Electron Backscatter Diffraction Studies on the Formation of Superlattice Metal Hydride Alloys
Abstract
:1. Introduction
2. Experimental Setup
3. Results and Discussion
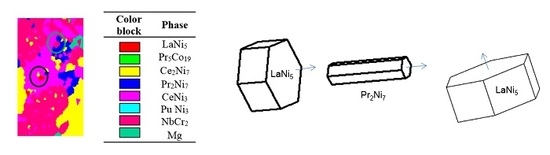
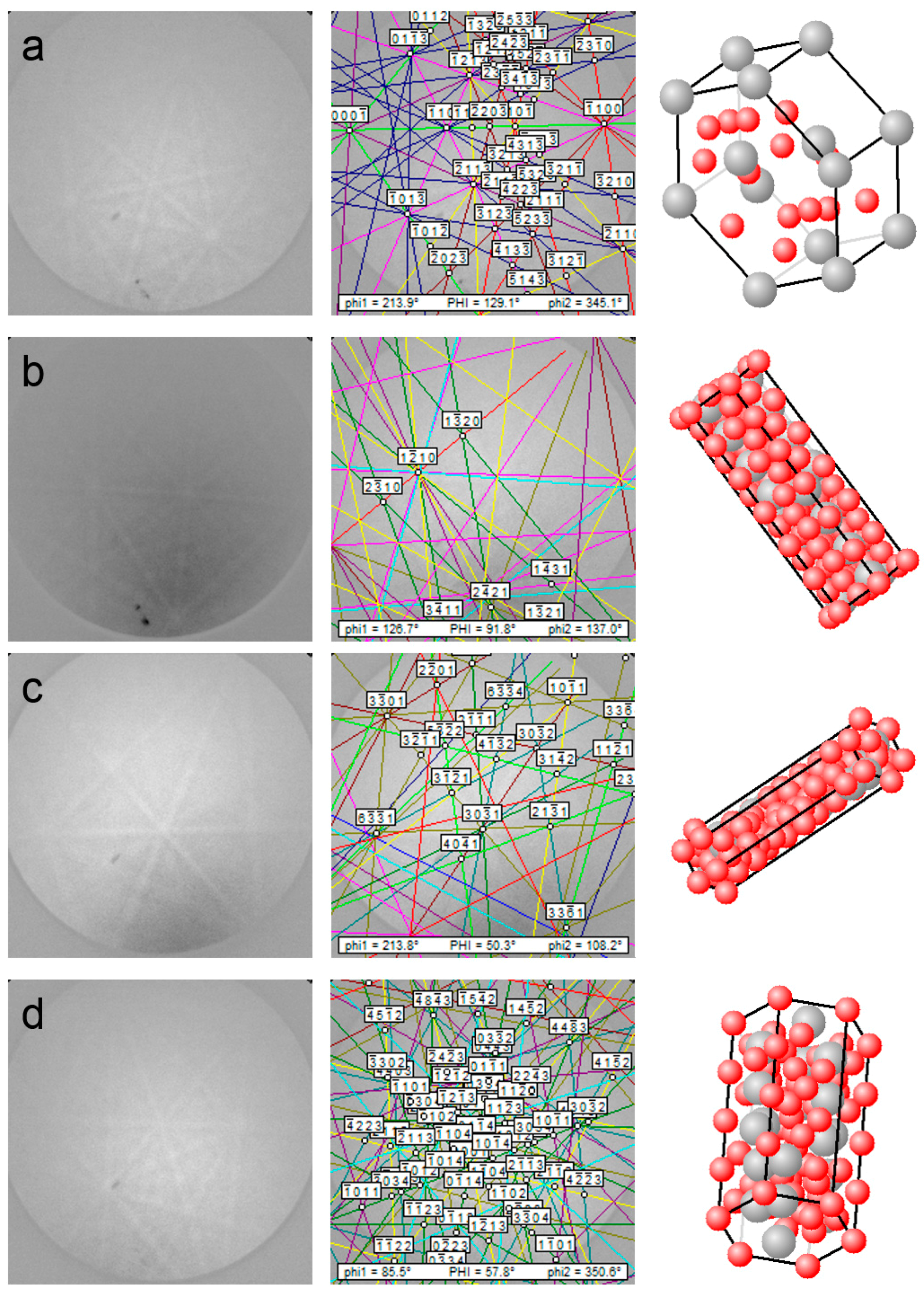
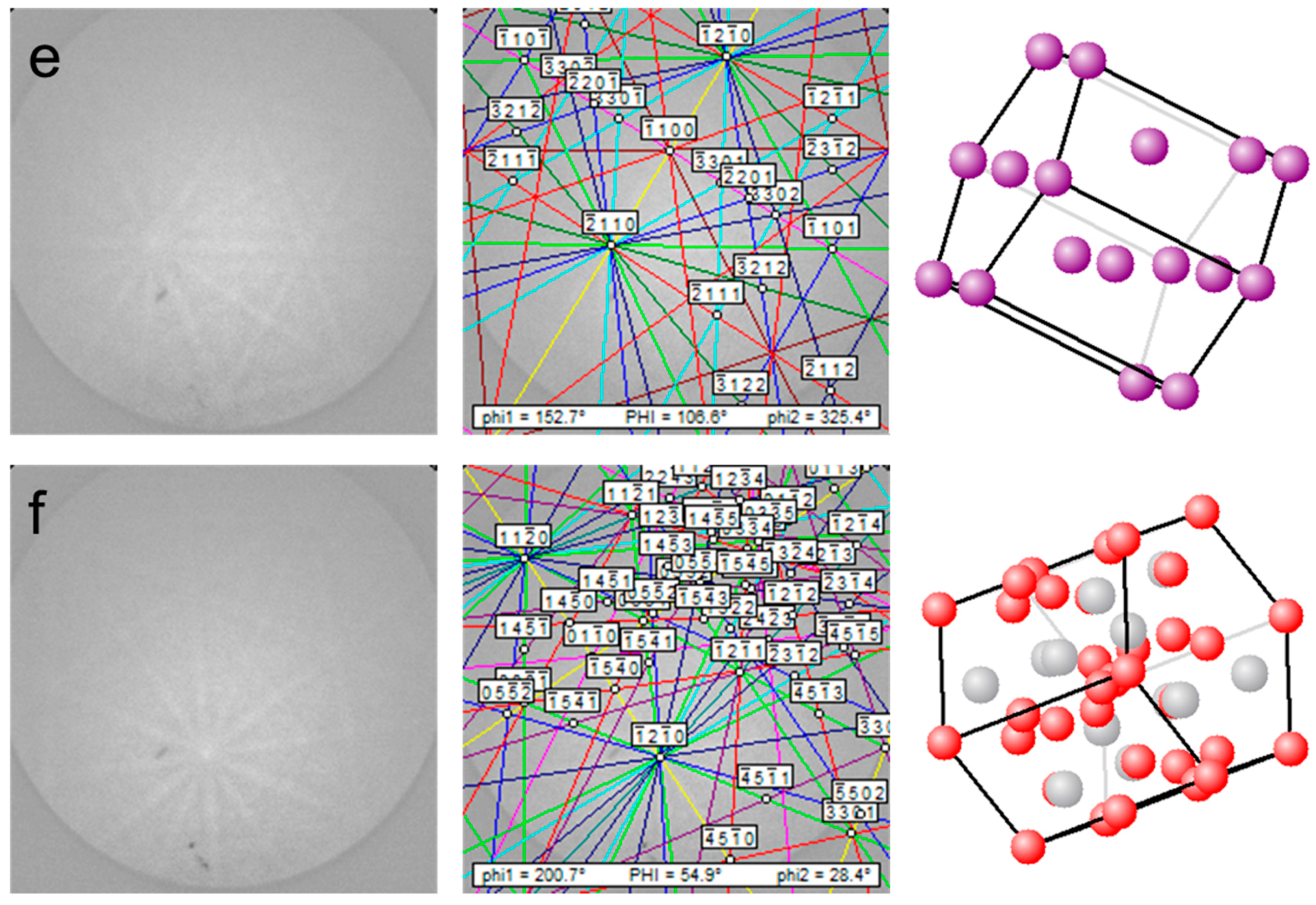
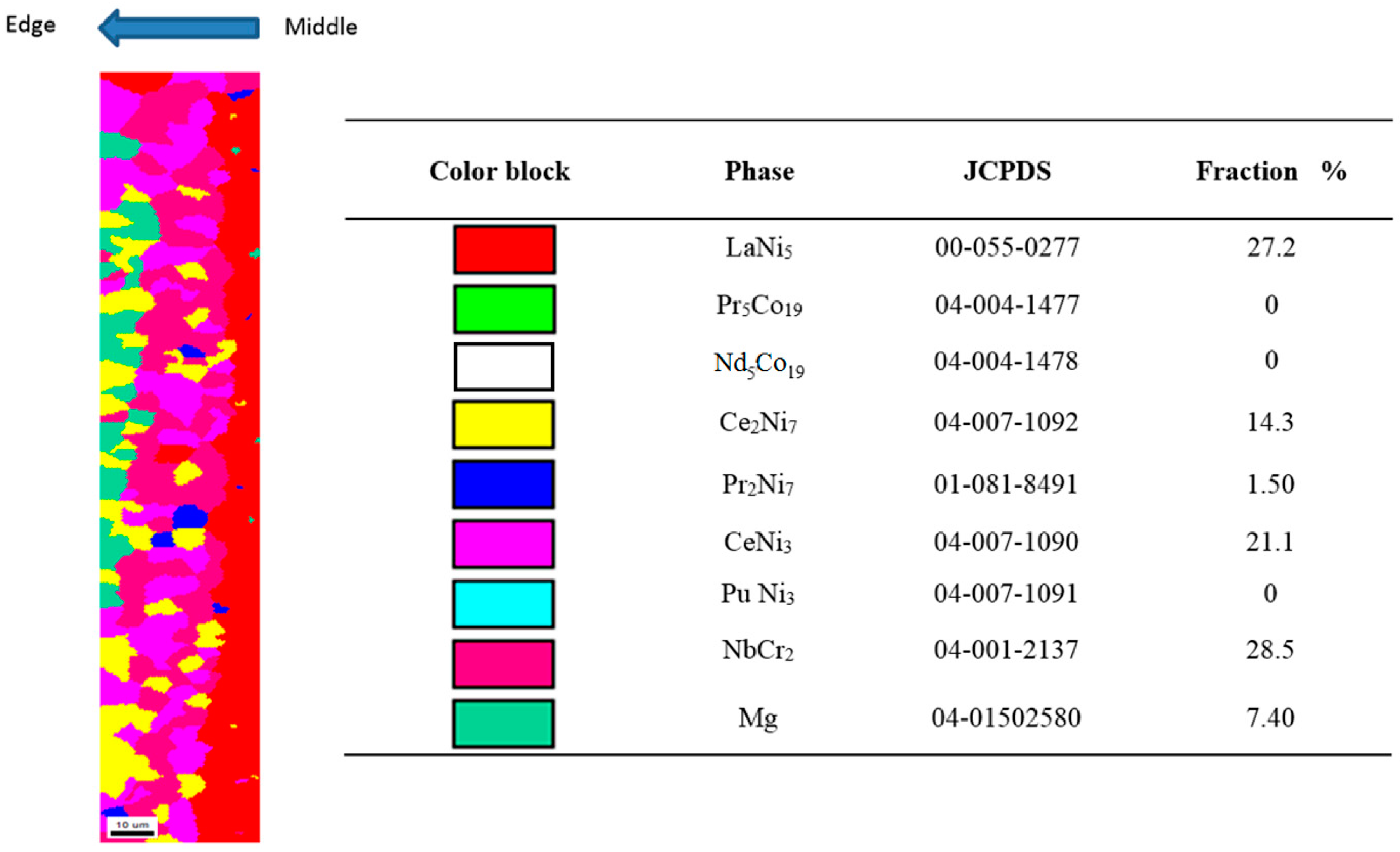
