Sodium Rechargeable Batteries with Electrolytes Based on Nafion Membranes Intercalated by Mixtures of Organic Solvents
Abstract
1. Introduction
2. Results
2.1. The Composition of the Materials Obtained
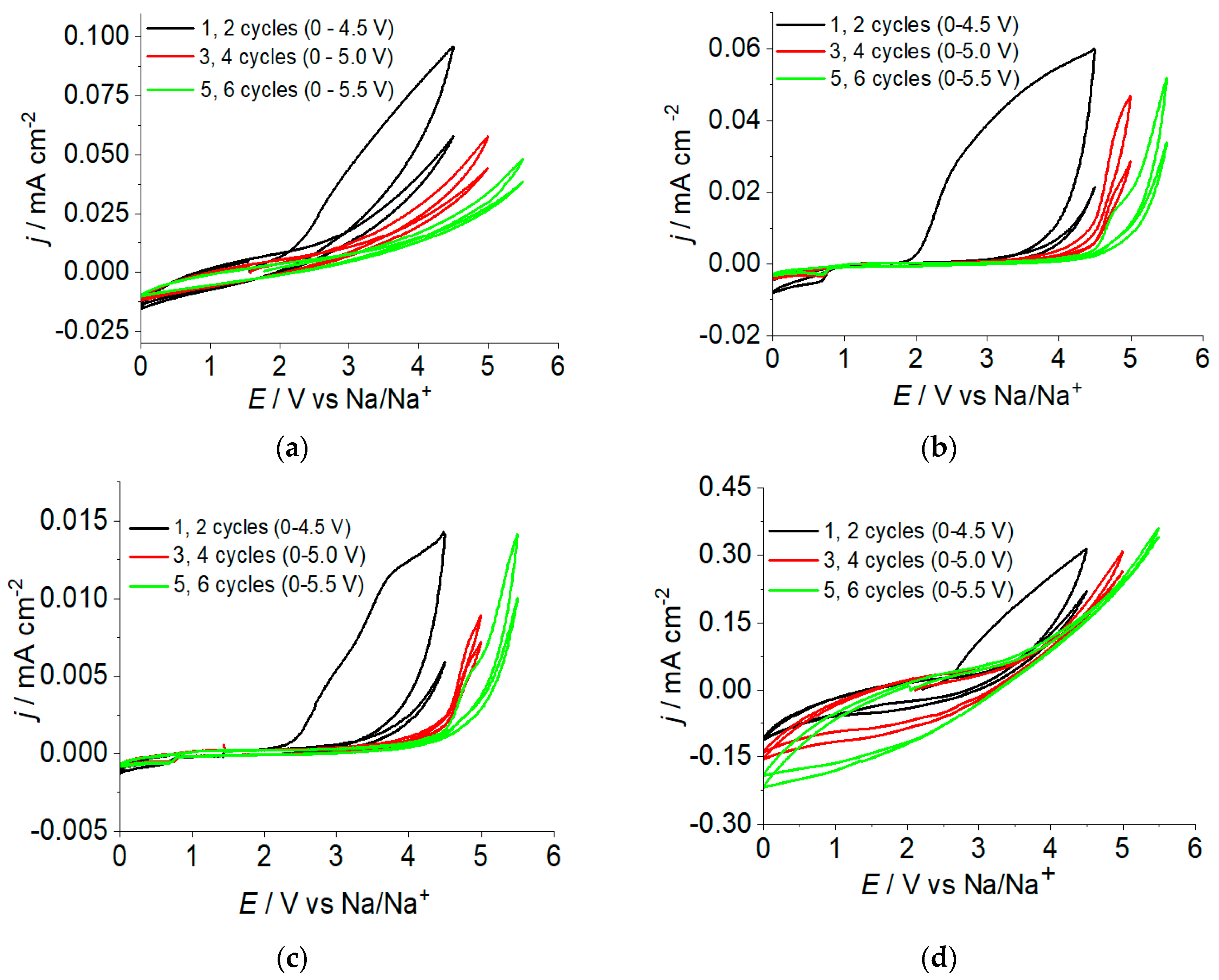
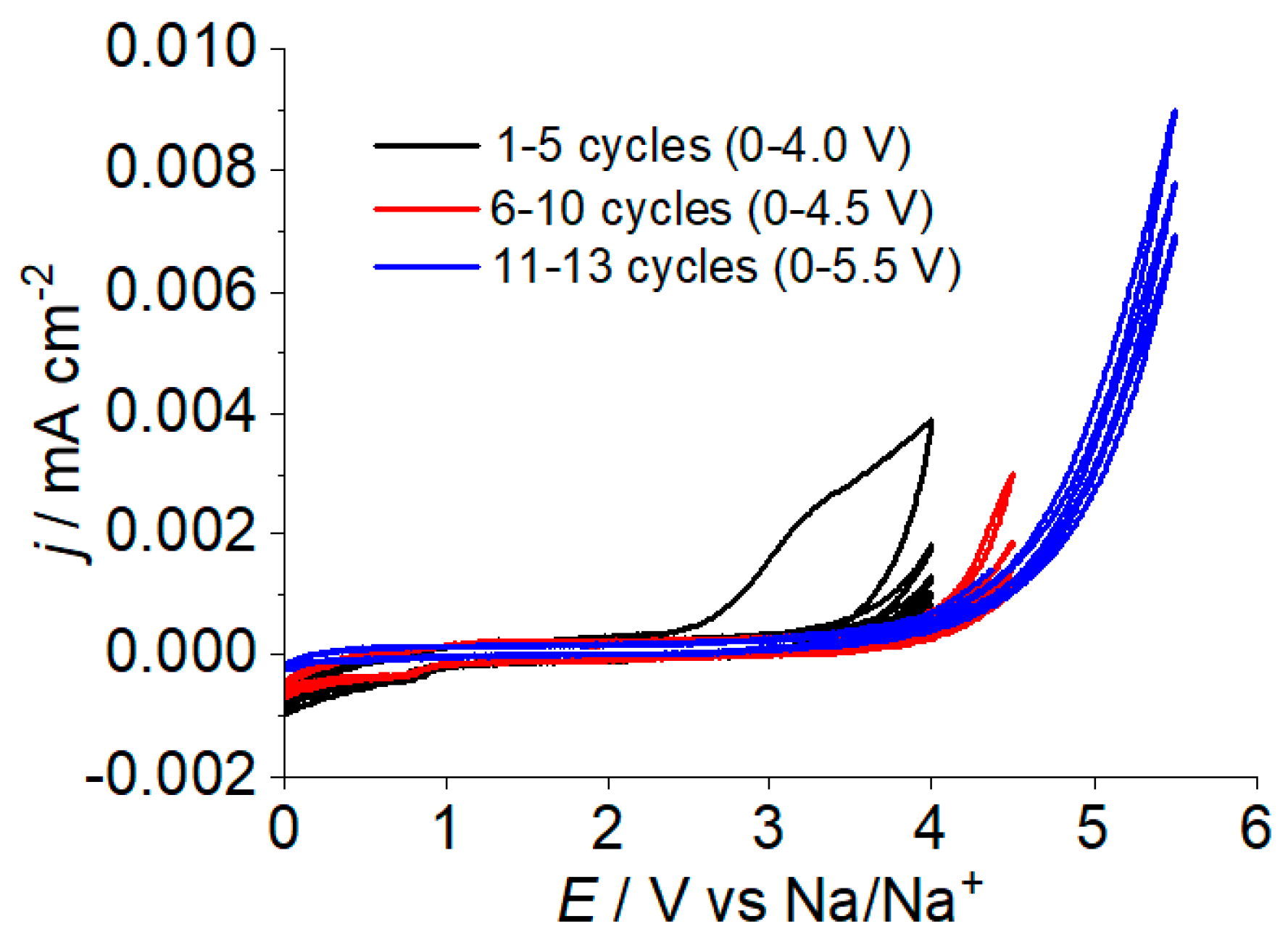
2.2. The Electrochemical Stability of Polymer Electrolyte Liquid Phase
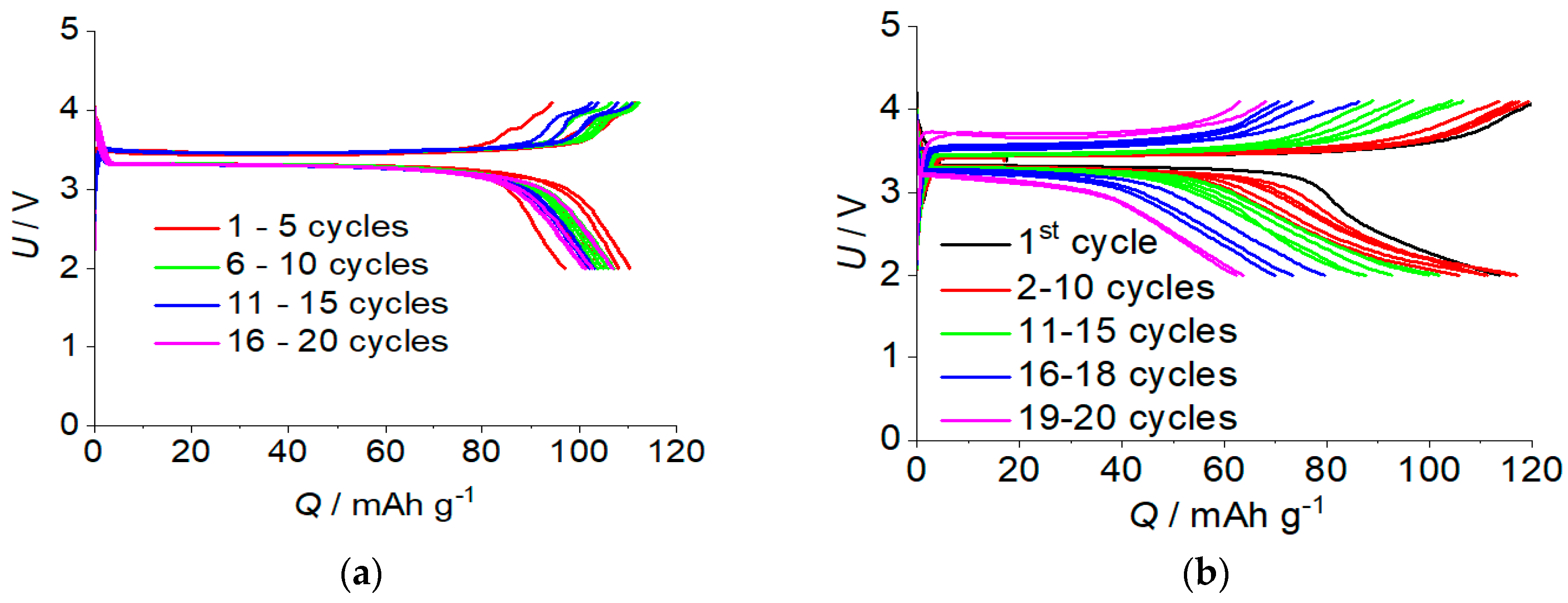
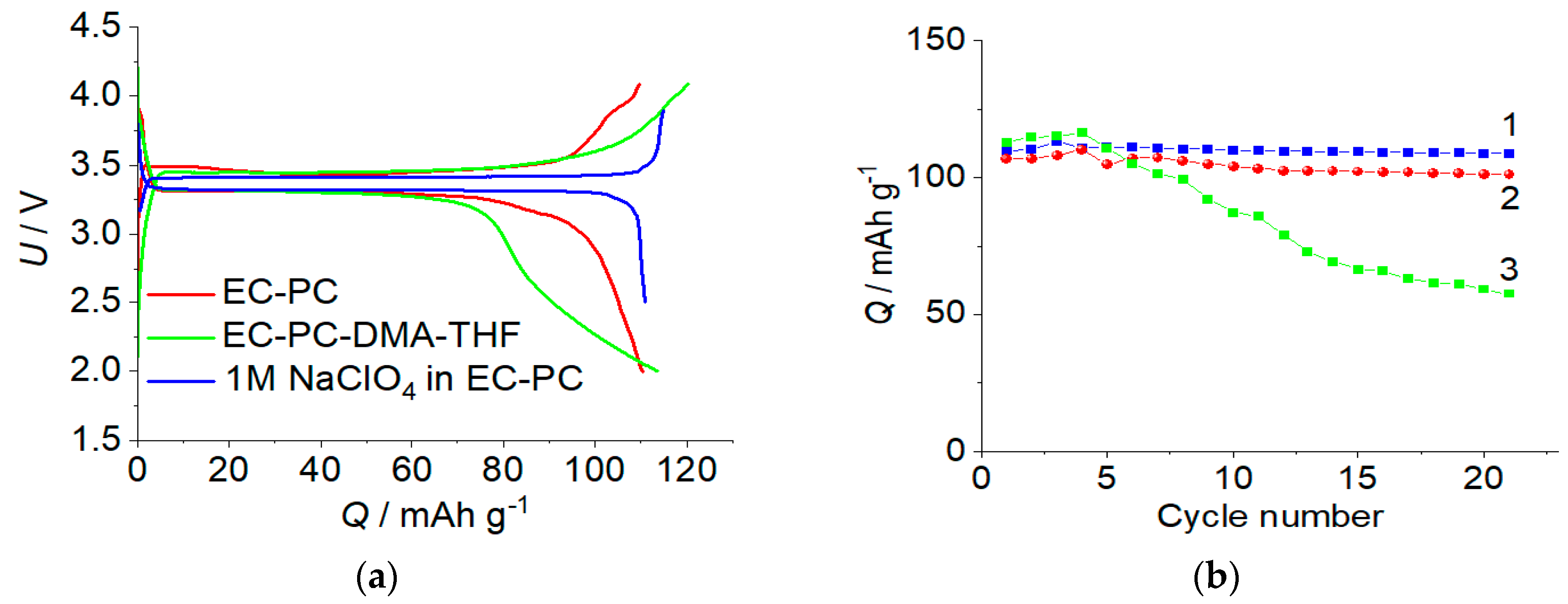
2.3. The Rechargeable Battery with a Polymer Electrolyte
3. Discussions
4. Materials and Methods
4.1. Preparation of Electrolytes Based on a Nafion Membrane
4.2. The Binder Preparation
4.3. The Cathode Material Synthesis
4.4. The Materials Characterization
4.5. The Electrochemical Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kundu, D.; Talaie, E.; Duffort, V.; Nazar, L.F. The emerging chemistry of sodium ion batteries for electrochemical energy storage. Angew. Chem. Int. Ed. Engl. 2015, 54, 3431–3448. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y.; Myung, S.T.; Sun, Y.K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef] [PubMed]
- Skundin, A.M.; Kulova, T.L.; Yaroslavtsev, A.B. Sodium-ion batteries (a review). Russ. J. Electrochem. 2018, 54, 113–152. [Google Scholar] [CrossRef]
- Wang, L.P.; Yu, L.; Wang, X.; Srinivasan, M.; Xu, Z.J. Recent developments in electrode materials for sodium-ion batteries. J. Mater. Chem. A 2015, 3, 9353–9378. [Google Scholar] [CrossRef]
