From Thermosetting Resins to Energy Devices: A Review on Polybenzoxazine-Derived Materials for Supercapacitors
Abstract
1. Introduction
2. Supercapacitor Performance of Novel Benzoxazines and Their Copolymers
3. Supercapacitor Performance of Polybenzoxazine/Bimetal Oxides
4. Supercapacitor Performance of Polybenzoxazine Composites
5. Supercapacitor Performance of Polybenzoxazine with Other Polymers
6. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Murugan, E.; Munusamy, K.; Babu, A.V. Development of Aryl Ether-Free Cross-Linked Polymer Membranes for Sustainable Electrochemical Energy Conversion and Storage Applications. Chem. Eng. J. 2024, 501, 157473. [Google Scholar] [CrossRef]
- Raja, M.; Sadhasivam, B.; Janraj Naik, R.; Dhamodharan, R.; Ramanujam, K. A Chitosan/Poly(Ethylene Glycol)-Ran-Poly(Propylene Glycol) Blend as an Eco-Benign Separator and Binder for Quasi-Solid-State Supercapacitor Applications. Sustain. Energy Fuels 2019, 3, 760–773. [Google Scholar] [CrossRef]
- Murugan, E.; Govindaraju, S.; Santhoshkumar, S. Hydrothermal Synthesis, Characterization and Electrochemical Behavior of NiMoO4 Nanoflower and NiMoO4/RGO Nanocomposite for High-Performance Supercapacitors. Electrochim. Acta 2021, 392, 138973. [Google Scholar] [CrossRef]
- Kesava, M.; Velautham, S.; Krishnan, S.; Kannaiyan, D. Graphene Nanosheets Dispersed Hydrophobic and Flexible Aliphatic Chain Containing Multifunctional Poly(Benzoxazines) Nanocomposites for Medium Temperature Proton Exchange Membrane Fuel Cell Applications. Int. J. Energy Res. 2022, 46, 18162–18178. [Google Scholar] [CrossRef]
- Cao, X.; Cui, L.; Liu, B.; Liu, Y.; Jia, D.; Yang, W.; Razal, J.M.; Liu, J. Reverse Synthesis of Star Anise-like Cobalt Doped Cu-MOF/Cu2+1 O Hybrid Materials Based on a Cu(OH)2 Precursor for High Performance Supercapacitors. J. Mater. Chem. A 2019, 7, 3815–3827. [Google Scholar] [CrossRef]
- Kesava, M.; Dinakaran, K. SnO2 Nanoparticle Assisted Enhanced Proton Exchange Membrane Fuel Cell Performance of Sulfuric Acid-Doped Porous Poly (Triphenylpyridine-Aliphatic Ethers). J. Phys. Chem. C 2021, 125, 130–142. [Google Scholar] [CrossRef]
- Lade, H.; Kumar, V.; Arthanareeswaran, G.; Ismail, A.F. Sulfonated Poly(Arylene Ether Sulfone) Nanocomposite Electrolyte Membrane for Fuel Cell Applications: A Review. Int. J. Hydrogen Energy 2017, 42, 1063–1074. [Google Scholar] [CrossRef]
- Le, Q.B.; Nguyen, T.H.; Fei, H.; Bubulinca, C.; Munster, L.; Bugarova, N.; Micusik, M.; Kiefer, R.; Dao, T.T.; Omastova, M.; et al. Electrochemical Performance of Composite Electrodes Based on RGO, Mn/Cu Metal–Organic Frameworks, and PANI. Sci. Rep. 2022, 12, 664. [Google Scholar] [CrossRef]
- Selvaraj, K.; Yu, B.; Spontón, M.E.; Kumar, P.; Veerasamy, U.S.; Arulraj, A.; Mangalaraja, R.V.; Almarhoon, Z.M.; Sayed, S.R.M.; Kannaiyan, D. Nonylphenol Polybenzoxazines-Derived Nitrogen-Rich Porous Carbon (NRPC)-Supported g-C3N4/Fe3O4 Nanocomposite for Efficient High-Performance Supercapacitor Application. Soft Matter 2024, 20, 7957–7969. [Google Scholar] [CrossRef]
- Selvaraj, K.; Spontón, M.E.; Estenoz, D.A.; Casarino, A.F.; Veerasamy, U.S.; Kumar, M.; Al-Mohaimeed, A.M.; Al-onazi, W.A.; Kannaiyan, D. Development of Quinoline-Based Heteroatom Polybenzoxazines Reinforced Graphitic Carbon Nitride (GCN) Carbonisation Composites for Emerging Supercapacitor Applications. Soft Matter 2024, 20, 1210–1223. [Google Scholar] [CrossRef]
- Wang, S.; Ma, J.; Shi, X.; Zhu, Y.; Wu, Z.S. Recent Status and Future Perspectives of Ultracompact and Customizable Micro-Supercapacitors. Nano Res. Energy 2022, 1, e9120018. [Google Scholar] [CrossRef]
- Amir, M.; Deshmukh, R.G.; Khalid, H.M.; Said, Z.; Raza, A.; Muyeen, S.M.; Nizami, A.S.; Elavarasan, R.M.; Saidur, R.; Sopian, K. Energy Storage Technologies: An Integrated Survey of Developments, Global Economical/Environmental Effects, Optimal Scheduling Model, and Sustainable Adaption Policies. J. Energy Storage 2023, 72, 108694. [Google Scholar] [CrossRef]
- Wang, H.; Shao, Y.; Mei, S.; Lu, Y.; Zhang, M.; Sun, J.K.; Matyjaszewski, K.; Antonietti, M.; Yuan, J. Polymer-Derived Heteroatom-Doped Porous Carbon Materials. Chem. Rev. 2020, 120, 9363–9419. [Google Scholar] [CrossRef]
- Rajendran, K.; Lolupiman, K.; Okhawilai, M.; Therese, H.A.; Kheawhom, S.; Tan, P.; Qin, J. Synthesis, Formation Mechanism and Supercapacitor Performance of MoS2/Mo2C/C Nanofibers. J. Alloys Compd. 2024, 980, 173549. [Google Scholar] [CrossRef]
- Asrafali, S.P.; Periyasamy, T.; Kim, S.C. Enhanced Electrochemical Performance of HC/NiCo@800C//HC Using Redox-Active Electrolytes Showing Increased Energy Density. J. Alloys Compd. 2024, 972, 172753. [Google Scholar] [CrossRef]