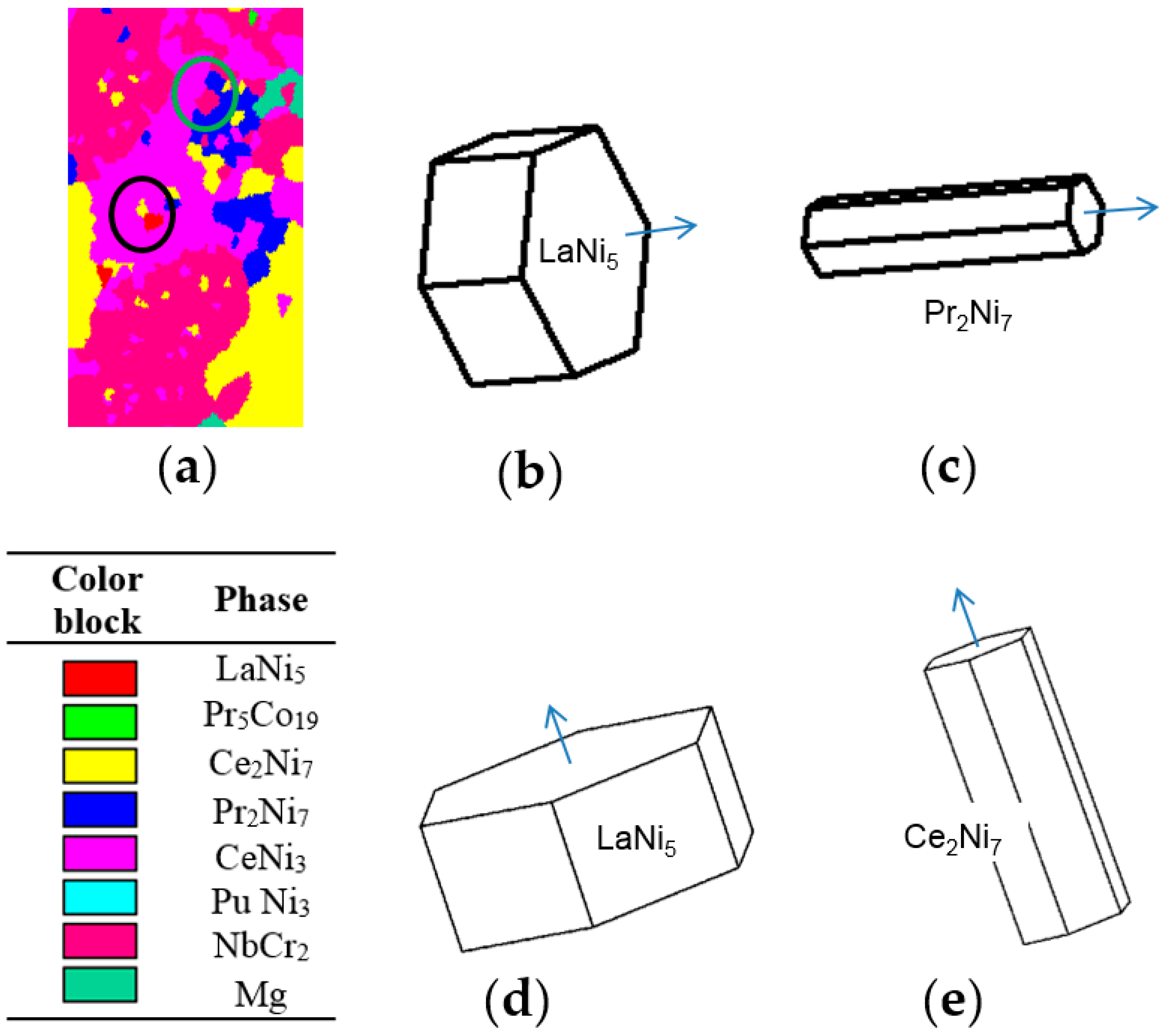
3.1. Phase Identification
3.2. Phase Distribution
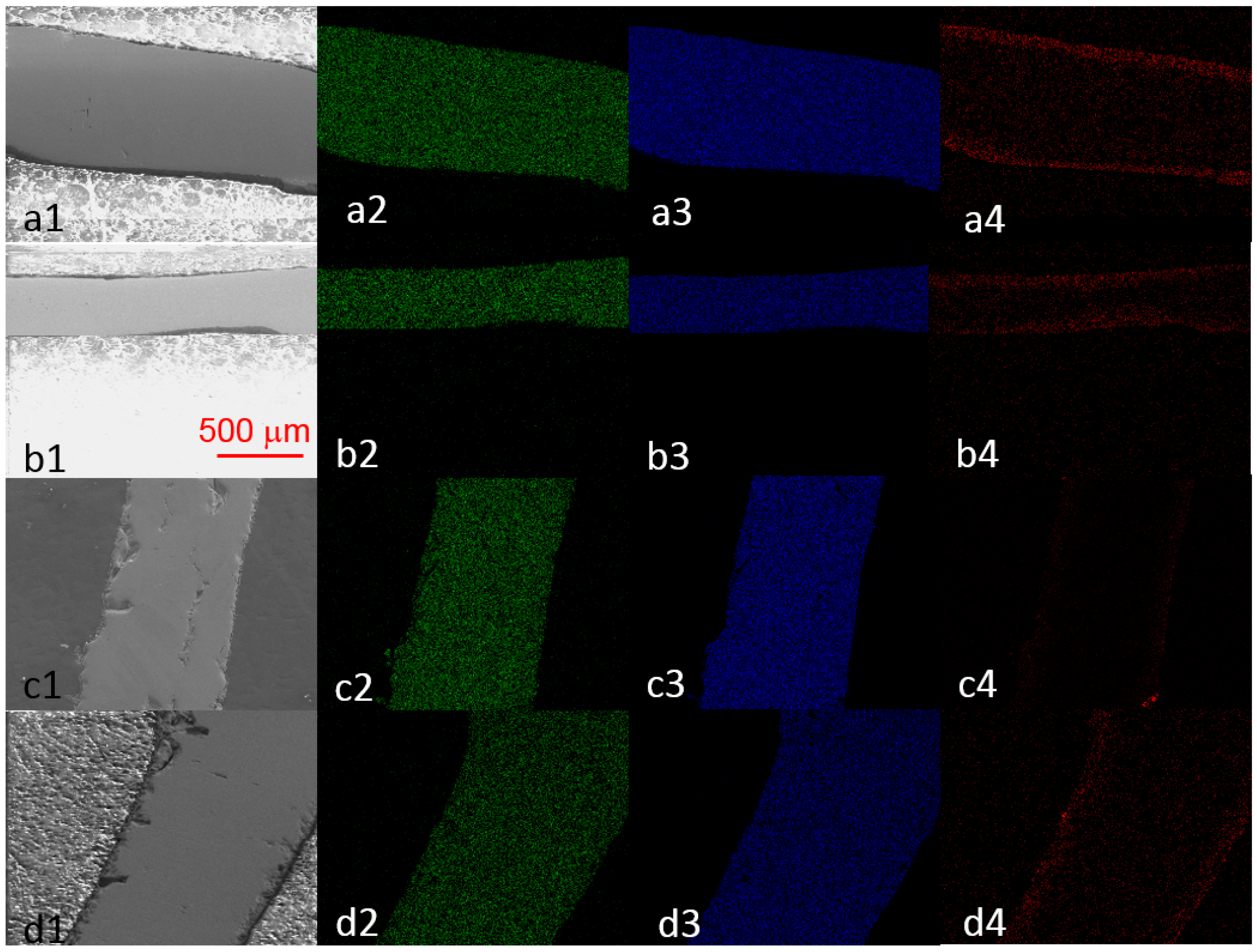
3.2.1. Element Distribution
3.2.2. Phase Distribution
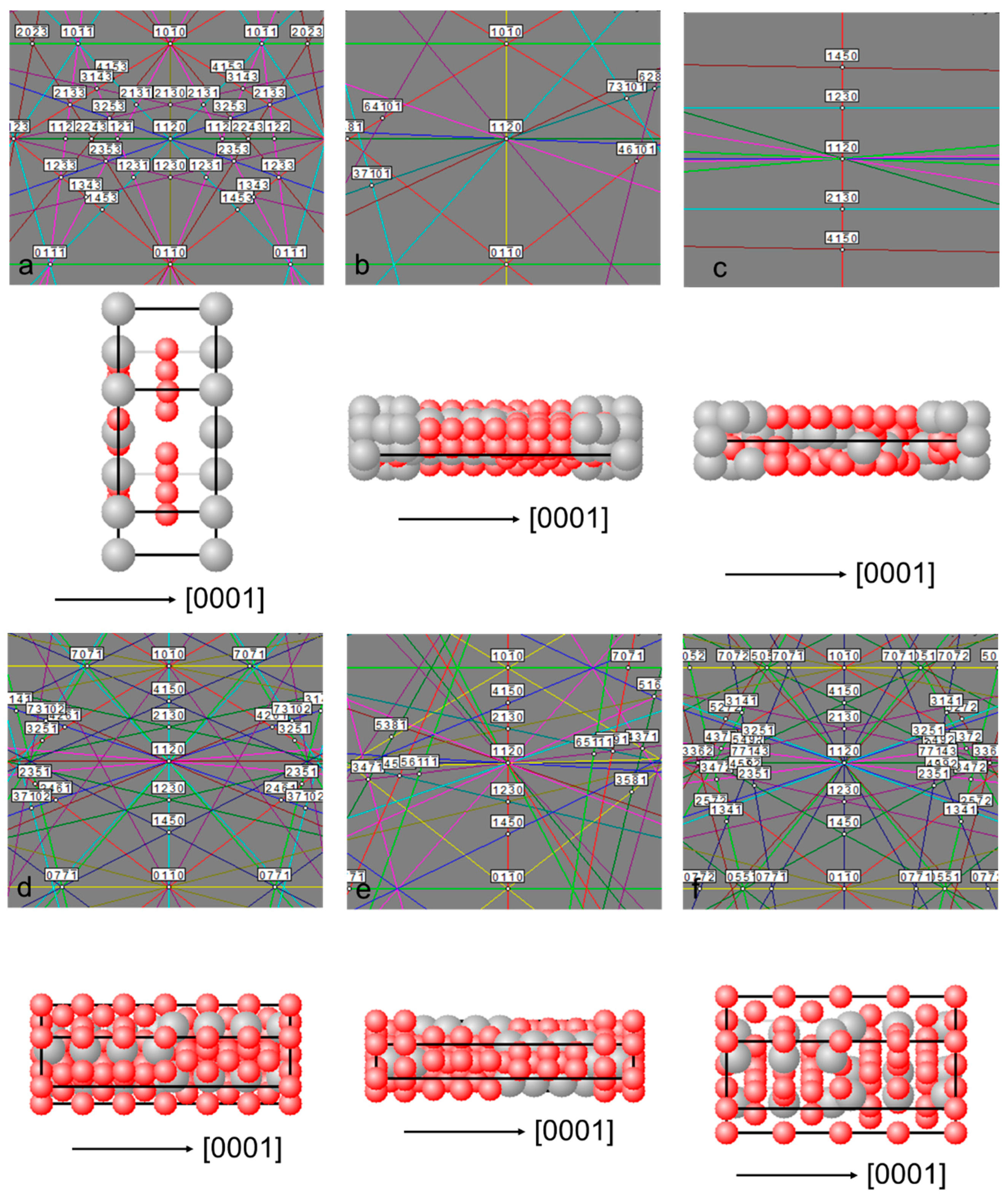
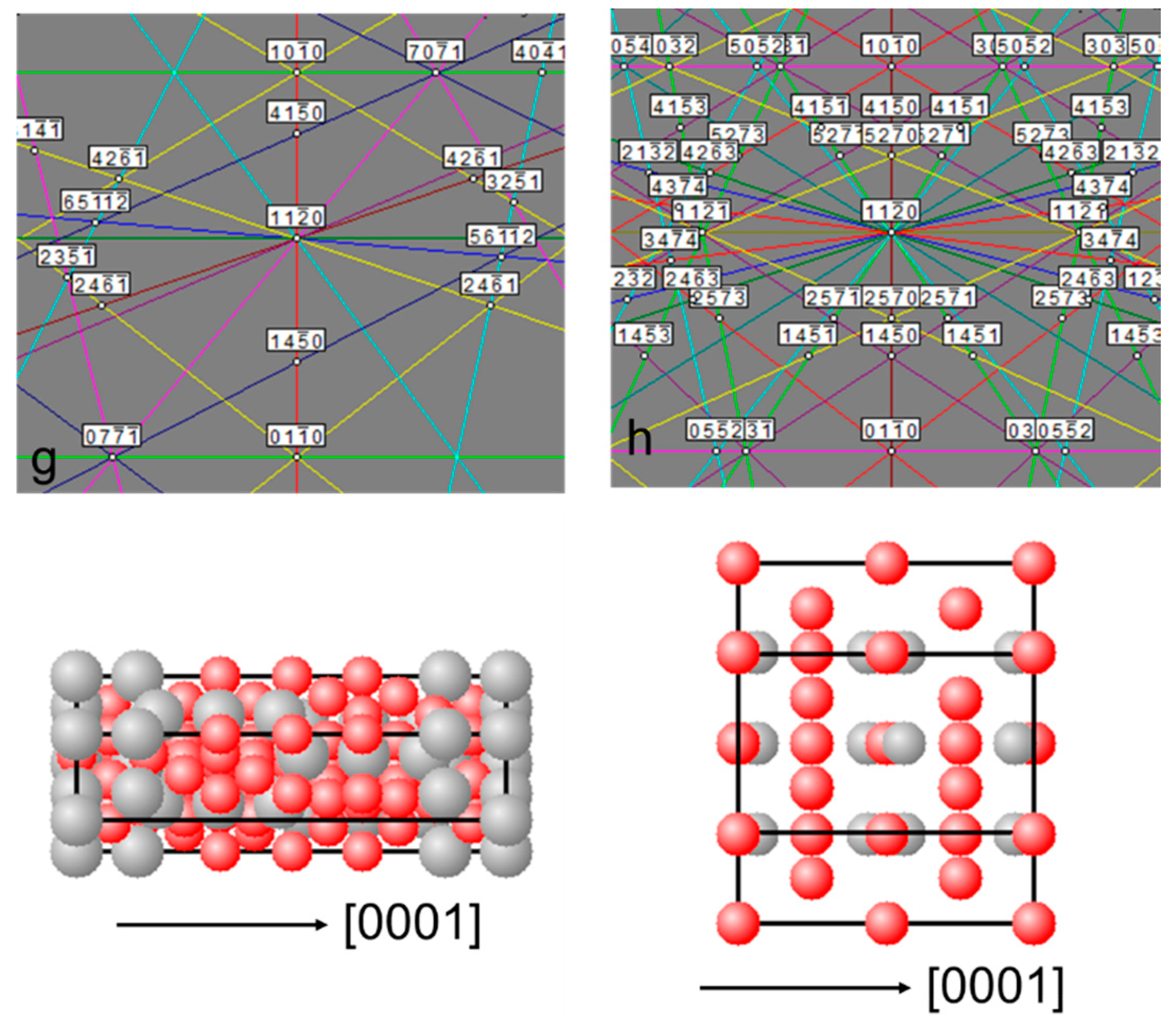
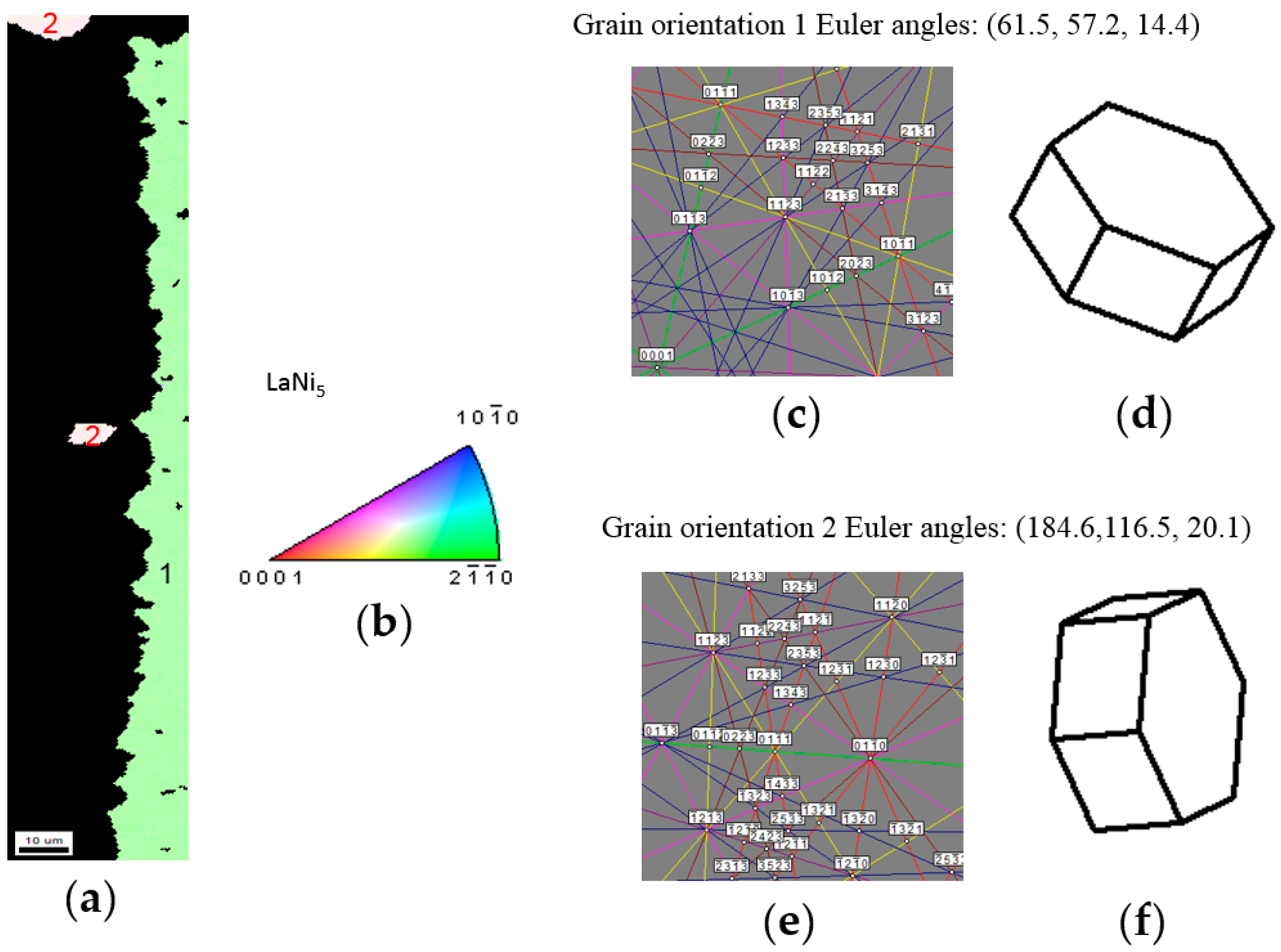
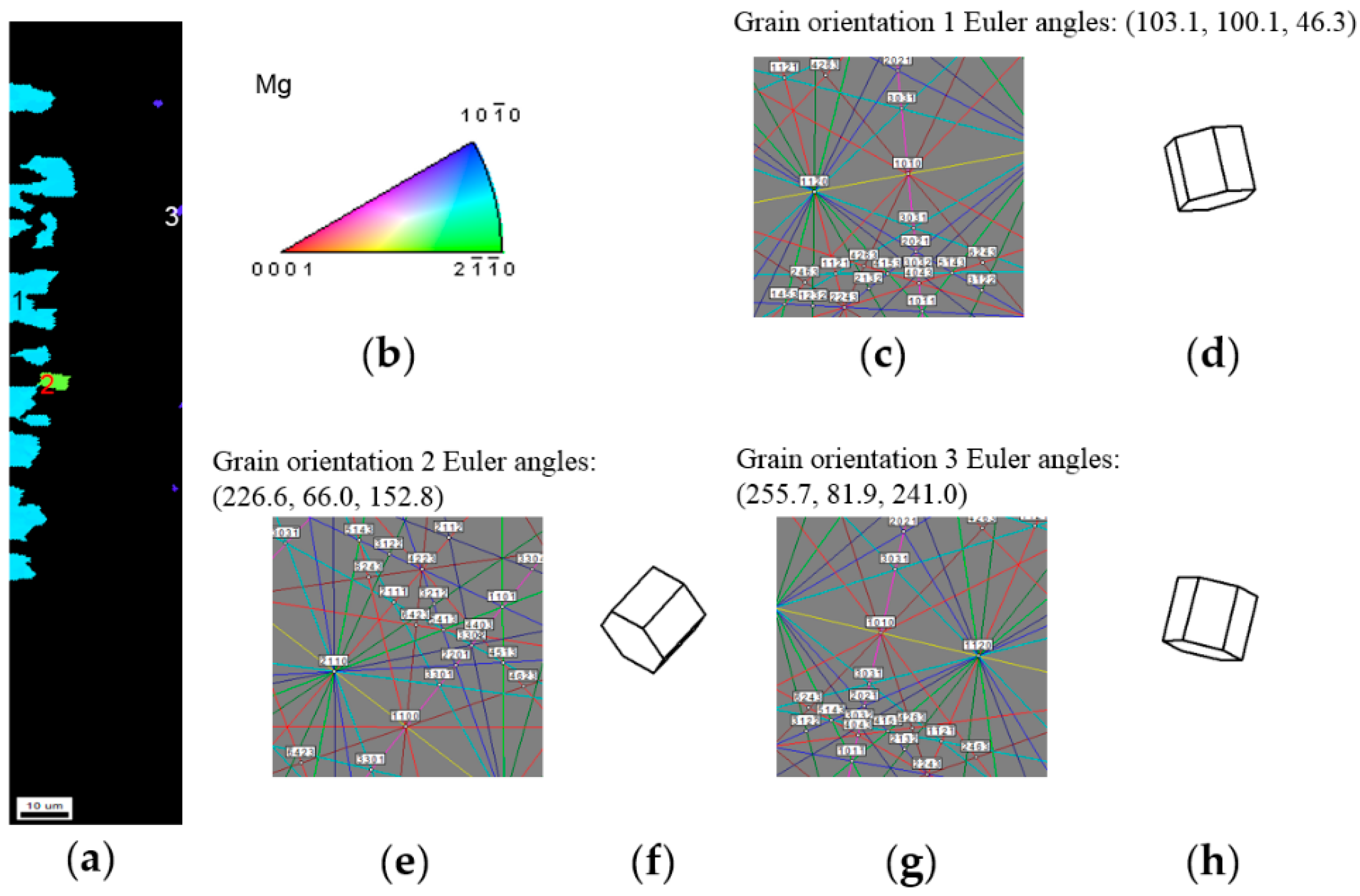