- Agrawal, R.C.; Pandey, G.P. Solid polymer electrolytes: Materials designing and all-solid-state battery applications: an overview. J. Phys. D Appl. Phys. 2008, 41, 223001. [Google Scholar] [CrossRef]
- Gao, H.; Xue, L.; Xin, S.; Park, K.; Goodenough, J.B. A plastic-crystal electrolyte interphase for all-solid-state sodium batteries. Angew. Chem. Int. Ed. Engl. 2017, 56, 5541–5545. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Liu, W.; Tan, L.; Liao, X.; Li, L. Sodium-ion batteries using ion exchange membranes as electrolytes and separator. Chem. Commun. 2013, 49, 11740–11742. [Google Scholar] [CrossRef] [PubMed]
- Kulova, T.L.; Skundin, A.M. Polymer electrolytes for sodium-ion batteries. Electrochem. Energy 2018, 18, 22–48. [Google Scholar]
- Vignarooban, K.; Kushagra, R.; Elango, A.; Badami, P.; Mellander, B.E.; Xu, X.; Tucker, T.G.; Nam, C.; Kannan, A.M. Current trends and future challenges of electrolytes for sodium-ion batteries. Int. J. Hydrogen Energy 2016, 41, 2829–2846. [Google Scholar] [CrossRef]
- Cao, C.; Liu, W.; Tan, L.; Liao, X.; Li, L. Sodium-ion batteries using ion exchange membranes as electrolytes and separators. Chem. Commun. (Camb.) 2013, 49, 11740–11742. [Google Scholar] [CrossRef]
- Yaroslavtsev, A.B. Ion transport in heterogeneous solid systems. Russ. J. Inorg. Chem. 2000, 45, S249–S267. [Google Scholar]
