Nanostructured Networks for Energy Storage: Vertically Aligned Carbon Nanotubes (VACNT) as Current Collectors for High-Power Li4Ti5O12(LTO)//LiMn2O4(LMO) Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experimental Section
2.1. VACNT Synthesis
2.2. Synthesis of the LTO Precursor
2.3. Synthesis of the LMO Precursor
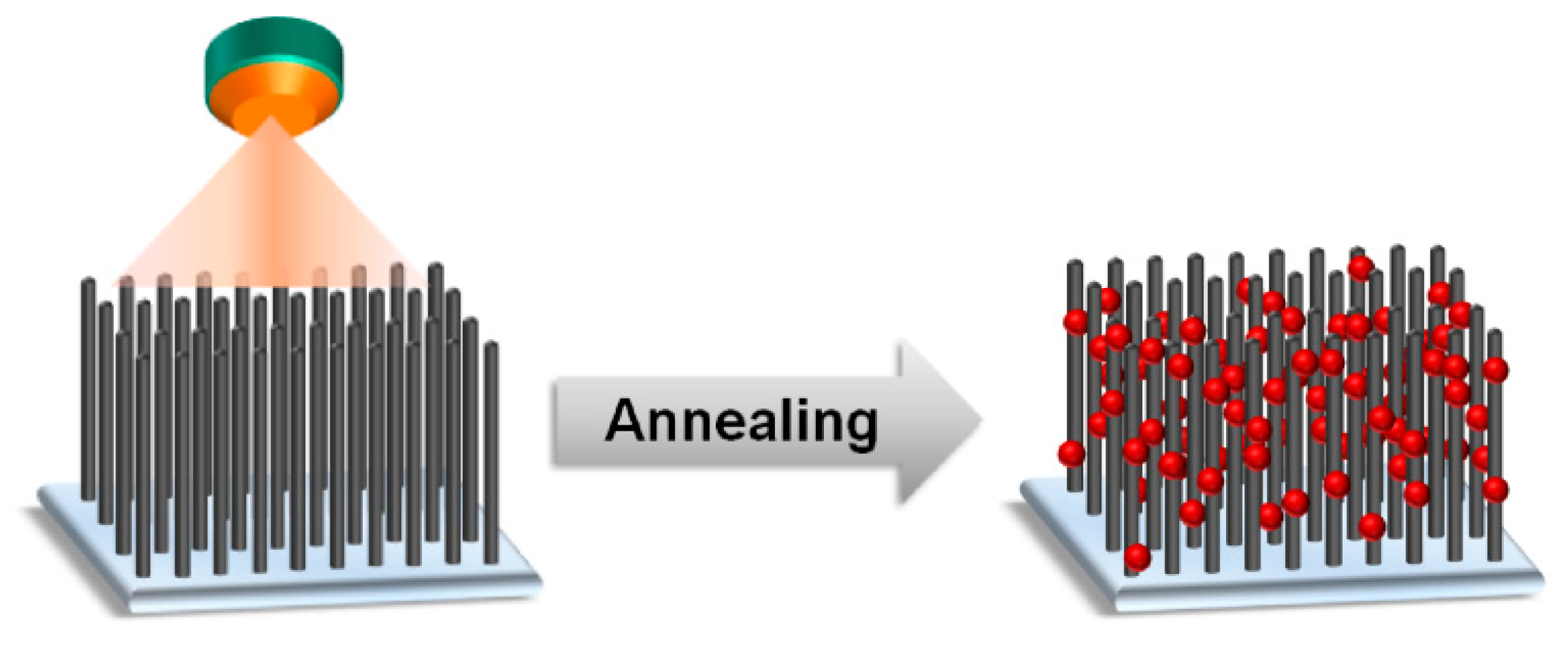
2.4. Preparation of the LTO-/LMO-VACNT Composite
2.5. Material Characterization
2.6. Electrochemical Characterization
3. Results and Discussion
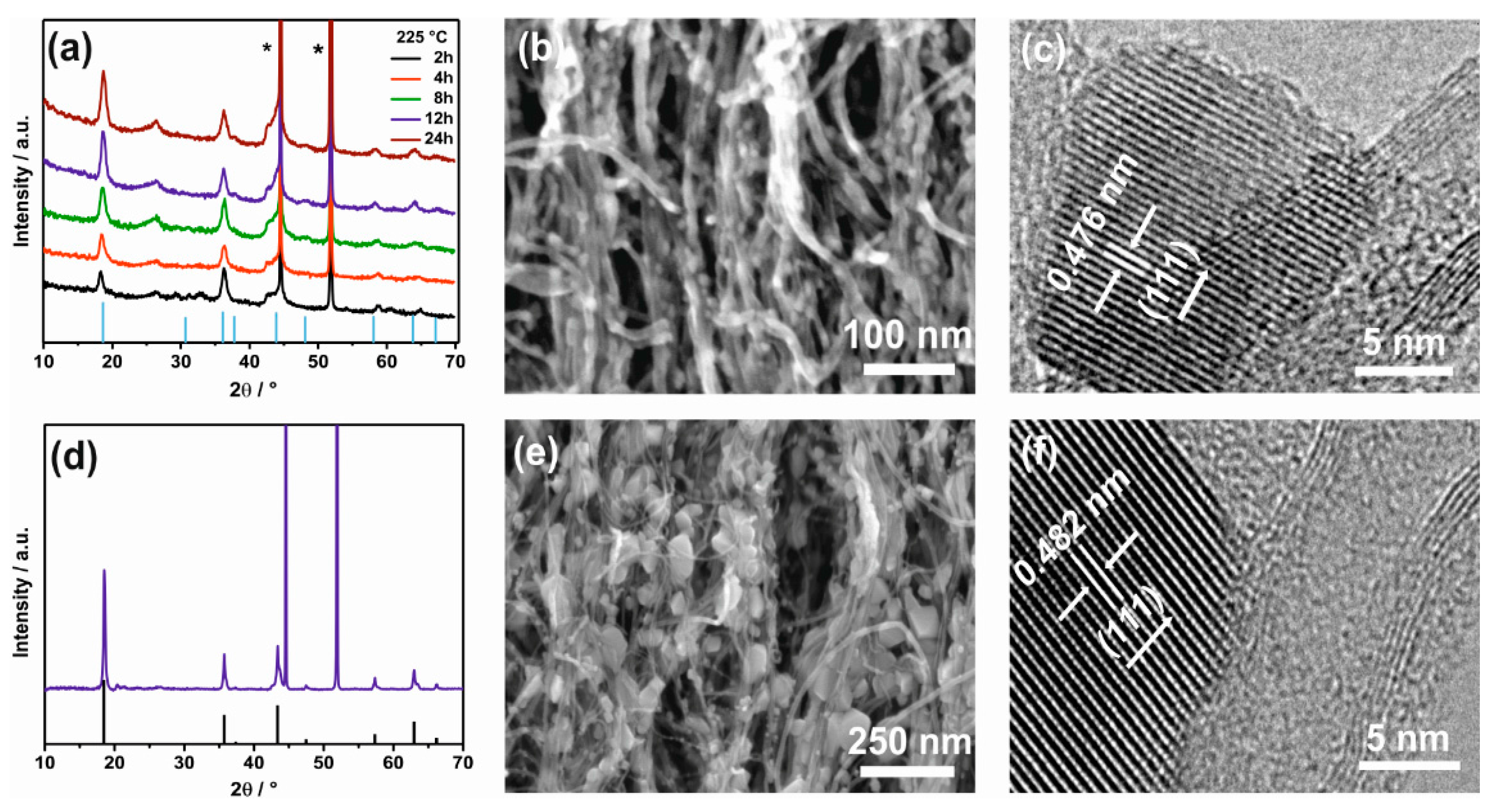
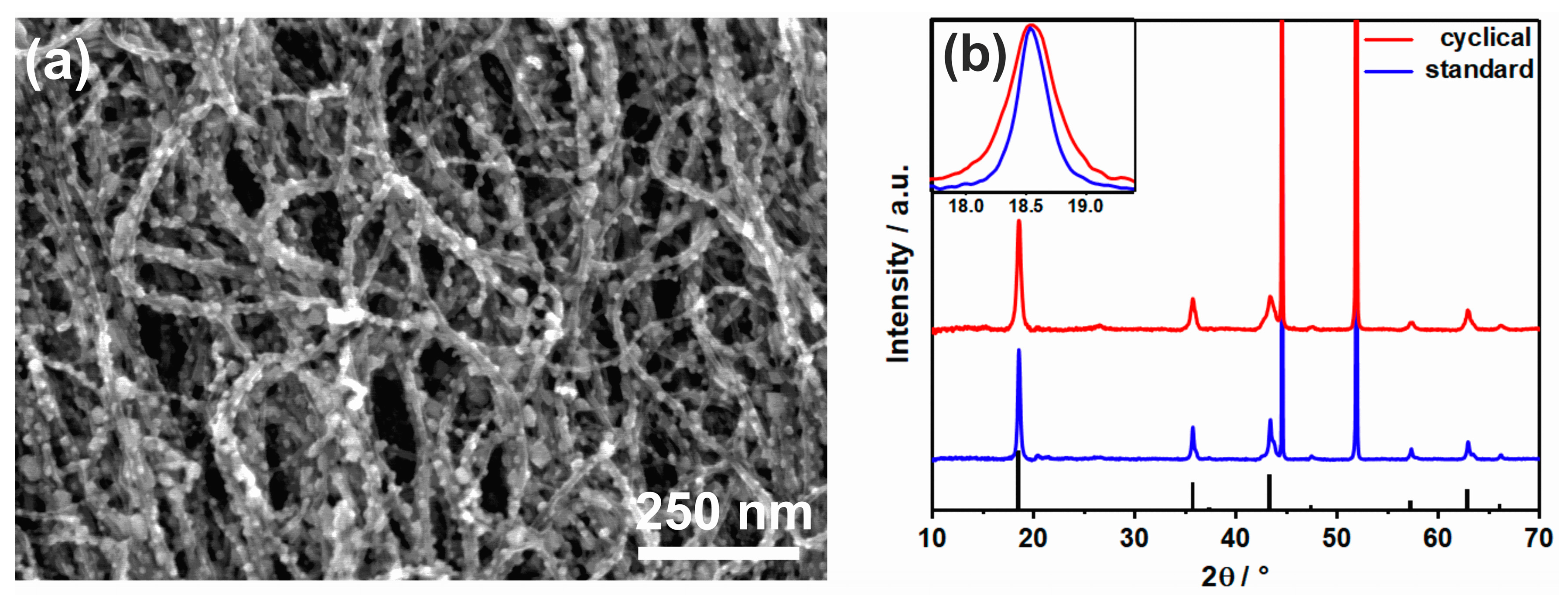
3.1. Structure Characterization of LTO– and LMO–VACNT Nanoarrays
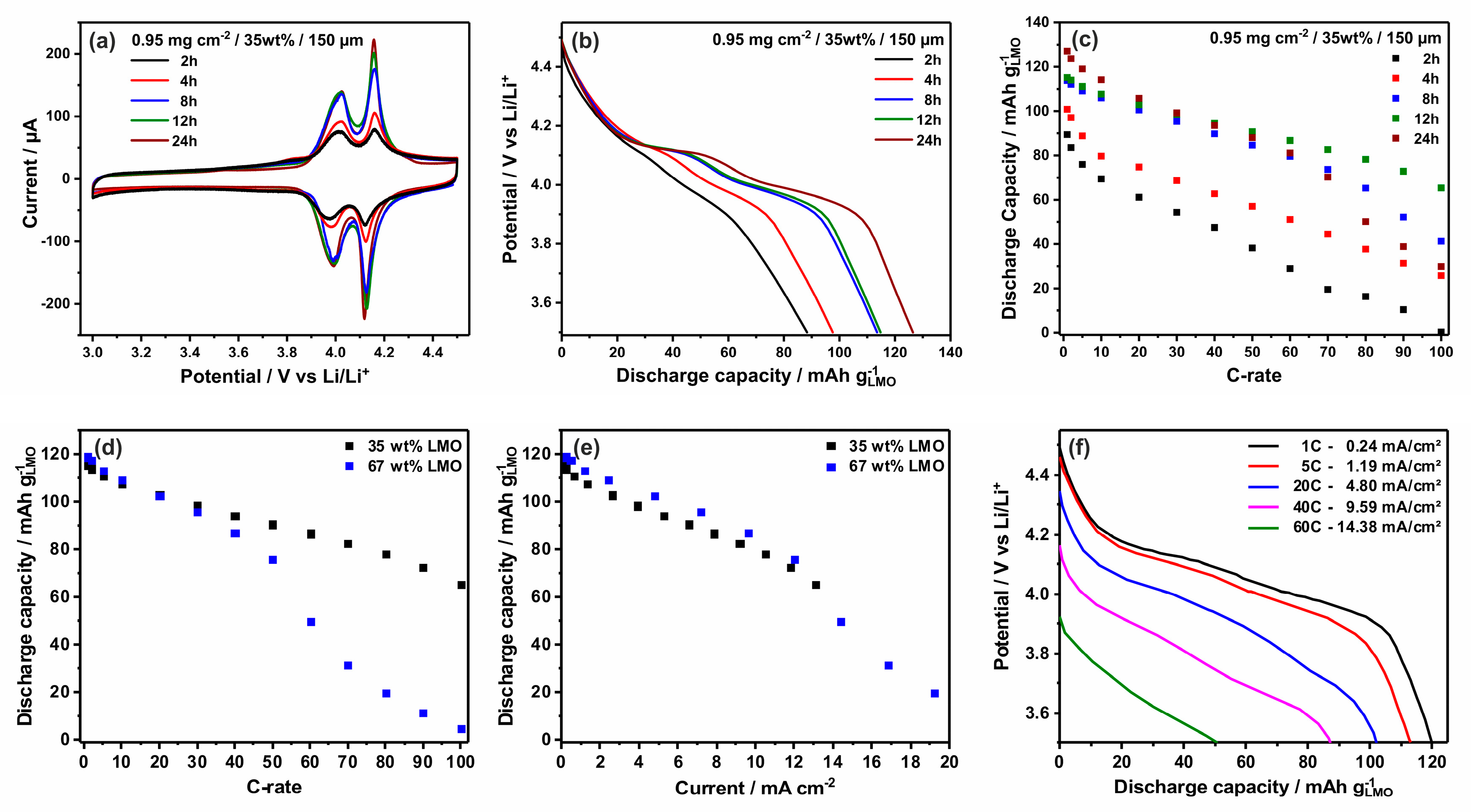
3.2. Electrochemical Characterization of LMO-VACNT Nanoarrays
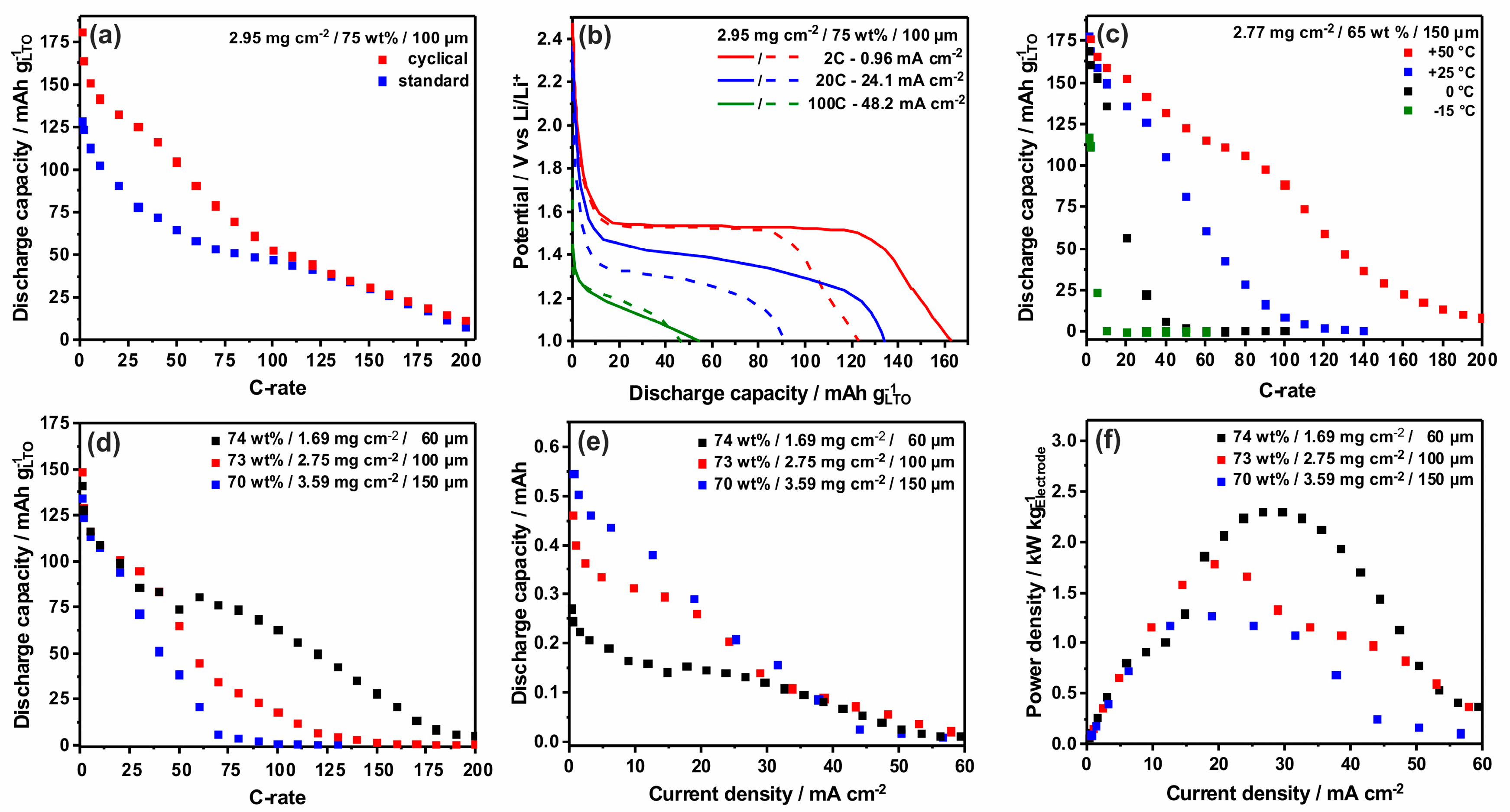
3.3. Structure Property Relationship in LTO-VACNT Nanoarrays
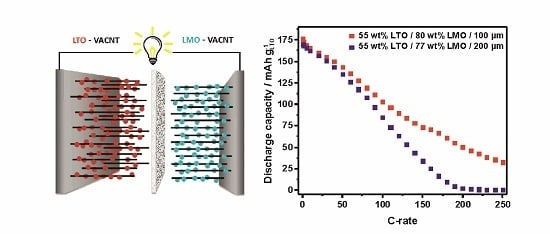
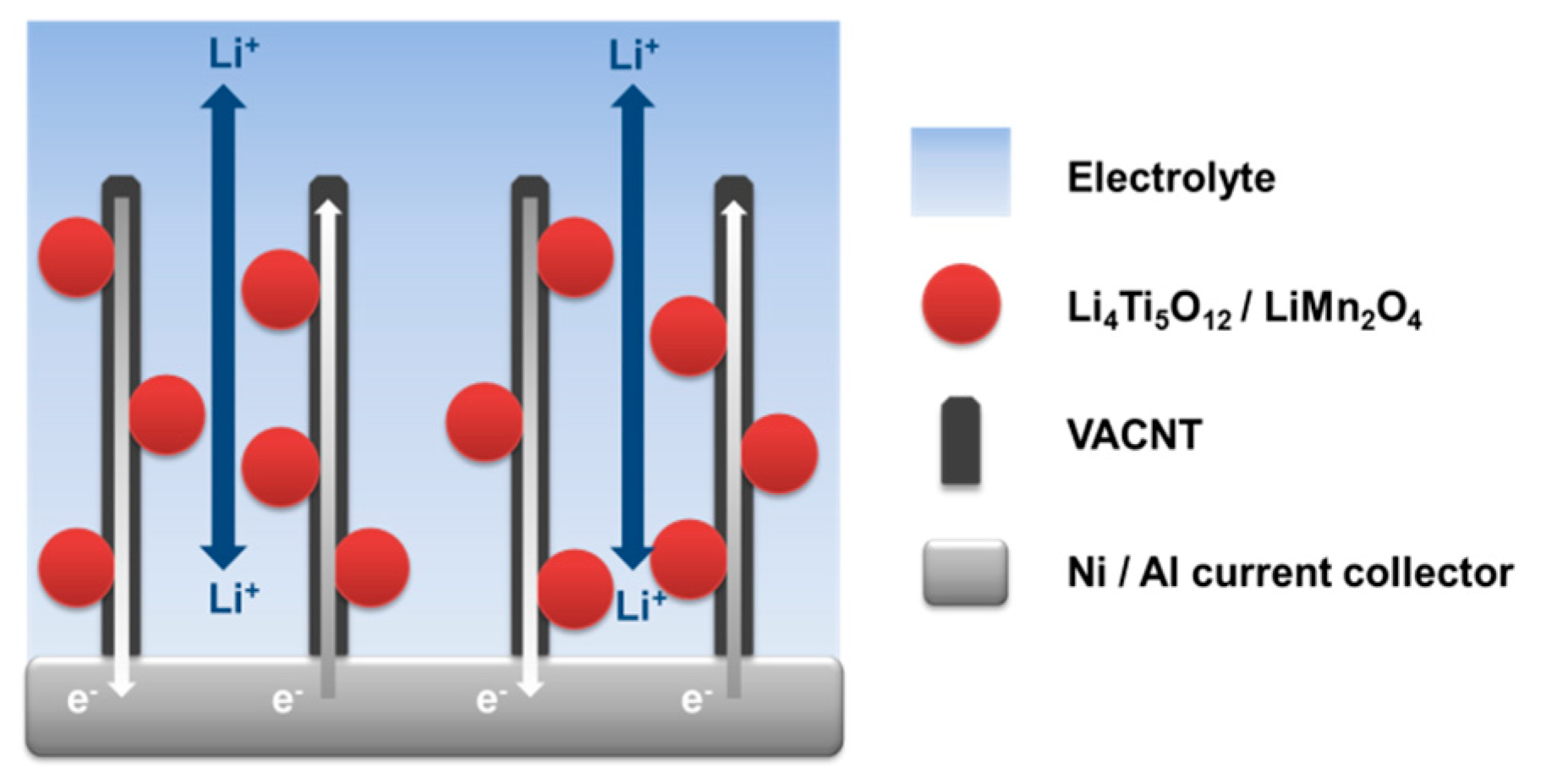
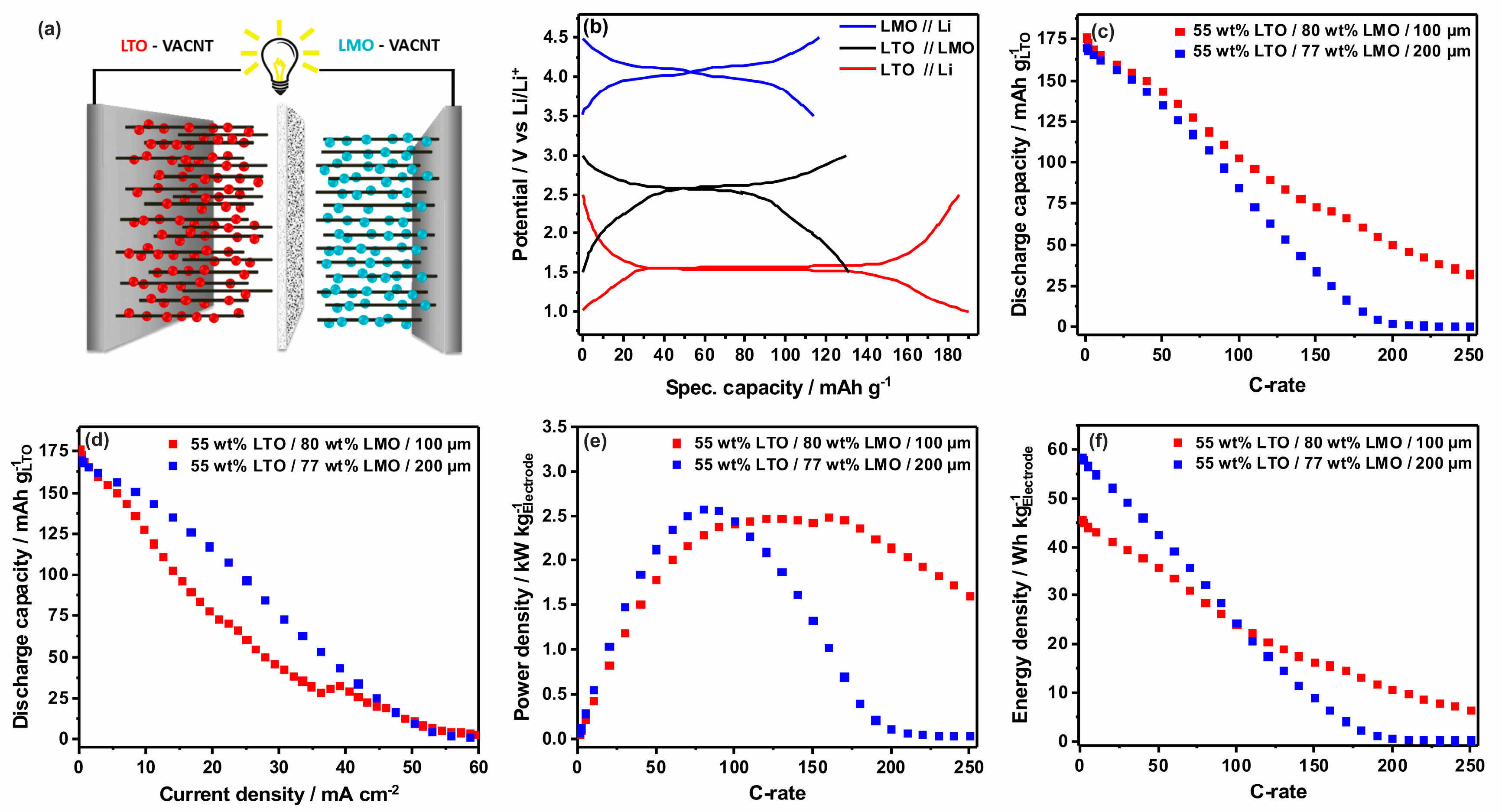
3.4. Electrochemical Characterization of LTO//LMO-VACNT Full Cells
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Van Noorden, R. The rechargeable revolution: A better battery. Nature 2014, 507, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Cho, J. Critical Requirements for Rapid Charging of Rechargeable Al- and Li-Ion Batteries. Angew. Chem. Int. Ed. Engl. 2015, 54, 9452–9455. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Tatsuyama, Y.; Tsuji, M.; Ishida, K.; Okada, S.; Yamaki, J. Preparation of LiMn2O4 nanoparticles for Li ion secondary batteries by laser ablation in water. Mater. Lett. 2007, 61, 2062–2065. [Google Scholar] [CrossRef]
- Mohammadi, M.R.; Fray, D.J. Low temperature nanostructured lithium titanates. J. Sol-Gel Sci. Technol. 2010, 55, 19–35. [Google Scholar] [CrossRef]
