Factors Affecting the Effectiveness of Bioelectrochemical System Applications: Data Synthesis and Meta-Analysis
Abstract
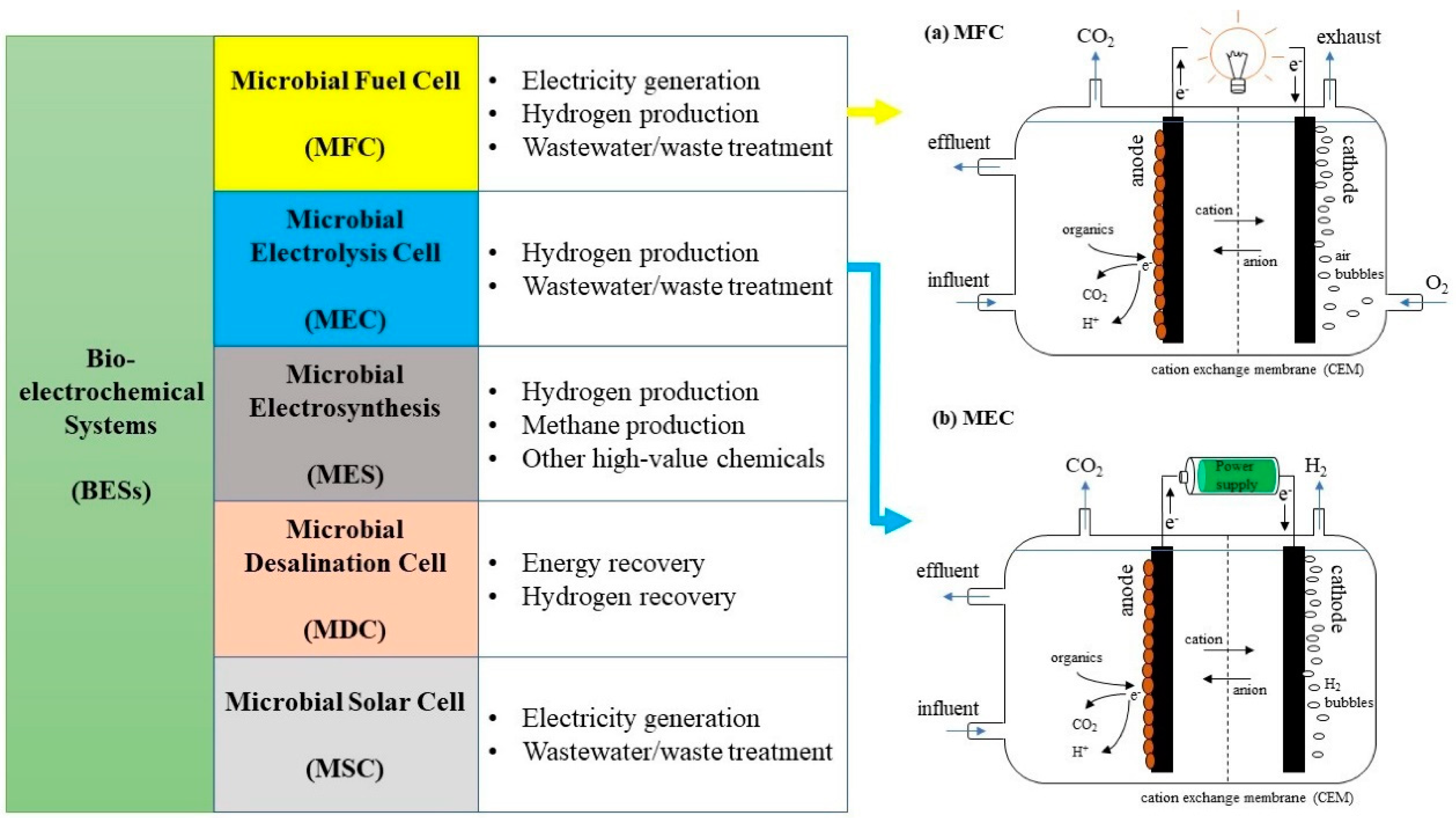
1. Introduction
2. Results and Discussion
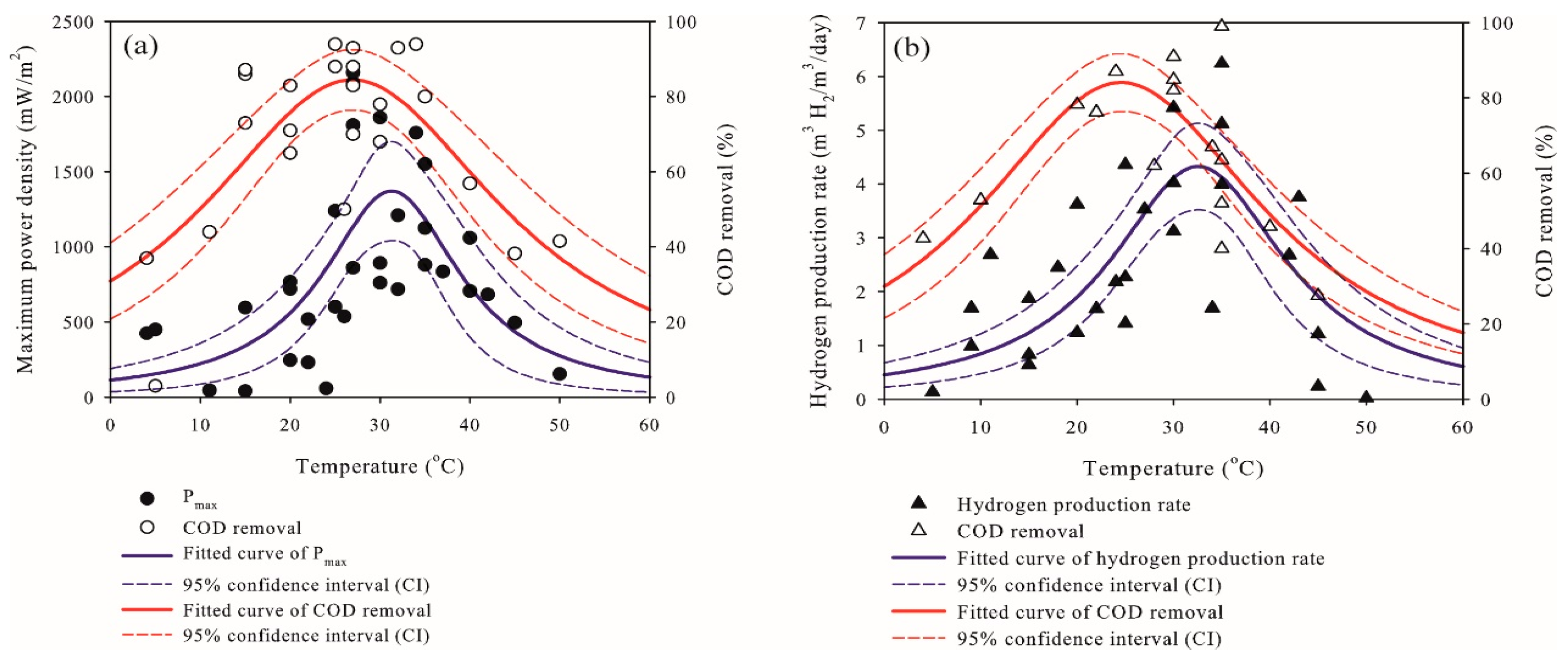
2.1. Effects of Temperature on the Performance of Microbial Fuel Cells and Microbial Electrolysis Cells
2.2. Effects of pH on the Performance of Microbial Fuel Cells and Microbial Electrolysis Cells
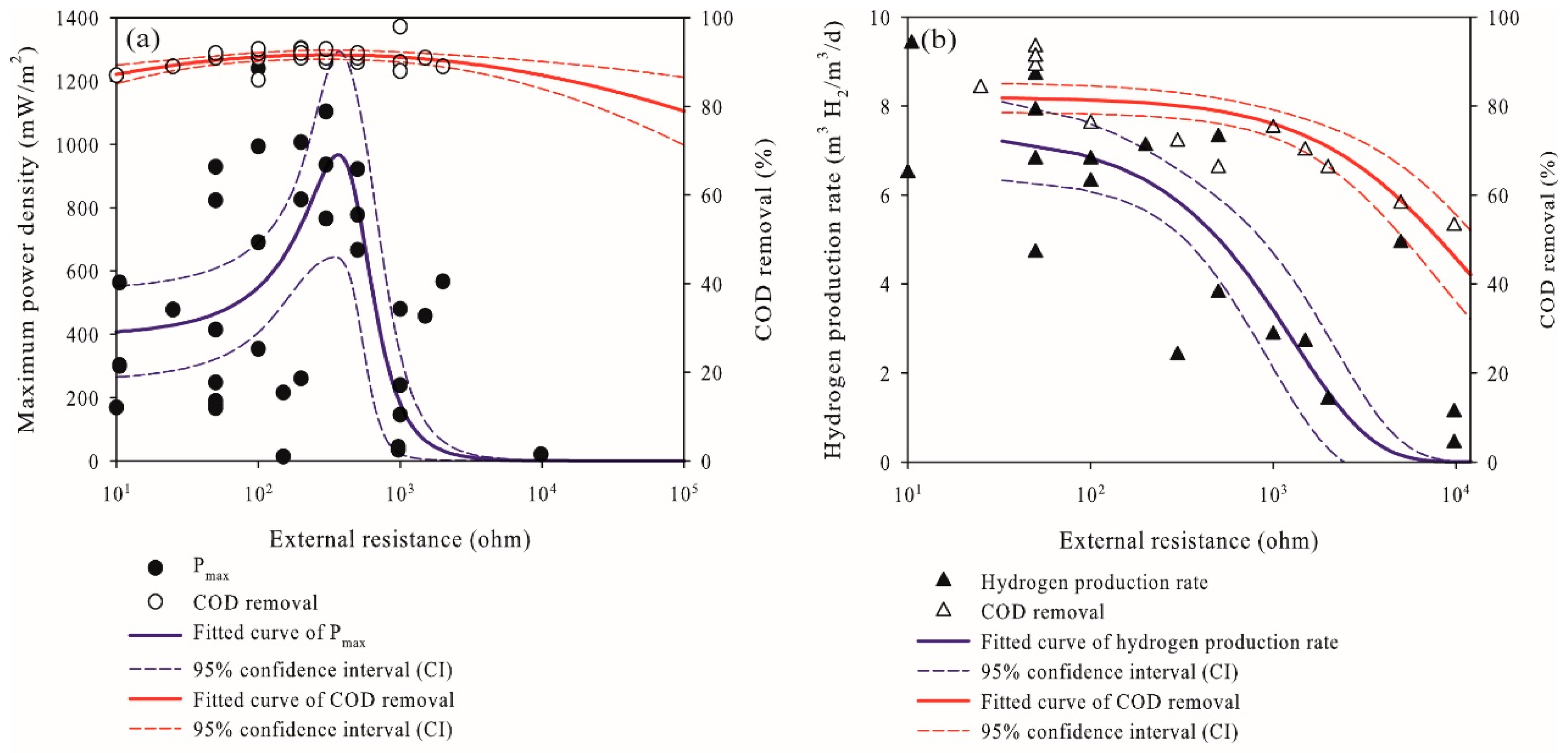
2.3. Effects of External Resistance on the Performance of Microbial Fuel Cells and Microbial Electrolysis Cells
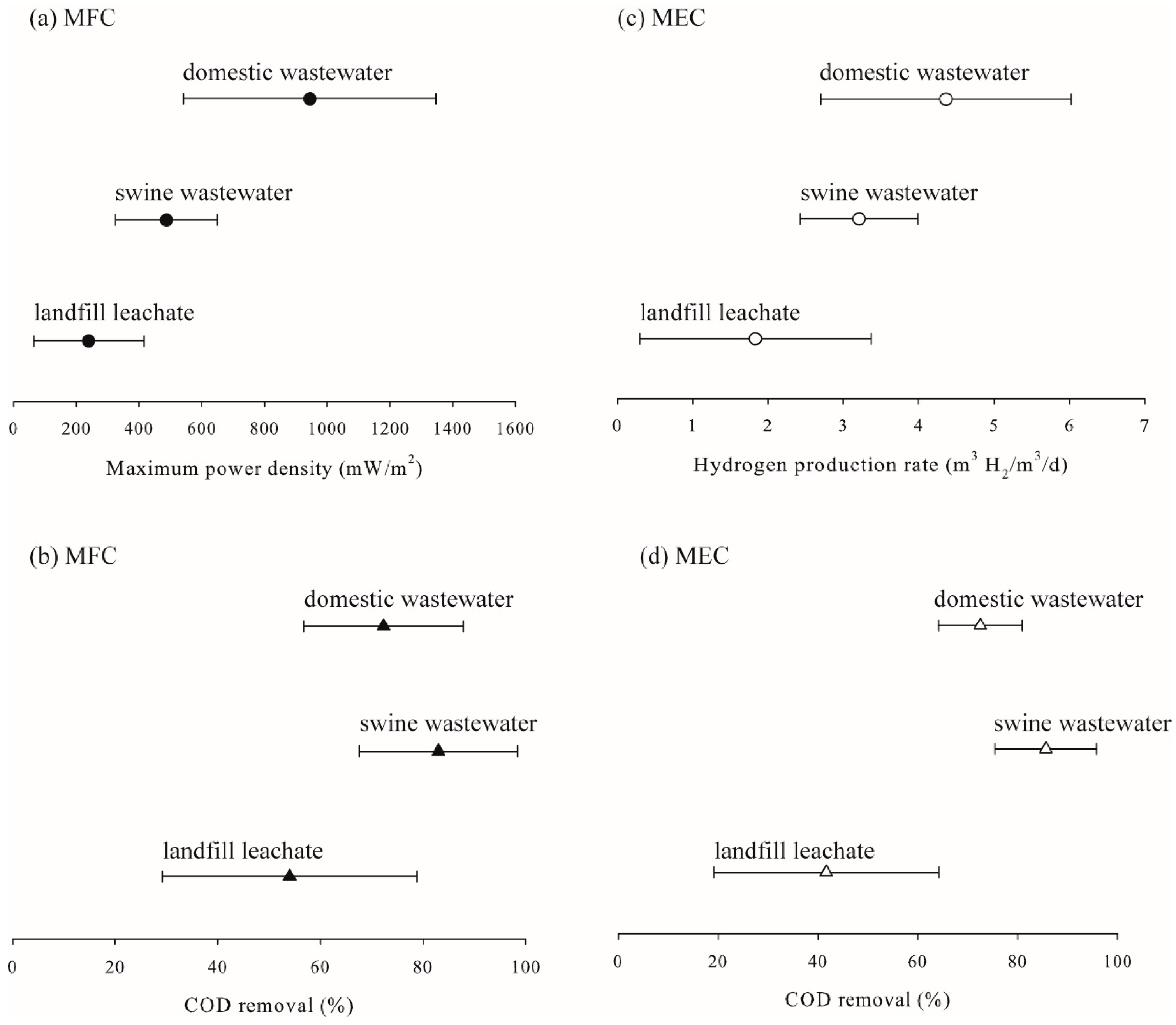
2.4. Effects of Substrate Type on the Performance of Microbial Fuel Cells and Microbial Electrolysis Cells
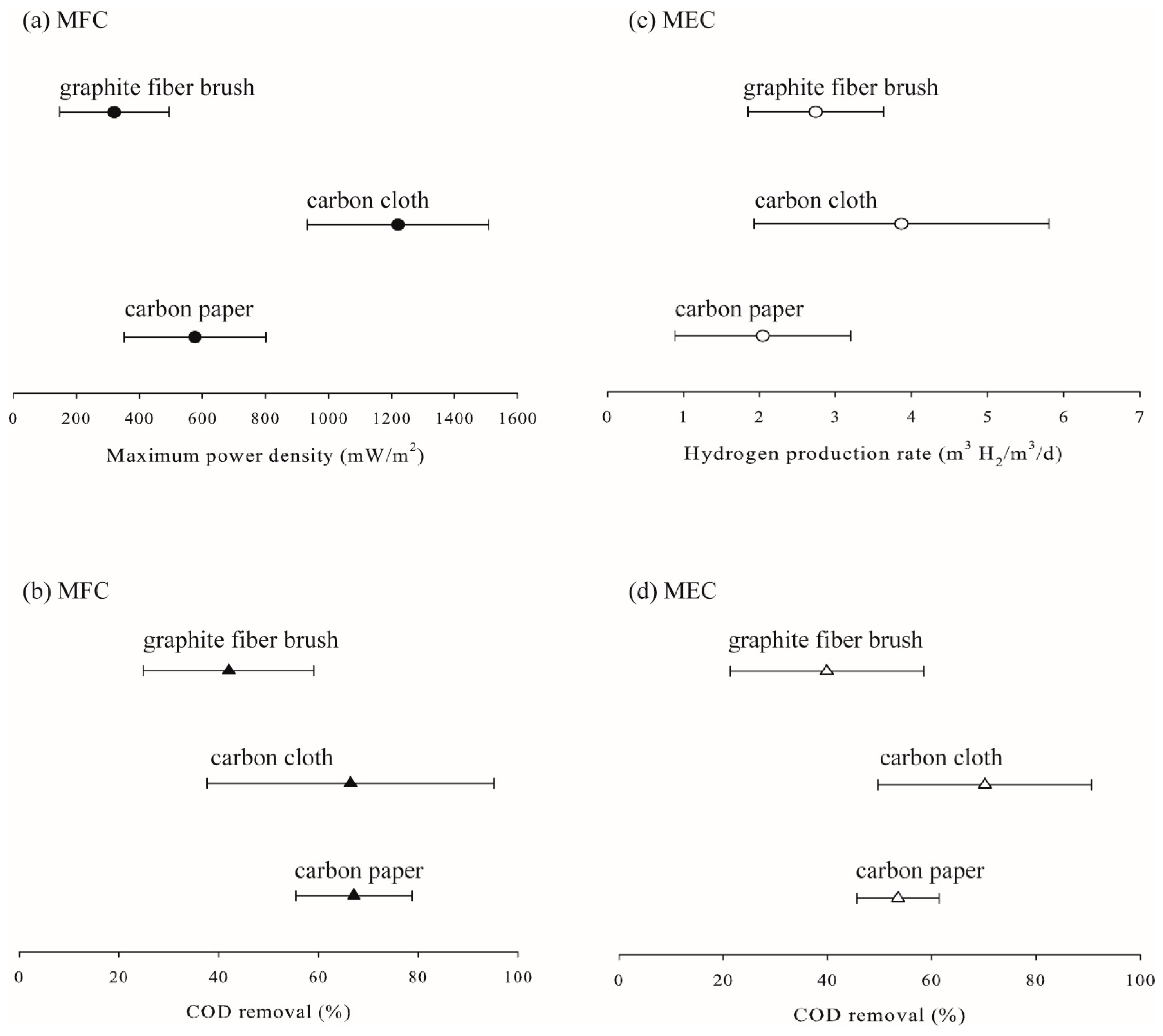
2.5. Effects of Electrode Type on the Performance of Microbial Fuel Cells and Microbial Electrolysis Cells
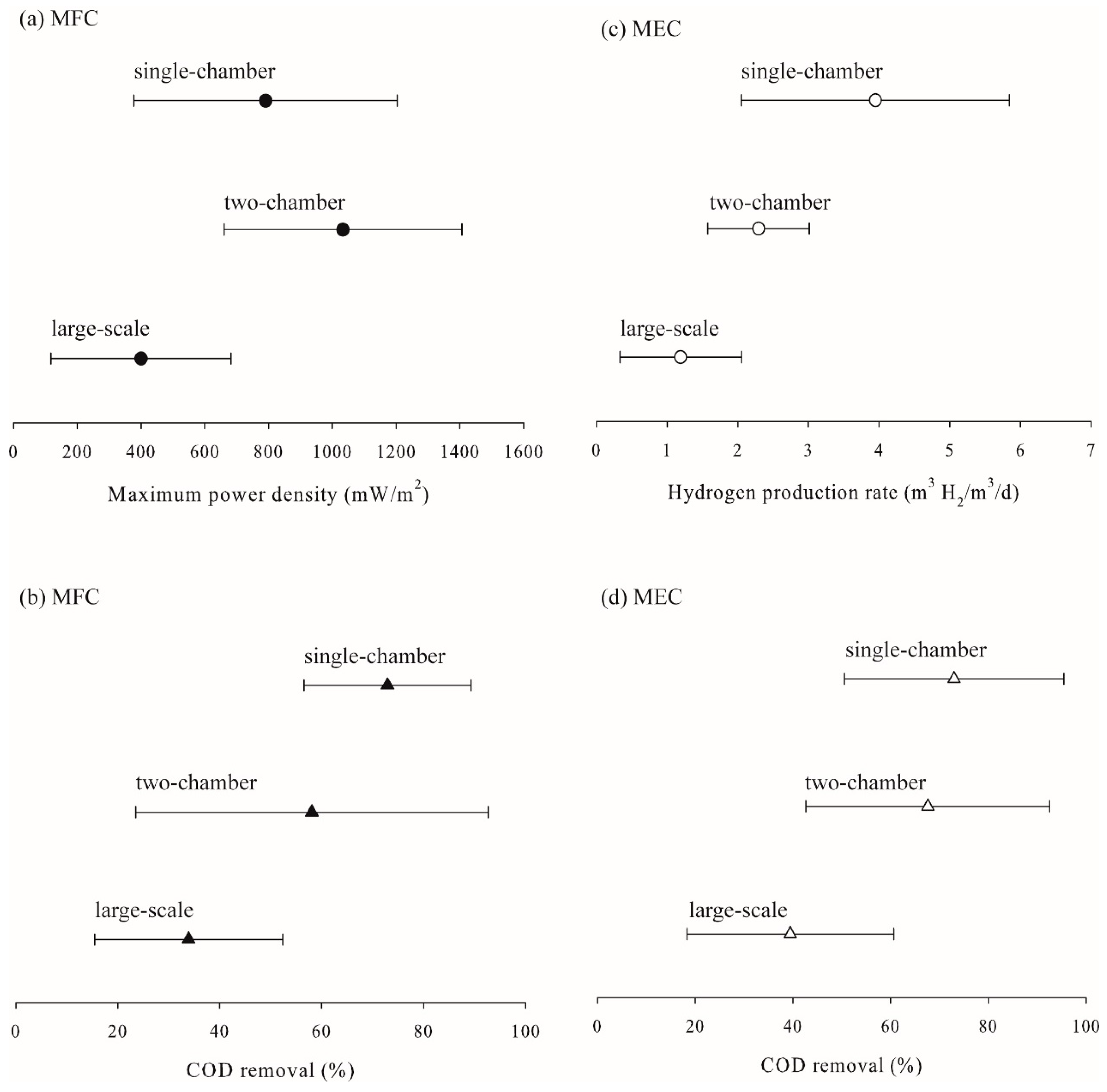
2.6. Effects of Reactor Configuration on the Performance of Microbial Fuel Cells and Microbial Electrolysis Cells
3. Implications for Commercial-Scale Applications
4. Methods
4.1. Data Sources
4.2. Data Grouping and Processing
4.3. Curve Fitting
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bajracharya, S.; Srikanth, S.; Mohanakrishna, G.; Zacharia, R.; Strik, D.P.B.T.B.; Pant, D. Biotransformation of carbon dioxide in bioelectrochemical systems: State of the art and future prospects. J. Power Sources 2017, 356, 256–273. [Google Scholar] [CrossRef]
- Wang, H.M.; Ren, Z.Y.J. A comprehensive review of microbial electrochemical systems as a platform technology. Biotechnol. Adv. 2013, 31, 1796–1807. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E. Exoelectrogenic bacteria that power microbial fuel cells. Nat. Rev. Microbiol. 2009, 7, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Li, S.M.; Chen, G. Effects of evolving quality of landfill leachate on microbial fuel cell performance. Waste Manag. Res. 2018, 36, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Call, D.; Logan, B.E. Hydrogen production in a single chamber microbial electrolysis cell lacking a membrane. Environ. Sci. Technol. 2008, 42, 3401–3406. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Sheng, G.P.; Zhang, L.; Xia, C.R.; Mu, Z.X.; Liu, X.W.; Wang, H.L.; Yu, H.Q.; Qi, R.; Yu, T.; et al. An mec-mr-coupled system for biohydrogen production from acetate. Environ. Sci. Technol. 2008, 42, 8095–8100. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Shinde, V.N.; Deopurkar, R.L.; Kale, S.P.; Patil, S.A.; Pant, D. Recent advances in the use of different substrates in microbial fuel cells toward wastewater treatment and simultaneous energy recovery. Appl. Energy 2016, 168, 706–723. [Google Scholar] [CrossRef]
- Kumar, P.; Chandrasekhar, K.; Kumari, A.; Sathiyamoorthi, E.; Kim, B.S. Electro-fermentation in aid of bioenergy and biopolymers. Energies 2018, 11, 343. [Google Scholar] [CrossRef]
- Min, B.; Logan, B.E. Continuous electricity generation from domestic wastewater and organic substrates in a flat plate microbial fuel cell. Environ. Sci. Technol. 2004, 38, 5809–5814. [Google Scholar] [CrossRef] [PubMed]
- Ichihashi, O.; Hirooka, K. Removal and recovery of phosphorus as struvite from swine wastewater using microbial fuel cell. Bioresour. Technol. 2012, 114, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, X.; Logan, B.E.; Lee, H. Brewery wastewater treatment using air-cathode microbial fuel cells. Appl. Microbiol. Biotechnol. 2008, 78, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Anam, M.; Yousaf, S.; Maleeha, S.; Bangash, Z. Characterization of the electric current generation potential of the pseudomonas aeruginosa using glucose, fructose, and sucrose in double chamber microbial fuel cell. Iran. J. Biotechnol. 2017, 15, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zheng, P.; Qaisar, M.; Sun, P. Effect of electrode types on simultaneous anaerobic sulfide and nitrate removal in microbial fuel cell. Sep. Purif. Technol. 2014, 134, 20–25. [Google Scholar] [CrossRef]
- Yu, J.; Park, Y.; Lee, T. Effect of separator and inoculum type on electricity generation and microbial community in single-chamber microbial fuel cells. Bioprocess Biosyst. Eng. 2014, 37, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cheng, S.A.; Logan, B.E. Power generation in fed-batch microbial fuel cells as a function of ionic strength, temperature, and reactor configuration. Environ. Sci. Technol. 2005, 39, 5488–5493. [Google Scholar] [CrossRef] [PubMed]
- Vicari, F.; Albamonte, M.; Galia, A.; Scialdone, O. Effect of mode of operation, substrate and final electron acceptor on single-chamber membraneless microbial fuel cell operating with a mixed community. J. Electroanal. Chem. 2018, 814, 104–110. [Google Scholar] [CrossRef]
