Nano-Alloy FeSb Wrapped in Three-Dimensional Honeycomb Carbon for High-Performance Lithium-Ion Batteries
Abstract
1. Introduction
2. Experimental Section
2.1. Material Synthesis
2.2. Material Characterization
2.3. Electrochemical Characterization
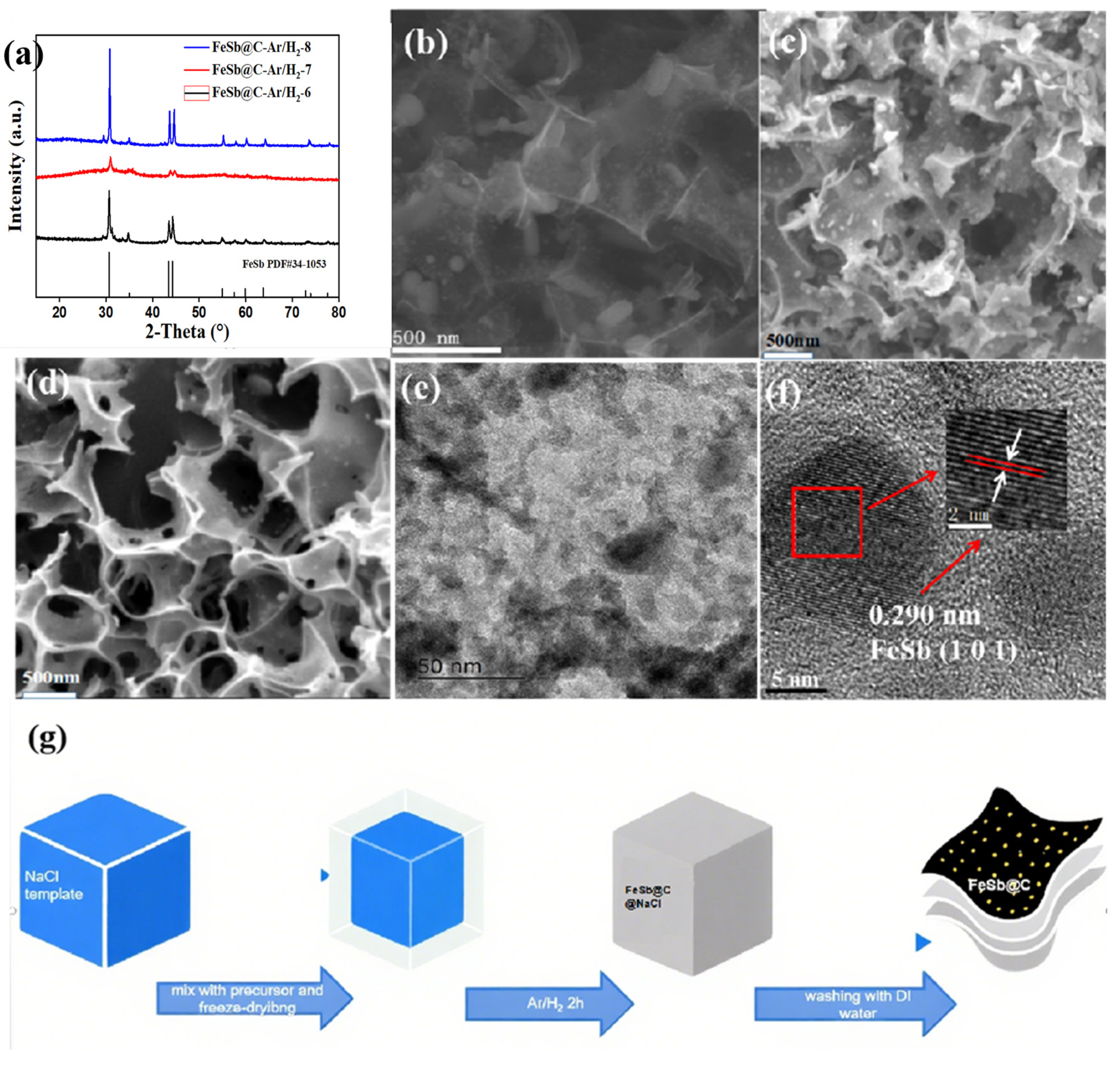
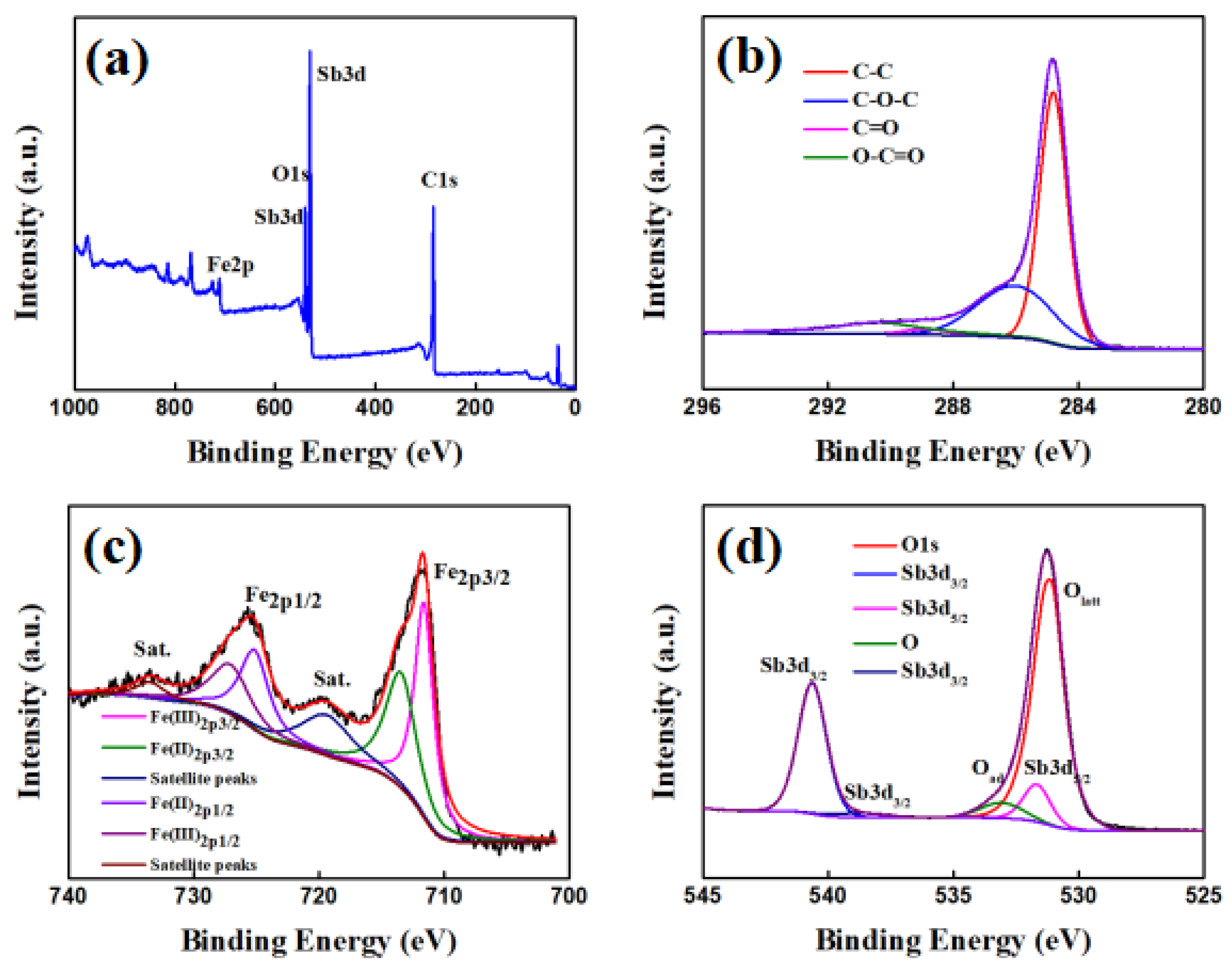
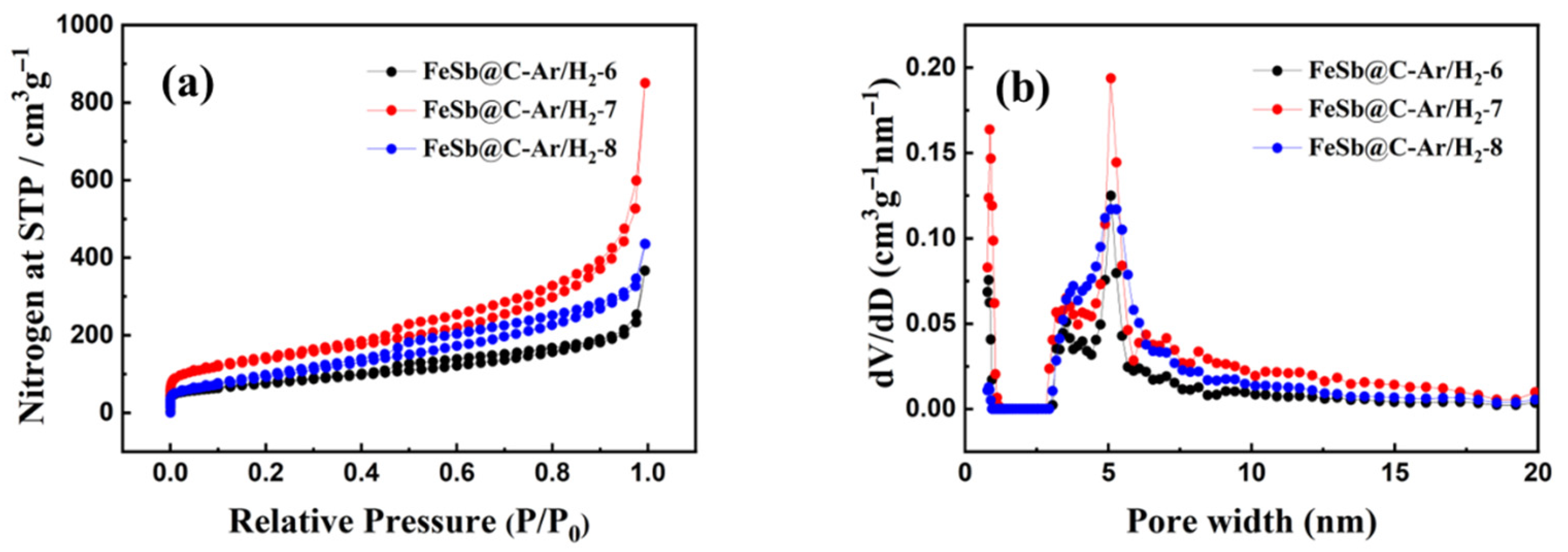
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bruce Dunn, H.K.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Ramasubramanian, B.; Ling, J.; Jose, R.; Ramakrishna, S. Ten major challenges for sustainable lithium-ion batteries. Cell Rep. Phys. Sci. 2024, 5, 102032. [Google Scholar] [CrossRef]
- Kaygusuz, K. Energy for sustainable development: A case of developing countries. Renew. Sustain. Energy Rev. 2012, 16, 1116–1126. [Google Scholar] [CrossRef]
- Wagner, R.; Preschitschek, N.; Passerini, S.; Leker, J.; Winter, M. Current research trends and prospects among the various materials and designs used in lithium-based batteries. J. Appl. Electrochem. 2013, 43, 481–496. [Google Scholar] [CrossRef]
- Nishi, Y. The Development of Lithium Ion Secondary Batteries. Chem. Rec. 2001, 1, 406–413. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Ding, F.; Xu, W.; Xiao, J.; Cao, Y.; Meduri, P.; Liu, J.; Graff, G.L.; Zhang, J.G. Conductive rigid skeleton supported silicon as high-performance Li-ion battery anodes. Nano Lett. 2012, 12, 4124–4130. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous Liquid Electrolytes for Lithium-Based Rechargeable Batteries. Chem. Rev. 2004, 104, 4303–4417. [Google Scholar] [CrossRef]
- Li, B.; Yang, S.; Li, S.; Wang, B.; Liu, J. From Commercial Sponge Toward 3D Graphene-Silicon Networks for Superior Lithium Storage. Adv. Energy Mater. 2015, 5, 1500289. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nat. Vol. 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Fong, R.; von Sacken, U.; Dahn, J.R. Studies of Lithium Intercalation into Carbons Using Nonaqueous Electrochemical Cells. J. Electrochem. Soc. 2019, 137, 2009. [Google Scholar] [CrossRef]