- Armand, M. Polymer electrolytes—An overview. Solid State Ion. 1983, 9–10, 745–754. [Google Scholar] [CrossRef]
- Yaroslavtsev, A.B. Solid electrolytes: Main prospects of research and development. 2016, 85, 1255–1276. Russ. Chem. Rev. 2016, 85, 1255–1276. [Google Scholar] [CrossRef]
- Phair, J.W.; Badwal, S.P.S. Review of proton conductors for hydrogen separation. Ionics 2006, 12, 103–115. [Google Scholar] [CrossRef]
- Karpenko-Jereb, L.V.; Kelterer, A.-M.; Berezina, N.P.; Pimenov, A.V. Conductometric and computational study of cationic polymer membranes in H+ and Na+-forms at various hydration levels. J. Membr. Sci. 2013, 444, 127–138. [Google Scholar] [CrossRef]
- Safronova, E.; Golubenko, D.; Pourcelly, G.; Yaroslavtsev, A. Mechanical properties and influence of straining on ion conductivity of perfluorosulfonic acid Nafion®-type membranes depending on water uptake. J. Membr. Sci. 2015, 473, 218–225. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, Y.; Liu, S.; Li, L.; Zhang, Y. High performance of lithium-ion polymer battery based on non-aqueous lithiated perfluorinated sulfonic ion-exchange membranes. Energy Environ. Sci. 2012, 5, 5690–5693. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, Z.; Tan, L.; Li, L. Ion exchange membranes as electrolyte for high performance Li-ion batteries. Energy Environ. Sci. 2012, 5, 9007–9013. