Sulfurized Polyacrylonitrile for Rechargeable Batteries: A Comprehensive Review
Abstract
1. Introduction
2. Dataset Construction and Research Progress
3. Chemical Structure

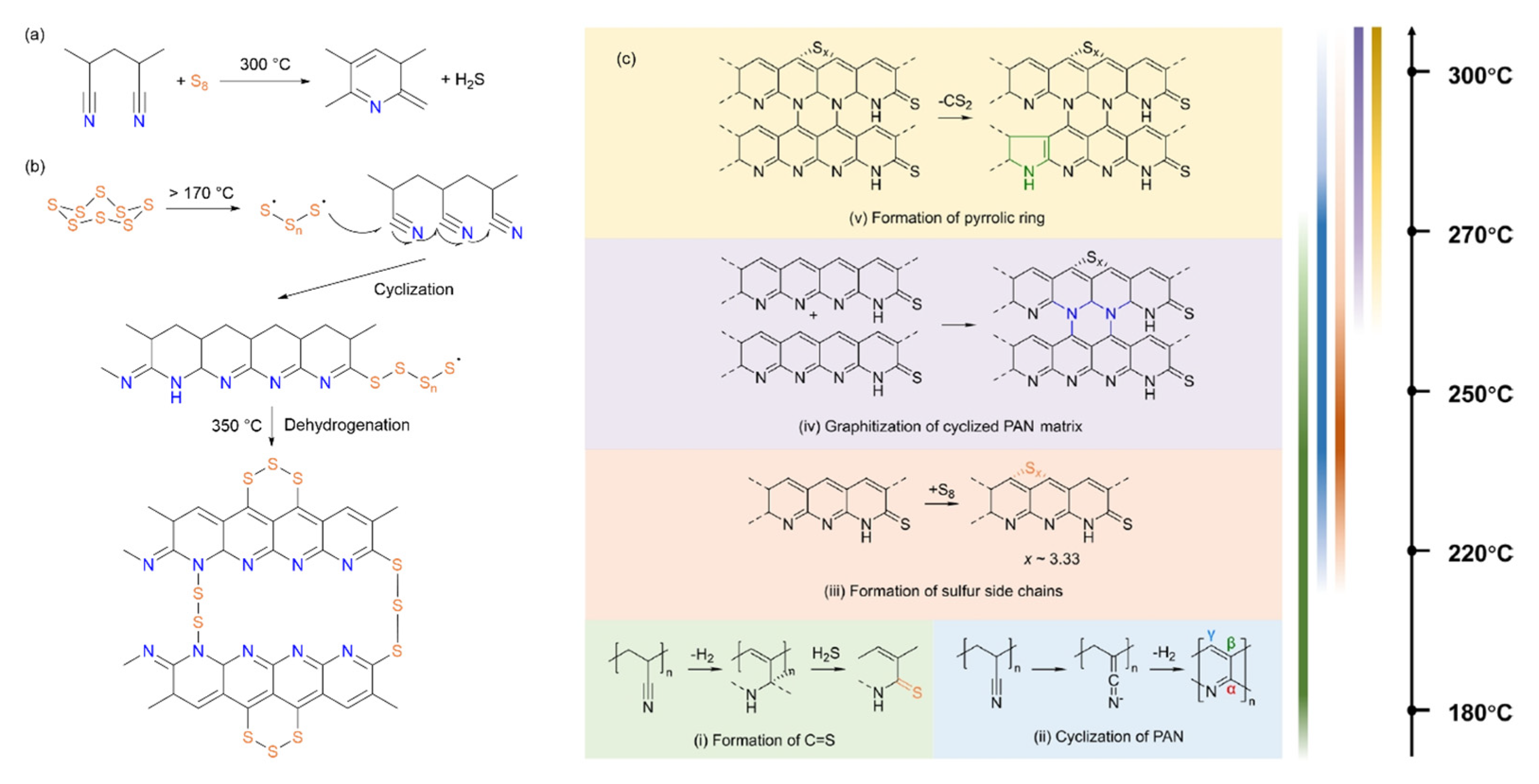
4. Structural Evolution During Synthesis
5. Electrochemical Properties
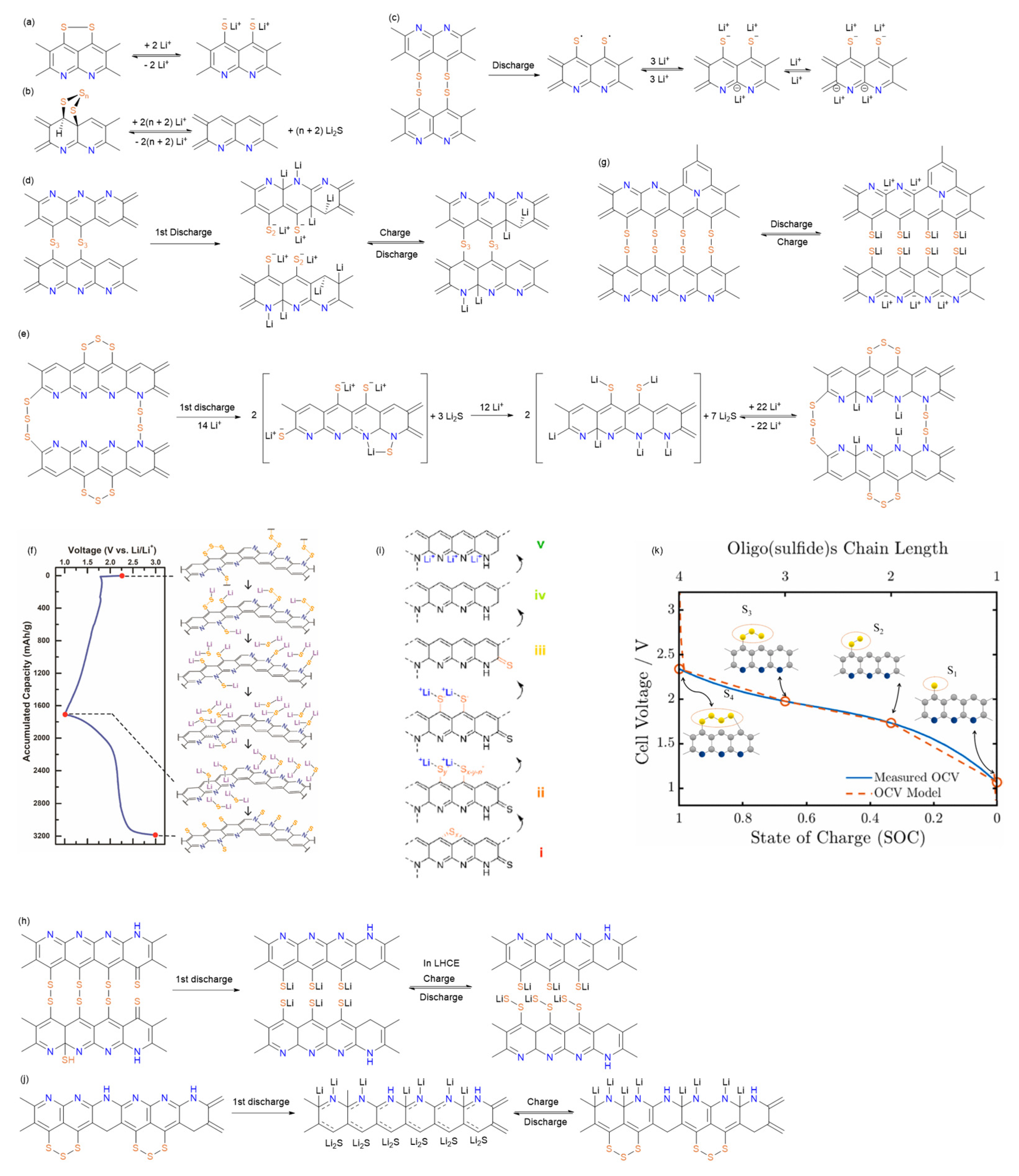
6. Redox Reaction Mechanism
7. SPAN Synthetic Conditions
7.1. Temperature and Timespan
7.2. PAN to Sulfur Ratio
7.3. Powder Processing
7.4. Solvent
7.5. Vapor Pressure
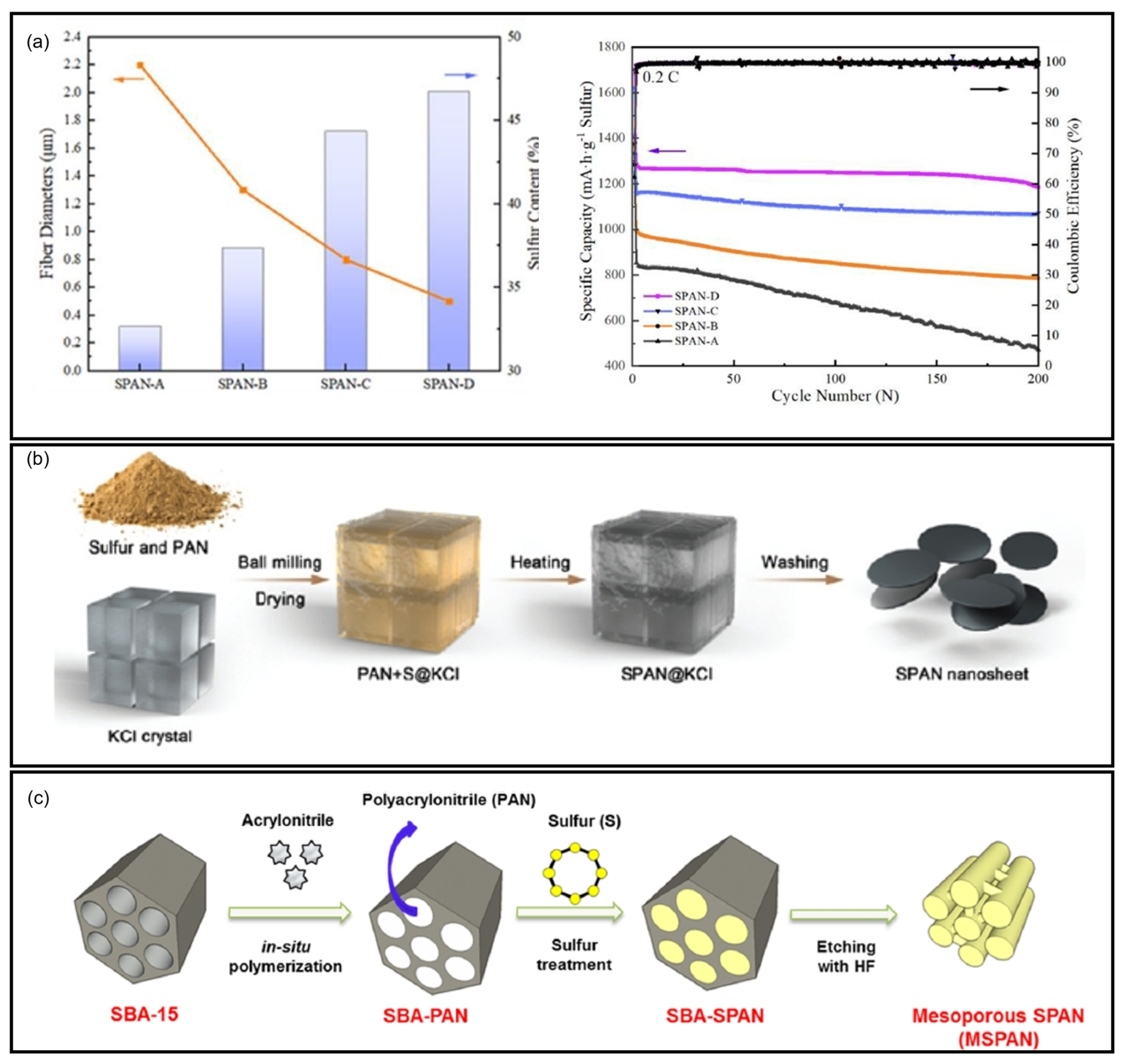
7.6. PAN Precursors
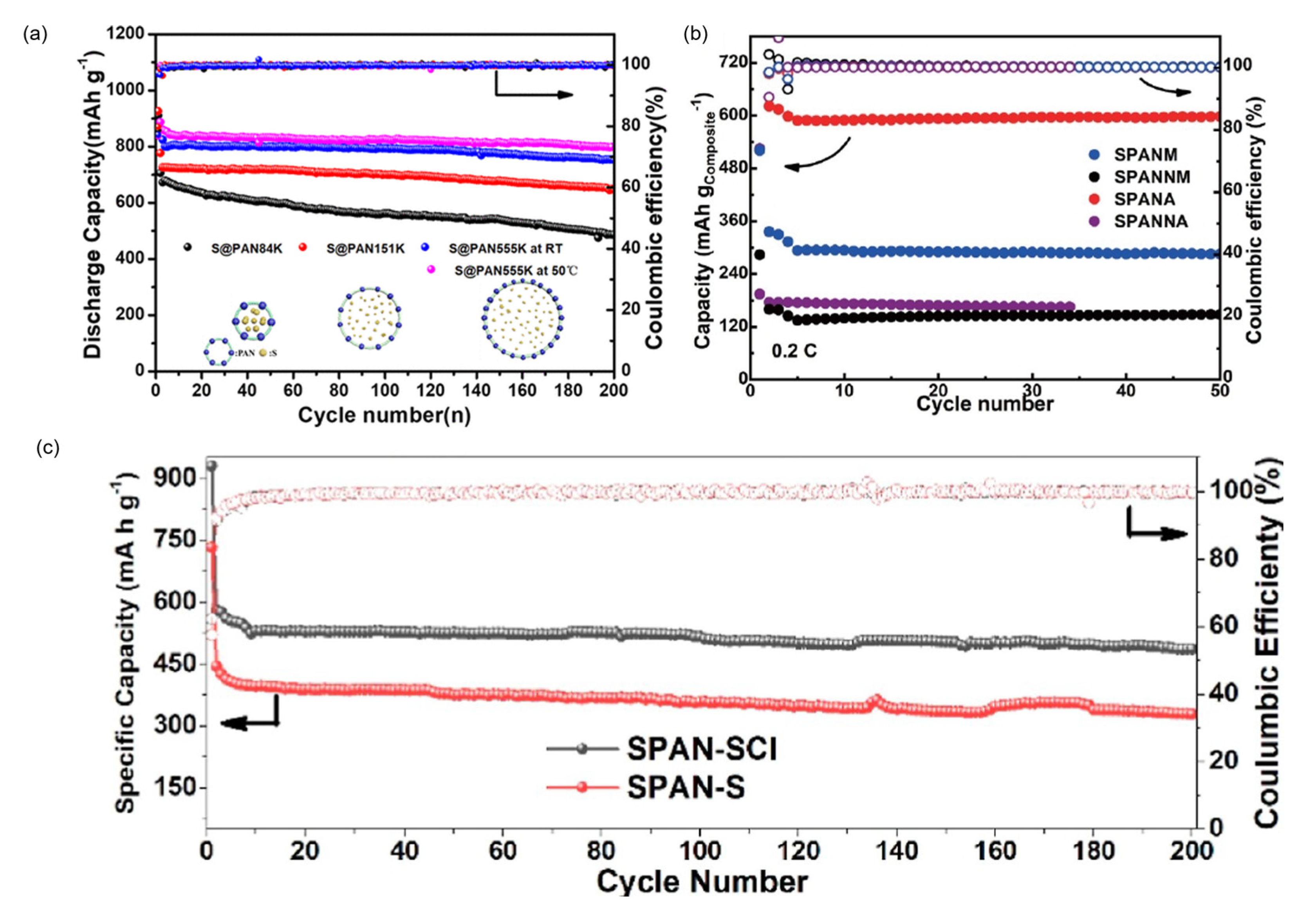
7.7. Vulcanization Agents
8. Cathode Modification
8.1. Conductive Carbon Additives
8.1.1. Carbon Nanotubes
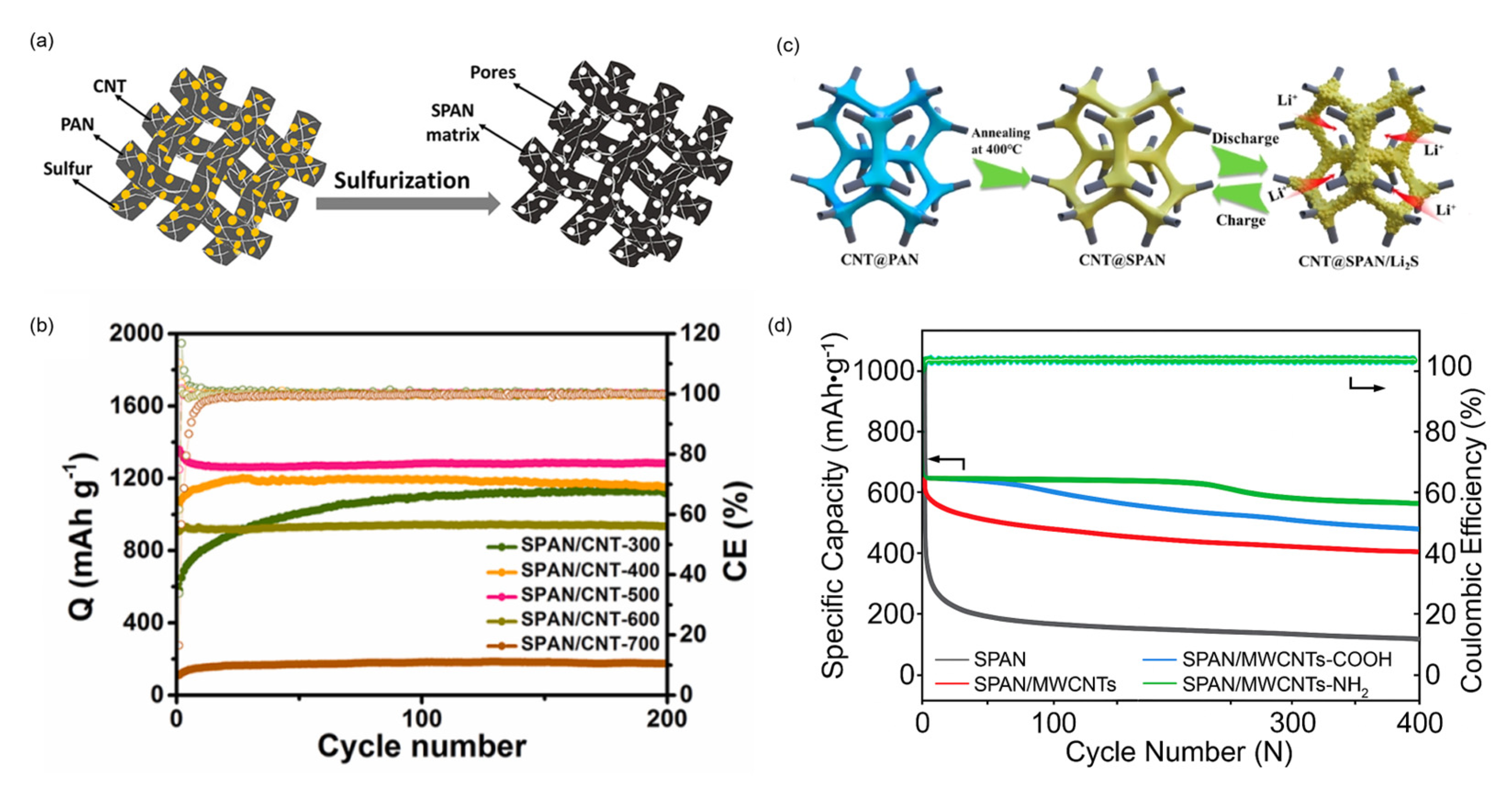
8.1.2. Graphene

8.1.3. Porous Carbon
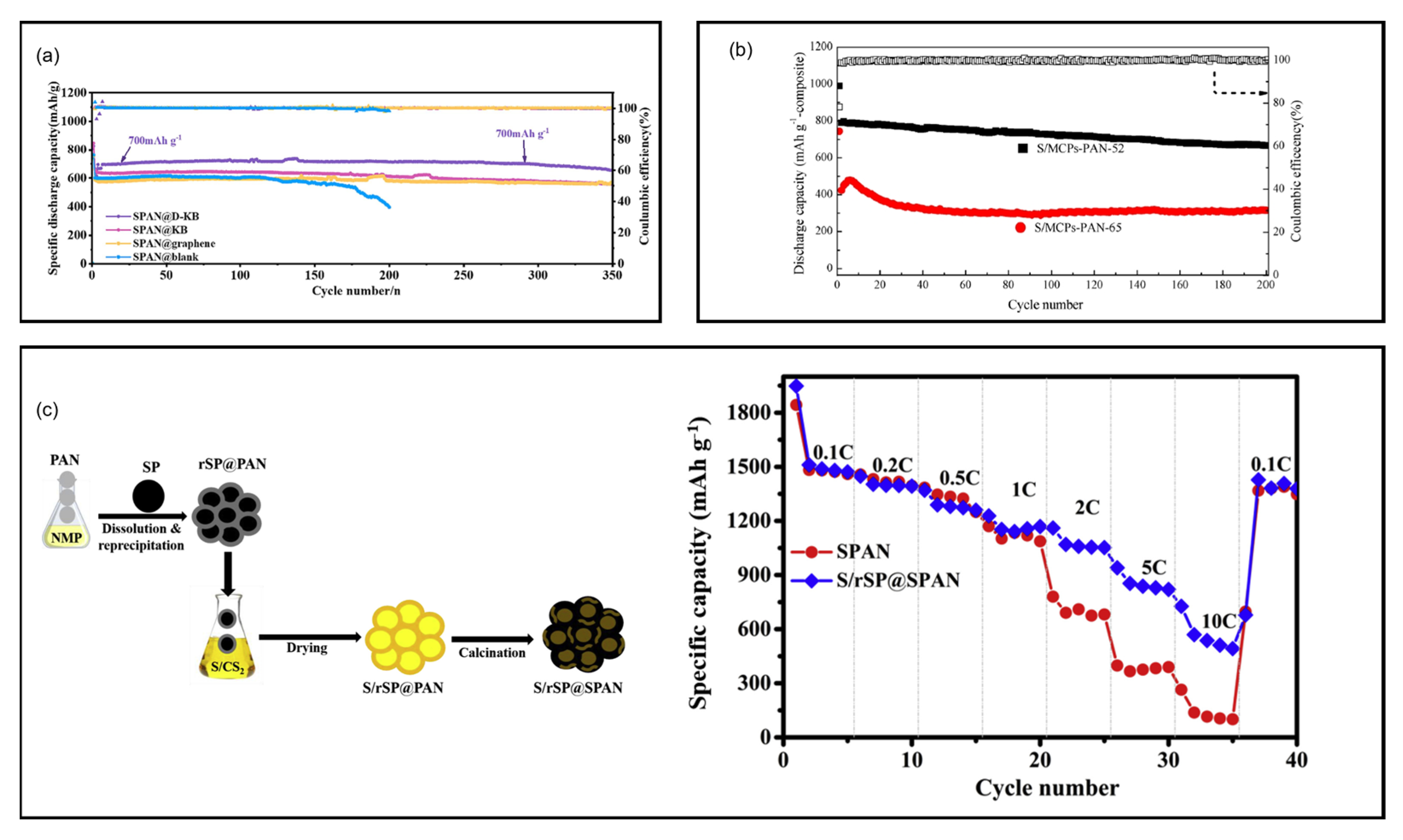
8.1.4. Graphite
8.1.5. Carbon Fibers

8.1.6. Dense Carbon
8.2. Vulcanization Accelerators and Vulcanization Agents
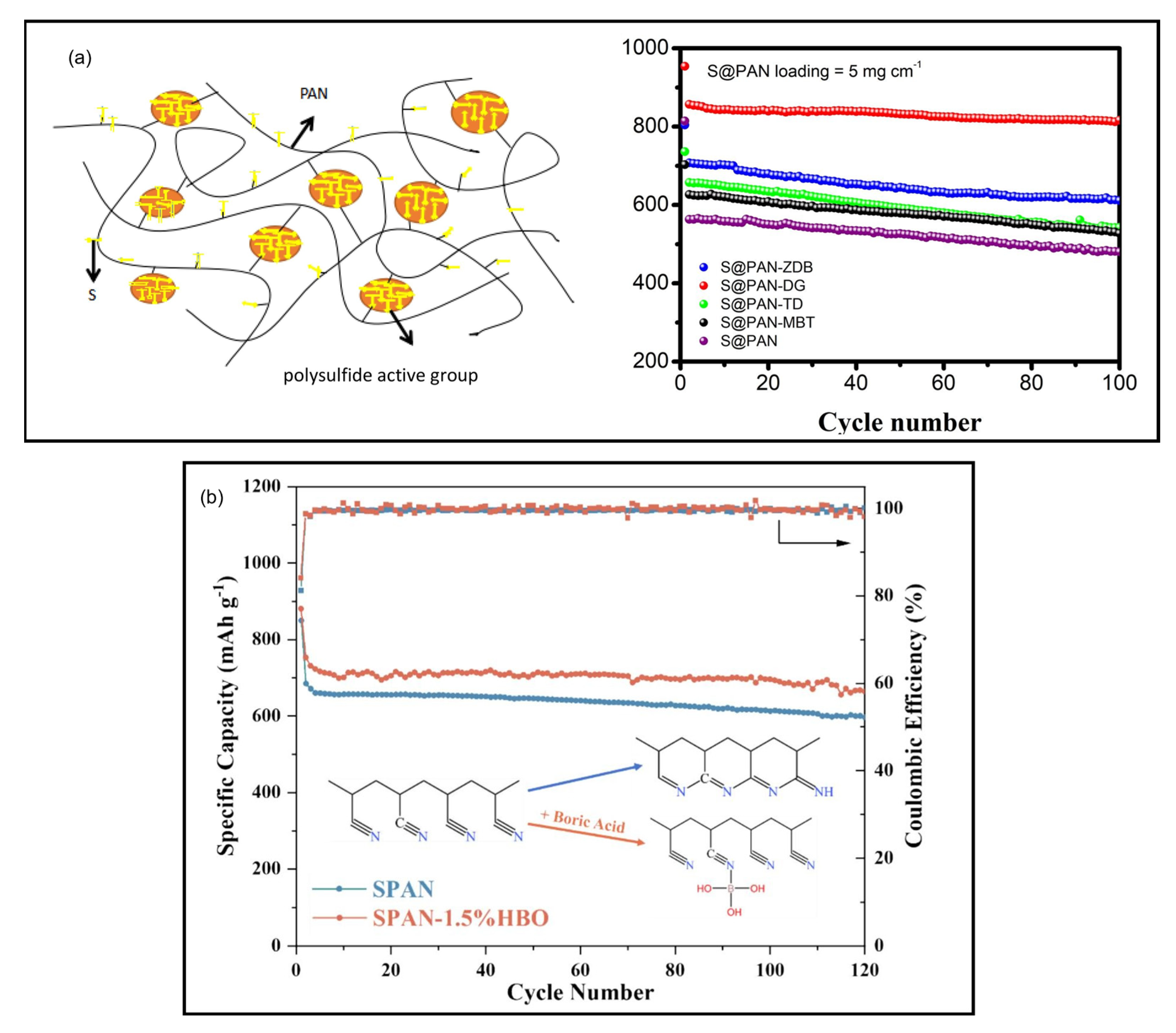
8.3. Morphology and Structure Engineering

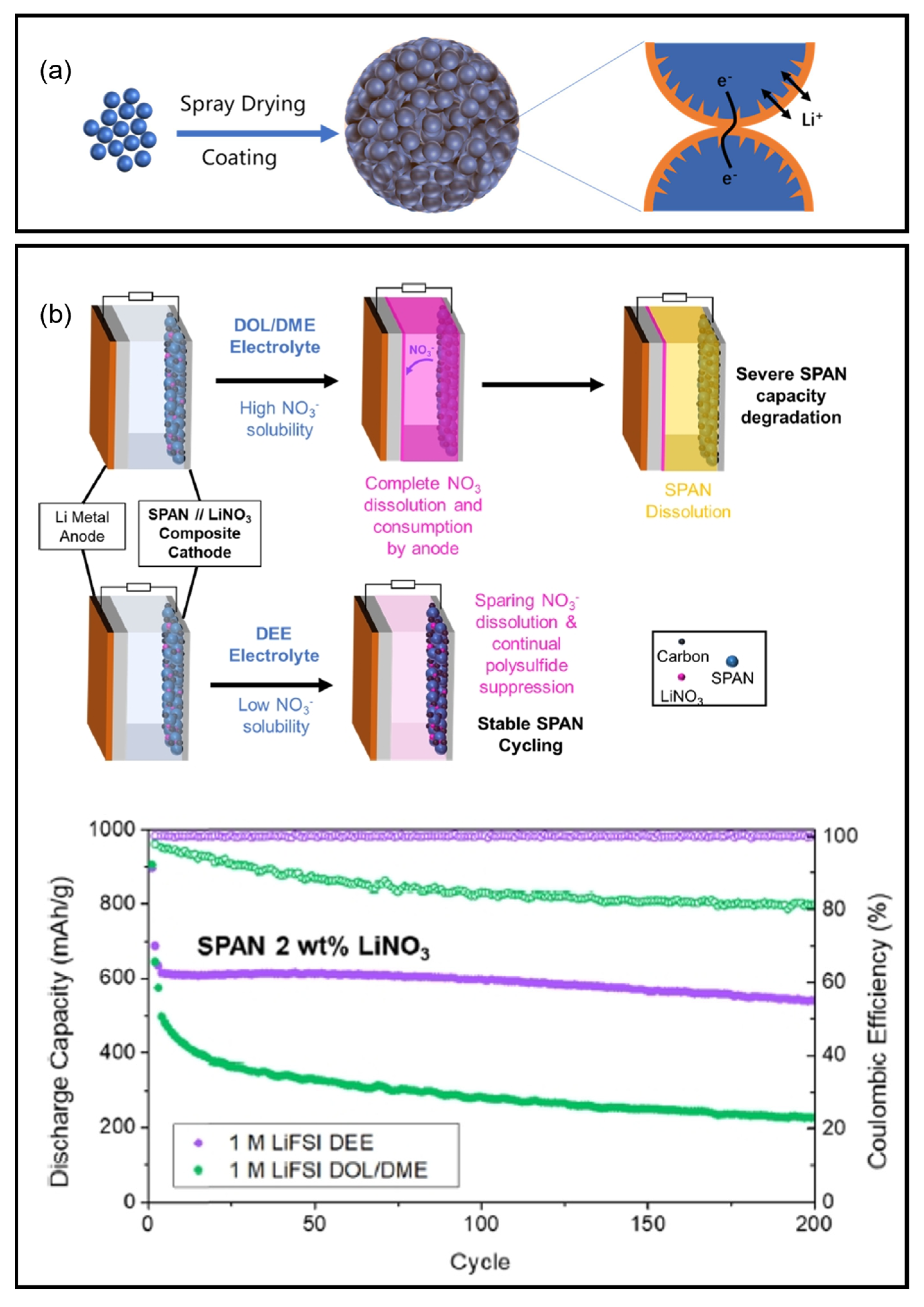
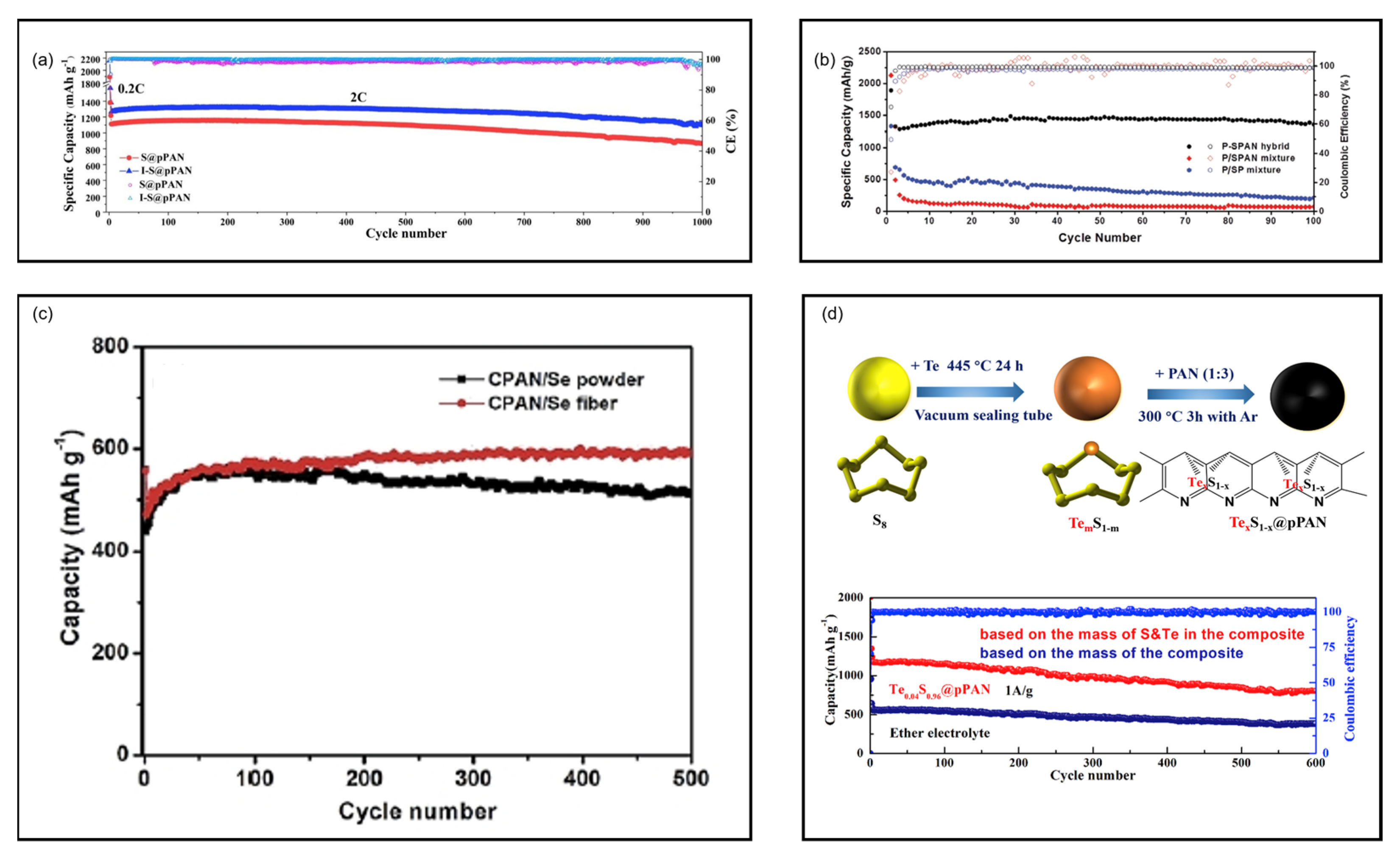
8.4. Redox Accelerators

