Abstract
We report on the effect state of charge (SoC), cell format, and chemistry have on the volume and composition (H2, CO2, CO, CH4, C2H4, C2H6, C3H6, and C3H8) of cell failure gas from Li-ion cells. Nickel manganese cobalt oxide (NMC) 21700 cells with a 5 Ah capacity were externally heated to failure at a 5–100% SoC under an inert atmosphere. This showed that the volume of gas increased with cell SoC (1.8 L at 5% SoC vs. 8.3 L at 100% SoC). The effect of the cell chemistry format and abuse method was also investigated using 18650, pouch, and prismatic cells (2.3–50 Ah) with Ni-based or lithium cobalt oxide (LCO) cathodes or lithium titanium oxide (LTO) anodes. The results showed that at higher SoCs, larger quantities of gas were generated; however, there was no correlation between the cell SoC and the composition of gases produced. Tests on the other cells found that the Ni-based cell generated 1.29–1.89 L/Ah of gas. The main constituents of this were H2, CO, and CO2; however, all other hydrocarbons were identified in varying quantities. The LTO cells generated lower volumes of gas, 0.8 L/Ah compared to Ni-based cells, and the gas was found to contain lower H2 concentrations but higher concentrations of CO2. The LCO cell was found to generate a gas volume of 1.2 L/Ah. This forms the final of four papers which cover a total of 213 tests on 29 cell types with six different chemistries, all tested using a single robust testing method.
1. Introduction
The increasing popularity of Li-ion batteries for use in electric vehicles and battery energy storage, according to Chen et al., has the potential to lead to an accelerated number of battery failure events—a result of unwanted cell chemical reactions within the cell or exposure to conditions outside of the cell’s normal operating range (thermal, electrical, or mechanical) [1]. Exposure to a range of conditions can lead to the cell entering thermal runaway (TR), which leads to self-heating of the cell [2,3].
An increase in temperature can result in decomposition of the cell electrolyte. The decomposition products are often gaseous compositions and cause an increase in pressure within the cell casing, which eventually causes a failure event, often involving the release of smoke, flame, or sparks, which have the potential to create safety concerns.
Several factors can impact the volume and composition of gas generated during cell failure—including the cell chemistry, state of charge (SoC), and atmosphere in which the cell is failed. There are reports that give data for the volumes of gas generated for different format cells, tested under varying conditions [4,5]. To allow for comparison, gas volumes have been given as standard atmospheric temperature and pressure (SATP).
Studies into cells with nickel manganese cobalt oxide (NMC) chemistry, with various formats and capacities, have been outlined in the literature. Cells that failed via external heating under an air atmosphere at a 100% SoC have been found to be consistent, generating, on average, gas volumes in the range of 1.81 to 3.5 L/Ah [6,7,8]. Gas volumes given in L/Ah refer to the volume of gas generated per capacity (Ah) stored in the cell.
The testing of NMC 18650 cells, via the same method and within an inert atmosphere, by Golubkov and Zhang found that a similar volume of gas was generated (1.96 L/Ah and 1.87 L/Ah, respectively). These gas volumes were higher compared to those reported by Diaz in N2 and air atmospheres, from 3 Ah NMC 18650 cells. Gas volumes under air generated 0.68 L/Ah and under N2, they generated 0.66 L/Ah [6]. The opposite was observed by Howard, in 5 Ah NMC pouch cells, who reported an average volume of 1.3 L/Ah under N2 compared to 0.9 L/Ah under air [9]. These differences were attributed to the O2 availability for combustion in an air atmosphere.
The gas composition of failed NMC cells is typically found to contain H2, CO, CO2, and other small hydrocarbons, with any other trace gases deemed negligible [10]. Work by Laurelle identified the presence of CO, CO2, C2H4, and CH4 in gas samples of cells that failed under an air atmosphere and also identified that the confinement of the cell reduced the concentrations of these gases [11]. The quantification of gases by Shen and Koch found that most of the gas contained proportions of CO2 (12–37%), CO (17–28%), and H2 (13–27%), with small hydrocarbons also identified [4,8]. Similar gas compositions were observed by Zhang. Gas collected from cells under a N2 atmosphere were composed of H2 (33%), CO (30%), CO2 (25%), and other hydrocarbons contributing >5% each [12].
Adding lithium manganese oxide (LMO) to the cathode of a NMC cell appears to affect the gas volume and composition produced during failure. Golubkov observed that NMC/LMO cells generated almost double the gas, 2.63 L/Ah, compared to NMC cells, 1.96 L/Ah, when failed under an air atmosphere [13]. The CO2 and CO composition also differed; the NMC cells produced 24.9 mol% and 41.2 mol%, whereas the NMC/LMO cells generated 27.6 mol% and 13 mol%, respectively. There was little difference, however, in the concentration of other gases identified (H2, CH4, C2H4, C2H6). CO (1.2 mg/m3), CO2 (0.9 mg/m3), and CH4 (0.16 mg/m3) were also reported and quantified by Zhong under similar conditions [14]. Interestingly the gas volume reported by Sturk, from the failure of a 14 Ah five cell block of NMC/LMO cells, reported a total of 1500 L, (21 L/Ah) of gas under a N2 atmosphere [10]. However, the temperature was not reported, which may explain the large volume of gas compared to similar tests.