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, L.; Li, L. Ion exchange membranes as electrolyte to improve high temperature capacity retention of LiMn2O4 cathode lithium-ion batteries. Chem. Commun. 2012, 48, 9858–9860. [Google Scholar] [CrossRef]
- Liang, H.Y.; Qiu, X.P.; Zhang, S.C.; Zhu, W.T.; Chen, L.Q. Study of lithiated Nafion ionomer for lithium batteries. J. Appl. Electrochem. 2004, 34, 1211–1214. [Google Scholar] [CrossRef]
- Voropaeva, D.Y.; Novikova, S.A.; Kulova, T.L.; Yaroslavtsev, A.B. Conductivity of Nafion-117 membranes intercalated by polar aprotonic solvents. Ionics 2018, 24, 1685–1692. [Google Scholar] [CrossRef]
- Jin, Z.; Xie, K.; Hong, X.; Hu, Z.; Liu, X. Application of lithiated Nafion ionomer film as functional separator for lithium sulfur cells. J. Power Sources 2012, 218, 163–167. [Google Scholar] [CrossRef]
- Jin, Z.; Xie, K.; Hong, X. Electrochemical performance of lithium/sulfur batteries using perfluorinated ionomer electrolyte with lithium sulfonyl dicyanomethide functional groups as functional separator. RSC Adv. 2013, 3, 8889–8898. [Google Scholar] [CrossRef]
- Gao, J.; Sun, C.; Xu, L.; Chen, J.; Wang, C.; Guo, D.; Chen, H. Lithiated Nafion as polymer electrolyte for solid-state lithium sulfur batteries using carbon-sulfur composite cathode. J. Power Sources 2018, 382, 179–189. [Google Scholar] [CrossRef]
