Coefficients of Thermal Expansion of Al- and Y-Substituted NaSICON Solid Solution Na3+2xAlxYxZr2−2xSi2PO12
Abstract
:1. Introduction
2. Experimental
3. Results and Discussions
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Song, S.F.; Duong, H.M.; Korsunsky, A.M.; Hu, N.; Lu, L. A Na+ Superionic Conductor for Room-Temperature Sodium Batteries. Sci. Rep. 2016, 6, 32330. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.; Noi, K.; Sakuda, A.; Tatsumisago, M. Superionic glass-ceramic electrolytes for room-temperature rechargeable sodium batteries. Nat. Commun. 2012, 3, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Guin, M.; Tietz, F. Survey of the transport properties of sodium superionic conductor materials for use in sodium batteries. J. Power Sources 2015, 273, 1056–1064. [Google Scholar] [CrossRef]
- Hueso, K.B.; Armand, M.; Rojo, T. High temperature sodium batteries: Status, challenges and future trends. Energy Environ. Sci. 2013, 6, 734–749. [Google Scholar] [CrossRef]
- Ma, Q.; Guin, M.; Naqash, S.; Tsai, C.L.; Tietz, F.; Guillon, O. Scandium-Substituted Na3Zr2(SiO4)2(PO4) Prepared by a Solution Assisted Solid-State Reaction Method as Sodium-Ion. Conductors. Chem. Mater. 2016, 28, 4821–4828. [Google Scholar] [CrossRef]