- Zhang, W.; Qian, M.; Luo, G.; Feng, X.; Wu, C.; Qin, W. Improved lithium ion storage performance of Ti3C2Tx MXene@S composite with carboxymethyl cellulose binder. J. Colloid Interface Sci. 2023, 641, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, X.; Qi, X.; Zhao, W.; Ju, Z. Sb nanoparticles encapsulated in 3D porous carbon as anode material for lithium-ion and potassium-ion batteries. Mater. Res. Bull. 2018, 103, 32–37. [Google Scholar] [CrossRef]
- Wang, X.; Jia, N.; Li, J.; Liu, P.; Zhao, X.; Lin, Y.; Sun, C.; Qin, W. Sb Nanoparticles Embedded in the N-Doped Carbon Fibers as Binder-Free Anode for Flexible Li-Ion Batteries. Nanomaterials 2022, 12, 3093. [Google Scholar] [CrossRef]
- Qian, M.; Zhang, W.; Luo, G.; Wu, C.; Qin, W. Air-stabilized pore structure engineering of antimony-based anode by electrospinning for potassium ion batteries. J. Colloid Interface Sci. 2023, 633, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yu, L.; Wu, C.; Wen, Y.; Yin, K.; Chiang, F.K.; Hu, R.; Liu, J.; Sun, L.; Gu, L.; et al. New Nanoconfined Galvanic Replacement Synthesis of Hollow Sb@C Yolk-Shell Spheres Constituting a Stable Anode for High-Rate Li/Na-Ion Batteries. Nano Lett. 2017, 17, 2034–2042. [Google Scholar] [CrossRef]
- Xiong, P.; Wu, J.; Zhou, M.; Xu, Y. Bismuth-Antimony Alloy Nanoparticle@Porous Carbon Nanosheet Composite Anode for High-Performance Potassium-Ion Batteries. ACS Nano 2020, 14, 1018–1026. [Google Scholar] [CrossRef]
- Antitomaso, P.; Fraisse, B.; Stievano, L.; Biscaglia, S.; Aymé-Perrot, D.; Girard, P.; Sougrati, M.T.; Monconduit, L. SnSb electrodes for Li-ion batteries: The electrochemical mechanism and capacity fading origins elucidated by using operando techniques. J. Mater. Chem. A 2017, 5, 6546–6555. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, R.; Luo, T.; Ma, G.; Hu, G.; Lyu, J.; Zhou, L.; Wu, J. Solid Solution of Bi and Sb for Robust Lithium Storage Enabled by Consecutive Alloying Reaction. Small 2021, 17, 2102915. [Google Scholar] [CrossRef]
- He, J.; Wei, Y.; Zhai, T.; Li, H. Antimony-based materials as promising anodes for rechargeable lithium-ion and sodium-ion batteries. Mater. Chem. Front. 2018, 2, 437–455. [Google Scholar] [CrossRef]
- Li, Z.; Gan, Q.; Zhang, Y.; Hu, J.; Liu, P.; Xu, C.; Wu, X.; Ge, Y.; Wang, F.; Yao, Q.; et al. FeSb@N-doped carbon quantum dots anchored in 3D porous N-doped carbon with pseudocapacitance effect enabling fast and ultrastable potassium storage. Nano Res. 2021, 15, 217–224. [Google Scholar] [CrossRef]