- Cao, C.; Wang, H.; Liu, W.; Liao, X.; Li, L. Nafion membranes as electrolyte and separator for sodium-ion battery. Int. J. Hydrogen Energy 2014, 39, 16110–16115. [Google Scholar] [CrossRef]
- Voropaeva, D.Y.; Novikova, S.A.; Kulova, T.L.; Yaroslavtsev, A.B. Solvation and sodium conductivity of nonaqueous polymer electrolytes based on Nafion-117 membranes and polar aprotic solvents. Solid State Ion. 2018, 324, 28–32. [Google Scholar] [CrossRef]
- Roux, B.; Karplus, M. Ion transport in a gramicidin-like channel: Dynamics and mobility. J. Phys. Chem. 1991, 95, 4856–4868. [Google Scholar] [CrossRef]
- Roux, B.; Karplus, M. Ion-transport in the gramicidin channel-free-energy of the solvated right-handed dimer in a model membrane. J. Am. Chem. Soc. 1993, 115, 3250–3262. [Google Scholar] [CrossRef]
- Klassen, J. S.; Anderson, S.G.; Blades, A.T.; Kebarle, P. Reaction enthalpies for M+L = M+ + L, Where M+ = Na+ and K+ and L = Acetamide, N-Methylacetamide, N,N-Dimethylacetamide, Glycine, and Glycylglycine, from determinations of the collision-induced dissociation thresholds. J. Phys. Chem. 1996, 100, 14218–14227. [Google Scholar] [CrossRef]
- Aboulaich, A.; Bouchet, R.; Delaizir, G.; Seznec, V.; Tortet, L.; Morcrette, M.; Rozier, P.; Tarascon, J.-M.; Viallet, V.; Dollé, M. A new approach to develop safe all-inorganic monolithic Li-ion batteries. Adv. Energy Mater. 2011, 1, 179–183. [Google Scholar] [CrossRef]