- Logan, B.E. Scaling up microbial fuel cells and other bioelectrochemical systems. Appl. Microbiol. Biotechnol. 2010, 85, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.L.; He, Y.T.; Yu, P.F.; Sun, H.; Fu, J.X. Effect of temperature on electricity generation of single-chamber microbial fuel cells with proton exchange membrane. Adv. Mater. Res. 2012, 393–395, 1169–1172. [Google Scholar] [CrossRef]
- Behera, M.; Murthy, S.S.R.; Ghangrekar, M.M. Effect of operating temperature on performance of microbial fuel cell. Water Sci. Technol. 2011, 64, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Logan, B.E. Effectiveness of domestic wastewater treatment using microbial fuel cells at ambient and mesophilic temperatures. Bioresour. Technol. 2010, 101, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.J.; Lee, H.; Wang, X.; Liu, Y.L. Electricity generation in microbial fuel cells at different temperature and isolation of electrogenic bacteria. In Proceedings of the 2009 Asia-Pacific Power and Energy Engineering Conference (APPEEC), Wuhan, China, 27–31 March 2009; Volumes 1–7, p. 530. [Google Scholar]
- Trinh, N.T.; Park, J.H.; Kim, B.W. Increased generation of electricity in a microbial fuel cell using geobacter sulfurreducens. Korean J. Chem. Eng. 2009, 26, 748–753. [Google Scholar] [CrossRef]
- Liu, Y.; Climent, V.; Berna, A.; Feliu, J.M. Effect of temperature on the catalytic ability of electrochemically active biofilm as anode catalyst in microbial fuel cells. Electroanalysis 2011, 23, 387–394. [Google Scholar] [CrossRef]
- Heidrich, E.S.; Dolfing, J.; Wade, M.; Sloan, W.T.; Quince, C.; Curtis, T.P. Temperature, inocula and substrate: Contrasting electroactive consortia, diversity and performance in microbial fuel cells. Bioelectrochemistry 2018, 119, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Im, S.; Chung, J.W. Optimizing the operating temperature for microbial electolysis cell treating sewage sludge. Int. J. Hydrogen Energy 2017, 42, 27784–27791. [Google Scholar] [CrossRef]
- Gil, G.C.; Chang, I.S.; Kim, B.H.; Kim, M.; Jang, J.K.; Park, H.S.; Kim, H.J. Operational parameters affecting the performance of a mediator-less microbial fuel cell. Biosens. Bioelectron. 2003, 18, 327–334. [Google Scholar] [CrossRef]
- Strik, D.P.B.T.B.; Picot, M.; Buisman, C.J.N.; Barriere, F. pH and temperature determine performance of oxygen reducing biocathodes. Electroanalysis 2013, 25, 652–655. [Google Scholar] [CrossRef]
- Jia, Q.B.; Wei, L.L.; Han, H.L.; Shen, J.Q. Factors that influence the performance of two-chamber microbial fuel cell. Int. J. Hydrogen Energy 2014, 39, 13687–13693. [Google Scholar] [CrossRef]
- Jannelli, N.; Nastro, R.A.; Cigolotti, V.; Minutillo, M.; Falcucci, G. Low pH, high salinity: Too much for microbial fuel cells? Appl. Energy 2017, 192, 543–550. [Google Scholar] [CrossRef]
- Rismani-Yazdi, H.; Christy, A.D.; Carver, S.M.; Yu, Z.T.; Dehority, B.A.; Tuovinen, O.H. Effect of external resistance on bacterial diversity and metabolism in cellulose-fed microbial fuel cells. Bioresour. Technol. 2011, 102, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Mench, M.M.; Regan, J.M. Impedance characteristics and polarization behavior of a microbial fuel cell in response to short-term changes in medium pH. Environ. Sci. Technol. 2011, 45, 9069–9074. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Regan, J.M. Influence of external resistance on electrogenesis, methanogenesis, and anode prokaryotic communities in microbial fuel cells. Appl. Environ. Microbiol. 2011, 77, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.P.; Srinivasan, B.; Guiot, S.R.; Tartakousky, B. The effect of real-time external resistance optimization on microbial fuel cell performance. Water Res. 2011, 45, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.J.; Cheng, S.A.; Yu, L.L.; Huang, H.B. Effective swine wastewater treatment by combining microbial fuel cells with flocculation. Chemosphere 2017, 182, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Wei, H.W.; Qiu, H.J.; Su, Y.L.; Jaafry, S.W.H.; Zhan, L.; Xie, B. Power generation and pollutants removal from landfill leachate in microbial fuel cell: Variation and influence of anodic microbiomes. Bioresour. Technol. 2018, 247, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.C.; Liang, P.; Huang, X. Recent progress in electrodes for microbial fuel cells. Bioresour. Technol. 2011, 102, 9335–9344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Saito, T.; Cheng, S.A.; Hickner, M.A.; Logan, B.E. Microbial fuel cell cathodes with poly(dimethylsiloxane) diffusion layers constructed around stainless steel mesh current collectors. Environ. Sci. Technol. 2010, 44, 1490–1495. [Google Scholar] [CrossRef] [PubMed]