8.5. Heteroatoms Doping
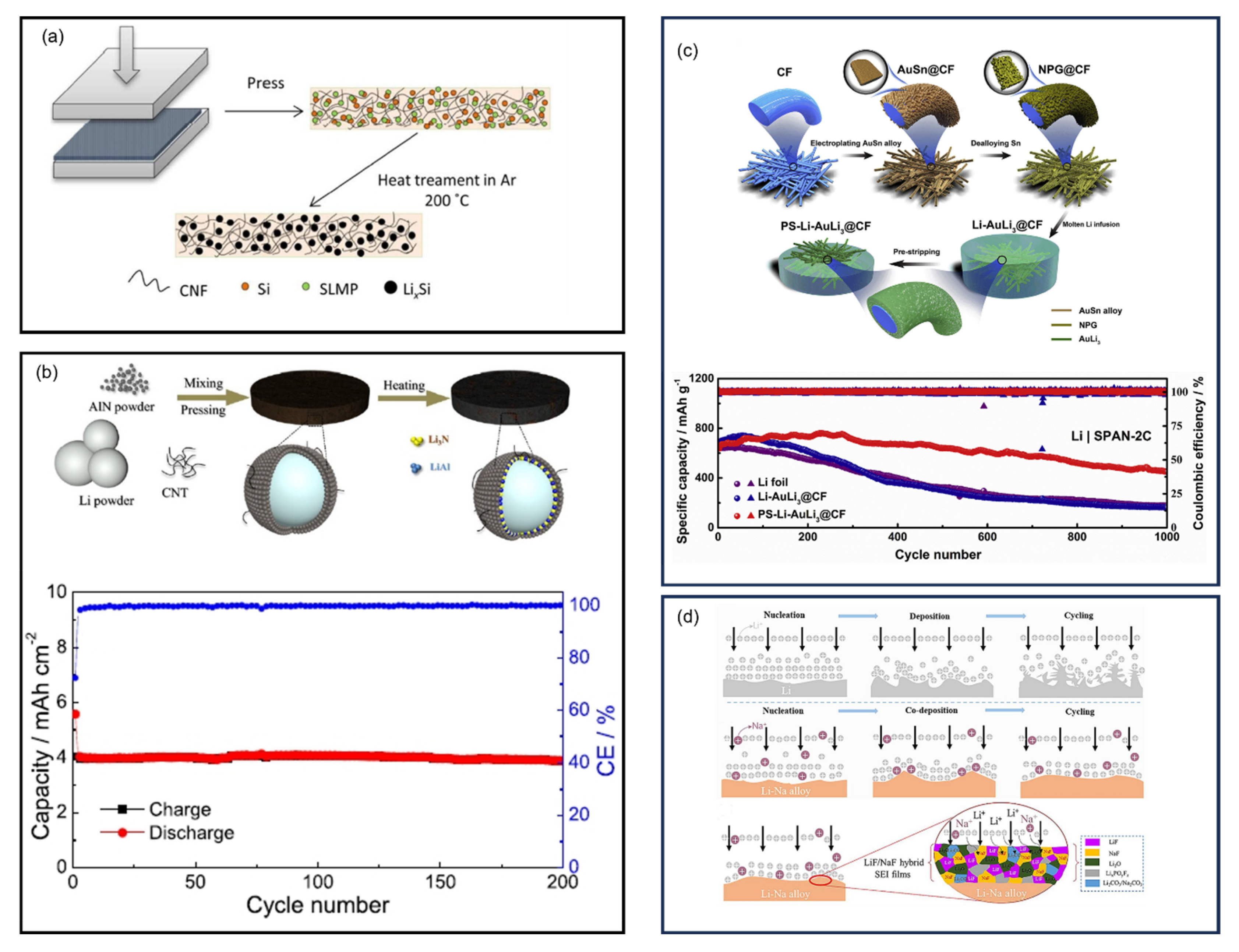
8.6. Pre-Lithiation of SPAN
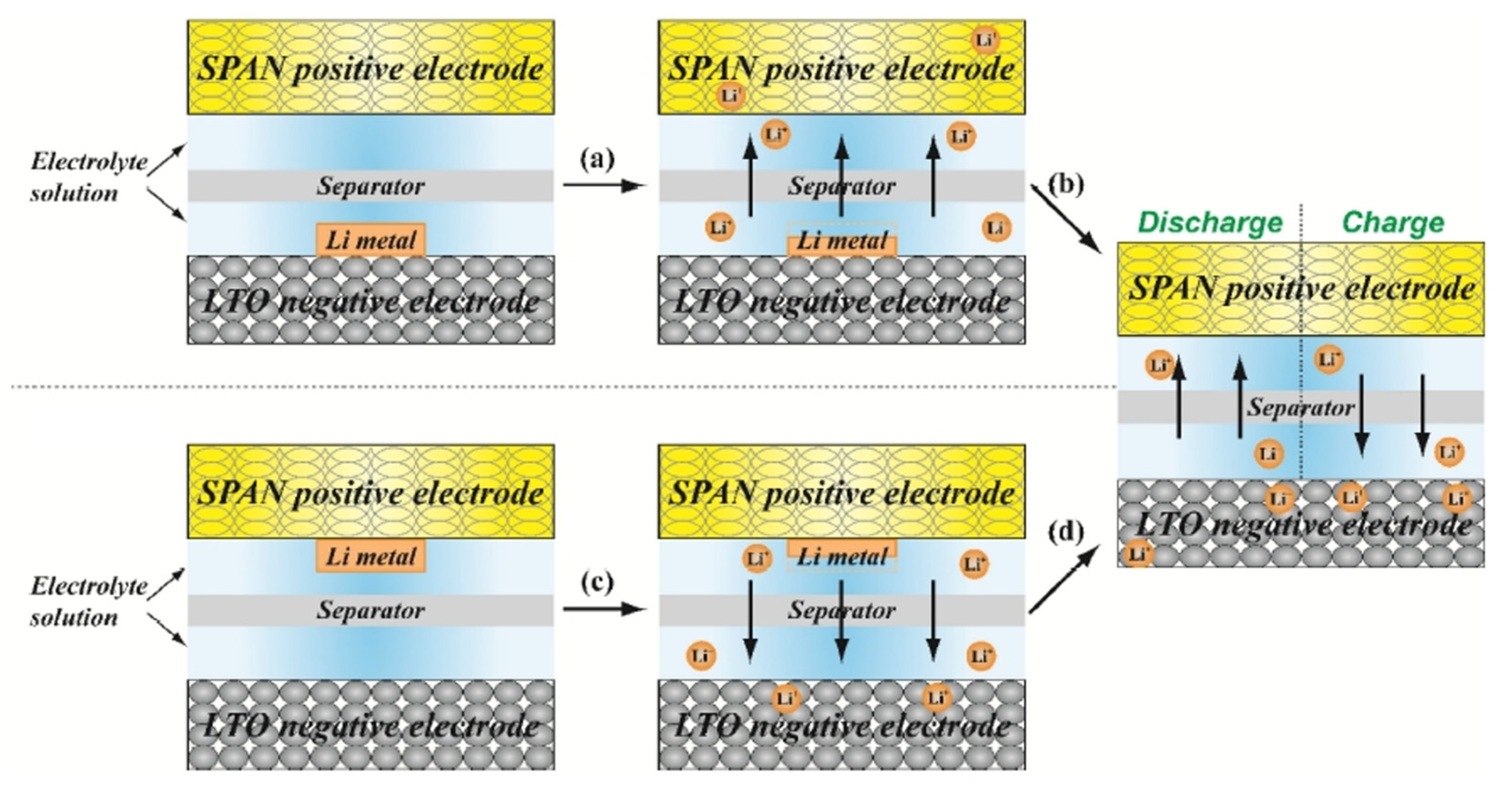
8.6.1. Half-Cell Electrochemical Method

8.6.2. Short-Circuit Electrochemical Method
8.6.3. Chemical Method

8.6.4. Li-Containing Additives
9. Electrolytes
9.1. Carbonate-Based Electrolytes
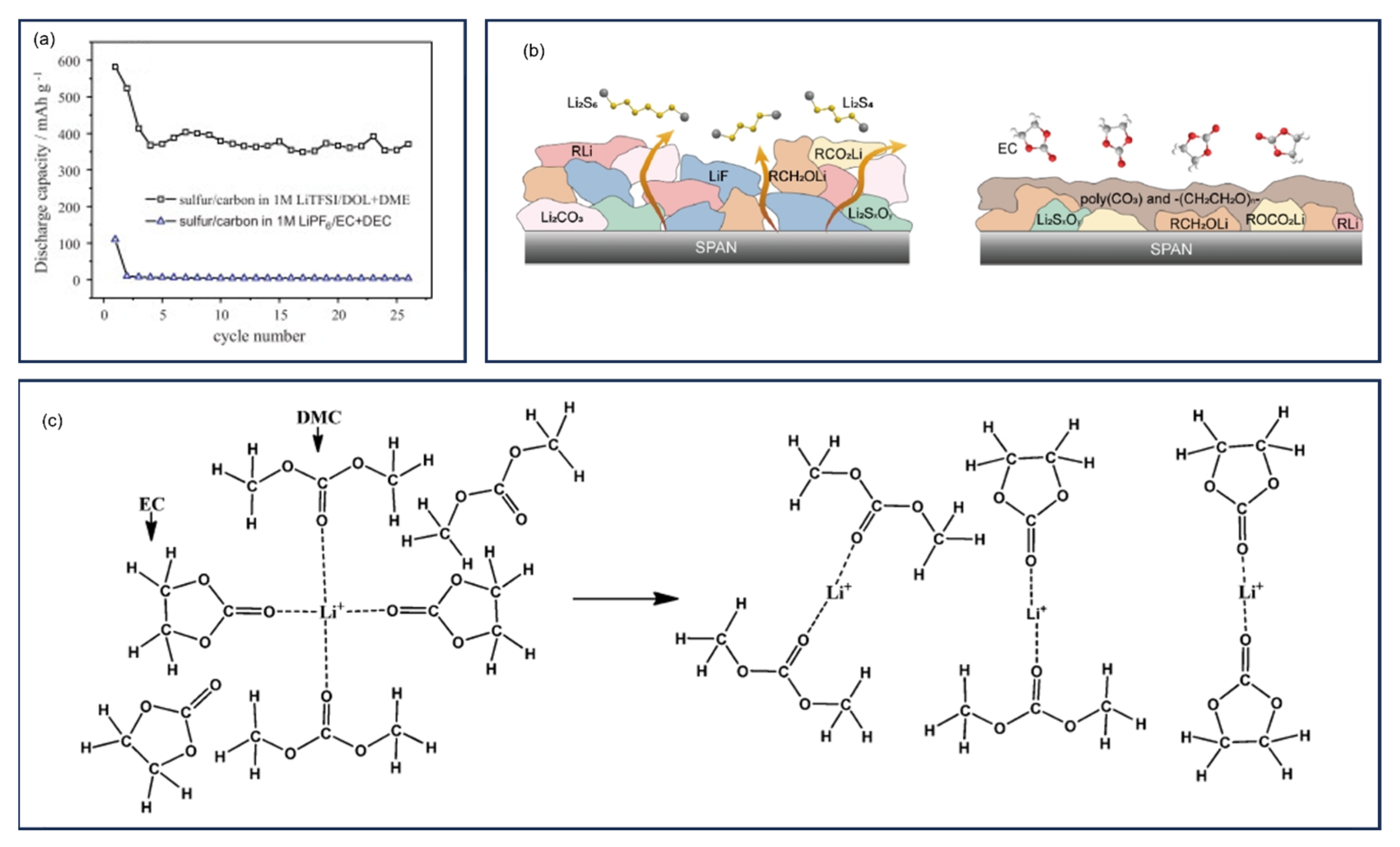
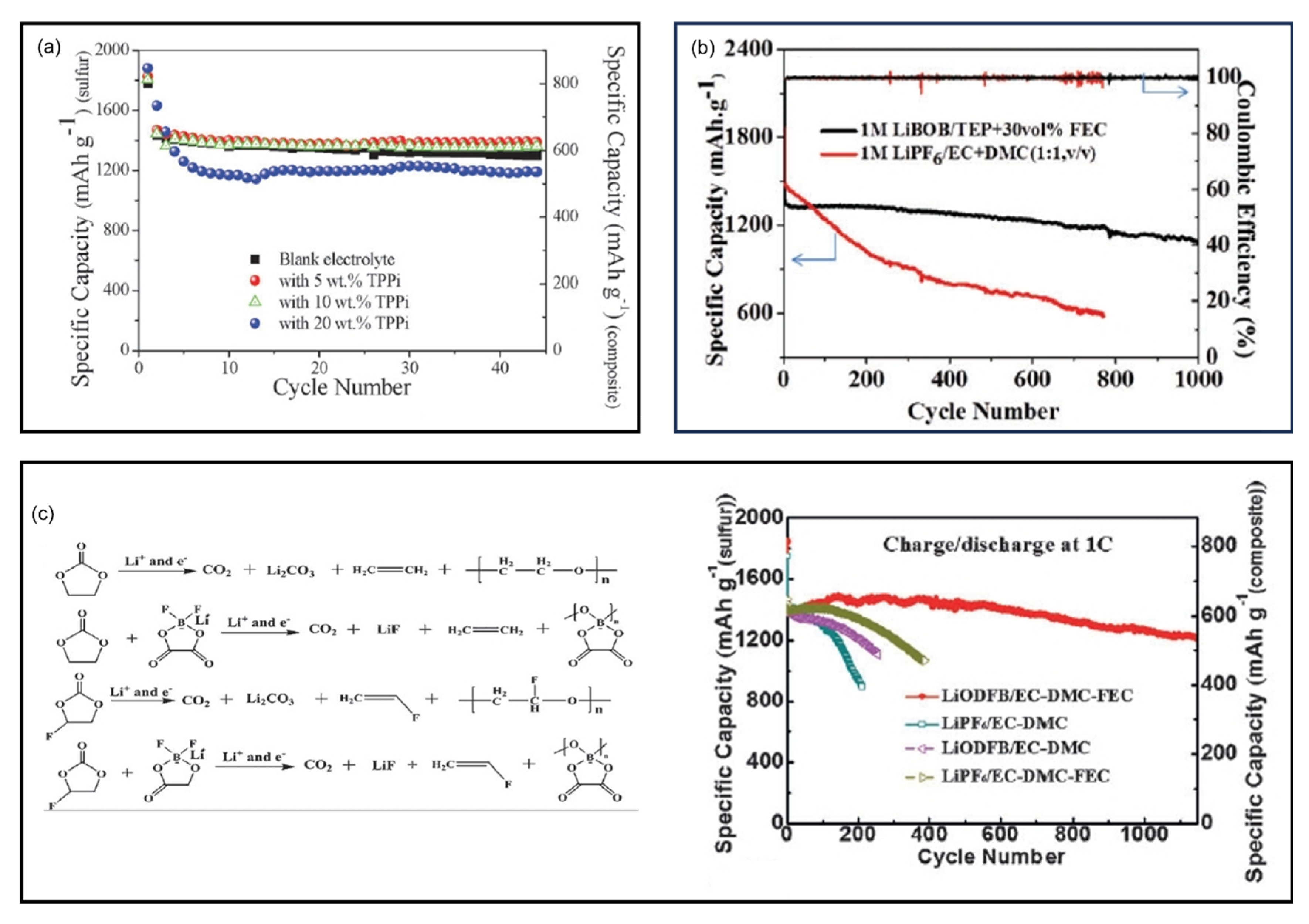
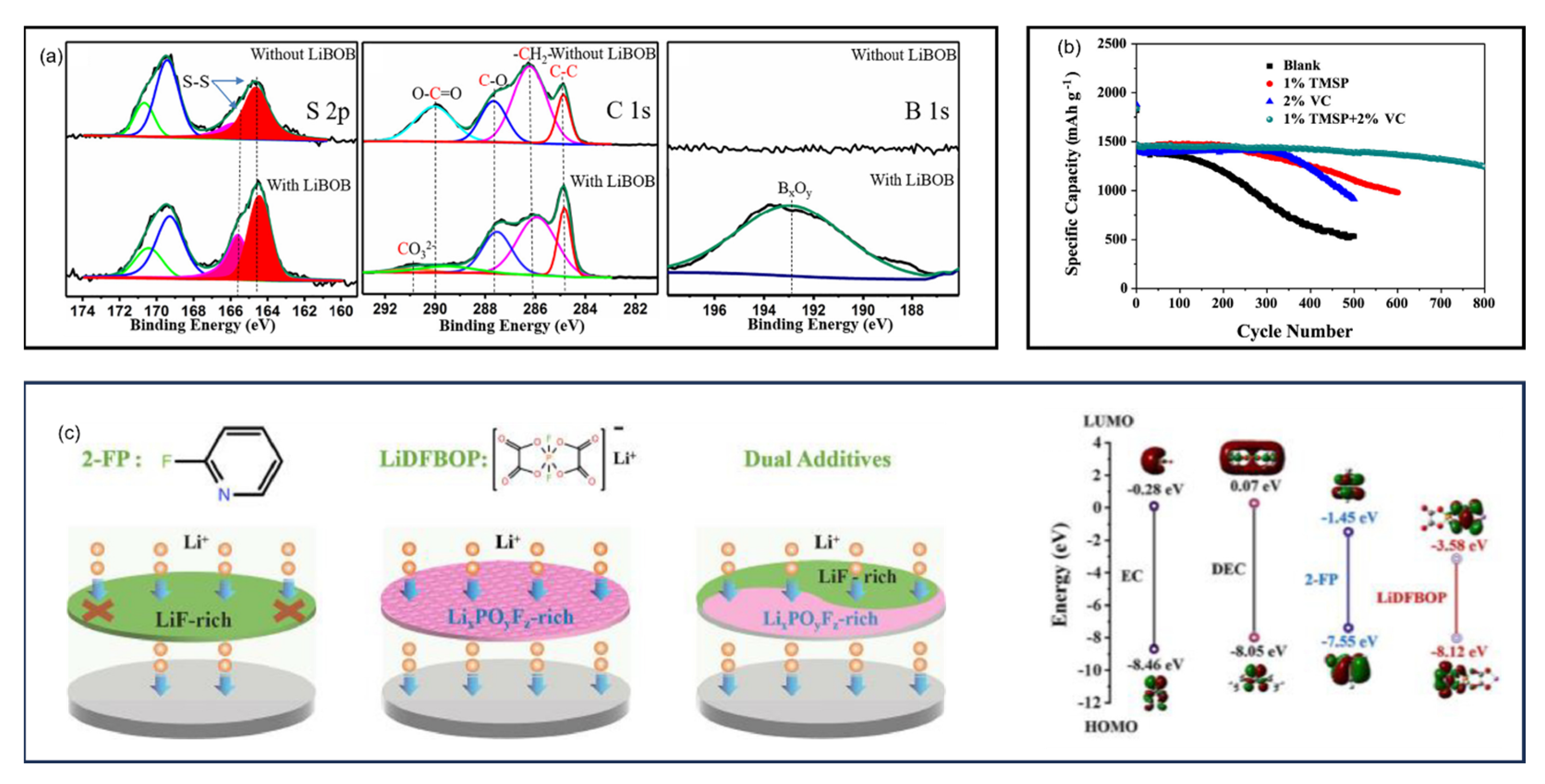
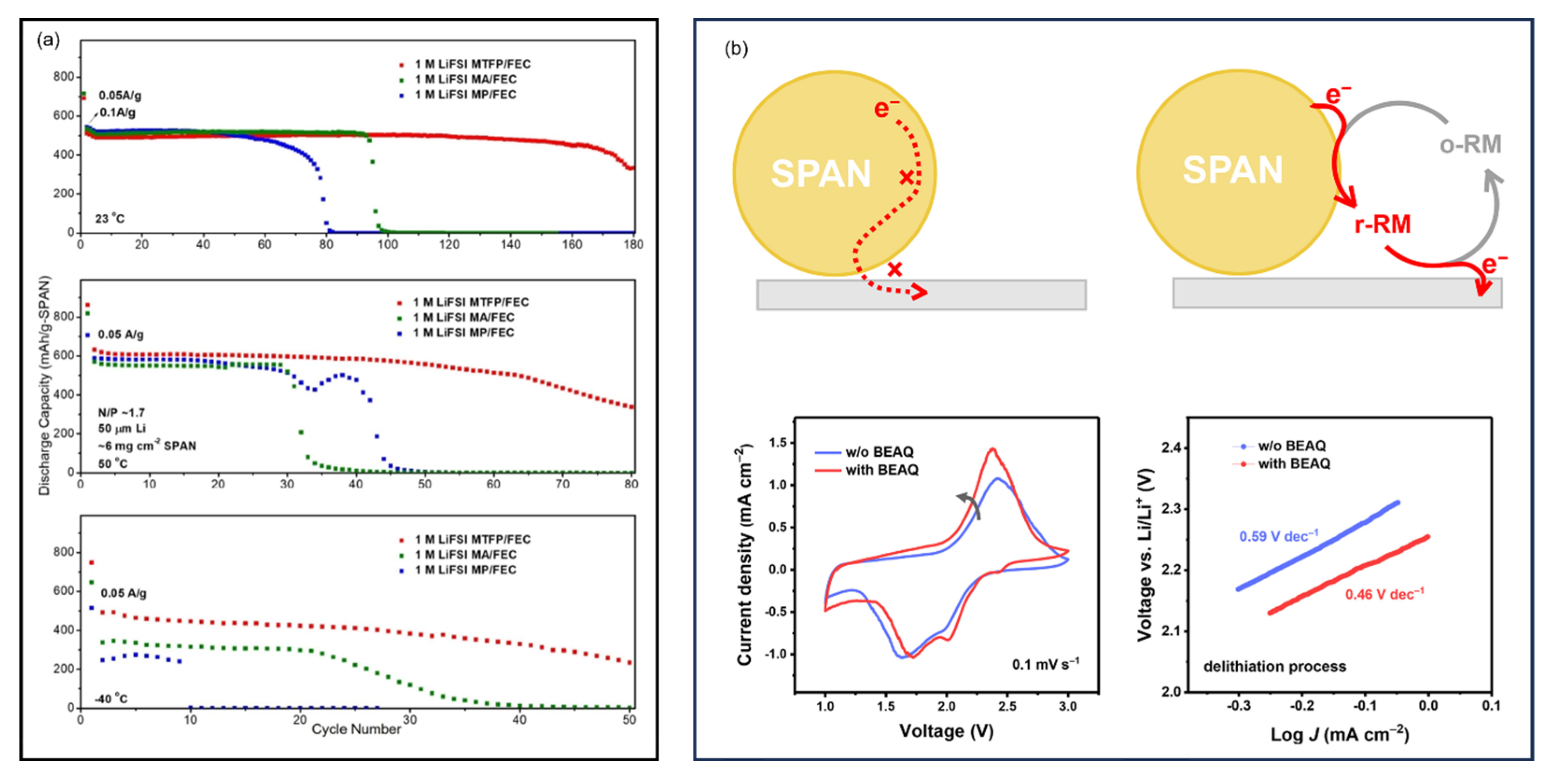
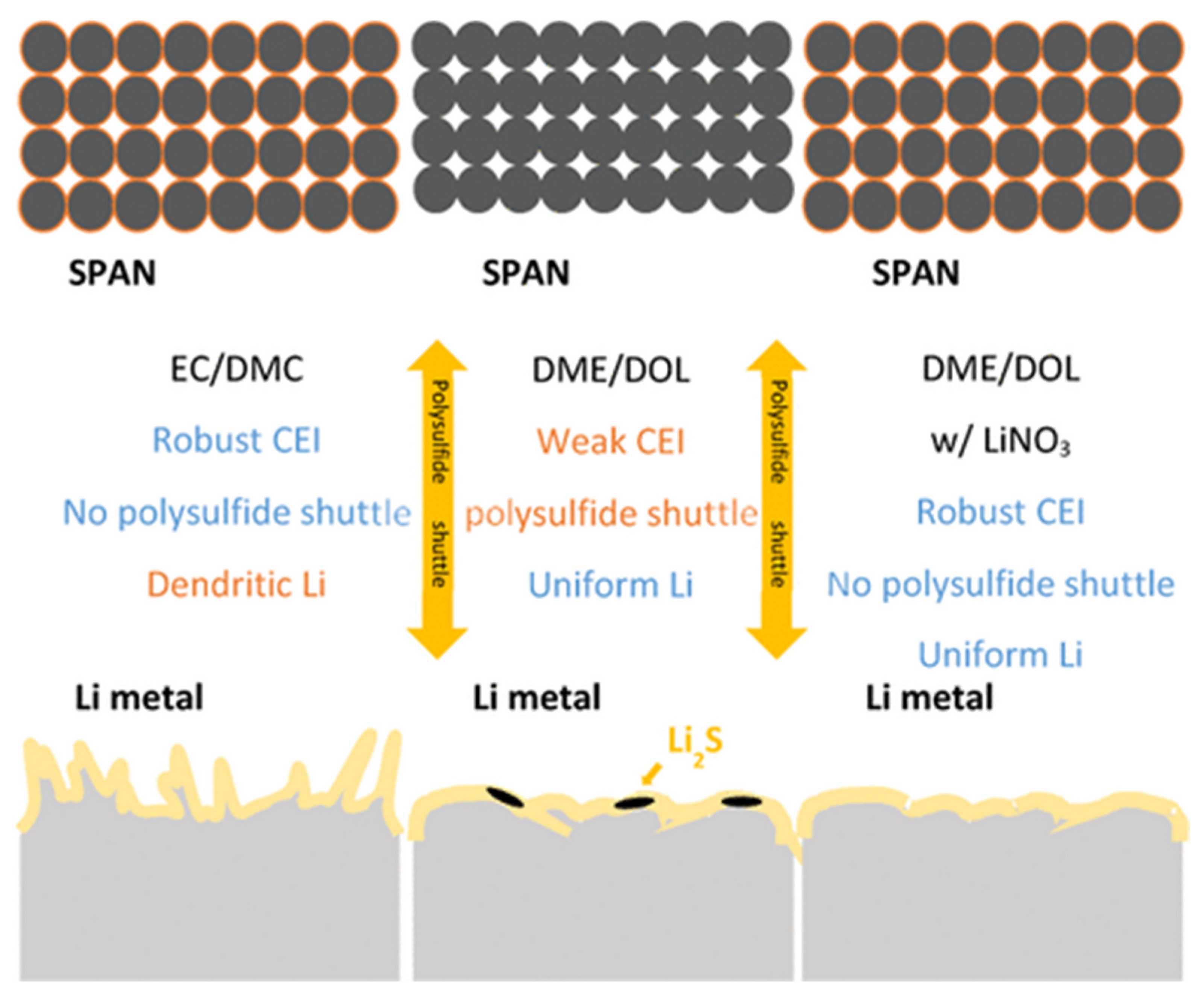
9.2. Dilute Ether-Based Electrolytes
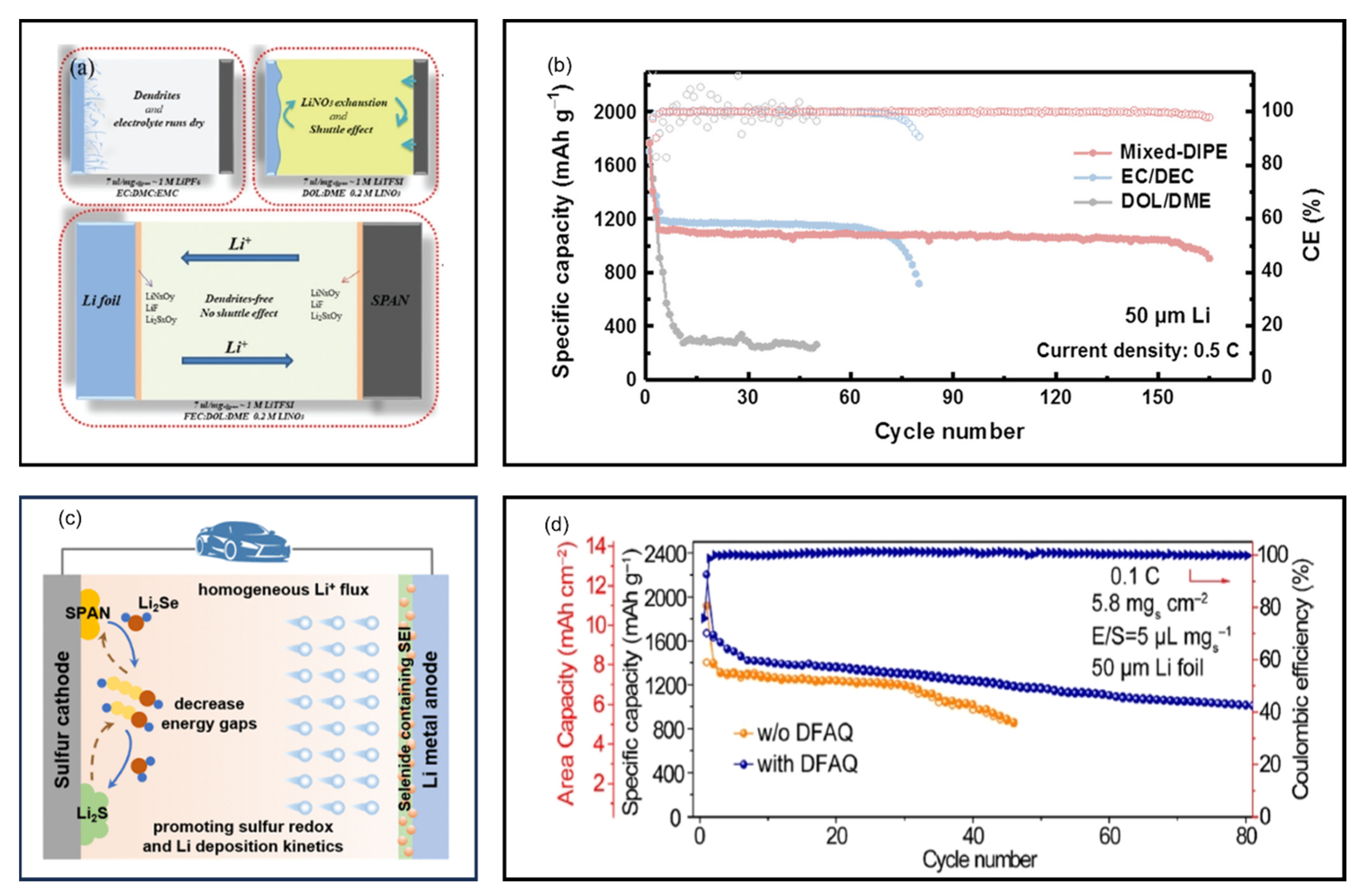
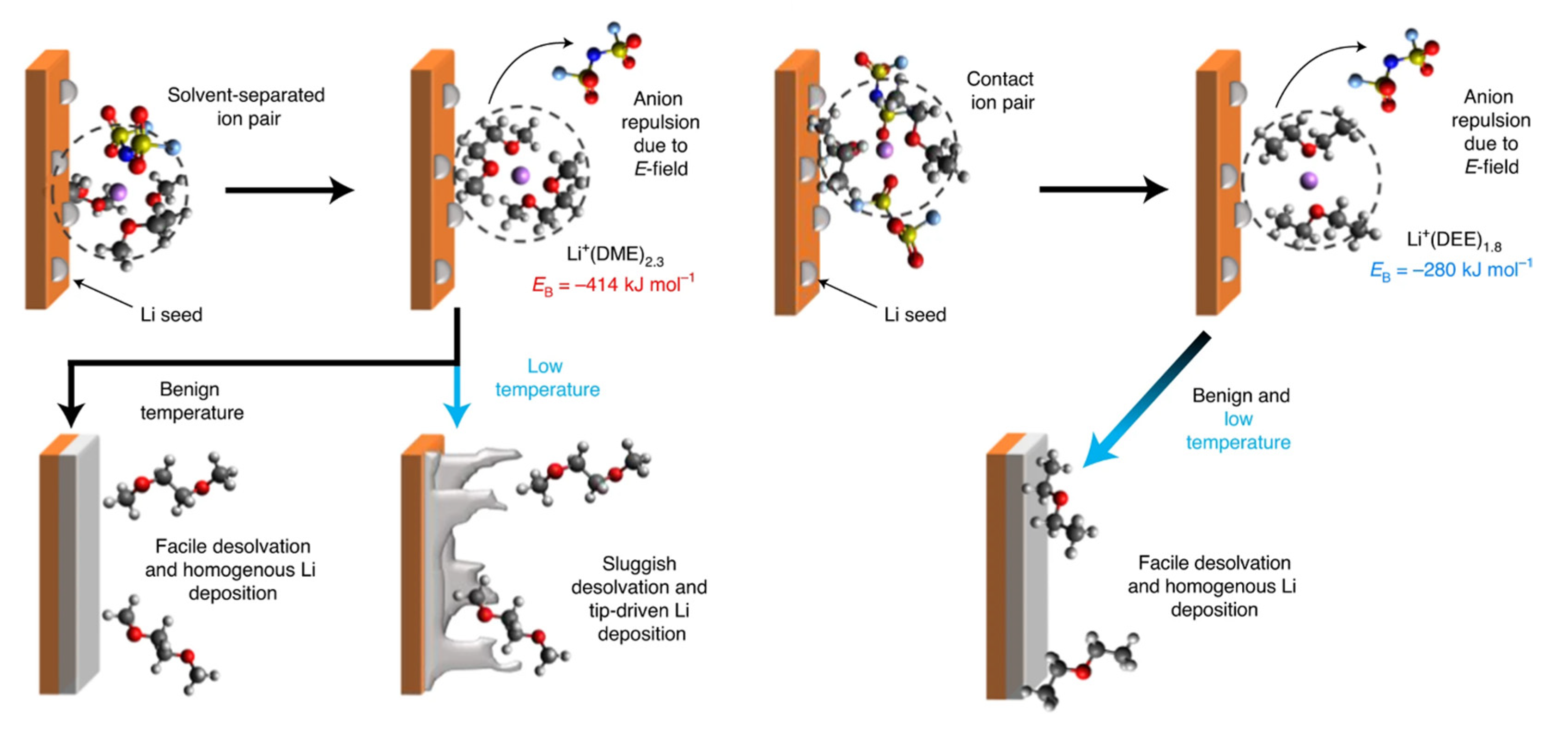
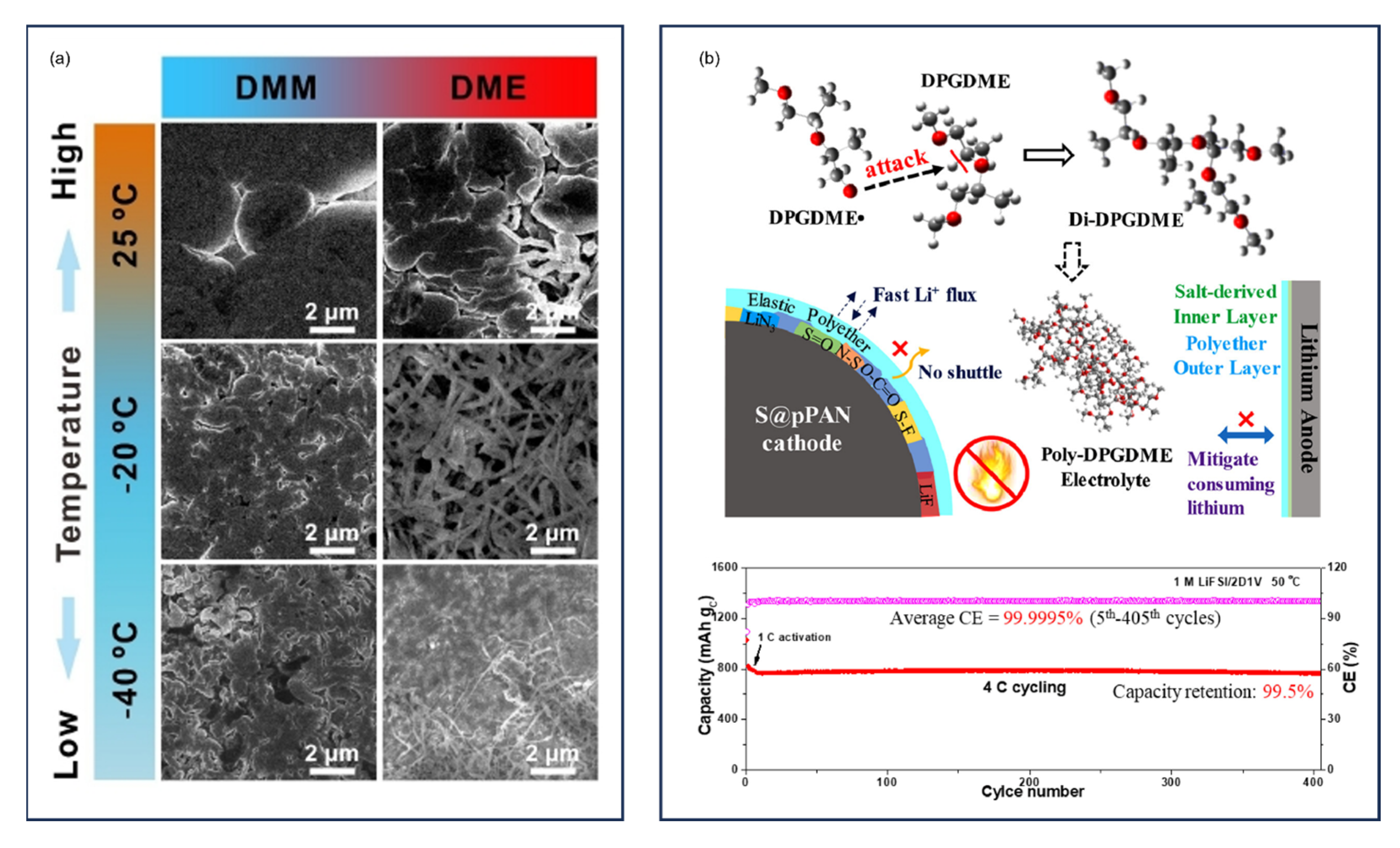
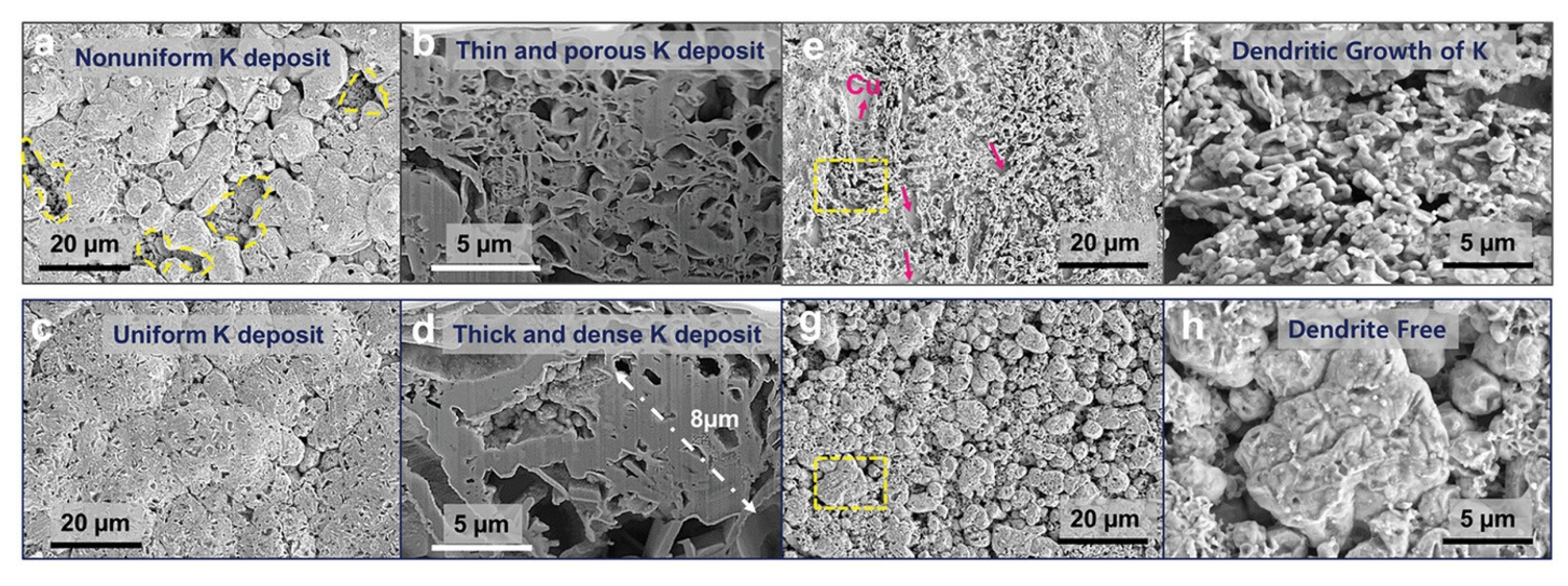
9.3. High-Concentration Electrolytes and Localized High-Concentration Electrolytes
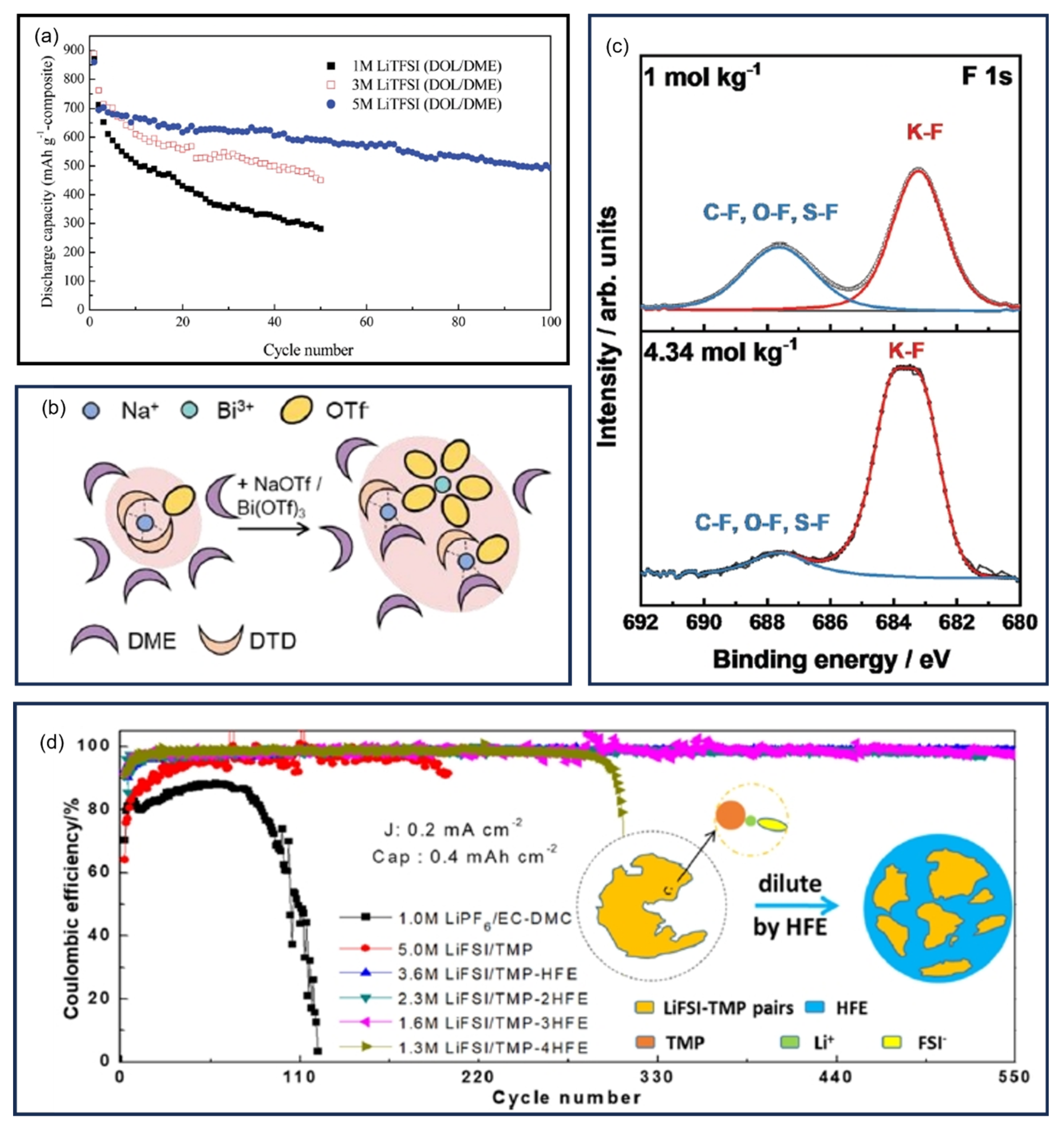