Observations by Xu found that the gas volume decreased with the cell’s SoC, suggesting a correlation between these properties. The decrease in SoC also affected the gas composition, with an increase in CO2 but a decrease in CO concentration. Interestingly, the H2 concentration was highest at 50% SoC [15]. Slight variations in this general trend were observed by Willstrad, reporting that both CO2 and CO concentrations increased with SoC [16].
The abuse method can also impact the volume of gas generated. Diaz reported that mechanically failed cells generated a smaller gas volume compared to externally heated cells, regardless of atmosphere [6]. Gas analysis comparisons on different abuse methods (external heat and nail penetration), tested under a N2 atmosphere, found H2, CO, and CO2 to be the most abundant, with CH4, C2H4, C2H6, C3H6, and C3H8 also detected [17]. Quantification of gases generated by a 156 Ah prismatic cell was as follows: CO (25–35 vol%), CO2 (18–32 vol%), and H2 (25–35 vol%). The pouch cell produced CO (22–24 vol%), CO2 (29–39 vol%), and H2 (20–30 vol%) [18]. The presence of CO and CO2 was also observed, in air atmosphere, for 40 Ah prismatic cells failed via nail penetration [19].
Gas volume and composition data are more limited for cells with a lithium titanium oxide (LTO) anode. Yuan failed a 1.3 Ah LTO 18650, which generated 2.46 L/Ah, with the gaseous species quantified [7]. The following gas concentrations were given: CO2 (37.6%), H2 (8.41%), CO (5.3%), C2H4 (1.38%), CH4 (1.23%), C2H6 (0.4%), and C2H2 (0.0008%). However, it is not clear what the rest of the gas composition contained.
There are numerous factors that can affect the gas volume and composition measurements, which include the abuse method used to fail the cell, the atmosphere in which cell failure took place, and cell chemistry or SoC. The reactions that generate the gas during failure events can be influenced by the surrounding environment. For example, testing cells under an air atmosphere can result in variation in gas volumes, due to the O2 concentration available for combustion. Many studies referenced also rely on a single data point, which does not show variation in the gas volume and composition values.
We have previously reported numerous test campaigns that demonstrate the factors that can affect the gas volume generated during cell failure. The first paper indicated that the cell SoC can affect the volume of gas produced—cells at a 100% SoC generated more gas compared to those at a 25% SoC [20]. There was also a difference in the gas composition. Further testing of Ni-based 18650 and pouch cells indicated that the volume and composition of gas was affected by the atmosphere in which the cell was failed. The cells failed under N2 or Ar often generated a larger gas volume than those failed under air. The gas was generally found to contain less H2 and CO in air but more CO2 [9,21,22]. Testing of nickel cobalt aluminium oxide (NCA) cylindrical cells with different SoCs and different cell capacities, given in Ah, found that as these two factors increase the volume of gas generated also increases, and that at increased SoCs, the amount of flammable gas constituents is increased [23].
Lithium iron phosphate (LFP) cells have been tested, including 26,650, pouch, and prismatic cells ranging from 3 to 230 Ah. These tests found that there is little difference in gas volume, when calculated as L/Ah, accounting for the cell capacity (Ah) at the time of failure. The gas composition was also similar between cell formats and the atmosphere in which the cell had failed. These LFP cells typically have a higher percentage of H2 (40–50%) generated by LFP due to differences in failure mechanisms between LFP cells and Ni-based cells [24].
All our previously reported data provides a comprehensive dataset for the gas generation for cell failure, using the same test set-up, analysis methods, and calculations.
We have continued to expand the dataset through the results presented in the following paper and provide data points for less commonly reported cell chemistries, including lithium cobalt oxide (LCO) and cells with an LTO anode, as well as a broader dataset on a NMC cell. This paper is therefore the fourth in a series which now encompasses over 210 individual tests on 29 different cell types. All tests have been repeated and a variety of SoCs and abuse methods have been used. We believe that this is the largest publicly available collection of such data which uses a single methodology and equipment and therefore provides directly comparable results.
2. Materials and Methods
The test series aimed to understand two key influencing factors that affect the failure gas volume and composition, cell SoC, and failure method:
- External heating of a single cell type at SoC ranging from 5 to 100%.
- External heating and nail penetration of different capacity cells (2.3–50 Ah) with Ni-based, LCO cathodes, or LTO anodes.
2.1. Cell Types Tested
Testing was performed on eight commercially available cells with different capacities, formats, and chemistries. All cells were charged according to the manufacturer’s specification. Each cell was given a cell ID to identify it throughout testing. Prior to the experiment, where possible, the average cell weight, dimensions, and internal resistance (IR) were recorded (Table 1).

Table 1.
The details for the cells failed including the capacity, format, dimensions, chemistry, average weight, and internal resistance (IR). NMC (nickel manganese cobalt oxide), LMO (lithium manganese oxide), LTO (lithium titanium oxide), LCO (lithium cobalt oxide). (Note: where marked as ‘N/A’, data was not relevant and/or unable to be collected.)
