschin, A.; Steen, B.; Johansson, P.; Palacín, M.R. Non-aqueous electrolytes for sodium-ion batteries. J. Mater. Chem. A 2014, 3, 22–42. [Google Scholar] [CrossRef]
- Alibaba Group. 18650, 26650, 32650 Cylinder Cell Case with PTC for Lithium Battery. Alibaba.com. 6 March 2018. Available online: https://www.alibaba.com/product-detail/18650-26650-32650-Cylinder-Cell-Case_60485442268.html?spm=a2700.7724857/B.main07.18.38f76528zEF7cY&s=p (accessed on 30 November 2018).
- Alibaba Group. 18650 Cylinder Cell Case with Anti-Explosive Cap and Insulation O-Ring—100 Pcs/package—EQ-Lib-18650. Alibaba.com. 6 March 2018. Available online: https://www.alibaba.com/product-detail/18650-Cylinder-Cell-Case-with-Anti_60342001362.html?spm=a2700.7724838.2017115.100.530523e2fcGCg1 (accessed on 30 November 2018).
- ICIS Chemical Commodities Prices, Markets & Analysis. 2017. Available online: https://www.icis.com/chemicals/ (accessed on 10 May 2018).
- Golubkov, A.W.; Fuchs, D.; Wagner, J.; Wiltsche, H.; Stangl, C.; Fauler, G.; Voitic, G.; Thaler, A.; Hacker, V. Thermal-runaway experiments on consumer Li-ion batteries with metal-oxide and olivin-type cathodes. RSC Adv. 2014, 4, 3633–3642. [Google Scholar] [CrossRef]
- Majeau-Bettez, G.; Hawkins, T.R.; Strømman, A.H. Life Cycle Environmental Assessment of Lithium-Ion and Nickel Metal Hydride Batteries for Plug-In Hybrid and Battery Electric Vehicles. Environ. Sci. Technol. 2011, 45, 4548–4554. [Google Scholar] [CrossRef] [PubMed]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Eurostat. Wages and Labour Costs; Statistical Office of the European Union, European Commission: Brussels, Belgium, 2017. [Google Scholar]
- Roberts, S.; Kendrick, E. The re-emergence of sodium ion batteries: Testing, processing, and manufacturability. Nanotechnol. Sci. Appl. 2018, 11, 23–33. [Google Scholar]
- Berckmans, G.; Messagie, M.; Smekens, J.; Omar, N.; Vanhaverbeke, L.; Van Mierlo, J.; Berckmans, G.; Messagie, M.; Smekens, J.; Omar, N.; et al. Cost Projection of State of the Art Lithium-Ion Batteries for Electric Vehicles Up to 2030. Energies 2017, 10, 1314. [Google Scholar] [CrossRef]
- Sripad, S.; Viswanathan, V. Evaluation of Current, Future, and Beyond Li-Ion Batteries for the Electrification of Light Commercial Vehicles: Challenges and Opportunities. J. Electrochem. Soc. 2017, 164, E3635–E3646. [Google Scholar] [CrossRef]
- Wu, L.; Buchholz, D.; Vaalma, C.; Giffin, G.A.; Passerini, S. Apple-Biowaste-Derived Hard Carbon as a Powerful Anode Material for Na-Ion Batteries. ChemElectroChem 2015, 292–298. [Google Scholar] [CrossRef]
- Chen, L.; Fiore, M.; Wang, J.E.; Ruffo, R.; Kim, D.-K.; Longoni, G. Readiness Level of Sodium-Ion Battery Technology: A Materials Review. Adv. Sustain. Syst. 2018, 2, 1700153. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).