3.2.3. LaNi5 and Mg Grain Distributions and Orientations
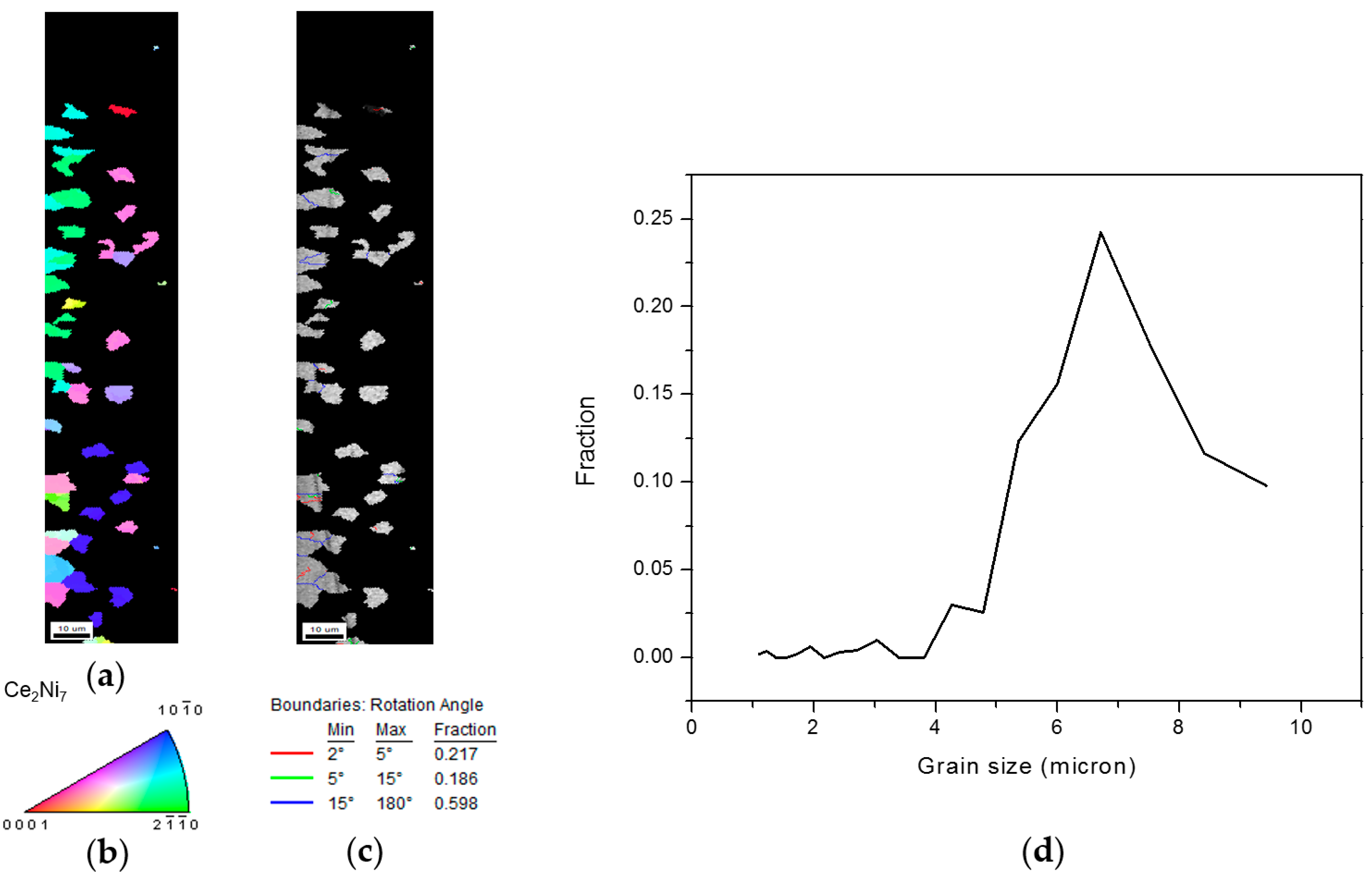
3.2.4. Ce2Ni7 and Pr2Ni7 Grain Distributions and Orientations
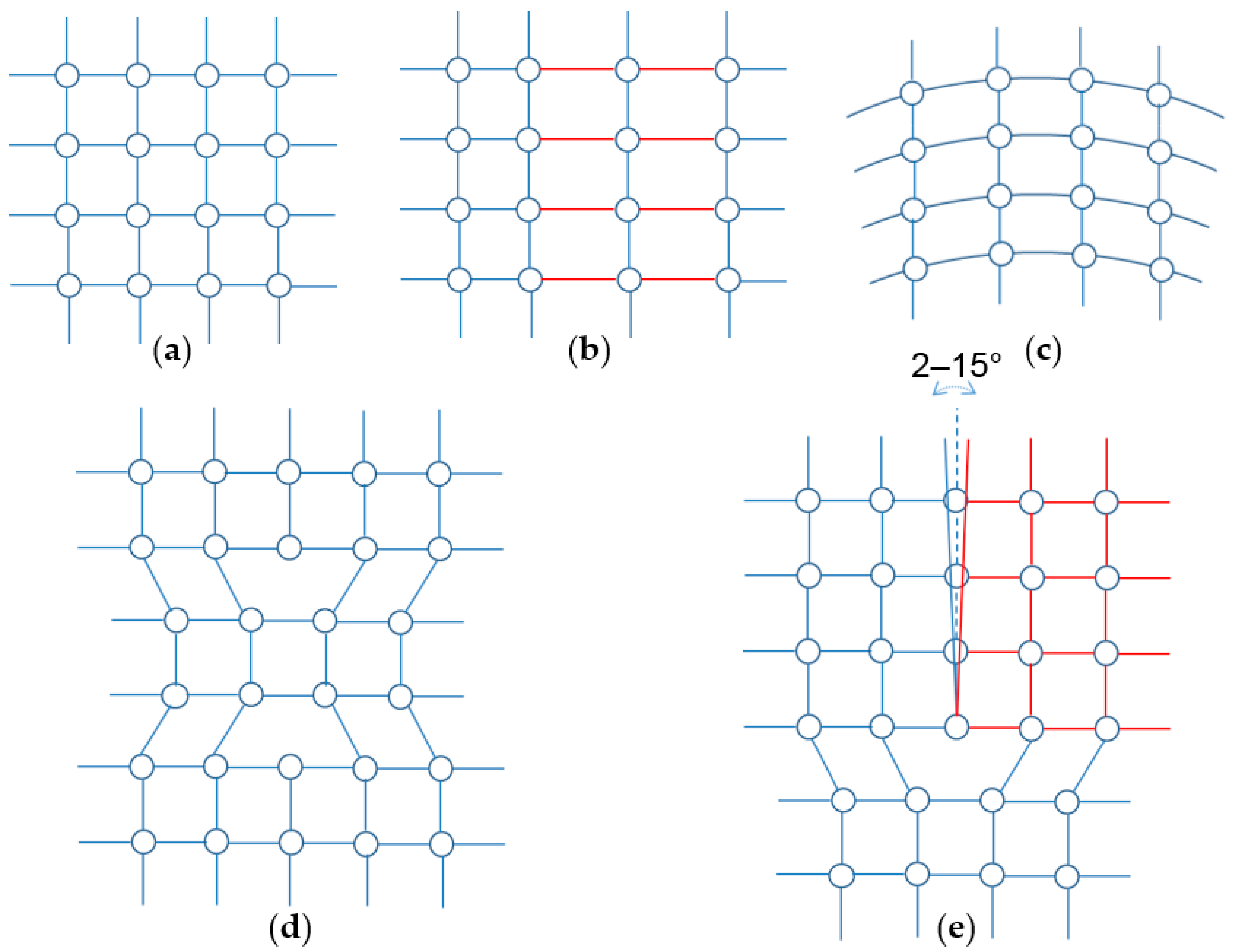
3.3. Alignment in Crystallographic Orientations
3.4. Effect of Process Temperature on Phase Development
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| RE | Rare earth |
| MH | Metal hydride |
| Ni/MH | Nickel/metal hydride |
| HRD | High-rate dischargeability |
| EBSD | Electron backscatter diffraction |
| SEM | Scanning electron microscope |
| EDS | Energy dispersive spectroscopy |
| BEI | Backscattered electron image |
| SSD | Statistically stored dislocations |
| GND | Geometrically necessary dislocations |
| IPF | Inverse pole figure |
| IQ | Image quality |
| LAGB | Low-angle grain boundary |
| HAGB | High-angle grain boundary |
References
- Yasuoka, S.; Magari, Y.; Murata, T.; Tanaka, T.; Ishida, J.; Nakamura, H.; Nohma, T.; Kihara, M.; Baba, Y.; Teraoka, H. Development of high-capacity nickel-metal hydride batteries using superlattice hydrogen-absorbing alloys. J. Power Sources 2006, 156, 662–666. [Google Scholar] [CrossRef]
- Teraoka, H. Development of Low Self-Discharge Nickel-Metal Hydride Battery. Available online: http://www.scribd.com/doc/9704685/Teraoka-Article-En (accessed on 9 April 2016).