- Zhu, X.; Ji, C.; Meng, Q.; Mi, H.; Yang, Q.; Li, Z.; Yang, N.; Qiu, J. Freeze-Tolerant Hydrogel Electrolyte with High Strength for Stable Operation of Flexible Zinc-Ion Hybrid Supercapacitors. Small 2022, 18, 2200055. [Google Scholar] [CrossRef]
- Silva, R.J.; Klobukoski, V.; de Paula, J.I.S.; Riegel-Vidotti, I.C.; Vidotti, M. Assembly of Symmetric Supercapacitor Based on Alginate Hydrogel Electrolyte and Polyaniline Modified Electrodes. Electrochim. Acta 2022, 429, 140914. [Google Scholar] [CrossRef]
- Liu, J.; Ye, Z.; Hu, X.; Ahmed, S.; Song, S. High-Performance Na-Ion Conducting Polymer Gel Membrane for Supercapacitor Applications. ACS Appl. Polym. Mater. 2022, 4, 280–288. [Google Scholar] [CrossRef]
- Jin, T.; Su, J.; Luo, Q.; Zhu, W.; Lai, H.; Huang, D.; Wang, C. Preparation of N,P Co-Doped Porous Carbon Derived from Daylily for Supercapacitor Applications. ACS Omega 2022, 7, 37564–37571. [Google Scholar] [CrossRef]
- Zhang, P.; Mu, J.; Guo, Z.; Wong, S.I.; Sunarso, J.; Zhao, Y.; Xing, W.; Zhou, J.; Zhuo, S. Watermelon Peel-Derived Heteroatom-Doped Hierarchical Porous Carbon as a High-Performance Electrode Material for Supercapacitors. ChemElectroChem 2021, 8, 1196–1203. [Google Scholar] [CrossRef]
- Ghosh, S.; Barg, S.; Jeong, S.M.; Ostrikov, K. Heteroatom-Doped and Oxygen-Functionalized Nanocarbons for High-Performance Supercapacitors. Adv. Energy Mater. 2020, 10, 2001239. [Google Scholar] [CrossRef]
- Zuo, W.; Li, R.; Zhou, C.; Li, Y.; Xia, J.; Liu, J. Battery-Supercapacitor Hybrid Devices: Recent Progress and Future Prospects. Adv. Sci. 2017, 4, 1600539. [Google Scholar] [CrossRef]
- Lukatskaya, M.; Dunn, B.; Gogotsi, Y. Multidimensional materials and device architectures for future hybrid energy storage. Nat. Commun. 2016, 7, 12647. [Google Scholar] [CrossRef]
- Wang, D.-G.; Liang, Z.; Gao, S.; Qu, C.; Zou, R. Metal-organic framework-based materials for hybrid supercapacitor application. Coord. Chem. Rev. 2020, 404, 213093. [Google Scholar] [CrossRef]
- Tiwari, I.; Tanwar, V.; Ingole, P.P.; Nebhani, L. Heteroatom-Enriched Carbon Particles Derived from Multifunctional Polybenzoxazine Particles for High-Performance Supercapacitors. ACS Appl. Energy Mater. 2024, 7, 7185–7204. [Google Scholar] [CrossRef]
- Ye, X.; Fan, Q.; Shang, L.; Ye, F. Adsorptive Carbon-Based Materials for Biomedical Applications. Eng. Regen. 2022, 3, 352–364. [Google Scholar] [CrossRef]
- Saida, T.; Sakakibara, K.; Igami, R.; Maruyama, T. Synthesis of a Pt/Carbon-Sphere Catalyst and Evaluation of Its Oxygen Reduction Reaction Activity in Acidic Environments. Energy Fuels 2022, 36, 1027–1033. [Google Scholar] [CrossRef]
- Sobczuk, K.S.; Pełech, I.; Narkiewicz, U.; Staciwa, P.; Sibera, D.; Moszyński, D. The Influence of the Synthesis PH on the Morphology and Adsorption Properties of Carbon Spheres. Appl. Surf. Sci. 2023, 608, 155196. [Google Scholar] [CrossRef]
- Kakani, V.; Ramesh, S.; Yadav, H.M.; Bathula, C.; Basivi, P.K.; Palem, R.R.; Kim, H.S.; Pasupuletti, V.R.; Lee, H.; Kim, H. Hydrothermal Synthesis of CuO@MnO2 on Nitrogen-Doped Multiwalled Carbon Nanotube Composite Electrodes for Supercapacitor Applications. Sci. Rep. 2022, 12, 12951. [Google Scholar] [CrossRef]
- Bîru, E.I.; Gârea, S.A.; Iovu, H. Developing Polybenzoxazine Composites Based on Various Carbon Structures. Macromol. Chem. Phys. 2019, 220, 1800322. [Google Scholar] [CrossRef]
- Le, T.H.; Yoon, H. Strategies for Fabricating Versatile Carbon Nanomaterials from Polymer Precursors. Carbon 2019, 152, 796–817. [Google Scholar] [CrossRef]
- Al Aiti, M.; Jehnichen, D.; Fischer, D.; Brünig, H.; Heinrich, G. On the Morphology and Structure Formation of Carbon Fibers from Polymer Precursor Systems. Prog. Mater. Sci. 2018, 98, 477–551. [Google Scholar] [CrossRef]
- Liu, J.; Qiao, S.Z.; Liu, H.; Chen, J.; Orpe, A.; Zhao, D.; Lu, G.Q. Extension of the Stöber Method to the Preparation of Monodisperse Resorcinol-Formaldehyde Resin Polymer and Carbon Spheres. Angew. Chem.—Int. Ed. 2011, 50, 5947–5951. [Google Scholar] [CrossRef]
- Wu, H.; Qin, Y.; Zong, S.; Hu, Y.; Xaba, M.S.; Liu, X.; Chen, A. Porous Yolk–Shell-Structured Carbon Nanospheres for Electrochemical Energy Storage. J. Mater. Sci. Mater. Electron. 2020, 31, 13321–13329. [Google Scholar] [CrossRef]
- Konnola, R.; Anirudhan, T.S. Efficient Carbon Dioxide Capture by Nitrogen and Sulfur Dual-Doped Mesoporous Carbon Spheres from Polybenzoxazines Synthesized by a Simple Strategy. J. Environ. Chem. Eng. 2020, 8, 103614. [Google Scholar] [CrossRef]
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Morishita, T. Nitrogen-Doped Carbon Materials. Carbon 2018, 132, 104–140. [Google Scholar] [CrossRef]
