A Ni/MH Pouch Cell with High-Capacity Ni(OH)2
Abstract
:1. Introduction
2. Experimental Setup
2.1. Cell Assembely
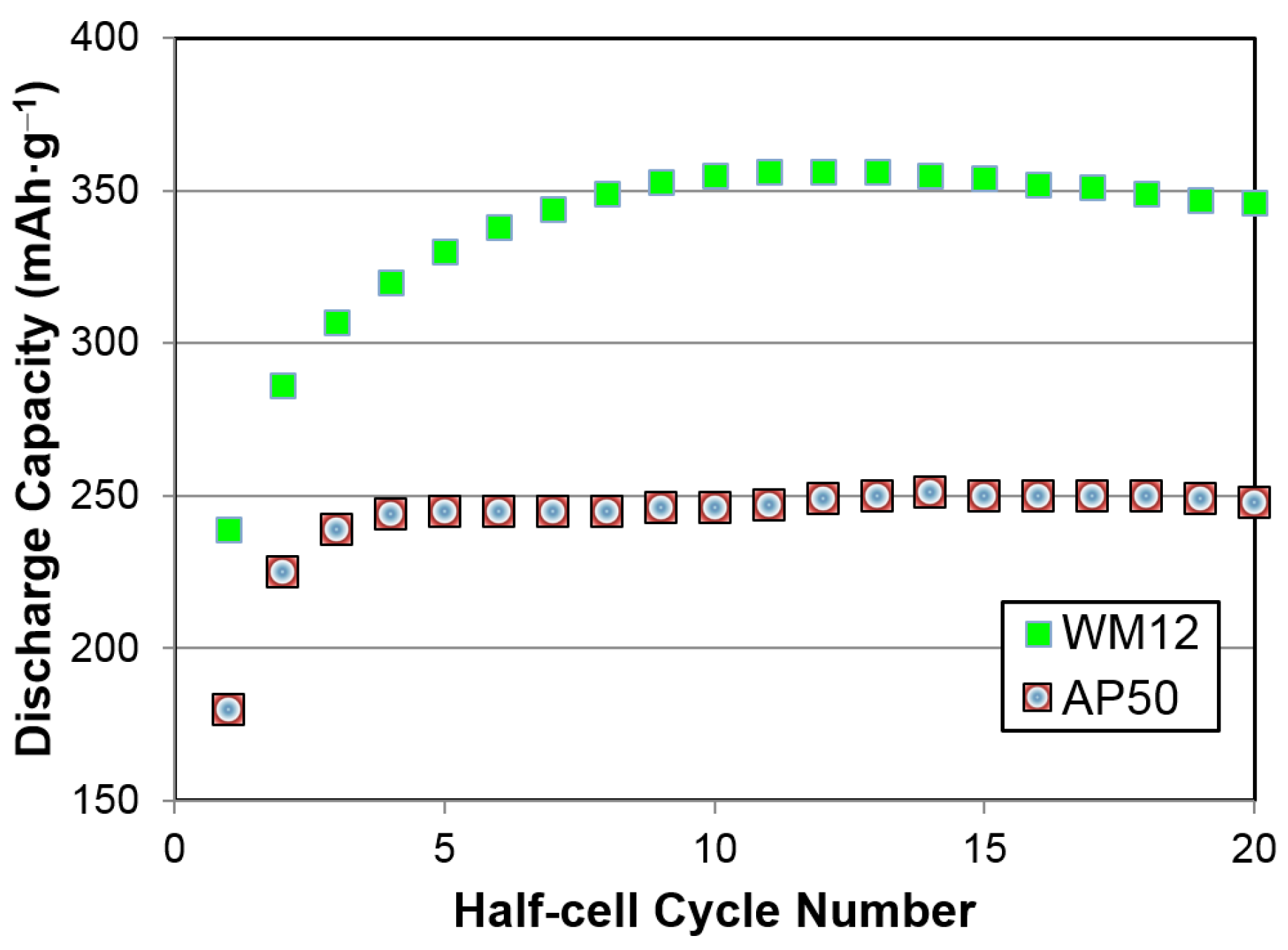
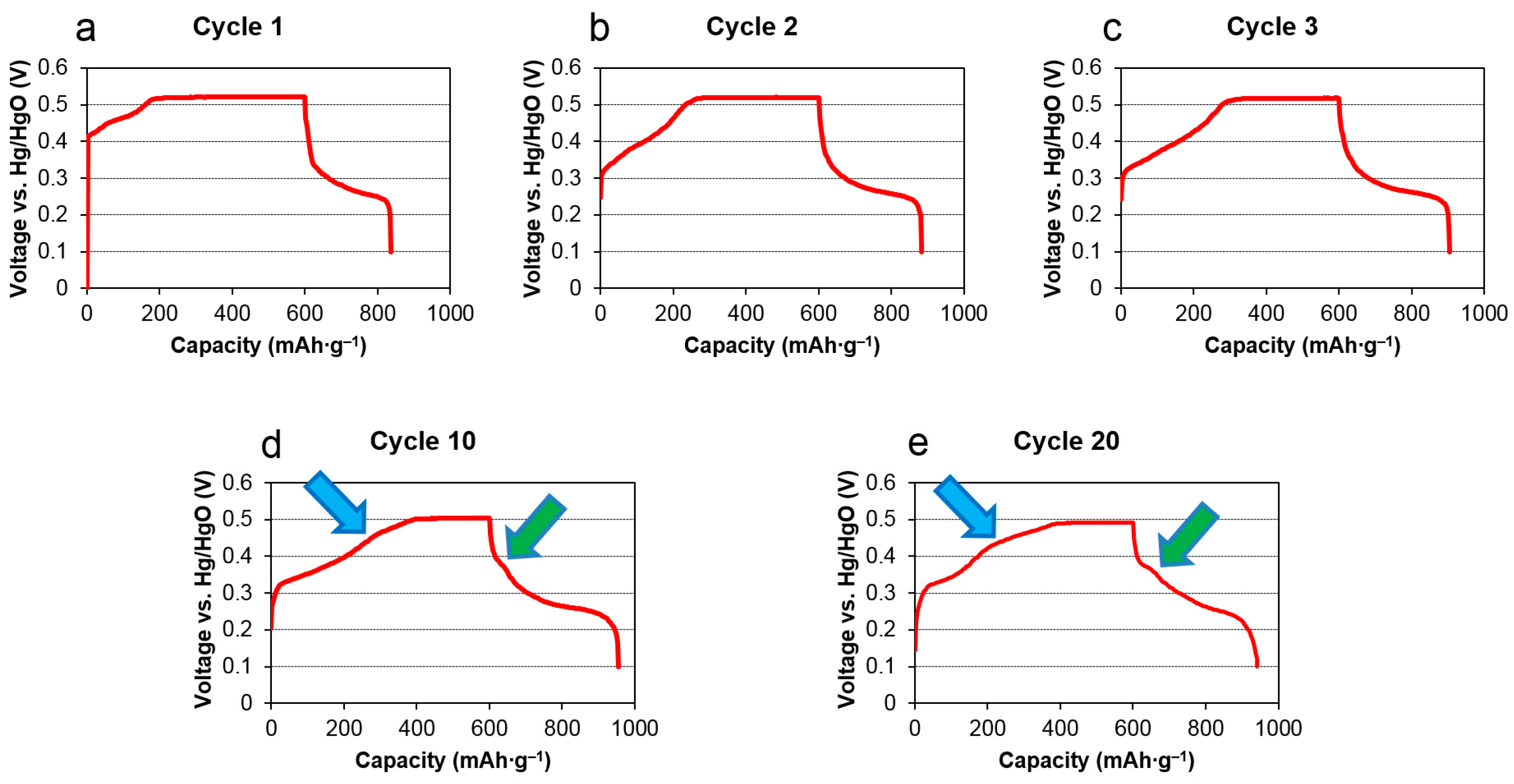
2.2. Activation Process
2.3. Charge Rate Capability