- Delaizir, G.; Viallet, V.; Aboulaich, A.; Bouchet, R.; Tortet, L.; Seznec, V.; Morcrette, M.; Tarascon, J.M.; Rozier, P.; Dollé, M. The stone age revisited: Building a monolithic inorganic lithium-ion battery. Adv. Funct. Mater. 2012, 22, 2140–2147. [Google Scholar] [CrossRef]
- Noguchi, Y.; Kobayashi, E.; Plashnitsa, L. S.; Okada, S.; Yamaki, J. Fabrication and performances of all solid-state symmetric sodium battery based on NASICON-related compounds. Electrochim. Acta 2013, 101, 59–65. [Google Scholar] [CrossRef]
- Berezina, N.P.; Timofeev, S.V.; Kononenko, N.A. Effect of conditioning techniques of perfluorinated sulphocationic membranes on their hydrophylic and electrotransport properties. J. Membr. Sci. 2002, 209, 509–518. [Google Scholar] [CrossRef]
- Chekannikov, A.; Kapaev, R.; Novikova, S.; Tabachkova, N.; Kulova, T.; Skundin, A.; Yaroslavtsev, A. Na3V2(PO4)3/C/Ag nanocomposite materials for Na-ion batteries obtained by the modified Pechini method. J. Solid State Electrochem. 2017, 21, 1615–1624. [Google Scholar] [CrossRef]
- Novikova, S.A.; Larkovich, R.V.; Chekannikov, A.A.; Kulova, T.L.; Skundin, A.M.; Yaroslavtsev, A.B. Electrical conductivity and electrochemical characteristics of Na3V2(PO4)3-based NASICON-type materials. Inorg. Mater. 2018, 54, 794–804. [Google Scholar] [CrossRef]
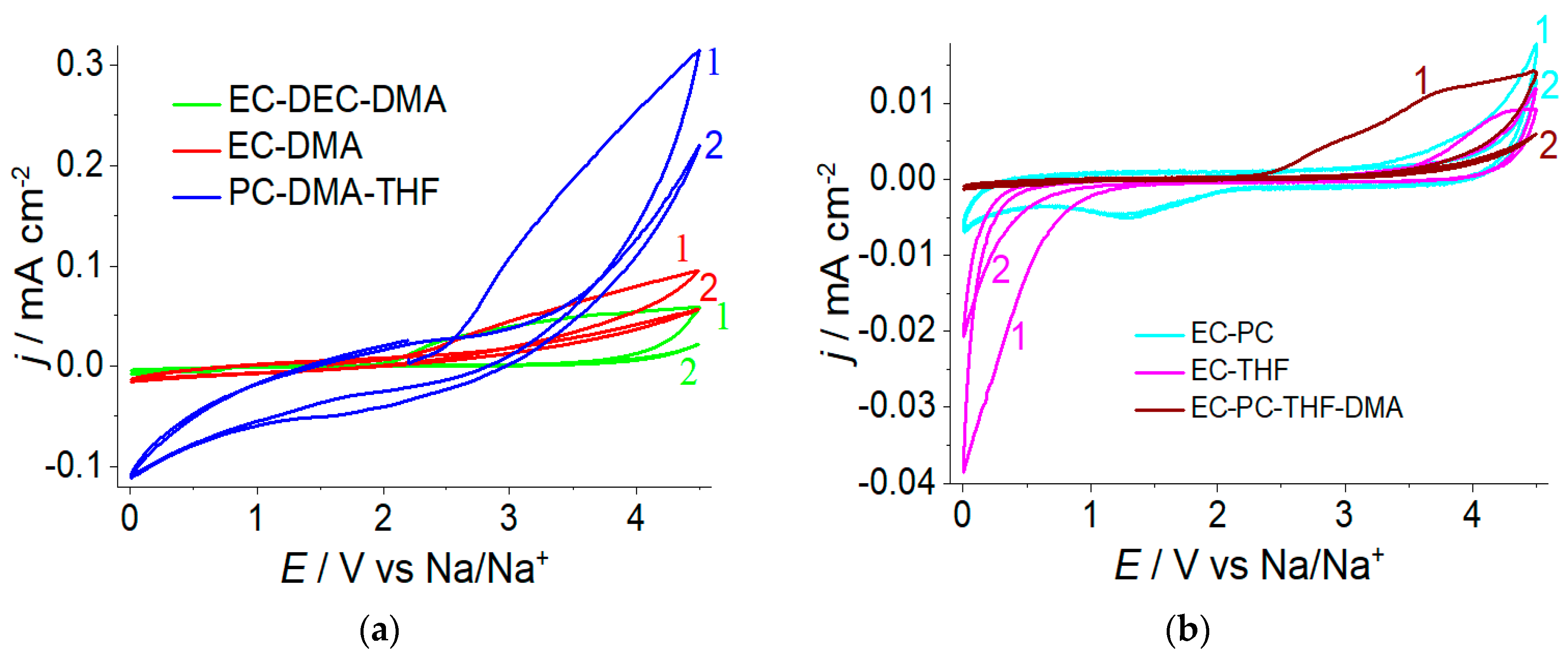
