1. Introduction
Among polyanionic compounds suitable for utilization as the cathode materials in the Na-ion batteries, the mixed-valence sodium vanadium fluorophosphates with a general formula of Na
3V
23+xO
2x(PO
4)
2F
3−2x (0 ≤ x ≤ 1) [
1] are the most attractive, due to their high average operating voltage 3.8–3.9 V vs. Na
+/Na, high theoretical capacity of 128 mAh·g
−1, and exceptional electrochemical stability upon cycling. Na
3V
2(PO
4)
2F
3 is the end member of this series with x = 0; it has the highest average operating voltage of 3.89 V vs. Na
+/Na [
2]. Theoretical energy density of Na
3V
2(PO
4)
2F
3 is 507 Wh/kg, which is comparable with that of LiFePO
4 (580 Wh·kg
−1) and LiMn
2O
4 (480 Wh·kg
−1). However, the polyanionic cathode materials usually exhibit lower electrical conductivity than the corresponding oxides, that worsens their high-rate capability. Indeed, the electrical conductivity of Na
3V
2(PO
4)
2F
3 is only σ = 1.2·10
−7 S·cm
−1 with the electronic conductivity being much lower than the ionic one (2·10
−11 S·cm
−1 vs. 1.2·10
−7 S·cm
−1) [
3]. Therefore, the enhancement of the electronic conductivity of Na
3V
2(PO
4)
2F
3 is of higher priority.
Several approaches are known to improve electrical conductivity, and hence, the electrochemical properties of cathode materials such as surface and bulk modification. Surface modification is usually carried out by electroconductive carbon. However, the carbon coating approach has no beneficial effects on the bulk electronic conductivity and alkali ion mobility. Meanwhile, the bulk modification realized by homo- or heterovalent doping can influence the conductivity, voltage, capacity, and the rate capability of the electrode materials due to the changes in their intrinsic characteristics, including crystal and electronic structure, and affect the size of the alkali-ion diffusion channels. Depending on the chosen atom-dopant, one can consider n-doping, which introduces occupied electronic states close to the conduction band (CB), and p-doping, which introduces empty states close to the valence band (VB), thus changing the Fermi level, and the chemical and physical properties.
In recent years, a lot of experimental studies have been made on electrode materials for lithium-ion batteries tuned with rare earth elements (RE)—Y, La, Ce, Nd, Sm, Yb, etc., known for their large radius, high charge, and strong self-polarization ability. Among studied materials there are Li
3V
2(PO
4)
3 [
4], LiCoO
2 [
5], LiNi
1/3Co
1/3Mn
1/3O
2 [
6], LiFePO
4 [
7], LiMn
2O
4 [
8,
9], etc. The introduction of the RE ions into the structure of the electrode materials leads to an increase in the unit cell volume due to the significant difference between ionic radii of d-metal (about 0.53–0.78 Å) and RE (about 0.87–1.03 Å), and the sizes of the alkali ion diffusion channels. Such changes cause a facile Li-ion diffusion, and as a result, increase electrical conductivity. In general, in order to form an extended region of substitutional solid solutions, the difference in ionic radii of atoms must not exceed 15% (the Hume-Rothery rule). However, in some cases, metal ions are able to replace one another in a limited range of compositions, although the difference in their radii exceeds 60% [
10]. The influence of the Ce
4+- and La
3+-doping on the crystal and electronic structure of LiCoO
2 has been investigated by the first-principles calculations [
11]. The results indicated that Ce
4+ and La
3+-doped LiCoO
2 has expanded Li
+ slab distances, thus decreasing the Li
+ migration energy barrier; the diffusion coefficient was improved by 4 and 7 orders of magnitude, respectively. The calculations of the electronic structure showed that the doped samples possess a smaller band gap compared to pure LiCoO
2.
In the case of sodium-vanadium fluorophosphates, the surface modification with different carbon materials was widely studied and some promising results in the improvement of their electrochemical properties were achieved [
12,
13,
14]. In contrast, there are only a few works on the effect of the coating by inorganic materials on their electrochemical properties. It has been shown that the RuO
2-coated Na
3V
2O
2(PO
4)
2F displays the charge-transfer resistance which is more than 4 times lower compared to the bare material, higher reversible capacity, and better rate capability [
15]. On the other hand, the bulk modification of Na
3V
2(PO
4)
2F
3 was conducted by a partial substitution of V
3+ (r
V3+ = 0.64 Å) by Al
3+ (r
Al3+ = 0.54 Å) [
16,
17], which stabilizes its crystal structure and increases the operating voltage [
16]. Interestingly, the Al-doped Na
3V
2−zAl
z(PO
4)
2F
3 material exhibits improved kinetics and an additional Na
+ ion insertion at low voltages up to the Na
4V
2−zAl
z(PO
4)
2F
3 composition due to the decreased energy barrier [
17]. A successful doping of Na
3V
2(PO
4)
2F
3 by the Y
3+ ions (r
Y3+ = 0.9 Å) leads to an enhancement of its electrical conductivity and electrochemical properties [
18]. The excellent rate performance of Na
3V
1.9Y
0.1(PO
4)
2F
3/C could be a consequence of two factors: the enlarged diffusion channels for the Na
+ ions due to the expanded NVPF lattice parameters by the larger Y
3+ ions, and the improvement of the intrinsic electronic conductivity due to the weaker Y–O bond compared to the V–O bond [
18].
In both cases, surface modification with RuO2 and ion doping with RE (Y3+), the mechanism of the improvement of the Na3V2(PO4)2F3 behavior is not well understood yet. Study of doping and coating strategies is of great significance for the further improvement of the electrochemical performance of Na3V2(PO4)2F3. Based on these considerations, in the present work, we studied the effect of the La3+ modification on the conductive and electrochemical properties of the Na3V2(PO4)2F3 cathode material.