- Kavan, L.; Grätzel, M. Facile Synthesis of Nanocrystalline Li4Ti5O12 (Spinel) Exhibiting Fast Li Insertion. Electrochem. Solid State Lett. 2002, 5, A39. [Google Scholar] [CrossRef]
- Li, J.; Tang, Z.; Zhang, Z. Controllable formation and electrochemical properties of one-dimensional nanostructured spinel Li4Ti5O12. Electrochem. Commun. 2005, 7, 894–899. [Google Scholar] [CrossRef]
- Lee, S.C.; Lee, S.M.; Lee, J.W.; Lee, J.B.; Lee, S.M.; Han, S.S.; Lee, H.C.; Kim, H.J. Spinel Li4Ti5O12 Nanotubes for Energy Storage Materials. J. Phys. Chem. C 2009, 113, 18420–18423. [Google Scholar] [CrossRef]
- Ding, Y.L.; Xie, J.; Cao, G.S.; Zhu, T.J.; Yu, H.M.; Zhao, X.B. Single-Crystalline LiMn2O4 Nanotubes Synthesized Via Template-Engaged Reaction as Cathodes for High-Power Lithium Ion Batteries. Adv. Funct. Mater. 2011, 21, 348–355. [Google Scholar] [CrossRef]
- Wang, X.; Liu, B.; Hou, X.; Wang, Q.; Li, W.; Chen, D.; Shen, G. Ultralong-life and high-rate web-like Li4Ti5O12 anode for high-performance flexible lithium-ion batteries. J. Nano Res. 2014, 7, 1073–1082. [Google Scholar] [CrossRef]
- Hosono, E.; Kudo, T.; Honma, I.; Matsuda, H.; Zhou, H. Synthesis of single crystalline spinel LiMn2O4 nanowires for a lithium ion battery with high power density. Nano Lett. 2009, 9, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Cao, F.; Liu, Y.; Zhao, X.; Liu, X.; Yuan, J. Nanoporous LiMn2O4 nanosheets with exposed {111} facets as cathodes for highly reversible lithium-ion batteries. J. Mater. Chem. 2012, 22, 20952. [Google Scholar] [CrossRef]
- Liu, J.; Wei, X.; Liu, X.W. Two-dimensional wavelike spinel lithium titanate for fast lithium storage. Sci. Rep. 2015, 5, 9782. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Mei, T.; Zhu, Y.; Wang, L.; Liang, J.; Zhang, X.; Qian, Y.; Tang, K. Hydrothermal synthesis of layered Li1.81H0.19Ti2O5·xH2O nanosheets and their transformation to single-crystalline Li4Ti5O12 nanosheets as the anode materials for Li-ion batteries. CrystEngComm 2012, 14, 6435. [Google Scholar] [CrossRef]