- Zheng, W.; Yu, X.; Guo, Z.; Song, G.; Hu, F. Magnetron sputtering deposition of MSb(M=Fe, Ni, Co) thin films as negative electrodes for Li-ion and Na-ion batteries. Mater. Res. Express 2019, 6, 056410. [Google Scholar] [CrossRef]
- Guo, S.; Bai, Y.; Geng, Z.; Wu, F.; Wu, C. Facile synthesis of Li3V2(PO4)3/C cathode material for lithium-ion battery via freeze-drying. J. Energy Chem. 2019, 32, 159–165. [Google Scholar] [CrossRef]
- Wang, S.; Wang, R.; Zhao, Q.; Ren, L.; Wen, J.; Chang, J.; Fang, X.; Hu, N.; Xu, C. Freeze-drying induced self-assembly approach for scalable constructing MoS2/graphene hybrid aerogels for lithium-ion batteries. J. Colloid Interface Sci. 2019, 544, 37–45. [Google Scholar] [CrossRef]
- Jakubowska, E.; Lulek, J. The application of freeze-drying as a production method of drug nanocrystals and solid dispersions—A review. J. Drug Deliv. Sci. Technol. 2021, 62, 102357. [Google Scholar] [CrossRef]
- Xu, Y.; Li, W.; Zhang, F.; Zhang, X.; Zhang, W.; Lee, C.-S.; Tang, Y. In situ incorporation of FeS nanoparticles/carbon nanosheets composite with an interconnected porous structure as a high-performance anode for lithium ion batteries. J. Mater. Chem. A 2016, 4, 3697–3703. [Google Scholar] [CrossRef]
- Ren, X.; Ai, D.; Zhan, C.; Lv, R.; Kang, F.; Huang, Z.-H. NaCl-template-assisted freeze-drying synthesis of 3D porous carbon-encapsulated V2O3 for lithium-ion battery anode. Electrochim. Acta 2019, 318, 730–736. [Google Scholar] [CrossRef]
- Allcorn, E.; Manthiram, A. High-rate, high-density FeSb–TiC–C nanocomposite anodes for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 3891–3900. [Google Scholar] [CrossRef]
- Liang, J.-C.; Zhou, K.-Y.; Chen, G.-Y.; Zhang, W.-X.; Liu, J.-A.; Zhang, W.-Z.; Zhang, Z.-P.; Hou, W.; Zhou, M.; Liu, G.-F.; et al. Embedding FeSb alloy nanoparticles in N-doped carbon layers as an efficient bifunctional electrocatalyst for zinc-air battery. J. Solid State Electrochem. 2017, 21, 3315–3324. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Li, H.; Liu, J.; Song, J.; Fusaro, L.; Hu, Z.-Y.; Chen, Y.; Li, Y.; Su, B.