2.2. Pressure Vessel Set-Up
All tests within the experimental series were performed inside a 47 L pressure vessel rated to 10 bar at 200 °C, contained within a blast chamber and remotely operated. Temperature data was collected using type-N thermocouples (accuracy ± 2.2 °C) placed above the cell, inside the pressure vessel (ambient temperature), and on the cell’s surface (cell temperature). Pressure data measurements were taken using a 0–2 bar pressure transducer for lower capacity cells and 0–7 bar for higher capacity cells. The pressure transducer was located on the back wall of the pressure vessel and data measurements were taken every 10 ms. Depending on the test requirements, the pressure vessel has either an air or an inert (N2 or Ar) atmosphere. To achieve the inert atmosphere, the vessel was purged seven times, bringing the O2 concentration to >0.05%. When required, a gas sample was collected, via a sampling vent to a 5 L Tedlar bag, once the vessel temperature had returned to ambient temperature.
2.3. Gas Volume Calculations
The volume of gas generated during cell failure was calculated using the ideal gas law. During the test, the ambient temperature and pressure were recorded and used in the gas volume calculations. By calculating and normalising the initial moles, the additional gas volume could be attributed to the cell failure. The number of moles was subsequently used to calculate the final gas volume. The values were standardised to achieve a gas volume at standard atmosphere temperature and pressure (298 K, 101,325 Pa), allowing for easier comparison between tests [9,20].
2.4. Gas Composition Analysis
The composition of gas collected after testing was analysed by mass spectrometry using a Hiden Analytical™ HPR-20 quadrupole mass spectrometer with a heated capillary tube (Hiden, Warrington, UK). Raw data was converted into % volume by subtracting the contribution of overlapping fragment ions and converting them using the instrument’s relative sensitivity factors for the target gas. A background subtract was applied to the data prior to conversion. Data was collected over a 0–150 m/z mass range and specifically quantified for the following target gases based on our literature review: H2, CO2, CO, C2H6, C2H4, C3H6, C3H8, and CH4. If any other gases were present in the samples, it was assumed to be at very low concentrations and therefore negligible [10]. Only permanent gas was included in the final analysis results, which therefore excluded vapour concentration. Gas analysis was carried out on the same day as testing. Further details for the gas composition analysis calculations have previously been reported. Although we present gas composition data, we do not explore the mechanisms behind this formation, which has been previously reported and assessed to be due to the decomposition reactions of the solid electrolyte interphase (SEI) layer or electrolyte [25,26].
2.5. External Heat Test Set-Up (All Cells)
All cells were heated to failure by the attachment of an adhesive heater to the cell’s surface. The exact size and power supplied to the heaters can be found in the supplementary data (Table S4). All cells were tested under an inert (Ar or N2) atmosphere at a 100% SoC. The cylindrical cells (Cell A and I) were held in place by a cell holder placed inside a baffle box to prevent flame impingement on the pressure vessel’s inner surface. The pouch and prismatic cells were placed between calcium silicate fireboard and stainless-steel plates, which were bolted together to prevent cell swelling. At least two tests were performed for each cell type, with gas samples collected from each of these tests.
Additional tests were performed on Cell A, under Ar at a 5, 25, 50, and 75% SoC and at a 100% SoC under an air atmosphere. It should be noted that the cells at each SoC were new and had only undergone 3 ‘wake-up’ cycles to get them to their required SoC; therefore, this cannot be correlated to cell state of health (SoH).
2.6. Nail Penetration Test Set-Up
Cell C, Cell D, and Cell F were also failed using nail penetration inside the pressure vessel with an inert atmosphere. Tests were performed using a custom-built rig, which used an actuator with a nail moving at 0.1 m/s to puncture the centre of the cell. The cell was held in place using steel cable ties and the nail entered the cell about half of the overall depth. The cell surface temperature was measured throughout the test using a type-K thermocouple, and once the cell had failed, gas samples were collected. Three tests were performed on Cell C and Cell F, and two on Cell D.
The test set-up for each cell type is shown in (Figure 1).
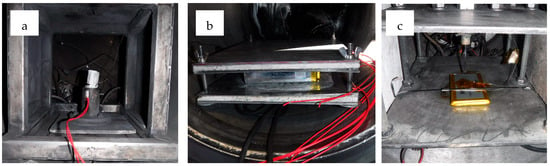
Figure 1.
(a) External heat test set-up for Cell A (5 Ah NMC 21700). (b) Test set-up for external heat tests on pouch and prismatic cells, constrained between calcium silicate board and stainless-steel plates. (c) Test set-up for pouch cell nail penetration tests.
A summary of all the tests performed in this test series is given in Table 2.

Table 2.
A summary of the number of each type of test performed for this test series.
3. Results and Discussion
3.1. 21700 NMC (Cell A) 100% External Heat
At a 100% SoC, Cell A (21700 NMC), cells tested under air generated half the gas, resulted in 4.2 L, compared to those under an inert atmosphere, 8.3 L. The results for Cell A have been previously reported but are included here for easier comparison [20]. This has been seen in previous tests on other Ni-based cells and is likely due to the combustion of H2 resulting in the formation of water [9,21]. This is supported by the gas composition, which shows a H2 concentration of 4.9% in air compared to 22.0% in inert conditions. The difference in O2 availability also results in a much higher percentage volume of CO2 in air due to combustion compared to inert atmospheres. The gas volume and composition for Cell A at 100% SoC is shown in (Table 3).

Table 3.
Average gas volume and composition data for Cell A (NMC 21700) at 100% SoC failed via external heat under air and inert atmosphere.