- An, B.M.; Song, Y.H.; Shin, J.W.; Park, J.Y. Two-chamber microbial fuel cell to simultaneously remove ethanolamine and nitrate. Desalination Water Treat. 2016, 57, 7866–7873. [Google Scholar] [CrossRef]
- Antonopoulou, G.; Stamatelatou, K.; Bebelis, S.; Lyberatos, G. Electricity generation from synthetic substrates and cheese whey using a two chamber microbial fuel cell. Biochem. Eng. J. 2010, 50, 10–15. [Google Scholar] [CrossRef]
- Kim, J.R.; Cheng, S.; Oh, S.E.; Logan, B.E. Power generation using different cation, anion, and ultrafiltration membranes in microbial fuel cells. Environ. Sci. Technol. 2007, 41, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Xing, D.F.; Xie, T.H.; Ren, N.Q.; Logan, B.E. Hydrogen production from proteins via electrohydrogenesis in microbial electrolysis cells. Biosens. Bioelectron. 2010, 25, 2690–2695. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Ge, Z.; Grimaud, J.; Hurst, J.; He, Z. Long-term performance of liter-scale microbial fuel cells treating primary effluent installed in a municipal wastewater treatment facility. Environ. Sci. Technol. 2013, 47, 4941–4948. [Google Scholar] [CrossRef] [PubMed]
- Asensio, Y.; Mansilla, E.; Fernandez-Marchante, C.M.; Lobato, J.; Canizares, P.; Rodrigo, M.A. Towards the scale-up of bioelectrogenic technology: Stacking microbial fuel cells to produce larger amounts of electricity. J. Appl. Electrochem. 2017, 47, 1115–1125. [Google Scholar] [CrossRef]
- Hsu, L.; Chadwick, B.; Kagan, J.; Thacher, R.; Wotawa-Bergen, A.; Richter, K. Scale up considerations for sediment microbial fuel cells. RSC Adv. 2013, 3, 15947–15954. [Google Scholar] [CrossRef]
- Rothstein, H.R.; Sutton, A.J.; Borenstein, M. Publication Bias in Meta-Analysis: Prevention, Assessment and Adjustments; Wiley: London, UK, 2005; pp. 1–7. [Google Scholar]
- Asztalos, J.R.; Kim, Y. Enhanced digestion of waste activated sludge using microbial electrolysis cells at ambient temperature. Water Res. 2015, 87, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Catal, T.; Kavanagh, P.; O’Flaherty, V.; Leech, D. Generation of electricity in microbial fuel cells at sub-ambient temperatures. J. Power Sources 2011, 196, 2676–2681. [Google Scholar] [CrossRef]
- Cheng, S.A.; Xing, D.F.; Logan, B.E. Electricity generation of single-chamber microbial fuel cells at low temperatures. Biosens. Bioelectron. 2011, 26, 1913–1917. [Google Scholar] [CrossRef] [PubMed]
- Cotterill, S.E.; Dolfing, J.; Jones, C.; Curtis, T.P.; Heidrich, E.S. Low temperature domestic wastewater treatment in a microbial electrolysis cell with 1 m(2) anodes: Towards system scale-up. Fuel Cells 2017, 17, 584–592. [Google Scholar] [CrossRef]
- Jadhav, G.S.; Ghangrekar, M.M. Performance of microbial fuel cell subjected to variation in pH, temperature, external load and substrate concentration. Bioresour. Technol. 2009, 100, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Larrosa-Guerrero, A.; Scott, K.; Head, I.M.; Mateo, F.; Ginesta, A.; Hernandez-Fernandez, F.J.; Godinez, C. Low temperature performance of microbial fuel cells. In Proceedings of the PRES 2010: 13th International Conference on Process Integration, Modelling and Optimisation for Energy Saving and Pollution Reduction, Prague, Czech Republic, 28 August–1 September 2010; Volume 21, pp. 463–468. [Google Scholar]
- Liu, L.H.; Tsyganova, O.; Lee, D.J.; Chang, J.S.; Wang, A.J.; Ren, N.Q. Double-chamber microbial fuel cells started up under room and low temperatures. Int. J. Hydrogen Energy 2013, 38, 15574–15579. [Google Scholar] [CrossRef]
- Lu, L.; Xing, D.F.; Ren, N.Q.; Logan, B.E. Syntrophic interactions drive the hydrogen production from glucose at low temperature in microbial electrolysis cells. Bioresour. Technol. 2012, 124, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.U.; Kim, M.; Kim, D.J. Selective inhibition of ammonia oxidation and nitrite oxidation linked to n2o emission with activated sludge and enriched nitrifiers. J. Microbiol. Biotechnol. 2013, 23, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Barbato, R.A.; Foley, K.L.; Toro-Zapata, J.A.; Jones, R.M.; Reynolds, C.M. The power of soil microbes: Sustained power production in terrestrial microbial fuel cells under various temperature regimes. Appl. Soil Ecol. 2017, 109, 14–22. [Google Scholar] [CrossRef]