-L. Weaving 3D highly conductive hierarchically interconnected nanoporous web by threading MOF crystals onto multi walled carbon nanotubes for high performance Li–Se battery. J. Energy Chem. 2021, 59, 396–404. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Y.; Huang, R.; Ji, J.; Yao, J.; Xiao, S. One-pot hydrothermal synthesis of N-rGO supported Fe2O3 nanoparticles as a superior anode material for lithium-ion batteries. Solid State Ion. 2021, 368, 115693. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, Y.; Sun, X.; Liu, H.; Cui, J.; Zhang, Y.; He, W. N, S co-doped modified graphene/Fe2O3 composites synthesized via microwave-assisted method for Na-ion batteries. Inorg. Chem. Commun. 2020, 121, 108188. [Google Scholar] [CrossRef]
- Wang, J.; Yang, X.; Wang, Y.; Jin, S.; Cai, W.; Liu, B.; Ma, C.; Liu, X.; Qiao, W.; Ling, L. Rational design and synthesis of sandwich-like reduced graphene oxide/Fe2O3/N-doped carbon nanosheets as high-performance anode materials for lithium-ion batteries. Chem. Eng. Sci. 2021, 231, 116271. [Google Scholar] [CrossRef]
- Birchall, T.; Connor, J.A.; Hillier, L.H. High-energy Photoelectron Spectroscopy of some Antimony Compounds. J. Chem. Soc. Dalton Trans. 1975, 20, 2003–2006. [Google Scholar] [CrossRef]
- Deng, M.; Li, S.; Hong, W.; Jiang, Y.; Xu, W.; Shuai, H.; Zou, G.; Hu, Y.; Hou, H.; Wang, W.; et al. Octahedral Sb2O3 as high-performance anode for lithium and sodium storage. Mater. Chem. Phys. 2019, 223, 46–52. [Google Scholar] [CrossRef]
- Feng, X.; Qian, M.; Guo, Z.; Luo, G.; Wu, C.; Qin, W. Graphene modified Sb2O3/porous carbon nanofibers via electrospinning treated in air for potassium-ion batteries with enhanced cycling stability. Int. J. Hydrogen Energy 2024, 83, 326–334. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, K.; Wang, D.; Luo, S.; Liu, X.; Liu, Y.; Wang, Q.; Zhang, Y.; Hao, A.; He, C.; et al. Constructing N-Doped porous carbon confined FeSb alloy nanocomposite with Fe-N-C coordination as a universal anode for advanced Na/K-ion batteries. Chem. Eng. J. 2020, 384, 123327. [Google Scholar] [CrossRef]
- Chandrasekaran Nithya, G.T. Morphology oriented CuS nanostructures: Superior K-ion storage by surface enhanced pseudocapacitive effects. Sustain. Energy Fuels 2020, 4, 3574–3587. [Google Scholar] [CrossRef]