3.2. 21700 NMC (Cell A) Other SoC External Heat
3.2.1. Temperature Data and Failure Characteristics (Cell A)
The failure characteristics for NMC 21700 (Cell A) showed no distinct pattern in the maximum temperature reached by the cell during failure, with maximum temperatures falling between 350 and 560 °C. The temperature reached by the cell did also not seem to be impacted by the amount of jelly roll ejected during failure. In general, the jelly roll was partially expelled from the cell, except for one test at a 25% SoC (A-15). The results for all Cell A tests are summarised in (Table 4).

Table 4.
Characteristics of Cell A failure including maximum temperature, if jelly roll was ejected, and mass loss for each state of charge (SoC) tested.
The different visual failures of the cell are summarised in Figure 2. Figure 2a shows the complete ejection of the cell jelly roll. The vent cap can also be seen next to the holder, which became detached from the cell due to the amount of pressure released during the failure event. The jelly roll remains partially contained and joined to the cell casing, as demonstrated in Figure 2b. Figure 2c shows a cell where no jelly roll was ejected, and metal deposits can also be seen around the vent cap. These events were all in cells of the same type, and the jelly roll’s position was unpredictable after cell failure.
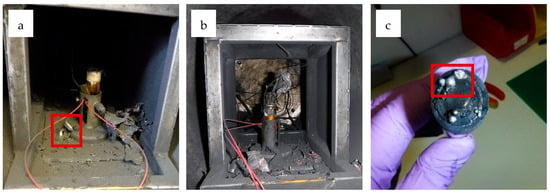
Figure 2.
(a) Cell A-15, tested at a 25% SoC under Ar, was the only cell to fully eject the jelly roll and remove the vent cap from the cell (highlighted in red). (b) Cell A-9, tested at a 50% SoC under Ar, showed partial ejection of the jelly roll, with part remaining stuck in the top of the cell. (c) Cell A-14, tested at a 5% SoC under Ar, showed no ejection of jelly roll or bursting of the vent cap. Solid metal deposits were formed around the top of the cell (highlighted in red).
3.2.2. Gas Volume and Composition (Cell A)
Gas volume appeared to be affected by the cell SoC, with the volume of gas generated increasing with the cell SoC. A seemingly linear trend was observed until the lowest SoC, 5%, was reached, which is consistent with the results observed by Xu. Analysis of the gas composition for the cells at different SoCs under inert conditions shows no consistent pattern for H2, CO2, CO, C2H6, and C2H4; however, there is an increase in the percentage volume of C3H8, C3H6, and CH4 with decreasing SoC. The average values for gas volume and composition for these tests are found in Figure 3.
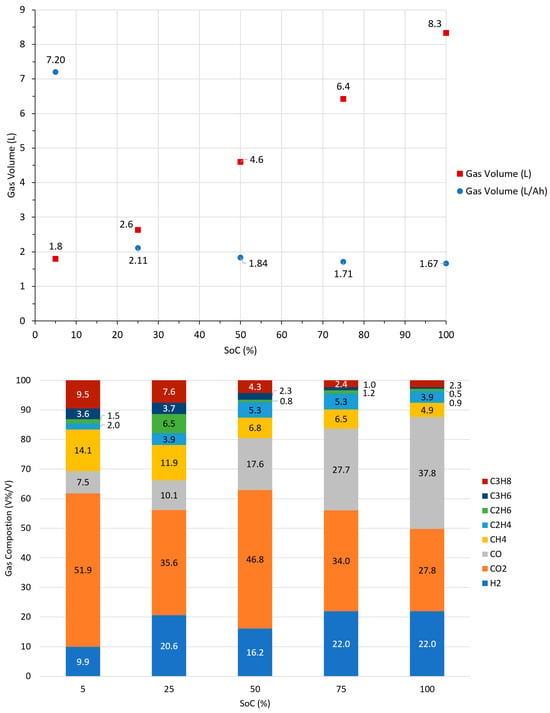
Figure 3.
Average gas volumes at each state of charge (SoC) (5, 25, 50, 75, and 100%) for Cell A failed via external heating under Ar atmosphere (top). Gas volumes are given in L and L/Ah. Average gas composition for same tests (bottom).
The gas produced during failure was collected for the cells at different SoCs and the composition analysed. The results showed no consistent pattern for H2, CO2, CO, or C2H4. However, there was an increase in percentage volume for C3H8, C3H6, and CH4 as the SoC decreased. Figure 3 shows the average gas volume and compositions for the test series. A full breakdown of all tests performed on Cell A is provided in the supplementary data (Table S1).
The data presented in Figure 3 showed general consistency between cells of the same type and configuration, with the gas composition values falling within a 5% range. The Cell B dataset showed a higher variation between CO levels. Two of the tests gave 21.8 and 21.3%, but the third was lower at 14.5%. The supplementary data gives the full data for individual tests.
3.3. Ni-Based Pouch and Prismatic External Heat and Nail Penetration
3.3.1. Temperature Data and Failure Characteristics
A record of the maximum observed temperature during all external heat and nail penetration tests on Ni-based pouch and prismatic cells is summarised in Table 5.

Table 5.
Maximum observed temperatures reached across all external heat and nail penetration tests performed on Ni-based pouch and prismatic cells. (* Maximum temperature was much lower due to thermocouple becoming detached during cell failure.)