- Cusick, R.D.; Bryan, B.; Parker, D.S.; Merrill, M.D.; Mehanna, M.; Kiely, P.D.; Liu, G.L.; Logan, B.E. Performance of a pilot-scale continuous flow microbial electrolysis cell fed winery wastewater. Appl. Microbiol. Biotechnol. 2011, 89, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Michie, I.S.; Kim, J.R.; Dinsdale, R.M.; Guwy, A.J.; Premier, G.C. The influence of psychrophilic and mesophilic start-up temperature on microbial fuel cell system performance. Energy Environ. Sci. 2011, 4, 1011–1019. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Sun, J.; Hu, Y.Y.; Wang, Z.Y.; Li, S.Z. Effects of periodically alternating temperatures on performance of single-chamber microbial fuel cells. Int. J. Hydrogen Energy 2014, 39, 8048–8054. [Google Scholar] [CrossRef]
- Chae, K.J.; Choi, M.J.; Kim, K.Y.; Ajayi, F.F.; Chang, I.S.; Kim, I.S. Selective inhibition of methanogens for the improvement of biohydrogen production in microbial electrolysis cells. Int. J. Hydrogen Energy 2010, 35, 13379–13386. [Google Scholar] [CrossRef]
- Omidi, H.; Sathasivan, A. Optimal temperature for microbes in an acetate fed microbial electrolysis cell (MEC). Int. Biodeterior. Biodegrad. 2013, 85, 688–692. [Google Scholar] [CrossRef]
- Abbas, S.Z.; Rafatullah, M.; Ismail, N.; Nastro, R.A. Enhanced bioremediation of toxic metals and harvesting electricity through sediment microbial fuel cell. Int. J. Energy Res. 2017, 41, 2345–2355. [Google Scholar] [CrossRef]
- Adelaja, O.; Keshavarz, T.; Kyazze, G. The effect of salinity, redox mediators and temperature on anaerobic biodegradation of petroleum hydrocarbons in microbial fuel cells. J. Hazard. Mater. 2015, 283, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Anam, M.; Yousaf, S.; Sharafat, I.; Zafar, Z.; Ayaz, K.; Ali, N. Comparing natural and artificially designed bacterial consortia as biosensing elements for rapid non—Specific detection of organic pollutant through microbial fuel cell. Int. J. Electrochem. Sci. 2017, 12, 2836–2851. [Google Scholar] [CrossRef]
- Carver, S.M.; Vuoriranta, P.; Tuovinen, O.H. A thermophilic microbial fuel cell design. J. Power Sources 2011, 196, 3757–3760. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, M.; Shen, N.; Zeng, R.J. H-2 production by the thermoelectric microconverter coupled with microbial electrolysis cell. Int. J. Hydrogen Energy 2016, 41, 22760–22768. [Google Scholar] [CrossRef]
- Hussain, A.; Mehta, P.; Raghavan, V.; Wang, H.; Guiot, S.R.; Tartakovsky, B. The performance of a thermophilic microbial fuel cell fed with synthesis gas. Enzyme Microb. Technol. 2012, 51, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Cerrillo, M.; Vinas, M.; Bonmati, A. Unravelling the active microbial community in a thermophilic anaerobic digester-microbial electrolysis cell coupled system under different conditions. Water Res. 2017, 110, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Daud, W.R.W.; Mokhtarian, N.; Mayahi, A.; Ismail, M.; Anisi, F.; Sedighi, M.; Alam, J. The effect of nitric acid, ethylenediamine, and diethanolamine modified polyaniline nanoparticles anode electrode in a microbial fuel cell. Int. J. Hydrogen Energy 2013, 38, 9525–9532. [Google Scholar] [CrossRef]
- Huang, L.P.; Angelidaki, I. Effect of humic acids on electricity generation integrated with xylose degradation in microbial fuel cells. Biotechnol. Bioeng. 2008, 100, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.W.; Deshusses, M. Application of the finite difference method to model pH and substrate concentration in a double-chamber microbial fuel cell. Environ. Technol. 2014, 35, 1064–1076. [Google Scholar] [CrossRef] [PubMed]
- Thung, W.E.; Ong, S.A.; Ho, L.N.; Wong, Y.S.; Ridwan, F.; Lehl, H.K.; Oon, Y.L.; Oon, Y.S. Biodegradation of acid orange 7 in a combined anaerobic-aerobic up-flow membrane-less microbial fuel cell: Mechanism of biodegradation and electron transfer. Chem. Eng. J. 2018, 336, 397–405. [Google Scholar] [CrossRef]
- Ye, Y.J.; Wang, L.Y.; Chen, Y.W.; Zhu, S.M.; Shen, S.B. High yield hydrogen production in a single-chamber membrane-less microbial electrolysis cell. Water Sci. Technol. 2010, 61, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Li, J.T.; Zhang, S.H.; Hua, Y.M. Performance of denitrifying microbial fuel cell subjected to variation in pH, cod concentration and external resistance. Water Sci. Technol. 2013, 68, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Tremouli, A.; Martinos, M.; Lyberatos, G. The effects of salinity, pH and temperature on the performance of a microbial fuel cell. Waste Biomass Valorization 2017, 8, 2037–2043. [Google Scholar] [CrossRef]
- Feng, Y.L.; Cheng, Y.L.; Du, Y.L.; Teng, Q.; Li, H.R. Hydrogen production from acetate in a sleeve shape microbial electrolysis cell with a mipor cathode. Int. J. Electrochem. Sci. 2014, 9, 6993–7002. [Google Scholar]