- Pet’kov, V.I.; Asabina, E.A.; Shchelokov, I.A. Thermal Expansion of NASICON Materials. Inorg. Mater. 2013, 49, 502–506. [Google Scholar] [CrossRef]
- Woodcock, D.A.; Lightfoot, P.; Ritter, C. Mechanism of low thermal expansion in the cation-ordered Nasicon structure. Chem. Commun. 1998, 1, 107–108. [Google Scholar] [CrossRef]
- Oota, T.; Yamai, I. Thermal Expansion Behavior of NaZr2(PO4)3Type Compounds. J. Am. Ceram. Soc. 1986, 69, 1–6. [Google Scholar] [CrossRef]
- Kaus, M.; Guin, M.; Yavuz, M.; Knapp, M.; Tietz, F.; Guillon, O.; Ehrenberg, H.; Indris, S. Fast Na+ Ion. Conduction in NASICON-Type Na3.4Sc2(SiO4)0.4(PO4)2.6 Observed by 23Na NMR Relaxometry. J. Phys. Chem. C 2017, 121, 1449–1454. [Google Scholar] [CrossRef]
- Guin, M. Chemical and Physical Properties of Sodium Ionic Conductors for Solid-State Batteries; Rheinisch Westfälischen Technischen Hochschule (RWTH): Aachen, Germany, 2016. [Google Scholar]
- Srikanth, V.; Subbarao, E.C.; Agrawal, D.K.; Huang, C.-Y.; Roy, R.; Rao, G.V. Thermal Expansion Anisotropy and Acoustic Emission of NaZr2P3O12 Family Ceramics. J. Am. Ceram. Soc. 1991, 74, 365–368. [Google Scholar] [CrossRef]