When failed via external heat, the pouch cells generally reached higher temperatures compared to the prismatic cells. After failure, the prismatic cells remained intact, with only the vent cap appearing to burst on both Cell B and Cell G (Figure 4a,c). Despite Cell G being constrained, there was also slight swelling to the case (Figure 4a). The outer casing of all the pouch cells was damaged during the external heat tests, often having been melted, leaving the electrode foil exposed, which crumbled very easily when moved (Figure 4b).

Figure 4.
(a) Cell G-1 showing swollen casing after external heat test. (b) Cell H-2 with melted outer foil and exposed electrode foils after external heating. (c) Cell B-1 after external heat test with burst vent cap. (d) Cell F swollen after nail penetration test. (e) Cell C after nail penetration test with destroyed outer foil layer and exposed electrode foils.
Exposing cells to external heat provided an insight into the failure events in different cell types. The pouch cells appeared to generally reach a higher temperature than prismatic cells. The consequences of a failure event were also variable. The prismatic cells remained intact, which was demonstrated by Cell B and Cell G (Figure 4a,c). The vent caps burst, and Cell G had slight swelling to the casing, despite being constrained (Figure 4a). The pouch cells appeared to sustain more damage, with the outer casing melting to expose the electrode foil that crumbled when disturbed.
Utilising nail penetration demonstrated that pouch cells can undergo failure using this as a mechanism. Cell F’s outer foil showed signs of splitting along the side seam (Figure 4d); however, the smaller, lower capacity pouch cell (Cell C) had opened completely (Figure 4e).
Cell C and Cell F were tested using both external heat and nail penetration as failure mechanisms to compare the effects. The cells reached a higher surface temperature when subjected to nail penetration compared to external heat tests. Localised heating around the entry point, as the temperature measurements were taken from the outer foil layer, could be reasons for the observation. Interestingly, Cell D was also tested but did not appear to fail and only reached a maximum temperature of 36 °C across both tests.
3.3.2. Gas Volumes and Composition
Ni-based pouch (Cell C, Cell D, Cell F, and Cell H) and prismatic (Cell B and Cell G) cells that failed using an external heat source demonstrated consistent gas production, which ranged from 1.29 to 1.89 L/Ah (Figure 5). Analysis of the composition found that CO2 (19.3–29.3%), CO (19.2–28.5%), and H2 (23.2–33.3%) were the most abundant gases (Table 4). Small hydrocarbons were also identified within the mixture and typically contributed <5% of the total volume. C2H4 was detected at a higher level, particularly for Cell G, which could be attributed to variations in electrolyte mixtures between the cells.
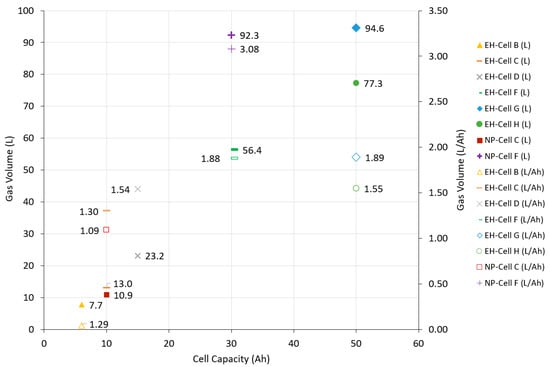
Figure 5.
Average gas volumes for Ni-based pouch and prismatic cells failed by either external heat (EH) or nail penetration (NP) given in L and L/Ah.
Cell C and Cell F, both pouch cells with NMC chemistries, were subjected to external heat and nail penetration to observe the effects of these stimuli. These were the only cells to reach failure where gas samples could be collected. Cell C generated a large gas volume when heated, 1.3 L/Ah, compared to nail penetration at 1.09 L/Ah, which was consistent with the results observed by Diaz [6]. In contrast, Cell F generated a larger gas volume in nail penetration tests (3.08 L/Ah) than the external heat tests (1.88 L/Ah) (Figure 5). There was an observed variation between tests with different failure mechanisms, but the gas composition percentage remained constant (Table 6). All full breakdown of all external heat tests for Cells B–I are given in the supplementary data (Table S2).

Table 6.
Gas composition for Ni-based pouch and prismatic cells. All pouch and prismatic cells were failed via external heat (EH), with additional nail penetration (NP) tests performed on ‘Cell C’ (10 Ah pouch) and ‘Cell F’ (30 Ah pouch) cells.
3.4. Cell E (23 Ah LTO Prismatic) External Heat
Three external heat tests were performed on the 23 Ah LTO prismatic cell inside the pressure vessel.
3.4.1. Temperature Data and Failure Characteristics
When removed from the pressure vessel after a failure event, each cell tested appeared to have slight swelling of the outer casing and a burst vent, which was similar to what was seen with Cell G during the external heat tests; the maximum temperatures recorded were 365 (Cell E-1), 377 (Cell E-3), and 383 °C (Cell E-2). These temperatures are lower than those recorded for 50 Ah Ni-based pouch and prismatic cells (Cell G and Cell H). A comparison of the temperatures for each of the cells is shown in Figure 6.