- Montpart, N.; Rago, L.; Baeza, J.A.; Guisasola, A. Hydrogen production in single chamber microbial electrolysis cells with different complex substrates. Water Res. 2015, 68, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Raghavulu, S.V.; Mohan, S.V.; Goud, R.K.; Sarma, P.N. Effect of anodic pH microenvironment on microbial fuel cell (MFC) performance in concurrence with aerated and ferricyanide catholytes. Electrochem. Commun. 2009, 11, 371–375. [Google Scholar] [CrossRef]
- Rozendal, R.A.; Hamelers, H.V.M.; Buisman, C.J.N. Effects of membrane cation transport on pH and microbial fuel cell performance. Environ. Sci. Technol. 2006, 40, 5206–5211. [Google Scholar] [CrossRef] [PubMed]
- Puig, S.; Serra, M.; Coma, M.; Cabre, M.; Balaguer, M.D.; Colprim, J. Effect of pH on nutrient dynamics and electricity production using microbial fuel cells. Bioresour. Technol. 2010, 101, 9594–9599. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, F.F.; Kim, K.Y.; Chae, K.J.; Choi, M.J.; Kim, S.Y.; Chang, I.S.; Kim, I.S. Study of hydrogen production in light assisted microbial electrolysis cell operated with dye sensitized solar cell. Int. J. Hydrogen Energy 2009, 34, 9297–9304. [Google Scholar] [CrossRef]
- Aelterman, P.; Versichele, M.; Marzorati, M.; Boon, N.; Verstraete, W. Loading rate and external resistance control the electricity generation of microbial fuel cells with different three-dimensional anodes. Bioresour. Technol. 2008, 99, 8895–8902. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.F.; Geng, J.F.; Pu, K.B.; Ma, Q.; Jing, D.W.; Wang, Y.H.; Chen, Q.Y.; Liu, H. Investigation of a two-dimensional model on microbial fuel cell with different biofilm porosities and external resistances. Chem. Eng. J. 2018, 333, 572–582. [Google Scholar] [CrossRef]
- Katuri, K.P.; Scott, K.; Head, I.M.; Picioreanu, C.; Curtis, T.P. Microbial fuel cells meet with external resistance. Bioresour. Technol. 2011, 102, 2758–2766. [Google Scholar] [CrossRef] [PubMed]
- Rago, L.; Monpart, N.; Cortes, P.; Baeza, J.A.; Guisasola, A. Performance of microbial electrolysis cells with bioanodes grown at different external resistances. Water Sci. Technol. 2016, 73, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.J.; Lee, H.; Wang, X.; Liu, Y.L.; He, W.H. Continuous electricity generation by a graphite granule baffled air-cathode microbial fuel cell. Bioresour. Technol. 2010, 101, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Coronado, J.; Perrier, M.; Tartakovsky, B. Pulse-width modulated external resistance increases the microbial fuel cell power output. Bioresour. Technol. 2013, 147, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Wu, Y.C.; Zhang, F.; Huang, Z.C.; Chen, Z.; Xu, H.J.; Zhao, F. Factors affecting the performance of single-chamber soil microbial fuel cells for power generation. Pedosphere 2014, 24, 330–338. [Google Scholar] [CrossRef]
- Huang, H.; Wang, X.H.; Gong, X.B.; You, S.J. Numerical anodic mass transfer of redox mediators in microbial fuel cell. In Proceedings of the 2013 International Conference on Materials for Renewable Energy and Environment (ICMREE), Chengdu, China, 19–21 August 2014; IEEE: Piscataway, NJ, USA, 2013; Volumes 1–3, pp. 298–302. [Google Scholar]
- Sukkasem, C.; Xua, S.T.; Park, S.; Boonsawang, P.; Liu, H. Effect of nitrate on the performance of single chamber air cathode microbial fuel cells. Water Res. 2008, 42, 4743–4750. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Sheng, G.P.; Mu, Z.X.; Liu, X.W.; Chen, Y.Z.; Wang, H.L.; Yu, H.Q. Manipulating the hydrogen production from acetate in a microbial electrolysis cell-microbial fuel cell-coupled system. J. Power Sources 2009, 191, 338–343. [Google Scholar] [CrossRef]
- Yan, H.J.; Yates, M.D.; Regan, J.M. Effects of constant or dynamic low anode potentials on microbial community development in bioelectrochemical systems. Appl. Microbiol. Biotechnol. 2015, 99, 9319–9329. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.K.; Harnisch, F.; Dockhorn, T.; Schroder, U. Examining sludge production in bioelectrochemical systems treating domestic wastewater. Bioresour. Technol. 2015, 198, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Yang, W.L.; Logan, B.E. Impact of electrode configurations on retention time and domestic wastewater treatment efficiency using microbial fuel cells. Water Res. 2015, 80, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Stager, J.L.; Zhang, X.Y.; Logan, B.E. Addition of acetate improves stability of power generation using microbial fuel cells treating domestic wastewater. Bioelectrochemistry 2017, 118, 154–160. [Google Scholar] [CrossRef] [PubMed]
- You, S.J.; Zhao, Q.L.; Jiang, J.Q.; Zhang, J.N. Treatment of domestic wastewater with simultaneous electricity generation in microbial fuel cell under continuous operation. Chem. Biochem. Eng. Q. 2006, 20, 407–412. [Google Scholar]