2.4. Discharge Rate Capability
2.5. Self-Discharge
2.6. Internal Resistance Measurement
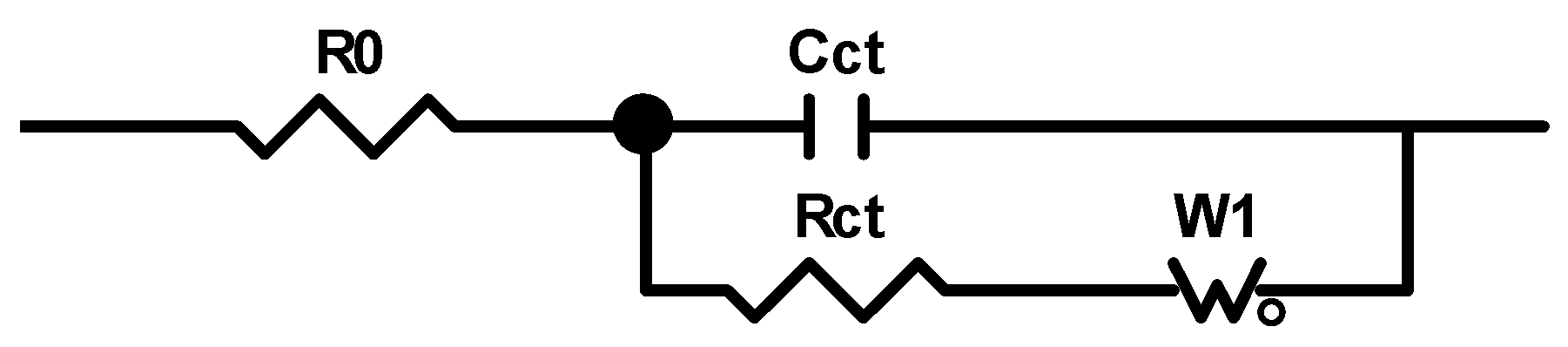
2.7. Charge-Transfer Resistance Measurement
3. Results and Discussion
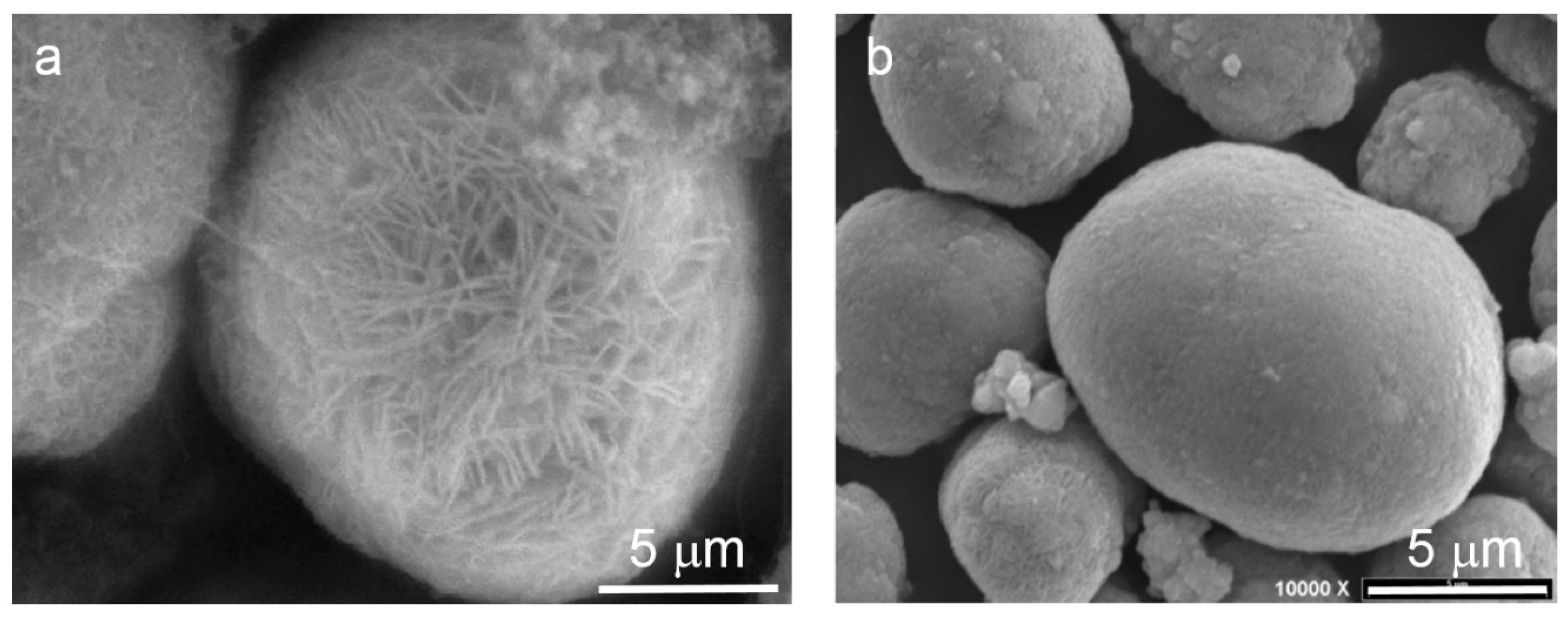