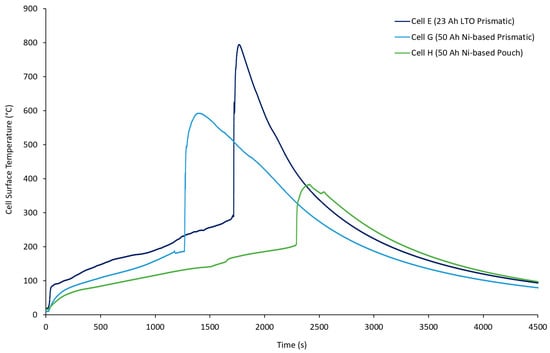
Figure 6.
Temperature data for Cell E (23 Ah LTO prismatic) (dark blue), Cell G (50 Ah Ni-based prismatic) (light blue), and Cell H (green).
3.4.2. Gas Volume and Compositon
Gas volume calculations for the LTO cells were shown to generate smaller volumes of gas, producing on average 0.8 L/Ah compared to the average 1.09 L/Ah, the lowest volume of gas produced for Ni-based cells. This is consistent with tests performed by Yuan [7]. They also had slightly different gas compositions. One observation was that less H2 was produced, 12.9%, compared with 33.3%, the maximum concentration recorded in the Ni-based cells. More CO2 was also observed 47.7% compared with a maximum of 29.3% in the Ni-based cells (Figure 7). According to Haung, the difference in the gas formation is understood to be due to the lithium intercalation differences between LTO anodes and NMC cathodes, which affects how this reacts with the electrolyte during thermal runaway [27]. The LTO cells appeared to generate smaller gas volumes (giving an average of 0.8 L/Ah), compared to the lowest value for Ni-based cells (1.09 L/Ah), a finding that was consistent with Yuan [7]. The gas compositions also varied between cell types, with the maximum percentage of H2 (LTO: 12.9% vs. Ni-based: 33.3%) and CO2 (LTO: 47.7% vs. Ni-based: 29.3%) being distinctly different (Figure 7).
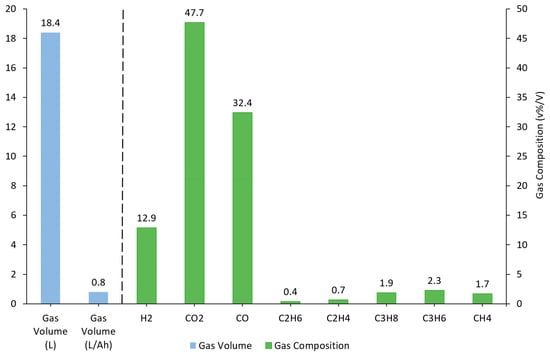
Figure 7.
Average gas volume (L, L/Ah) and gas composition for external heat tests on Cell E.
The effect of the cell failure method on the compostion of gas has been discussed in more detail in our previously published paper, which found that OC made the biggest difference to gas composition [22]. Only two cells in this test series were tested using more than one failure method, Cell C and Cell F, which were subjected to external heat and nail penetration, both of which did not seem to affect gas composition. All individul test data for the nail penetration tests on Cell C, Cell F and Cell D is given in Table S3.
3.5. LCO 18650 (Cell I) Gas Volume and Composition
When failed via external heating, ‘Cell I’ generated an average gas volume of 2.8 L, with the main gas composition comprising CO2, 38.6%, and H2, 29.1% (Table 7). Gas analysis and temperature data from our previously reported external heat tests on a 3 Ah NMC 18650 are included for comparison (note: these tests did not account for the presence of C3H6). The full test results for the NMC cell are presented in a paper by Abbott et al. [21].

Table 7.
Average gas volume and composition and temperature data for Cell I. Same data is also included for 3 Ah NMC 18650 cell taken from our previous publication [21].
Cell I, a LCO 18650 cell, was subjected to an external heat test and 2.8 L of gas was collected at the failure event. The main composition consisted of CO2 (38.6%) and H2 (29.1%), which is outlined in Table 7; the results for a similar 3 Ah NMC 18650 cell are included for comparison.
The NMC cell was tested using the same test method and generated an average gas volume of 1.3 L/Ah, which is like the LCO cell. The H2 and CO2 concentrations were also comparable; however, the CO proportion was significantly larger for the NMC cell (24.4%) compared to the LCO (0.54%). Generally, hydrocarbon ratios vary between the cell types, demonstrated by the higher percentage of CH4 but lower C2H4 for NMC when assessed against the LCO values.
4. Conclusions
An experimental campaign was conducted to study the failure events when a series of Li-ion cells, with different properties and chemistries, were subjected to external heat or nail penetration. The data highlights the changes to the gas volume and composition when different failure mechanisms are used; however, we have no further evidence to report why this has the potential to affect failure mechanisms. Other results, within the published literature, corroborate the findings presented within this paper. A full dataset was compiled for all cell cathode and additional LTO anode chemistries that are commonly used in commercial applications. Completing the tests using the same methodology as we have previously reported allowed for a direct comparison between the 29 different cell types covered withing this and our previous papers [21,22,23,24]. These cover all currently available common Li-ion cell chemistries and formats and cells between 2 and 230 Ah. Over 210 individual tests are reported, each experiment has been repeated at least once, and for the most part, results from three tests are given. We believe this to be the largest and broadest such dataset within the open literature.