- Leng, J.; Wang, Z.; Wang, J.; Wu, H.H.; Yan, G.; Li, X.; Guo, H.; Liu, Y.; Zhang, Q.; Guo, Z. Advances in Nanostructures Fabricated: Via Spray Pyrolysis and Their Applications in Energy Storage and Conversion. Chem. Soc. Rev. 2019, 48, 3015–3072. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Atchudan, R.; Parveen, A.S.; Lee, Y.R.; Kim, S.C. Polybenzoxazine Originated N-Doped Mesoporous Carbon Ropes as an Electrode Material for High-Performance Supercapacitors. J. Alloys Compd. 2018, 750, 384–391. [Google Scholar] [CrossRef]
- Yang, Y.; Du, H.; Sun, D.; Lu, C.; Lu, C.; Gao, J.; Xu, C.; Ma, X. Boosting Capacitive Performance of S-Doped Carbon Fibers via Substrate-Oriented Activation Methodology. Ind. Eng. Chem. Res. 2025, 64, 2745–2757. [Google Scholar] [CrossRef]
- Wang, H.; Wang, P.; Li, J.; Ran, Q. Facile Preparation and Improved Electrochemical Performance of Oxygen-Enriched Porous Carbon Materials Based on Diacetal-Containing Polybenzoxazine. Macromol. Mater. Eng. 2023, 308, 2200508. [Google Scholar] [CrossRef]
- Hulicova-Jurcakova, D.; Kodama, M.; Shiraishi, S.; Hatori, H.; Zhu, Z.H.; Lu, G.Q. Nitrogen-Enriched Nonporous Carbon Electrodes with Extraordinary Supercapacitance. Adv. Funct. Mater. 2009, 19, 1800–1809. [Google Scholar] [CrossRef]
- Wang, L.; Sun, J.; Zhang, H.; Xu, L.; Liu, G. Preparation of Benzoxazine-Based N-Doped Mesoporous Carbon Material and Its Electrochemical Behaviour as Supercapacitor. J. Electroanal. Chem. 2020, 868, 114196. [Google Scholar] [CrossRef]
- Cheng, Q.; Tang, J.; Ma, J.; Zhang, H.; Shinya, N.; Qin, L.C. Graphene and Carbon Nanotube Composite Electrodes for Supercapacitors with Ultra-High Energy Density. Phys. Chem. Chem. Phys. 2011, 13, 17615–17624. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.G.; Su, B.X.; Kuo, S.W. Robust Nitrogen-Doped Microporous Carbon via Crown Ether-Functionalized Benzoxazine-Linked Porous Organic Polymers for Enhanced CO2 Adsorption and Supercapacitor Applications. ACS Appl. Mater. Interfaces 2024, 16, 40858–40872. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Ishida, H. Polymerization of an AB-Type Benzoxazine Monomer toward Different Polybenzoxazine Networks: When Diels-Alder Reaction Meets Benzoxazine Chemistry in a Single-Component Resin. Macromolecules 2019, 52, 7386–7395. [Google Scholar] [CrossRef]
- Samy, M.M.; Mohamed, M.G.; Kuo, S.W. Directly Synthesized Nitrogen-and-Oxygen–Doped Microporous Carbons Derived from a Bio-Derived Polybenzoxazine Exhibiting High-Performance Supercapacitance and CO2 Uptake. Eur. Polym. J. 2020, 138, 109954. [Google Scholar] [CrossRef]
- Mukherjee, S.; Amarnath, N.; Lochab, B. Oxazine Ring-Substituted 4th Generation Benzoxazine Monomers & Polymers: Stereoelectronic Effect of Phenyl Substituents on Thermal Properties. Macromolecules 2021, 54, 9510–9525. [Google Scholar] [CrossRef]
- Pei, F.; An, T.H.; Zang, J.; Zhao, X.J.; Fang, X.L.; Zheng, M.S.; Dong, Q.F.; Zheng, N.F. From Hollow Carbon Spheres to N-Doped Hollow Porous Carbon Bowls: Rational Design of Hollow Carbon Host for Li-S Batteries. Adv. Energy Mater. 2016, 6, 1502539. [Google Scholar] [CrossRef]
- Zhao, J.; Gilani, M.R.H.S.; Lai, J.; Nsabimana, A.; Liu, Z.; Luque, R.; Xu, G. Autocatalysis Synthesis of Poly(Benzoxazine- Co-Resol)-Based Polymer and Carbon Spheres. Macromolecules 2018, 51, 5494–5500. [Google Scholar] [CrossRef]
- Majumdar, D.; Mandal, M.; Bhattacharya, S.K. V2O5 and Its Carbon-Based Nanocomposites for Supercapacitor Applications. ChemElectroChem 2019, 6, 1623–1648. [Google Scholar] [CrossRef]
- Chen, N.; Younis, A.; Huang, S.; Chu, D.; Li, S. Advanced Three-Dimensional Hierarchical Pr6O11@Ni-Co Oxides-Based Core-Shell Electrodes for Supercapacitance Application. J. Alloys Compd. 2019, 783, 772–778. [Google Scholar] [CrossRef]
- Das, T.K.; Ghosh, P.; Das, N.C. Preparation, Development, Outcomes, and Application Versatility of Carbon Fiber-Based Polymer Composites: A Review. Adv. Compos. Hybrid Mater. 2019, 2, 214–233. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, S.; Wang, D.; Qing, Y.; Yan, G.; Li, L.; Wu, Y. Low-Tortuosity Carbon Electrode Derived from Wood@ZIF-67 for Supercapacitor Applications. Chem. Eng. J. 2023, 454, 140410. [Google Scholar] [CrossRef]
- Wang, S.; Li, W.C.; Zhang, L.; Jin, Z.Y.; Lu, A.H. Polybenzoxazine-based monodisperse carbon spheres with low-thermal shrinkage and their CO2 adsorption properties. J. Mater. Chem. A 2014, 2, 4406–4412. [Google Scholar] [CrossRef]
- Wang, S.; Li, W.C.; Hao, G.P.; Hao, Y.; Sun, Q.; Zhang, X.Q.; Lu, A.H. Temperature-Programmed Precise Control over the Sizes of Carbon Nanospheres Based on Benzoxazine Chemistry. J. Am. Chem. Soc. 2011, 133, 15304–15307. [Google Scholar] [CrossRef]
- Lu, N.; He, G.; Liu, J.; Liu, G.; Li, J. Combustion synthesis of graphene for water treatment. Ceram. Int. 2018, 44, 2463–2469. [Google Scholar] [CrossRef]