3.1. Ni(OH)2 Selection
3.2. Charge Rate Capability
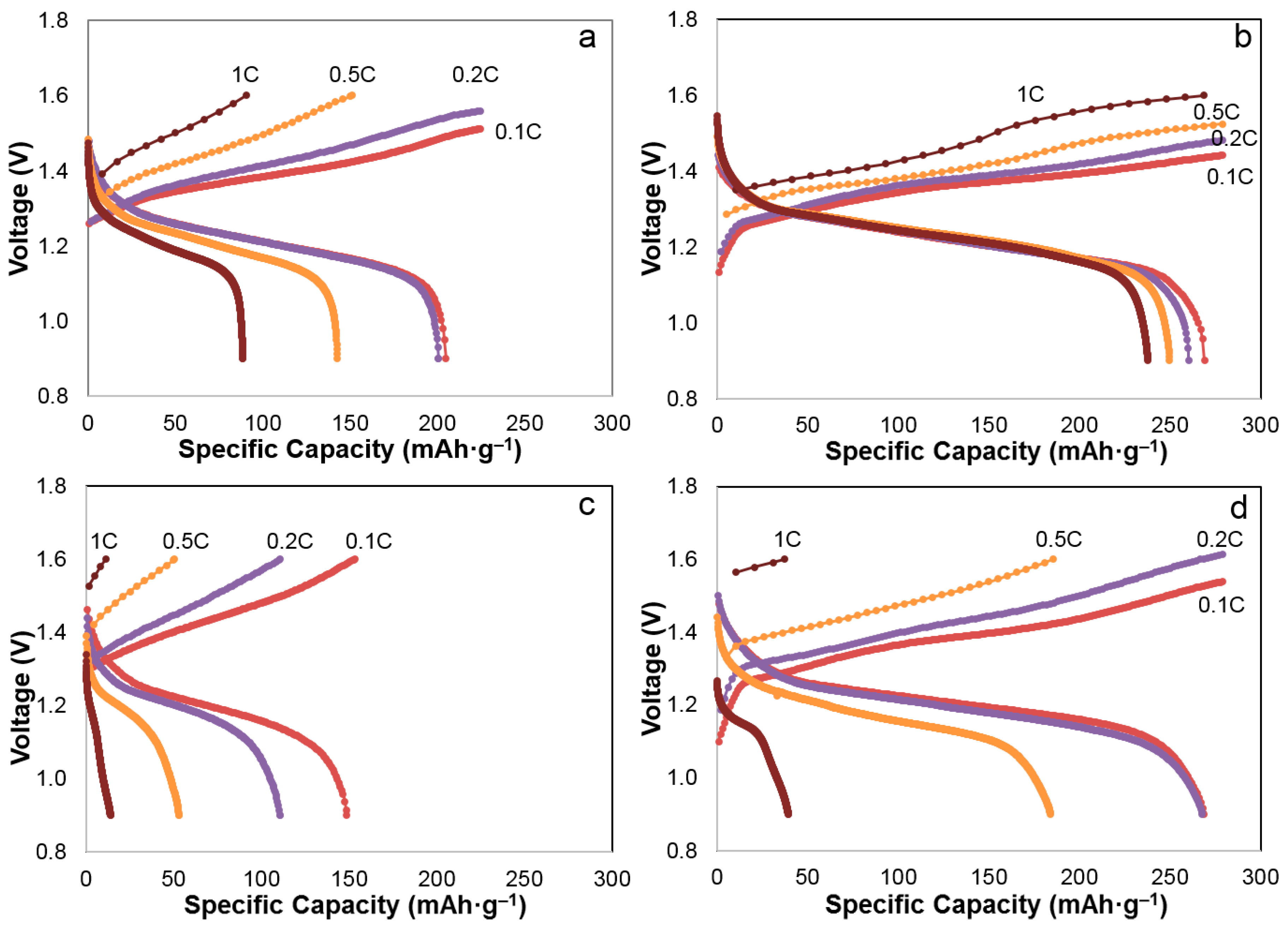
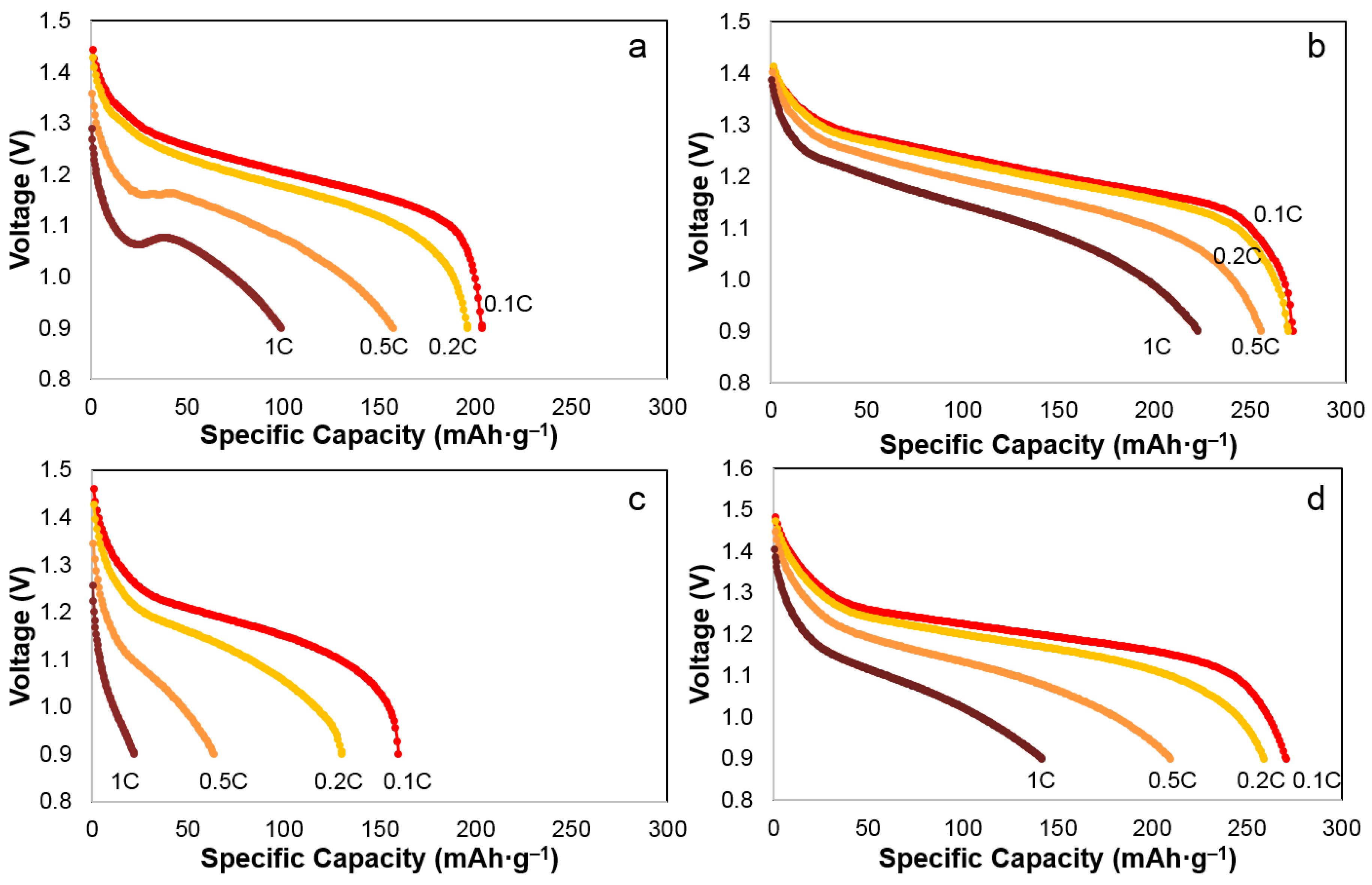
3.3. Discharge Rate Capability
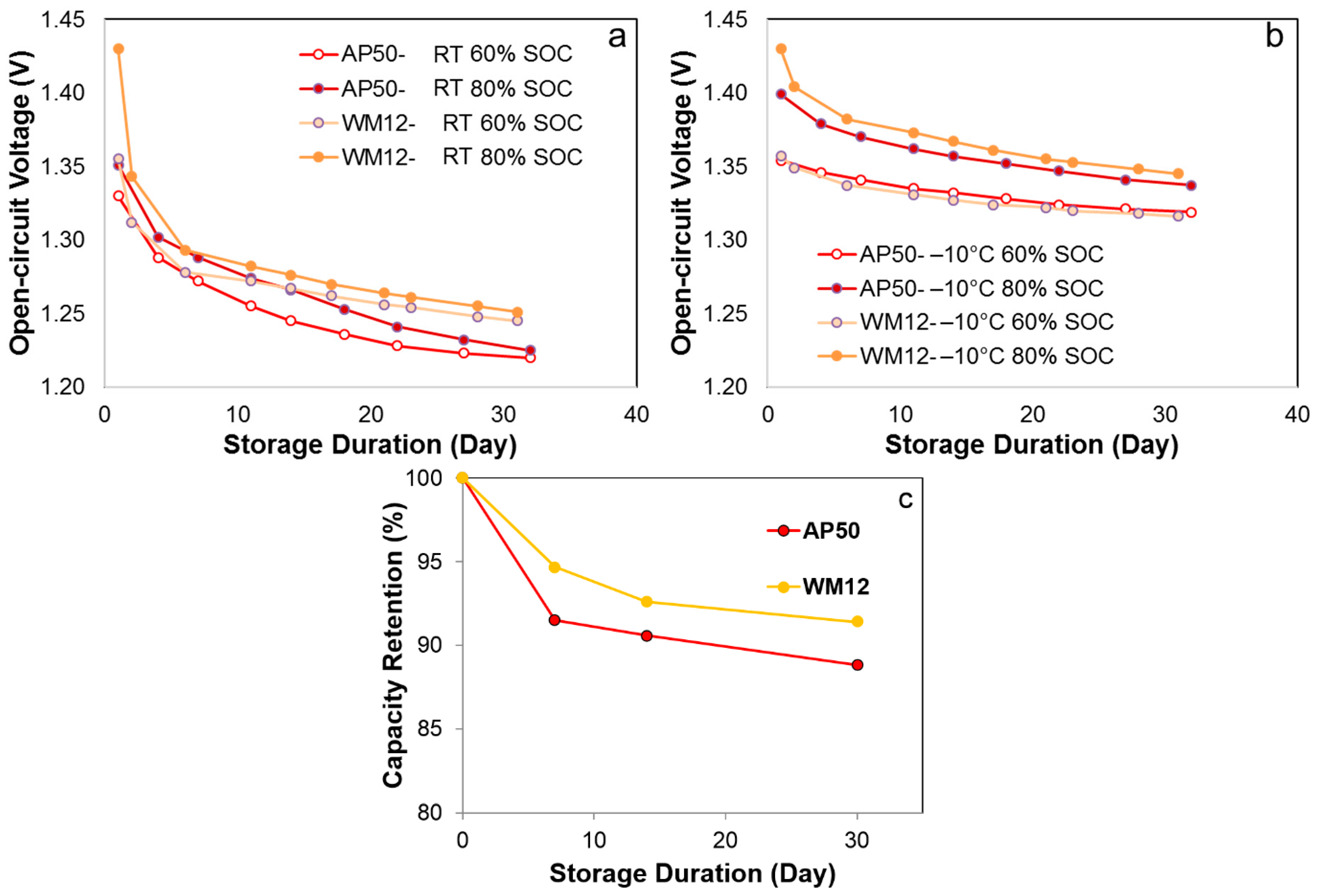
3.4. Self-Discharge
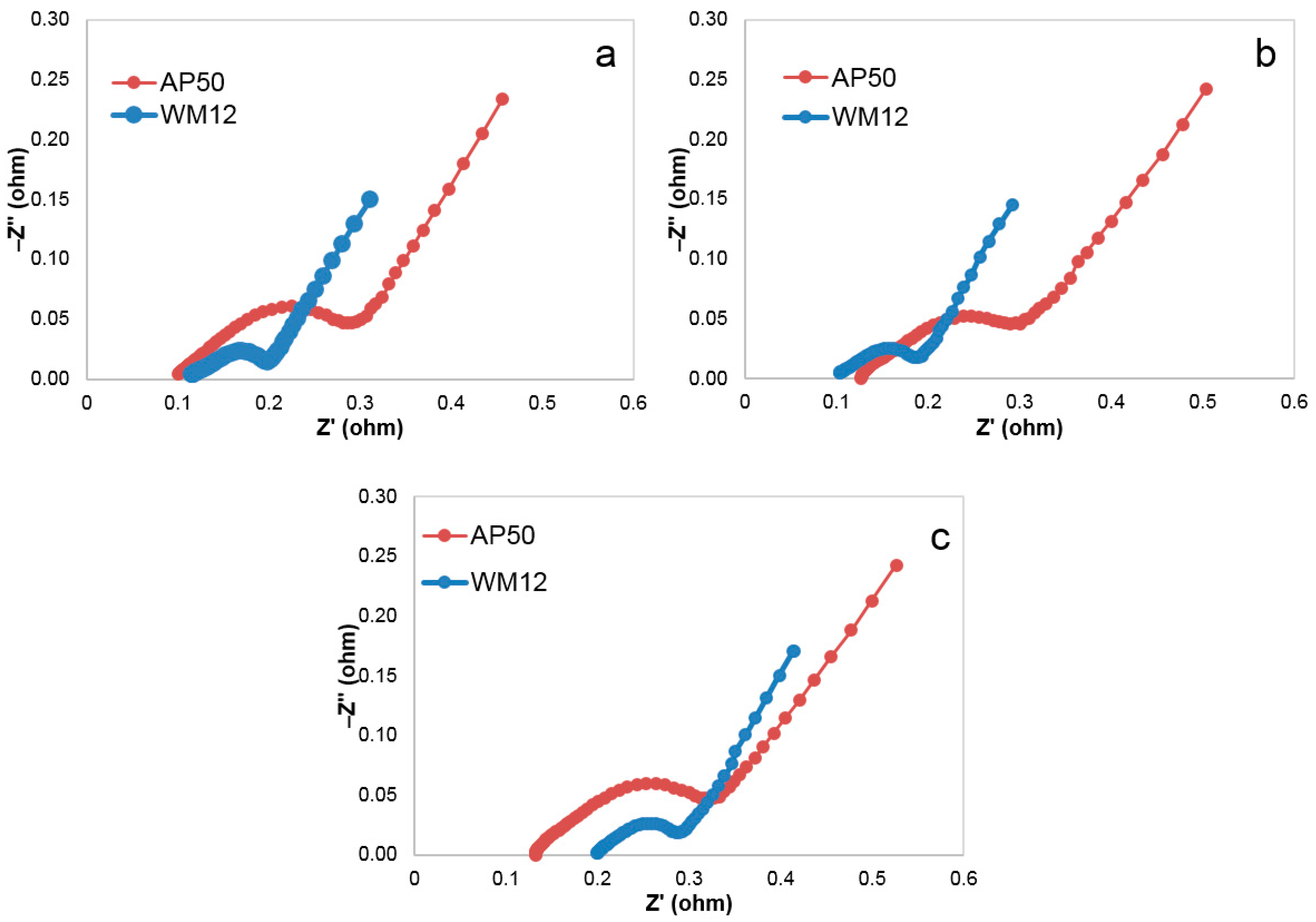