Within this paper, we show that Ni-based cells gave a gas volume of 1.3–1.8 L/Ah when exposed to external heat, which is higher than the tests on LCO cells (1.2 L/Ah) and LTO (0.8 L/Ah). Interestingly, the gas mixtures also varied between the cell chemistries. For example, the sample collected for LTO failure contained a lower concentration of H2 compared to similar Ni-based cells.
The method impacts the overall volume of gas generated when a cell fails, which is highlighted by the lower values for nail penetration tests when compared to external heat. The ratio of gas remained proportional in both cases.
The SoC for a cell at the time of failure also impacts the gas volume, with both showing a linear decrease. However, clear correlation was not observed for SoC and gas composition.
Overall, it is important to understand that many factors can influence the volume and composition of gas generated when different Li-ion cells fail; therefore, it should not be assumed that a single cell failure will give a result that is completely representative of all cells.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/batteries11090320/s1. Table S1: Full set of gas analysis and temperature data for Cell A external heat tests at a 5, 25, 50, 75, and 100% SoC under Ar and 100% SoC under air. (Note: Where gas volumes are given in L/Ah, these were calculated using the nominal cell capacity.). Table S2: Full set of gas analysis and temperature data for Cells B—I external heat tests at a 100% SoC under Ar. (Note: Where gas volumes are given in L/Ah, these were calculated using the nominal cell capacity.). Table S3: Full set of gas analysis and temperature data for Cell C, Cell F, and Cell D nail penetration tests at a 100% SoC under Ar. (Note: Where gas volumes are given in L/Ah, these were calculated using the nominal cell capacity.). Table S4: The number of heaters, heater type, and power supplied for all external heat tests.
Author Contributions
G.E.H.: Conceptualization, Formal Analysis, Investigation, Visualisation, Writing—Original Draft. J.E.H.B.: Conceptualization, Funding Acquisition, Supervision, Writing—Review and Editing. J.G.: Conceptualization. S.L.G.: Data Curation, Formal Analysis, Investigation, Methodology, Validation. J.W.M.: Investigation. P.A.P.R.: Formal Analysis, Investigation. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Health and Safety Executive, UK.
Institutional Review Board Statement
The contents of this publication, including any opinions and/or conclusions expressed, are those of the authors alone and do not necessarily reflect HSE policy.
Data Availability Statement
The data presented in this study are available in the article and supplementary data.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SoC | State of charge |
| NMC | Nickel manganese cobalt oxide |
| LCO | Lithium cobalt oxide |
| LTO | Lithium titanium oxide |
| TR | Thermal runaway |
| SATP | Standard atmospheric temperature and pressure |
| LMO | Lithium manganese oxide |
| NMC/LMO | Nickel manganese cobalt oxide/Lithium manganese oxide |
| NCA | Nickel cobalt aluminium oxide |
| LFP | Lithium iron phosphate |
| IR | Internal resistance |
| EH | External heat |
| NP | Nail penetration |
| SoH | State of health |
References
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Dai, Y.; Panahi, A. Thermal runaway process in lithium-ion batteries: A review. Next Energy 2025, 6, 100186. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Shen, H.; Wang, H.; Li, M.; Li, C.; Zhang, Y.; Li, Y.; Yang, X.; Yang, X.; Feng, X.; Ouyang, M. Thermal Runaway Characteristics and Gas Composition Analysis of Lithium-Ion Batteries with Different LFP and NCM Cathode Materials under Inert Atmosphere. Electronics 2023, 12, 1603. [Google Scholar] [CrossRef]
- Huang, W.; Feng, X.; Han, X.; Zhang, W.; Jiang, F. Questions and Answers Relating to Lithium-Ion Battery Safety Issues. Cell Rep. Phys. Sci. 2021, 2, 100285. [Google Scholar] [CrossRef]
- Diaz, F.; Wang, Y.; Weyhe, R.; Friedrich, B. Gas generation measurement and evaluation during mechanical processing and thermal treatment of spent Li-ion batteries. Waste Manag. 2019, 84, 102–111. [Google Scholar] [CrossRef]
- Yuan, L.; Dubaniewicz, T.; Zlochower, I.; Thomas, R.; Rayyan, N. Experimental study on thermal runaway and vented gases of lithium-ion cells. Process Saf. Environ. Prot. 2020, 144, 186–192. [Google Scholar] [CrossRef]
- Koch, S.; Fill, A.; Birke, K.P. Comprehensive gas analysis on large scale automotive lithium-ion cells in thermal runaway. J. Power Sources 2018, 398, 106–112. [Google Scholar] [CrossRef]