2. Results and Discussion
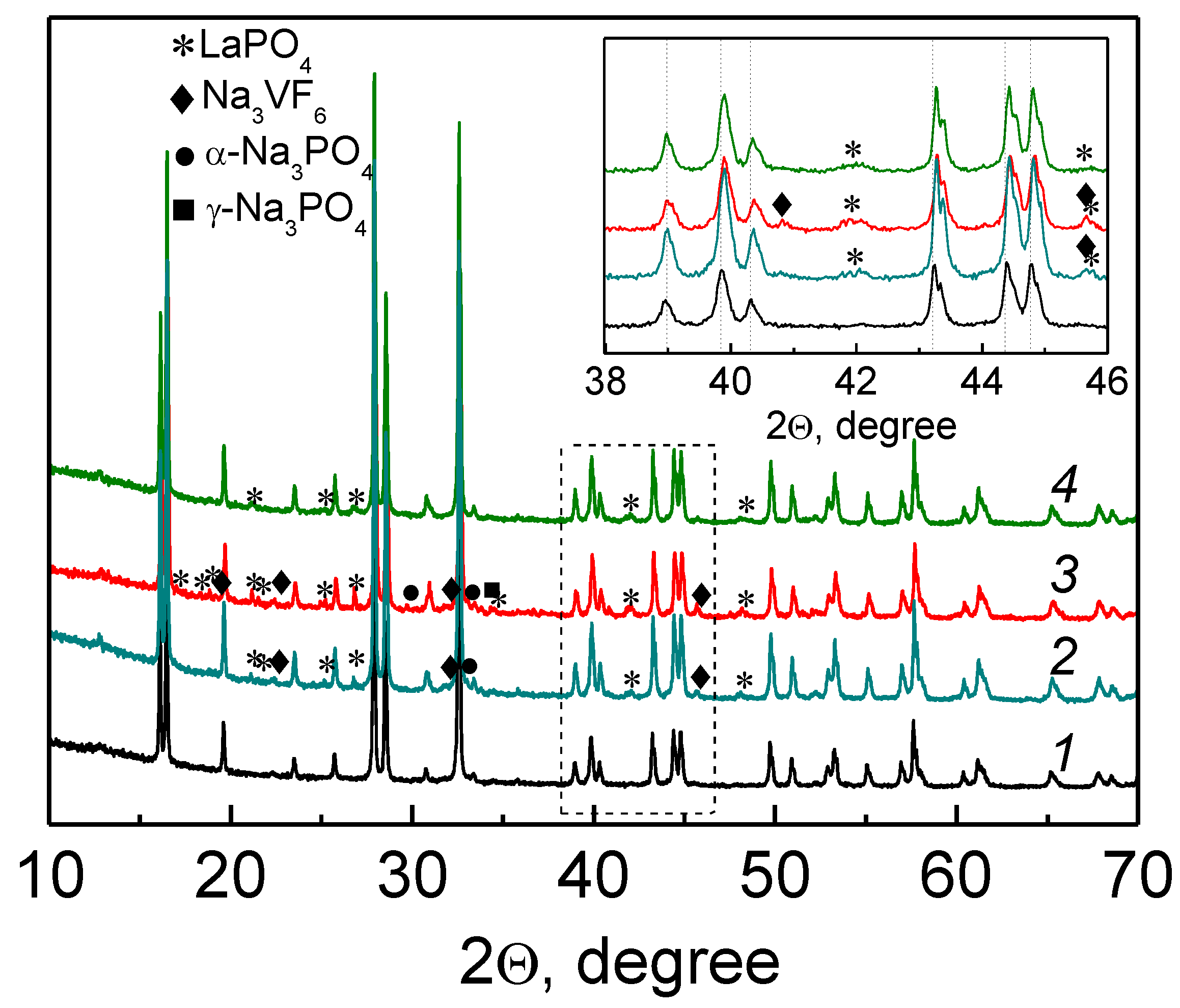
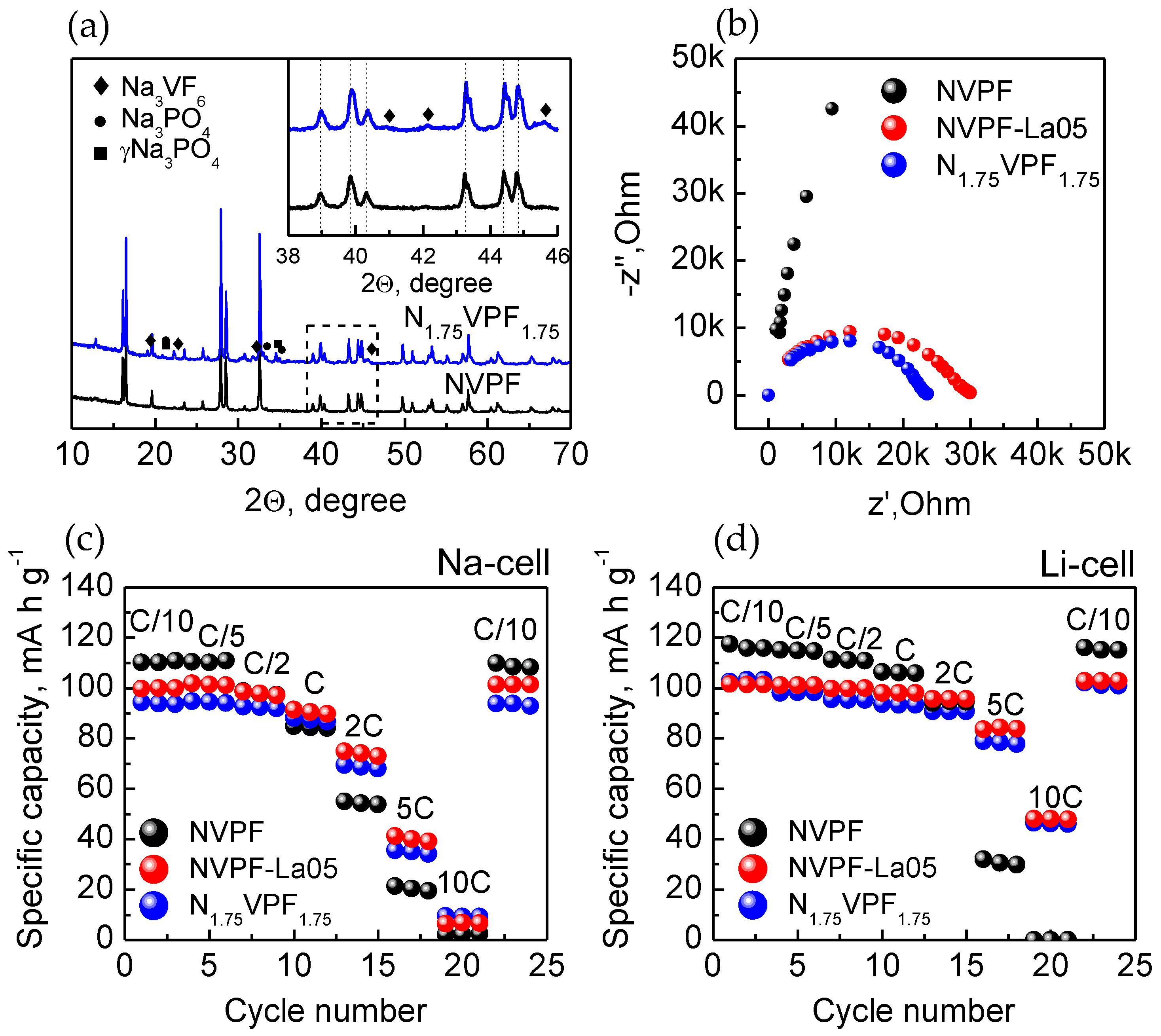
Figure 1 shows the XRD patterns of the initial Na
3V
2(PO
4)
2F
3 (further referred as NVPF) and the La-modified products Na
3V
2−xLa
x(PO
4)
2F
3 with x = 0.02, 0.05 (hereinafter, NVPF-La02 and NVPF-La05, respectively) prepared by the mechanochemically assisted solid-state synthesis (see
Section 3). All the samples are well-crystallized; however, only NVPF is a single-phase material ascribed to Na
3V
2(PO
4)
2F
3. On the X-ray patterns of the other two samples, the reflections of LaPO
4 [PDF 00-035-0731], Na
3VF
6 [PDF 00-029-1286], and Na
3PO
4 (including the cubic γ-Na
3PO
4 [PDF 01-031-1323] and the tetragonal Na
3PO
4 [PDF 01-072-7303] modifications) are present. The inset in
Figure 1 clearly demonstrates that the position of the reflections of the main Na
3V
2(PO
4)
2F
3 phase do not change for the La-modified samples, evidencing that doping has not been realized because of a large difference in the ionic radii of La
3+ (1.03 Å) and V
3+ (0.64 Å).
The structure of Na
3V
2(PO
4)
2F
3 is composed of the VO
4F
2 octahedra, which are bridged together by one F atom forming the V
2O
8F
3 bioctahedra, alternately connected by the PO
4 tetrahedra. This results in a stable 3D framework, in which the Na
+ ions can migrate along the [110] and [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10] directions. The structure can be described using two space groups: the tetragonal