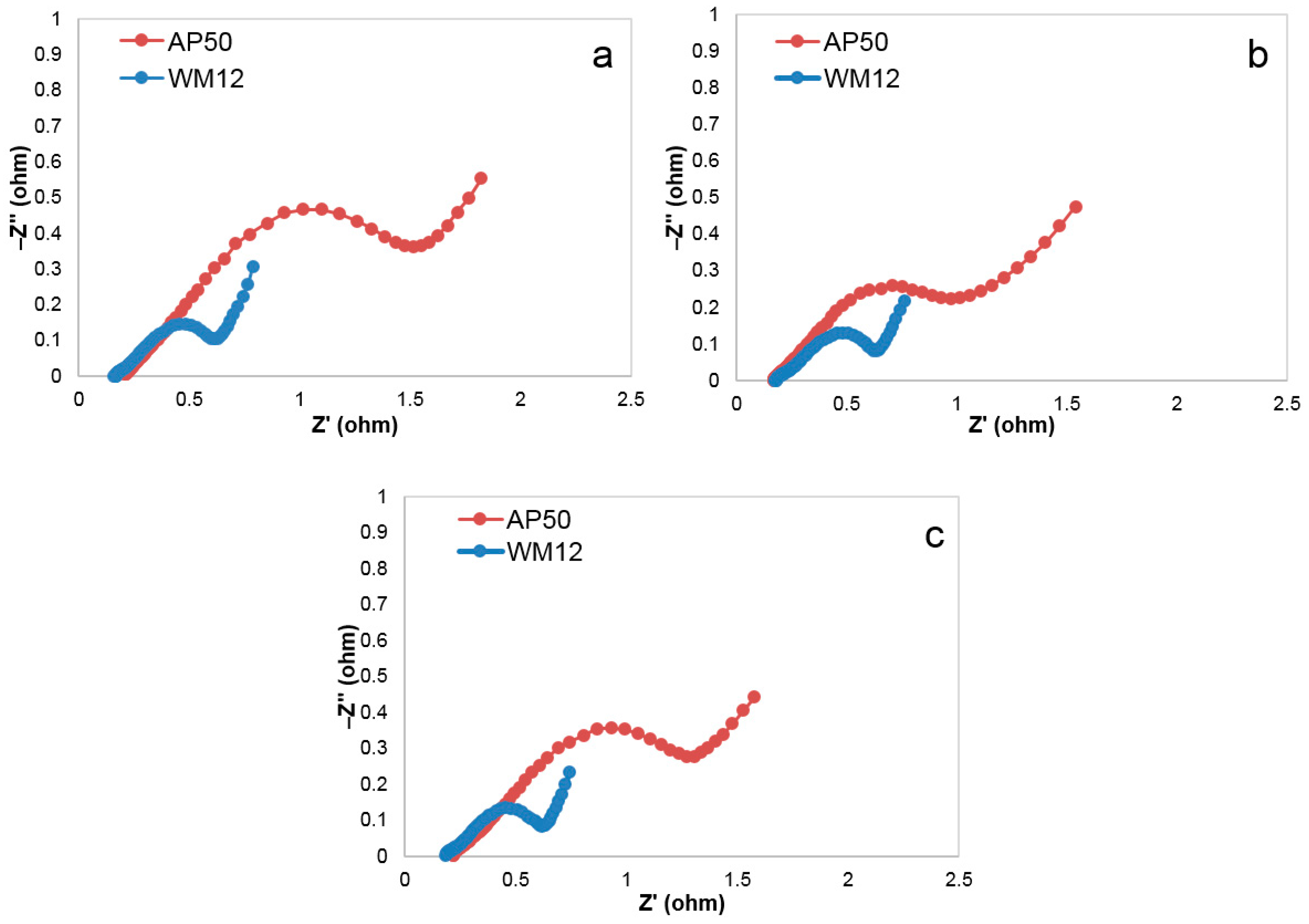
3.5. Internal Resistance
3.6. Charge-Transfer Resistance
4. Summary
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| EV | Electric vehicle |
| G | Graphite |
| LTO | Li-titanate |
| LMO | Li-spinel |
| NCA | LiNiCoAl oxide |
| NMC | LiNiMnCo oxide |
| LFP | LiFePO4 |
| Ni/MH | Nickel/metal hydride |
| HEV | Hybrid electric vehicle |
| MH | Metal hydride |
| N/P ratio | Negative-to-positive capacity ratio |
| RT | Room temperature |
| SOC | State of charge |
| Rint | Internal resistance |
| Rct | Charge transfer resistance |
| R0 | Solution resistance |
References
- Tesla Model S Battery. Available online: http://enipedia.tudelft.nl/wiki/Tesla_Model_S_Battery (accessed on 11 July 2017).