- Maca, K.; Pouchy, V.; Boccaccini, R. Sintering Densification Curve–A Practical Approach for its Construction from Dilatometric Shrinkage Data. Sci. Sinering 2008, 40, 117–122. [Google Scholar] [CrossRef]
- Shiratori, Y.; Tietz, F.; Buchkremer, H.P.; Stöver, D. YSZ-MgO composite electrolyte with adjusted thermal expansion coefficient to other SOFC components. Solid State Ion. 2003, 164, 27–33. [Google Scholar] [CrossRef]
- Biswas, M.; Kumbhar, C.S.; Gowtam, D.S. Characterisation of Nanocrystalline Yttria-Stabilized Zirconia: An. In Situ HTXRD Study. ISRN Nanotechnol. 2011, 2011, 305687. [Google Scholar] [CrossRef]
- Agrawal, D.K.; Roy, R. Composite route to “zero” expansion ceramics. J. Mater. Sci. 1985, 20, 4617–4623. [Google Scholar] [CrossRef]
- Alamo, J.; Roy, R. Ultralow-Expansion Ceramics in the System Na2O-ZrO2-P2O5-SiO2. J. Am. Ceram. Soc. 1984, 67, c78–c80. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Agrawal, D.K.; McKinstry, H.A. Thermal expansion behaviour of M′Ti2P3O12 (M′ = Li, Na, K, Cs) and M″Ti4P6O24 (M″ = Mg, Ca, Sr, Ba) compounds. J. Mater. Sci. 1995, 30, 3509–3514. [Google Scholar] [CrossRef]