- Nagaraju, P.; Alsalme, A.; Alswieleh, A.; Jayavel, R. Facile in-situ microwave irradiation synthesis of TiO2/graphene nanocomposite for high-performance supercapacitor applications. J. Electroanal. Chem. 2018, 808, 90–100. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Li, Y.-L.; Liu, L.-N.; Xu, Z.-W.; Xie, G.; Wang, Y.; Zhao, F.-G.; Gao, T.; Li, W.-S. Microporous N- and O-Codoped Carbon Materials Derived from Benzoxazine for Supercapacitor Application. Inorganics 2023, 11, 269. [Google Scholar] [CrossRef]
- Shaer, C.; Oppenheimer, L.; Lin, A.; Ishida, H. Advanced Carbon Materials Derived from Polybenzoxazines: A Review. Polymers 2021, 13, 3775. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Atchudan, R.; Parveen, A.S.; Lee, Y.R.; Kim, S.C. The Synthesis of Mechanically Stable Polybenzoxazine-Based Porous Carbon and Its Application as High-Performance Supercapacitor Electrodes. New J. Chem. 2021, 45, 8738–8746. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, J.; Nagaraju, D.H.; Jiang, L.; Marinov, V.R.; Lubineau, G.; Alshareef, H.N.; Oh, M. Flexible, Highly Graphitized Carbon Aerogels Based on Bacterial Cellulose/Lignin: Catalyst-Free Synthesis and its Application in Energy Storage Devices. Adv. Funct. Mater. 2015, 25, 3193–3202. [Google Scholar] [CrossRef]
- Jin, Y.; Tian, K.; Wei, L.; Zhang, X.; Guo, X. Hierarchical porous microspheres of activated carbon with a high surface area from spores for electrochemical double-layer capacitors. J. Mater. Chem. A 2016, 4, 15968–15979. [Google Scholar] [CrossRef]
- Sudhan, N.; Subramani, K.; Karnam, M.; Ilayaraja, N.; Sathish, M. Biomass-Derived Activated Porous Carbon from Rice Straw for a High-Energy Symmetric Supercapacitor in Aqueous and Non-aqueous Electrolytes. Energy Fuels 2017, 31, 977–985. [Google Scholar] [CrossRef]
- Thubsuang, U.; Chotirut, S.; Thongnok, A.; Promraksa, A.; Nisoa, M.; Manmuanpom, N.; Wongkasemjit, S.; Chaisuwan, T. Facile Preparation of Polybenzoxazine-Based Carbon Microspheres with Nitrogen Functionalities: Effects of Mixed Solvents on Pore Structure and Supercapacitive Performance. Front. Chem. Sci. Eng. 2020, 14, 1072–1086. [Google Scholar] [CrossRef]
- Lei, W.; Guo, J.; Wu, Z.; Xuan, C.; Xiao, W.; Wang, D. Highly nitrogen and sulfur dual-doped carbon microspheres for supercapacitors. Sci. Bull. 2017, 62, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, X.; Tu, M.; Cheng, J.; Zhang, J. Resin-derived activated carbons with in-situ nitrogen doping and high specific surface area for high-performance supercapacitors. Mater. Lett. 2017, 191, 178–181. [Google Scholar] [CrossRef]
- Zhu, D.; Jiang, J.; Sun, D.; Qian, X.; Wang, Y.; Li, L.; Wang, Z.; Chai, X.; Gan, L.; Liu, M. A general strategy to synthesize high-level N-doped porous carbons via Schiff-base chemistry for supercapacitors. J. Mater. Chem. A 2018, 6, 12334–12343. [Google Scholar] [CrossRef]
- Song, Z.; Duan, H.; Zhu, D.; Lv, Y.; Xiong, W.; Cao, T.; Li, L.; Liu, M.; Gan, L. Ternary-doped carbon electrodes for advanced aqueous solid-state supercapacitors based on a “water-in-salt” gel electrolyte. J. Mater. Chem. A 2019, 7, 15801–15811. [Google Scholar] [CrossRef]
- Li, Q.; Lu, T.; Wang, L.; Pang, R.; Shao, J.; Liu, L.; Hu, X. Biomass based N-doped porous carbons as efficient CO2 adsorbents and high-performance supercapacitor electrodes. Sep. Purif. Technol. 2021, 275, 119204. [Google Scholar] [CrossRef]
- Li, J.; Zou, Y.; Xiang, C.; Xu, F.; Sun, L.; Li, B.; Zhang, J. Osmanthus fragrans-derived N-doped porous carbon for supercapacitor applications. J. Energy Storage 2021, 42, 103017. [Google Scholar] [CrossRef]
- Wan, L.; Wang, J.; Sun, Y.; Feng, C.; Li, K. Polybenzoxazine-Based Nitrogen-Containing Porous Carbons for High-Performance Supercapacitor Electrodes and Carbon Dioxide Capture. RSC Adv. 2015, 5, 5331–5342. [Google Scholar] [CrossRef]
- Wang, J.C.; Liu, Q. An efficient one-step condensation and activation strategy to synthesize porous carbons with optimal micropore sizes for highly selective CO2 adsorption. Nanoscale 2014, 6, 4148–4156. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.P.; Li, W.C.; Qian, D.; Lu, A.H. Rapid Synthesis of Nitrogen-Doped Porous Carbon Monolith for CO2 Capture. Adv. Mater. 2010, 22, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Tian, C.; Fu, Y.; Yang, Y.; Yin, J.; Wang, L.; Fu, H. Nitrogen-Doped Porous Graphitic Carbon as an Excellent Electrode Material for Advanced Supercapacitors. Chem.–Eur. J. 2014, 20, 564–574. [Google Scholar] [CrossRef]
- Xu, B.; Zheng, D.; Jia, M.; Cao, G.; Yang, Y. Nitrogen-doped porous carbon simply prepared by pyrolyzing a nitrogen-containing organic salt for supercapacitors. Electrochim. Acta 2013, 98, 176–182. [Google Scholar] [CrossRef]