- General Motors EV1. Available online: https://en.wikipedia.org/wiki/General_Motors_EV1 (accessed on 11 July 2017).
- Young, K.; Nei, J.; Meng, T. Alkaline and Non-Aqueous Proton-Conducting Pouch-Cell Batteries. U.S. Patent Application 20160233461, 11 August 2016. [Google Scholar]
- Young, K.; Ng, K.Y.S.; Bendersky, L.A. A technical report of the Robust Affordable Next Generation Energy Storage System-BASF program. Batteries 2016, 2, 2. [Google Scholar] [CrossRef]
- Meng, T.; Young, K.; Beglau, D.; Yan, S.; Zeng, P.; Cheng, M. Hydrogenated amorphous silicon thin film anode for proton conducting batteries. J. Power Sources 2016, 302, 31–38. [Google Scholar] [CrossRef]
- Meng, T.; Young, K.; Wong, D.F.; Nei, J. Ionic liquid-based non-aqueous electrolytes for nickel/metal hydride batteries. Batteries 2017, 3, 4. [Google Scholar] [CrossRef]
- Young, K.; Wang, L.; Yan, S.; Liao, X.; Meng, T.; Shen, H.; Mays, W.C. Fabrications of high-capacity alpha-Ni(OH)2. Batteries 2017, 3, 6. [Google Scholar] [CrossRef]
- Meng, T.; Young, K.; Hu, C.; Reichman, B. Effects of alkaline pre-etching to metal hydride alloys. Batteries 2017, 3, 30. [Google Scholar] [CrossRef]
- Young, K.; Ng, K.Y.S. Reviews on the Chinese Patents regarding nickel/metal hydride battery. Batteries 2017, 3, 24. [Google Scholar] [CrossRef]
- Chang, S.; Young, K.; Nei, J.; Fierro, C. Reviews on the US Patents regarding nickel/metal hydride battery. Batteries 2016, 1, 10. [Google Scholar] [CrossRef]
- Corrigan, D.A.; Knight, S.L. Electrochemical and spectroscopic evidence on the participation of quadrivalent nickel in the nickel hydroxide redox reaction. J. Electrochem. Soc. 1989, 136, 613–619. [Google Scholar] [CrossRef]
- Corrigan, D.A.; Bendert, R.M. Effect of coprecipitated metal ions on the electrochemistry of nickel hydroxide thin films: cyclic voltammetry in 1M KOH. J. Electrochem. Soc. 1989, 136, 723–728. [Google Scholar] [CrossRef]
- Fierro, C.; Zallen, A.; Koch, J.; Fetcenko, M.A. The influence of nickel-hydroxide composition and microstructure on the high-temperature performance of nickel metal hydride batteries. J. Electrochem. Soc. 2006, 153, A492–A496. [Google Scholar] [CrossRef]
- Fierro, C.; Fetcenko, M.A.; Young, K.; Ovshinsky, S.R.; Sommers, B.; Harrison, C. Nickel Hydroxide Positive Electrode Material Exhibiting Improved Conductivity and Engineered Activation Energy. U.S. Patent 6,228,535, 8 May 2001. [Google Scholar]
- Zhang, L. AC impedance studies on sealed nickel metal hydride batteries over cycle life in analog and digital operations. Electrochim. Acta 1998, 43, 3333–3342. [Google Scholar] [CrossRef]
- Zhang, W.; Kumar, M.P.S.; Srinivasan, S. AC impedance studies on metal hydride electrodes. J. Electrochem. Soc. 1995, 142, 2935–2943. [Google Scholar] [CrossRef]

| Cell Maker | Chemistry | Configuration | Energy Density (Wh·L−1) | Energy Density (Wh·kg−1) | Used in |
|---|---|---|---|---|---|
| AESC | G/LMO-NCA | Pouch | 309 | 155 | Leaf by Nissan |
| LG-Chem | G/NMC-LMO | Pouch | 275 | 157 | Zoe by Renault |
| Li-Tec | G/NMC | Pouch | 316 | 152 | Smart by Daimler |
| LG-Chem | G/Ni-rich | Prismatic | 208 | 136 | Bolt by General Motor |
| Li-Energy | G/LMO-NMC | Prismatic | 218 | 109 | i-MiEV by Mitsubishi |
| Samsung | G/NMC-LMO | Prismatic | 243 | 132 | 500 by Fiat |
| Lishen | G/LFP | Prismatic | 226 | 116 | EV by Coda |
| Toshiba | LTO/NMC | Prismatic | 200 | 89 | Fit by Honda |
| Panasonic | G/NMC | Cylindrical | 630 | 233 | Model S by Tesla |
| BASF–Ovonic | Ni/MH | Pouch | 427 | 145 | Not yet |
| Type | Capacity (Ah) | Case Material | Vent Cap | Pros | Cons |
|---|---|---|---|---|---|
| Coin | 0.02–0.4 | Stainless steel | No | High volume mass-production | Only for low-rate application |
| Cylindrical | 0.3–10 | Stainless steel | Yes | High volume mass-production | Limited capacity |