P4
2/
mnm and the orthorhombic
Amam ones. The latter was proposed by Bianchini et al. [
19] using synchrotron radiation; it is the most accurate to consider a small orthorhombic distortion in Na
3V
2(PO
4)
2F
3 (
b/
a = 1.002). This structure preserves the framework but modifies the distribution of the Na
+ ions between three crystallographic positions in contrast to the
P4
2/
mnm S.G. with two sodium positions.
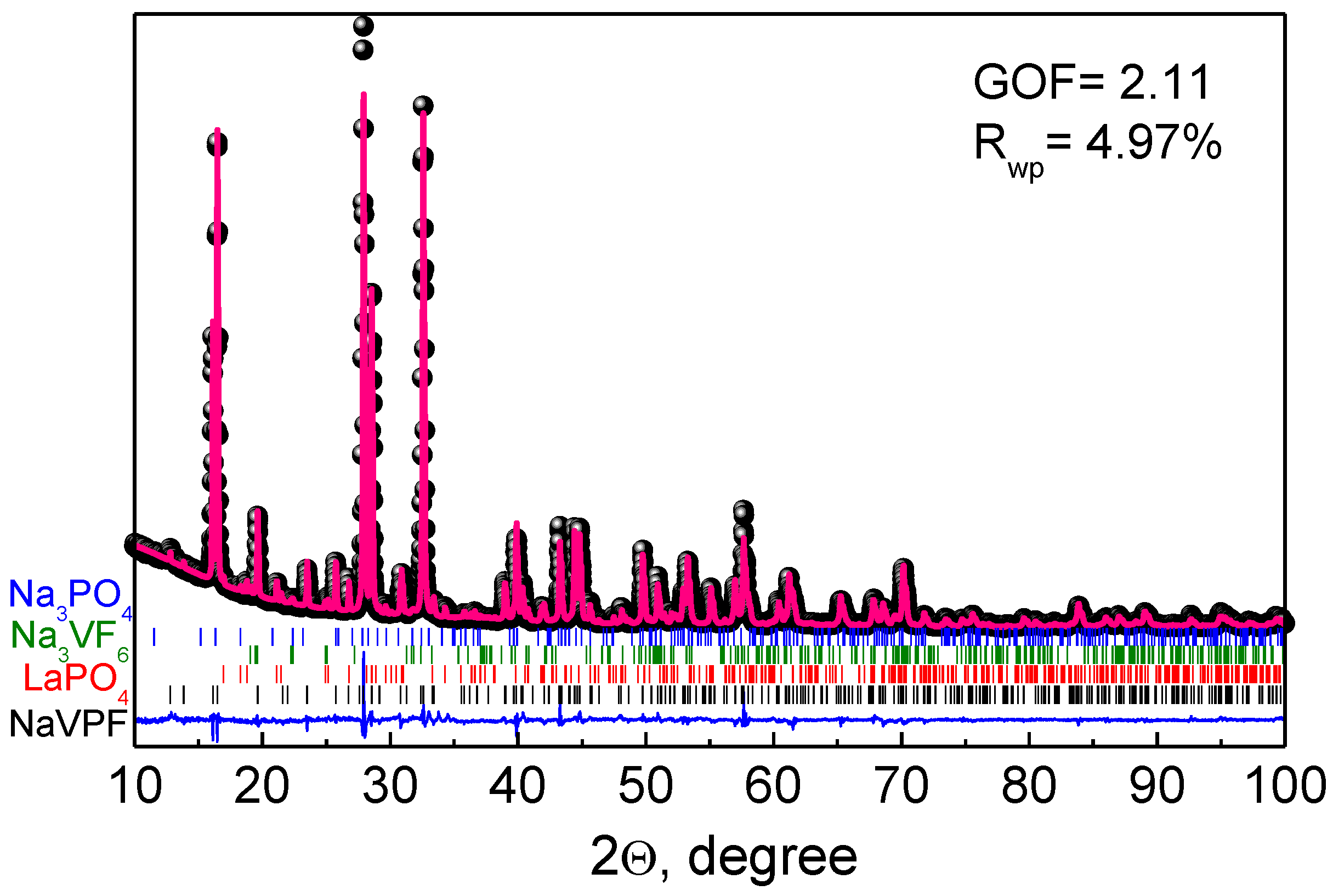
Figure 2 displays the Rietveld refined XRD pattern of NVPF-La05 with the quantitative phase analysis using the
P4
2/
mnm S.G., which is commonly used to describe the structure of Na
3V
2(PO
4)
2F
3 using the data of the regular XRD. The refined lattice parameters of the Na
3V
2(PO
4)
2F
3 main phase in all the samples along with the mass content of the impurities are shown in
Table 1. As can be seen, the lattice parameters of Na
3V
2(PO
4)
2F
3 in NVPF-La02 and NVPF-La05 coincide with that for pristine NVPF and are close to the literature data [
20]. Moreover, the estimated mass content of the LaPO
4 impurity is very close to the experimental amounts of LaPO
4 (1.1 wt % (2.1 mol %) and 2.7 wt % (5 mol %)) introduced into the NVPF-La02 and NVPF-La05 reagent mixtures, respectively. A slight deviation from the expected values comes from the presence of the other impurities: Na
3VF
6 and Na
3PO