9.4. Other Liquid Electrolytes

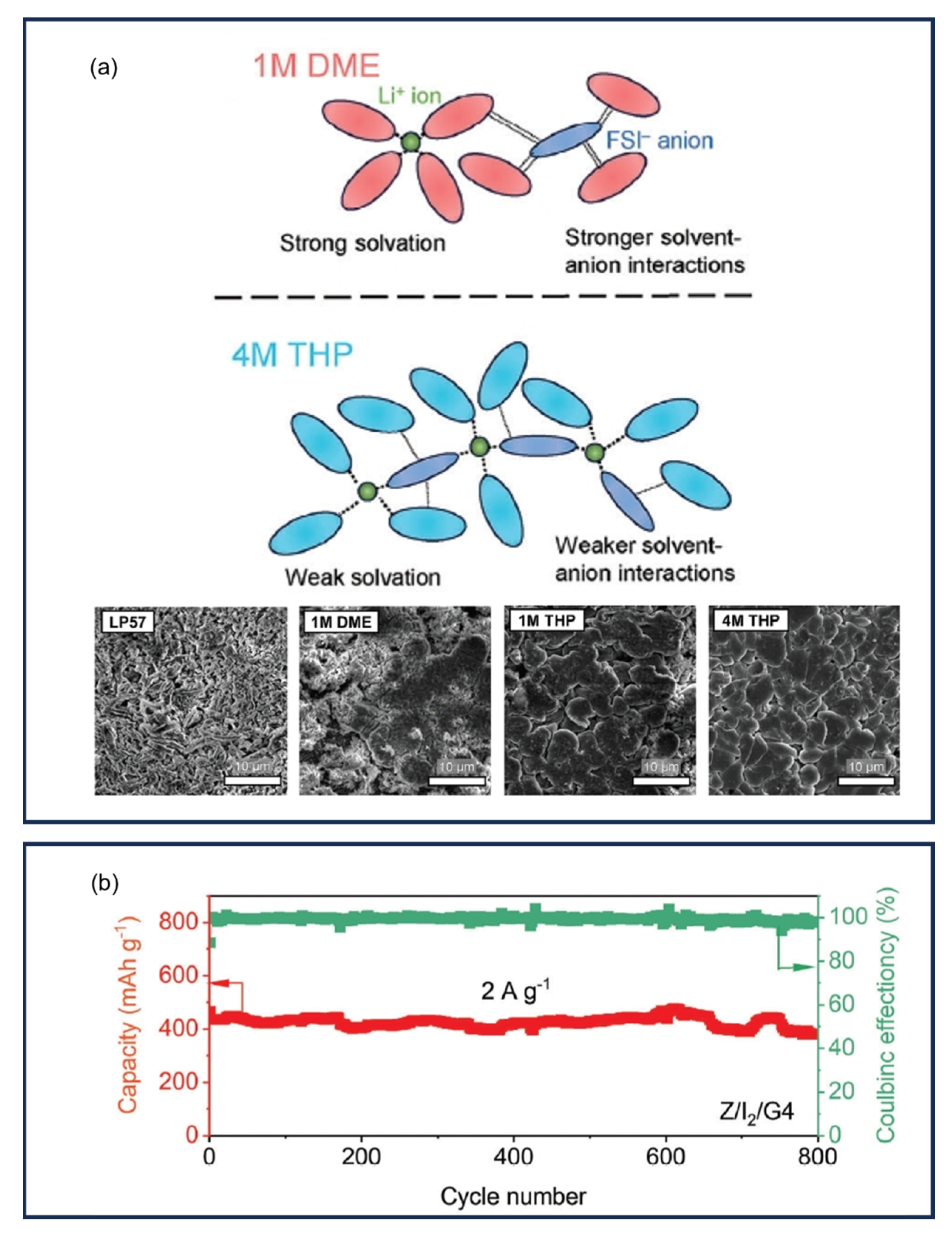
9.5. Gel Polymer Electrolytes

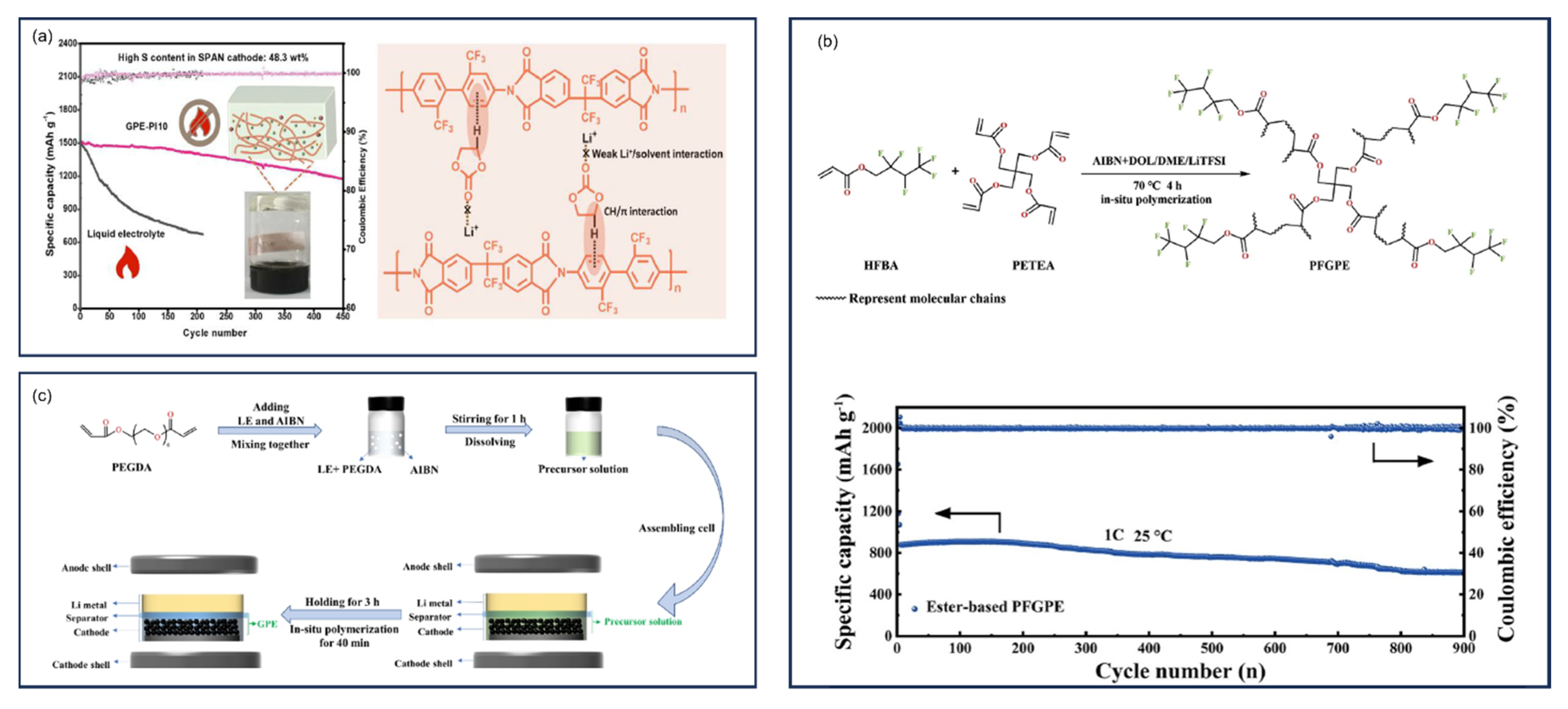
9.6. Solid-State Electrolytes
9.6.1. Solid-State Polymer Electrolytes
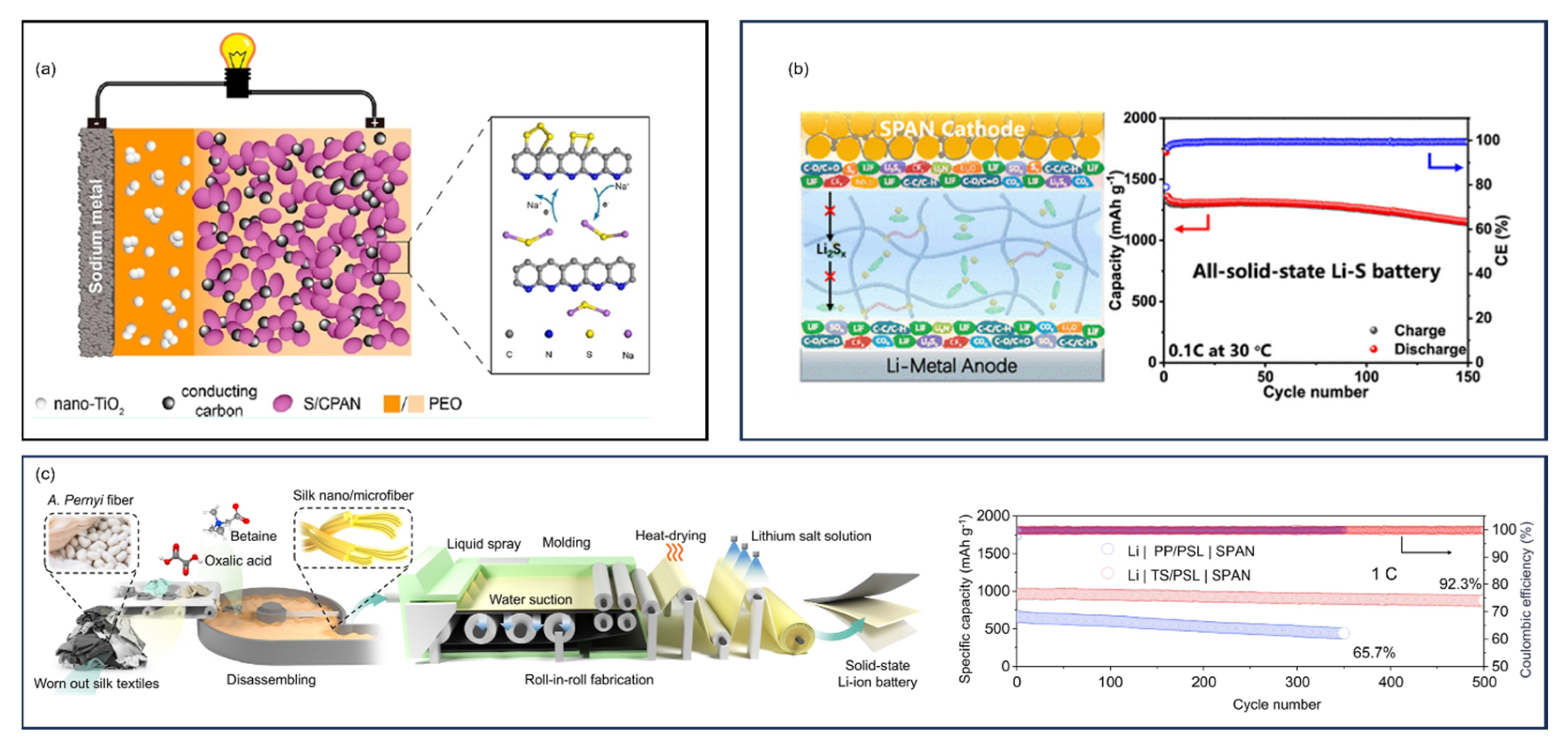

9.6.2. Solid-State Ceramic Electrolytes
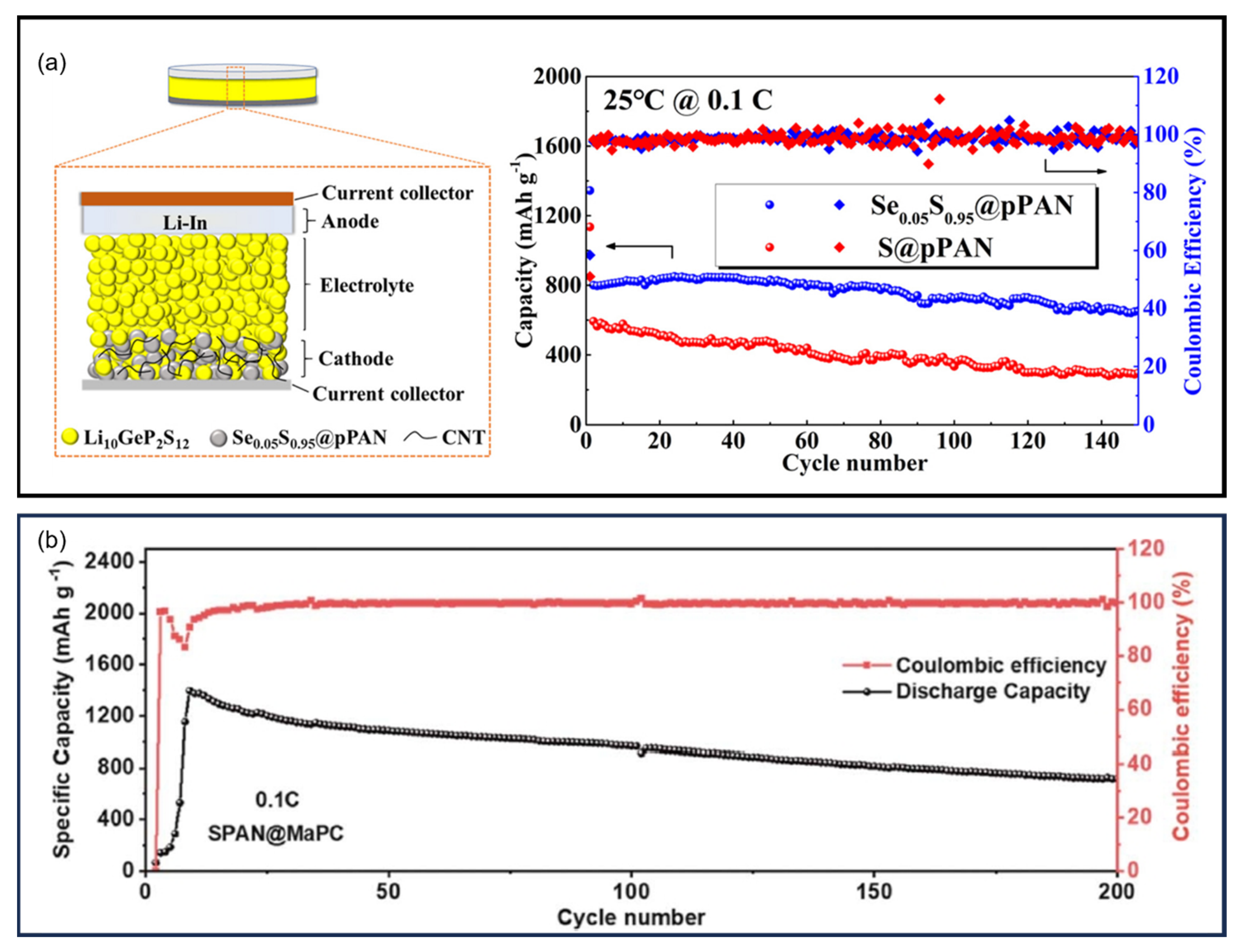

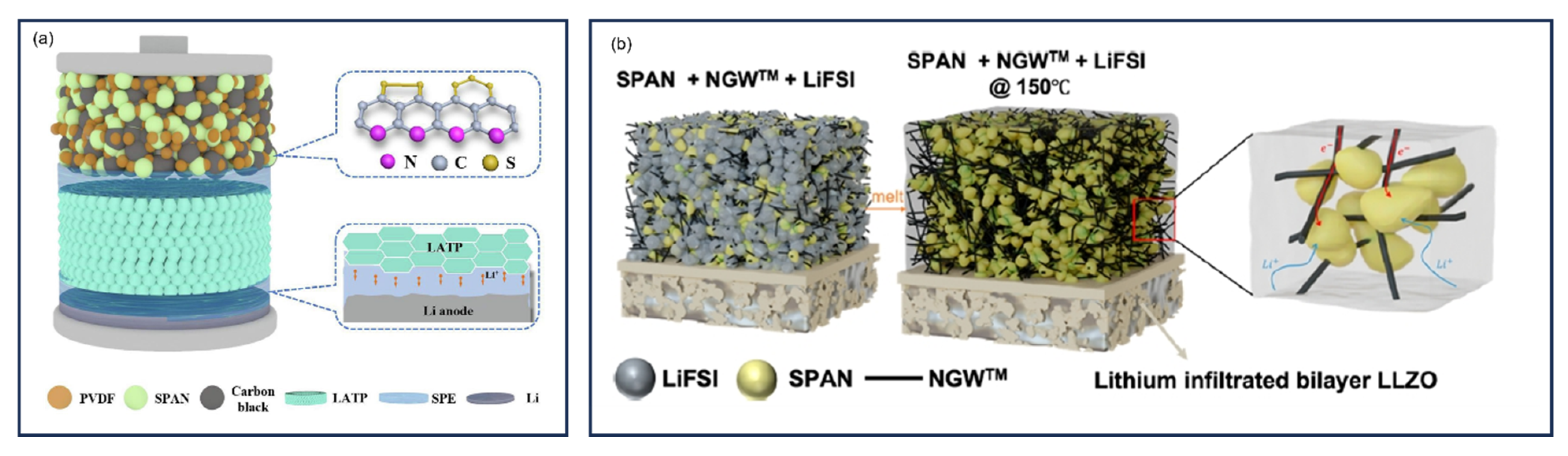
9.6.3. Solid-State Composite Electrolytes and Hybrid Electrolytes
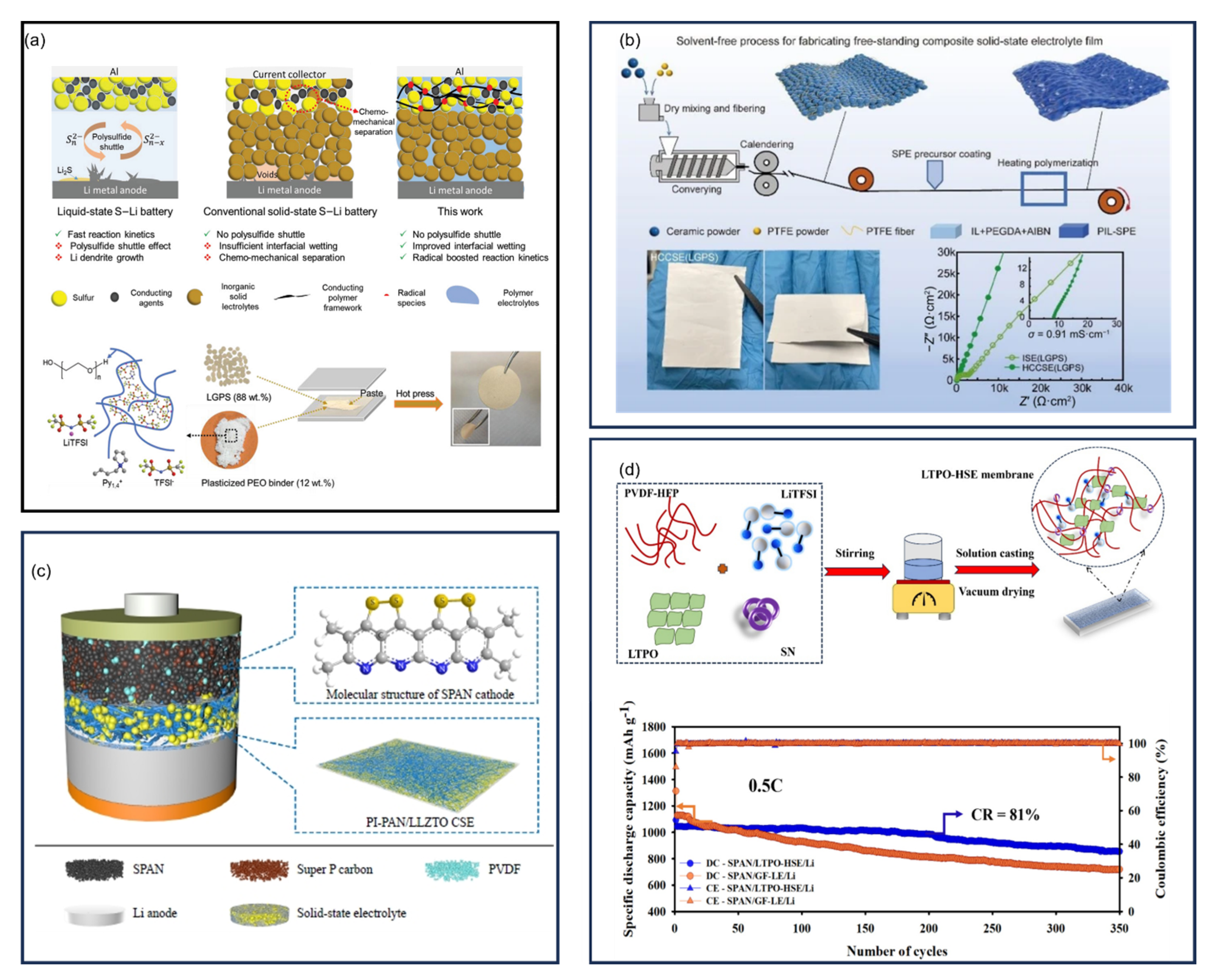
10. Binders