- Chen, L.F.; Huang, Z.H.; Liang, H.W.; Yao, W.T.; Yu, Z.Y.; Yu, S.H. Flexible all-solid-state high-power supercapacitor fabricated with nitrogen-doped carbon nanofiber electrode material derived from bacterial cellulose. Energy Environ. Sci. 2013, 6, 3331–3338. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, L.; Luo, J.; Peng, Y.; Ji, Q.; Dai, J.; Zhu, J.; Liu, X. Biobased Nitrogen- and Oxygen-Codoped Carbon Materials for High-Performance Supercapacitor. ACS Sustain. Chem. Eng. 2019, 7, 2763–2773. [Google Scholar] [CrossRef]
- Huang, C.H.; Zhang, Q.; Chou, T.C.; Chen, C.M.; Su, D.S.; Doong, R.A. Three dimensional hierarchically ordered porous carbons with partially graphitic nanostructures for electrochemical capacitive energy storage. ChemSusChem 2012, 5, 563–571. [Google Scholar] [CrossRef]
- Chen, L.F.; Zhang, X.D.; Liang, H.W.; Kong, M.G.; Guan, Q.F.; Chen, P.; Wu, Z.Y.; Yu, S.H. Synthesis of nitrogen-doped porous carbon nanofibers as an efficient electrode material for supercapacitors. ACS Nano 2012, 6, 7092–7102. [Google Scholar] [CrossRef]
- Li, J.G.; Lee, P.Y.; Ahmed, M.M.M.; Mohamed, M.G.; Kuo, S.W. Varying the hydrogen bonding strength in phenolic/PEO-b -PLA blends provides mesoporous carbons having large accessible pores suitable for energy storage. Macromol. Chem. Phys. 2020, 221, 2000040. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Hung, W.S.; EL-Mahdy, A.F.M.; Ahmed, M.M.M.; Dai, L.; Chen, T.; Kuo, S.W. High-molecular-weight PLA-b-PEO-b-PLA triblock copolymer templated large mesoporous carbons for supercapacitors and CO2 capture. Polymers 2020, 12, 1193. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, C.; Wang, Y.; Gao, F.; Cheng, J.; Zhang, J. High-Performance Supercapacitor Based on Nitrogen and Phosphorus Co-Doped Nonporous Polybenzoxazine-Based Carbon Electrodes. J. Electrochem. Soc. 2018, 165, A3313–A3320. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Lee, Y.-F.; Chang, K.-H.; Hu, C.-C. Synthesis of N-doped carbon nanosheets from collagen for electrochemical energy storage/conversion systems. Electrochem. Commun. 2011, 13, 50. [Google Scholar] [CrossRef]
- Wang, C.; Sun, L.; Zhou, Y.; Wan, P.; Zhang, X.; Qiu, J. P/N co-doped microporous carbons from H3PO4-doped polyaniline by in situ activation for supercapacitors. Carbon 2013, 59, 537. [Google Scholar] [CrossRef]
- Bardestani, R.; Patience, G.S.; Kaliaguine, S. Experimental methods in chemical engineering: Specific surface area and pore size distribution measurements—BET, BJH, and DFT. Can. J. Chem. Eng. 2019, 97, 2781–2791. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, Z.; Zhou, J.; Jin, L.; Wei, B.O.; He, X. Facile synthesis of MOF-derived concave cube nanocomposite by self-templated toward lightweight and wideband microwave absorption. Carbon 2022, 186, 574–588. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Z.; Huang, Z.; Zhang, H. Effects of oxygen-containing functional groups on the supercapacitor performance of incompletely reduced graphene oxides. Int. J. Hydrogen Energy 2017, 42, 7186–7194. [Google Scholar] [CrossRef]
- Bai, L.; Ge, Y.; Bai, L. Boron and Nitrogen Co-Doped Porous Carbons Synthesized from Polybenzoxazines for High-Performance Supercapacitors. Coatings 2019, 9, 657. [Google Scholar] [CrossRef]
- Puthusseri, D.; Aravindan, V.; Madhavi, S.; Ogale, S. 3D micro-porous conducting carbon beehive by single step polymer carbonization for high performance supercapacitors: The magic of in situ porogen formation. Energy Environ. Sci. 2014, 7, 728–735. [Google Scholar] [CrossRef]
- Wang, Y.G.; Song, Y.F.; Xia, Y.Y. Electrochemical capacitors: Mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 2016, 45, 5925–5950. [Google Scholar] [CrossRef]
- Periyasamy, T.; Asrafali, S.P.; Kim, S.C. Nitrogen-Rich Porous Carbon/NiMn Hybrids as Electrode Materials for High-Performance Supercapacitors. ACS Appl. Energy Mater. 2022, 5, 15605–15614. [Google Scholar] [CrossRef]
- Aasen, D.A.; Shen, Y.; Ivey, D.G. Zn-Based Oxides Anchored to Nitrogen-Doped Carbon Nanotubes as Efficient Bifunctional Catalysts for Zn-Air Batteries. ChemElectroChem. 2020, 7, 2283. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Zhang, F.; Yang, Z.; Huang, S. One-pot hydrothermal synthesis of reduced graphene oxide/carbon nanotube/α-Ni(OH)2 composites for high performance electrochemical supercapacitor. J. Power Sources 2013, 243, 555–561. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Atchudan, R.; Shakila Parveen, A.; Santhamoorthy, M.; Ramkumar, V.; Kim, S.-C. N-Doped Mesoporous Carbon Prepared from a Polybenzoxazine Precursor for High Performance Supercapacitors. Polymers 2021, 13, 2048. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Wen, H.; Ma, M.; Wang, W.; Yang, L.; Wang, L.; Shi, X.; Cheng, X.; Sun, X.; Yao, Y. A self-supported hierarchical Co-MOF as a supercapacitor electrode with ultrahigh areal capacitance and excellent rate performance. Chem. Commun. 2018, 54, 10499–10502. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhu, T.; Chi, C.; Liu, L.; Zheng, J.; Gong, X. All-Solid-State Asymmetric Supercapacitors with Novel Ionic Liquid Gel Electrolytes. ACS Appl. Electron. Mater. 2020, 2, 3906–3914. [Google Scholar] [CrossRef]