4, which were not included in the refinement process in the case of NVPF-La02, because of their negligible quantities. Based on the observed results, we can conclude that the crystalline LaPO
4 and sodium-containing impurities are formed in the NVPF-La0.02 and NVPF-La0.05 samples, instead of the expected substitution V
3+ for La
3+.
The LaPO
4 surface coating of NVPF was performed according to the procedure described in Refs. [
21,
22] with minor changes (see Materials and Methods). Based on the XRD results (
Figure 1) and the quantitative phase analysis of the coated sample (hereinafter referred as NVPF/LPO) using the Rietveld method (
Table 1), it was found that the as-obtained composite material contains only two phases: Na
3V
2(PO
4)
2F
3 and LaPO
4. The reflection positions of the main phase maintain unchanged (see the insert in
Figure 1), and the estimated mass amount of LaPO
4 corresponds to the theoretical value (2.5 wt % (4.5 mol %)) (
Table 1).
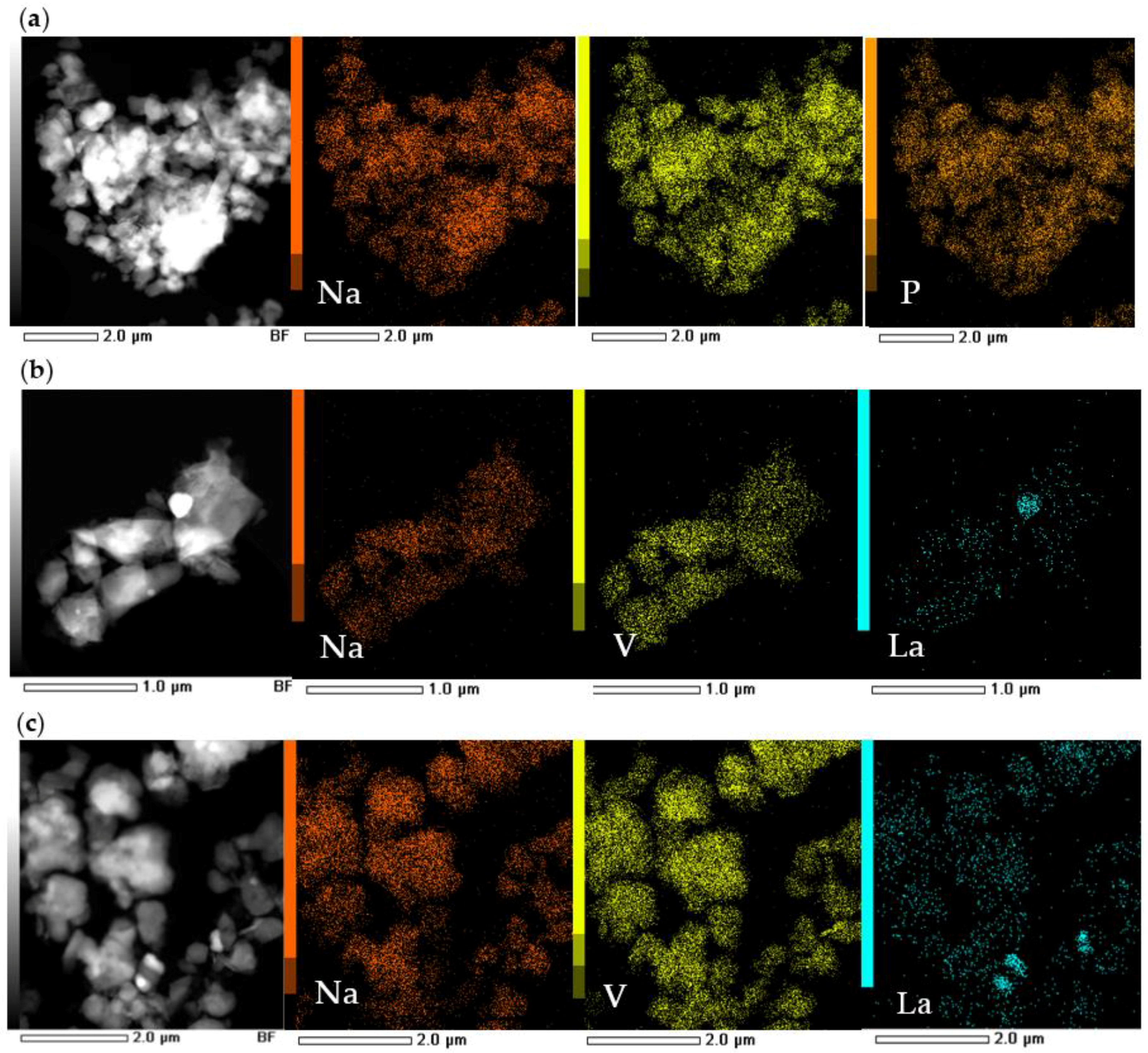
The spatial distribution of the elements in the NVPF-La05 and NVPF/LPO samples was studied by STEM combined with the EDX element mapping. As follows from
Figure 3, the sodium and vanadium are uniformly distributed in all the samples. LaPO
4 forms the individual crystallites with an average particle size of 200–300 nm, which are randomly distributed on the surface of the particles of the NVPF-La05 sample. For the coated sample, the LaPO
4 surface distribution is also not uniform.