11. Current Collectors

12. Separators
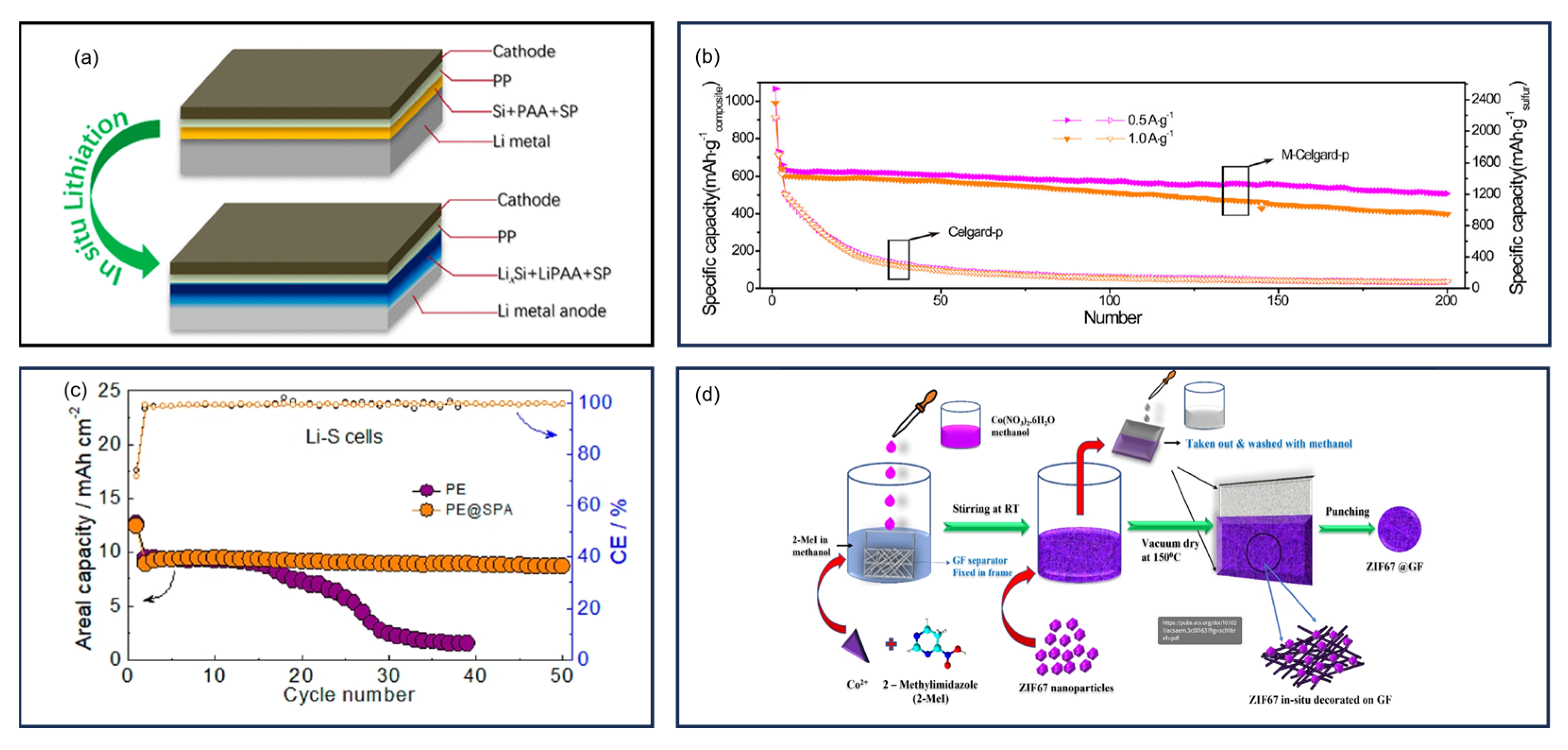
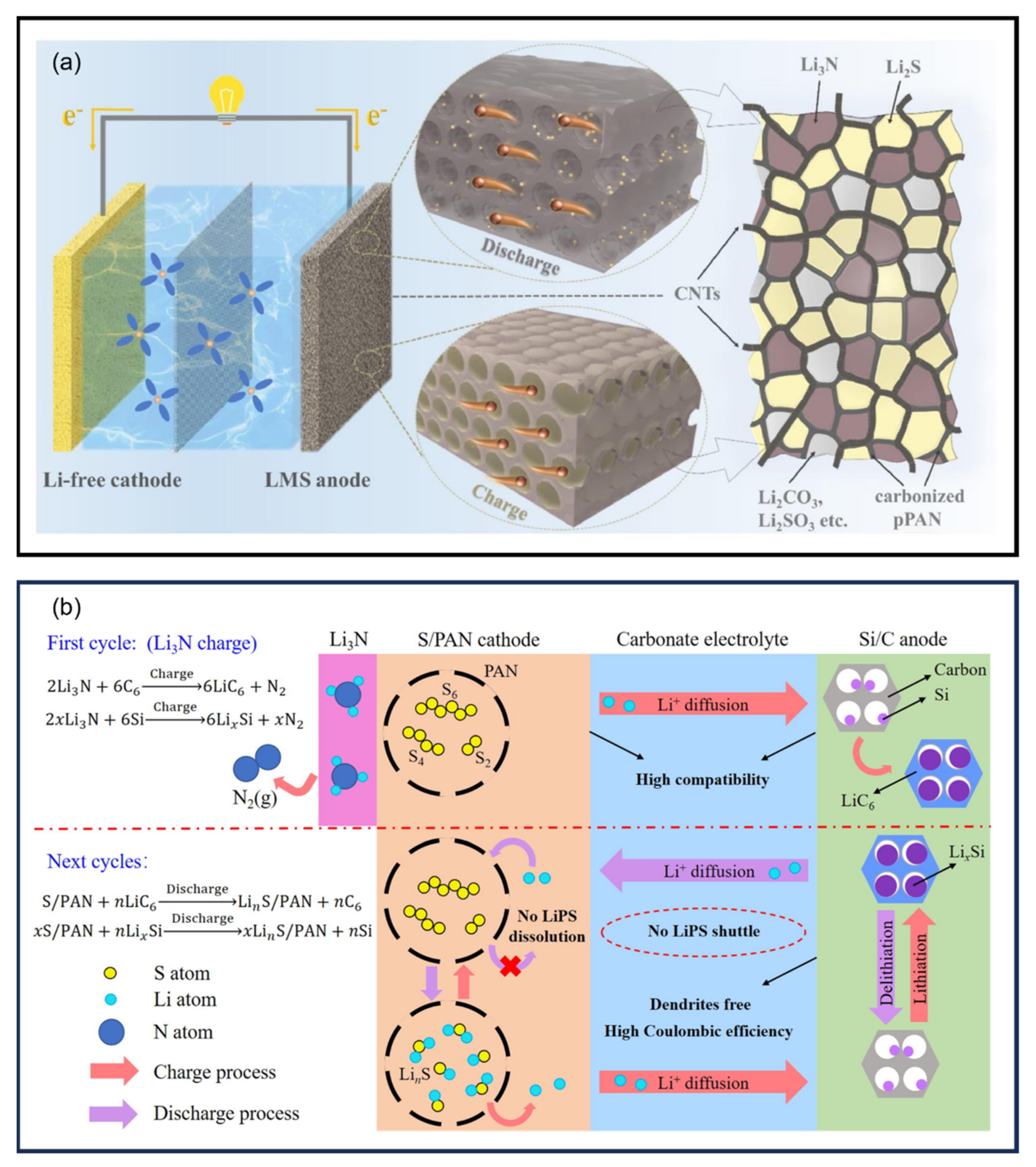
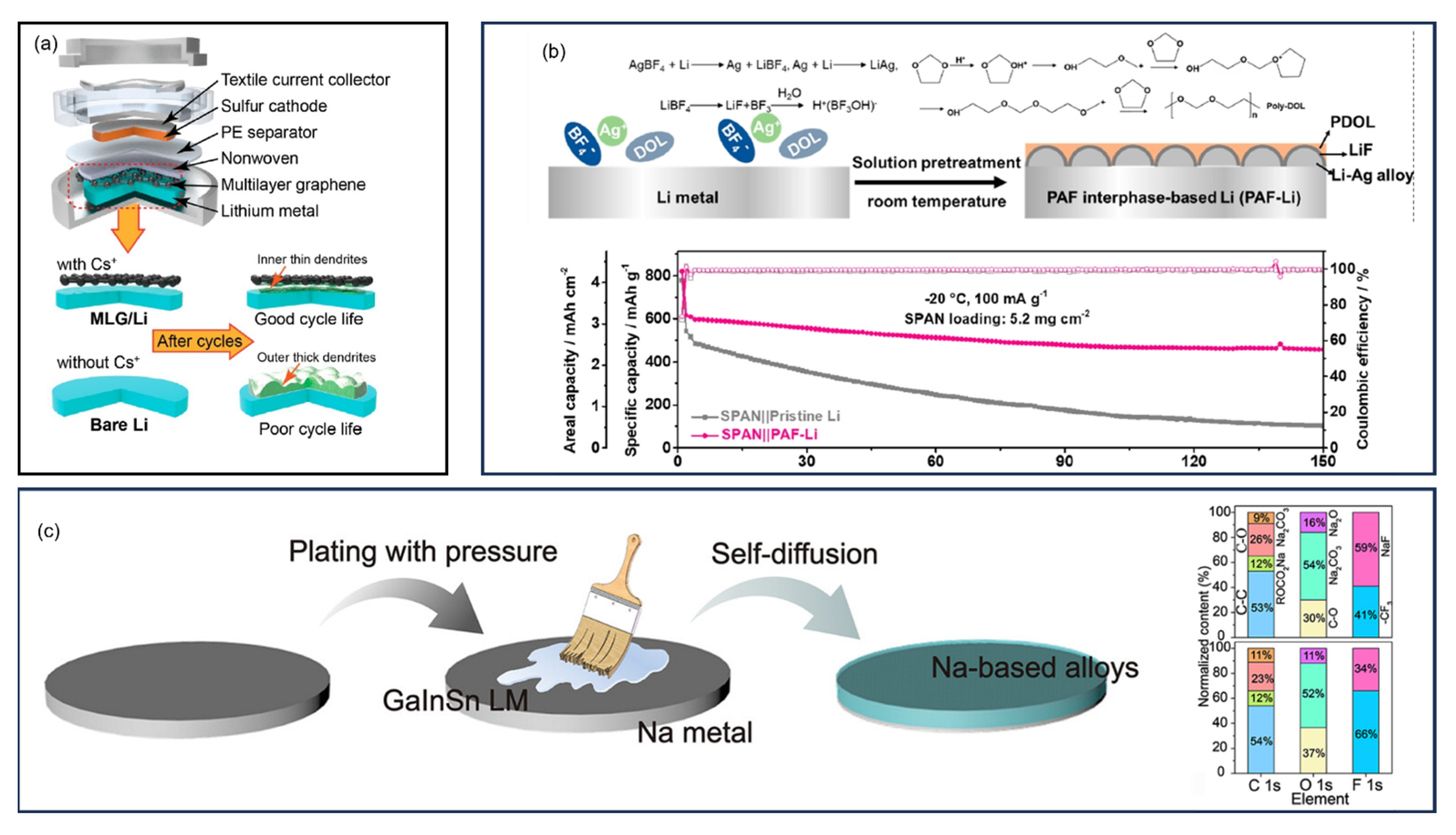
13. Anodes