- Zawawi, M.H.; Mimit, M.N.; Rosli, N.A.; Hossain, M.D.; Zainal, N.A.; Kamarudin, M.A. Efficiency of single chamber microbial fuel cell (MFC) in wastewater treatment. Int. J. Adv. Appl. Sci. 2017, 4, 47–51. [Google Scholar] [CrossRef]
- Heidrich, E.S.; Edwards, S.R.; Dolfing, J.; Cotterill, S.E.; Curtis, T.P. Performance of a pilot scale microbial electrolysis cell fed on domestic wastewater at ambient temperatures for a 12 month period. Bioresour. Technol. 2014, 173, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Angosto, J.M.; Fernandez-Lopez, J.A.; Godinez, C. Brewery and liquid manure wastewaters as potential feedstocks for microbial fuel cells: A performance study. Environ. Technol. 2015, 36, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Egbadon, E.O.; Akujobi, C.O.; Nweke, C.O.; Braide, W.; Akaluka, C.K.; Adeleye, S.A. Simultaneous generation of bioelectricity and treatment of swine wastewater in a microbial fuel cell. Int. Lett. Nat. Sci. 2016, 54, 100–107. [Google Scholar] [CrossRef]
- Lin, H.J.; Wu, X.; Nelson, C.; Miller, C.; Zhu, J. Electricity generation and nutrients removal from high-strength liquid manure by air-cathode microbial fuel cells. J. Environ. Sci. Health Part A 2016, 51, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Ogugbue, C.J.; Ebode, E.E.; Leera, S. Electricity generation from swine wastewater using microbial fuel cell. J. Ecol. Eng. 2015, 16, 26–33. [Google Scholar] [CrossRef]
- Min, B.; Kim, J.R.; Oh, S.E.; Regan, J.M.; Logan, B.E. Electricity generation from swine wastewater using microbial fuel cells. Water Res. 2005, 39, 4961–4968. [Google Scholar] [CrossRef] [PubMed]
- Cetinkaya, A.Y.; Ozkaya, B.; Taskan, E.; Karadag, D.; Cakmakci, M. The production of electricity from dual-chambered microbial fuel cell fueled by old age leachate. Energy Source Part A 2016, 38, 1544–1552. [Google Scholar] [CrossRef]
- Greenman, J.; Galvez, A.; Giusti, L.; Ieropoulos, L. Electricity from landfill leachate using microbial fuel cells: Comparison with a biological aerated filter. Enzyme Microb. Technol. 2009, 44, 112–119. [Google Scholar] [CrossRef]
- Hassan, M.; Pous, N.; Xie, B.; Colprim, J.; Balaguer, M.D.; Puig, S. Influence of iron species on integrated microbial fuel cell and electro-fenton process treating landfill leachate. Chem. Eng. J. 2017, 328, 57–65. [Google Scholar] [CrossRef]
- Rikame, S.S.; Mungray, A.A.; Mungray, A.K. Electricity generation from acidogenic food waste leachate using dual chamber mediator less microbial fuel cell. Int. Biodeterior. Biodegrad. 2012, 75, 131–137. [Google Scholar] [CrossRef]
- Yuan, H.R.; Deng, L.F.; Chen, Y. Optimization of biodrying pretreatment of municipal solid waste and microbial fuel cell treatment of leachate. Biotechnol. Bioproc. Eng. 2014, 19, 668–675. [Google Scholar] [CrossRef]
- Huang, L.; Li, X.C.; Cai, T.; Huang, M.H. Electrochemical performance and community structure in three microbial fuel cells treating landfill leachate. Process Saf. Environ. Protect. 2018, 113, 378–387. [Google Scholar] [CrossRef]
- Shehzad, A.; Bashir, M.J.K.; Sethupathi, S.; Lim, J.W.; Younas, M. Bioelectrochemical system for landfill leachate treatment—Challenges, opportunities, and recommendations. Geosyst. Eng. 2016, 19, 337–345. [Google Scholar] [CrossRef]
- Ahn, Y.T.; Logan, B.E. Altering anode thickness to improve power production in microbial fuel cells with different electrode distances. Energy Fuel 2013, 27, 271–276. [Google Scholar] [CrossRef]
- Chen, S.L.; Patil, S.A.; Schroder, U. A high-performance rotating graphite fiber brush air-cathode for microbial fuel cells. Appl. Energy 2018, 211, 1089–1094. [Google Scholar] [CrossRef]
- Hutchinson, A.J.; Tokash, J.C.; Logan, B.E. Analysis of carbon fiber brush loading in anodes on startup and performance of microbial fuel cells. J. Power Sources 2011, 196, 9213–9219. [Google Scholar] [CrossRef]
- Wagner, R.C.; Regan, J.M.; Oh, S.E.; Zuo, Y.; Logan, B.E. Hydrogen and methane production from swine wastewater using microbial electrolysis cells. Water Res. 2009, 43, 1480–1488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.D.; Zhao, Q.L.; Jiao, Y.; Zhang, J.N.; Jiang, J.Q.; Ren, N.; Kim, B.H. Improved performance of microbial fuel cell using combination biocathode of graphite fiber brush and graphite granules. J. Power Sources 2011, 196, 6036–6041. [Google Scholar] [CrossRef]
- Nam, T.; Son, S.; Koo, B.; Tran, H.V.H.; Kim, J.R.; Choi, Y.; Jung, S.P. Comparative evaluation of performance and electrochemistry of microbial fuel cells with different anode structures and materials. Int. J. Hydrogen Energy 2017, 42, 27677–27684. [Google Scholar] [CrossRef]
- Zhen, G.Y.; Lu, X.Q.; Kumar, G.; Bakonyi, P.; Xu, K.Q.; Zhao, Y.C. Microbial electrolysis cell platform for simultaneous waste biorefinery and clean electrofuels generation: Current, situation, challenges and future perspectives. Prog. Energy Combust. Sci. 2017, 63, 119–145. [Google Scholar] [CrossRef]