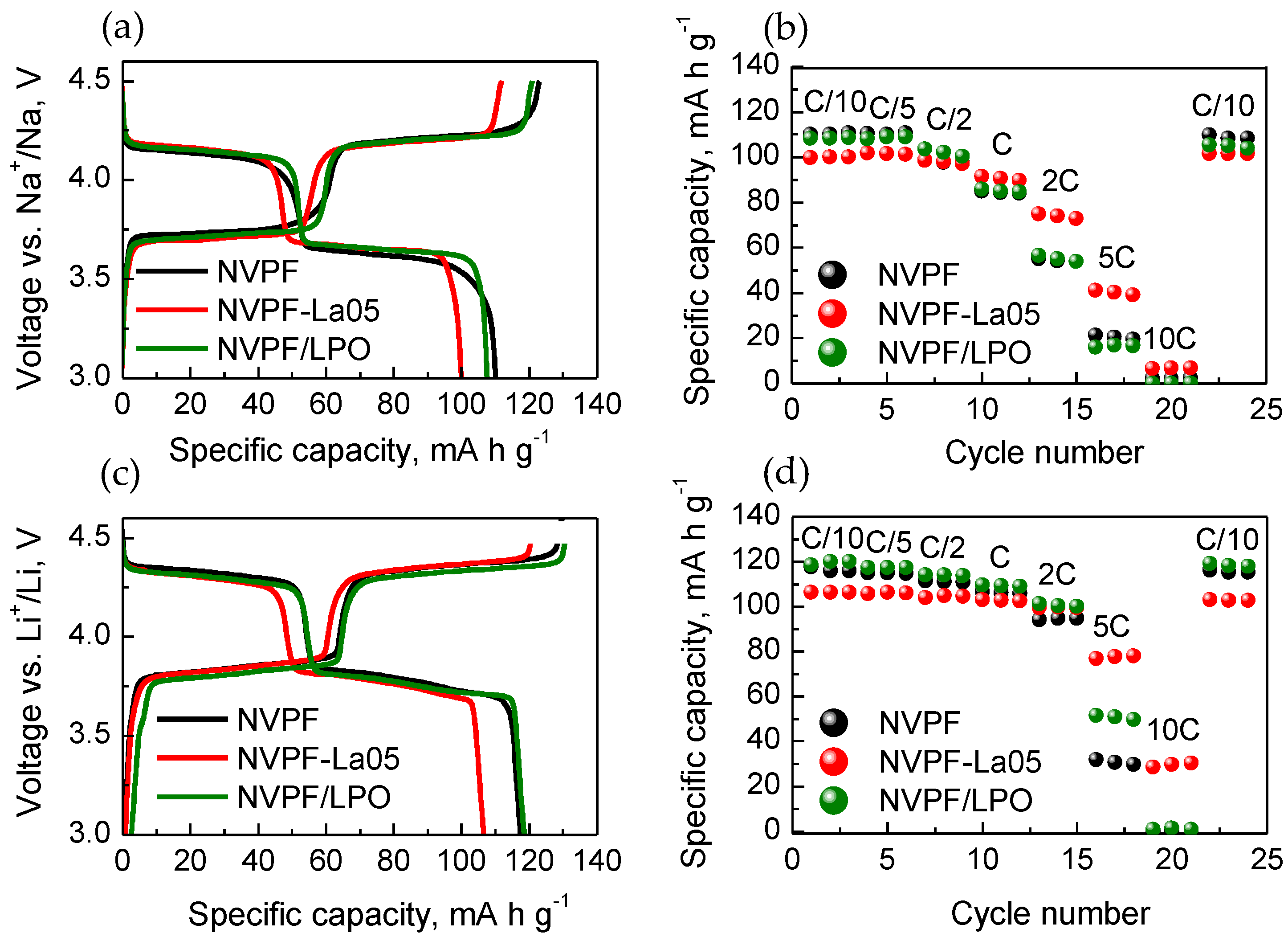
Figure 4a,c show the initial charge and discharge profiles, and
Figure 4b,d display the high-rate performance of pristine NVPF and the LaPO
4-modified samples prepared by direct solid-state synthesis (NVPF-La05) and by the coating procedure (NVPF/LPO) in Na and Li cells. The charge-discharge profiles of all the samples in the 3.0–4.5 V range exhibit a rather similar shape. The curves consist of two pseudo-plateaus at the average voltages of 3.6–3.7 V and 4.2 V for a Na cell, and at a voltage ~0.2 V higher for a Li cell. This similarity evidences that NVPF is a single electrochemically active phase within this voltage range. It is seen that polarization is rather small for all three samples, indicating facile alkali-ion (de)insertion reactions. The discharge capacity of the NVPF, NVPF-La05, and NVPF/LPO samples at the C/10 rate are equal to 111 mAh·g
−1, 100 mAh·g
−1, 108 mAh·g
−1 in a Na cell and to 118 mAh·g
−1, 107 mAh·g
−1, 117 mAh·g
−1 in a Li cell, respectively. Although the initial discharge capacity of NVPF-La05 is smaller than that of the pristine sample, this value corresponds to the mass quantity of the electrochemically active component—Na
3V
2(PO
4)
2F
3—in this sample. Vice versa, this sample is characterized by the best high-rate performance. When the cycling rate is increased to 1C, the specific discharge capacity of NVPF-La05 is 92 mAh·g
−1 when cycled in a Na-cell, and 103 mAh·g
−1 when cycled in a Li-cell. At the 5C rate, the material shows the capacity of 41 mAh·g
−1 and 77 mAh·g
−1 when cycled in Na and Li cells, respectively. For comparison, the specific discharge capacity of the pristine sample NVPF is 85 mAh·g
−1 in a Na cell and 106 mAh·g
−1 in a Li cell at 1C; 21 mAh·g
−1 and 32 mAh·g
−1 in Na and Li cells at 5C. The behavior of the LaPO
4-coated sample is close to that of the pristine material: the specific discharge capacity is 16 mAh·g
−1 and 51 mAh·g
−1 at the 5C rate when cycled in Na and Li cells, respectively.
The improved cyclability of NVPF-La05 at high rates is associated with an increase in its electrical conductivity. There are some publications devoted to the use of LaPO
4 as a coating material to increase the high-rate capability of the lithium-rich cathode materials. However, the improvement was related to the protective activity of LaPO
4 from the electrode-electrolyte interaction [
21,
22]. On the other hand, some authors associate it with the increase in electrical conductivity due to the high ionic conductivity of LaPO
4 [