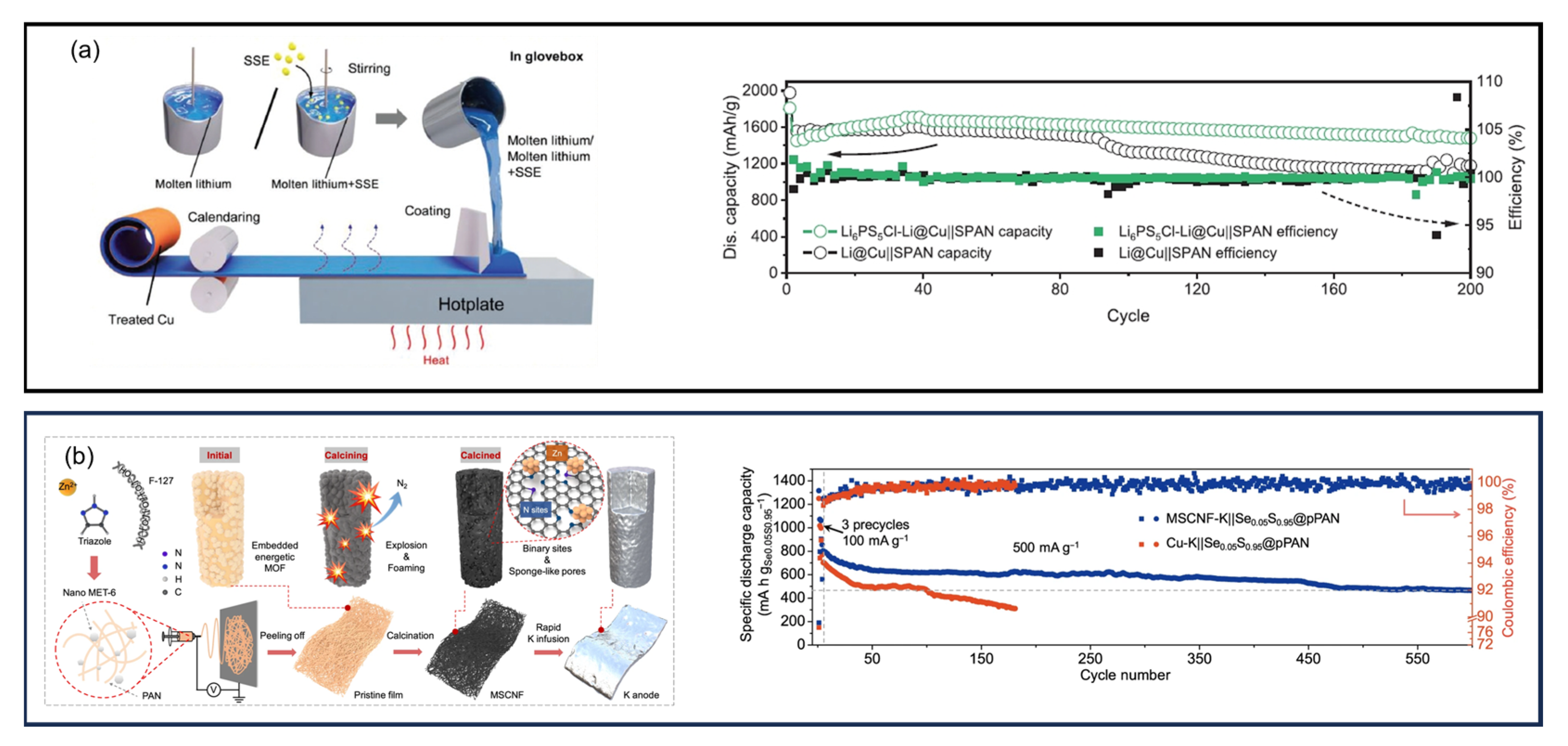
14. SPAN as a Cathode Additive

15. SPAN as Anode
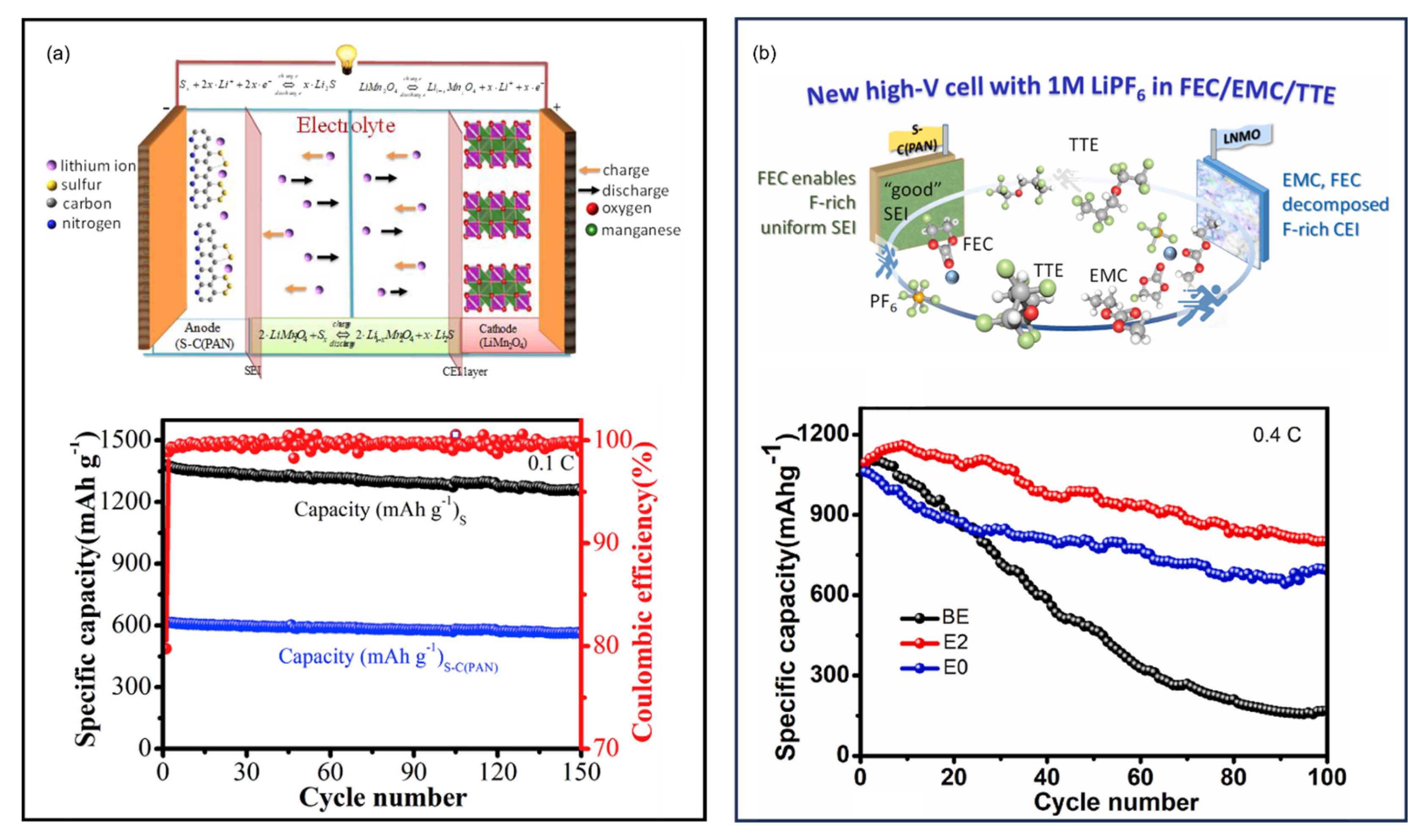
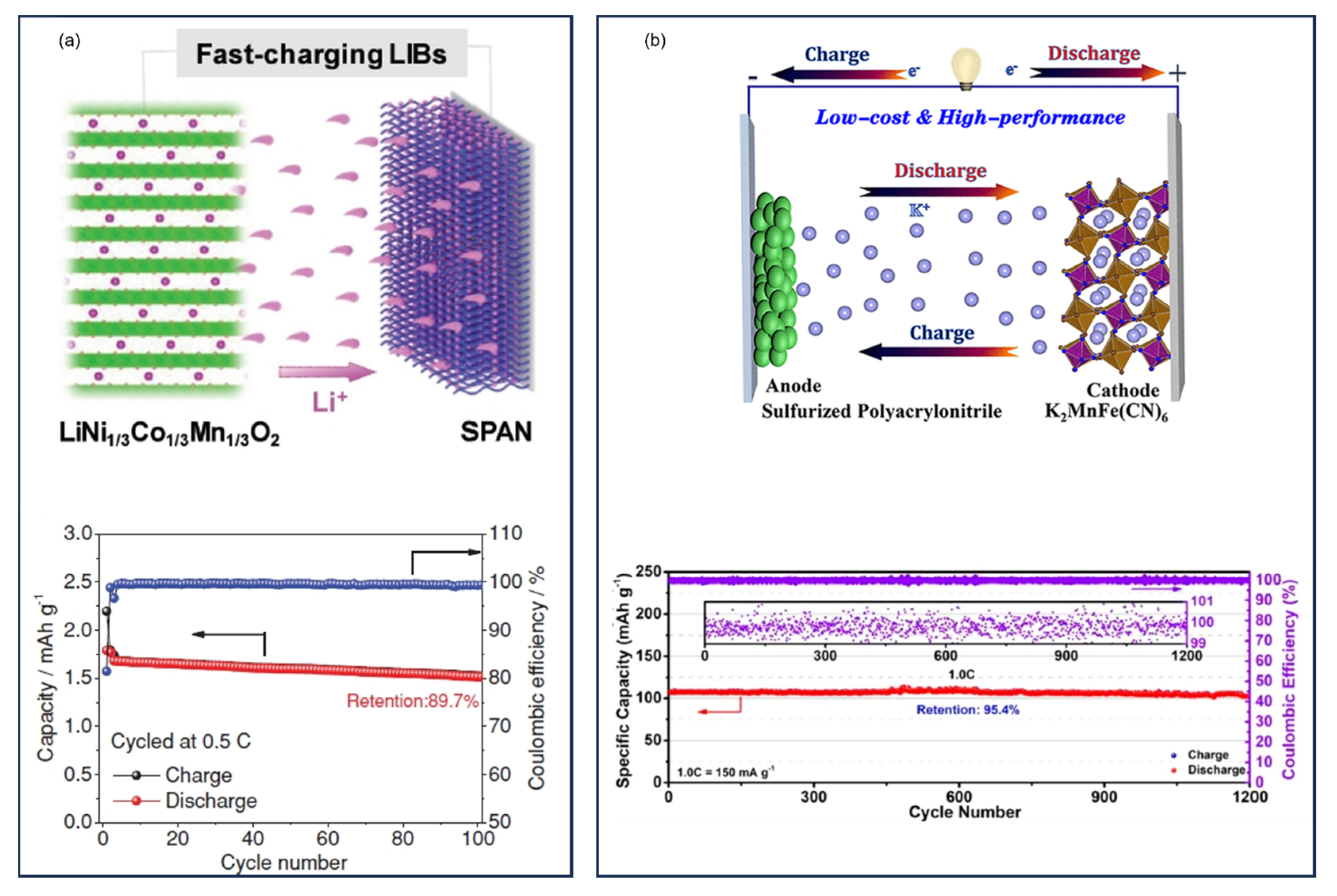
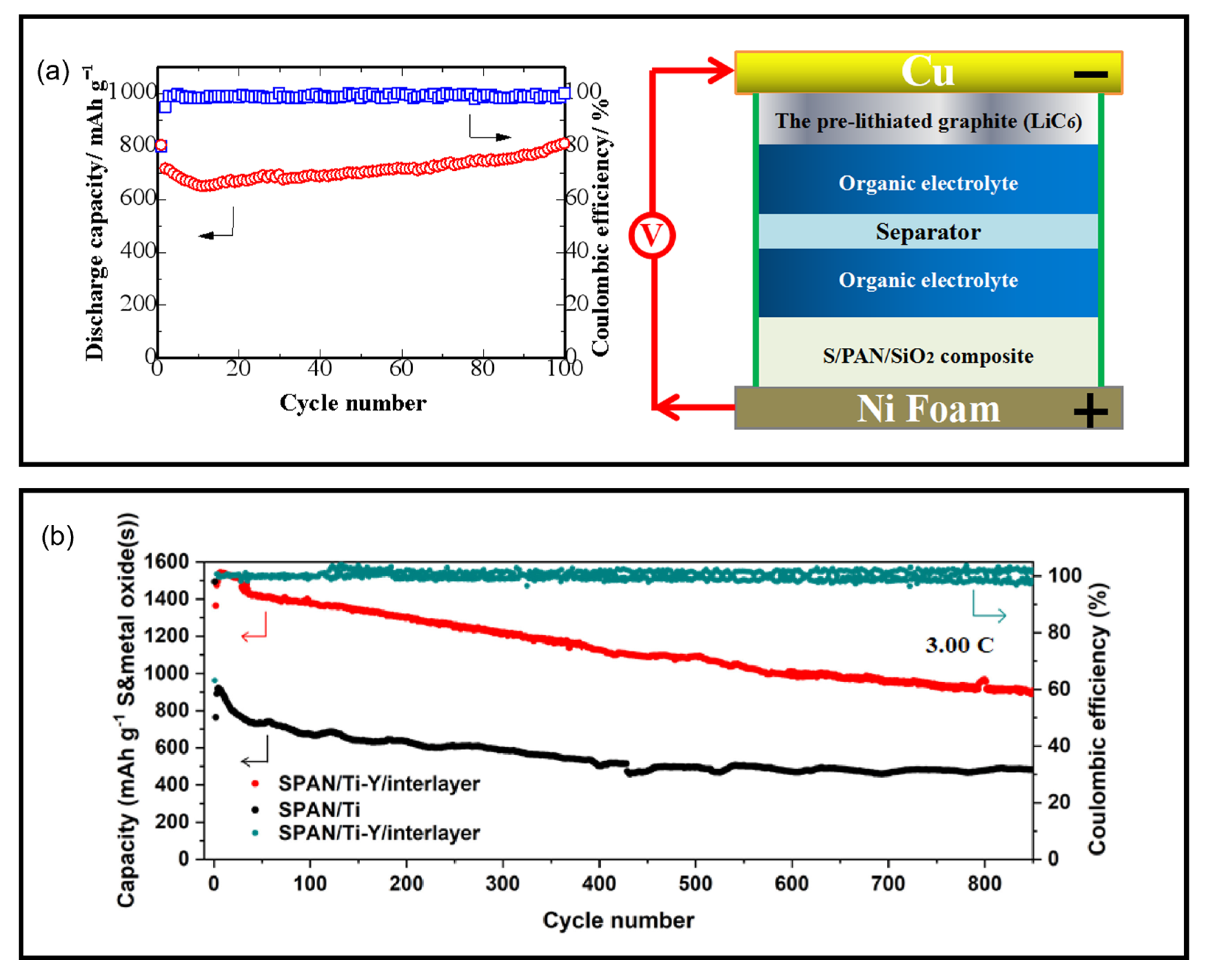
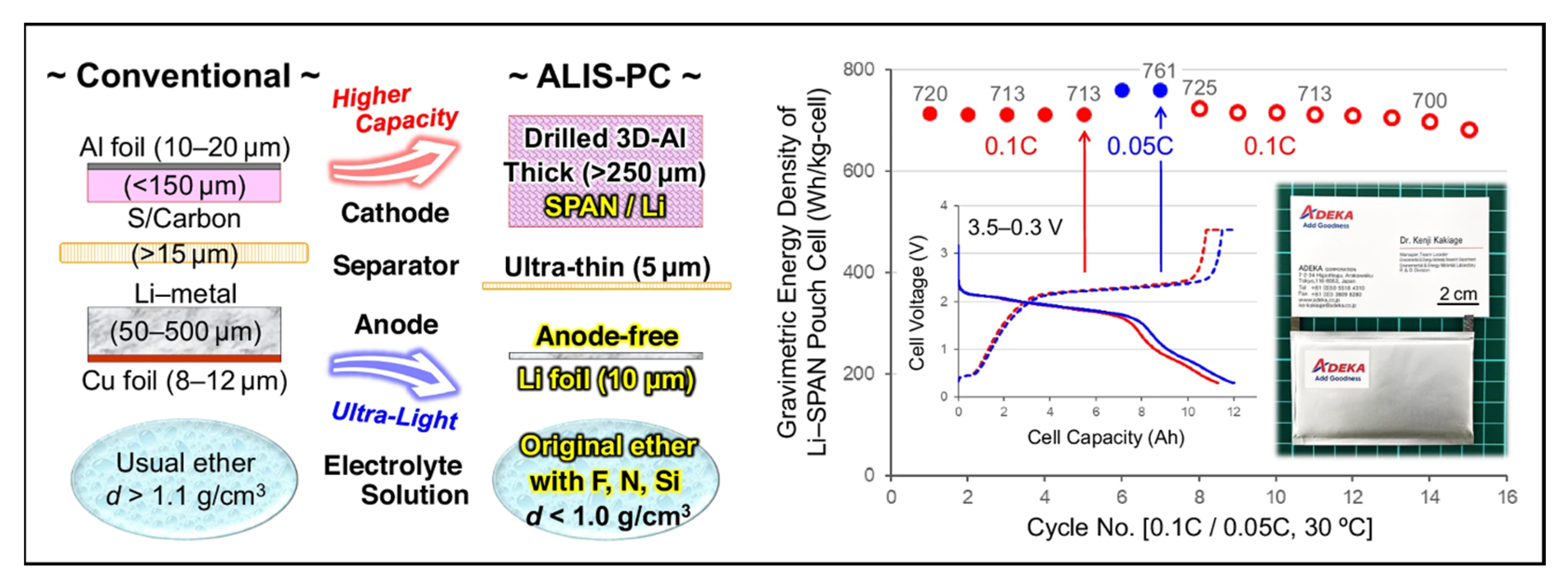
16. High-Energy Li||SPAN Batteries
- A lightweight 3D-Al foam sheet, Al-CELMET, was chosen as the current collector for the SPAN cathode. A high areal loading up to 32.4 mgS cm−2 (68.0 mgSPAN cm−2) on both sides could be achieved, corresponding to an areal capacity up to 46.6 mAh cm−2.
- The 3D-Al foam was laser-drilled with homogeneous hole of φ = 1.0 mm to save the weight by 31%.
- SWCNT and CNF were used as the conducting agent and the binder to achieve higher SPAN weight ratio in the cathode due to their superior electrical conductivity with high surface area and mechanical strength. A cathode fabricated using these materials achieved up to 98.0 wt% SPAN.
- SWCNT was dispersed in water using a soft dispersing method, Nihon Spindle Manufacturing’s JET PASTER technique. The SPAN cathode fabricated via this method exhibited a low ohmic resistance (Rohm).
- A unique shape design of the SPAN composite was employed. SPAN particles and fibers were mixed in a blend ratio of 90/10 to obtain the cathode to improve the electronic and ionic conductivities. As a result, the SPAN cathode exhibited reduced ion diffusion resistance (Rion).
- A porous SPAN fiber prepared via using PAN/PMMA as the electrospinning precursor was applied and exhibited excellent electrolyte absorbency.
- An expanded charge/discharge window was implemented in a potential range of 3.5–0.3 V to fully utilize the sulfur and the backbone redox capacities in SPAN.
- A novel ether-based electrolyte solution (Light-Ele) with properties of lightweight (0.98 g cm−3), high ionic conductivity, and low viscosity was designed. Light-Ele was composed of 0.2 M LiTFSI + 0.2 M LiFSI + 0.1 M LiNO3 + 0.1 M lithium1,1,2,2,3,3-hexafluoropropane-1,3-disulfonimide (LiHFDF) in DME/DOL/(trifluoromethyl)trimethylsilane (TFMTMS) (75/5/20, v/v/v). However, the activity of SPAN with the Light-Ele was lower than those with the conventional carbonate-based electrolytes, leading to a poorer CEI. Therefore, a two-step charge/discharge method using two different electrolytes was applied. First, a carbonate-based electrolyte with FEC and LiBOB additives was used to form a stable CEI. Second, Light-Ele was used to reduce the cell weight after removing the electrolyte from the first step.
- To reduce the cell weight, a thin separator, SETELA PE-type separator film, with a thickness of 5 µm and 35% porosity was used. In addition, a thinner pouch of an aluminum laminated film with a thickness under 80 µm (thin-type DNP Battery Pouch) was applied.
- An anode-free configuration design was implemented to maximize the energy density of the Li||SPAN cells. The SPAN cathode was electrochemically pre-lithiated using the half-cell method in carbonated-based electrolytes with FEC and LiBOB additives (for robust CEI formation). An ultra-thin Li foil ca. 10 µm thickness was used as the negative current collector instead of the conventional Cu foil.
17. Conclusions and Outlook
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AB | acetylene black |
| AN | acrylonitrile |
| ANFs | aramid nanofibers films |
| BEAQ | 1,5-bis(2-(2-(2-methoxyethoxy)ethoxy)ethoxy)anthra-9,10-quinon |
| BME | butyl methyl ether |
| BP | black phosphorus |
| BTFE | bis-(2,2,2-trifluorosulfonyl)imide |
| CDW | carbonized delignified wood |
| CE | Coulombic efficiency |
| CF | carbon foam |
| CFs | carbon fibers |
| CNFs | carbon nanofibers |
| CNTs | carbon nanotubes |
| CPME | cyclopentyl methyl ether |
| CP-MAS | cross-polarization/magic angle spinning |
| CTAC | hexadecyl trimethylammonium chloride |
| CV | cyclic voltammetry |
| C-β-CD | carbonyl-β-cyclodextrin |
| DBB | 2,2-dithiobis(benzothiazole) |
| DBE | dibutyl ether |
| DEC | diethylene carbonate |
| DEE | diethyl ether |
| DEMS | diethoxydimethylsilane |
| DFAQ | 1,4-difluoroanthraquinone |
| DFT | density functional theory |
| DG | diphenylguanidine |
| DGE | desolvated gel electrolyte |
| DIG | diglyme |
| DIPE | diisopropyl ether |
| DMC | dimethyl carbonate |
| DME | dimethoxyethane |
| DMM | dimethoxymethane |
| DMMP | dimethyl methylphosphonate |
| DMMS | dimethyldimethoxysilane |
| DMP | 1,2-dimethyoxypropane |
| DMSO | dimethyl sulfoxide |
| DOD | depth of discharge |
| DOL | 1,3-dioxolane |
| DPC | dipropyl carbonate |
| DPE | dipropyl ether |
| DPGDME | dipropyleneglycol dimethyl ether |
| DSC | differential scanning calorimetric |
| DTD | 1,3,2-dioxathiolane 2,2-dioxide |
| EA | elemental analysis |
| EC | ethylene carbonate |
| EC-AFM | electrochemical atomic force microscopy |
| EGBMC | ethylene glycol bis(methyl carbonate) |
| EIS | electrochemical impedance spectroscopy |
| EMC | ethyl methyl carbonate |
| EPR | electron paramagnetic resonance |
| ET | ethlenethiourea |
| EVs | electric vehicles |
| FB | fluorobenzene |
| FEC | fluoroethylene carbonate |
| FT-IR | Fourier transform infrared |
| GCN | graphitic carbon nitride |
| GE | gel electrolyte |
| GF | glass fiber |
| GFs | graphene foams |
| GG | guar gum |
| GNS | graphene nanosheet |
| GO | graphene oxide |
| GPEs | gel polymer electrolytes |
| Gr | graphite |
| G4 | tetraglyme |
| HBO | boric acid |
| HC-EM | half-cell electrochemical method |
| HCEs | high-concentration electrolytes |
| HFBA | hexafluorobutyl acrylate |
| HFE | hexafluoropropylene |
| IA | itaconic acid |
| IL | ionic liquid |
| IL-GPE | ionic liquid gel polymer electrolyte |
| KB | Ketjen black |
| LA133 | polyacrylic latex |
| LBG | locust beam gum |
| LHCEs | localized high concentration electrolytes |
| LiBOB | lithium bis(oxalate) borate |
| LIBs | lithium-ion batteries |
| LiDFBOP | lithium difluorobis (oxalate) phosphate |
| LiDFOB | lithium difluoro(oxalate)borate |
| LiFSI | lithium bis(fluorosulfonyl)imide |
| LiHFDF | lithium1,1,2,2,3,3-hexafluoropropane-1,3-disulfonimide |
| LiODFB | lithium oxalyldifluoroborate |
| LiPSs | lithium polysulfides |
| LiTFSI | lithium bis(trifluoromethanesulfonyl)imide |
| LMA | lithium metal anode |
| LMO | lithium manganese oxide |
| LSBs | lithium–sulfur batteries |
| MaPC | macro-porous carbon |
| MBA | magnesium bis(diisopropyl)amide |
| MBT | 2-mercaptobenzothiazoles |
| MCPs | microporous carbon polyhedrons |
| MD | molecular dynamics |
| MeIM | 2-methylimidazole |
| MLG | multilayered graphene |
| MOFs | metal–organic frameworks |
| MP | methyl propionate |
| MSBs | metal–sulfur batteries |
| MTFP | methyl 3,3,3-trifluoropionate |
| MWCNTs | multi-walled carbon nanotube |
| NaCMC | sodium carboxymethyl cellulose |
| NaTPB | sodium tetraphenylborate |
| NaTFSI | sodium trifluoromethanesulfonimide |
| NaPPB | sodium bis(perfluoropinacol)borate |
| NCM | lithium nickel manganese cobalt |
| NER | nitrogen evolution reaction |
| NMP | N-methyl-2-pyrrolidone |
| NMR | nuclear magnetic resonance |
| PAA | polyacrylic acid |
| PAAS | sodium polyacrylate |
| PAF | polymer-alloy-fluoride |
| PAN | polyacrylonitrile |
| PC | propylene carbonate |
| PCE | plastic crystal electrolyte |
| PDA | polydopamine |
| pair distribution function | |
| PDSe | phenyl diselenide |
| PE | polyethylene |
| PEG | polyethylene glycol |
| PEGDA | poly(ethylene glycol) diacrylate |
| PEO | polyethylene oxide |
| PEOEC | poly(ethylene oxide-co-ethylene carbonate) |
| PETA | pentaerythritol triacrylate |
| PETEA | pentaerythritol tetraacrylate |
| PI | polyimide |
| PIBs | potassium-ion batteries |
| PMMA | poly(methyl methacrylate) |
| PP | polypropylene separator |
| Ppy | polypyrole |
| PS | polystyrene |
| PuA | pulutan-graft-sodium polyacrylic acid |
| PVA | polyvinyl alcohol |
| PVDF | polyvinylidenefluoride |
| PVP | polyvinyl pyrrolidone |
| RAFT | addition-fragmentation chain transfer |
| Rsf | interfacial impedance |
| Rct | charge transfer resistance |
| rGO | reduced graphene oxide |
| RP | red phosphorus |
| sAXS | soft X-ray absorption spectroscopy |
| SBR | styrene butadiene rubber |
| SCMC | carboxymethyl cellulose |
| SCR | carboxylated styrene butadiene rubber |
| SC-EM | short-circuit electrochemical method |
| Se | selenium |
| SEI | solid-electrolyte interface |
| SEM | scanning electron microscopy |
| SIPS | solvent-induced phase separation |
| SLMP | stable lithium metal powder |
| SPAN | sulfurized polyacrylonitrile |
| SPEs | Solid-state polymer electrolytes |
| SPVac | sulfurized poly(vinylacetylene) |
| SWCNTs | single-walled carbon nanotubes |
| TD | tetrathylthiuruam disulfide |
| Te | tellurium |
| TEGDME | tetraethylene glycol dimethyl ether |
| TEM | transmission electron microscopy |
| TEP | triethyl phosphate |
| TFMTMS | (trifluoromethyl)trimethylsilane |
| TG | thermogravimetry |
| THF | tetrahydrofuran |
| THP | tetrahydropyran |
| TI | triallyl isocyanurate |
| TIPS | thermally induced phase separation |
| TMP | trimethyl phosphate |
| TMSB | tris(trimethylsilyl) borate |
| TMSP | tris(trimethylsilyl) phosphite |
| TMS-N3 | trimethylsilyl azide |
| TPA | terephthalic acid |
| TPOS | tetrapropoxysilane |
| TOF-SIMS | time-of flight secondary ion mass spectrometry |
| TPPi | triphenyl phosphite |
| TTCA | trithiocyanuric acid |
| TTE | 1,1,2,2-tetrafluoroethyl-2,2,3,3-tetrafluoropropylether |
| TTFP | tris(2,2,2-trifluoroethyl) phosphite |
| VAs | vulcanization accelerators |
| VC | vinylene carbonate |
| VGCF | vapor grown carbon fiber |
| XAS | X-ray absorption spectroscopy |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
| ZDB | zinc N-ethyl-N-phenyldithiocarbamate |
| 2-FP | 2-fluoropyridine |
References
- Chung, S.-H.; Manthiram, A. Current Status and Future Prospects of Metal–Sulfur Batteries. Adv. Mater. 2019, 31, 1901125. [Google Scholar] [CrossRef]
- Yu, X.; Manthiram, A. A Progress Report on Metal–Sulfur Batteries. Adv. Funct. Mater. 2020, 30, 2004084. [Google Scholar] [CrossRef]
- Phan, A.L.; Le, P.M.L.; Wang, C. Realizing High-Energy and Long-Life Li/SPAN Batteries. Joule 2024, 8, 1601–1618. [Google Scholar] [CrossRef]
- Wagenfeld, J.-G.; Al-Ali, K.; Almheiri, S.; Slavens, A.F.; Calvet, N. Sustainable Applications Utilizing Sulfur, a by-Product from Oil and Gas Industry: A State-of-the-Art Review. Waste Manag. 2019, 95, 78–89. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, H.; Liu, J.; Chen, J.; Lu, H.; Huang, Y.; Wang, J. Structure and Reactions Mechanism of Sulfurized Polyacrylonitrile as Cathodes for Rechargeable Li-S Batteries. Nano Res. 2023, 16, 8159–8172. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, C.; Li, Z.; Hu, X.; Razzaq, A.A.; Deng, Z. Sulfurized Polyacrylonitrile for High-Performance Lithium Sulfur Batteries: Advances and Prospects. J. Mater. Chem. A 2021, 9, 19282–19297. [Google Scholar] [CrossRef]
- Shaibani, M.; Mirshekarloo, M.S.; Singh, R.; Easton, C.D.; Cooray, M.C.D.; Eshraghi, N.; Abendroth, T.; Dörfler, S.; Althues, H.; Kaskel, S.; et al. Expansion-Tolerant Architectures for Stable Cycling of Ultrahigh-Loading Sulfur Cathodes in Lithium-Sulfur Batteries. Sci. Adv. 2020, 6, eaay2757. [Google Scholar] [CrossRef]
- Li, J.; Gao, L.; Pan, F.; Gong, C.; Sun, L.; Gao, H.; Zhang, J.; Zhao, Y.; Wang, G.; Liu, H. Engineering Strategies for Suppressing the Shuttle Effect in Lithium–Sulfur Batteries. Nano-Micro Lett. 2023, 16, 12. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Xie, J.; Xu, N. A Novel Conductive Polymer–Sulfur Composite Cathode Material for Rechargeable Lithium Batteries. Adv. Mater. 2002, 14, 963–965. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Wan, C.; Du, K.; Xie, J.; Xu, N. Sulfur Composite Cathode Materials for Rechargeable Lithium Batteries. Adv. Funct. Mater. 2003, 13, 487–492. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; He, X.; Ren, J.; Jiang, C.; Wan, C. Electrochemical Characteristics of Sulfur Composite Cathode Materials in Rechargeable Lithium Batteries. J. Power Sources 2004, 138, 271–273. [Google Scholar] [CrossRef]
- Miao, Q.; Solan, N.; Hyun, G.; Holoubek, J.; Liu, P. Electrolyte Engineering for Long-Life Li-SPAN Batteries. ACS Energy Lett. 2023, 8, 4818–4830. [Google Scholar] [CrossRef]
- Wei, M. SPAN Dataset 2025. Available online: https://github.com/weimufeng/SPAN (accessed on 23 July 2025).
- Yu, X.; Xie, J.; Yang, J.; Huang, H.; Wang, K.; Wen, Z. Lithium Storage in Conductive Sulfur-Containing Polymers. J. Electroanal. Chem. 2004, 573, 121–128. [Google Scholar] [CrossRef]
- Fanous, J.; Wegner, M.; Grimminger, J.; Andresen, Ä.; Buchmeiser, M.R. Structure-Related Electrochemistry of Sulfur-Poly(Acrylonitrile) Composite Cathode Materials for Rechargeable Lithium Batteries. Chem. Mater. 2011, 23, 5024–5028. [Google Scholar] [CrossRef]
- Fanous, J.; Wegner, M.; Grimminger, J.; Rolff, M.; Spera, M.B.M.; Tenzer, M.; Buchmeiser, M.R. Correlation of the Electrochemistry of Poly(Acrylonitrile)–Sulfur Composite Cathodes with Their Molecular Structure. J. Mater. Chem. 2012, 22, 23240–23245. [Google Scholar] [CrossRef]
- Doan, T.N.L.; Ghaznavi, M.; Zhao, Y.; Zhang, Y.; Konarov, A.; Sadhu, M.; Tangirala, R.; Chen, P. Binding Mechanism of Sulfur and Dehydrogenated Polyacrylonitrile in Sulfur/Polymer Composite Cathode. J. Power Sources 2013, 241, 61–69. [Google Scholar] [CrossRef]
- Zhang, S.S. Understanding of Sulfurized Polyacrylonitrile for Superior Performance Lithium/Sulfur Battery. Energies 2014, 7, 4588–4600. [Google Scholar] [CrossRef]
- Wei, S.; Ma, L.; Hendrickson, K.E.; Tu, Z.; Archer, L.A. Metal–Sulfur Battery Cathodes Based on PAN–Sulfur Composites. J. Am. Chem. Soc. 2015, 137, 12143–12152. [Google Scholar] [CrossRef]
- Wang, W.; Cao, Z.; Elia, G.A.; Wu, Y.; Wahyudi, W.; Abou-Hamad, E.; Emwas, A.-H.; Cavallo, L.; Li, L.-J.; Ming, J. Recognizing the Mechanism of Sulfurized Polyacrylonitrile Cathode Materials for Li–S Batteries and beyond in Al–S Batteries. ACS Energy Lett. 2018, 3, 2899–2907. [Google Scholar] [CrossRef]
- Jin, Z.-Q.; Liu, Y.-G.; Wang, W.-K.; Wang, A.-B.; Hu, B.-W.; Shen, M.; Gao, T.; Zhao, P.-C.; Yang, Y.-S. A New Insight into the Lithium Storage Mechanism of Sulfurized Polyacrylonitrile with No Soluble Intermediates. Energy Storage Mater. 2018, 14, 272–278. [Google Scholar] [CrossRef]
- Weret, M.A.; Jeffrey Kuo, C.-F.; Zeleke, T.S.; Beyene, T.T.; Tsai, M.-C.; Huang, C.-J.; Berhe, G.B.; Su, W.-N.; Hwang, B.-J. Mechanistic Understanding of the Sulfurized-Poly(Acrylonitrile) Cathode for Lithium-Sulfur Batteries. Energy Storage Mater. 2020, 26, 483–493. [Google Scholar] [CrossRef]
- Huang, C.-J.; Lin, K.-Y.; Hsieh, Y.-C.; Su, W.-N.; Wang, C.-H.; Brunklaus, G.; Winter, M.; Jiang, J.-C.; Hwang, B.J. New Insights into the N–S Bond Formation of a Sulfurized-Polyacrylonitrile Cathode Material for Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2021, 13, 14230–14238. [Google Scholar] [CrossRef]
- Huang, C.-J.; Cheng, J.-H.; Su, W.-N.; Partovi-Azar, P.; Kuo, L.-Y.; Tsai, M.-C.; Lin, M.-H.; Panahian Jand, S.; Chan, T.-S.; Wu, N.-L.; et al. Origin of Shuttle-Free Sulfurized Polyacrylonitrile in Lithium-Sulfur Batteries. J. Power Sources 2021, 492, 229508. [Google Scholar] [CrossRef]
- Zhu, T.; Mueller, J.E.; Hanauer, M.; Sauter, U.; Jacob, T. Structural Motifs for Modeling Sulfur-Poly(Acrylonitrile) Composite Materials in Sulfur-Lithium Batteries. ChemElectroChem 2017, 4, 2494–2499. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, Y.; Li, H.; Zhou, C.; Wang, X.; Du, L. Sulfurized Polyacrylonitrile as Cathodes for Advanced Lithium–Sulfur Batteries: Advances in Modification Strategies. Nanoscale 2024, 16, 5060–5078. [Google Scholar] [CrossRef]
- He, X.-M.; Wang, L.; Pu, W.-H.; Ren, J.-G.; Wu, W.; Jiang, C.-Y.; Wan, C.-R. Thermal Analysis of Sulfurization of Polyacrylonitrile with Elemental Sulfur. J. Therm. Anal. Calorim. 2008, 94, 151–155. [Google Scholar] [CrossRef]
- Xie, J.; Chen, J.; Guo, L.; Li, Y.; Wang, Y.; Zheng, S.; Zhang, N.; Meng, J.; Zhang, K.; Li, Q.; et al. Deciphering the Sulfur-Involved Bonding Interactions in Sulfurized Polyacrylonitrile: The Formation Thermodynamics and the Roles in Electrochemical Characteristics. ACS Nano 2025, 19, 3931–3943. [Google Scholar] [CrossRef]
- He, X.; Pu, W.; Ren, J.; Wang, L.; Wang, J.; Jiang, C.; Wan, C. Charge/Discharge Characteristics of Sulfur Composite Cathode Materials in Rechargeable Lithium Batteries. Electrochim. Acta 2007, 52, 7372–7376. [Google Scholar] [CrossRef]
- He, X.; Pu, W.; Ren, J.; Wang, L.; Wang, J.; Jiang, C.; Wan, C. Charge/Discharge Characteristics of Sulfur Composite Electrode at Different Temperature and Current Density in Rechargeable Lithium Batteries. Ionics 2008, 14, 335–337. [Google Scholar] [CrossRef]
- He, X.; Ren, J.; Wang, L.; Pu, W.; Jiang, C.; Wan, C. Expansion and Shrinkage of the Sulfur Composite Electrode in Rechargeable Lithium Batteries. J. Power Sources 2009, 190, 154–156. [Google Scholar] [CrossRef]
- Wang, L.; He, X.; Ren, J.; Pu, W.; Li, J.; Gao, J. The Electrochemical Characteristics of Sulfur Composite Cathode. Ionics 2010, 16, 689–695. [Google Scholar] [CrossRef]
- Niesen, S.; Trück, J.; Seidl, C.; Renger, K.; Buchmeiser, M.R. Lithium-Sulfur Batteries Based on Sulfurized Poly(Acrylonitrile) Cathodes: Impact of Electrode Density on Cell Performance. J. Electrochem. Soc. 2021, 168, 110513. [Google Scholar] [CrossRef]
- Moschner, R.; Gerle, M.; Danner, T.; Simanjuntak, E.K.; Michalowski, P.; Latz, A.; Nojabaee, M.; Kwade, A.; Friedrich, K.A. Impact of the Sulfurized Polyacrylonitrile Cathode Microstructure on the Electrochemical Performance of Lithium–Sulfur Batteries. Adv. Sci. 2025, 12, 2415436. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, J.; He, X.; Wan, C. Kinetic Investigation of Sulfurized Polyacrylonitrile Cathode Material by Electrochemical Impedance Spectroscopy. Electrochim. Acta 2011, 56, 5252–5256. [Google Scholar] [CrossRef]
- Wang, L.; He, X.; Sun, W.; Li, J.; Gao, J.; Tian, G.; Wang, J.; Fan, S. Organic Polymer Material with a Multi-Electron Process Redox Reaction: Towards Ultra-High Reversible Lithium Storage Capacity. RSC Adv. 2013, 3, 3227–3231. [Google Scholar] [CrossRef]
- Jiang, Z.; Guo, H.-J.; Zeng, Z.; Chen, X.; Lei, Y.; Liang, X.; Han, Z.; Hu, W.; Feng, J.; Wen, R.; et al. In Situ Characterization of Over-Lithiation of Organosulfide-Based Lithium Metal Anodes. ACS Appl. Mater. Interfaces 2021, 13, 41555–41562. [Google Scholar] [CrossRef]
- Dominko, R.; Patel, M.U.M.; Bele, M.; Pejovnik, S. Sulphured Polyacrylonitrile Composite Analysed by in Operando UV-Visible Spectroscopy and 4-Electrode Swagelok Cell. Acta Chim. Slov. 2016, 63, 569–577. [Google Scholar] [CrossRef]
- Warneke, S.; Eusterholz, M.; Zenn, R.K.; Hintennach, A.; Dinnebier, R.E.; Buchmeiser, M.R. Differences in Electrochemistry between Fibrous SPAN and Fibrous S/C Cathodes Relevant to Cycle Stability and Capacity. J. Electrochem. Soc. 2017, 165, A6017. [Google Scholar] [CrossRef]
- Kappler, J.; Klostermann, S.V.; Lange, P.L.; Dyballa, M.; Veith, L.; Schleid, T.; Weil, T.; Kästner, J.; Buchmeiser, M.R. Sulfur-Composites Derived from Poly(Acrylonitrile) and Poly(Vinylacetylene)—A Comparative Study on the Role of Pyridinic and Thioamidic Nitrogen. Batter. Supercaps 2023, 6, e202200522. [Google Scholar] [CrossRef]
- Wang, S.; Lu, B.; Cheng, D.; Wu, Z.; Feng, S.; Zhang, M.; Li, W.; Miao, Q.; Patel, M.; Feng, J.; et al. Structural Transformation in a Sulfurized Polymer Cathode to Enable Long-Life Rechargeable Lithium–Sulfur Batteries. J. Am. Chem. Soc. 2023, 145, 9624–9633. [Google Scholar] [CrossRef]
- Tan, S.; Rahman, M.M.; Wu, Z.; Liu, H.; Wang, S.; Ghose, S.; Zhong, H.; Waluyo, I.; Hunt, A.; Liu, P.; et al. Structural and Interphasial Stabilities of Sulfurized Polyacrylonitrile (SPAN) Cathode. ACS Energy Lett. 2023, 8, 2496–2504. [Google Scholar] [CrossRef]
- Pereira, R.; Sarode, K.K.; Rafie, A.; Fafarman, A.; Kalra, V. In-Operando FTIR Study on the Redox Behavior of Sulfurized Polyacrylonitrile as Cathode Material for Li–S Batteries. J. Phys. Chem. C 2023, 127, 19356–19365. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Z.; Lyu, L.; Qin, N.; Li, Y.; Gu, S.; Wang, Z.; Chen, J.; Yuan, H.; Yang, M.; et al. New Insights into the Reaction Mechanism of Sulfurized Polyacrylonitrile Cathode Material for Li–S Batteries. Compos. Commun. 2024, 49, 101971. [Google Scholar] [CrossRef]
- Liu, J.; Lu, H.; Wang, Q.; Kong, X.; Hao, C.; Yang, J.; NuLi, Y.; Duan, H.; Wang, J. Roles of the Polymer Backbone for Sulfurized Polyacrylonitrile Cathodes in Rechargeable Lithium Batteries. J. Am. Chem. Soc. 2025, 147, 426–435. [Google Scholar] [CrossRef]
- Kappler, J.; Tonbul, G.; Schoch, R.; Murugan, S.; Nowakowski, M.; Lange, P.L.; Klostermann, S.V.; Bauer, M.; Schleid, T.; Kästner, J.; et al. Understanding the Redox Mechanism of Sulfurized Poly(Acrylonitrile) as Highly Rate and Cycle Stable Cathode Material for Sodium-Sulfur Batteries. J. Electrochem. Soc. 2023, 170, 010526. [Google Scholar] [CrossRef]
- Zhu, T.; Mueller, J.E.; Hanauer, M.; Sauter, U.; Jacob, T. Theoretical Studies on the Charging and Discharging of Poly(Acrylonitrile)-Based Lithium-Sulfur Batteries. ChemElectroChem 2017, 4, 2975–2980. [Google Scholar] [CrossRef]
- Bertolini, S.; Jacob, T. Atomistic Discharge Studies of Sulfurized-Polyacrylonitrile through Ab Initio Molecular Dynamics. Electrochim. Acta 2022, 403, 139538. [Google Scholar] [CrossRef]
- Beltran, S.P.; Balbuena, P.B. First-Principles Explorations of the Electrochemical Lithiation Dynamics of a Multilayer Graphene Nanosheet-Based Sulfur–Carbon Composite. J. Mater. Chem. A 2018, 6, 18084–18094. [Google Scholar] [CrossRef]
- Perez Beltran, S.; Balbuena, P.B. Sulfurized Polyacrylonitrile for High-Performance Lithium–Sulfur Batteries: In-Depth Computational Approach Revealing Multiple Sulfur’s Reduction Pathways and Hidden Li+ Storage Mechanisms for Extra Discharge Capacity. ACS Appl. Mater. Interfaces 2021, 13, 491–502. [Google Scholar] [CrossRef]
- Perez Beltran, S.; Balbuena, P.B. Sulfurized Polyacrylonitrile (SPAN): Changes in Mechanical Properties during Electrochemical Lithiation. J. Phys. Chem. C 2021, 125, 13185–13194. [Google Scholar] [CrossRef]
- Bertolini, S.; Jacob, T. Density Functional Theory Studies on Sulfur–Polyacrylonitrile as a Cathode Host Material for Lithium–Sulfur Batteries. ACS Omega 2021, 6, 9700–9708. [Google Scholar] [CrossRef]
- Bertolini, S.; Jacob, T. Sulfurized-Polyacrylonitrile in Lithium-Sulfur Batteries: Interactions between Undercoordinated Carbons and Polymer Structure under Low Lithiation. J. Energy Chem. 2022, 66, 587–596. [Google Scholar] [CrossRef]
- Bertolini, S.; Jacob, T. Capturing Polysulfides by Sulfurized-Polyacrylonitrile in Lithium-Sulfur Batteries and the Sulfur-Chain Effects through Density Functional Theory. Electrochem. Sci. Adv. 2022, 2, e2100129. [Google Scholar] [CrossRef]
- Bertolini, S.; Venezuela, P.; Delcorte, A. The Effect of Lithium Battery Overpotential on Sulfurized-Polyacrylonitrile (SPAN): A Theoretical Approach. J. Energy Storage 2024, 78, 110049. [Google Scholar] [CrossRef]
- Simanjuntak, E.K.; Danner, T.; Wang, P.; Buchmeiser, M.R.; Latz, A. A Novel Modeling Approach for Sulfurized Polyacrylonitrile (SPAN) Electrodes in Li Metal Batteries. Electrochim. Acta 2024, 497, 144571. [Google Scholar] [CrossRef]
- Yu, X.; Xie, J.; Li, Y.; Huang, H.; Lai, C.; Wang, K. Stable-Cycle and High-Capacity Conductive Sulfur-Containing Cathode Materials for Rechargeable Lithium Batteries. J. Power Sources 2005, 146, 335–339. [Google Scholar] [CrossRef]
- Wang, L.; He, X.; Li, J.; Gao, J.; Guo, J.; Jiang, C.; Wan, C. Analysis of the Synthesis Process of Sulphur–Poly(Acrylonitrile)-Based Cathode Materials for Lithium Batteries. J. Mater. Chem. 2012, 22, 22077–22081. [Google Scholar] [CrossRef]
- Langrud, S.; Razzaq, A.A.; Santhanagopalan, S.; Brow, R.; Xing, W. Comprehensive Characterization of Multi-Phase Sulfurized Polyacrylonitrile Cathodes for Lithium-Sulfur Batteries. J. Electrochem. Soc. 2022, 169, 070514. [Google Scholar] [CrossRef]
- Pan, J.; Nie, J.; Zeng, B.; Chen, X.; Chen, Z.; Kang, D.; Miao, Y.; Liu, W.; Zhang, W. Optimized Preparation of Polyacrylonitrile/Sulfur Composite as Cathode for Lithium Sulfur Batteries. Int. J. Electrochem. Sci. 2022, 17, 22021. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, Y.; Liu, M.; Ma, J.; Yue, H.; Hu, Z.; Yang, S.-T.; Yin, Y. Synthesis of Highly Cyclized Polyacrylonitrile via Liquid-Phase Cyclization for Advanced Cathode Materials. ACS Sustain. Chem. Eng. 2024, 12, 17806–17816. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Wang, A.; Jin, Z.; Zhao, H.; Yang, Y. Effect of Vapor Pressure on Performance of Sulfurized Polyacrylonitrile Cathodes for Li/S Batteries. RSC Adv. 2016, 6, 106625–106630. [Google Scholar] [CrossRef]
- Páez Jerez, A.L.; Chemes, D.M.; Sham, E.L.; Davies, L.E.; Tesio, A.Y.; Flexer, V. Low-Temperature Synthesis of a Sulfur-Polyacrylonitrile Composite Cathode for Lithium-Sulfur Batteries. ChemistrySelect 2020, 5, 5465–5472. [Google Scholar] [CrossRef]
- Fanous, J.; Wegner, M.; Spera, M.B.M.; Buchmeiser, M.R. High Energy Density Poly(Acrylonitrile)-Sulfur Composite-Based Lithium-Sulfur Batteries. J. Electrochem. Soc. 2013, 160, A1169. [Google Scholar] [CrossRef]
- Cho, G.-B.; Park, H.-B.; Jeong, J.-S.; Chae, M.-R.; Im, Y.-M.; Han-Gyeol, L.; Sang-Hui, P.; Kim, K.-W. Effect of Ball Milling on Electrochemical Properties of Sulfur/Polyacrylonitrile (SPAN) Cathode in Li/S Battery. J. Nanosci. Nanotechnol. 2018, 18, 6431–6436. [Google Scholar] [CrossRef]
- Konarov, A.; Gosselink, D.; Doan, T.N.L.; Zhang, Y.; Zhao, Y.; Chen, P. Simple, Scalable, and Economical Preparation of Sulfur–PAN Composite Cathodes for Li/S Batteries. J. Power Sources 2014, 259, 183–187. [Google Scholar] [CrossRef]
- Du, K.; Zhang, Q. Facile Fabrication of Sulfur Entrapped Carbonized Material as a Cathode for High-Performance Lithium Batteries. RSC Adv. 2016, 6, 87690–87695. [Google Scholar] [CrossRef]
- Sarode, K.; Yim, T.; Pereira, R.; Cardoza, N.; Kalra, V. Solid–Liquid–Solid Mediated Artificial SEI Coated Stable Lithium and High-Sulfur Percentage SPAN for High Performance Li–S Batteries. Energy Adv. 2024, 3, 584–591. [Google Scholar] [CrossRef]
- Zhu, G. A Novel Suspension Polymerization Process to Prepare Sulfur Composite Cathode Materials for Lithium/Sulfur Batteries. World Electr. Veh. J. 2010, 4, 332–334. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Bakenov, Z.; Konarov, A.; Chen, P. Preparation of Novel Network Nanostructured Sulfur Composite Cathode with Enhanced Stable Cycle Performance. J. Power Sources 2014, 270, 326–331. [Google Scholar] [CrossRef]
- Pu, W.; He, X.; Wang, L.; Tian, Z.; Jiang, C.; Wan, C. Sulfur Composite Cathode Materials: Comparative Characterization of Polyacrylonitrile Precursor. Ionics 2007, 13, 273–276. [Google Scholar] [CrossRef]
- Lei, J.; Chen, J.; Zhang, H.; Naveed, A.; Yang, J.; Nuli, Y.; Wang, J. High Molecular Weight Polyacrylonitrile Precursor for S@pPAN Composite Cathode Materials with High Specific Capacity for Rechargeable Lithium Batteries. ACS Appl. Mater. Interfaces 2020, 12, 33702–33709. [Google Scholar] [CrossRef]
- Liu, H.; He, R.; Li, Y.; Jin, Y.; Liu, H.; Zhang, X. Effect of Sulfurized Polyacrylonitrile-g-rGO Composition on the Specific Capacity and Cycling Stability of Lithium Sulfur Batteries. J. Electroanal. Chem. 2023, 939, 117465. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Shi, C.; Shen, Y. Diphenylguanidine Combined with High Molecular Weight Polyacrylonitrile Precursor for High-Conductivity and High-Sulfur-Loading Cathode of Lithium-Sulfur Battery. Mater. Lett. 2023, 336, 133831. [Google Scholar] [CrossRef]
- Yi, Y.; Hai, F.; Chen, W.; Gao, X.; Guo, J.; Xue, W.; Li, M. A Universal Strategy for the Refined Frameworks and Improved Performance of Distinct Commercial Polyacrylonitriles in Sulfur Cathodes. Sci. China Mater. 2024, 67, 2915–2924. [Google Scholar] [CrossRef]
- Geller, B.E. Status and Prospects for Development of Polyacrylonitrile Fibre Production. A Review. Fibre Chem. 2002, 34, 151–161. [Google Scholar] [CrossRef]
- Huang, X. Fabrication and Properties of Carbon Fibers. Materials 2009, 2, 2369–2403. [Google Scholar] [CrossRef]