- Sorensen, E.M.; Barry, S.J.; Jung, H.-K.; Rondinelli, J.M.; Vaughey, J.T.; Poeppelmeier, K.R. Three-Dimensionally Ordered Macroporous Li4Ti5O12. Chem. Mater. 2006, 18, 482–489. [Google Scholar] [CrossRef]
- Luo, J.Y.; Wang, Y.G.; Xiong, H.M.; Xia, Y.Y. Ordered Mesoporous Spinel LiMn2O4 by a Soft-Chemical Process as a Cathode Material for Lithium-Ion Batteries. Chem. Mater. 2007, 19, 4791–4795. [Google Scholar] [CrossRef]
- Lai, C.; Wu, Z.; Zhu, Y.; Wu, Q.; Li, L.; Wang, C. Ball-milling assisted solid-state reaction synthesis of mesoporous Li4Ti5O12 for lithium-ion batteries anode. J. Power Sources 2013, 226, 71–74. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Y.; Li, W.; Ma, B.; Chen, X. Rational material design for ultrafast rechargeable lithium-ion batteries. Chem. Soc. Rev. 2015, 44, 5926–5940. [Google Scholar] [CrossRef] [PubMed]
- Arthur, T.S.; Bates, D.J.; Cirigliano, N.; Johnson, D.C.; Malati, P.; Mosby, J.M.; Perre, E.; Rawls, M.T.; Prieto, A.L.; Dunn, B. Three-dimensional electrodes and battery architectures. MRS Bull. 2011, 36, 523–531. [Google Scholar] [CrossRef]
- Wang, Y.; Rong, H.; Li, B.; Xing, L.; Li, X.; Li, W. Microemulsion-assisted synthesis of ultrafine Li4Ti5O12/C nanocomposite with oleic acid as carbon precursor and particle size controller. J. Power Sources 2014, 246, 213–218. [Google Scholar] [CrossRef]
- Patey, T.J.; Büchel, R.; Ng, S.H.; Krumeich, F.; Pratsinis, S.E.; Novák, P. Flame co-synthesis of LiMn2O4 and carbon nanocomposites for high power batteries. J. Power Sources 2009, 189, 149–154. [Google Scholar] [CrossRef]
- Xia, H.; Ragavendran, K.R.; Xie, J.; Lu, L. Ultrafine LiMn2O4/carbon nanotube nanocomposite with excellent rate capability and cycling stability for lithium-ion batteries. J. Power Sources 2012, 212, 28–34. [Google Scholar] [CrossRef]