- Aelterman, P.; Versichele, M.; Genettello, E.; Verbeken, K.; Verstraete, W. Microbial fuel cells operated with iron-chelated air cathodes. Electrochim. Acta 2009, 54, 5754–5760. [Google Scholar] [CrossRef]
- Butler, C.S.; Nerenberg, R. Performance and microbial ecology of air-cathode microbial fuel cells with layered electrode assemblies. Appl. Microbiol. Biotechnol. 2010, 86, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Wang, C.T.; Yang, Y.C.; Chen, W.J. Application of aluminum-alloy mesh composite carbon cloth for the design of anode/cathode electrodes in escherichia coli microbial fuel cell. Int. J. Hydrogen Energy 2013, 38, 11131–11137. [Google Scholar] [CrossRef]
- Tsan, W.C.; Jung, C.W.; Yao, H.R. Effect of culture time on the growth curve and power performance in a microbial fuel cell at a fixed amount of liquid culture. Int. J. Green Energy 2016, 13, 695–702. [Google Scholar] [CrossRef]
- Cai, W.F.; Geng, D.L.; Wang, Y.H. Assessment of cathode materials for Ni(ii) reduction in microbial electrolysis cells. RSC Adv. 2016, 6, 31732–31738. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Ye, D.D.; Zhu, X.; Liao, Q.; Zhang, B.A. Enhanced performances of microbial fuel cells using surface-modified carbon cloth anodes: A comparative study. Int. J. Hydrogen Energy 2014, 39, 19148–19155. [Google Scholar] [CrossRef]
- Amade, R.; Moreno, H.A.; Hussain, S.; Vila-Costa, M.; Bertran, E. Vertically aligned carbon nanotubes as anode and air-cathode in single chamber microbial fuel cells. Appl. Phys. Lett. 2016, 109. [Google Scholar] [CrossRef]
- Chen, Y.W.; Xu, Y.; Chen, L.L.; Li, P.W.; Zhu, S.M.; Shen, S.B. Microbial electrolysis cells with polyaniline/multi-walled carbon nanotube-modified biocathodes. Energy 2015, 88, 377–384. [Google Scholar] [CrossRef]
- Erbay, C.; Yang, G.; de Figueiredo, P.; Sadr, R.; Yu, C.H.; Han, A. Three-dimensional porous carbon nanotube sponges for high-performance anodes of microbial fuel cells. J. Power Sources 2015, 298, 177–183. [Google Scholar] [CrossRef]
- Wang, L.Y.; Chen, Y.W.; Huang, Q.; Feng, Y.Y.; Zhu, S.M.; Shen, S.B. Hydrogen production with carbon nanotubes based cathode catalysts in microbial electrolysis cells. J. Chem. Technol. Biotechnol. 2012, 87, 1150–1156. [Google Scholar] [CrossRef]
- Ghasemi, M.; Daud, W.R.W.; Hassan, S.H.A.; Jafary, T.; Rahimnejad, M.; Ahmad, A.; Yazdi, M.H. Carbon nanotube/polypyrrole nanocomposite as a novel cathode catalyst and proper alternative for Pt in microbial fuel cell. Int. J. Hydrogen Energy 2016, 41, 4872–4878. [Google Scholar] [CrossRef]
- Bo, T.; Zhu, X.Y.; Zhang, L.X.; Tao, Y.; He, X.H.; Li, D.P.; Yan, Z.Y. A new upgraded biogas production process: Coupling microbial electrolysis cell and anaerobic digestion in single-chamber, barrel-shape stainless steel reactor. Electrochem. Commun. 2014, 45, 67–70. [Google Scholar] [CrossRef]
- Hu, H.Q.; Fan, Y.Z.; Liu, H. Hydrogen production using single-chamber membrane-free microbial electrolysis cells. Water Res. 2008, 42, 4172–4178. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.T.; Zhu, G.F.; Jha, A.K.; Zou, R.; Liu, L.; Huang, X.; Liu, C.X. Hydrogen production with effluent from an anaerobic baffled reactor (ABR) using a single-chamber microbial electrolysis cell (MEC). Int. J. Hydrogen Energy 2013, 38, 11117–11123. [Google Scholar] [CrossRef]
- Cui, K.P.; Wang, Y.; Sun, S.Q. Electricity generation and wastewater treatment using an air-cathode single chamber microbial fuel cell. In Proceedings of the 2010 Asia-Pacific Power and Energy Engineering Conference (APPEEC), Chengdu, China, 28–31 March 2010. [Google Scholar]
- Kim, H.; Kim, B.; Kim, J.; Lee, T.; Yu, J. Electricity generation and microbial community in microbial fuel cell using low-pH distillery wastewater at different external resistances. J. Biotechnol. 2014, 186, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Abourached, C.; Catal, T.; Liu, H. Efficacy of single-chamber microbial fuel cells for removal of cadmium and zinc with simultaneous electricity production. Water Res. 2014, 51, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Ren, N.Q.; Zhao, X.; Wang, H.A.; Wu, D.; Xing, D.F. Hydrogen production, methanogen inhibition and microbial community structures in psychrophilic single-chamber microbial electrolysis cells. Energy Environ. Sci. 2011, 4, 1329–1336. [Google Scholar] [CrossRef]
- Chae, K.J.; Choi, M.J.; Lee, J.; Ajayi, F.F.; Kim, I.S. Biohydrogen production via biocatalyzed electrolysis in acetate-fed bioelectrochemical cells and microbial community analysis. Int. J. Hydrogen Energy 2008, 33, 5184–5192. [Google Scholar] [CrossRef]