- Wei, W.; Wang, J.; Zhou, L.; Yang, J.; Schumann, B.; NuLi, Y. CNT Enhanced Sulfur Composite Cathode Material for High Rate Lithium Battery. Electrochem. Commun. 2011, 13, 399–402. [Google Scholar] [CrossRef]
- Shi, Z.; Bi, D.; Wang, L.; Xu, H.; Yue, H.; Yin, Y.; Yang, S. Disulfide Dichloride: A High Efficiency Vulcanizing Agent for Sulfurized Polyacrylonitrile. ACS Appl. Energy Mater. 2022, 5, 8015–8022. [Google Scholar] [CrossRef]
- Liu, X.-M.; Huang, Z.D.; Oh, S.W.; Zhang, B.; Ma, P.-C.; Yuen, M.M.F.; Kim, J.-K. Carbon Nanotube (CNT)-Based Composites as Electrode Material for Rechargeable Li-Ion Batteries: A Review. Compos. Sci. Technol. 2012, 72, 121–144. [Google Scholar] [CrossRef]
- Tong, Z.; Lv, C.; Bai, G.-D.; Yin, Z.-W.; Zhou, Y.; Li, J.-T. A Review on Applications and Challenges of Carbon Nanotubes in Lithium-Ion Battery. Carbon Energy 2025, 7, e643. [Google Scholar] [CrossRef]
- Yin, L.; Wang, J.; Yang, J.; Nuli, Y. A Novel Pyrolyzed Polyacrylonitrile-sulfur@MWCNT Composite Cathode Material for High-Rate Rechargeable Lithium/Sulfur Batteries. J. Mater. Chem. 2011, 21, 6807–6810. [Google Scholar] [CrossRef]
- Electrochemical properties of lithium/sulfur-polyacrylonitrile-carbon nanotube composite cells using ether-based electrolyte at high rate. J. Ceram. Process. Res. 2015, 16, 199–202. [CrossRef]
- Abdul Razzaq, A.; Yao, Y.; Shah, R.; Qi, P.; Miao, L.; Chen, M.; Zhao, X.; Peng, Y.; Deng, Z. High-Performance Lithium Sulfur Batteries Enabled by a Synergy between Sulfur and Carbon Nanotubes. Energy Storage Mater. 2019, 16, 194–202. [Google Scholar] [CrossRef]
- Razzaq, A.A.; Yuan, X.; Chen, Y.; Hu, J.; Mu, Q.; Ma, Y.; Zhao, X.; Miao, L.; Ahn, J.-H.; Peng, Y.; et al. Anchoring MOF-Derived CoS2 on Sulfurized Polyacrylonitrile Nanofibers for High Areal Capacity Lithium–Sulfur Batteries. J. Mater. Chem. A 2020, 8, 1298–1306. [Google Scholar] [CrossRef]
- Abdul Razzaq, A.; Chen, G.; Zhao, X.; Yuan, X.; Hu, J.; Li, Z.; Chen, Y.; Xu, J.; Shah, R.; Zhong, J.; et al. Cobalt Coordination with Pyridines in Sulfurized Polyacrylonitrile Cathodes to Form Conductive Pathways and Catalytic M-N4S Sites for Accelerated Li-S Kinetics. J. Energy Chem. 2021, 61, 170–178. [Google Scholar] [CrossRef]
- Wang, X.; Qian, Y.; Wang, L.; Yang, H.; Li, H.; Zhao, Y.; Liu, T. Sulfurized Polyacrylonitrile Cathodes with High Compatibility in Both Ether and Carbonate Electrolytes for Ultrastable Lithium–Sulfur Batteries. Adv. Funct. Mater. 2019, 29, 1902929. [Google Scholar] [CrossRef]
- Li, H.; Xue, W.; Xu, W.; Wang, L.; Liu, T. Controllable Synthesis of Sulfurized Polyacrylonitrile Nanofibers for High Performance Lithium–Sulfur Batteries. Compos. Commun. 2021, 24, 100675. [Google Scholar] [CrossRef]
- Hu, X.; Jiang, H.; Hou, Q.; Yu, M.; Jiang, X.; He, G.; Li, X. Scalable SPAN Membrane Cathode with High Conductivity and Hierarchically Porous Framework for Enhanced Ion Transfer and Cycling Stability in Li–S Batteries. ACS Mater. Lett. 2023, 5, 2047–2057. [Google Scholar] [CrossRef]
- Shao, J.; Huang, C.; Zhu, Q.; Sun, N.; Zhang, J.; Wang, R.; Chen, Y.; Zhang, Z. Flexible CNT-Interpenetrating Hierarchically Porous Sulfurized Polyacrylonitrile (CIHP-SPAN) Electrodes for High-Rate Lithium-Sulfur (Li-S) Batteries. Nanomaterials 2024, 14, 1155. [Google Scholar] [CrossRef]
- Zuo, W.; Guo, Y.; Zhang, C.; Zhang, L.; Zhang, S. Mussel and Cobweb Inspired High Areal Capacity SPAN Electrode. Small 2024, 20, 2309126. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Li, Y.; Liu, H.; Han, N.; Zhang, X. Loadings of Functionalized Multiwalled Carbon Nanotubes for Enhancing Sulfurized Polyacrylonitrile Performance in Lithium–Sulfur Batteries. ACS Appl. Nano Mater. 2023, 6, 21058–21067. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The Rise of Graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Yin, L.; Wang, J.; Lin, F.; Yang, J.; Nuli, Y. Polyacrylonitrile/Graphene Composite as a Precursor to a Sulfur-Based Cathode Material for High-Rate Rechargeable Li–S Batteries. Energy Environ. Sci. 2012, 5, 6966–6972. [Google Scholar] [CrossRef]
- Yin, L.; Wang, J.; Yu, X.; Monroe, C.W.; NuLi, Y.; Yang, J. Dual-Mode Sulfur-Based Cathode Materials for Rechargeable Li–S Batteries. Chem. Commun. 2012, 48, 7868–7870. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Bakenov, Z.; Babaa, M.-R.; Konarov, A.; Ding, C.; Chen, P. Effect of Graphene on Sulfur/Polyacrylonitrile Nanocomposite Cathode in High Performance Lithium/Sulfur Batteries. J. Electrochem. Soc. 2013, 160, A1194. [Google Scholar] [CrossRef]
- Wang, J.; Yin, L.; Jia, H.; Yu, H.; He, Y.; Yang, J.; Monroe, C.W. Hierarchical Sulfur-Based Cathode Materials with Long Cycle Life for Rechargeable Lithium Batteries. ChemSusChem 2014, 7, 563–569. [Google Scholar] [CrossRef]
- Konarov, A.; Bakenov, Z.; Yashiro, H.; Sun, Y.-K.; Myung, S.-T. Effect of Carbon-Sulphur Bond in a Sulphur/Dehydrogenated Polyacrylonitrile/Reduced Graphene Oxide Composite Cathode for Lithium-Sulphur Batteries. J. Power Sources 2017, 355, 140–146. [Google Scholar] [CrossRef]
- Krishnaveni, K.; Subadevi, R.; Sivakumar, M. Graphene Oxide-Crowned Poly(Acrylonitrile)/Sulfur as a Lithium–Sulfur Battery Cathode: Performance and Characterization. SN Appl. Sci. 2020, 2, 766. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Q.; Zhong, J.; Chen, M.; Deng, H.; Cao, J.; Wang, L.; Peng, L.; Zhu, J.; Lu, B. 3D Holey Graphene/Polyacrylonitrile Sulfur Composite Architecture for High Loading Lithium Sulfur Batteries. Adv. Energy Mater. 2021, 11, 2100448. [Google Scholar] [CrossRef]
- Kim, H.; Hwang, J.-Y.; Bang, S.; Jung, H.-G.; Sun, Y.-K. Geometrical Engineering of a SPAN–Graphene Composite Cathode for Practical Li–S Batteries. J. Mater. Chem. A 2022, 10, 10844–10853. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, Y.; Huang, J.; Jiang, H.; Liang, B.; Wang, B.; He, D.; Chen, H. A Free-Standing Sulfide Polyacrylonitrile/Reduced Graphene Oxide Film Cathode with Nacre-like Architecture for High-Performance Lithium-Sulfur Batteries. J. Power Sources 2025, 629, 235916. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, Q.; Gentle, I.R.; Wang, D.-W. “Soft” Graphene Oxide-Organopolysulfide Nanocomposites for Superior Pseudocapacitive Lithium Storage. Chin. Chem. Lett. 2018, 29, 603–605. [Google Scholar] [CrossRef]
- Kumar, A.; Verma, C.J.; Singh, M.K.; Prakash, R. 5—Porous Carbon from Conducting Polymers for Electrochemical Applications. In Conjugated Polymers for Next-Generation Applications; Kumar, V., Sharma, K., Sehgal, R., Kalia, S., Eds.; Woodhead Publishing Series in Electronic and Optical Materials; Woodhead Publishing: Sawston, UK, 2022; Volume 1, pp. 147–180. ISBN 978-0-12-823442-6. [Google Scholar]
- Wen, Z.; Lu, D. Fabrication and Electrochemical Performance of Polyacrylonitrile-S/Carbon Composite as Cathode for Lithium Ion Batteries. J. Electrochem. Soc. 2013, 160, A2311. [Google Scholar] [CrossRef]
- Sohn, H.; Gordin, M.L.; Regula, M.; Kim, D.H.; Jung, Y.S.; Song, J.; Wang, D. Porous Spherical Polyacrylonitrile-Carbon Nanocomposite with High Loading of Sulfur for Lithium–Sulfur Batteries. J. Power Sources 2016, 302, 70–78. [Google Scholar] [CrossRef]
- Xu, A.; Jin, Z.; Wang, B.; Xie, X.; Xiao, X.; Wang, A.; Zhang, J.; Wang, W.; Lu, J.; Zeng, F. Unraveling the Mechanism on Improved Kinetics Performance of Sulfurized Polyacrylonitrile with Defective Conductive Carbon Matrix. Chem. Eng. J. 2024, 484, 149558. [Google Scholar] [CrossRef]
- Chang, Z.; Dou, H.; Ding, B.; Wang, J.; Wang, Y.; Xu, G.; Li, C. Interconnected Core–Shell Pyrolyzed Polyacrylonitrile@sulfur/Carbon Nanocomposites for Rechargeable Lithium–Sulfur Batteries. New J. Chem. 2016, 40, 7680–7686. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Wu, Z.-Z.; Pan, G.-L.; Liu, S.; Gao, X.-P. Microporous Carbon Polyhedrons Encapsulated Polyacrylonitrile Nanofibers as Sulfur Immobilizer for Lithium–Sulfur Battery. ACS Appl. Mater. Interfaces 2017, 9, 12436–12444. [Google Scholar] [CrossRef]
- Kuo, C.J.; Weret, M.A.; Hung, H.; Tsai, M.; Huang, C.; Su, W.; Hwang, B. Sulfurized−poly(Acrylonitrile) Wrapped Carbonsulfur Composite Cathode Material for High Performance Rechargeable Lithiumsulfur Batteries. J. Power Sources 2019, 412, 670–676. [Google Scholar] [CrossRef]
- Zhang, W.; Pan, J.; Liu, P.; Ouyang, Z.; Fang, R.; Zhang, S.; Lian, J. A Li+-Conductive Porous Carbon/Polyacrylonitrile/Sulfur Composite for Li-S Batteries. Int. J. Electrochem. Sci. 2020, 15, 7925–7934. [Google Scholar] [CrossRef]
- Qin, F.; Zhang, K.; Hong, B.; Zhang, L.; Zhang, Z.; Li, J.; Wang, X.; Lai, Y. A New Insight into Capacity Fading of Sulfurized Polyacrylonitrile Composite in Carbonate Electrolyte. J. Electroanal. Chem. 2021, 882, 114964. [Google Scholar] [CrossRef]
- Insinna, T.; Bassey, E.N.; Märker, K.; Collauto, A.; Barra, A.-L.; Grey, C.P. Graphite Anodes for Li-Ion Batteries: An Electron Paramagnetic Resonance Investigation. Chem. Mater. 2023, 35, 5497–5511. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, P.; Chen, Z.; Fang, R.; Kang, D.; Ouyang, Z.; Zhang, S. Polyacrylonitile/Sulphur (PAN-S) Cathode with KS6 Graphite as the Conductive Agent for Li-S Battery. Int. J. Electrochem. Sci. 2021, 16, 210247. [Google Scholar] [CrossRef]
- Liu, Y.; Haridas, A.K.; Lee, Y.; Cho, K.-K.; Ahn, J.-H. Freestanding Porous Sulfurized Polyacrylonitrile Fiber as a Cathode Material for Advanced Lithium Sulfur Batteries. Appl. Surf. Sci. 2019, 472, 135–142. [Google Scholar] [CrossRef]
- Han, Z.; Li, S.; Xiong, R.; Jiang, Z.; Sun, M.; Hu, W.; Peng, L.; He, R.; Zhou, H.; Yu, C.; et al. Low Tortuosity and Reinforced Concrete Type Ultra-Thick Electrode for Practical Lithium–Sulfur Batteries. Adv. Funct. Mater. 2022, 32, 2108669. [Google Scholar] [CrossRef]
- Jo, C.-H.; Yu, J.H.; Kim, H.J.; Hwang, J.-Y.; Kim, J.-Y.; Jung, H.-G.; Myung, S.-T. Sulfurized Carbon Composite with Unprecedentedly High Tap Density for Sodium Storage. Adv. Energy Mater. 2022, 12, 2102836. [Google Scholar] [CrossRef]
- Dogadkin, B.A.; Shershnev, V.A. Vulcanization of Rubber in the Presence of Organic Accelerators. Rubber Chem. Technol. 1962, 35, 1–56. [Google Scholar] [CrossRef]
- Chen, H.; Wang, C.; Hu, C.; Zhang, J.; Gao, S.; Lu, W.; Chen, L. Vulcanization Accelerator Enabled Sulfurized Carbon Materials for High Capacity and High Stability of Lithium–Sulfur Batteries. J. Mater. Chem. A 2014, 3, 1392–1395. [Google Scholar] [CrossRef]
- Wang, Y.; Shuai, Y.; Chen, K. Diphenyl Guanidine as Vulcanization Accelerators in Sulfurized Polyacrylonitrile for High Performance Lithium-Sulfur Battery. Chem. Eng. J. 2020, 388, 124378. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Shuai, Y.; Chen, K. Diphenyl Guanidine Vulcanization Accelerators Enable Sulfurized Polyacrylonitrile Cathode for High Capacity and Ether-Compatible by Fast Kinetic. Energy 2021, 233, 121160. [Google Scholar] [CrossRef]
- Qin, J.; Lu, Y.; Wang, R.; Li, Z.; Shen, T.; Wang, D. Sulfurization Accelerator Coupled Fe1−xS Electrocatalyst Boosting SPAN Cathode Performance. Nano Res. 2023, 16, 9231–9239. [Google Scholar] [CrossRef]
- Wu, Q.; Qin, M.; Wu, Y.; Zhu, H.; Cheng, S.; Xie, J. Triallyl Isocyanurate Enabled SPAN-Based Organosulfur Featuring High Sulfur & Selenium Loading for Advanced Li/Na–S Batteries. J. Mater. Chem. A 2023, 11, 22913–22921. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, H.-C.; Hao, C.-R.; Liu, J.-Q.; Kong, X.-R.; Wang, J.-L. Delaying Cyclization of Polyacrylonitrile by Boric Acid for Sulfurized Poly(Acrylonitrile) Cathode Materials. Chem. Eng. J. 2024, 500, 156857. [Google Scholar] [CrossRef]
- Ouyang, Q.; Wang, H.; Cheng, L.; Sun, Y. Effect of Boric Acid on the Stabilization of Poly(Acrylonitrile-Co-Itaconic Acid). J. Polym. Res. 2007, 14, 497–503. [Google Scholar] [CrossRef]
- Ouyang, Q.; Cheng, L.; Wang, H.; Li, K. FTIR Study of the Retardation Effect of Boric Acid on the Cyclization Reaction of Polyacrylonitrile. e-Polym. 2009, 9, 015. [Google Scholar] [CrossRef][Green Version]
- Xiang, J.; Guo, Z.; Yi, Z.; Zhang, Y.; Yuan, L.; Cheng, Z.; Shen, Y.; Huang, Y. Facile Synthesis of Sulfurized Polyacrylonitrile Composite as Cathode for High-Rate Lithium-Sulfur Batteries. J. Energy Chem. 2020, 49, 161–165. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Liu, H.; Zhang, Y.; Zhang, X. Effects of Fiber Diameter on Sulfur Loading and Lithium–Sulfur Battery Performance of Semicarbonized and Sulfurized Polyacrylonitrile Cathode Materials. ACS Appl. Energy Mater. 2023, 6, 8511–8520. [Google Scholar] [CrossRef]
- Hwang, T.H.; Jung, D.S.; Kim, J.-S.; Kim, B.G.; Choi, J.W. One-Dimensional Carbon–Sulfur Composite Fibers for Na–S Rechargeable Batteries Operating at Room Temperature. Nano Lett. 2013, 13, 4532–4538. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, T.; Zhang, N.; Feng, T.; Li, L.; Wu, F.; Chen, R. Powering Lithium–Sulfur Batteries by Ultrathin Sulfurized Polyacrylonitrile Nanosheets. Nanoscale 2021, 13, 16690–16695. [Google Scholar] [CrossRef]
- Frey, M.; Zenn, R.K.; Warneke, S.; Müller, K.; Hintennach, A.; Dinnebier, R.E.; Buchmeiser, M.R. Easily Accessible, Textile Fiber-Based Sulfurized Poly(Acrylonitrile) as Li/S Cathode Material: Correlating Electrochemical Performance with Morphology and Structure. ACS Energy Lett. 2017, 2, 595–604. [Google Scholar] [CrossRef]
- Liu, Y.; Haridas, A.K.; Cho, K.-K.; Lee, Y.; Ahn, J.-H. Highly Ordered Mesoporous Sulfurized Polyacrylonitrile Cathode Material for High-Rate Lithium Sulfur Batteries. J. Phys. Chem. C 2017, 121, 26172–26179. [Google Scholar] [CrossRef]
- Warneke, S.; Zenn, R.K.; Lebherz, T.; Müller, K.; Hintennach, A.; Starke, U.; Dinnebier, R.E.; Buchmeiser, M.R. Hybrid Li/S Battery Based on Dimethyl Trisulfide and Sulfurized Poly(Acrylonitrile). Adv. Sustain. Syst. 2018, 2, 1700144. [Google Scholar] [CrossRef]
- Wang, K.; Ju, S.; Gao, Q.; Xia, G.; Wang, G.; Yan, H.; Dong, L.; Yang, Z.; Yu, X. Porous Sulfurized Poly(Acrylonitrile) Nanofiber as a Long-Life and High-Capacity Cathode for Lithium–Sulfur Batteries. J. Alloys Compd. 2021, 860, 158445. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Lu, Y.; Lou, X.W. (David) A Pyrolyzed Polyacrylonitrile/Selenium Disulfide Composite Cathode with Remarkable Lithium and Sodium Storage Performances. Sci. Adv. 2018, 4, eaat1687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, W.; Zhu, Z.; Huang, Q.; Liu, X.; Zhang, M.; Pei, W.-B.; Wu, J. Multi-Channel Sulfurized Polyacrylonitrile with Hollow Structure as Cathode for Room Temperature Sodium–Sulfur Batteries. J. Solid State Chem. 2021, 301, 122359. [Google Scholar] [CrossRef]
- Huang, X.; Liu, J.; Huang, Z.; Ke, X.; Liu, L.; Wang, N.; Liu, J.; Guo, Z.; Yang, Y.; Shi, Z. Flexible Free-Standing Sulfurized Polyacrylonitrile Electrode for Stable Li/Na Storage. Electrochim. Acta 2020, 333, 135493. [Google Scholar] [CrossRef]
- Song, Y.; Lai, J.; Li, X.; Huo, J.; Zhao, C.; He, C. Steaming Inspired 3D Porous Architecture for Improving the Capability and Stability of Sulfurized Polyacrylonitrile Cathode. Mater. Lett. 2021, 296, 129933. [Google Scholar] [CrossRef]
- Lebherz, T.; Frey, M.; Hintennach, A.; Buchmeiser, M.R. Influence of Morphology of Monolithic Sulfur–Poly(Acrylonitrile) Composites Used as Cathode Materials in Lithium–Sulfur Batteries on Electrochemical Performance. RSC Adv. 2019, 9, 7181–7188. [Google Scholar] [CrossRef]
- He, R.; Li, Y.; Mu, Z.; Liu, H.; Zhang, Y.; Zhang, X. Flower-Shaped Sulfurized Polyacrylonitrile Nanostructures as Cathode Materials for High-Performance Lithium–Sulfur Batteries. ACS Appl. Nano Mater. 2023, 6, 23163–23172. [Google Scholar] [CrossRef]
- Lei, J.; Chen, J.; Naveed, A.; Zhang, H.; Yang, J.; Nuli, Y.; Wang, J. Sulfurized Polyacrylonitrile Cathode Derived from Intermolecular Cross-Linked Polyacrylonitrile for a Rechargeable Lithium Battery. ACS Appl. Energy Mater. 2021, 4, 5706–5712. [Google Scholar] [CrossRef]
- Lei, J.; Lu, H.; Chen, J.; Yang, J.; Nuli, Y.; Wang, J. Crosslinked Polyacrylonitrile Precursor for S@pPAN Composite Cathode Materials for Rechargeable Lithium Batteries. J. Energy Chem. 2022, 65, 186–193. [Google Scholar] [CrossRef]
- Moschner, R.; Cavers, H.; Michalowski, P.; Kwade, A. Investigation of an Industrially Scalable Production of Sulfur-Polyacrylonitrile Based Cathodes. Batter. Supercaps 2024, 7, e202400154. [Google Scholar] [CrossRef]
- Wang, W.; Xu, W.; Xia, S.; Xue, W.; Wang, J.; Wang, X.; Li, H.; Lin, S.; Zhao, Y.; Wang, L.; et al. A New Insight into the Molecular Rearrangement of Sulfurized Polyacrylonitrile Cathode in Ether Electrolyte. Chem. Eng. J. 2023, 470, 144142. [Google Scholar] [CrossRef]
- Xue, W.; Xu, W.; Wang, W.; Gao, G.; Wang, L. Iodine-Doped Fibrous Sulfurized Polyacrylonitrile with Accelerated Reaction Kinetics. Compos. Commun. 2022, 30, 101078. [Google Scholar] [CrossRef]
- Li, H.; Xue, W.; Wang, L.; Liu, T. Two Competing Reactions of Sulfurized Polyacrylonitrile Produce High-Performance Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2021, 13, 25002–25009. [Google Scholar] [CrossRef]
- Yuan, X.; Zhu, B.; Feng, J.; Wang, C.; Cai, X.; Qin, R. Feasible Catalytic-Insoluble Strategy Enabled by Sulfurized Polyacrylonitrile with In Situ Built Electrocatalysts for Ultrastable Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2021, 13, 50936–50947. [Google Scholar] [CrossRef]
- Haridas, A.K.; Heo, J.; Li, X.; Ahn, H.-J.; Zhao, X.; Deng, Z.; Agostini, M.; Matic, A.; Ahn, J.-H. A Flexible and Free-Standing FeS/Sulfurized Polyacrylonitrile Hybrid Anode Material for High-Rate Sodium-Ion Storage. Chem. Eng. J. 2020, 385, 123453. [Google Scholar] [CrossRef]
- Weret, M.A.; Kuo, C.-F.J.; Su, W.-N.; Zeleke, T.S.; Huang, C.-J.; Sahalie, N.A.; Zegeye, T.A.; Wondimkun, Z.T.; Fenta, F.W.; Jote, B.A.; et al. Fibrous Organosulfur Cathode Materials with High Bonded Sulfur for High-Performance Lithium-Sulfur Batteries. J. Power Sources 2022, 541, 231693. [Google Scholar] [CrossRef]
- Yi, Y.; Hai, F.; Guo, J.; Gao, X.; Chen, W.; Tian, X.; Tang, W.; Hua, W.; Li, M. Electrochemical Enhancement of Lithium-Ion Diffusion in Polypyrrole-Modified Sulfurized Polyacrylonitrile Nanotubes for Solid-to-Solid Free-Standing Lithium–Sulfur Cathodes. Small 2023, 19, 2303781. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Li, Y.; Han, N.; Liu, H.; Zhang, X. Solid-State Transformations of Active Materials in the Pores of Sulfurized-Polyacrylonitrile Fiber Membranes via Nucleophilic Reactions for High-Loading and Free-Standing Lithium–Sulfur Battery Cathodes. Adv. Fiber Mater. 2024, 6, 772–785. [Google Scholar] [CrossRef]
- Gao, G.; Wang, J.; Zhang, X.; Li, H.; Wang, L.; Liu, T. An Ionic Liquid Enhanced Gel Polymer Electrolyte for High Performance Lithium-Metal Batteries Based on Sulfurized Polyacrylonitrile Cathode. Compos. Commun. 2022, 31, 101100. [Google Scholar] [CrossRef]
- Kim, I.; Kim, C.H.; Choi, S.h.; Ahn, J.-P.; Ahn, J.-H.; Kim, K.-W.; Cairns, E.J.; Ahn, H.-J. A Singular Flexible Cathode for Room Temperature Sodium/Sulfur Battery. J. Power Sources 2016, 307, 31–37. [Google Scholar] [CrossRef]
- Kim, H.; Sadan, M.K.; Kim, C.; Jo, J.; Seong, M.; Cho, K.-K.; Kim, K.-W.; Ahn, J.-H.; Ahn, H.-J. Enhanced Reversible Capacity of Sulfurized Polyacrylonitrile Cathode for Room-Temperature Na/S Batteries by Electrochemical Activation. Chem. Eng. J. 2021, 426, 130787. [Google Scholar] [CrossRef]
- Mentbayeva, A.; Belgibayeva, A.; Umirov, N.; Zhang, Y.; Taniguchi, I.; Kurmanbayeva, I.; Bakenov, Z. High Performance Freestanding Composite Cathode for Lithium-Sulfur Batteries. Electrochim. Acta 2016, 217, 242–248. [Google Scholar] [CrossRef]
- Kim, H.; Kim, C.; Sadan, M.K.; Yeo, H.; Cho, K.-K.; Kim, K.-W.; Ahn, J.-H.; Ahn, H.-J. Binder-Free and High-Loading Sulfurized Polyacrylonitrile Cathode for Lithium/Sulfur Batteries. RSC Adv. 2021, 11, 16122–16130. [Google Scholar] [CrossRef]
- Yang, K.; Kim, S.; Yang, X.; Cho, M.; Lee, Y. Binder-Free and High-Loading Cathode Realized by Hierarchical Structure for Potassium–Sulfur Batteries. Small Methods 2022, 6, 2100899. [Google Scholar] [CrossRef] [PubMed]
- Sabet, S.M.; Sapkota, N.; Chiluwal, S.; Zheng, T.; Clemons, C.M.; Rao, A.M.; Pilla, S. Sulfurized Polyacrylonitrile Impregnated Delignified Wood-Based 3D Carbon Framework for High-Performance Lithium–Sulfur Batteries. ACS Sustain. Chem. Eng. 2023, 11, 2314–2323. [Google Scholar] [CrossRef]
- Peng, H.; Wang, X.; Zhao, Y.; Tan, T.; Mentbayeva, A.; Bakenov, Z.; Zhang, Y. Enhanced Electrochemical Performance of Sulfur/Polyacrylonitrile Composite by Carbon Coating for Lithium/Sulfur Batteries. J. Nanopart. Res. 2017, 19, 348. [Google Scholar] [CrossRef]
- Wang, S.-L.; Hong, J.-L. Polydopamine as an Interfacial Layer to Enhance Mechanical and Adhesive Properties of the Active Materials in a Sulfur Cathode of Sodium-Sulfur Batteries. Chem. Eng. J. Adv. 2022, 11, 100352. [Google Scholar] [CrossRef]
- Zuo, W.; Li, R.; Wu, X.; Guo, Y.; Zhou, S.; Wen, B.; Luo, J.; Zhang, L. SPAN Secondary Particles Enabled High Energy Density Lithium-Sulfur Battery. Chem. Eng. J. 2024, 491, 151977. [Google Scholar] [CrossRef]
- Holoubek, J.; Yan, Q.; Liu, H.; Wu, Z.; Xing, X.; Zhou, H.; Luo, J.; Chen, Z.; Liu, P. Low-Cost Li||SPAN Batteries Enabled by Sustained Additive Release. ACS Appl. Energy Mater. 2021, 4, 6422–6429. [Google Scholar] [CrossRef]
- Qin, R.; Wang, Z.; Li, J.; Deng, L. Sulfureted Polyacrylonitrile Derived Carbon Encapsulated Silicon as High-Performance Anode Material for Lithium-Ion Batteries. J. Alloys Compd. 2022, 929, 167355. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Wang, A.; Jin, Z.; Zhao, H.; Yang, Y. A Polysulfide Reduction Accelerator—NiS2-Modified Sulfurized Polyacrylonitrile as a High Performance Cathode Material for Lithium–Sulfur Batteries. J. Mater. Chem. A 2017, 5, 22120–22124. [Google Scholar] [CrossRef]
- Haridas, A.K.; Heo, J.; Liu, Y.; Ahn, H.-J.; Zhao, X.; Deng, Z.; Agostini, M.; Matic, A.; Cho, K.-K.; Ahn, J.-H. Boosting High Energy Density Lithium-Ion Storage via the Rational Design of an FeS-Incorporated Sulfurized Polyacrylonitrile Fiber Hybrid Cathode. ACS Appl. Mater. Interfaces 2019, 11, 29924–29933. [Google Scholar] [CrossRef]
- Li, Y.; He, R.; Liu, H.; Zhang, Y.; Liu, H.; Han, N.; Zhang, X. Construction of CoS2 Reduction Accelerator-Modified Sulfurized Polyacrylonitrile Nanofibers as High-Performance Cathode Materials for Practical Lithium–Sulfur Batteries. ACS Appl. Energy Mater. 2023, 6, 8466–8478. [Google Scholar] [CrossRef]
- Xu, Z.-Q.; Zou, R.; Liu, W.-W.; Liu, G.-L.; Cui, Y.-S.; Lei, Y.-X.; Zheng, Y.-W.; Niu, W.-J.; Wu, Y.-Z.; Gu, B.-N.; et al. Design of Atomic Cobalt Selenide-Doped Sulfurized Polyacrylonitrile Cathode with Enhanced Electrochemical Kinetics for High Performance Lithium-SPAN Batteries. Chem. Eng. J. 2023, 471, 144581. [Google Scholar] [CrossRef]
- Wang, L.; Shi, H.; Xie, Y.; Wu, Z.-S. Boosting Solid–Solid Conversion Kinetics of Sulfurized Polyacrylonitrile via MoS2 Doping for High-Rate and Long-Life Li-S Batteries. Carbon Neutralization 2023, 2, 262–270. [Google Scholar] [CrossRef]
- Lei, Z.; Zheng, J.; He, X.; Wang, Y.; Yang, X.; Xiao, F.; Xue, H.; Xiong, P.; Wei, M.; Chen, Q.; et al. Defect-Rich WS2–SPAN Nanofibers for Sodium/Potassium-Ion Batteries: Ultralong Lifespans and Wide-Temperature Workability. Inorg. Chem. Front. 2023, 10, 1187–1196. [Google Scholar] [CrossRef]
- Xiao, F.; Zhang, J.; Zhou, W.; Fang, Y.; He, X.; Lai, W.; Lin, C.; Ge, M.; Fan, H.; Qian, Q.; et al. Defect-Engineered WSxSe2−x Nanocrystals Anchored on Selenized Polyacrylonitrile Fibers toward High-Performance Sodium/Potassium-Ion Batteries with a Wide Working Temperature Range. Inorg. Chem. Front. 2024, 11, 2164–2177. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Lin, C.; Wang, Y.; Lei, Z.; Xiong, P.; Luo, Y.; Chen, Q.; Zeng, L.; Wei, M.; et al. Structure Engineering of BiSbS Nanocrystals Embedded within Sulfurized Polyacrylonitrile Fibers for High Performance of Potassium-Ion Batteries. Chem.—Eur. J. 2022, 28, e202200028. [Google Scholar] [CrossRef]
- Li, R.; Tong, L.; Jiang, Y.; Wang, Y.; Long, J.; Chen, X.; Wu, J.; Li, X.; Chen, Y. SnS2 Nanoparticles Embedded in Sulfurized Polyacrylonitrile Composite Fibers for High-Performance Potassium-Ion Batteries. Interdiscip. Mater. 2024, 3, 150–159. [Google Scholar] [CrossRef]
- Wang, Y.; Guan, Z.; Zhang, Y.; Qu, B.; Sa, B.; Zhou, X.; Wang, J.; Peng, D.-L.; Xie, Q.; Pan, F. Li+/Mg2+ Co-Intercalation SnS2-SPAN Cathode for Super-Stable Magnesium-Based Batteries. J. Magnes. Alloys 2024, in press. [CrossRef]
- Zhang, Y.; Zhao, Y.; Yermukhambetova, A.; Bakenov, Z.; Chen, P. Ternary Sulfur/Polyacrylonitrile/Mg0.6Ni0.4O Composite Cathodes for High Performance Lithium/Sulfur Batteries. J. Mater. Chem. A 2012, 1, 295–301. [Google Scholar] [CrossRef]
- He, Y.; Shan, Z.; Tan, T.; Chen, Z.; Zhang, Y. Ternary Sulfur/Polyacrylonitrile/SiO2 Composite Cathodes for High-Performance Sulfur/Lithium Ion Full Batteries. Polymers 2018, 10, 930. [Google Scholar] [CrossRef] [PubMed]
- Páez Jerez, A.L.; Davies, L.E.; Tesio, A.Y.; Flexer, V. Synergistic Combination of TiO2 and S-PAN for Li-S Batteries with Long-Term Cyclability at High C-Rates. J. Electrochem. Soc. 2021, 168, 120536. [Google Scholar] [CrossRef]
- Fang, L.; Xu, W.; Lyu, X.; Liu, Y.; Reinhart, B.; Nguyen, H.; Li, T. Suppressing the Shuttle Effects with FeCo/SPAN Cathodes and High-Concentration Electrolytes for High-Performance Lithium–Sulfur Batteries. ACS Appl. Energy Mater. 2023, 6, 795–801. [Google Scholar] [CrossRef]
- Blázquez-Moreno, J.M.; Páez Jerez, A.L.; Tesio, A.Y.; Benítez, A.; Caballero, Á. Stable Long-Term Cycling of Room-Temperature Sodium-Sulfur Batteries Based on Non-Complex Sulfurised Polyacrylonitrile Cathodes. Batter. Supercaps 2025, 8, e202400640. [Google Scholar] [CrossRef]
- Páez Jerez, A.L.; Vera, M.L.; Sham, E.L.; Tesio, A.Y.; Flexer, V. Novel Multicomponent Composite with TiO2/Y2O3 as Cathode Material and Interlayer for Li-SPAN Batteries Cycled at High C-Rates. Electrochim. Acta 2023, 463, 142876. [Google Scholar] [CrossRef]
- Yuan, X.; Zhu, B.; Feng, J.; Wang, C.; Cai, X.; Qin, R. An In-Situ Catalytic-Insoluble Strategy Enabled by Sulfurized Polyacrylonitrile-Based Composite Cathode for Potassium–Sulfur Batteries. Funct. Mater. Lett. 2021, 14, 2143003. [Google Scholar] [CrossRef]
- Ma, S.; Zuo, P.; Zhang, H.; Yu, Z.; Cui, C.; He, M.; Yin, G. Iodine-Doped Sulfurized Polyacrylonitrile with Enhanced Electrochemical Performance for Room-Temperature Sodium/Potassium Sulfur Batteries. Chem. Commun. 2019, 55, 5267–5270. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, Z.; Wang, Y.; Yu, Z.; Cui, C.; He, M.; Huo, H.; Yin, G.; Zuo, P. Iodine-Doped Sulfurized Polyacrylonitrile with Enhanced Electrochemical Performance for Lithium Sulfur Batteries in Carbonate Electrolyte. Chem. Eng. J. 2021, 418, 129410. [Google Scholar] [CrossRef]
- Ma, S.; Wang, Y.; Fu, C.; Ma, Y.; Gao, Y.; Yin, G.; Zuo, P. Black Phosphorus-Modified Sulfurized Polyacrylonitrile with High C-Rate and Cycling Performance in Ether-Based Electrolyte for Lithium Sulfur Batteries. Chem. Commun. 2020, 56, 12797–12800. [Google Scholar] [CrossRef]
- Wang, Y.; Zuo, P.; Ma, S.; Xie, B.; Yu, Z.; Yin, G. DFT and Experimental Study of Nano Red Phosphorus Anchoring on Sulfurized Polyacrylonitrile for Lithium-Ion Batteries. Chem. Commun. 2020, 56, 12857–12860. [Google Scholar] [CrossRef] [PubMed]
- Rabiei Baboukani, A.; Khakpour, I.; Adelowo, E.; Drozd, V.; Shang, W.; Wang, C. High-Performance Red Phosphorus-Sulfurized Polyacrylonitrile Composite by Electrostatic Spray Deposition for Lithium-Ion Batteries. Electrochim. Acta 2020, 345, 136227. [Google Scholar] [CrossRef]
- Hu, Y.; Li, B.; Jiao, X.; Zhang, C.; Dai, X.; Song, J. Stable Cycling of Phosphorus Anode for Sodium-Ion Batteries through Chemical Bonding with Sulfurized Polyacrylonitrile. Adv. Funct. Mater. 2018, 28, 1801010. [Google Scholar] [CrossRef]
- Abouimrane, A.; Dambournet, D.; Chapman, K.W.; Chupas, P.J.; Weng, W.; Amine, K. A New Class of Lithium and Sodium Rechargeable Batteries Based on Selenium and Selenium–Sulfur as a Positive Electrode. J. Am. Chem. Soc. 2012, 134, 4505–4508. [Google Scholar] [CrossRef]