| Electrolyte | Thickness, µm | Solvent Uptake (n(Solvent/n(SO3−)) | Conductivity at 30 °C, mS·cm−1 |
|---|---|---|---|
| Nafion 117-EC-PC | 240 | 19.8 | 0.7 |
| Nafion 117-EC-PC-THF-DMA | 260 | 24.3 | 4.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulova, T.; Skundin, A.; Chekannikov, A.; Novikova, S.; Voropaeva, D.; Yaroslavtsev, A. Sodium Rechargeable Batteries with Electrolytes Based on Nafion Membranes Intercalated by Mixtures of Organic Solvents. Batteries 2018, 4, 61. https://doi.org/10.3390/batteries4040061
Kulova T, Skundin A, Chekannikov A, Novikova S, Voropaeva D, Yaroslavtsev A. Sodium Rechargeable Batteries with Electrolytes Based on Nafion Membranes Intercalated by Mixtures of Organic Solvents. Batteries. 2018; 4(4):61. https://doi.org/10.3390/batteries4040061
Chicago/Turabian StyleKulova, Tatiana, Alexander Skundin, Andrey Chekannikov, Svetlana Novikova, Daria Voropaeva, and Andrey Yaroslavtsev. 2018. "Sodium Rechargeable Batteries with Electrolytes Based on Nafion Membranes Intercalated by Mixtures of Organic Solvents" Batteries 4, no. 4: 61. https://doi.org/10.3390/batteries4040061
APA StyleKulova, T., Skundin, A., Chekannikov, A., Novikova, S., Voropaeva, D., & Yaroslavtsev, A. (2018). Sodium Rechargeable Batteries with Electrolytes Based on Nafion Membranes Intercalated by Mixtures of Organic Solvents. Batteries, 4(4), 61. https://doi.org/10.3390/batteries4040061