- Ge, J.; Qu, Y.; Cao, L.; Wang, F.; Dou, L.; Yu, J.; Ding, B. Polybenzoxazine-Based Highly Porous Carbon Nanofibrous Membranes Hybridized by Tin Oxide Nanoclusters: Durable Mechanical Elasticity and Capacitive Performance. J. Mater. Chem. A 2016, 4, 7795–7804. [Google Scholar] [CrossRef]
- Ma, C.; Li, Y.J.; Shi, J.L.; Song, Y.; Liu, L. High-performance supercapacitor electrodes based on porous flexible carbon nanofiber paper treated by surface chemical etching. Chem. Eng. J. 2014, 249, 216–225. [Google Scholar] [CrossRef]
- Ike, I.S.; Sigalas, I.; Iyuke, S. Understanding performance limitation and suppression of leakage current or self-discharge in electrochemical capacitors: A review. Phys. Chem. Chem. Phys. 2016, 18, 661–680. [Google Scholar] [CrossRef]
- Wang, G.; Wang, R.; Liu, L.; Zhang, H.; Du, J.; Zhang, Y.; Liu, M.; Liang, K.; Chen, A. Synthesis of hollow mesoporous carbon spheres via Friedel-Crafts reaction strategy for supercapacitor. Mater. Lett. 2017, 197, 71–74. [Google Scholar] [CrossRef]
- Chen, A.; Xia, K.; Zhang, L.; Yu, Y.; Li, Y.; Sun, H.; Wang, Y.; Li, Y.; Li, S. Fabrication of Nitrogen-Doped Hollow Mesoporous Spherical Carbon Capsules for Supercapacitors. Langmuir 2016, 32, 8934–8941. [Google Scholar] [CrossRef]
- Du, J.; Chen, A.; Liu, L.; Li, B.; Zhang, Y. N-Doped Hollow Mesoporous Carbon Spheres Prepared by Polybenzoxazines Precursor for Energy Storage. Carbon 2020, 160, 265–272. [Google Scholar] [CrossRef]
- Ding, B.; Fan, Z.; Lin, Q.; Wang, J.; Chang, Z.; Li, T.; Henzie, J.; Kim, J.; Dou, H.; Zhang, X.; et al. Confined pyrolysis of ZIF-8 polyhedrons wrapped with graphene oxide nanosheets to prepare 3D porous carbon heterostructures. Small Methods 2019, 3, 1900277. [Google Scholar] [CrossRef]
- Sobrinho, R.A.L.; Andrade, G.R.S.; Costa, L.P.; de Souza, M.J.B.; de Souza, A.; Gimenez, I.F. Ordered micro-mesoporous carbon from palm oil cooking waste via nanocasting in HZSM-5/SBA-15 composite: Preparation and adsorption studies. J. Hazard. Mater. 2019, 362, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhou, Y.; Li, L.; Li, Y.; Liu, X.; Zhao, P.; Gao, B. Amino acid protic ionic liquids: Multifunctional carbon precursor for N/S codoped hierarchically porous carbon materials toward supercapacitive energy storage. ACS Sustain. Chem. Eng. 2019, 7, 9281–9290. [Google Scholar] [CrossRef]
- Shen, H.; Xia, X.; Ouyang, Y.; Jiao, X.; Mutahir, S.; Mandler, D.; Hao, Q. Preparation of high specific capacitance biomass based porous carbons for its application in supercapacitors. ChemElectroChem 2019, 15, 3599–3605. [Google Scholar] [CrossRef]
- Liu, F.; Gao, Y.; Zhang, C.; Huang, H.; Yan, C.; Chu, X.; Xu, Z.; Wang, Z.; Zhang, H.; Xiao, X.; et al. Highly microporous carbon with nitrogen-doping derived from natural biowaste for highperformance flexible solid-state supercapacitor. J. Colloid Interface Sci. 2019, 548, 322–332. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, Z.; Yu, C.; Zhong, W. Heteroatom-doped sheet-like and hierarchical porous carbon based on natural biomass small molecule peach gum for high performance supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 3389–3403. [Google Scholar] [CrossRef]
- Wan, L.; Du, C.; Yang, S. Synthesis of Graphene Oxide/Polybenzoxazine-Based Nitrogen-Containing Porous Carbon Nanocomposite for Enhanced Supercapacitor Properties. Electrochim. Acta 2017, 251, 12–24. [Google Scholar] [CrossRef]
- Zhang, H.; Bhat, V.V.; Gallego, N.C.; Contescu, C.I. Thermal Treatment Effects on Charge Storage Performance of Graphene-Based Materials for Supercapacitors. ACS Appl. Mater. Interfaces 2012, 4, 3239–3246. [Google Scholar] [CrossRef]
- Xu, J.; Gai, S.; He, F.; Niu, N.; Gao, P.; Chen, Y.; Yang, P. A Sandwich-Type Three-Dimensional Layered Double Hydroxide Nanosheet Array/Graphene Composite: Fabrication and High Supercapacitor Performance. J. Mater. Chem. A 2014, 2, 1022–1031. [Google Scholar] [CrossRef]
- Shi, K.; Zhitomirsky, I. Polypyrrole Nanofiber-Carbon Nanotube Electrodes for Supercapacitors with High Mass Loading Obtained Using an Organic Dye as a Co-Dispersant. J. Mater. Chem. A 2013, 1, 11614–11622. [Google Scholar] [CrossRef]
- Kotz, R.; Carlen, M. Principles and Applications of the Electrochemical Capacitors. Electrochim. Acta 2000, 45, 2483–2498. [Google Scholar] [CrossRef]
- Mohamed, M.; Gamal, M.; Kuo, S. Pyrene-functionalized tetraphenylethylene polybenzoxazine for dispersing single-walled carbon nanotubes and energy storage. Compos. Sci. Technol. 2020, 199, 108360. [Google Scholar] [CrossRef]