- Howard, G.; Buston, J.; Gill, J. Experimental understanding of gas volumes and forces generated due to swelling during lithium-ion pouch cell failure. In Proceedings of the Hazards 31, Virtual, 16–18 November 2021. [Google Scholar]
- Sturk, D.; Rosell, L.; Blomqvist, P.; Tidblad, A.A. Analysis of Li-Ion Battery Gases Vented in an Inert Atmosphere Thermal Test Chamber. Batteries 2019, 5, 61. [Google Scholar] [CrossRef]
- Laruelle, S.; Forestier, C.; Lecocq, A.; Zantman, A.; Grugeon, S.; Sannier, L.; Marlair, G. Study of the Role of LiNi1/3Mn1/3Co1/3O2/Graphite Li-Ion Pouch Cells Confinement, Electrolyte Composition and Separator Coating on Thermal Runaway and Off-Gas Toxicity. J. Electrochem. Soc. 2020, 167, 090513. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Li, W.; Li, C. Quantitative identification of emissions from abused prismatic Ni-rich lithium-ion batteries. eTransportation 2019, 2, 100031. [Google Scholar] [CrossRef]
- Golubkov, A.W.; Fuchs, D.; Wagner, J.; Wiltsche, H.; Stangl, C.; Fauler, G.; Voitic, G.; Thaler, A.; Hacker, V. Thermal-runaway experiments on consumer Li-ion batteries with metal-oxide and olivin-type cathodes. RSC Adv. 2014, 4, 3633–3642. [Google Scholar] [CrossRef]
- Zhong, G.; Mao, B.; Wang, C.; Jiang, L.; Xu, K.; Sun, J.; Wang, Q. Thermal runaway and fire behavior investigation of lithium ion batteries using modified cone calorimeter. J. Therm. Anal. Calorim. 2018, 135, 2879–2889. [Google Scholar] [CrossRef]
- Xu, L.; Wang, S.; Li, Y.; Li, Y.; Sun, J.; Zhao, F.; Wang, H.; Wang, Y.; Xu, C.; Feng, X. Thermal runaway propagation behavior and gas production characteristics of NCM622 battery modules at different state of charge. Process Saf. Environ. Prot. 2024, 185, 267–276. [Google Scholar] [CrossRef]
- Willstrand, O.; Pushp, M.; Andersson, P.; Brandell, D. Impact of different Li-ion cell test conditions on thermal runaway characteristics and gas release measurements. J. Energy Storage 2023, 68, 107785. [Google Scholar] [CrossRef]
- Essl, C.; Golubkov, A.W.; Fuchs, A. Comparing Different Thermal Runaway Triggers for Two Automotive Lithium-Ion Battery Cell Types. J. Electrochem. Soc. 2020, 167, 130542. [Google Scholar] [CrossRef]
- Xu, C.; Fan, Z.; Zhang, M.; Wang, P.; Wang, H.; Jin, C.; Peng, Y.; Jiang, F.; Feng, X.; Ouyang, M. A comparative study of the venting gas of lithium-ion batteries during thermal runaway triggered by various methods. Cell Rep. Phys. Sci. 2023, 4, 101705. [Google Scholar] [CrossRef]
- Nedjalkov, A.; Meyer, J.; Köhring, M.; Doering, A.; Angelmahr, M.; Dahle, S.; Sander, A.; Fischer, A.; Schade, W. Toxic Gas Emissions from Damaged Lithium Ion Batteries—Analysis and Safety Enhancement Solution. Batteries 2016, 2, 5. [Google Scholar] [CrossRef]
- Abbott, K.C.; Buston, J.E.H.; Gill, J.; Goddard, S.L.; Howard, D.; Howard, G.; Read, E.; Williams, R.C.E. Comprehensive gas analysis of a 21700 Li(Ni0.8Co0.1Mn0.1O2) cell using mass spectrometry. J. Power Sources 2022, 539, 231585. [Google Scholar] [CrossRef]
- Abbott, K.C.; Buston, J.E.H.; Gill, J.; Goddard, S.L.; Howard, D.; Howard, G.E.; Read, E.; Williams, R.C.E. Experimental Study of Three Commercially Available 18650 Lithium Ion Batteries using Multiple Abuse Methods. J. Energy Storage 2023, 65, 107293. [Google Scholar] [CrossRef]
- Howard, G.E.; Abbott, K.C.; Buston, J.E.H.; Gill, J.; Goddard, S.L.; Howard, D. Comprehensive Study of the Gas Volume and Composition Generated by 5 Ah Nickel Manganese Cobalt Oxide (NMC) Li-Ion Pouch Cells Through Different Failure Mechanisms at Varying States of Charge. Batteries 2025, 11, 197. [Google Scholar] [CrossRef]
- Reeve, P.A.P.; Buston, J.E.H.; Gill, J.; Goddard, S.L.; Howard, G.E.; Mellor, J.W. Failure Gas Analysis of Lithium-Nickel-Cobalt-Aluminium Oxide Cells From Different Manufacturers. RSC Adv. 2025, 15, 5084–5095. [Google Scholar] [CrossRef] [PubMed]
- Howard, G.E.; Buston, J.E.H.; Gill, J.; Goddard, S.L.; Mellor, J.W.; Reeve, P.A.P. Comprehensive Study of the Gas Volume and Composition Produced by Different 3–230 Ah Lithium Iron Phosphate (LFP) Cells Failed Using External Heat, Overcharge and Nail Penetration Under Air and Inert Atmospheres. Batteries 2025, 11, 267. [Google Scholar] [CrossRef]
- Rowden, B.; Garcia-Araez, N. A review of gas evolution in lithium ion batteries. Energy Rep. 2020, 6, 10–18. [Google Scholar] [CrossRef]
- Gong, T.; Duan, X.; Shan, Y.; Huang, L. Gas Generation in Lithium-Ion Batteries: Mechanisms, Failure Pathways, and Thermal Safety Implications. Batteries 2025, 11, 152. [Google Scholar] [CrossRef]
- Huang, P.; Wang, Q.; Li, K.; Ping, P.; Sun, J. The combustion behavior of large scale lithium titanate battery. Sci. Rep. 2015, 5, 7788. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).