- Li, X.; Qu, M.; Yu, Z. Preparation and electrochemical performance of Li4Ti5O12/graphitized carbon nanotubes composite. Solid State Ion. 2010, 181, 635–639. [Google Scholar] [CrossRef]
- Shi, Y.; Wen, L.; Li, F.; Cheng, H.-M. Nanosized Li4Ti5O12/graphene hybrid materials with low polarization for high rate lithium ion batteries. J. Power Sources 2011, 196, 8610–8617. [Google Scholar] [CrossRef]
- Han, S.Y.; Kim, I.Y.; Jo, K.Y.; Hwang, S.-J. Solvothermal-Assisted Hybridization between Reduced Graphene Oxide and Lithium Metal Oxides. J. Phys. Chem. C 2012, 116, 7269–7279. [Google Scholar] [CrossRef]
- Zhao, H.; Zhou, M.; Wen, L.; Lei, Y. Template-directed construction of nanostructure arrays for highly-efficient energy storage and conversion. Nano Energy 2015, 13, 790–813. [Google Scholar] [CrossRef]
- Zhang, F.; Qi, L. Recent Progress in Self-Supported Metal Oxide Nanoarray Electrodes for Advanced Lithium-Ion Batteries. Adv. Sci. 2016, 3, 1600049. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Xia, Q.; Lin, B.; Zhu, J.; Seo, J.K.; Meng, Y.S. Self-standing porous LiMn2O4 nanowall arrays as promising cathodes for advanced 3D microbatteries and flexible lithium-ion batteries. Nano Energy 2016, 22, 475–482. [Google Scholar] [CrossRef]
- Tang, X.; Lin, B.; Ge, Y.; Ge, Y.; Lu, C.; Savilov, S.V.; Aldoshin, S.M.; Xia, H. LiMn2O4 nanorod arrays. Mater. Res. Bull. 2015, 69, 2–6. [Google Scholar] [CrossRef]
- Liu, J.; Song, K.; van Aken, P.A.; Maier, J.; Yu, Y. Self-supported Li4Ti5O12-C nanotube arrays as high-rate and long-life anode materials for flexible Li-ion batteries. Nano Lett. 2014, 14, 2597–2603. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Li, Y.; Liu, J.; Huang, X.; Yuan, C.; Lou, X.W.D. Recent advances in metal oxide-based electrode architecture design for electrochemical energy storage. Adv. Mater. 