- Kyazze, G.; Popov, A.; Dinsdale, R.; Esteves, S.; Hawkes, F.; Premier, G.; Guwy, A. Influence of catholyte pH and temperature on hydrogen production from acetate using a two chamber concentric tubular microbial electrolysis cell. Int. J. Hydrogen Energy 2010, 35, 7716–7722. [Google Scholar] [CrossRef]
- Nam, J.Y.; Logan, B.E. Optimization of catholyte concentration and anolyte pHs in two chamber microbial electrolysis cells. Int. J. Hydrogen Energy 2012, 37, 18622–18628. [Google Scholar] [CrossRef]
- Rivera, I.; Buitron, G.; Bakonyi, P.; Nemestothy, N.; Belafi-Bako, K. Hydrogen production in a microbial electrolysis cell fed with a dark fermentation effluent. J. Appl. Electrochem. 2015, 45, 1223–1229. [Google Scholar] [CrossRef]
- Esfandyari, M.; Fanaei, M.A.; Gheshlaghi, R.; Mandavi, M.A. Dynamic modeling of a continuous two-chamber microbial fuel cell with pure culture of shewanella. Int. J. Hydrogen Energy 2017, 42, 21198–21202. [Google Scholar] [CrossRef]
- Xiao, B.Y.; Yang, F.; Liu, J.X. Evaluation of electricity production from alkaline pretreated sludge using two-chamber microbial fuel cell. J. Hazard. Mater. 2013, 254, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Escapa, A.; Mateos, R.; Martinez, E.J.; Blanes, J. Microbial electrolysis cells: An emerging technology for wastewater treatment and energy recovery. From laboratory to pilot plant and beyond. Renew. Sustain. Energy Rev. 2016, 55, 942–956. [Google Scholar] [CrossRef]
- Kadier, A.; Logrono, W.; Rai, P.K.; Kalil, M.S.; Mohamed, A.; Abu Hasan, H.; Hamid, A.A. None-platinum electrode catalysts and membranes for highly efficient and inexpensive h-2 production in microbial electrolysis cells (MECS): A review. Iran. J. Catal. 2017, 7, 89–102. [Google Scholar]
- Heidrich, E.S.; Dolfing, J.; Scott, K.; Edwards, S.R.; Jones, C.; Curtis, T.P. Production of hydrogen from domestic wastewater in a pilot-scale microbial electrolysis cell. Appl. Microbiol. Biotechnol. 2013, 97, 6979–6989. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liang, P.; Zhang, C.Y.; Bian, Y.H.; Sun, X.L.; Zhang, H.L.; Yang, X.F.; Zhao, F.; Huang, X. Periodic polarity reversal for stabilizing the pH in two-chamber microbial electrolysis cells. Appl. Energy 2016, 165, 670–675. [Google Scholar] [CrossRef]
- Jiang, D.Q.; Curtis, M.; Troop, E.; Scheible, K.; McGrath, J.; Hu, B.X.; Suib, S.; Raymond, D.; Li, B.K. A pilot-scale study on utilizing multi-anode/cathode microbial fuel cells (MAC MFCS) to enhance the power production in wastewater treatment. Int. J. Hydrogen Energy 2011, 36, 876–884. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]

| Groups | Subgroups | Number of Studies | References |
|---|---|---|---|
| Temperature (Temp.) | Low (<25 °C) | 13 | [15,19,20,21,24,46,47,48,49,50,51,52,53] |
| Moderate (25–35 °C) | 12 | [15,19,21,50,51,54,55,56,57,58,59,60] | |
| High (>35 °C) | 9 | [19,60,61,62,63,64,65,66,67] | |
| pH | Low (<6) | 5 | [68,69,70,71,72] |
| Moderate (6–8) | 6 | [5,61,73,74,75,76] | |
| High (>8) | 5 | [73,77,78,79,80] | |
| External resistance (Rext) | Low (<100 Ω) | 7 | [30,50,81,82,83,84,85] |
| Moderate (100–1000 Ω) | 9 | [30,50,82,83,84,86,87,88,89] | |
| High (>1000 Ω) | 5 | [50,83,88,90,91] | |
| Substrate (S) | Domestic wastewater | 10 | [20,21,49,79,92,93,94,95,96,97] |
| Swine wastewater | 7 | [10,41,98,99,100,101,102] | |
| Landfill leachate | 8 | [4,103,104,105,106,107,108,109] | |
| Electrode material (Electro.) | Graphite fiber brush | 7 | [110,111,112,113,114,115,116] |
| Carbon cloth | 7 | [15,117,118,119,120,121,122] | |
| Carbon paper | 5 | [123,124,125,126,127] | |
| Reactor configuration (Config.) | Single-chamber | 10 | [48,87,123,128,129,130,131,132,133,134] |
| Two-chamber | 9 | [20,52,122,135,136,137,138,139,140] | |
| Large-scale | 7 | [49,56,141,142,143,144,145] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Chen, G. Factors Affecting the Effectiveness of Bioelectrochemical System Applications: Data Synthesis and Meta-Analysis. Batteries 2018, 4, 34. https://doi.org/10.3390/batteries4030034
Li S, Chen G. Factors Affecting the Effectiveness of Bioelectrochemical System Applications: Data Synthesis and Meta-Analysis. Batteries. 2018; 4(3):34. https://doi.org/10.3390/batteries4030034
Chicago/Turabian StyleLi, Simeng, and Gang Chen. 2018. "Factors Affecting the Effectiveness of Bioelectrochemical System Applications: Data Synthesis and Meta-Analysis" Batteries 4, no. 3: 34. https://doi.org/10.3390/batteries4030034
APA StyleLi, S., & Chen, G. (2018). Factors Affecting the Effectiveness of Bioelectrochemical System Applications: Data Synthesis and Meta-Analysis. Batteries, 4(3), 34. https://doi.org/10.3390/batteries4030034