- Zhu, W.; Kierzek, K.; Wang, S.; Li, S.; Holze, R.; Chen, X. Improved performance in lithium ion battery of CNT-Fe3O4@graphene induced by three-dimensional structured construction. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 126014. [Google Scholar] [CrossRef]
- Peng, Y.-T.; Lo, C.-T. Effect of Microstructure and Morphology of Electrospun Ultra-Small Carbon Nanofibers on Anode Performances for Lithium Ion Batteries. J. Electrochem. Soc. 2015, 162, A1085–A1093. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Wang, W.; Li, D.; Wang, C.; Wang, P.; Zhu, K.; Li, Z. Synthesis of N-doped multi-cavity Sn/C composite and utilization to anode in lithium ion batteries. Mater. Chem. Phys. 2021, 260, 124199. [Google Scholar] [CrossRef]
- He, H.; Sun, D.; Tang, Y.; Wang, H.; Shao, M. Understanding and improving the initial Coulombic efficiency of high-capacity anode materials for practical sodium ion batteries. Energy Storage Mater. 2019, 23, 233–251. [Google Scholar] [CrossRef]
- Xiao, J.; Li, Q.; Bi, Y.; Cai, M.; Dunn, B.; Glossmann, T.; Liu, J.; Osaka, T.; Sugiura, R.; Wu, B.; et al. Understanding and applying coulombic efficiency in lithium metal batteries. Nat. Energy 2020, 5, 561–568. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, X.-Y.; Lou, X.W.; Paik, U. Sb@C coaxial nanotubes as a superior long-life and high-rate anode for sodium ion batteries. Energy Environ. Sci. 2016, 9, 2314–2318. [Google Scholar] [CrossRef]
- Yang, X.; Ma, J.; Wang, H.; Chai, Y.; Yuan, R. Partially reduced Sb/Sb2O3@C spheres with enhanced electrochemical performance for lithium ion storage. Mater. Chem. Phys. 2018, 213, 208–212. [Google Scholar] [CrossRef]
- Wang, H.-G.; Zhou, Y.; Shen, Y.; Li, Y.; Zuo, Q.; Duan, Q. Fabrication, formation mechanism and the application in lithium-ion battery of porous Fe2O3 nanotubes via single-spinneret electrospinning. Electrochim. Acta 2015, 158, 105–112. [Google Scholar] [CrossRef]
- He, C.; Wu, S.; Zhao, N.; Shi, C.; Liu, E.; Li, J. Carbon-encapsulated Fe3O4 nanoparticles as a high-rate lithium ion battery anode material. ACS Nano 2013, 7, 4459–4469. [Google Scholar] [CrossRef]