23], but this statement is contradictory [
24]. We were unable to find information on the high ionic conductivity of LaPO
4. Moreover, the precipitation of LaPO
4 on the surface of the pristine NVPF material does not result in an improvement of its cyclability at high rates (
Figure 4b,d).
Interestingly, the effect is more pronounced upon cycling the materials in a Li cell, when the Li
+/Na
+ mixed electrolyte is formed after the first charge. This phenomenon may indicate a better kinetic of the mixed intercalation of the Li
+ and Na
+ ions. The authors [
25] have shown that for the alkali ions of different size the variation in the reaction energetics for insertion/extraction in AVPO
4F may arise from the different contributions of the ion desolvation on the one hand, and transition of ions through the adsorbate layer/electrode interface on the other. Since the ionic radius of the Li
+ ions is smaller than that of the Na
+ ions, they have higher desolvation energy, but at the same time, they can more easily penetrate through the SEI. Earlier, we showed that Na
3V
2(PO
4)
2F
3 displayed good cycleability and high-rate capability when cycled in a hybrid Na/Li cell [
26]. The study of the structure and the composition of the charged and discharged samples pointed to the preservation of the initial structure, the occurrence of a negligible Na/Li electrochemical exchange, and a predominant sodium-based cathode reaction. Thus, a better kinetics of the cooperative Na/Li (de)intercalation in the mixed electrolyte might be explained by a competitive effect of the desolvation energy and a penetration rate through the SEI.
In order to find the reason of the high-rate capability improvement of NVPF-La05, EIS measurements were performed.
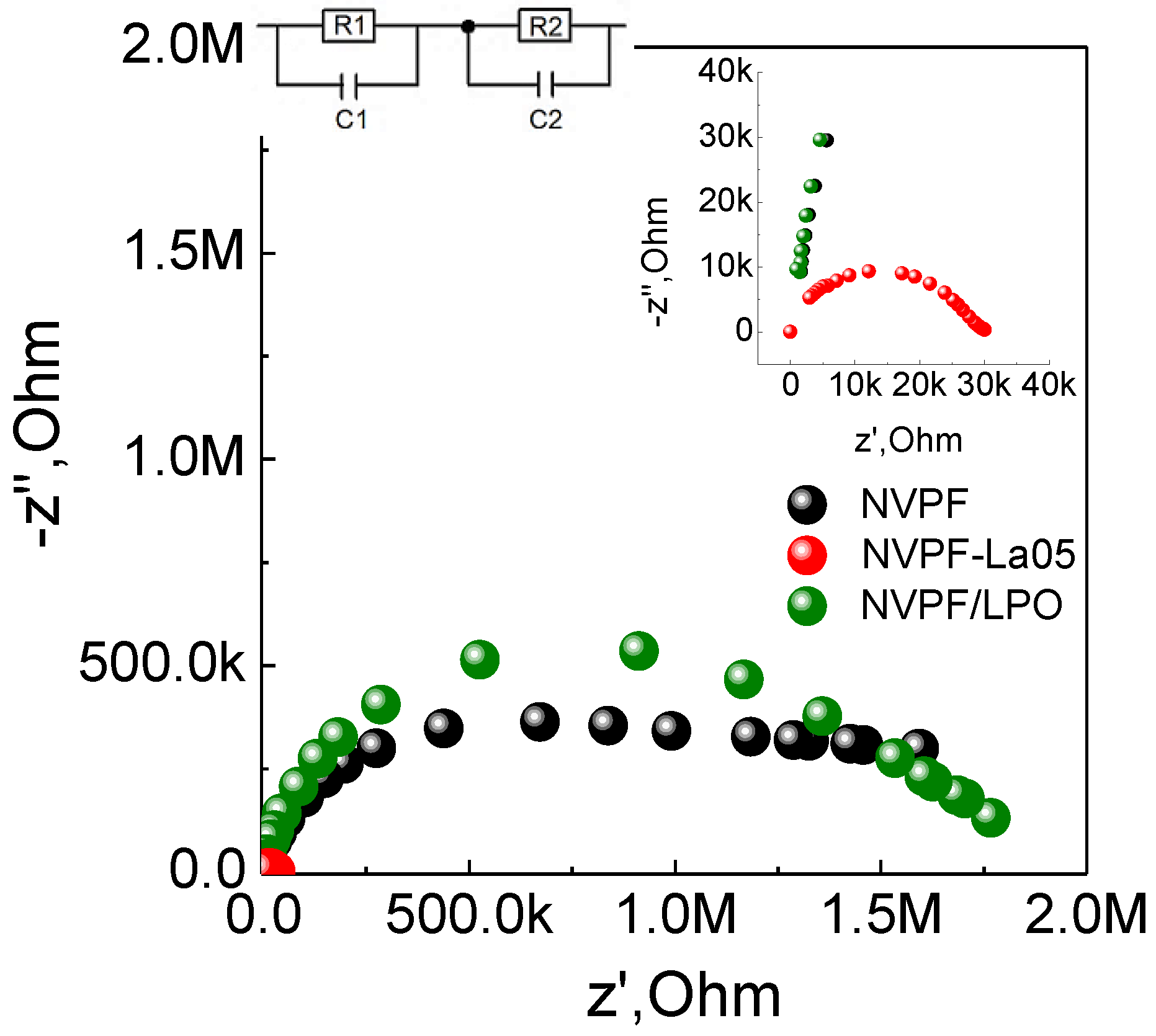
Figure 5 represents the Nyquist plots of the pristine NVPF, the La-modified NVPF-La05 and coated NVPF/LPO samples, and the inset in
Figure 5 shows the high-frequency region. The impedance spectra consist of two semicircles; the corresponding equivalent circuit scheme is presented in
Figure 5. The first part (R
1C
1) corresponds to the bulk resistance and the capacity of the individual grains (crystallites) of the polycrystalline samples, and the second part (R
2C
2) to the resistance and capacity of the grain boundaries. NVPF-La05 possesses the lowest total resistance, which is about two orders of magnitude lower than those of the pristine NVPF and NVPF/LPO samples. This correlates with the nice high-rate cycling performance of NVPF-La05, and the worse performance of NVPF/LPO, thereby confirming the low electrical conductivity of LaPO