- Lenain, G.E.; McKinstry, H.A.; Limaye, S.Y.; Woodward, A. Low thermal expansion of alkali-zirconium phosphates. Mater. Res. Bull. 1984, 19, 1451–1456. [Google Scholar] [CrossRef]
- Colomban, P.; Mouchon, E. Phase transition in, thermal history and expansion of NASICON, solid solution and lithium derivative ceramics and of SiC (mullite) fibers-NASICON composites. Solid State Ion. 1994, 73, 209–220. [Google Scholar] [CrossRef]
- Yousef, S.G. Mikrorissbildung Durch Anisotrope Thermische Ausdehnung: Experiment und Numerische Simulation, in Material-Und Geowissenschaften. Ph.D. Thesis, Technischen Universität Darmstadt, Darmstadt, Germany, 2004. [Google Scholar]
- Naqash, S.; Tietz, F.; Guillon, O. Synthesis and characterization of equimolar Al/Y-substituted NASICON solid solution Na1+2x+yAlxYxZr2−2xSiyP3−yO12. Solid State Ion. 2018, 319, 13–21. [Google Scholar] [CrossRef]
- Jolley, A.G.; Taylor, D.D.; Schreiber, N.J.; Wachsman, E.D. Structural Investigation of Monoclinic-Rhombohedral Phase Transition in Na3Zr2Si2PO12 and Doped NASICON. J. Am. Ceram. Soc. 2015, 98, 2902–2907. [Google Scholar] [CrossRef]
- Patil, R.N.; Subbarao, E.C. Axial thermal expansion of ZrO2 and HfO2 in the range room temperature to 1400 °C. J. Appl. Crystallogr. 1969, 2, 281–288. [Google Scholar] [CrossRef]