2012, 24, 5166–5180. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Li, Y.; Liu, J.; Huang, X. Building one-dimensional oxide nanostructure arrays on conductive metal substrates for lithium-ion battery anodes. Nanoscale 2011, 3, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Ellis, B.L.; Knauth, P.; Djenizian, T. Three-dimensional self-supported metal oxides for advanced energy storage. Adv. Mater. 2014, 26, 3368–3397. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Xie, H.; Li, J. Hierarchical Structures Based on Two-Dimensional Nanomaterials for Rechargeable Lithium Batteries. Adv. Energy Mater. 2017, 7, 1601906. [Google Scholar] [CrossRef]
- Chen, S.; Xin, Y.; Zhou, Y.; Ma, Y.; Zhou, H.; Qi, L. Self-supported Li4Ti5O12 nanosheet arrays for lithium ion batteries with excellent rate capability and ultralong cycle life. Energy Environ. Sci. 2014, 7, 1924. [Google Scholar] [CrossRef]
- Cha, G.; Lee, H.J.; Choi, J. Preparation of binder-free thin film Li4Ti5O12 anode with an adjustable thickness through anodic TiO2 nanotubes. Curr. Appl. Phys. 2013, 13, 1788–1795. [Google Scholar] [CrossRef]
- Pawlitzek, F.; Pampel, J.; Schmuck, M.; Althues, H.; Schumm, B.; Kaskel, S. High-power lithium ion batteries based on preorganized necklace type Li4Ti5O12/VACNT nano-composites. J. Power Sources 2016, 325, 1–6. [Google Scholar] [CrossRef]
- Dörfler, S.; Felhösi, I.; Kék, I.; Marek, T.; Althues, H.; Kaskel, S.; Nyikos, L. Tailoring structural and electrochemical properties of vertical aligned carbon nanotubes on metal foil using scalable wet-chemical catalyst deposition. J. Power Sources 2012, 208, 426–433. [Google Scholar] [CrossRef]
- Dörfler, S.; Meier, A.; Thieme, S.; Németh, P.; Althues, H.; Kaskel, S. Wet-chemical catalyst deposition for scalable synthesis of vertical aligned carbon nanotubes on metal substrates. Chem. Phys. Lett. 2011, 511, 288–293. [Google Scholar] [CrossRef]