4.
It has been shown that, besides the formation of the surface LaPO4 phase, NVPF-La05 has additional impurity phases containing sodium, vanadium and phosphorous ions, while NVPF/LPO does not. Since the substitution of V3+ by La3+ in the Na3V2(PO4)2F3 structure does not occur, the NaF/VPO4 ratio in NVPF-La05 becomes slightly higher than in the stoichiometric one (>3/2). We assumed that such non-stoichiometry could lead to the formation of surface Na-V-P impurity phases, probably with high conductivity, which influences the electrical conductivity of the cathode material, rather than that of LaPO4.
To confirm this assumption, we prepared another sample with a highly stoichiometric composition Na
1.75V(PO
4)F
1.75 (N
1.75VPF
1.75) corresponding to Na
3.5V
2(PO
4)
2F
3.5 with the NaF/VPO
4 ratio close to that in NVPF-La05, using the same synthesis procedure.
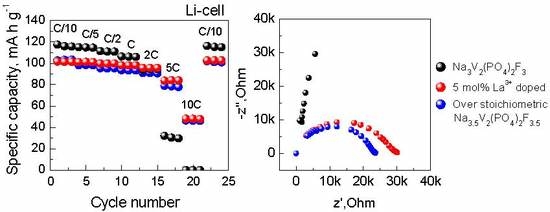
Figure 6 represents the results of XRD, EIS, and the discharge capacity vs. cycling rate (C/10−10C) plots for N
1.75VPF
1.75 cycled in the Na and Li cells in comparison with the pristine NVPF and NVPF-La05. According to the XRD data, the sample contains the same impurity phases as those observed in NVPF-La05, except LaPO
4: Na
3VF
6—7.1(3)% and Na
3PO
4—12.2(7)%. The Rietveld refined lattice parameters of the main phase—Na
3V
2(PO
4)
2F
3 (80.7(6)%) are as follows:
a = 9.0389(2) Å,
b = 10.7533(3) Å,
V = 878.55(4) Å
3, which match well with those of the pristine NVPF sample. In
Figure 6b, the Nyquist plots of the NVPF-La05 and N
1.75VPF
1.75 samples are compared in the high-frequency region. Both spectra have similar semicircles. The capacitance calculated from the maximum frequency (ω = 1/RC) is about 4·10
−10 F for both NVPF-La05 and N
1.75VPF
1.75, which is within the typical values for the grain boundaries capacitance [
27]. Thus, this determines that the grain boundary resistance dominates the overall impedance. It can be seen that the total resistance for both samples is of the same order of magnitude (2.3·10
4 Ω for N
1.75VPF
1.75 and 2.9·10
4 Ω for NVPF-La05), which is much lower than those of the pristine (NVPF) and the LaPO
4-coated (NVPF/LPO) samples. The higher conductivity of N
1.75VPF
1.75 results in its superior high-rate performance, as well as for NVPF-La05. When the cycling rate is increased from C/10 to C, the specific discharge capacity of N
1.75VPF
1.75 decreases by 7% only; this is comparable with the results obtained for NVPF-La05.