- Cao, K.; Jiao, L.; Liu, H.; Liu, Y.; Wang, Y.; Guo, Z.; Yuan, H. 3D Hierarchical Porous α-Fe2O3Nanosheets for High-Performance Lithium-Ion Batteries. Adv. Energy Mater. 2015, 5, 1401421. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, S.; Du, J.; Jin, Q.; Zhang, T.; Cheng, F.; Chen, J. Ultrasmall Sn nanoparticles embedded in nitrogen-doped porous carbon as high-performance anode for lithium-ion batteries. Nano Lett. 2014, 14, 153–157. [Google Scholar] [CrossRef]
- Rai, A.K.; Anh, L.T.; Gim, J.; Mathew, V.; Kang, J.; Paul, B.J.; Song, J.; Kim, J. Simple synthesis and particle size effects of TiO2 nanoparticle anodes for rechargeable lithium ion batteries. Electrochim. Acta 2013, 90, 112–118. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, Y.; Liu, Y.; Wang, C. Electrochemical Performance of Porous Carbon/Tin Composite Anodes for Sodium-Ion and Lithium-Ion Batteries. Adv. Energy Mater. 2013, 3, 128–133. [Google Scholar] [CrossRef]
- Lu, G.; Qiu, S.; Liu, J.; Wang, X.; He, C.; Bai, Y.-J. Enhanced Electrochemical Performance of Zn-Doped Fe3O4 with Carbon Coating. Electrochim. Acta 2014, 117, 230–238. [Google Scholar] [CrossRef]
- Luo, G.; Zhou, N.; Feng, X.; Guo, Z.; Wu, C.; Qin, W. Dual-carbon confined Sb2Se3 nanorods for potassium ion batteries anode with improved capacity and cycling stability. J. Energy Storage 2024, 87, 111520. [Google Scholar] [CrossRef]
- Xiong, S.; Lin, X.; Liu, S.; Weng, S.; Jiang, S.; Jiao, Y.; Xu, Y.; Cheng, J. Metal-organic framework derived α-Fe2O3 nano-octahedron with oxygen vacancies for realizing outstanding energy storage performance. Vacuum 2020, 182, 109692. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, Y.; Guo, Y.; Liu, S.; Liu, H.; Wu, H. Flakelike LiCoO2 with Exposed {010} Facets As a Stable Cathode Material for Highly Reversible Lithium Storage. ACS Appl. Mater. Interfaces 2016, 8, 2723–2731. [Google Scholar] [CrossRef]
- Gao, G.; Dang, W.; Wu, H.; Zhang, G.; Feng, C. Synthesis of MnWO4@C as novel anode material for lithium ion battery. J. Mater. Sci. Mater. Electron. 2018, 29, 12804–12812. [Google Scholar] [CrossRef]