- Wang, H.; Li, S.; Chen, Z.; Liu, H.K.; Guo, Z. A Novel Type of One-Dimensional Organic Selenium-Containing Fiber with Superior Performance for Lithium–Selenium and Sodium–Selenium Batteries. RSC Adv. 2014, 4, 61673–61678. [Google Scholar] [CrossRef]
- Guo, J.; Wen, Z.; Wang, Q.; Jin, J.; Ma, G. A Conductive Selenized Polyacrylonitrile Cathode Material for Re-Chargeable Lithium Batteries with Long Cycle Life. J. Mater. Chem. A 2015, 3, 19815–19821. [Google Scholar] [CrossRef]
- Zhu, T.; Pang, Y.; Wang, Y.; Wang, C.; Xia, Y. S0.87Se0.13/CPAN Composites as High Capacity and Stable Cycling Performance Cathode for Lithium Sulfur Battery. Electrochim. Acta 2018, 281, 789–795. [Google Scholar] [CrossRef]
- Liu, Y.; Tai, Z.; Zhang, Q.; Wang, H.; Pang, W.K.; Liu, H.K.; Konstantinov, K.; Guo, Z. A New Energy Storage System: Rechargeable Potassium-Selenium Battery. Nano Energy 2017, 35, 36–43. [Google Scholar] [CrossRef]
- Chen, X.; Peng, L.; Wang, L.; Yang, J.; Hao, Z.; Xiang, J.; Yuan, K.; Huang, Y.; Shan, B.; Yuan, L.; et al. Ether-Compatible Sulfurized Polyacrylonitrile Cathode with Excellent Performance Enabled by Fast Kinetics via Selenium Doping. Nat. Commun. 2019, 10, 1021. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, K.; Gao, S.; Wang, R.; Han, J.; Yan, J.; Cheng, S.; Jiang, K. Selenium as Extra Binding Site for Sulfur Species in Sulfurized Polyacrylonitrile Cathodes for High Capacity Lithium-Sulfur Batteries. ChemElectroChem 2019, 6, 1365–1370. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Zhang, N.; Liu, H.; Chen, Z.; Zhang, L.; Guo, S.; Li, D.; Xu, J. One-Step In Situ Preparation of Polymeric Selenium Sulfide Composite as a Cathode Material for Enhanced Sodium/Potassium Storage. ACS Appl. Mater. Interfaces 2019, 11, 29807–29813. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, Y.; Peng, L.; Yang, J.; Jia, H.; Zhang, Z.; Shan, B.; Xie, J. Se as Eutectic Accelerator in Sulfurized Polyacrylonitrile for High Performance All-Solid-State Lithium-Sulfur Battery. Energy Storage Mater. 2019, 21, 287–296. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Li, S.; Yang, J.; Sun, Y.; Peng, L.; Shan, B.; Xie, J. Effect of Eutectic Accelerator in Selenium-Doped Sulfurized Polyacrylonitrile for High Performance Room Temperature Sodium–Sulfur Batteries. J. Mater. Chem. A 2019, 7, 12732–12739. [Google Scholar] [CrossRef]
- Pham, V.H.; Boscoboinik, J.A.; Stacchiola, D.J.; Self, E.C.; Manikandan, P.; Nagarajan, S.; Wang, Y.; Pol, V.G.; Nanda, J.; Paek, E.; et al. Selenium-Sulfur (SeS) Fast Charging Cathode for Sodium and Lithium Metal Batteries. Energy Storage Mater. 2019, 20, 71–79. [Google Scholar] [CrossRef]
- Jia, H.; Liang, X.; An, T.; Peng, L.; Feng, J.; Xie, J. Effect of Halogen Doping in Sodium Solid Electrolytes Based on the Na–Sn–Si–P–S Quinary System. Chem. Mater. 2020, 32, 4065–4071. [Google Scholar] [CrossRef]
- Jia, H.; Peng, L.; Zhang, Z.; An, T.; Xie, J. Na3.8 [Sn0.67Si0.33]0.8Sb0.2S4: A Quinary Sodium Fast Ionic Conductor for All-Solid-State Sodium Battery. J. Energy Chem. 2020, 48, 102–106. [Google Scholar] [CrossRef]
- An, T.; Jia, H.; Peng, L.; Xie, J. Material and Interfacial Modification toward a Stable Room-Temperature Solid-State Na–S Battery. ACS Appl. Mater. Interfaces 2020, 12, 20563–20569. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, H.; Yang, M.; Yan, X.; Yu, C.; Liu, D.; Zhang, L. High Performance Room Temperature All-Solid-State Na-SexS Battery with Na3SbS4–Coated Cathode via Aqueous Solution. J. Energy Chem. 2020, 48, 250–258. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, C.; Xu, R.; Peng, L.; Ren, H.; Zhang, J.; Zhang, L.; Cheng, S.; Xie, J. Iodine-Rich Lithium Argyrodite with Enhanced Ionic Conductivity for Solid-State Batteries. Scr. Mater. 2022, 210, 114475. [Google Scholar] [CrossRef]
- Jiang, Z.; Guo, H.-J.; Zeng, Z.; Han, Z.; Hu, W.; Wen, R.; Xie, J. Reconfiguring Organosulfur Cathode by Over-Lithiation to Enable Ultrathick Lithium Metal Anode toward Practical Lithium–Sulfur Batteries. ACS Nano 2020, 14, 13784–13793. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zeng, Z.; Liang, X.; Yang, L.; Hu, W.; Zhang, C.; Han, Z.; Feng, J.; Xie, J. Fluorobenzene, A Low-Density, Economical, and Bifunctional Hydrocarbon Cosolvent for Practical Lithium Metal Batteries. Adv. Funct. Mater. 2021, 31, 2005991. [Google Scholar] [CrossRef]
- Jiang, Z.; Zeng, Z.; Hu, W.; Han, Z.; Cheng, S.; Xie, J. Diluted High Concentration Electrolyte with Dual Effects for Practical Lithium-Sulfur Batteries. Energy Storage Mater. 2021, 36, 333–340. [Google Scholar] [CrossRef]
- Zhang, W.; Li, S.; Wang, L.; Wang, X.; Xie, J. Insight into Sulfur-Rich Selenium Sulfide/Pyrolyzed Polyacrylonitrile Cathodes for Li–S Batteries. Sustain. Energy Fuels 2020, 4, 3588–3596. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, D.; Wang, W.; Hu, K.; Huang, Q.; Zhang, Y.; Miao, Y.; Fu, L.; Wu, M.; Wu, Y. Toward Heat-Tolerant Potassium Batteries Based on Pyrolyzed Selenium Disulfide/Polyacrylonitrile Positive Electrode and Gel Polymer Electrolyte. J. Mater. Chem. A 2020, 8, 4544–4551. [Google Scholar] [CrossRef]
- He, B.; Rao, Z.; Cheng, Z.; Liu, D.; He, D.; Chen, J.; Miao, Z.; Yuan, L.; Li, Z.; Huang, Y. Rationally Design a Sulfur Cathode with Solid-Phase Conversion Mechanism for High Cycle-Stable Li–S Batteries. Adv. Energy Mater. 2021, 11, 2003690. [Google Scholar] [CrossRef]
- Teng, W.; Li, Y.; Ma, T.; Ren, X.; Nan, D.; Liu, J.; Wang, X.; Yang, Q.; Deng, J. Uniform Lithium Deposition Induced by ZnFx(OH)y for High-Performance Sulfurized Polyacrylonitrile-Based Lithium-Sulfur Batteries. Polymers 2022, 14, 4494. [Google Scholar] [CrossRef]
- Kong, X.; Kong, Y.; He, L.; Zhang, W.; Song, Y.; Liu, S.; Zhao, Y. A New Ether-Based Medium-Concentrated Electrolyte for Lithium–Sulfur Battery with Lean Li Anode. J. Power Sources 2022, 551, 232211. [Google Scholar] [CrossRef]
- Kong, X.; Kong, Y.; Liao, X.; Liu, S.; Zhao, Y. A Novel Mixed Ether-Based Electrolyte for Lithium–Sulfur Batteries with Li Anode Protection by Dual Salts. Sustain. Energy Fuels 2022, 6, 3658–3668. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, H.; Wu, Q.; Li, S.; Zhong, W.; He, R.; Cheng, S.; Xie, J. High-Performance Prelithiated Si-S Full Cell Enabled by Trifluorobenzene Modified Diluted High-Concentration Electrolyte. Mater. Today Energy 2022, 28, 101069. [Google Scholar] [CrossRef]
- Li, S.; Zhu, H.; Liu, Y.; Han, Z.; Peng, L.; Li, S.; Yu, C.; Cheng, S.; Xie, J. Codoped Porous Carbon Nanofibres as a Potassium Metal Host for Nonaqueous K-Ion Batteries. Nat. Commun. 2022, 13, 4911. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Chen, X.; Lin, C.; Wang, H.-E.; Xiong, P.; Chen, Q.; Qian, Q.; Wei, M.; Zeng, L. Stabilizing Intermediate Phases via the Efficient Confinement Effects of the SnS2-SPAN Fibre Composite for Ultra-Stable Half/Full Sodium/Potassium-Ion Batteries. J. Mater. Chem. A 2022, 10, 11449–11457. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, F.; Chen, X.; Xiong, P.; Lin, C.; Wang, H.-E.; Wei, M.; Qian, Q.; Chen, Q.; Zeng, L. Extraordinarily Stable and Wide-Temperature Range Sodium/Potassium-Ion Batteries Based on 1D SnSe-SePAN Composite Nanofibers. InfoMat 2023, 5, e12467. [Google Scholar] [CrossRef]
- He, R.; Li, Y.; Wei, S.; Liu, H.; Zhang, S.; Han, N.; Liu, H.; Wang, X.; Zhang, X. Construction of High-Performance Sulfurized Poly(Acrylonitrile) Cathodes for Lithium-Sulfur Batteries via Catalytic and Conductive Regulation. J. Alloys Compd. 2022, 919, 165838. [Google Scholar] [CrossRef]
- He, R.; Li, Y.; Yin, Z.; Liu, H.; Jin, Y.; Zhang, Y.; Liu, H.; Zhang, X. Selenium-Doped Sulfurized Poly(Acrylonitrile) Composites as Ultrastable and High-Volumetric-Capacity Cathodes for Lithium–Sulfur Batteries. ACS Appl. Energy Mater. 2023, 6, 3903–3914. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, W.; Li, S.; Zhong, W.; Zhu, H.; Zeng, Z.; Yu, C.; Cheng, S.; Xie, J. Electrospun Sulfurized Polyacrylonitrile Nanofibers for Long-Term Cycling Stability and High-Rate Lithium–Sulfur Batteries. ACS Appl. Energy Mater. 2022, 5, 5212–5218. [Google Scholar] [CrossRef]
- Xu, L.; Ye, J.; Guo, W.; Chen, T.; Chen, X.; Qian, Q.; Zhang, J.; Wei, M.; Peng, X.; Zeng, L. Rational Construction of VSe2 Encapsulated in Selenized Polyacrylonitrile toward a High-Rate Capacity and Wide Temperature Tolerance for Potassium-Ion Batteries. Inorg. Chem. Front. 2023, 10, 5053–5063. [Google Scholar] [CrossRef]
- Yuan, Z.; Zheng, J.; Chen, X.; Xiao, F.; Yang, X.; Luo, L.; Xiong, P.; Lai, W.; Lin, C.; Qin, F.; et al. In Situ Encapsulation of MoSxSe2–x Nanocrystals with the Synergistic Function of Anion Doping and Physical Confinement with Chemical Bonding for High-Performance Sodium/Potassium-Ion Batteries with Wide Temperature Workability. ACS Sustain. Chem. Eng. 2023, 11, 13050–13061. [Google Scholar] [CrossRef]
- Yang, F.; Yun, Z.; Gao, H.; Gao, S.; Tian, Z.; Huang, J. Se as Eutectic Accelerator SPAN Cathode for K−S Batteries with Improved Specific Capacity and Reaction Kinetics. ChemistrySelect 2023, 8, e202302227. [Google Scholar] [CrossRef]
- Liu, H.; Yan, T.; Xu, Q.; Zhang, Y.; Li, Y.; Han, N.; Liu, H.; Zhang, X. Enhancing the Performance of a Lithium-Sulfur Battery with Spatially Confined Mesoporous Nanoreactors in Sulfurized Polyacrylonitrile Cathodes. J. Colloid Interface Sci. 2025, 678, 829–840. [Google Scholar] [CrossRef]
- Ma, T.; Ren, X.; Hu, L.; Teng, W.; Wang, X.; Wu, G.; Liu, J.; Nan, D.; Li, B.; Yu, X. A Diluted Electrolyte for Long-Life Sulfurized Polyacrylonitrile-Based Anode-Free Li-S Batteries. Polymers 2022, 14, 3312. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Deng, J.; Lin, Y.; Liang, Q.; Hu, L.; Wang, X.; Liu, J.; Zhao, X.; Li, Y.; Nan, D.; et al. Li-Rich Organosulfur Cathode with Boosted Kinetics for High-Energy Lithium-Sulfur Batteries. Energy Environ. Mater. 2024, 7, e12704. [Google Scholar] [CrossRef]
- Ma, S.; Yu, Z.; Wang, L.; Zuo, P. Selenium-Doped Sulfurized Polyacrylonitrile Hybrid Cathodes with Ultrahigh Sulfur Content for High-Performance Solid-State Lithium Sulfur Batteries. Langmuir 2024, 40, 9255–9264. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, T.; Liu, Y.; Yu, T.; Chen, G.; Tang, W.; Li, L.; Wu, F.; Chen, R. Accelerating Redox Kinetics of Sulfurized Polyacrylonitrile Nanosheets by Trace Doping of Element. Chem. Eng. J. 2024, 487, 150300. [Google Scholar] [CrossRef]
- Li, S.; Han, Z.; Hu, W.; Peng, L.; Yang, J.; Wang, L.; Zhang, Y.; Shan, B.; Xie, J. Manipulating Kinetics of Sulfurized Polyacrylonitrile with Tellurium as Eutectic Accelerator to Prevent Polysulfide Dissolution in Lithium-Sulfur Battery under Dissolution-Deposition Mechanism. Nano Energy 2019, 60, 153–161. [Google Scholar] [CrossRef]
- Li, S.; Zeng, Z.; Yang, J.; Han, Z.; Hu, W.; Wang, L.; Ma, J.; Shan, B.; Xie, J. High Performance Room Temperature Sodium–Sulfur Battery by Eutectic Acceleration in Tellurium-Doped Sulfurized Polyacrylonitrile. ACS Appl. Energy Mater. 2019, 2, 2956–2964. [Google Scholar] [CrossRef]
- Wang, K.; Guan, Y.; Jin, Z.; Wang, W.; Wang, A. Te0.045S0.955PAN Composite with High Average Discharge Voltage for Li–S Battery. J. Energy Chem. 2019, 39, 249–255. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Peng, L.; Li, S.; Wang, X.; Cheng, S.; Xie, J. Elevating Reactivity and Cyclability of All-Solid-State Lithium-Sulfur Batteries by the Combination of Tellurium-Doping and Surface Coating. Nano Energy 2020, 76, 105083. [Google Scholar] [CrossRef]
- Li, Y.; He, R.; Liu, H.; Zhang, X. Construction of High-Rate Performance Sulfurized Poly(Acrylonitrile) Nanofibers Cathodes for Practical Lithium–Sulfur Batteries via Tellurium Catalytic Regulation. Mater. Chem. Phys. 2023, 308, 128288. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, W.; Qin, M.; Zhong, W.; Yan, H.; Zhu, H.; Cheng, S.; Xie, J. Tellurium Doped Sulfurized Polyacrylonitrile Nanoflower for High-Energy-Density, Long-Lifespan Sodium-Sulfur Batteries. Nano Energy 2024, 129, 110049. [Google Scholar] [CrossRef]
- Fang, C.; Li, J.; Zhang, M.; Zhang, Y.; Yang, F.; Lee, J.Z.; Lee, M.-H.; Alvarado, J.; Schroeder, M.A.; Yang, Y.; et al. Quantifying Inactive Lithium in Lithium Metal Batteries. Nature 2019, 572, 511–515. [Google Scholar] [CrossRef]
- Liu, J.; Bao, Z.; Cui, Y.; Dufek, E.J.; Goodenough, J.B.; Khalifah, P.; Li, Q.; Liaw, B.Y.; Liu, P.; Manthiram, A.; et al. Pathways for Practical High-Energy Long-Cycling Lithium Metal Batteries. Nat. Energy 2019, 4, 180–186. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Zhou, Z. Towards Practical Lithium-Metal Anodes. Chem. Soc. Rev. 2020, 49, 3040–3071. [Google Scholar] [CrossRef] [PubMed]
- Albertus, P.; Babinec, S.; Litzelman, S.; Newman, A. Status and Challenges in Enabling the Lithium Metal Electrode for High-Energy and Low-Cost Rechargeable Batteries. Nat. Energy 2018, 3, 16–21. [Google Scholar] [CrossRef]
- Ding, J.-F.; Xu, R.; Yan, C.; Li, B.-Q.; Yuan, H.; Huang, J.-Q. A Review on the Failure and Regulation of Solid Electrolyte Interphase in Lithium Batteries. J. Energy Chem. 2021, 59, 306–319. [Google Scholar] [CrossRef]
- Jin, L.; Shen, C.; Wu, Q.; Shellikeri, A.; Zheng, J.; Zhang, C.; Zheng, J.P. Pre-Lithiation Strategies for Next-Generation Practical Lithium-Ion Batteries. Adv. Sci. 2021, 8, 2005031. [Google Scholar] [CrossRef]
- He, X.; Ren, J.; Wang, L.; Pu, W.; Wan, C.; Jiang, C. Electrochemical Characteristics of Sulfur Composite Cathode for Reversible Lithium Storage. Ionics 2009, 15, 477–481. [Google Scholar] [CrossRef]
- Machida, K.; Miyauchi, H.; Ushioda, Y.; Takahashi, K.; Seki, S. Investigation for Charge-Discharge Operations of Li4Ti5O12-Sulfur Batteries by Suitable Choice of Materials and Cell Preparation Processes. Electrochemistry 2022, 90, 067006. [Google Scholar] [CrossRef]
- Shen, C.; Ye, D.; Jin, L.; Andrei, P.; Zheng, J.P. Communication—A Simple and Scalable Pre-Lithiation Approach for High Energy and Low Cost Lithium Ion Sulfur Batteries. J. Electrochem. Soc. 2020, 167, 060517. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Zhan, R.; Chen, Z.; Tu, S.; Li, C.; Liu, X.; Seh, Z.W.; Sun, Y. Nanocomposite of Conducting Polymer and Li Metal for Rechargeable High Energy Density Batteries. ACS Appl. Mater. Interfaces 2022, 14, 37709–37715. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Cao, C.; Zhao, W.; Huang, K.; Zhang, Y.; Shen, Y.; Li, Z.; Huang, Y. Lithium–Sulfur Pouch Cells with 99% Capacity Retention for 1000 Cycles. Energy Environ. Sci. 2024, 17, 7047–7057. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, J.; Pu, Y.; Wang, H.; Wang, B.; Qian, J.; Cao, Y.; Zhong, F.; Ai, X.; Yang, H. Effective Chemical Prelithiation Strategy for Building a Silicon/Sulfur Li-Ion Battery. ACS Energy Lett. 2019, 4, 1717–1724. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, S.; Mu, X.; Li, R.; Yin, G.; Zuo, P. A Scalable Cathode Chemical Prelithiation Strategy for Advanced Silicon-Based Lithium Ion Full Batteries. ACS Appl. Mater. Interfaces 2021, 13, 11985–11994. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, T.; Kuai, Y.; Yang, J.; Wang, J.; Nuli, Y.; Guo, Y.; Liang, C. A Superb 3D Composite Lithium Metal Anode Prepared by in-Situ Lithiation of Sulfurized Polyacrylonitrile. Energy Storage Mater. 2020, 33, 452–459. [Google Scholar] [CrossRef]
- Guo, J.; Yang, Z.; Yu, Y.; Abruña, H.D.; Archer, L.A. Lithium–Sulfur Battery Cathode Enabled by Lithium–Nitrile Interaction. J. Am. Chem. Soc. 2013, 135, 763–767. [Google Scholar] [CrossRef]
- Wang, P.; Xia, C.; Yang, J.; He, X.; Lv, K.; Ren, S.; Song, H.; Wang, J.; He, P.; Zhou, H. High-Energy Silicon-Sulfurized Poly(Acrylonitrile) Battery Based on a Nitrogen Evolution Reaction. Sci. Bull. 2022, 67, 256–262. [Google Scholar] [CrossRef]
- Chen, W.-J.; Li, B.-Q.; Zhao, C.-X.; Zhao, M.; Yuan, T.-Q.; Sun, R.-C.; Huang, J.-Q.; Zhang, Q. Electrolyte Regulation towards Stable Lithium-Metal Anodes in Lithium–Sulfur Batteries with Sulfurized Polyacrylonitrile Cathodes. Angew. Chem. Int. Ed. 2020, 59, 10732–10745. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Lee, S.; Agostini, M.; Jeong, M.-G.; Jung, H.-G.; Ming, J.; Sun, Y.-K.; Kim, J.; Hwang, J.-Y. Multiscale Understanding of Covalently Fixed Sulfur–Polyacrylonitrile Composite as Advanced Cathode for Metal–Sulfur Batteries. Adv. Sci. 2021, 8, 2101123. [Google Scholar] [CrossRef]
- Ma, T.; Tao, Z. Challenges and Prospects of Electrolyte Design for Lithium-Sulfurized Polyacrylonitrile Batteries. Batter. Supercaps 2024, 7, e202400284. [Google Scholar] [CrossRef]
- Wang, L.; He, X.; Li, J.; Chen, M.; Gao, J.; Jiang, C. Charge/Discharge Characteristics of Sulfurized Polyacrylonitrile Composite with Different Sulfur Content in Carbonate Based Electrolyte for Lithium Batteries. Electrochim. Acta 2012, 72, 114–119. [Google Scholar] [CrossRef]
- Warneke, S.; Hintennach, A.; Buchmeiser, M.R. Communication—Influence of Carbonate-Based Electrolyte Composition on Cell Performance of SPAN-Based Lithium-Sulfur-Batteries. J. Electrochem. Soc. 2018, 165, A2093. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, W.; Mao, S.; Li, S.; Wang, X.; Lu, Y. Tailored Electrolytes Enabling Practical Lithium–Sulfur Full Batteries via Interfacial Protection. ACS Energy Lett. 2021, 6, 2673–2681. [Google Scholar] [CrossRef]
- Wu, B.; Liu, Q.; Mu, D.; Ren, Y.; Li, Y.; Wang, L.; Xu, H.; Wu, F. New Desolvated Gel Electrolyte for Rechargeable Lithium Metal Sulfurized Polyacrylonitrile (S-PAN) Battery. J. Phys. Chem. C 2014, 118, 28369–28376. [Google Scholar] [CrossRef]
- Beltran, S.P.; Balbuena, P.B. A Solid Electrolyte Interphase to Protect the Sulfurized Polyacrylonitrile (SPAN) Composite for Li–S Batteries: Computational Approach Addressing the Electrolyte/SPAN Interfacial Reactivity. J. Mater. Chem. A 2021, 9, 7888–7902. [Google Scholar] [CrossRef]
- Klostermann, S.V.; Kappler, J.; Waigum, A.; Buchmeiser, M.R.; Köhn, A.; Kästner, J. The Reduction Behavior of Sulfurized Polyacrylonitrile (SPAN) in Lithium–Sulfur Batteries Using a Carbonate Electrolyte: A Computational Study. Phys. Chem. Chem. Phys. 2024, 26, 9998–10007. [Google Scholar] [CrossRef]
- Lin, F.; Wang, J.; Jia, H.; Monroe, C.W.; Yang, J.; NuLi, Y. Nonflammable Electrolyte for Rechargeable Lithium Battery with Sulfur Based Composite Cathode Materials. J. Power Sources 2013, 223, 18–22. [Google Scholar] [CrossRef]
- Wang, J.; Lin, F.; Jia, H.; Yang, J.; Monroe, C.W.; NuLi, Y. Towards a Safe Lithium–Sulfur Battery with a Flame-Inhibiting Electrolyte and a Sulfur-Based Composite Cathode. Angew. Chem. Int. Ed. 2014, 53, 10099–10104. [Google Scholar] [CrossRef]
- Jia, H.; Wang, J.; Lin, F.; Monroe, C.W.; Yang, J.; NuLi, Y. TPPi as a Flame Retardant for Rechargeable Lithium Batteries with Sulfur Composite Cathodes. Chem. Commun. 2014, 50, 7011–7013. [Google Scholar] [CrossRef]
- Yang, H.; Li, Q.; Guo, C.; Naveed, A.; Yang, J.; Nuli, Y.; Wang, J. Safer Lithium–Sulfur Battery Based on Nonflammable Electrolyte with Sulfur Composite Cathode. Chem. Commun. 2018, 54, 4132–4135. [Google Scholar] [CrossRef]
- Wu, J.; Liu, J.; Lu, Z.; Lin, K.; Lyu, Y.-Q.; Li, B.; Ciucci, F.; Kim, J.-K. Non-Flammable Electrolyte for Dendrite-Free Sodium-Sulfur Battery. Energy Storage Mater. 2019, 23, 8–16. [Google Scholar] [CrossRef]
- Wu, B.; Chen, F.; Mu, D.; Liao, W.; Wu, F. Cycleability of Sulfurized Polyacrylonitrile Cathode in Carbonate Electrolyte Containing Lithium Metasilicate. J. Power Sources 2015, 278, 27–31. [Google Scholar] [CrossRef]
- Wang, L.; Li, Q.; Yang, H.; Yang, J.; Nuli, Y.; Wang, J. Superior Rate Capability of a Sulfur Composite Cathode in a Tris(Trimethylsilyl)Borate-Containing Functional Electrolyte. Chem. Commun. 2016, 52, 14430–14433. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, J.; Yang, J.; Miao, X.; Chen, R.; Qian, J.; Miao, R. Enhanced Performance of a Lithium–Sulfur Battery Using a Carbonate-Based Electrolyte. Angew. Chem. Int. Ed. 2016, 55, 10372–10375. [Google Scholar] [CrossRef]
- Kim, H.M.; Hwang, J.-Y.; Aurbach, D.; Sun, Y.-K. Electrochemical Properties of Sulfurized-Polyacrylonitrile Cathode for Lithium–Sulfur Batteries: Effect of Polyacrylic Acid Binder and Fluoroethylene Carbonate Additive. J. Phys. Chem. Lett. 2017, 8, 5331–5337. [Google Scholar] [CrossRef]
- Yang, H.; Naveed, A.; Li, Q.; Guo, C.; Chen, J.; Lei, J.; Yang, J.; Nuli, Y.; Wang, J. Lithium Sulfur Batteries with Compatible Electrolyte Both for Stable Cathode and Dendrite-Free Anode. Energy Storage Mater. 2018, 15, 299–307. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, J.; Guo, Y.; Liang, C.; Yang, J.; Wang, J.; Nuli, Y. A Compatible Carbonate Electrolyte with Lithium Anode for High Performance Lithium Sulfur Battery. Electrochim. Acta 2018, 282, 555–562. [Google Scholar] [CrossRef]
- Shuai, Y.; Zhang, Z.; Chen, K.; Lou, J.; Wang, Y. Highly Stable Lithium Plating by a Multifunctional Electrolyte Additive in a Lithium-Sulfurized Polyacrylonitrile Battery. Chem. Commun. 2019, 55, 2376–2379. [Google Scholar] [CrossRef]
- Cai, G.; Holoubek, J.; Xia, D.; Li, M.; Yin, Y.; Xing, X.; Liu, P.; Chen, Z. An Ester Electrolyte for Lithium–Sulfur Batteries Capable of Ultra-Low Temperature Cycling. Chem. Commun. 2020, 56, 9114–9117. [Google Scholar] [CrossRef]
- Park, H.; Kang, H.; Kim, H.; Kansara, S.; Allen, J.L.; Tran, D.; Sun, H.H.; Hwang, J.-Y. Strategy for High-Energy Li–S Battery Coupling with a Li Metal Anode and a Sulfurized Polyacrylonitrile Cathode. ACS Appl. Mater. Interfaces 2023, 15, 45876–45885. [Google Scholar] [CrossRef]
- Dai, H.; Xi, K.; Liu, X.; Lai, C.; Zhang, S. Cationic Surfactant-Based Electrolyte Additives for Uniform Lithium Deposition via Lithiophobic Repulsion Mechanisms. J. Am. Chem. Soc. 2018, 140, 17515–17521. [Google Scholar] [CrossRef]
- Jin, F.; Hu, C.; Liu, C.; Zheng, Y.; Chen, H.; Shen, Y.; Chen, L. Enhancing the Performance of Sulfurized Polyacrylonitrile Cathode by in-Situ Wrapping. J. Electroanal. Chem. 2019, 835, 156–160. [Google Scholar] [CrossRef]
- Li, Q.; Yang, H.; Naveed, A.; Guo, C.; Yang, J.; Nuli, Y.; Wang, J. Duplex Component Additive of Tris(Trimethylsilyl) Phosphite-Vinylene Carbonate for Lithium Sulfur Batteries. Energy Storage Mater. 2018, 14, 75–81. [Google Scholar] [CrossRef]
- Zhao, J.; Wan, Z.; Zeng, X.; Tian, M.; Wang, K.; Chen, X.; Ling, M.; Ni, W.; Liang, C. Solubilized LiNO3 by the Cationic Size Effect of CsF for Lithium Metal Anode Protection and Dendrite Formation Prevention in Carbonate Electrolyte. Inorg. Chem. Front. 2023, 10, 1809–1817. [Google Scholar] [CrossRef]
- Guo, Z.; Yang, H.; Wei, Q.; Xu, S.; Hu, G.; Bai, S.; Li, F. Dual-Additives Enable Stable Electrode-Electrolyte Interfaces for Long Life Li-SPAN Batteries. Chin. Chem. Lett. 2024, 35, 108622. [Google Scholar] [CrossRef]
- Lebherz, T.; Weldin, D.L.; Hintennach, A.; Buchmeiser, M.R. Synthesis of Ionic Dendrimers and Their Potential Use as Electrolytes for Lithium–Sulfur Batteries. Macromol. Chem. Phys. 2020, 221, 1900436. [Google Scholar] [CrossRef]
- Mayer, A.; Buchmeiser, M.R. Communication—Influence of Temperature and Electrolyte Viscosity on the Electrochemical Performance of SPAN-Based Lithium-Sulfur Cells. J. Electrochem. Soc. 2018, 165, A3943. [Google Scholar] [CrossRef]
- Cai, G.; Gao, H.; Li, M.; Gupta, V.; Holoubek, J.; Pascal, T.A.; Liu, P.; Chen, Z. Partially Ion-Paired Solvation Structure Design for Lithium-Sulfur Batteries under Extreme Operating Conditions. Angew. Chem. Int. Ed. 2024, 63, e202316786. [Google Scholar] [CrossRef]
- Zhao, C.-X.; Chen, W.-J.; Zhao, M.; Song, Y.-W.; Liu, J.-N.; Li, B.-Q.; Yuan, T.; Chen, C.-M.; Zhang, Q.; Huang, J.-Q. Redox Mediator Assists Electron Transfer in Lithium–Sulfur Batteries with Sulfurized Polyacrylonitrile Cathodes. EcoMat 2021, 3, e12066. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, Q.; Zeng, Z.; Yu, C.; Cheng, S.; Xie, J. An Organodiselenide Containing Electrolyte Enables Sulfurized Polyacrylonitrile Cathodes with Fast Redox Kinetics in Li–S Batteries. Chem. Commun. 2021, 57, 9688–9691. [Google Scholar] [CrossRef]
- Chen, S.; Wu, G.; Jiang, H.; Wang, J.; Chen, T.; Han, C.; Wang, W.; Yang, R.; Zhao, J.; Tang, Z.; et al. External Li Supply Reshapes Li Deficiency and Lifetime Limit of Batteries. Nature 2025, 638, 676–683. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Nuli, Y.; Holze, R. Room Temperature Na/S Batteries with Sulfur Composite Cathode Materials. Electrochem. Commun. 2007, 9, 31–34. [Google Scholar] [CrossRef]
- Murugan, S.; Niesen, S.; Kappler, J.; Küster, K.; Starke, U.; Buchmeiser, M.R. Ultra-Stable Cycling of High Capacity Room Temperature Sodium-Sulfur Batteries Based on Sulfurated Poly(Acrylonitrile). Batter. Supercaps 2021, 4, 1636–1646. [Google Scholar] [CrossRef]
- Murugan, S.; Klostermann, S.V.; Frey, W.; Kästner, J.; Buchmeiser, M.R. A Sodium Bis(Perfluoropinacol) Borate-Based Electrolyte for Stable, High-Performance Room Temperature Sodium-Sulfur Batteries Based on Sulfurized Poly(Acrylonitrile). Electrochem. Commun. 2021, 132, 107137. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Wang, J.; Zhang, Y.; Zhu, Y.; Chen, Y.; Fu, L.; Wu, Y. Sulfur Nanocomposite as a Positive Electrode Material for Rechargeable Potassium–Sulfur Batteries. Chem. Commun. 2018, 54, 2288–2291. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Zhang, Y.; Shuai, Y.; Chen, K.; Chen, S. Na+/K+ Hybrid Battery Based on a Sulfurized Polyacrylonitrile Cathode. Materials 2019, 12, 969. [Google Scholar] [CrossRef]
- Wu, Z.; Bak, S.-M.; Shadike, Z.; Yu, S.; Hu, E.; Xing, X.; Du, Y.; Yang, X.-Q.; Liu, H.; Liu, P. Understanding the Roles of the Electrode/Electrolyte Interface for Enabling Stable Li∥Sulfurized Polyacrylonitrile Batteries. ACS Appl. Mater. Interfaces 2021, 13, 31733–31740. [Google Scholar] [CrossRef]
- Miao, R.; Yang, J.; Feng, X.; Jia, H.; Wang, J.; Nuli, Y. Novel Dual-Salts Electrolyte Solution for Dendrite-Free Lithium-Metal Based Rechargeable Batteries with High Cycle Reversibility. J. Power Sources 2014, 271, 291–297. [Google Scholar] [CrossRef]
- Kuai, D.; Wang, S.; Perez-Beltran, S.; Yu, S.; Real, G.A.; Liu, P.; Balbuena, P.B. Interfacial Electrochemical Lithiation and Dissolution Mechanisms at a Sulfurized Polyacrylonitrile Cathode Surface. ACS Energy Lett. 2024, 9, 810–818. [Google Scholar] [CrossRef]
- Shuai, Y.; Wang, D.; Chen, K.; Zhang, Z.; Wang, Y.; Lou, J. Highly Stable Performance of Lithium-Sulfurized Polyacrylonitrile Batteries Using a Lean Ether-Based Electrolyte. Chem. Commun. 2019, 55, 11271–11274. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kim, D.W.; Jung, H.T.; Choi, J.W. Controlled Lithium Dendrite Growth by a Synergistic Effect of Multilayered Graphene Coating and an Electrolyte Additive. Chem. Mater. 2015, 27, 2780–2787. [Google Scholar] [CrossRef]
- Kim, J.-S.; Yoo, D.-J.; Min, J.; Shakoor, R.A.; Kahraman, R.; Choi, J.W. Poreless Separator and Electrolyte Additive for Lithium–Sulfur Batteries with High Areal Energy Densities. ChemNanoMat 2015, 1, 240–245. [Google Scholar] [CrossRef]
- Jia, P.; Wang, J.; Zheng, T.; Tao, C.; Yila, G.; Wang, L.; Wang, Y.; Liu, T. Boosting Cathode Activity and Anode Stability of Lithium–Sulfur Batteries with Vigorous Iodic Species Triggered by Nitrate. Angew. Chem. Int. Ed. 2024, 63, e202401055. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Chen, Z.; Zhang, R.; Lu, C.; Zhu, J.; Zhuang, X. Realizing Sulfurized Polyacrylonitrile Cathode in Ether-Based Electrolyte. Electrochim. Acta 2024, 474, 143533. [Google Scholar] [CrossRef]
- Chen, W.-J.; Zhao, C.-X.; Li, B.-Q.; Jin, Q.; Zhang, X.-Q.; Yuan, T.-Q.; Zhang, X.; Jin, Z.; Kaskel, S.; Zhang, Q. A Mixed Ether Electrolyte for Lithium Metal Anode Protection in Working Lithium–Sulfur Batteries. Energy Environ. Mater. 2020, 3, 160–165. [Google Scholar] [CrossRef]
- Zheng, X.; Zou, F.; Yang, H.; Su, X.; Hu, X.; Liu, X. 4-Aminobenzoic Acid as an Electrolyte Additive for Enhancing the Electrochemical Properties of the Sulfurized Polyacrylonitrile Cathode in Ether Electrolyte. Ionics 2023, 29, 3663–3671. [Google Scholar] [CrossRef]