- Achary, S.N.; Jayakumar, O.D.; Tyagi, A.K.; Kulshresththa, S.K. Preparation, phase transition and thermal expansion studies on low-cristobalite type Al1−xGaxPO4 (x = 0.0, 0.20, 0.50, 0.80 and 1.00). J. Solid State Chem. 2003, 176, 37–46. [Google Scholar] [CrossRef]
- Smirnova, O.A.; Fuentes, R.O.; Figueiredo, F.; Kharton, V.V.; Marques, F.M.B. Stability and thermal expansion of Na+-conducting ceramics. J. Electroceram. 2003, 11, 179–189. [Google Scholar] [CrossRef]
- Luo, Y.R.; Lide, D.R. Bond. Dissociation Energies, CRC Handbook of Chemistry and Physics, 97th ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Hong, H.Y.P. Crystal structures and crystal chemistry in the system Na1+xZr2SixP3−xO12. Mater. Res. Bull. 1976, 11, 173–182. [Google Scholar] [CrossRef]
- Guin, M.; Tietz, F.; Guillon, O. New promising NASICON material as solid electrolyte for sodium-ion batteries: Correlation between composition, crystal structure and ionic conductivity of Na3+xSc2SixP3−xO12. Solid State Ion. 2016, 293, 18–26. [Google Scholar] [CrossRef]
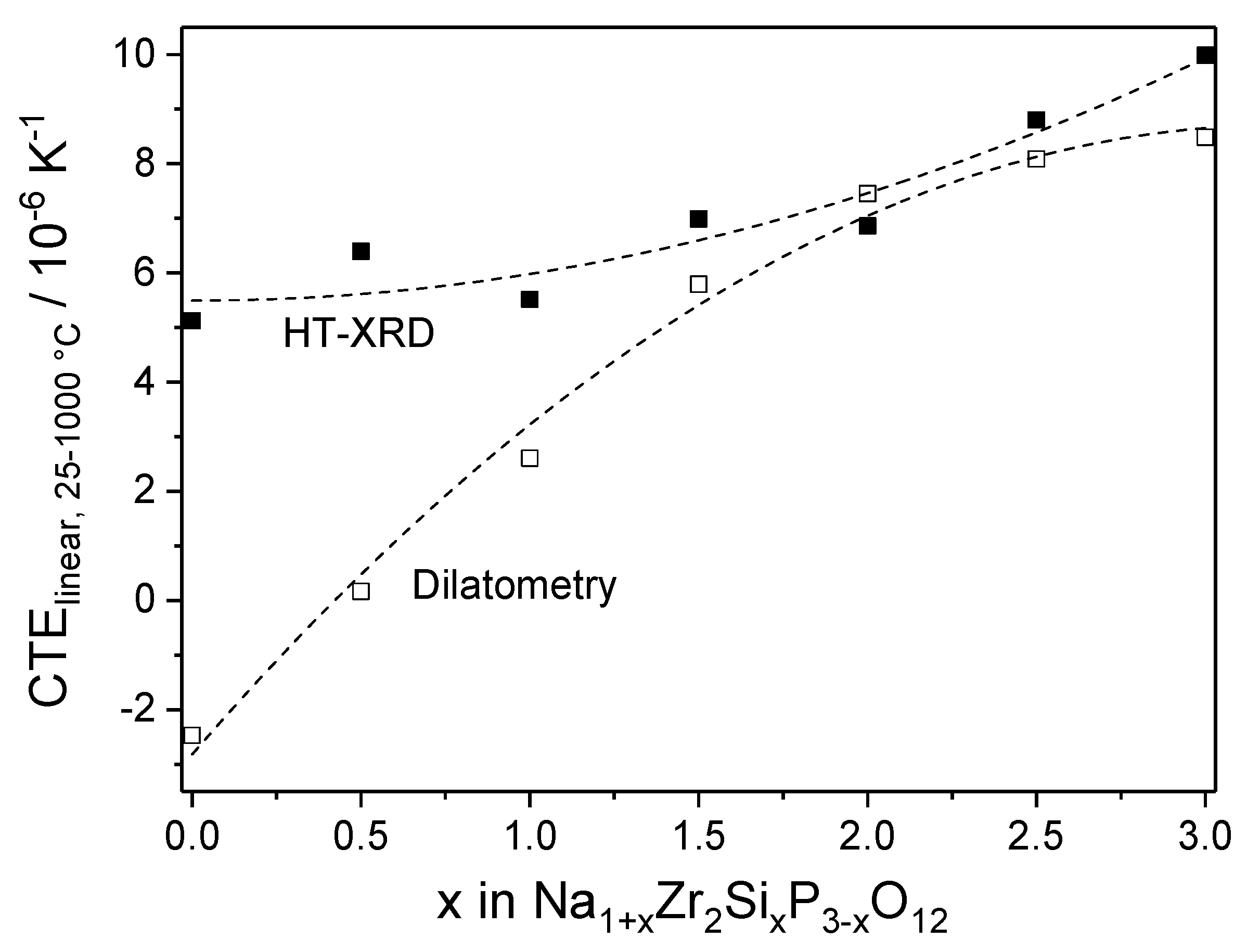

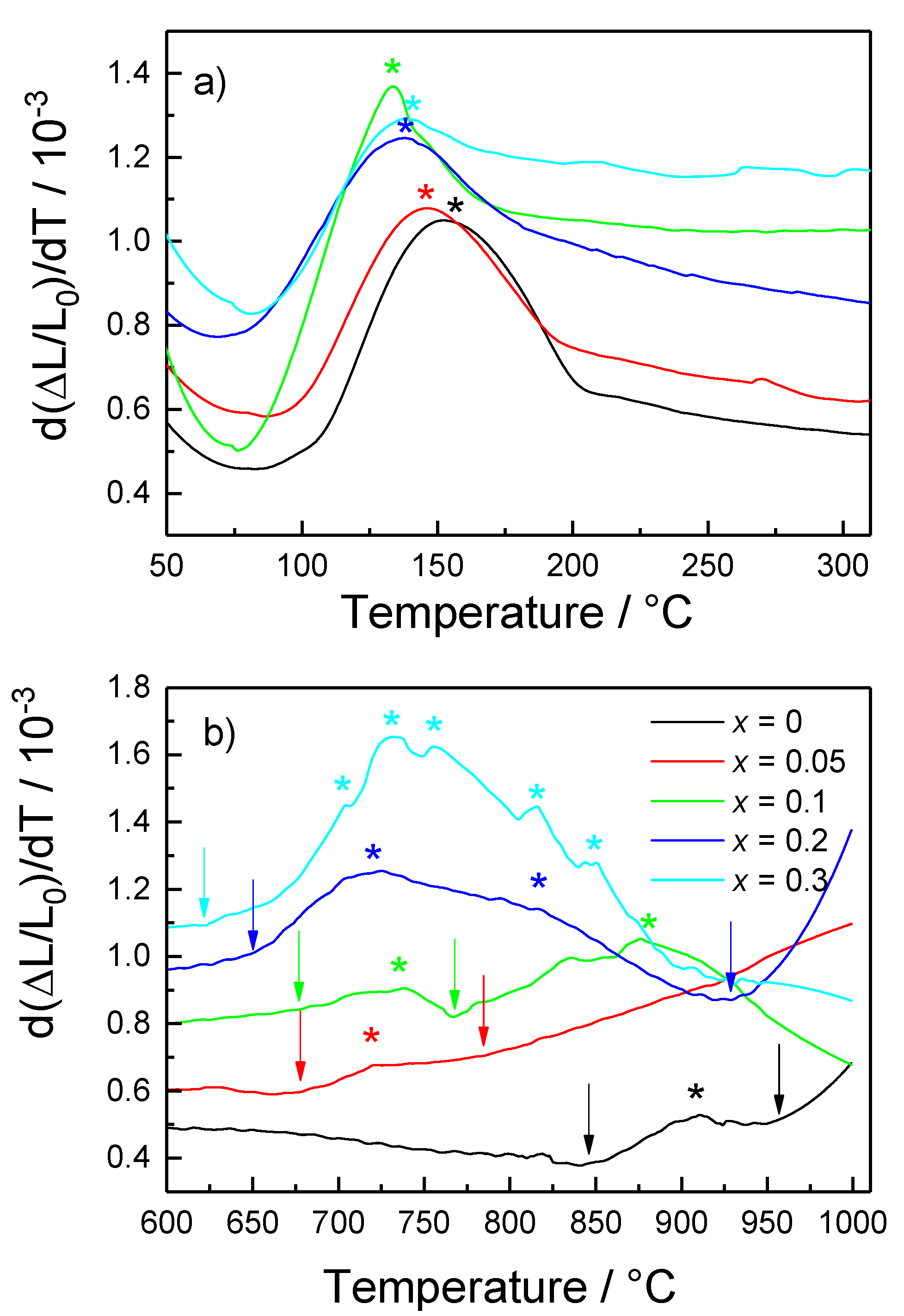
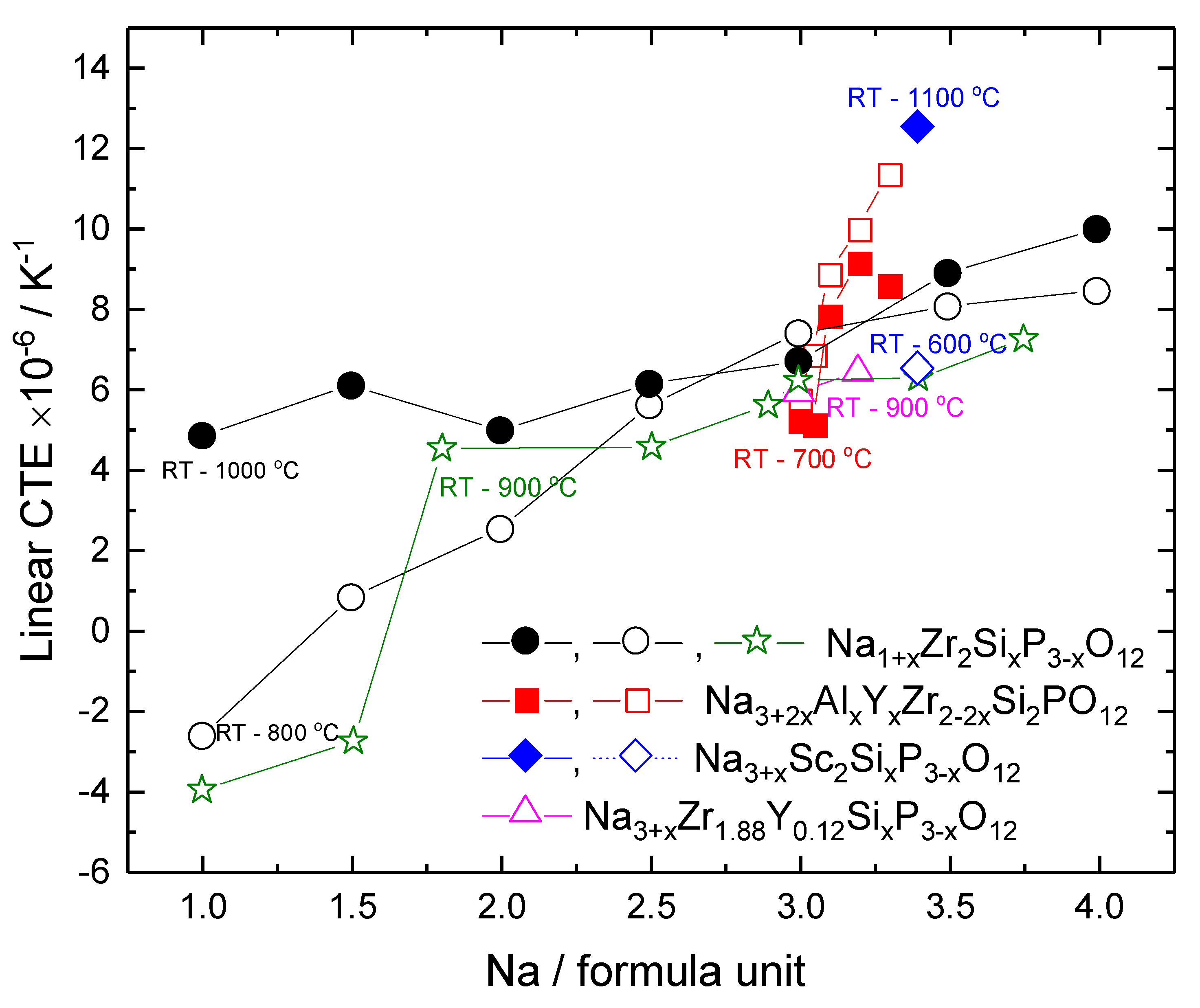