- Wen, Y.; Liu, X.; Wen, X.; Chen, X.; Szymanska, K.; Dobrzynska, R.; Mijowska, E. Na3PO4 assistant dispersion of nano-CaCO3 template to enhance electrochemical interface: N/O/P co-doped porous carbon hybrids towards high-performance flexible supercapacitors. Compos. Part B 2020, 199, 108256. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, K.; Guo, J.; Li, J.; Peng, X.; Hong, B.; Wang, X.; Ge, H. Facile fabrication of Au/Fe3O4 nanocomposites as excellent nanocatalyst for ultrafast recyclable reduction of 4-nitropheol. Chem. Eng. J. 2020, 381, 122596. [Google Scholar] [CrossRef]
- Li, H.; Lun, N.; Bai, Y.J. N-doped carbon-coated TiN exhibiting excellent electrochemical performance for supercapacitors. Electrochim. Acta 2017, 257, 56–63. [Google Scholar]
- Wang, S.; Wang, B.; He, S.; Wang, Y.; Cheng, J.; Li, Y. Enhancing the photovoltaic performance of planar heterojunction perovskite solar cells via introducing binary-mixed organic electron transport layers. New J. Chem. 2023, 47, 5048–5055. [Google Scholar] [CrossRef]
- Sharma, P.; Tanwar, V.; Tiwari, I.; Ingole, P.P.; Nebhani, L. Sustainable Upcycling of Nitrogen-Enriched Polybenzoxazine Thermosets into Nitrogen-Doped Carbon Materials for Contriving High-Performance Supercapacitors. Energy Fuels 2023, 37, 7445–7467. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, D.; Wang, Y.; Hou, B. Electrocatalytic Activity of Nitrogen-Doped Graphene Synthesized via a One-Pot Hydrothermal Process towards Oxygen Reduction Reaction. J. Power Sources 2013, 227, 185–190. [Google Scholar] [CrossRef]
- Bhattacharjya, D.; Kim, M.-S.; Bae, T.-S.; Yu, J.-S. High Performance Supercapacitor Prepared from Hollow Mesoporous Carbon Capsules with Hierarchical Nanoarchitecture. J. Power Sources 2013, 244, 799–805. [Google Scholar] [CrossRef]
- Lau, S.C.; Lim, H.N.; Ravoof, T.B.S.A.; Yaacob, M.H.; Grant, D.M.; MacKenzie, R.C.I.; Harrison, I.; Huang, N.M. A Three-Electrode Integrated Photo-Supercapacitor Utilizing Graphene-Based Intermediate Bifunctional Electrode. Electrochim. Acta 2017, 238, 178–184. [Google Scholar] [CrossRef]
- Zhao, J.; Niu, W.; Zhang, L.; Cai, H.; Han, M.; Yuan, Y.; Majeed, S.; Anjum, S.; Xu, G. A Template-Free and Surfactant-Free Method for High-Yield Synthesis of Highly Monodisperse 3-Aminophenol-Formaldehyde Resin and Carbon Nano/Microspheres. Macromolecules 2013, 46, 140–145. [Google Scholar] [CrossRef]
- Silvestre-Albero, A.M.; Juárez-Galán, J.M.; Silvestre-Albero, J.; Rodríguez-Reinoso, F. Low-Pressure Hysteresis in Adsorption: An Artifact? J. Phys. Chem. C 2012, 116, 16652–16655. [Google Scholar] [CrossRef]
- Wen, Y.; Chi, L.; Wenelska, K.; Wen, X.; Chen, X.; Mijowska, E. Eucalyptus Derived Heteroatom-Doped Hierarchical Porous Carbons as Electrode Materials in Supercapacitors. Sci. Rep. 2020, 10, 14631. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, G.Y.; Lv, Y.; Wang, L.Z.; Zhang, A.Q.; Song, Y.H.; Huang, B.L. Electrochemical Investigation of MnO2 Electrode Material for Supercapacitors. Int. J. Hydrogen Energy 2011, 36, 11760–11766. [Google Scholar] [CrossRef]
- Khan, M.S.; Shakya, P.; Bhardwaj, N.; Jhankal, D.; Sharma, A.K.; Banerjee, M.K.; Sachdev, K. Chemical Vapor Deposited Graphene-Based Quasi-Solid-State Ultrathin and Flexible Sodium-Ion Supercapacitor. J. Electrochem. Sci. Eng. 2022, 12, 799–813. [Google Scholar] [CrossRef]
- Li,, D.-J.; Lei, S.; Wang, Y.-Y.; Chen, S.; Kang, Y.; Gu, Z.-G; Zhang, J. Helical carbon tubes derived from epitaxial Cu-MOF coating on textile for enhanced supercapacitor performance. Dalton Trans. 2018, 47, 5558–5563. [Google Scholar] [CrossRef] [PubMed]
- Chameh, B.; Pooriraj, M.; Keyhan, M.; Moradi, M. Cu-MOF-derived CuO/NiO/Ni3(VO4)2 composite materials with improved electrochemical performance for supercapacitor. J. Mater. Sci Mater. Electron. 2023, 34, 525. [Google Scholar] [CrossRef]
- Deng, Y.; Xie, Y.; Zou, K.; Ji, X. Review on recent advances in nitrogen-doped carbons: Preparations and applications in supercapacitors. J. Mater. Chem. A 2016, 4, 1144–1173. [Google Scholar] [CrossRef]
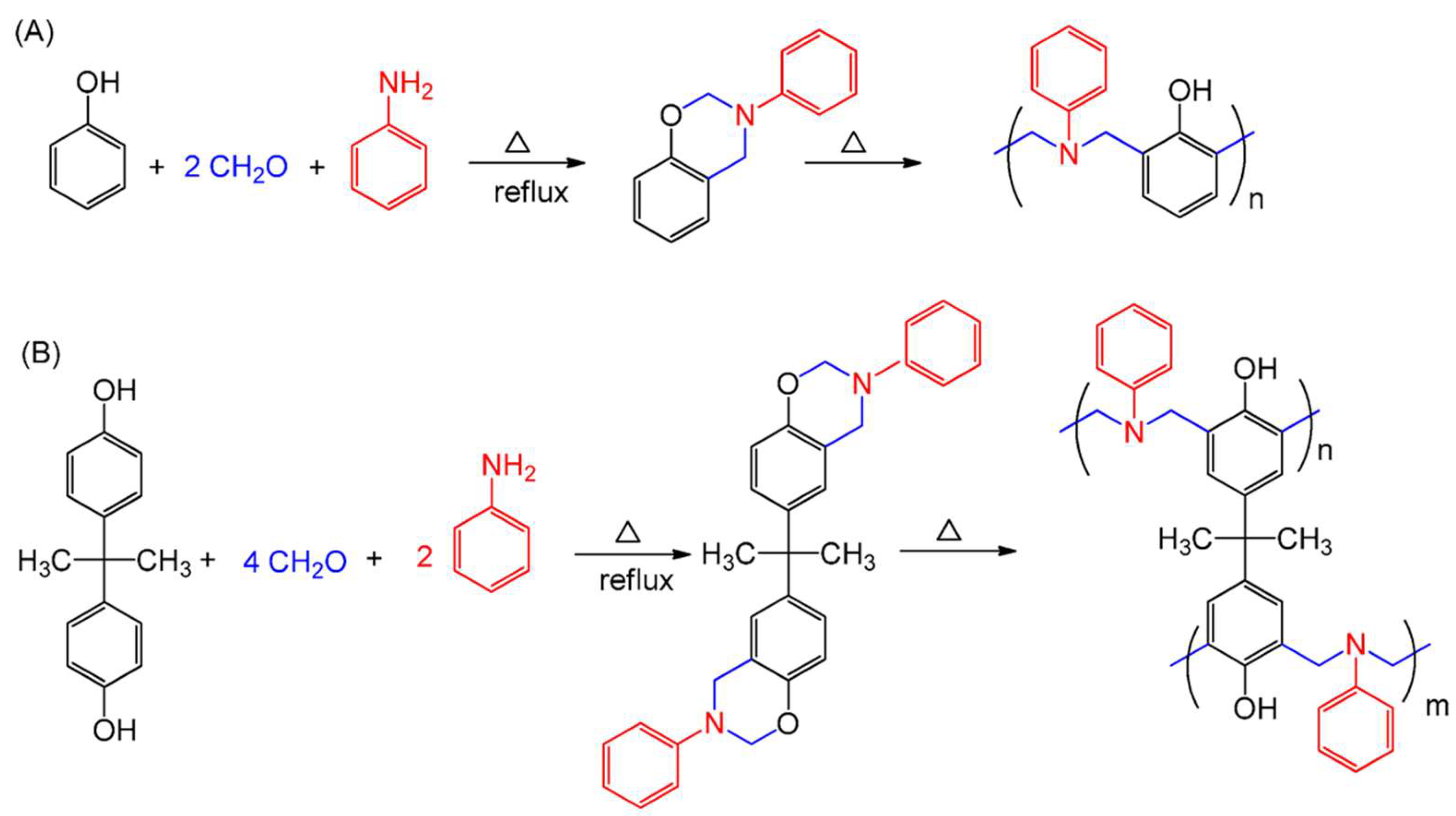

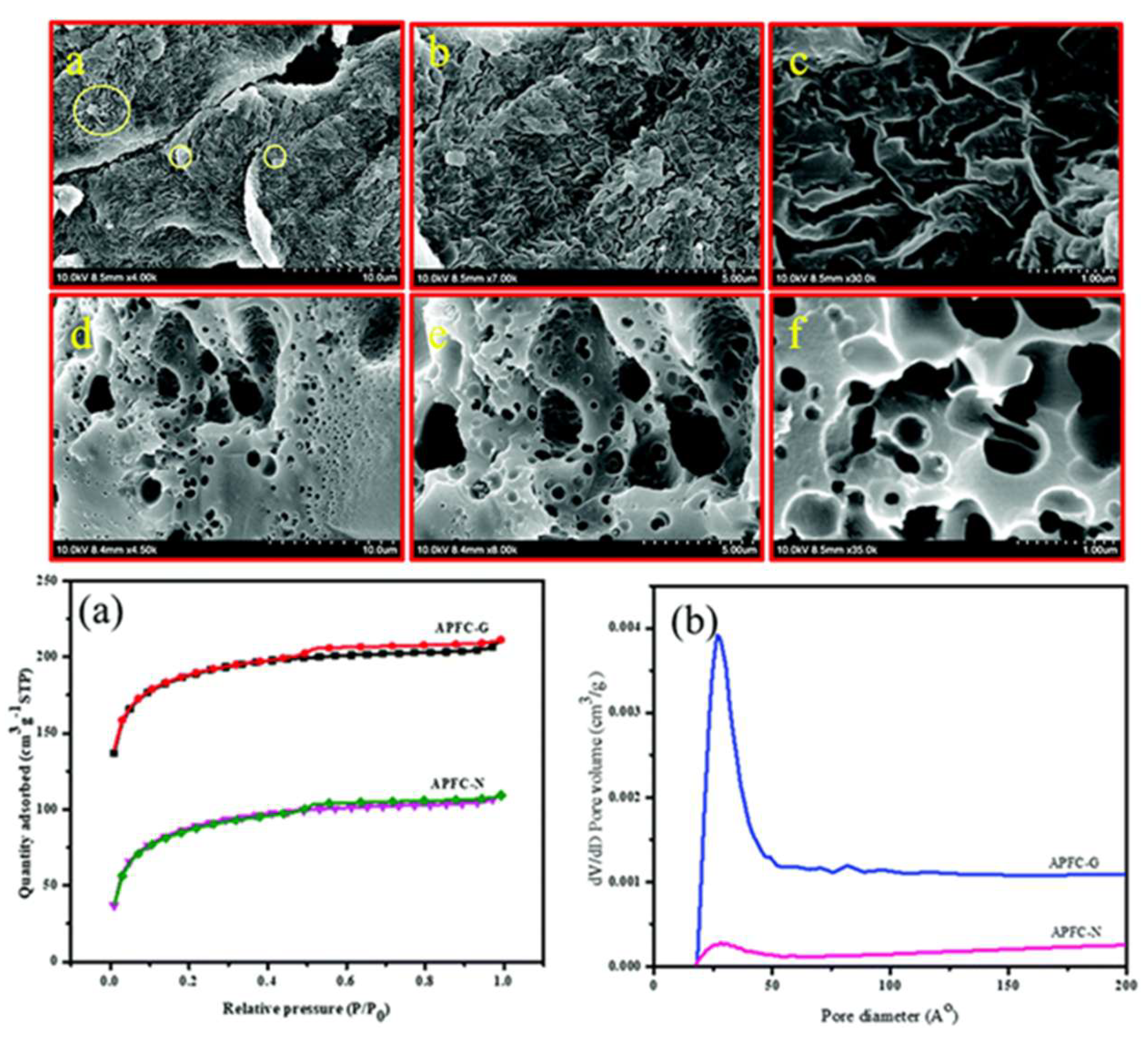
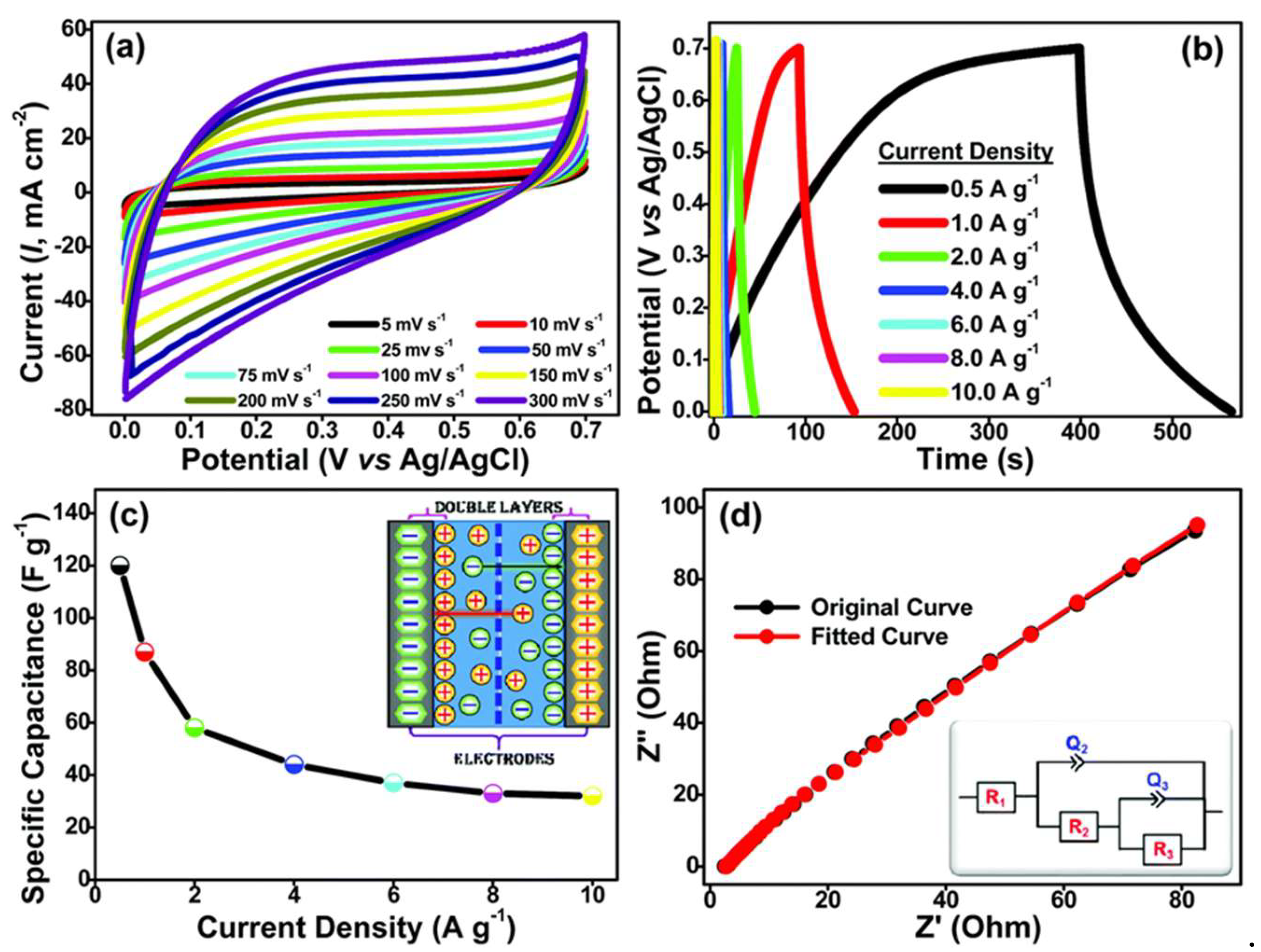
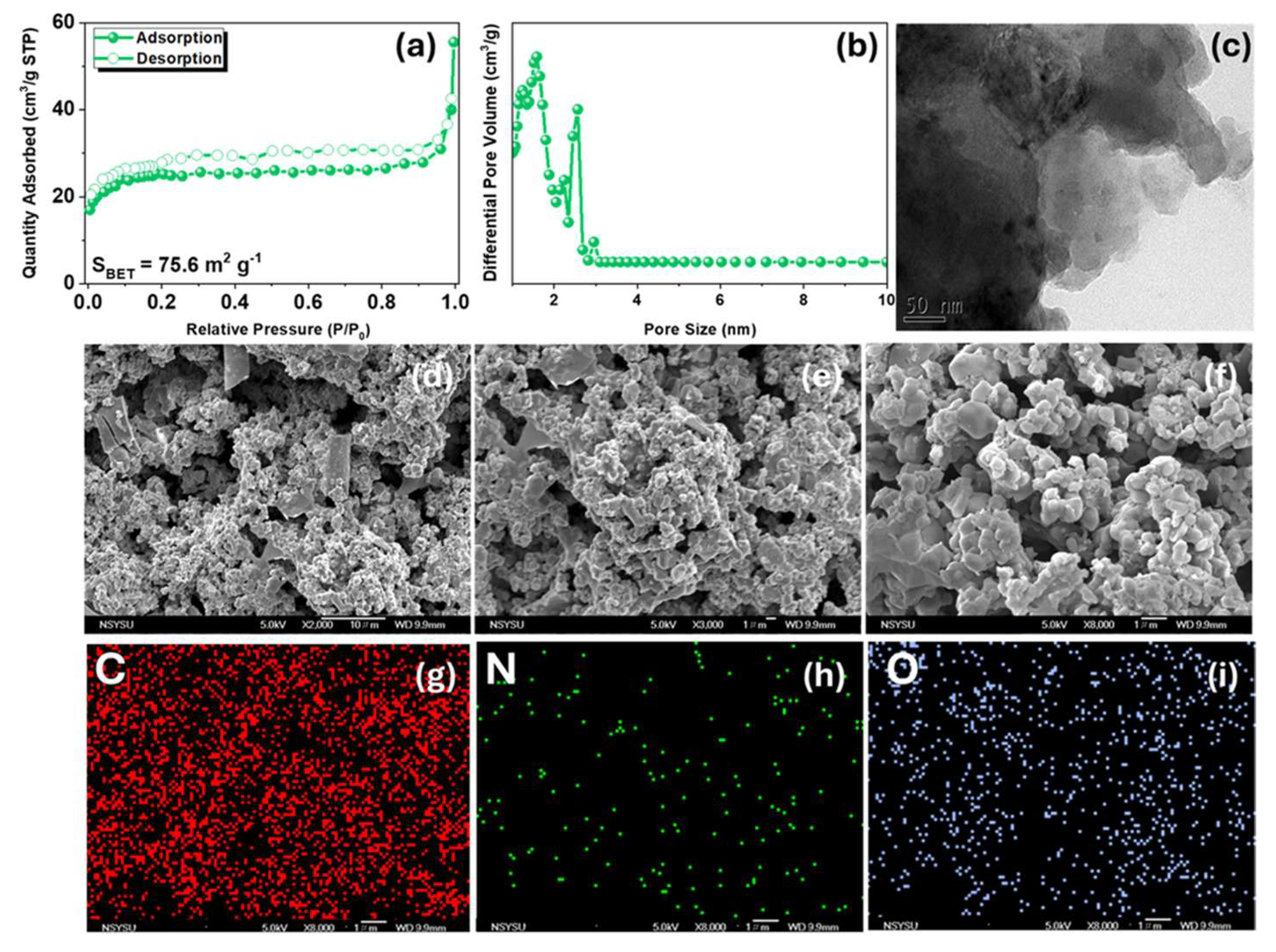

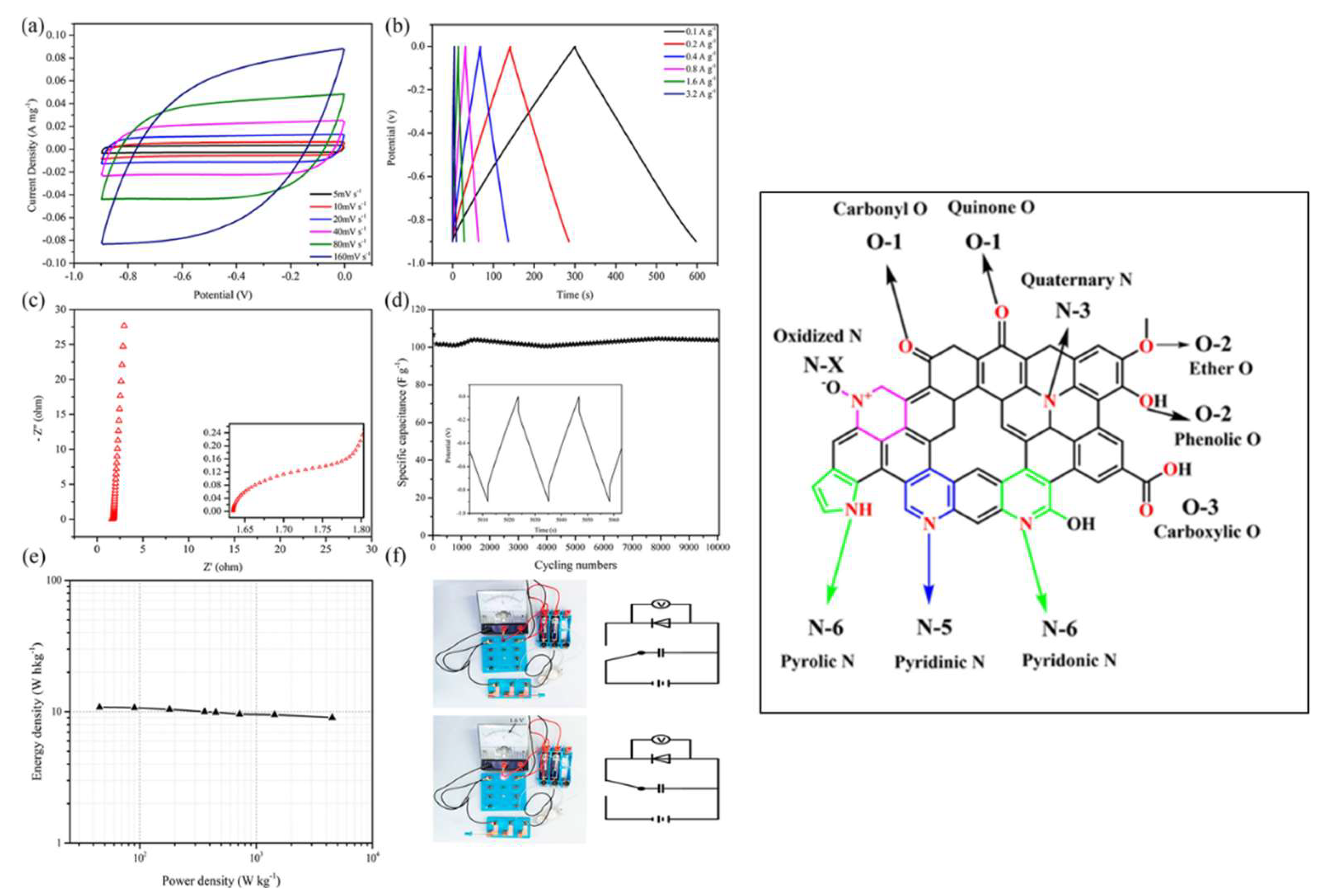
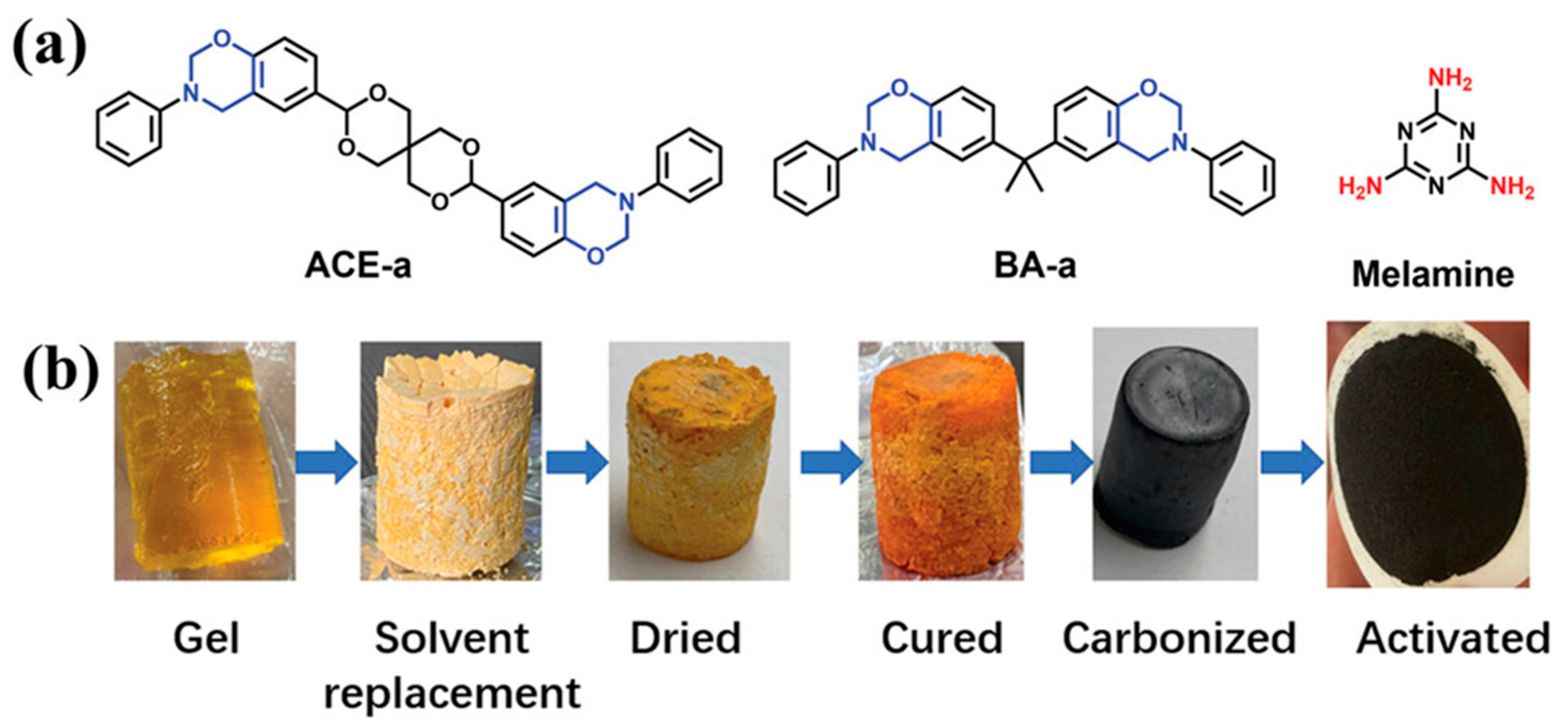
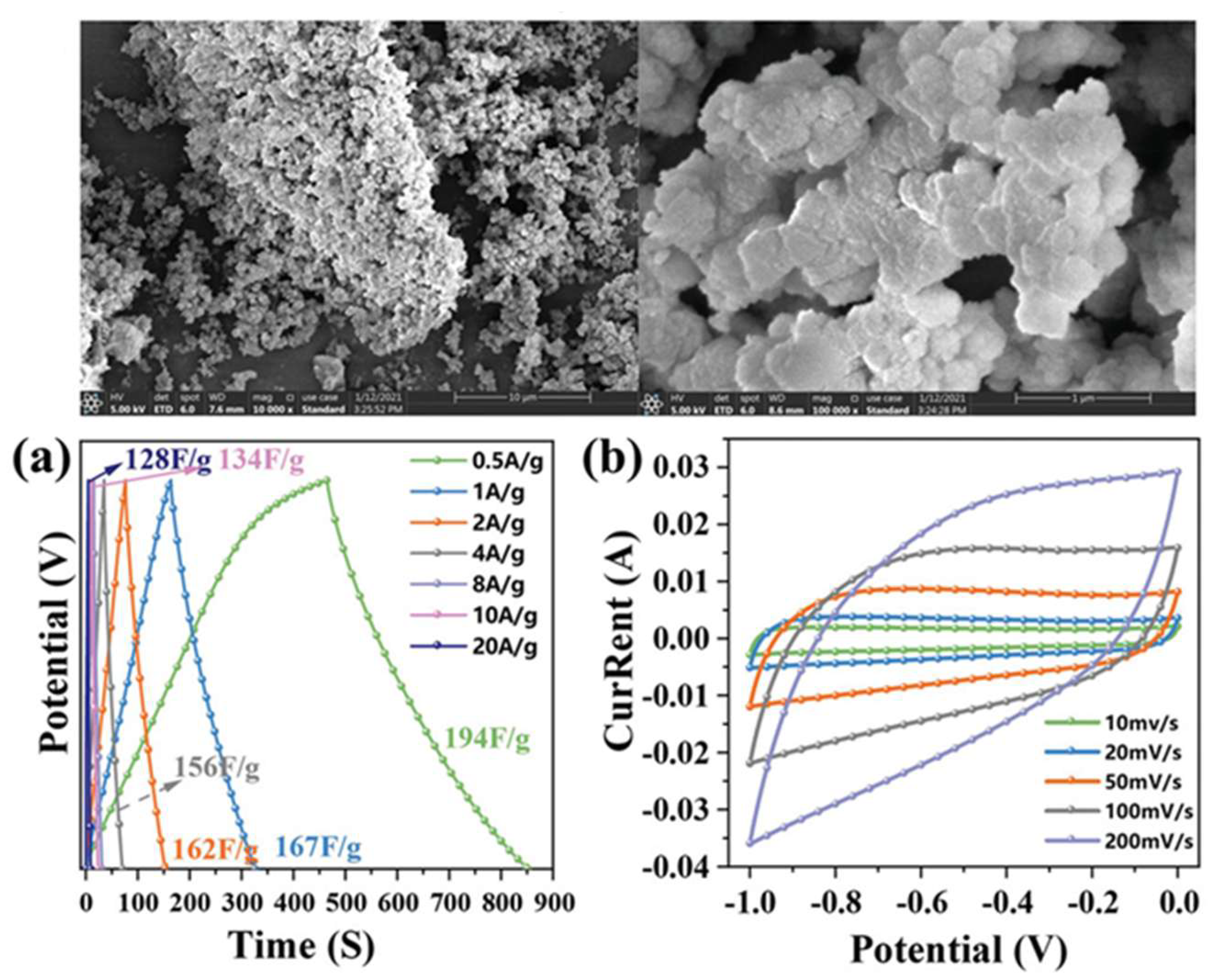

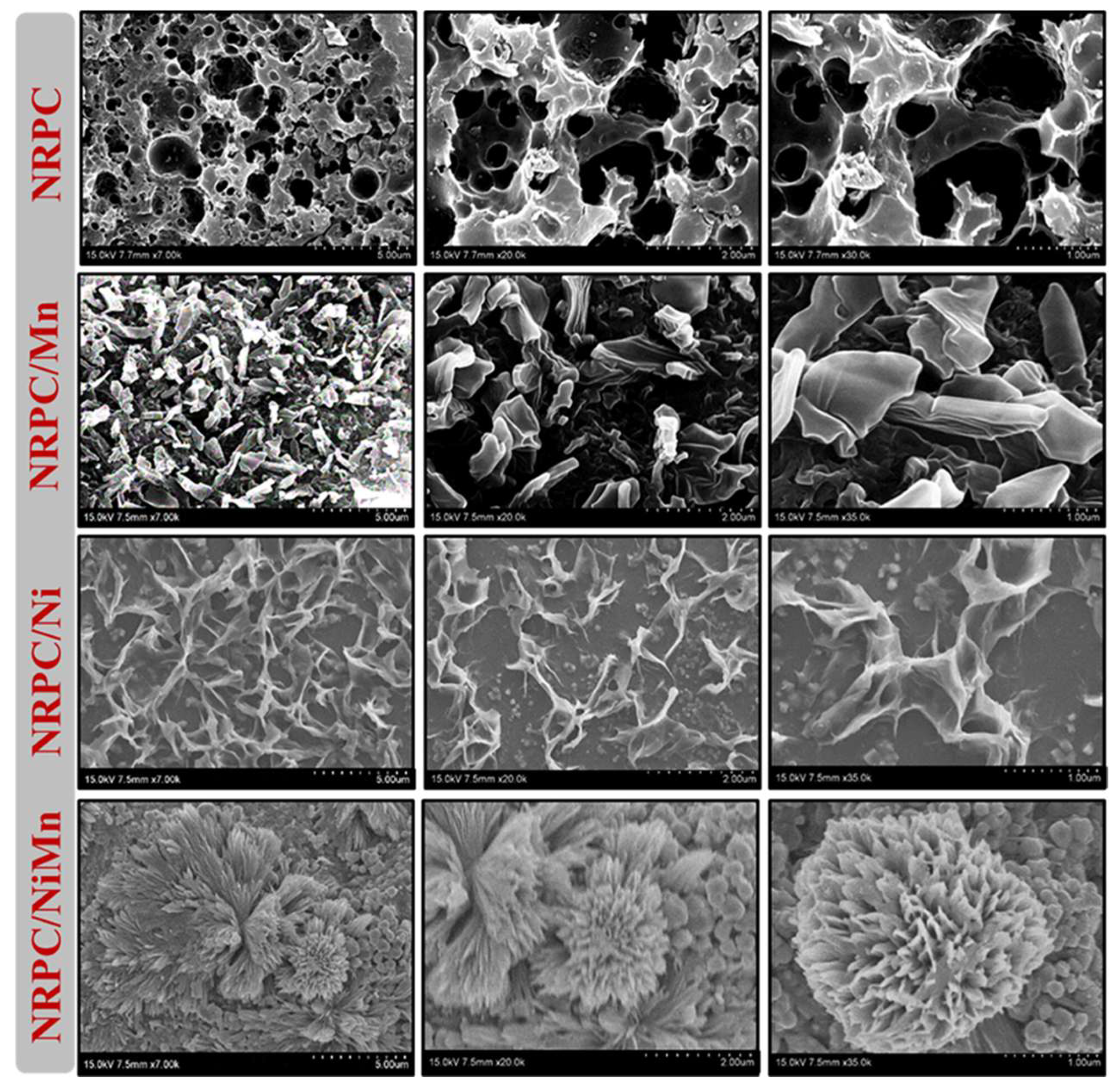
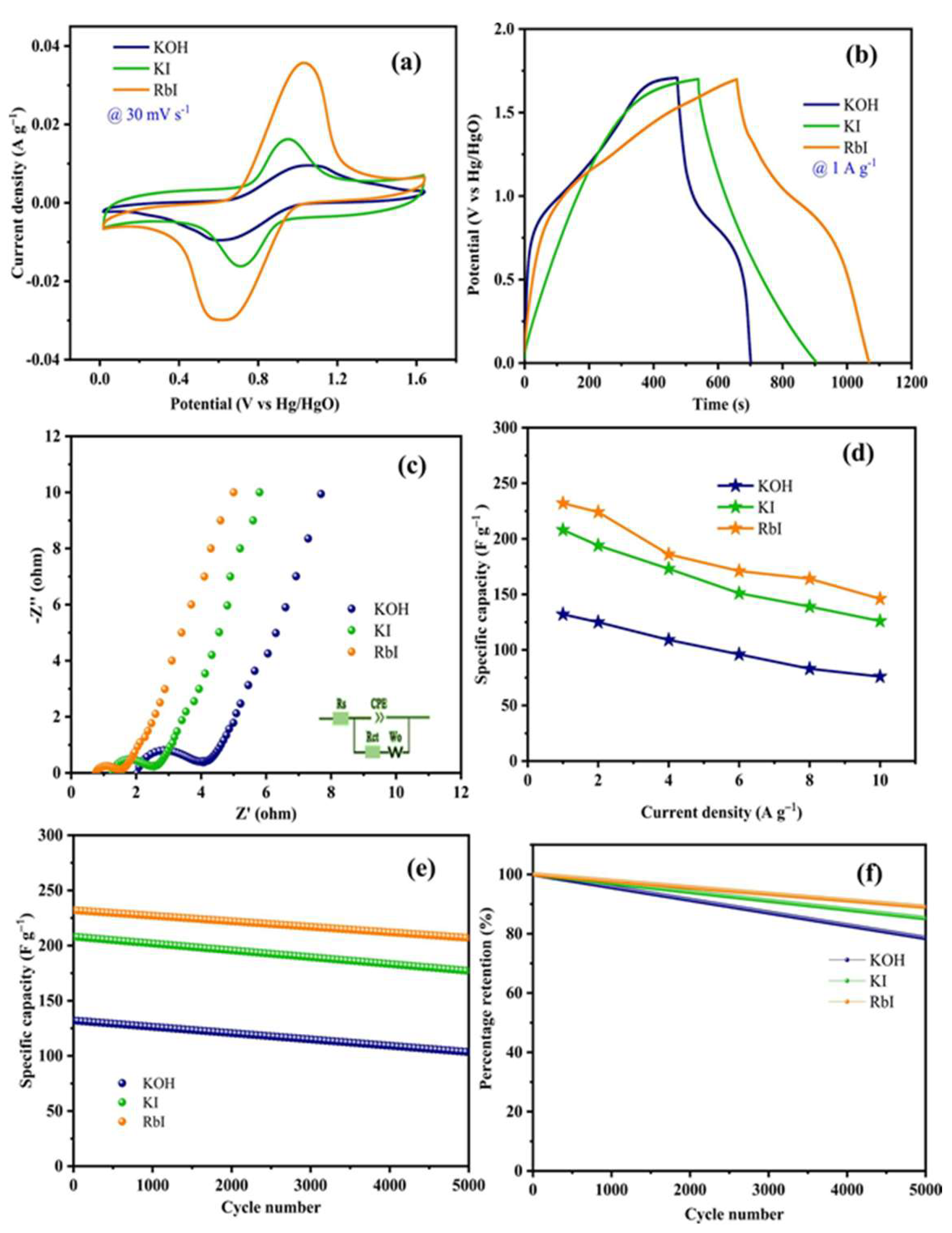
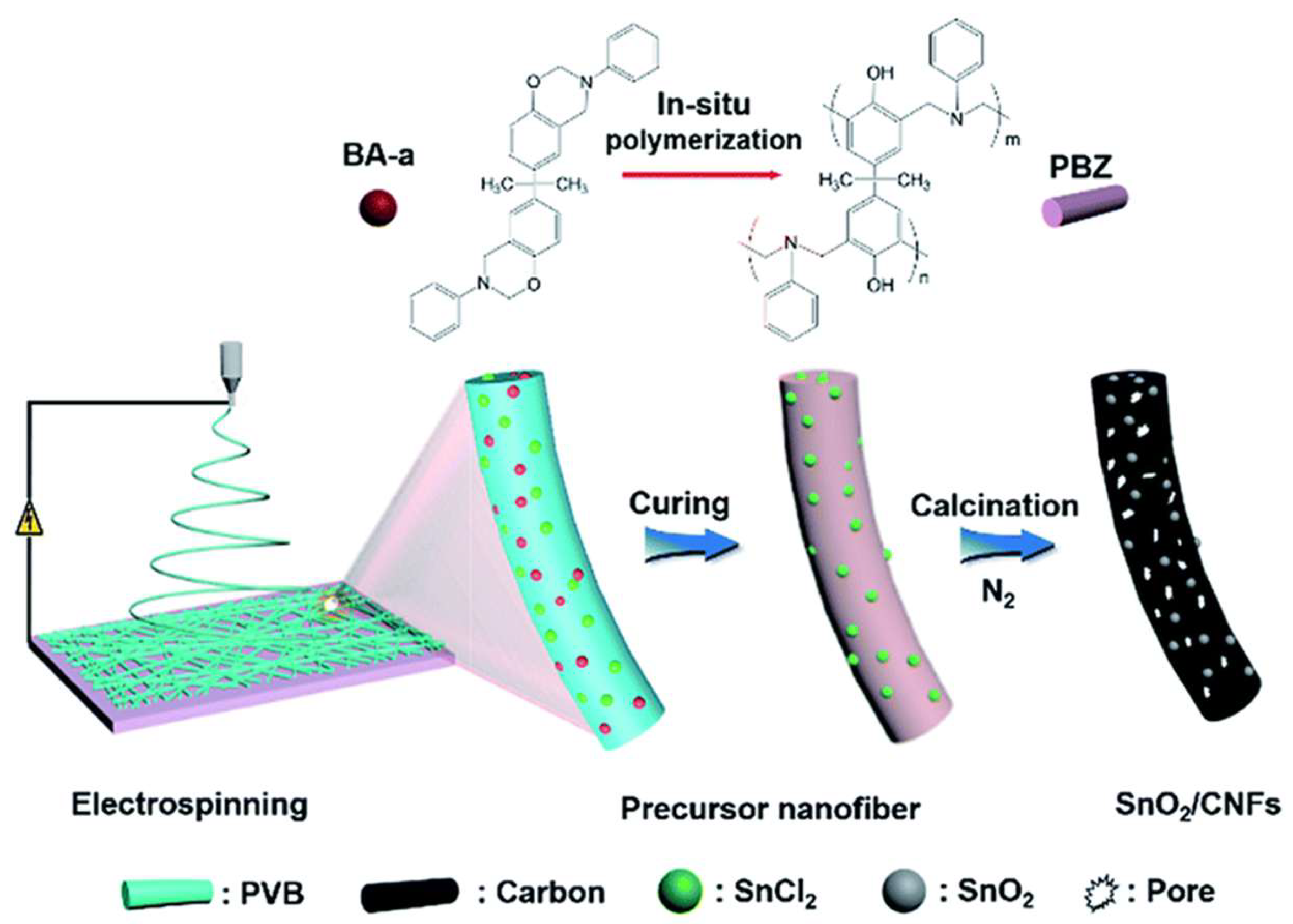

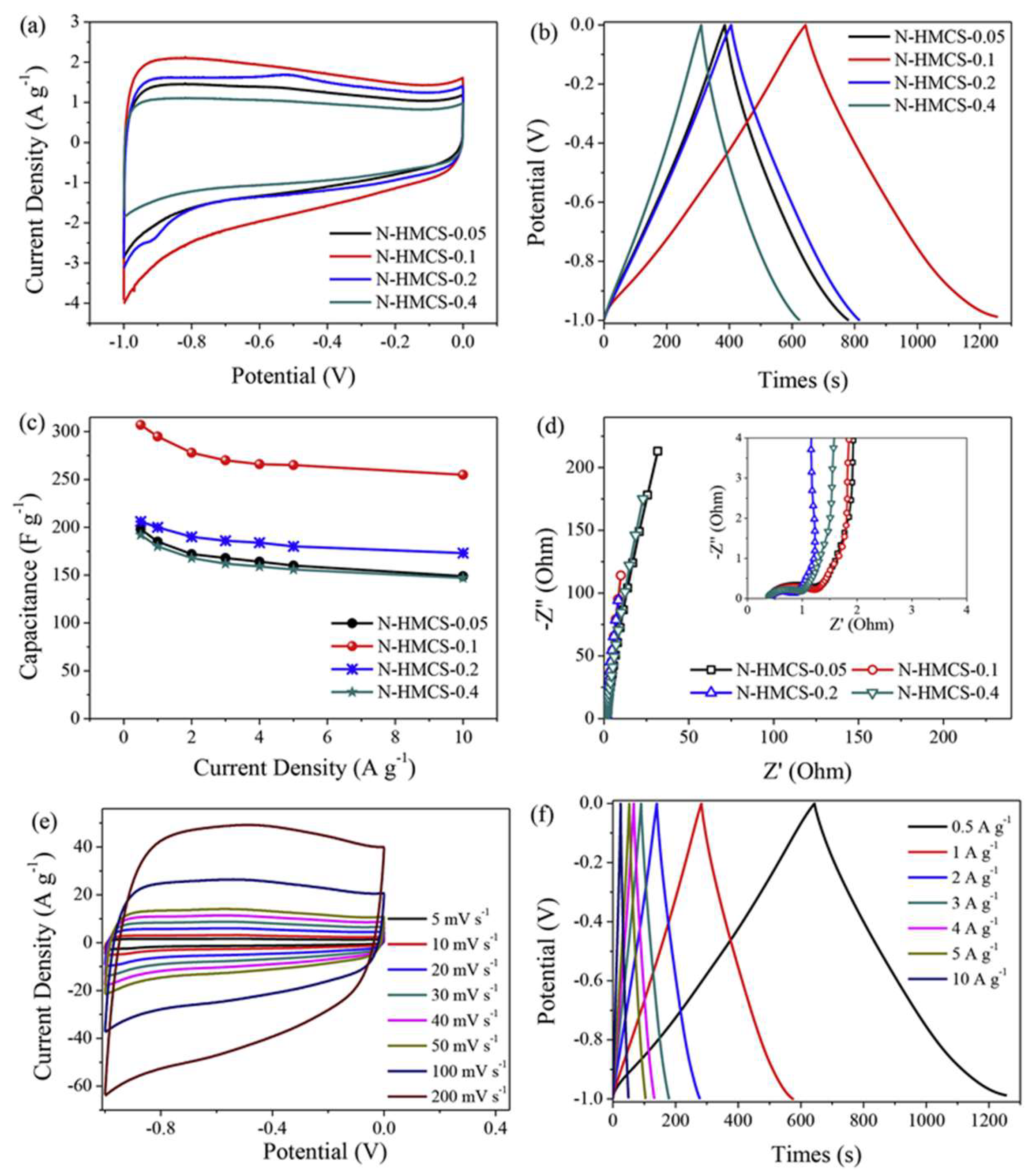
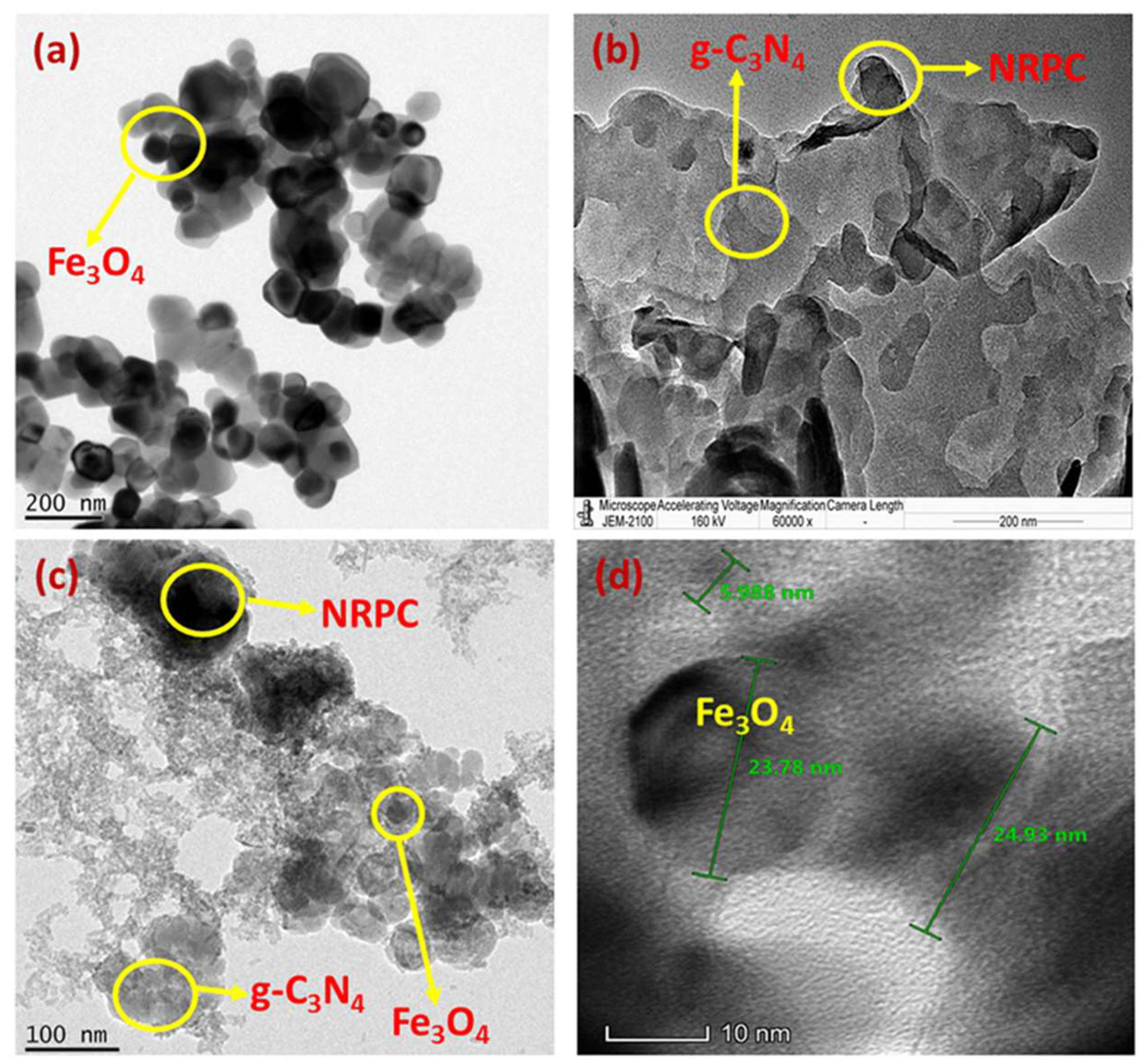
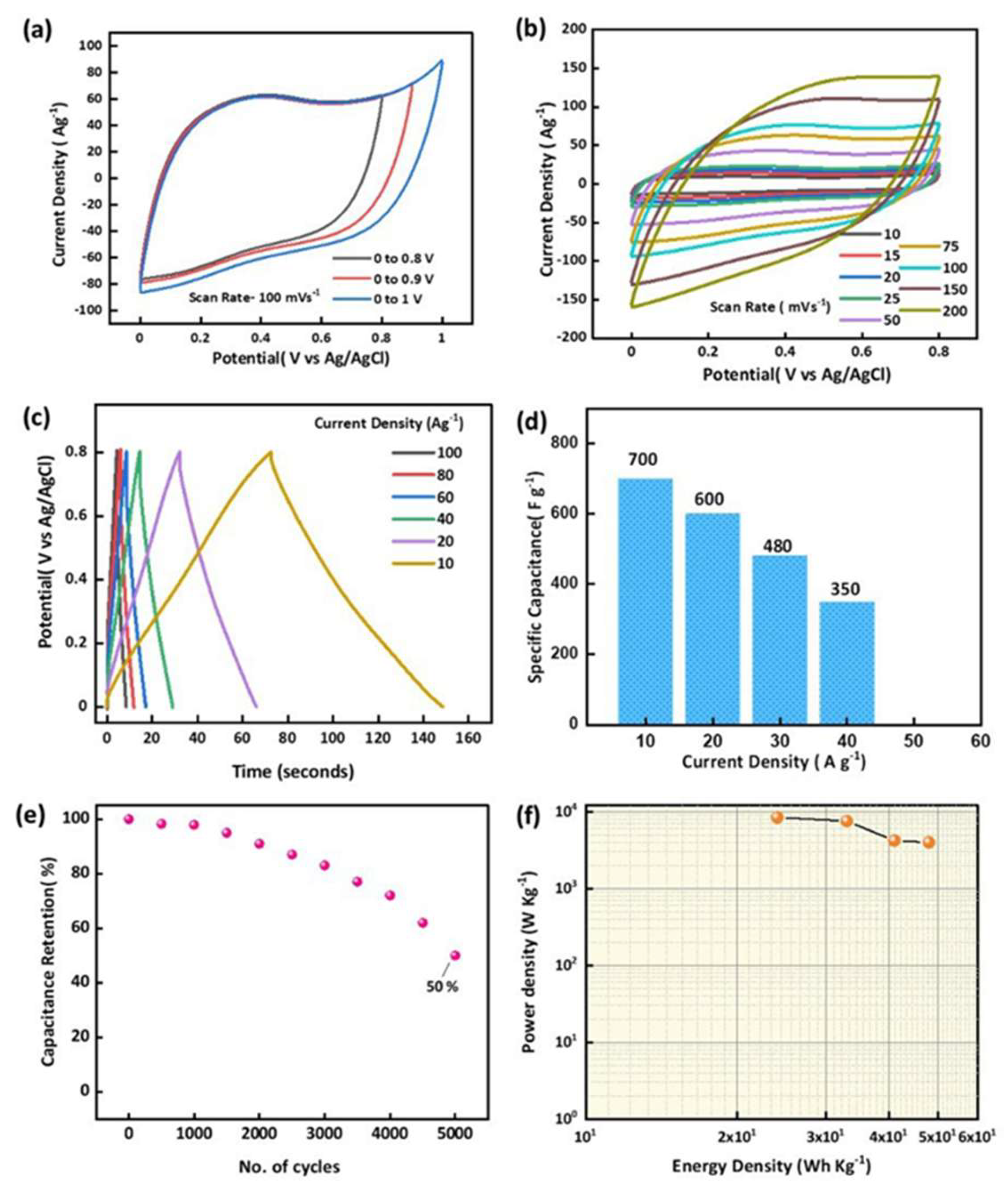
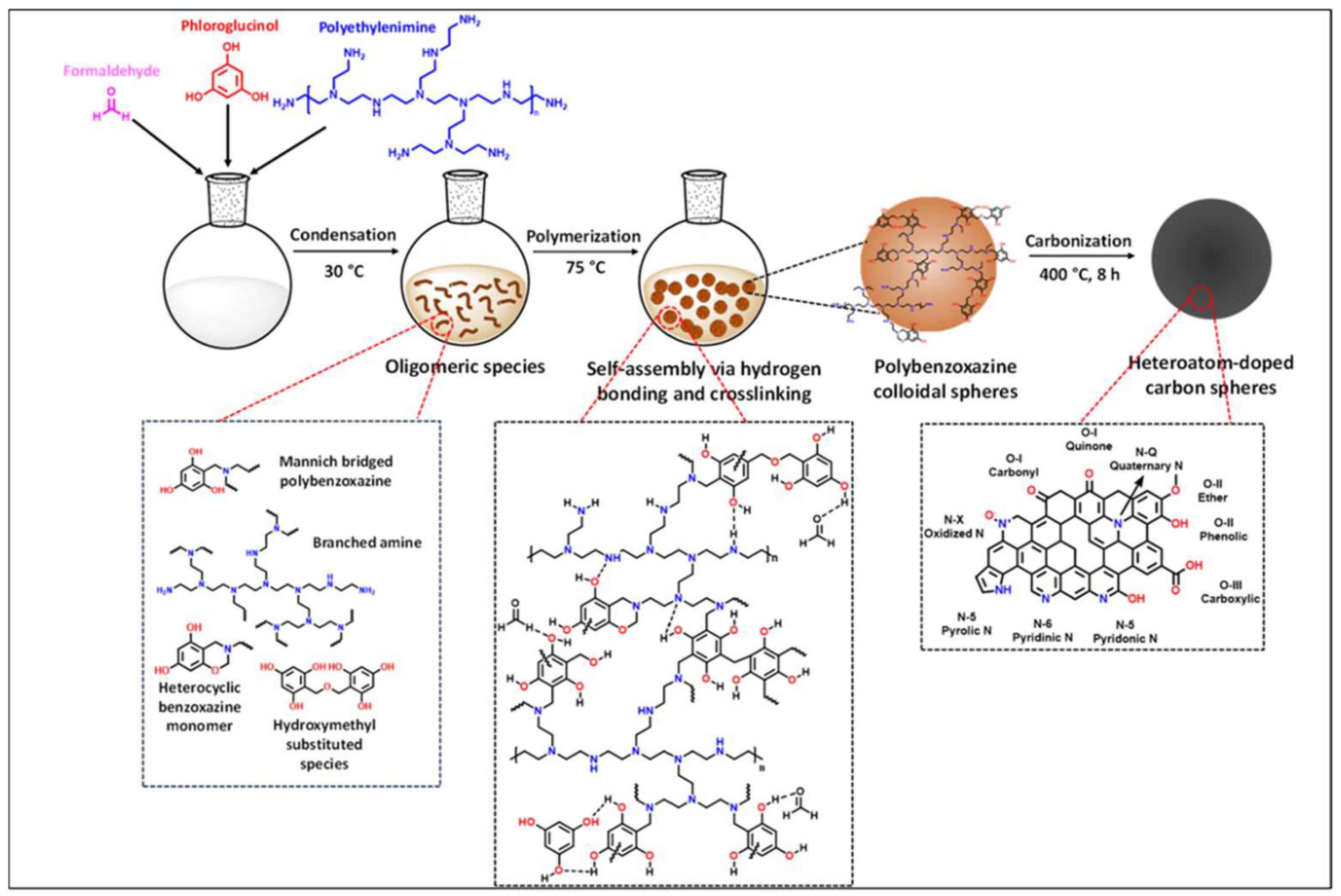
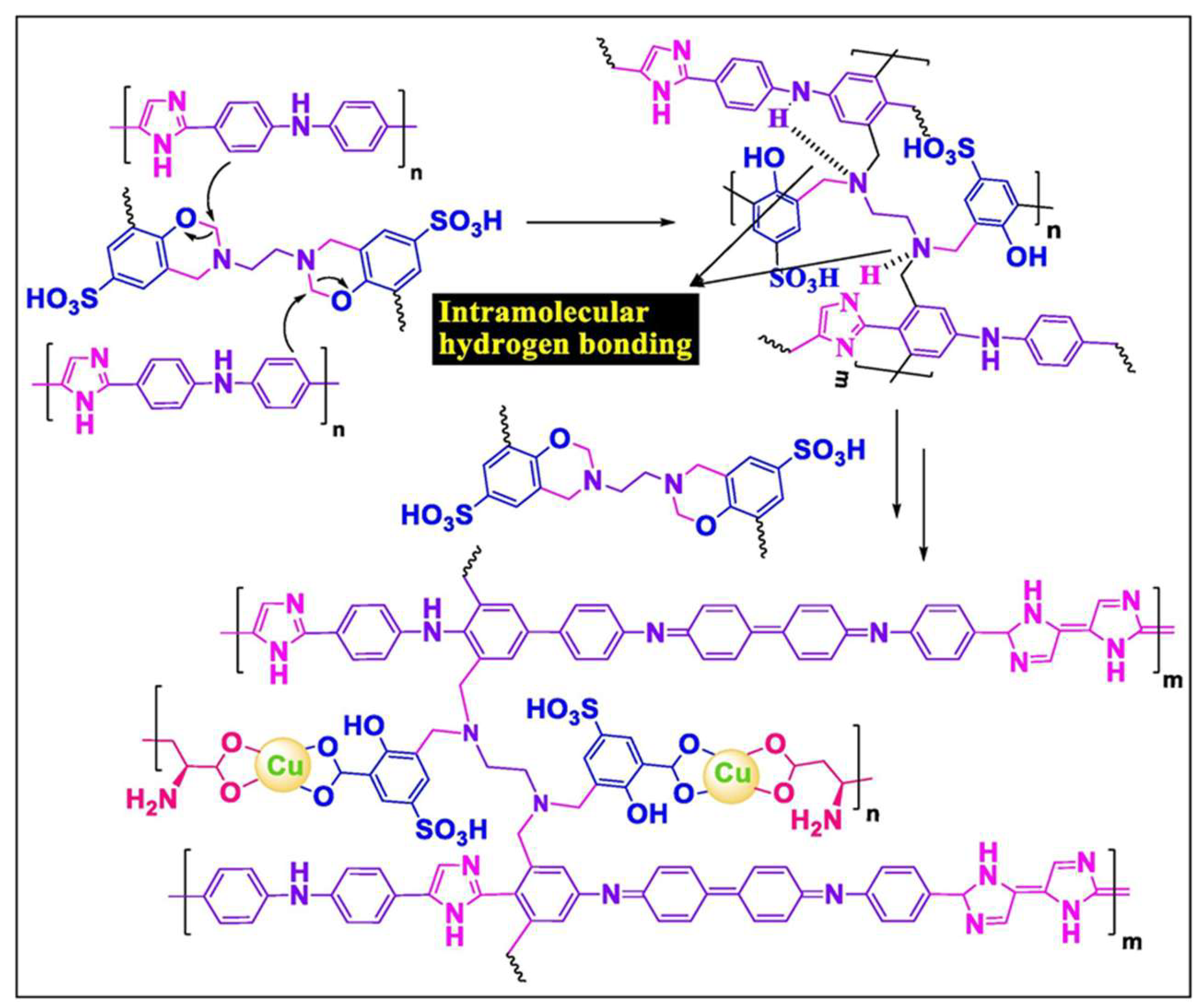