- Kai, T.; Ishida, J.; Yasuoka, S.; Takeno, K. The effect of nickel-metal hydride battery’s characteristics with structure of the alloy. In Proceedings of the 54th Battery Symposium in Japan, Osaka, Japan, 7–9 October 2013; p. 210. [Google Scholar]
- Takasaki, T.; Nishimura, K.; Saito, M.; Fukunaga, H.; Iwaki, T.; Sakai, T. Cobalt-free nickel-metal hydride battery for industrial applications. J. Alloys Compd. 2013, 580, S378–S381. [Google Scholar] [CrossRef]
- Teraoka, H. Development of Ni-MH EThSS with Lifetime and Performance Estimation Technology. In Proceedings of the 34th International Battery Seminar & Exhibit, Fort Lauderdale, FL, USA, 20–23 March 2017. [Google Scholar]
- Teraoka, H. Ni-MH Stationary Energy Storage: Extreme Temperature & Long Life Developments. In Proceedings of the 33th International Battery Seminar & Exhibit, Fort Lauderdale, FL, USA, 21–24 March 2016. [Google Scholar]
- Teraoka, H. Development of Highly Durable and Long Life Ni-MH Batteries for Energy Storage Systems. In Proceedings of the 32th International Battery Seminar & Exhibit, Fort Lauderdale, FL, USA, 9–12 March 2015. [Google Scholar]
- Ouchi, T.; Young, K.; Moghe, D. Reviews on the Japanese Patent Applications regarding nickel/metal hydride batteries. Batteries 2016, 2, 21. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Nei, J.; Koch, J.M.; Lien, Y. Comparison among constituent phases in superlattice metal hydride alloys for batter applications. Batteries 2017, 3, 34. [Google Scholar] [CrossRef]
- Young, K.; Yasuoka, S. Past, present, and future of metal hydride alloys in nickel-metal hydride batteries. In Proceedings of the 14th International Symposium on Metal-Hydrogen Systems, Manchester, UK, 21–25 July 2014. [Google Scholar]
- Hayakawa, H.; Enoki, H.; Akiba, E. Annealing conditions with Mg vapor-pressure control and hydrogen storage characteristic of La4MgNi19 hydrogen storage alloy. Jpn. Inst. Met. 2006, 70, 158–161. (In Japanese) [Google Scholar] [CrossRef]
- Crivello, J.-C.; Zhang, J.; Latroche, M. Structural stability of ABy phases in the (La,Mg)–Ni system obtained by density functional theory calculations. J. Phys. Chem. 2011, 115, 25470–25478. [Google Scholar]
- Crivello, J.-C.; Gupta, M.; Latroche, M. First principles calculations of (La,Mg)2Ni7 hydrides. J. Alloys Compd. 2015, 645, S5–S8. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Huang, B. Effects of annealing and stoichiometry to (Nd, Mg)(Ni, Al)3.5 metal hydride alloys. J. Power Sources 2012, 215, 152–159. [Google Scholar] [CrossRef]
- Zhao, X.; Li, B.; Zhu, X.; Han, S.; Yan, H.; Ji, L.; Wang, L.; Li, J.; Xiong, W.; Jia, T. Preparation Method of Low-Melting Point Metal Alloy. Chinese Patent Application 201,511,015,059, 31 December 2015. [Google Scholar]
- Young, K.; Chang, S.; Lin, X. C14 Laves phase metal hydride alloys for Ni/MH batteries applications. Batteries 2017, 3, 27. [Google Scholar] [CrossRef]
- Matiland, T.; Sitzman, S. Electron backscatter diffraction (EBSD) technique and materials characterizations examples. In Scanning Microscopy for Nanotechnology Techniques and Applications; Zhou, W., Wang, Z.L., Eds.; Springer: New York, NY, USA, 2007. [Google Scholar]
- Young, K.; Ouchi, T.; Liu, Y.; Reichman, B.; Mays, W.; Fetcenko, M.A. Structural and electrochemical properties of TixZr7‒xNi10. J. Alloy. Compd. 2009, 480, 521–528. [Google Scholar] [CrossRef]
- Liu, Y.; Young, K. Microstructure investigation on metal hydride alloys by electron backscatter diffraction technique. Batteries 2016, 2, 26. [Google Scholar] [CrossRef]
- Shen, H.; Young, K.; Meng, T.; Bendersky, L.A. Clean grain boundary found in C14/body-center-cubic multi-phase metal hydride alloys. Batteries 2016, 2, 22. [Google Scholar] [CrossRef]