- Piwko, M.; Althues, H.; Schumm, B.; Kaskel, S. Confocal Microscopy for Process Monitoring and Wide-Area Height Determination of Vertically-Aligned Carbon Nanotube Forests. Coatings 2015, 5, 477–487. [Google Scholar] [CrossRef]
- Brückner, J.; Thieme, S.; Grossmann, H.T.; Dörfler, S.; Althues, H.; Kaskel, S. Lithium–sulfur batteries. J. Power Sources 2014, 268, 82–87. [Google Scholar] [CrossRef]
- Baldi, M.; Finocchio, E.; Milella, F.; Busca, G. Catalytic combustion of C3 hydrocarbons and oxygenates over Mn3O4. Appl. Catal. B 1998, 16, 43–51. [Google Scholar] [CrossRef]
- Lin, B.; Yin, Q.; Hu, H.; Lu, F.; Xia, H. LiMn2O4 nanoparticles anchored on graphene nanosheets as high-performance cathode material for lithium-ion batteries. J. Solid State Chem. 2014, 209, 23–28. [Google Scholar] [CrossRef]
- Thackeray, M.M.; David, W.; Bruce, P.G.; Goodenough, J.B. Lithium insertion into manganese spinels. Mater. Res. Bull. 1983, 18, 461–472. [Google Scholar] [CrossRef]
- Tang, S.B.; Lai, M.O.; Lu, L. Electrochemical studies of low-temperature processed nano-crystalline LiMn2O4 thin film cathode at 55 °C. J. Power Sources 2007, 164, 372–378. [Google Scholar] [CrossRef]
- Striebel, K.A. Electrochemical Behavior of LiMn2O4 and LiCoO2 Thin Films Produced with Pulsed Laser Deposition. J. Electrochem. Soc. 1996, 143, 1821. [Google Scholar] [CrossRef]
- Djian, D.; Alloin, F.; Martinet, S.; Lignier, H. Macroporous poly(vinylidene fluoride) membrane as a separator for lithium-ion batteries with high charge rate capacity. J. Power Sources 2009, 187, 575–580. [Google Scholar] [CrossRef]