| Carbon Materials | Synthesis Method | Properties |
|---|---|---|
| Nitrogen-enriched mesoporous carbon ropes, NCMR [38] | Carbonization and KOH activation. | SA = 300 m2 g−1; pore size = 3 nm; pore volume = 0.003 cm3 g−1; Cs = 60 F g−1 @ 1 A g−1 (2 M KOH) |
| Apigenin and furfurylamine-based Bzo, APFC-N and APFC-G [60] | Gelation, calcination and KOH activation. | SA = 248 m2 g−1 (APFC-N) and 635 m2 g−1 (APFC-G); pore size = 2–5 nm; Cs (APFC-G) = 120 F g−1 @ 0.5 A g−1 (1 M H2SO4) |
| Carbon microspheres [64] | Gelation, carbonization and CO2 activation. | SA = 859 m2 g−1; pore size = 1 nm; Cs = 424.7 F g−1 @ 0.5 A g−1 (1 M H2SO4) |
| Porous organic polymer, Cr-TPA-4Bz-PY-POP [44] | Sonogashira–Hagihara cross-coupling and carbonization. | Pore size = 4.17 nm; Cs = 397.2 F g−1 @ 0.5 A g−1 (1 M KOH) |
| Nitrogen-doped porous carbon, NPC-2 [71] | Soft templating method and KOH activation. | SA = 2036 m2 g−1; Cs = 362.4 F g−1 @ 1 A g−1 (1 M KOH) |
| Nitrogen and oxygen-doped porous carbon, NOPC-bis-CN-3 [77] | Soft templating method and KOH activation. | SA = 2347 m2 g−1; pore size = 20–40 nm; Cs = 167.3 F g−1 @ 1 A g−1 (6 M KOH) |
| Vanillin-malonitrile-based PBz, poly(VFBZ-CN) 800 [46] | Carbonization and KOH activation. | SA = 560 m2 g−1; Cs = 506 F g−1 @ 0.5 A g−1 (1 M KOH) |
| Nitrogen and phosphorous co-doped carbon, C/P-20-1 [82] | Carbonization. | SA = 29 m2 g−1; Cs = 203 F g−1 @ 0.5 A g−1 (1 M H2SO4) |
| Diacetyl-type Bzo carbon, CA3MK [40] | Gelation and curing. | SA = 1383.9 m2 g−1; pore volume = 0.748 cm3 g−1; Cs = 430 F g−1 (0.5 M H2SO4) and 194 F g−1 (6 M KOH) @ 0.5 A g−1 |
| Boron and nitrogen co-doped porous carbon, BNPC-0.15 [88] | Carbonization and KOH activation. | Cs = 286 F g−1 @ 0.5 A g−1 (6 M KOH) |
| Nitrogen rich porous carbon, NRPC/NiMn [91] | Carbonization, KOH activation and hydrothermal reaction. | SA = 365 m2 g−1; pore volume = 0.42 cm3 g−1; Cs = 1825 F g−1 @ 1 A g−1 (1 M KOH) |
| Hetero atom-doped carbon, HC/NiCo@800C [15] | Carbonization, KOH activation and hydrothermal reaction. | Cs = 2334 F g−1 @ 1 A g−1 (1 M RbI) and 2076 F g−1 @ 1 A g−1 (1 M KI) |
| Carbon nano fibers, SnO2/CNF [97] | Template polymerization using PVB, electrospinning and carbonization. | SA = 1415 m2 g−1; pore volume = 0.82 cm3 g−1; Cs = 118 F g−1 @ 0.5 A g−1 (2 M HCl) |
| Porous yolk shell nanospheres, CPYCNs [34] | Layer-by-layer coating, ultra-sonication and carbonization. | SA = 486 m2 g−1; pore volume = 0.15 cm3 g−1; Cs = 236 F g−1 @ 0.5 A g−1 (6 M KOH) |
| Nitrogen-doped hollow mesoporous carbon spheres, N-HMCS (0.1) [102] | Stobber synthesis and polymerization. | SA = 636 m2 g−1; pore volume = 1.60 cm3 g−1; Cs = 307 F g−1 @ 0.5 A g−1 (6 M KOH) |
| Nitrogen-doped porous carbon/graphene oxide composites, GO/NC-2 [109] | Ring opening polymerization and KOH activation. | SA = 1345.8 m2 g−1; pore volume = 0.53 cm3 g−1; Cs = 405.6 F g−1 @ 1 A g−1 (6 M KOH) |
| Quinoline-based PBz/graphitic carbon nitride, poly(Q-xda) + 15 wt.% GCN [10] | Pyrolysis and ring-opening polymerization. | Cs = 370 F g−1 @ 6 A g−1 (1 M KOH) |
| Nitrogen rich porous carbon/graphitic carbon nitride/magnetite, NRPC/g-C3N4/Fe3O4 [9] | Carbonization, KOH activation, sonication and ageing. | SA = 497.6 m2 g−1; Cs = 385 F g−1 @ 1 A g−1 (1 M KOH) |
| Guaiacol-based PBz carbon, C-GP81 [119] | Carbonization. | Pore size = 200–300 µm; Cs = 700 F g−1 @ 10 A g−1 (0.1 M H2SO4) |
| Hetero-doped carbon spheres, C-P-PEI [25] | Sol-gel method and carbonization. | SA = 221 m2 g−1; pore size = 5.1 nm; pore volume = 0.28 cm3 g−1; Cs = 728 F g−1 @ 10 A g−1 (0.1 M H2SO4) |
| PBz and poly(imidazole diphosphoric acid)-based carbon, PABz-co-Cu MOFs-graft-PIPDA (50/50) [1] | Thermal curing. | Cs = 387 F g−1 @ 1 A g−1 (3 M KOH) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asrafali, S.P.; Periyasamy, T.; Lee, J. From Thermosetting Resins to Energy Devices: A Review on Polybenzoxazine-Derived Materials for Supercapacitors. Batteries 2025, 11, 345. https://doi.org/10.3390/batteries11090345
Asrafali SP, Periyasamy T, Lee J. From Thermosetting Resins to Energy Devices: A Review on Polybenzoxazine-Derived Materials for Supercapacitors. Batteries. 2025; 11(9):345. https://doi.org/10.3390/batteries11090345
Chicago/Turabian StyleAsrafali, Shakila Parveen, Thirukumaran Periyasamy, and Jaewoong Lee. 2025. "From Thermosetting Resins to Energy Devices: A Review on Polybenzoxazine-Derived Materials for Supercapacitors" Batteries 11, no. 9: 345. https://doi.org/10.3390/batteries11090345
APA StyleAsrafali, S. P., Periyasamy, T., & Lee, J. (2025). From Thermosetting Resins to Energy Devices: A Review on Polybenzoxazine-Derived Materials for Supercapacitors. Batteries, 11(9), 345. https://doi.org/10.3390/batteries11090345