- Liu, J.; Han, S.; Li, Y.; Zhang, L.; Zhao, Y.; Yang, S. Phase structures and electrochemical properties of La–Mg–Ni-based hydrogen storage alloys with superlattice structure. Int. J. Hydrogen Energy 2016, 41, 20261–20275. [Google Scholar] [CrossRef]
- Buschow, K.H.; Van Mal, H.H. Phase relations and hydrogen absorption in the lanthanum-nickel system. J. Less Common Met. 1972, 29, 203–210. [Google Scholar] [CrossRef]
- Young, K.; Nei, J. The current status of hydrogen storage alloy development for electrochemical applications. Materials 2013, 6, 4574–4608. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Young, K.-H.; Nei, J.; Fierro, C. Reviews on the US Patents regarding nickel/metal hydride batteries. Batteries 2016, 2, 10. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Nei, J.; Yasuoka, S. Fe-substitution for Ni in misch metal-based superlattice hydrogen absorbing alloys—Part 1. Structural, hydrogen storage, and electrochemical properties. Batteries 2016, 2, 34. [Google Scholar] [CrossRef]
- Wright, S.I.; Nowell, M.M.; Field, D.P. A review of strain analysis using electron backscatter diffraction. Microsc. Microanal. 2011, 17, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Winkelmann, A. Dynamical effects of anisotropic inelastic scattering in electron backscatter diffraction. Ultramicroscopy 2008, 108, 1546–1550. [Google Scholar] [CrossRef] [PubMed]
- Keller, R.R.; Roshko, A.; Geiss, R.H.; Bertness, K.A.; Quinn, T.P. EBSD measurement of strains in GaAs due to oxidation of buried AlGaAs layers. Microelecron. Eng. 2004, 75, 96–102. [Google Scholar] [CrossRef]
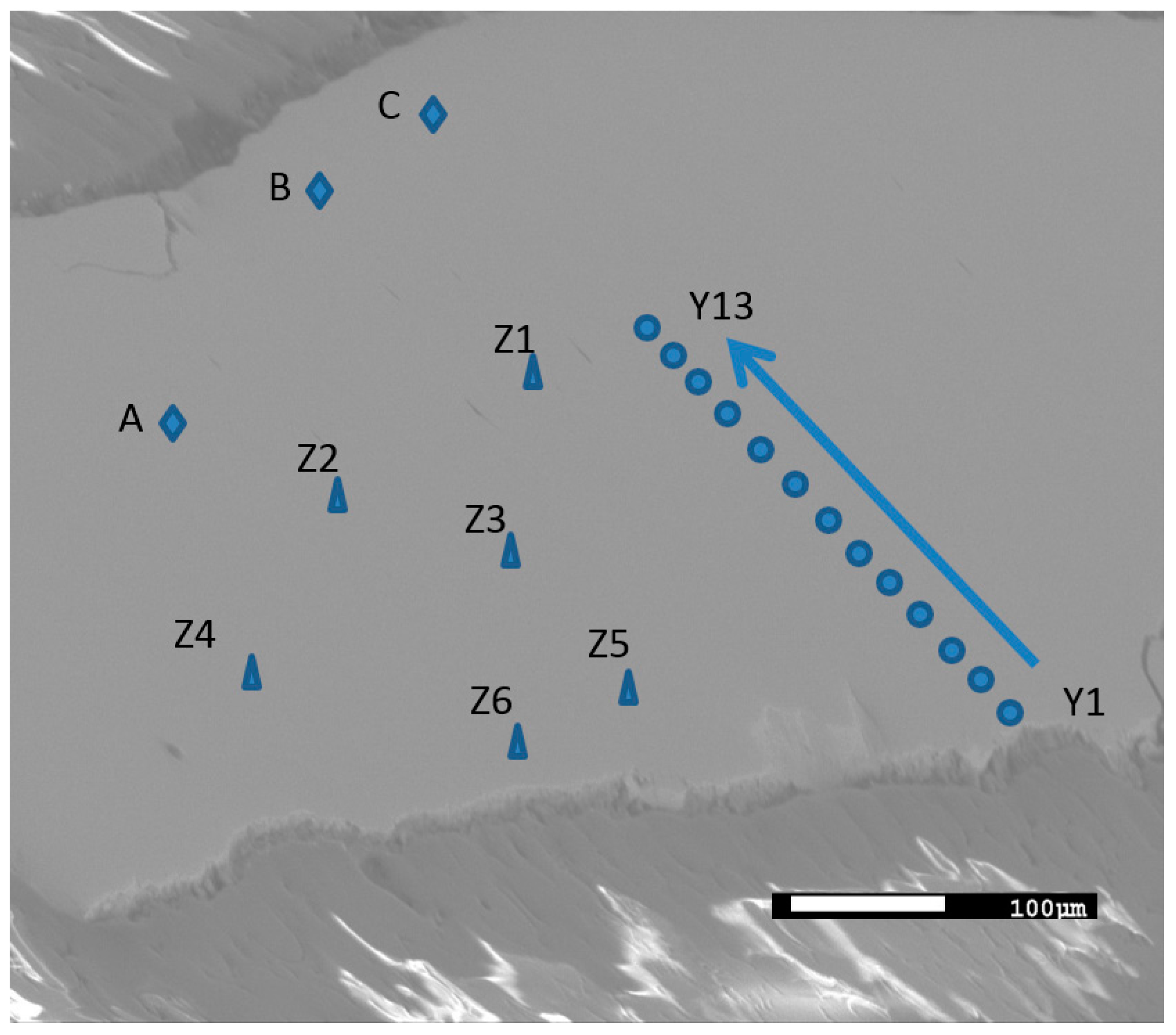


| Spot | Mg | La | Ni | Ni/(La + Mg) |
|---|---|---|---|---|
| Y1 | 24.01 | 16.19 | 59.79 | 1.49 |
| Y2 | 16.42 | 12.94 | 70.64 | 2.41 |
| Y3 | 13.12 | 14.09 | 72.78 | 2.67 |
| Y4 | 1.29 | 16.11 | 82.6 | 4.75 |
| Y5 | 0.00 | 16.45 | 83.55 | 5.08 |
| Y6 | 0.28 | 16.27 | 83.45 | 5.04 |
| Y7 | 0.29 | 16.10 | 83.61 | 5.10 |
| Y8 | 0.00 | 16.71 | 83.29 | 4.98 |
| Y9 | 0.00 | 15.82 | 84.18 | 5.32 |
| Y10 | 0.00 | 16.72 | 83.28 | 4.98 |
| Y11 | 0.00 | 16.24 | 83.76 | 5.16 |
| Y12 | 1.00 | 16.26 | 82.74 | 4.79 |
| Y13 | 8.32 | 15.16 | 76.54 | 3.26 |
| Phase | 4 h | 8 h | 16 h | 32 h |
|---|---|---|---|---|
| LaNi5 | 13.2 | 5.5 | 4.2 | 1.8 |
| Ce2Ni7 | 23.5 | 27.3 | 27.6 | 25.5 |
| Pr2Ni7 | 19.0 | 18.5 | 16.3 | 13.7 |
| CeNi3 | 22.6 | 19.5 | 26.4 | 21.0 |
| PuNi3 | 0.5 | 0.2 | 0.5 | 0.5 |
| NbCr2 | 16.2 | 25.5 | 22.3 | 30.3 |
| Mg | 4.9 | 3.4 | 2.8 | 7.2 |
| Grain Size (μm) | 4 h | 8 h | 16 h | 32 h |
|---|---|---|---|---|
| 1 to 5 | 24% | 79% | 35% | 37% |
| 5 to 12 | 61% | 16% | 48% | 49% |
| >12 | 15% | 5% | 16% | 14% |
| Grain Boundary (°) | 4 h | 8 h | 16 h | 32 h |
|---|---|---|---|---|
| 2 to 5 | 11.3% | 14.4% | 22.3% | 22.1% |
| 5 to 15 | 4.3% | 2.5% | 0.7% | 11.4% |
| 15 to 180 | 84.4% | 83.1% | 77.0% | 55.5% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, S.; Young, K.-H.; Zhao, X.; Mei, Z.; Ng, K.Y.S. Electron Backscatter Diffraction Studies on the Formation of Superlattice Metal Hydride Alloys. Batteries 2017, 3, 40. https://doi.org/10.3390/batteries3040040
Yan S, Young K-H, Zhao X, Mei Z, Ng KYS. Electron Backscatter Diffraction Studies on the Formation of Superlattice Metal Hydride Alloys. Batteries. 2017; 3(4):40. https://doi.org/10.3390/batteries3040040
Chicago/Turabian StyleYan, Shuli, Kwo-Hsiung Young, Xin Zhao, Zhi Mei, and K. Y. Simon Ng. 2017. "Electron Backscatter Diffraction Studies on the Formation of Superlattice Metal Hydride Alloys" Batteries 3, no. 4: 40. https://doi.org/10.3390/batteries3040040
APA StyleYan, S., Young, K.-H., Zhao, X., Mei, Z., & Ng, K. Y. S. (2017). Electron Backscatter Diffraction Studies on the Formation of Superlattice Metal Hydride Alloys. Batteries, 3(4), 40. https://doi.org/10.3390/batteries3040040