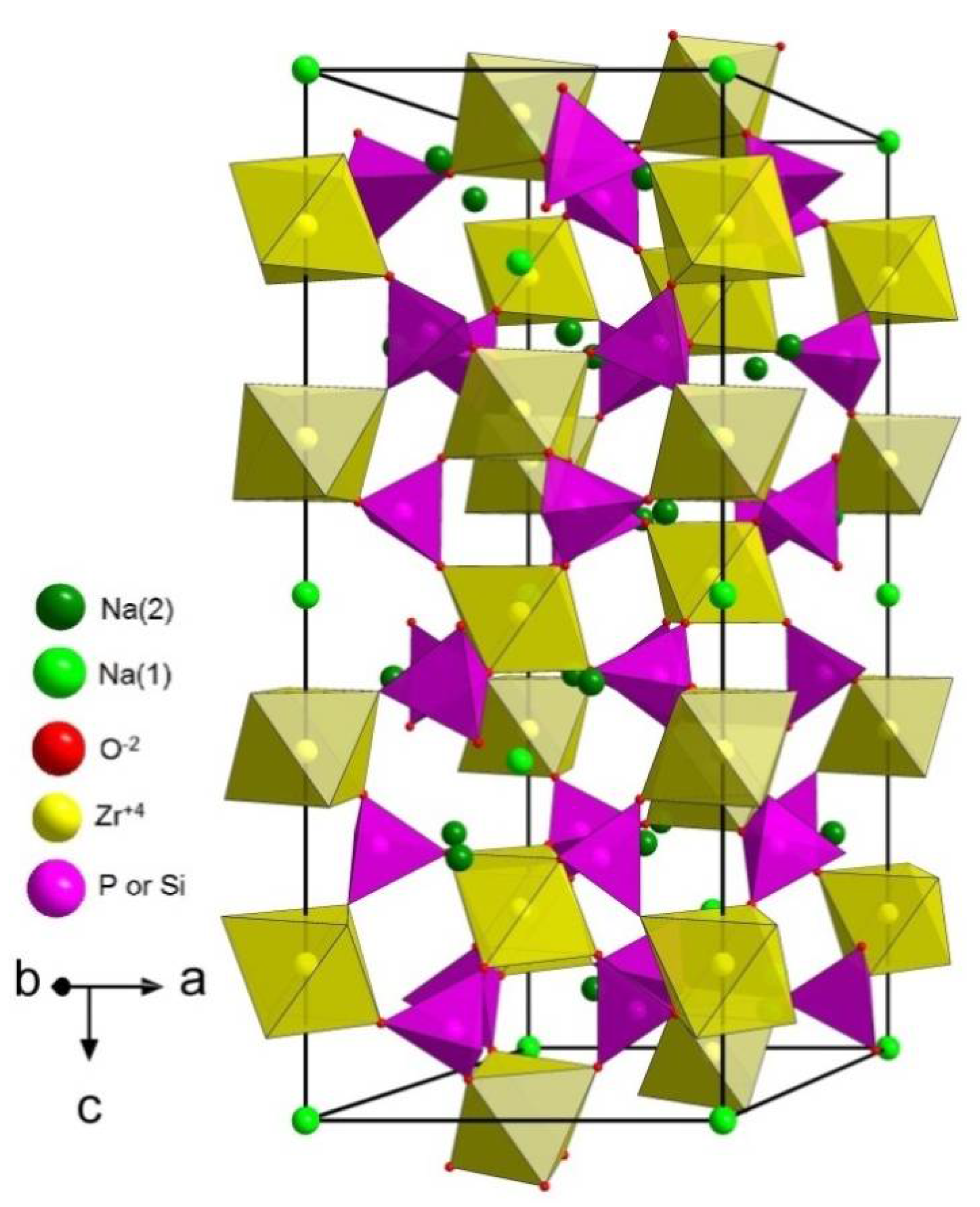
| Material | Lattice Parameters and Space Groups | |||
|---|---|---|---|---|
| 25 °C | Space Group | 700 °C | Space Group | |
| NAYZSiP0 | a: 15.637(1), b: 9.045(1), c: 9.225(1), β: 123.64(1)° | c2/c | a = b = 9.058(2), c: 23.173 (4) | Rc |
| NAYZSiP0.05 | a: 15.665(1) b: 9.058(1), c: 9.233(1), β: 123.79(1)° | c2/c | a = b = 9.070(1), c: 23.161(1) | Rc |
| NAYZSiP0.1 | a: 15.671(1) b: 9.065(1), c: 9.221(1), β: 123.95(1)° | c2/c | a = b = 9.090(9), c: 23.133(3) | Rc |
| NAYZSiP0.2 | a = b = 9.096(1), c: 22.741(2) | Rc | a = b = 9.120(9), c: 23.034(6) | Rc |
| NAYZSiP0.3 | a = b = 9.110(3), c: 22.671(1) | Rc | a = b = 9.139(6), c: 23.938(8) | Rc |
| x | Tmon-rhom/°C | Tonset1/°C | Tmax1/°C | Tonset2/°C | Tmax2/°C |
|---|---|---|---|---|---|
| 0 | 152 | 850 | 910 | 955 | >1000 |
| 0.05 | 146 | 675 | 720 | 780 | >1000 |
| 0.1 | 138 | 670 | 735 | 770 | 880 |
| 0.2 | 136 | 650 | 720 | 935 | 815 |
| 0.3 | 138 | 625 | 740 | - | 830 |
| x | CTEHT-XRD/K−1 10−6 | CTEdil./K−1 10−6 | CTEcalc./K−1 10−6 | /% | /% |
|---|---|---|---|---|---|
| 0 | 5.22 | 5.72 | 5.69 | 1.4 | 1.1 |
| 0.05 | 5.11 | 6.84 | 6.83 | 5.3 | 2.2 |
| 0.1 | 7.81 | 8.85 | 8.83 | 3.3 | 3.4 |