- Zhao, N.; Qin, J.; Chu, L.; Wang, L.; Xu, D.; Wang, X.; Yang, H.; Zhang, J.; Li, X. Heterogeneous interface of Se@Sb@C boosting potassium storage. Nano Energy 2020, 78, 105345. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, S.; Kang, Q.; Ni, L.; Chen, N.; Li, X.; Lu, C.; Wang, X.; Peng, L.; Guo, X.; et al. Iron oxide encapsulated in nitrogen-rich carbon enabling high-performance lithium-ion capacitor. Sci. China Mater. 2020, 63, 2289–2302. [Google Scholar] [CrossRef]
- Huang, C.; Xu, A.; Li, G.; Sun, H.; Wu, S.; Xu, Z.; Yan, Y. Alloyed BiSb Nanoparticles Confined in Tremella-Like Carbon Microspheres for Ultralong-Life Potassium Ion Batteries. Small 2021, 17, e2100685. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, Y.; Zhai, X.; Ding, C.; Zhao, X.; Li, J.; Jin, H. Graphene boosted pseudocapacitive lithium storage: A case of G-Fe2O3. Electrochim. Acta 2018, 282, 955–963. [Google Scholar] [CrossRef]


| FeSb@C-Ar/H2-6 | FeSb@C-Ar/H2-7 | FeSb@C-Ar/H2-8 | |
|---|---|---|---|
| Specific surface area/m2/g | 272.4 | 495.6 | 366.2 |
| Total pore volume/cm3/g | 0.367 | 0.851 | 0.506 |
| Samples | Specific Capacity/mAh g−1 | Current Density/mA g−1 | Cycle Numbers | References |
|---|---|---|---|---|
| Sb@PC | 280 | 1000 | 500 | [13] |
| Sb@NCFs | 480 | 400 | 300 | [14] |
| Sb@C | 405 | 1000 | 300 | [16] |
| Bi0.5Sb0.5@carbon | 489.4 | 1000 | 2000 | [19] |
| FeSb@C-Ar/H2-7 | 607.8 | 1000 | 600 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, N.; Nie, X.; Li, J.; Qin, W. Nano-Alloy FeSb Wrapped in Three-Dimensional Honeycomb Carbon for High-Performance Lithium-Ion Batteries. Batteries 2025, 11, 305. https://doi.org/10.3390/batteries11080305
Jia N, Nie X, Li J, Qin W. Nano-Alloy FeSb Wrapped in Three-Dimensional Honeycomb Carbon for High-Performance Lithium-Ion Batteries. Batteries. 2025; 11(8):305. https://doi.org/10.3390/batteries11080305
Chicago/Turabian StyleJia, Nanjun, Xinming Nie, Jianwei Li, and Wei Qin. 2025. "Nano-Alloy FeSb Wrapped in Three-Dimensional Honeycomb Carbon for High-Performance Lithium-Ion Batteries" Batteries 11, no. 8: 305. https://doi.org/10.3390/batteries11080305
APA StyleJia, N., Nie, X., Li, J., & Qin, W. (2025). Nano-Alloy FeSb Wrapped in Three-Dimensional Honeycomb Carbon for High-Performance Lithium-Ion Batteries. Batteries, 11(8), 305. https://doi.org/10.3390/batteries11080305