- Capiglia, C.; Saito, Y.; Kageyama, H.; Mustarelli, P.; Iwamoto, T.; Tabuchi, T.; Tukamoto, H. 7Li and 19F diffusion coefficients and thermal properties of non-aqueous electrolyte solutions for rechargeable lithium batteries. J. Power Sources 1999, 81–82, 859–862. [Google Scholar] [CrossRef]
- Rho, Y.H.; Kanamura, K. Li+ ion diffusion in Li4Ti5O12 thin film electrode prepared by PVP sol–gel method. J. Solid State Chem. 2004, 177, 2094–2100. [Google Scholar] [CrossRef]
- Kavan, L.; Procházka, J.; Spitler, T.M.; Kalbáč, M.; Zukalová, M.; Drezen, T.; Grätzel, M. Li Insertion into Li4Ti5O12 (Spinel). J. Electrochem. Soc. 2003, 150, A1000. [Google Scholar] [CrossRef]
- Bach, S.; Pereira-Ramos, J.; Baffier, N. Electrochemical properties of sol–gel Li4/3Ti5/3O4. J. Power Sources 1999, 81–82, 273–276. [Google Scholar] [CrossRef]
- Takai, S.; Yoshioka, K.; Iikura, H.; Matsubayashi, M.; Yao, T.; Esaka, T. Tracer diffusion coefficients of lithium ion in LiMn2O4 measured by neutron radiography. Solid State Ion. 2014, 256, 93–96. [Google Scholar] [CrossRef]
- Dickens, P.; Reynolds, G. Transport and equilibrium properties of some oxide insertion compounds. Solid State Ion. 1981, 5, 331–334. [Google Scholar] [CrossRef]
- Masarapu, C.; Subramanian, V.; Zhu, H.; Wei, B. Long-Cycle Electrochemical Behavior of Multiwall Carbon Nanotubes Synthesized on Stainless Steel in Li Ion Batteries. Adv. Funct. Mater. 2009, 19, 1008–1014. [Google Scholar] [CrossRef]
- Welna, D.T.; Qu, L.; Taylor, B.E.; Dai, L.; Durstock, M.F. Vertically aligned carbon nanotube electrodes for lithium-ion batteries. J. Power Sources 2011, 196, 1455–1460. [Google Scholar] [CrossRef]
- Yang, L.Y.; Li, H.Z.; Liu, J.; Tang, S.S.; Lu, Y.K.; Li, S.T.; Min, J.; Yan, N.; Lei, M. Li4Ti5O12 nanosheets as high-rate and long-life anode materials for sodium-ion batteries. J. Mater. Chem. A 2015, 3, 24446–24452. [Google Scholar] [CrossRef]
- Ohzuku, T. Zero-Strain Insertion Material of Li[Li[sub 1/3]Ti[sub 5/3]]O[sub 4] for Rechargeable Lithium Cells. J. Electrochem. Soc. 1995, 142, 1431. [Google Scholar] [CrossRef]