| 0.2 | 9.13 | 9.97 | 9.99 | 3.2 | 2.8 |
| 0.3 | 8.57 | 11.34 | 11.33 | 10.0 | 5.6 |
| A-B | D°/kJ mol−1 |
|---|---|
| Zr-O | 766.1 ± 10.6 |
| Al-O | 501.9 ± 10.6 |
| Y-O | 714.1 ± 10.2 |
| Sc-O | 671.4 ± 1.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naqash, S.; Gerhards, M.-T.; Tietz, F.; Guillon, O. Coefficients of Thermal Expansion of Al- and Y-Substituted NaSICON Solid Solution Na3+2xAlxYxZr2−2xSi2PO12. Batteries 2018, 4, 33. https://doi.org/10.3390/batteries4030033
Naqash S, Gerhards M-T, Tietz F, Guillon O. Coefficients of Thermal Expansion of Al- and Y-Substituted NaSICON Solid Solution Na3+2xAlxYxZr2−2xSi2PO12. Batteries. 2018; 4(3):33. https://doi.org/10.3390/batteries4030033
Chicago/Turabian StyleNaqash, Sahir, Marie-Theres Gerhards, Frank Tietz, and Olivier Guillon. 2018. "Coefficients of Thermal Expansion of Al- and Y-Substituted NaSICON Solid Solution Na3+2xAlxYxZr2−2xSi2PO12" Batteries 4, no. 3: 33. https://doi.org/10.3390/batteries4030033
APA StyleNaqash, S., Gerhards, M.-T., Tietz, F., & Guillon, O. (2018). Coefficients of Thermal Expansion of Al- and Y-Substituted NaSICON Solid Solution Na3+2xAlxYxZr2−2xSi2PO12. Batteries, 4(3), 33. https://doi.org/10.3390/batteries4030033