Summarizing all the above, our assumption regarding the positive effect of the conductive surface impurity phases on the electrochemical performance of the Na
3V
2(PO
4)
2F
3 cathode material has been proven. A similar effect has been previously observed in the studies of the LiFePO
4-based cathode materials. Ceder et al. [
28] created amorphous Fe-containing lithium phosphate coating with high Li
+ mobility on the surface of the LiFePO
4 particles and showed that this coating is responsible for the extremely high rate performance of LiFePO
4 due to removing anisotropy of the surface properties and enhancing the delivery of the Li
+ ions to the bulk of LiFePO
4. Nazar et al. [
29] studied the doped Li
xZr
0.01FePO
4 compositions and revealed that the surface metal-rich phosphide impurity phase could be responsible for the enhanced conductivity by formation of the percolating nano-network. The positive effect of the in situ formed surface impurity phase Li
3V
2(PO
4)
3 with high ionic conductivity on the improvement of high-rate performance of LiVPO
4F was also established in our earlier study [
30].
According to the literature data, the as-observed impurity phases Na
3PO
4 and Na
3VF
6 are characterized by high ionic and electronic conductivity, respectively [
30,
31,
32,
33]. Na
3PO
4 is a Na
+ ion conductor with σ
ion ~10
−7 S·cm
−1 at room temperature and σ
ion ~10
−3 S·cm
−1 at 300 °C, having high diffusion coefficient D
Na+ ~1.22·10
−6 cm
2·s
−1 [
31,
32], and thus, can improve the surface Na
+-ion conductivity of the Na
3V
2(PO
4)
2F
3 composite cathode and facilitate the Na
+-ion exchange at the electrode-electrolyte interface. On the other hand, Na
3VF
6 exhibits metal-like behavior; the resistivity of the material is 1.1·10
−18 Ω·cm at room temperature [
33]; therefore it can improve the electronic conductivity of the cathode material forming the percolating nano-network as the metal-rich phosphides do [
29]. At the same time, the formation of the amorphous sodium and lithium conductive phases cannot be excluded.
Thus, the positive effect of the surface phases on the conductivity and electrochemical performance of the Na3V2(PO4)2F3-based cathode material has been established; however, its mechanism requires further investigation.
3. Materials and Methods
The La
3+-modification of Na
3V
2(PO
4)
2F
3 (NVPF) was realized in two ways: by ‘doping’ and by surface coating. The ‘La-doped’ sodium vanadium fluorophosphates Na
3V
2−xLa
x(PO
4)
2F
3 with x = 0.02, 0.05 (NVPF-La02 and NVPF-La05, respectively) were prepared by a two-step mechanochemically-assisted solid-state synthesis using VPO
4 and LaPO
4 as the intermediates, according to the following reactions:
The preliminary solid-state mechanical activation (MA) of the reagent mixtures was performed by means of a high-energy AGO-2 planetary mill (~900 rpm) with stainless steel jars and balls for 5 min in an Ar atmosphere. The activated mixtures (1) and (2) were annealed at 800 °C for 4 h, while the mixture (3) was heat treated at 650 °C for 2 h in an Ar flow with slow cooling to room temperature.
To prepare the LaPO4-coated Na3V2(PO4)2F3, the pristine sample was dispersed in distilled water under magnetic stirring for 30 min. Then stoichiometric amounts of the water solutions of La(NO3)3 and (NH4)2HPO4 were consistently added to the suspension of Na3V2(PO4)2F3 under stirring for 30 min. The obtained solution was filtered, and the wet powder was dried at 90 °C in the air until the solvent was completely removed. Finally, the dried powders were further annealed at 400 °C for 4 h in the Ar atmosphere to get the LaPO4-coated Na3V2(PO4)2F3 (hereinafter referred as NVPF/LPO). The amount of LaPO4 in the composite material was 2.5 wt %, which is close to the La content in NVPF-La05.
X-ray powder diffraction (XRD) patterns of the as-prepared samples were recorded by a D8 Advance Bruker diffractometer (Bruker AXS Gmbl, Karlsruhe, Germany) with a high-rate detector Lynx Eye, Cu
Kα1,2 radiation (
α1 = 1.5406 Å,
α2 = 1.5445 Å) within the 10–100° (2
θ) range with a step of 0.02° and uptake time of 0.3–1.0 s. The structural refinement of the XRD data was carried out by the Rietveld method using the TOPAS software [
34]. The procedure was started with the refinement of the lattice parameters and atomic positions of the pristine NVPF. The thermal displacement parameters for all atoms were refined just once and then fixed at their final values; the thermal parameters for the atoms of the same elements were taken to be equal. The structural refinement of the main phase in the multi-phase samples was performed in a similar manner; all thermal parameters were kept fixed at the values extracted from the structural refinement of the pristine sample. STEM-EDS mapping was carried out using a JEM-2200FS transmission electron microscope (JEOL Co., Ltd., Tokyo, Japan) with an accelerating voltage of 200 kV and magnification of 0.1 nm.
Electrochemical impedance spectroscopy (EIS) study of the as-prepared samples was performed using an LCR-meter E7-25 (MNIPI, Minsk, Belarus) in the frequency range of 25 Hz–1 MHz in pellets with the Ag electrodes at room temperature. Electrochemical cycling was performed in a galvanostatic mode both in the Na and Li cells within the 3.0–4.5 V range at the C/10–10C rates. The composite cathode materials were fabricated by mixing 75 wt % active material with 20 wt % Super P (Timcal, Bodio, Switzerland) and 5 wt % PVDF/NMP binder. The mixed slurry was then pasted onto aluminum foil, dried in a vacuum oven at 90 °C, and then cut into small circular discs to obtain working electrodes. The total amount of carbon in the cathode mass was 20 wt %; mass loading ~1.5–2.0 mg·cm−2 and the electrode diameter of 10 mm were used throughout. Swagelok-type cells were assembled in an Ar-filled glove box with Na metal as an anode and 1 M NaPF6 solution in a mixture of ethylene carbonate and propylene carbonate 1:1 by weight as an electrolyte for the Na cells, and with Li metal as an anode and 1 M LiPF6 solution in a mixture of ethylene carbonate and dimethyl carbonate 2:1 by weight as an electrolyte for the Li cells. A glass fiber filter, Grade GF/C GE Healthcare UK Ltd., Little Chalfont, UK) was used as a separator.