| Stick | 1–2 | Stainless steel | Yes | High packing density | Higher cost and lower energy density |
| HEV-prismatic | 6.5 | Plastic or metal | Yes | High power density, easy packing | Lower pressure rating, poor heat transfer |
| EV-prismatic | 20–100 | Stainless steel | Yes | Large format (>100 Ah) | High manufacture cost |
| Pouch | 0.2–100 | Aluminum laminated with plastics | Yes/No | High gravimetric energy density | Low pressure rating |
| Materials | AP50 | WM12 |
|---|---|---|
| Composition | Ni0.91Co0.045Zn0.045(OH)2 | Ni0.84Co0.12Al0.04(OH)2 |
| Original structure | β-Ni(OH)2 | β-Ni(OH)2 |
| Structure after activation | β-Ni(OH)2 | α-β core-shell Ni(OH)2 |
| Tap density | 2.3 g·cc−1 | 0.9 g·cc−1 |
| Discharge capacity | 250 mAh·g−1 | 350 mAh·g−1 |
| BET surface area | 13.7 m2·g−1 | 51.98 m2·g−1 |
| Pore density | 0.022 cc·g−1 | 0.027 cc·g−1 |
| Average pore size | 19.7 Å | 24.6 Å |
| Test # | Temperature (°C) | Rate | Step | Cell AP50 Capacity (mAh·g−1) | Cell WM12 Capacity (mAh·g−1) |
|---|---|---|---|---|---|
| 1 | RT | 0.1C | Charge | 225 | 279 |
| 0.1C | Discharge | 205 | 269 | ||
| 2 | RT | 0.2C | Charge | 225 | 279 |
| 0.1C | Discharge | 201 | 260 | ||
| 3 | RT | 0.5C | Charge | 151 | 279 |
| 0.1C | Discharge | 143 | 250 | ||
| 4 | RT | 1C | Charge | 90 | 269 |
| 0.1C | Discharge | 89 | 238 | ||
| 5 | −10 | 0.1C | Charge | 153 | 279 |
| 0.1C | Discharge | 149 | 269 | ||
| 6 | −10 | 0.2C | Charge | 111 | 279 |
| 0.1C | Discharge | 110 | 268 | ||
| 7 | −10 | 0.5C | Charge | 53 | 186 |
| 0.1C | Discharge | 53 | 184 | ||
| 8 | −10 | 1C | Charge | 14 | 37 |
| 0.1C | Discharge | 14 | 39 |
| Test # | Temperature (°C) | Rate | Step | Cell AP50 Capacity (mAh·g−1) | Cell WM12 Capacity (mAh·g−1) |
|---|---|---|---|---|---|
| 1 | RT | 0.1C | Charge | 225 | 279 |
| 0.1C | Discharge | 203 | 272 | ||
| 2 | RT | 0.1C | Charge | 225 | 279 |
| 0.2C | Discharge | 196 | 270 | ||
| 3 | RT | 0.1C | Charge | 225 | 279 |
| 0.5C | Discharge | 157 | 256 | ||
| 4 | RT | 0.1C | Charge | 225 | 279 |
| 1C | Discharge | 99 | 223 | ||
| 5 | −10 | 0.1C | Charge | 225 | 279 |
| 0.1C | Discharge | 160 | 271 | ||
| 6 | −10 | 0.1C | Charge | 225 | 279 |
| 0.2C | Discharge | 130 | 259 | ||
| 7 | −10 | 0.1C | Charge | 225 | 279 |
| 0.5C | Discharge | 63 | 210 | ||
| 8 | −10 | 0.1C | Charge | 225 | 279 |
| 1C | Discharge | 22 | 142 |
| Temperature (°C) | Cell | Rint-Charge (Ω) | Rint-Charge (Ω) | Average Rint (Ω) |
|---|---|---|---|---|
| RT | AP50 | 0.36 | 0.40 | 0.38 |
| RT | WM12 | 0.17 | 0.23 | 0.20 |
| −10 | AP50 | 1.06 | 1.07 | 1.07 |
| −10 | WM12 | 0.45 | 0.49 | 0.47 |
| Temperature (°C) | Cycle # | Cell | R0 (Ω) | Rct (Ω) |
|---|---|---|---|---|
| RT | 0 | AP50 | 0.10 | 0.23 |
| RT | 0 | WM12 | 0.11 | 0.10 |
| RT | 20 | AP50 | 0.13 | 0.21 |
| RT | 20 | WM12 | 0.10 | 0.10 |
| RT | 50 | AP50 | 0.13 | 0.23 |
| RT | 50 | WM12 | 0.20 | 0.10 |
| −10 | 0 | AP50 | 0.21 | 1.64 |
| −10 | 0 | WM12 | 0.16 | 0.51 |
| −10 | 20 | AP50 | 0.17 | 1.18 |
| −10 | 20 | WM12 | 0.17 | 0.53 |
| −10 | 50 | AP50 | 0.20 | 1.30 |
| −10 | 50 | WM12 | 0.18 | 0.52 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, S.; Meng, T.; Young, K.-H.; Nei, J. A Ni/MH Pouch Cell with High-Capacity Ni(OH)2. Batteries 2017, 3, 38. https://doi.org/10.3390/batteries3040038
Yan S, Meng T, Young K-H, Nei J. A Ni/MH Pouch Cell with High-Capacity Ni(OH)2. Batteries. 2017; 3(4):38. https://doi.org/10.3390/batteries3040038
Chicago/Turabian StyleYan, Shuli, Tiejun Meng, Kwo-Hsiung Young, and Jean Nei. 2017. "A Ni/MH Pouch Cell with High-Capacity Ni(OH)2" Batteries 3, no. 4: 38. https://doi.org/10.3390/batteries3040038
APA StyleYan, S., Meng, T., Young, K.-H., & Nei, J. (2017). A Ni/MH Pouch Cell with High-Capacity Ni(OH)2. Batteries, 3(4), 38. https://doi.org/10.3390/batteries3040038