- Gallagher, K.G.; Trask, S.E.; Bauer, C.; Woehrle, T.; Lux, S.F.; Tschech, M.; Lamp, P.; Polzin, B.J.; Ha, S.; Long, B.; et al. Optimizing Areal Capacities through Understanding the Limitations of Lithium-Ion Electrodes. J. Electrochem. Soc. 2015, 163, A138–A149. [Google Scholar] [CrossRef]
- Johns, P.A.; Roberts, M.R.; Wakizaka, Y.; Sanders, J.H.; Owen, J.R. How the electrolyte limits fast discharge in nanostructured batteries and supercapacitors. Electrochem. Commun. 2009, 11, 2089–2092. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlitzek, F.; Althues, H.; Schumm, B.; Kaskel, S. Nanostructured Networks for Energy Storage: Vertically Aligned Carbon Nanotubes (VACNT) as Current Collectors for High-Power Li4Ti5O12(LTO)//LiMn2O4(LMO) Lithium-Ion Batteries. Batteries 2017, 3, 37. https://doi.org/10.3390/batteries3040037
Pawlitzek F, Althues H, Schumm B, Kaskel S. Nanostructured Networks for Energy Storage: Vertically Aligned Carbon Nanotubes (VACNT) as Current Collectors for High-Power Li4Ti5O12(LTO)//LiMn2O4(LMO) Lithium-Ion Batteries. Batteries. 2017; 3(4):37. https://doi.org/10.3390/batteries3040037
Chicago/Turabian StylePawlitzek, Fabian, Holger Althues, Benjamin Schumm, and Stefan Kaskel. 2017. "Nanostructured Networks for Energy Storage: Vertically Aligned Carbon Nanotubes (VACNT) as Current Collectors for High-Power Li4Ti5O12(LTO)//LiMn2O4(LMO) Lithium-Ion Batteries" Batteries 3, no. 4: 37. https://doi.org/10.3390/batteries3040037
APA StylePawlitzek, F., Althues, H., Schumm, B., & Kaskel, S. (2017). Nanostructured Networks for Energy Storage: Vertically Aligned Carbon Nanotubes (VACNT) as Current Collectors for High-Power Li4Ti5O12(LTO)//LiMn2O4(LMO) Lithium-Ion Batteries. Batteries, 3(4), 37. https://doi.org/10.3390/batteries3040037