- Wu, Q.; Ma, F.; Zhang, W.; Yan, H.; Chen, X.; Cheng, S.; Xie, J. Bifunctional Li2Se Mediator to Accelerate Sulfur Conversion and Lithium Deposition Kinetics in Lithium–Sulfurized Polyacrylonitrile Batteries. ACS Appl. Energy Mater. 2023, 6, 7138–7146. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, F.; Wu, Q.; Cai, Z.; Zhong, W.; Zeng, Z.; Cheng, S.; Xie, J. Bifunctional Fluorinated Anthraquinone Additive for Improving Kinetics and Interfacial Chemistry in Rechargeable Li–S Batteries. ACS Appl. Energy Mater. 2022, 5, 15719–15728. [Google Scholar] [CrossRef]
- Holoubek, J.; Liu, H.; Wu, Z.; Yin, Y.; Xing, X.; Cai, G.; Yu, S.; Zhou, H.; Pascal, T.A.; Chen, Z.; et al. Tailoring Electrolyte Solvation for Li Metal Batteries Cycled at Ultra-Low Temperature. Nat. Energy 2021, 6, 303–313. [Google Scholar] [CrossRef]
- Ma, T.; Ni, Y.; Wang, Q.; Zhang, W.; Jin, S.; Zheng, S.; Yang, X.; Hou, Y.; Tao, Z.; Chen, J. Optimize Lithium Deposition at Low Temperature by Weakly Solvating Power Solvent. Angew. Chem. 2022, 134, e202207927. [Google Scholar] [CrossRef]
- Liu, X.; Wu, S.; Hao, Z.; Shang, L.; Guo, M.; Hou, J.; Shao, S.; Li, H.; Li, Y.; Lu, Y.; et al. Regulating Interface Dipole Interaction between Ethers and Active Species Toward Highly Stable Li-SPAN Batteries. Angew. Chem. Int. Ed. 2025, 64, e202416731. [Google Scholar] [CrossRef]
- Cai, G.; Holoubek, J.; Li, M.; Gao, H.; Yin, Y.; Yu, S.; Liu, H.; Pascal, T.A.; Liu, P.; Chen, Z. Solvent Selection Criteria for Temperature-Resilient Lithium–Sulfur Batteries. Proc. Natl. Acad. Sci. USA 2022, 119, e2200392119. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, M.; Ma, L.; Li, X.; Chen, X.; Long, J.; Wang, Y.; Li, X.; Liu, J.; Guo, Z.; et al. Engineering Densely Packed Ion-Cluster Electrolytes for Wide-Temperature Lithium–Sulfurized Polyacrylonitrile Batteries. ACS Nano 2024, 18, 32984–32994. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Ni, Y.; Li, D.; Zha, Z.; Jin, S.; Zhang, W.; Jia, L.; Sun, Q.; Xie, W.; Tao, Z.; et al. Reversible Solid–Solid Conversion of Sulfurized Polyacrylonitrile Cathodes in Lithium–Sulfur Batteries by Weakly Solvating Ether Electrolytes. Angew. Chem. Int. Ed. 2023, 62, e202310761. [Google Scholar] [CrossRef]
- Liu, T.; Feng, J.; Shi, Z.; Li, H.; Zhao, W.; Mao, M.; Zhu, X.; Hu, Y.-S.; Li, H.; Huang, X.; et al. Diminishing Ether-Oxygen Content of Electrolytes Enables Temperature-Immune Lithium Metal Batteries. Sci. China Chem. 2023, 66, 2700–2710. [Google Scholar] [CrossRef]
- Pham, T.D.; Bin Faheem, A.; Kim, J.; Ma, S.-H.; Kwak, K.; Lee, K.-K. High-Efficiency Lithium Metal Stabilization and Polysulfide Suppression in Li-S Battery Enabled by Weakly Solvating Solvent. Small 2024, 20, 2307951. [Google Scholar] [CrossRef]
- Yin, Y.; Holoubek, J.; Kim, K.; Liu, A.; Bhamwala, B.; Wang, S.; Lu, B.; Yu, K.; Gao, H.; Li, M.; et al. Coulombic Condensation of Liquefied Gas Electrolytes for Li Metal Batteries at Ambient Pressure. Angew. Chem. Int. Ed. 2025, 64, e202420411. [Google Scholar] [CrossRef]
- Chen, J.; Lu, H.; Zhang, X.; Zhang, Y.; Yang, J.; Nuli, Y.; Huang, Y.; Wang, J. Electrochemical Polymerization of Nonflammable Electrolyte Enabling Fast-Charging Lithium-Sulfur Battery. Energy Storage Mater. 2022, 50, 387–394. [Google Scholar] [CrossRef]
- Zhou, L.; Cao, Z.; Zhang, J.; Sun, Q.; Wu, Y.; Wahyudi, W.; Hwang, J.-Y.; Wang, L.; Cavallo, L.; Sun, Y.-K.; et al. Engineering Sodium-Ion Solvation Structure to Stabilize Sodium Anodes: Universal Strategy for Fast-Charging and Safer Sodium-Ion Batteries. Nano Lett. 2020, 20, 3247–3254. [Google Scholar] [CrossRef]
- Liu, D.; Li, Z.; Li, X.; Chen, X.; Li, Z.; Yuan, L.; Huang, Y. Stable Room-Temperature Sodium–Sulfur Batteries in Ether-Based Electrolytes Enabled by the Fluoroethylene Carbonate Additive. ACS Appl. Mater. Interfaces 2022, 14, 6658–6666. [Google Scholar] [CrossRef]
- Soni, C.B.; Bera, S.; Sungjemmenla; Chandra, M.; K., V.S.; Kumar, S.; Kumar, H.; Kumar, V. Altering Na-Ion Solvation to Regulate Dendrite Growth for a Reversible and Stable Room-Temperature Sodium–Sulfur Battery. J. Mater. Chem. A 2024, 12, 21853–21863. [Google Scholar] [CrossRef]
- Basel, J.; Sapkota, N.; Parekh, M.; Rao, A.M. Electrolyte Optimization for Sodium-Sulfur Batteries. Appl. Phys. Lett. 2024, 124, 123901. [Google Scholar] [CrossRef]
- Li, A.-M.; Zavalij, P.Y.; Omenya, F.; Li, X.; Wang, C. Salt-in-Presalt Electrolyte Solutions for High-Potential Non-Aqueous Sodium Metal Batteries. Nat. Nanotechnol. 2025, 20, 388–396. [Google Scholar] [CrossRef]
- Park, J.; Oh, G.; Kim, U.-H.; Alfaruqi, M.H.; Xu, X.; Liu, Y.; Xiong, S.; Zikri, A.T.; Sun, Y.-K.; Kim, J.; et al. Regulating the Solvation Structure of Electrolyte via Dual–Salt Combination for Stable Potassium Metal Batteries. Adv. Sci. 2023, 10, 2301201. [Google Scholar] [CrossRef]
- Wang, P.; Buchmeiser, M.R. Rechargeable Magnesium–Sulfur Battery Technology: State of the Art and Key Challenges. Adv. Funct. Mater. 2019, 29, 1905248. [Google Scholar] [CrossRef]
- Wang, P.; Trück, J.; Niesen, S.; Kappler, J.; Küster, K.; Starke, U.; Ziegler, F.; Hintennach, A.; Buchmeiser, M.R. High-Performance Magnesium-Sulfur Batteries Based on a Sulfurated Poly(Acrylonitrile) Cathode, a Borohydride Electrolyte, and a High-Surface Area Magnesium Anode. Batter. Supercaps 2020, 3, 1239–1247. [Google Scholar] [CrossRef]
- Wang, P.; Kappler, J.; Sievert, B.; Häcker, J.; Küster, K.; Starke, U.; Ziegler, F.; Buchmeiser, M.R. Characteristics of Magnesium-Sulfur Batteries Based on a Sulfurized Poly(Acrylonitrile) Composite and a Fluorinated Electrolyte. Electrochim. Acta 2020, 361, 137024. [Google Scholar] [CrossRef]
- Shin, E.S.; Kim, K.; Oh, S.H.; Cho, W.I. Polysulfide Dissolution Control: The Common Ion Effect. Chem. Commun. 2013, 49, 2004–2006. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Liu, S.; Li, G.C.; Li, G.R.; Gao, X.P. Sulfur/Polyacrylonitrile/Carbon Multi-Composites as Cathode Materials for Lithium/Sulfur Battery in the Concentrated Electrolyte. J. Mater. Chem. A 2014, 2, 4652–4659. [Google Scholar] [CrossRef]
- Zhou, J.; Guo, Y.; Liang, C.; Cao, L.; Pan, H.; Yang, J.; Wang, J. A New Ether-Based Electrolyte for Lithium Sulfur Batteries Using a S@pPAN Cathode. Chem. Commun. 2018, 54, 5478–5481. [Google Scholar] [CrossRef]
- Xing, X.; Li, Y.; Wang, X.; Petrova, V.; Liu, H.; Liu, P. Cathode Electrolyte Interface Enabling Stable Li–S Batteries. Energy Storage Mater. 2019, 21, 474–480. [Google Scholar] [CrossRef]
- Ren, Y.; Lai, T.; Manthiram, A. Reversible Sodium–Sulfur Batteries Enabled by a Synergistic Dual-Additive Design. ACS Energy Lett. 2023, 8, 2746–2752. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, Z.; Zhou, L.; Park, G.-T.; Cavallo, L.; Wang, L.; Alshareef, H.N.; Sun, Y.-K.; Ming, J. Model-Based Design of Stable Electrolytes for Potassium Ion Batteries. ACS Energy Lett. 2020, 5, 3124–3131. [Google Scholar] [CrossRef]
- Lee, S.; Park, H.; Rizell, J.; Kim, U.-H.; Liu, Y.; Xu, X.; Xiong, S.; Matic, A.; Zikri, A.T.; Kang, H.; et al. High-Energy and Long-Lifespan Potassium–Sulfur Batteries Enabled by Concentrated Electrolyte. Adv. Funct. Mater. 2022, 32, 2209145. [Google Scholar] [CrossRef]
- Yoo, D.-J.; Yang, S.; Kim, K.J.; Choi, J.W. Fluorinated Aromatic Diluent for High-Performance Lithium Metal Batteries. Angew. Chem. Int. Ed. 2020, 59, 14869–14876. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, H.; Zhang, X.; Lei, J.; Zhang, H.; Yuan, H.; Yang, J.; Nuli, Y.; Wang, J. Highly Reversible Lithium-Metal Anode and Lithium–Sulfur Batteries Enabled by an Intrinsic Safe Electrolyte. ACS Appl. Mater. Interfaces 2019, 11, 33419–33427. [Google Scholar] [CrossRef]
- Yu, K.; Cai, G.; Li, M.; Wu, J.; Gupta, V.; Lee, D.J.; Holoubek, J.; Chen, Z. Effect of Electrolyte Chemistry and Sulfur Content in Li||Sulfurized Polyacrylonitrile (SPAN) Batteries. ACS Appl. Mater. Interfaces 2023, 15, 43724–43731. [Google Scholar] [CrossRef]
- Han, Z.; Li, S.; Sun, M.; He, R.; Zhong, W.; Yu, C.; Cheng, S.; Xie, J. Fluorobenzene Diluted Low-Density Electrolyte for High-Energy Density and High-Performance Lithium-Sulfur Batteries. J. Energy Chem. 2022, 68, 752–761. [Google Scholar] [CrossRef]
- Liu, H.; Holoubek, J.; Zhou, H.; Chen, A.; Chang, N.; Wu, Z.; Yu, S.; Yan, Q.; Xing, X.; Li, Y.; et al. Ultrahigh Coulombic Efficiency Electrolyte Enables Li||SPAN Batteries with Superior Cycling Performance. Mater. Today 2021, 42, 17–28. [Google Scholar] [CrossRef]
- He, Y.; Zou, P.; Bak, S.-M.; Wang, C.; Zhang, R.; Yao, L.; Du, Y.; Hu, E.; Lin, R.; Xin, H.L. Dual Passivation of Cathode and Anode through Electrode–Electrolyte Interface Engineering Enables Long-Lifespan Li Metal–SPAN Batteries. ACS Energy Lett. 2022, 7, 2866–2875. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, J. Dual Strategy with Li-Ion Solvation and Solid Electrolyte Interphase for High Coulombic Efficiency of Lithium Metal Anode. Energy Storage Mater. 2022, 44, 48–56. [Google Scholar] [CrossRef]
- Tan, S.; Liu, H.; Wu, Z.; Weiland, C.; Bak, S.-M.; Ronne, A.; Liu, P.; Whittingham, M.S.; Shadike, Z.; Hu, E.; et al. Isoxazole-Based Electrolytes for Lithium Metal Protection and Lithium-Sulfurized Polyacrylonitrile (SPAN) Battery Operating at Low Temperature. J. Electrochem. Soc. 2022, 169, 030513. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, P.; Wu, Z.; Engelhard, M.H.; Cao, X.; Jia, H.; Xu, Y.; Liu, H.; Wang, C.; Liu, J.; et al. Pinned Electrode/Electrolyte Interphase and Its Formation Origin for Sulfurized Polyacrylonitrile Cathode in Stable Lithium Batteries. ACS Appl. Mater. Interfaces 2022, 14, 52046–52057. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Diemant, T.; Mariani, A.; Dong, X.; Di Pietro, M.E.; Mele, A.; Passerini, S. Locally Concentrated Ionic Liquid Electrolyte with Partially Solvating Diluent for Lithium/Sulfurized Polyacrylonitrile Batteries. Adv. Mater. 2022, 34, 2207155. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Ma, Z.; Wang, Y.; Zhao, F.; Cao, Z.; Cavallo, L.; Li, Q.; Ming, J. Solvent–Solvent Interaction Mediated Lithium-Ion (De)Intercalation Chemistry in Propylene Carbonate Based Electrolytes for Lithium–Sulfur Batteries. ACS Nano 2023, 17, 18062–18073. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qi, X.; Zhou, H.; Yang, F.; Jin, X.; Jiang, R.; Zhu, Z.; Liang, C.; Li, Z.; Yuan, L.; et al. Stabilizing SPAN in Non-Flammable Acetonitrile Electrolytes for Long-Life Graphite||SPAN Batteries. Angew. Chem. Int. Ed. 2025, 64, e202419995. [Google Scholar] [CrossRef]
- Kim, J.-M.; Gao, P.; Miao, Q.; Zhao, Q.; Rahman, M.M.; Chen, P.; Zhang, X.; Hu, E.; Liu, P.; Zhang, J.-G.; et al. Tailoring Solvation Solvent in Localized High-Concentration Electrolytes for Lithium||Sulfurized Polyacrylonitrile. ACS Appl. Mater. Interfaces 2024, 16, 20618–20625. [Google Scholar] [CrossRef]
- Liu, X.; Mariani, A.; Diemant, T.; Di Pietro, M.E.; Dong, X.; Su, P.-H.; Mele, A.; Passerini, S. PFAS-Free Locally Concentrated Ionic Liquid Electrolytes for Lithium Metal Batteries. ACS Energy Lett. 2024, 9, 3049–3057. [Google Scholar] [CrossRef]
- Qin, K.; Li, S.; Yen, D.; Zhang, W.; Yang, Z.; Kamphaus, E.P.; Kim, E.Y.; Huang, J.; Shea, J.J.; Hu, E.; et al. A Coordinated-Anion-Enriched Electrolyte for Lean-Electrolyte Li–S Batteries. ACS Energy Lett. 2024, 9, 3869–3876. [Google Scholar] [CrossRef]
- Phan, A.L.; Nan, B.; Le, P.M.L.; Miao, Q.; Wu, Z.; Le, K.; Chen, F.; Engelhard, M.; Dan Nguyen, T.; Han, K.S.; et al. Lightweight Electrolyte Design for Li/Sulfurized Polyacrylonitrile (SPAN) Batteries. Adv. Mater. 2024, 36, 2406594. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Q.; Song, C.; Jiang, Y.; Sheng, X.; Pan, H.; Yang, L.; Wu, S.; Zeng, L.; Sun, D.; et al. A “Flexible” Solvent Molecule Enabling High-Performance Lithium Metal Batteries. Angew. Chem. Int. Ed. 2025, 64, e202422791. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, H.; Holoubek, J.; Anderson, C.; Shi, L.; Khemchandani, H.; Lu, D.; Liu, D.; Niu, C.; Xiao, J.; et al. The Role of Ion Transport in the Failure of High Areal Capacity Li Metal Batteries. ACS Energy Lett. 2022, 7, 2701–2710. [Google Scholar] [CrossRef]
- Pai, M.-H.; Lai, T.; Manthiram, A. Sodium–Sulfur Cells with a Sulfurized Polyacrylonitrile Cathode and a Localized High Concentration Electrolyte with Toluene as a Nonfluorinated Diluent. Adv. Funct. Mater. 2024, 34, 2407450. [Google Scholar] [CrossRef]
- Pai, M.-H.; Manthiram, A. Fluorine-Free Cosolvent Chemistry Empowering Sodium-Sulfurized Polyacrylonitrile Batteries. Adv. Energy Mater. 2025, 15, 2500026. [Google Scholar] [CrossRef]
- Lu, H.; Wang, Q.; Chen, J.; Zhang, H.; Ding, J.; Nuli, Y.; Yang, J.; Wang, J. Electrolyte Solvation Regulation Engineering Promotes Li-SPAN Battery without Esters. Energy Storage Mater. 2023, 63, 102994. [Google Scholar] [CrossRef]
- Li, M.; Chen, H.; Wang, Y.; Chen, X.; Wu, J.; Su, J.; Wang, M.; Li, X.; Li, C.; Ma, L.; et al. Two Birds with One Stone: Engineering Siloxane-Based Electrolytes for High-Performance Lithium–Sulfur Polyacrylonitrile Batteries. J. Mater. Chem. A 2023, 11, 11721–11729. [Google Scholar] [CrossRef]
- Ma, H.; Wang, Q.; Lu, H.; Si, Y.; Kong, X.; Wang, J. An Intrinsic Safe Siloxane Ether-Based Electrolyte for Lithium-Sulfur Batteries at High Temperatures. Chem. Eng. J. 2024, 479, 147557. [Google Scholar] [CrossRef]
- Tian, Y.; Li, M.; Chen, H.; Zhu, K.; Long, J.; Xie, W.; Chen, X.; Li, X.; Wu, J.; Chen, Y. Tailoring Eco-Friendly Siloxane-Based Electrolytes for High-Performance Lithium–Sulfur Batteries. Mater. Sci. Eng. B 2024, 310, 117773. [Google Scholar] [CrossRef]
- Liao, K.; Pai, M.-H.; Manthiram, A. Tuning the Solvation Structure of a Weakly Solvating Cyclic Ether Electrolyte for Wide-Temperature Cycling of Lithium-Sulfurized Polyacrylonitrile Batteries. Adv. Energy Mater. 2025, 15, 2403733. [Google Scholar] [CrossRef]
- Ren, W.; Wu, D.; NuLi, Y.; Zhang, X.; Yang, J.; Wang, J. A Chlorine-Free Electrolyte Based on Non-Nucleophilic Magnesium Bis(Diisopropyl)Amide and Ionic Liquid for Rechargeable Magnesium Batteries. ACS Appl. Mater. Interfaces 2021, 13, 32957–32967. [Google Scholar] [CrossRef]
- Wang, P.; Küster, K.; Starke, U.; Liang, C.; Niewa, R.; Buchmeiser, M.R. Performance Enhancement of Rechargeable Magnesium–Sulfur Batteries Based on a Sulfurized Poly(Acrylonitrile) Composite and a Lithium Salt. J. Power Sources 2021, 515, 230604. [Google Scholar] [CrossRef]
- Zhang, S.; Ren, W.; NuLi, Y.; Wang, B.; Yang, J.; Wang, J. Sulfurized-Pyrolyzed Polyacrylonitrile Cathode for Magnesium-Sulfur Batteries Containing Mg2+/Li+ Hybrid Electrolytes. Chem. Eng. J. 2022, 427, 130902. [Google Scholar] [CrossRef]
- Li, H.; Zhang, M.; Zheng, Z.; Wu, X.; Xiao, X.; Piao, Z.; Li, C.; Jia, Y.; Yang, J.; Zhou, G. High-Capacity and Long-Life Aqueous Zn-SPAN Batteries with Tandem Catalysis. Adv. Mater. 2025, 37, 2409771. [Google Scholar] [CrossRef]
- Cheng, X.; Pan, J.; Zhao, Y.; Liao, M.; Peng, H. Gel Polymer Electrolytes for Electrochemical Energy Storage. Adv. Energy Mater. 2018, 8, 1702184. [Google Scholar] [CrossRef]
- Qian, J.; Jin, B.; Li, Y.; Zhan, X.; Hou, Y.; Zhang, Q. Research Progress on Gel Polymer Electrolytes for Lithium-Sulfur Batteries. J. Energy Chem. 2021, 56, 420–437. [Google Scholar] [CrossRef]
- Jeddi, K.; Ghaznavi, M.; Chen, P. A Novel Polymer Electrolyte to Improve the Cycle Life of High Performance Lithium–Sulfur Batteries. J. Mater. Chem. A 2013, 1, 2769–2772. [Google Scholar] [CrossRef]
- Jeddi, K.; Zhao, Y.; Zhang, Y.; Konarov, A.; Chen, P. Fabrication and Characterization of an Effective Polymer Nanocomposite Electrolyte Membrane for High Performance Lithium/Sulfur Batteries. J. Electrochem. Soc. 2013, 160, A1052. [Google Scholar] [CrossRef]
- Jeddi, K.; Sarikhani, K.; Qazvini, N.T.; Chen, P. Stabilizing Lithium/Sulfur Batteries by a Composite Polymer Electrolyte Containing Mesoporous Silica Particles. J. Power Sources 2014, 245, 656–662. [Google Scholar] [CrossRef]
- Li, Z.; Lu, W.; Zhang, N.; Pan, Q.; Chen, Y.; Xu, G.; Zeng, D.; Zhang, Y.; Cai, W.; Yang, M.; et al. Single Ion Conducting Lithium Sulfur Polymer Batteries with Improved Safety and Stability. J. Mater. Chem. A 2018, 6, 14330–14338. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, D.; Yan, W.; Huang, Q.; Zhu, Y.; Fu, L.; Wu, Y. Synergy of Sulfur/Polyacrylonitrile Composite and Gel Polymer Electrolyte Promises Heat-Resistant Lithium-Sulfur Batteries. iScience 2019, 19, 316–325. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, H.; Chen, J.; Nuli, Y.; Wang, J. A Novel Filler for Gel Polymer Electrolyte with a High Lithium-Ion Transference Number toward Stable Cycling for Lithium-Metal Anodes in Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2021, 13, 48622–48633. [Google Scholar] [CrossRef]
- Huang, Y.; Ji, Y.; Zhang, L.; Cai, C.; Fu, Y. Integrated Configuration Design Strategy Via Cathode-Gel Electrolyte with Forged Solid Electrolyte Interface Toward Advanced Lithium-Sulfurized Polyacrylonitrile Batteries. Adv. Funct. Mater. 2023, 33, 2306484. [Google Scholar] [CrossRef]
- Huang, H.; Ding, F.; Zhong, H.; Li, H.; Zhang, W.; Liu, X.; Xu, Q. Nano-SiO2-Embedded Poly(Propylene Carbonate)-Based Composite Gel Polymer Electrolyte for Lithium–Sulfur Batteries. J. Mater. Chem. A 2018, 6, 9539–9549. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Fu, Y. All-Cellulose Gel Electrolyte with Black Phosphorus Based Lithium Ion Conductors toward Advanced Lithium-Sulfurized Polyacrylonitrile Batteries. Carbohydr. Polym. 2022, 296, 119950. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, J.; Liu, J.; Zhang, X.; Yang, J.; Nuli, Y.; Ma, H.; Wang, J. Gel Electrolyte with Flame Retardant Polymer Stabilizing Lithium Metal towards Lithium-Sulfur Battery. Energy Storage Mater. 2023, 61, 102885. [Google Scholar] [CrossRef]
- Hao, C.; Lu, H.; Liu, J.; Zhang, H.; Kong, X.; Yang, J.; Nuli, Y.; Wang, J. Construction of Ordered and Fast Lithium Ion Channels in Gel Electrolytes for Li-SPAN Batteries. ACS Appl. Mater. Interfaces 2024, 16, 60288–60297. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Pan, Y.; Yu, H.; Li, Z.; Li, C.; Wang, S.; Ma, Y.; Shi, X.; Zhang, H.; et al. Tailoring a Multi-System Adaptable Gel Polymer Electrolyte for the Realization of Carbonate Ester and Ether-Based Li-SPAN Batteries. Energy Environ. Sci. 2024, 17, 2576–2587. [Google Scholar] [CrossRef]
- Guo, Y.; Zuo, W.; Wu, X.; Zhang, L. Practical SPAN||Li Cells Enabled by in Situ Polymerized Electrolyte. Mater. Today Energy 2024, 45, 101662. [Google Scholar] [CrossRef]
- Zhang, M.; Xie, W.; Liu, M.; Liu, S.; Wang, W.; Jin, Z.; Wang, A.; Qiu, J.; Zhao, P.; Shi, Z. New Quasi-Solid-State Li-SPAN Battery Enhanced by In Situ Thermally Polymerized Gel Polymer Electrolytes. ACS Appl. Mater. Interfaces 2024, 16, 1578–1586. [Google Scholar] [CrossRef]
- Murugan, S.; Klostermann, S.V.; Schützendübe, P.; Richter, G.; Kästner, J.; Buchmeiser, M.R. Stable Cycling of Room-Temperature Sodium-Sulfur Batteries Based on an In Situ Crosslinked Gel Polymer Electrolyte. Adv. Funct. Mater. 2022, 32, 2201191. [Google Scholar] [CrossRef]
- She, C.; Shi, X.; Zhou, J.; Zhu, Z.; Lu, K.; Zheng, Z.; Zhu, Y. A ZIF-8-Enhanced PVDF/PEO Blending Polymer Gel Membrane for Quasi-Solid-State Na-S Batteries with Long Cycling Lifespan. Chem. Eng. J. 2024, 494, 153119. [Google Scholar] [CrossRef]
- Wang, P.; Trück, J.; Häcker, J.; Schlosser, A.; Küster, K.; Starke, U.; Reinders, L.; Buchmeiser, M.R. A Design Concept for Halogen-Free Mg2+/Li+-Dual Salt-Containing Gel-Polymer-Electrolytes for Rechargeable Magnesium Batteries. Energy Storage Mater. 2022, 49, 509–517. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.; He, Z.; Li, Y.; Mao, J.; Dai, K.; Yan, C.; Zheng, J. An Advance Review of Solid-State Battery: Challenges, Progress and Prospects. Sustain. Mater. Technol. 2021, 29, e00297. [Google Scholar] [CrossRef]
- Yu, X.; Xie, J.; Yang, J.; Wang, K. All Solid-State Rechargeable Lithium Cells Based on Nano-Sulfur Composite Cathodes. J. Power Sources 2004, 132, 181–186. [Google Scholar] [CrossRef]
- Zhu, T.; Dong, X.; Liu, Y.; Wang, Y.-G.; Wang, C.; Xia, Y.-Y. An All-Solid-State Sodium–Sulfur Battery Using a Sulfur/Carbonized Polyacrylonitrile Composite Cathode. ACS Appl. Energy Mater. 2019, 2, 5263–5271. [Google Scholar] [CrossRef]
- Fan, Z.; Ding, B.; Hu, B.; Li, Z.; Xiao, D.; Xu, C.; Dou, H.; Zhang, X. Failure Mechanisms Investigation of Ultra-Thin Composite Polymer Electrolyte-Based Solid-State Lithium Metal Batteries. Electrochim. Acta 2022, 436, 141441. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Q.; Sheng, J.; Chen, B.; Gao, R.; Piao, Z.; Zhong, X.; Han, Z.; Zhu, Y.; Wang, J.; et al. A Quasi-Intercalation Reaction for Fast Sulfur Redox Kinetics in Solid-State Lithium–Sulfur Batteries. Energy Environ. Sci. 2022, 15, 4289–4300. [Google Scholar] [CrossRef]
- You, D.; Wei, W.; Xiong, H. All-Solid-State Lithium–Sulfur Batteries with Robust Interphases by Utilizing Elastomeric Polymer-in-Salt Electrolytes. ACS Appl. Energy Mater. 2025, 8, 452–460. [Google Scholar] [CrossRef]
- Li, C.; Zhang, M.; Li, P.; Ren, H.-R.; Wu, X.; Piao, Z.; Xiao, X.; Zhang, M.; Liang, X.; Wu, X.; et al. Self-Assembly of Ultrathin, Ultrastrong Layered Membranes by Protic Solvent Penetration. J. Am. Chem. Soc. 2024, 146, 3553–3563. [Google Scholar] [CrossRef]
- Nie, L.; Li, Y.; Wu, X.; Zhang, M.; Wu, X.; Xiao, X.; Gao, R.; Piao, Z.; Wu, X.; Song, Y.; et al. Scalable Ultrathin Solid Electrolyte from Recycled Antheraea Pernyi Silk with Regulated Ion Transport for Solid-State Li–S Batteries. eScience 2025, 5, 100395. [Google Scholar] [CrossRef]
- Sapkota, N.; Sabet, M.; Parekh, M.; Chen, N.; Basel, J.; Koehler, K.C.; Ding, Y.; Rao, A.M. Commercial Cellulosic Paper-Based Solid Electrolyte for High-Temperature Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2025, 17, 1234–1243. [Google Scholar] [CrossRef]
- Huang, J.; Song, Z.; Wu, J.; Miao, Y.; Li, M.; Lin, D.; Zhu, K.; Chen, X.; Li, X.; Chen, Y. In Situ Polymerized Quasi-Solid Polymer Electrolytes Enabling Void-Free Interfaces for Room-Temperature Sodium–Sulfur Batteries. Energy Mater. Devices 2024, 2, 9370051. [Google Scholar] [CrossRef]
- Lao, Z.; Tao, K.; Xiao, X.; Qu, H.; Wu, X.; Han, Z.; Gao, R.; Wang, J.; Wu, X.; Chen, A.; et al. Data-Driven Exploration of Weak Coordination Microenvironment in Solid-State Electrolyte for Safe and Energy-Dense Batteries. Nat. Commun. 2025, 16, 1075. [Google Scholar] [CrossRef]
- Lei, D.; Shi, K.; Ye, H.; Wan, Z.; Wang, Y.; Shen, L.; Li, B.; Yang, Q.-H.; Kang, F.; He, Y.-B. Progress and Perspective of Solid-State Lithium–Sulfur Batteries. Adv. Funct. Mater. 2018, 28, 1707570. [Google Scholar] [CrossRef]
- Trevey, J.E.; Gilsdorf, J.R.; Stoldt, C.R.; Lee, S.-H.; Liu, P. Electrochemical Investigation of All-Solid-State Lithium Batteries with a High Capacity Sulfur-Based Electrode. J. Electrochem. Soc. 2012, 159, A1019. [Google Scholar] [CrossRef]
- Sun, Z.; Hu, Y.; Qin, F.; Lv, N.; Li, B.; Jiang, L.; Zhang, Z.; Liu, F. Sulfurized Polyacrylonitrile Cathodes with Electrochemical and Structural Tuning for High Capacity All-Solid-State Lithium–Sulfur Batteries. Sustain. Energy Fuels 2021, 5, 5603–5614. [Google Scholar] [CrossRef]
- Huang, J.; Shao, Y.; Liu, Z.; Lv, Y.; Guo, F.; Tu, Y.; Ichikawa, T.; Hu, Z.; Zhang, T. Nano Sulfurized Polyacrylonitrile Cathode for High Performance Solid-State Lithium–Sulfur Batteries. J. Power Sources 2023, 570, 233045. [Google Scholar] [CrossRef]
- Jin, X.; Fan, W.; Zhu, S.; Zhang, T. Synergistic Effects of Anion Substitution and Interfacial Modification to Enhance Ionic Conductivity in a Hydride Electrolyte. ACS Sustain. Chem. Eng. 2025, 13, 2924–2932. [Google Scholar] [CrossRef]
- Wang, C.; Adair, K.R.; Liang, J.; Li, X.; Sun, Y.; Li, X.; Wang, J.; Sun, Q.; Zhao, F.; Lin, X.; et al. Solid-State Plastic Crystal Electrolytes: Effective Protection Interlayers for Sulfide-Based All-Solid-State Lithium Metal Batteries. Adv. Funct. Mater. 2019, 29, 1900392. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, G.; He, P.; Hu, J.; Jiang, J.; Fan, L.-Z. Sandwich Structured NASICON-Type Electrolyte Matched with Sulfurized Polyacrylonitrile Cathode for High Performance Solid-State Lithium-Sulfur Batteries. Chem. Eng. J. 2020, 393, 124705. [Google Scholar] [CrossRef]
- Miao, X.; Wang, H.; Sun, R.; Ge, X.; Zhao, D.; Wang, P.; Wang, R.; Yin, L. Isotropous Sulfurized Polyacrylonitrile Interlayer with Homogeneous Na+ Flux Dynamics for Solid-State Na Metal Batteries. Adv. Energy Mater. 2021, 11, 2003469. [Google Scholar] [CrossRef]
- Shi, C.; Alexander, G.V.; O’Neill, J.; Duncan, K.; Godbey, G.; Wachsman, E.D. All-Solid-State Garnet Type Sulfurized Polyacrylonitrile/Lithium-Metal Battery Enabled by an Inorganic Lithium Conductive Salt and a Bilayer Electrolyte Architecture. ACS Energy Lett. 2023, 8, 1803–1810. [Google Scholar] [CrossRef]
- Shi, C.; Takeuchi, S.; Alexander, G.V.; Hamann, T.; O’Neill, J.; Dura, J.A.; Wachsman, E.D. High Sulfur Loading and Capacity Retention in Bilayer Garnet Sulfurized-Polyacrylonitrile/Lithium-Metal Batteries with Gel Polymer Electrolytes. Adv. Energy Mater. 2023, 13, 2301656. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, H.; Kong, X.; Yang, J.; Nuli, Y.; Wang, J. Graphitic Carbon Nitride Stabilized the Lithium Anode/Sulfide Electrolyte Interface for All-Solid-State Lithium-Sulfur Battery. Chem. Eng. J. 2024, 489, 150887. [Google Scholar] [CrossRef]
- Li, M.; Frerichs, J.E.; Kolek, M.; Sun, W.; Zhou, D.; Huang, C.J.; Hwang, B.J.; Hansen, M.R.; Winter, M.; Bieker, P. Solid-State Lithium–Sulfur Battery Enabled by Thio-LiSICON/Polymer Composite Electrolyte and Sulfurized Polyacrylonitrile Cathode. Adv. Funct. Mater. 2020, 30, 1910123. [Google Scholar] [CrossRef]
- Chen, D.; Hu, C.; Chen, Q.; Xue, G.; Tang, L.; Dong, Q.; Chen, B.; Zhang, F.; Gao, M.; Xu, J.; et al. High Ceramic Content Composite Solid-State Electrolyte Films Prepared via a Scalable Solvent-Free Process. Nano Res. 2023, 16, 3847–3854. [Google Scholar] [CrossRef]
- Temeche, E.; Zhang, X.; Laine, R.M. Solid Electrolytes for Li–S Batteries: Solid Solutions of Poly(Ethylene Oxide) with LixPON- and LixSiPON-Based Polymers. ACS Appl. Mater. Interfaces 2020, 12, 30353–30364. [Google Scholar] [CrossRef] [PubMed]
- Temeche, E.; Zhang, X.; Laine, R.M. Electrochemical Performance of LixSiON Polymer Electrolytes Derived from an Agriculture Waste Product, Rice Hull Ash. ACS Appl. Polym. Mater. 2021, 3, 2144–2152. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, T.; Shao, Y.; Cao, H.; Liu, Z.; Wang, S.; Zhang, X. Composite Electrolytes Based on Poly(Ethylene Oxide) and Lithium Borohydrides for All-Solid-State Lithium–Sulfur Batteries. ACS Sustain. Chem. Eng. 2021, 9, 5396–5404. [Google Scholar] [CrossRef]
- Luo, S.; Zhao, E.; Gu, Y.; Saito, N.; Zhang, Z.; Yang, L.; Hirano, S. Flexible, Solid-State, Fiber-Network-Reinforced Composite Solid Electrolyte for Long Lifespan Solid Lithium-Sulfurized Polyacrylonitrile Battery. Nano Res. 2022, 15, 3290–3298. [Google Scholar] [CrossRef]
- Nie, L.; Zhu, J.; Wu, X.; Zhang, M.; Xiao, X.; Gao, R.; Wu, X.; Zhu, Y.; Chen, S.; Han, Z.; et al. A Large-Scale Fabrication of Flexible, Ultrathin, and Robust Solid Electrolyte for Solid-State Lithium-Sulfur Batteries. Adv. Mater. 2024, 36, 2400115. [Google Scholar] [CrossRef]
- Zhu, Y.; Lao, Z.; Zhang, M.; Hou, T.; Xiao, X.; Piao, Z.; Lu, G.; Han, Z.; Gao, R.; Nie, L.; et al. A Locally Solvent-Tethered Polymer Electrolyte for Long-Life Lithium Metal Batteries. Nat. Commun. 2024, 15, 3914. [Google Scholar] [CrossRef]
- Unemoto, A.; Gambe, Y.; Komatsu, D.; Honma, I. Development of High Capacity All-Solid-State Lithium Battery Using Quasi-Solid-State Electrolyte Containing Tetraglyme–Li-TFSA Equimolar Complexes. Solid State Ion. 2014, 262, 765–768. [Google Scholar] [CrossRef]
- Yang, H.; Qiao, Y.; Chang, Z.; He, P.; Zhou, H. Designing Cation–Solvent Fully Coordinated Electrolyte for High-Energy-Density Lithium–Sulfur Full Cell Based on Solid–Solid Conversion. Angew. Chem. Int. Ed. 2021, 60, 17726–17734. [Google Scholar] [CrossRef] [PubMed]
- Anbunathan, A.; Walle, K.Z.; Wu, S.-H.; Wu, Y.-S.; Chang, J.-K.; Jose, R.; Yang, C.-C. Advanced Quasi-Solid-State Lithium-Sulfur Batteries: A High-Performance Flexible LiTa2PO8-Based Hybrid Solid Electrolyte Membrane with Enhanced Safety and Efficiency. J. Energy Storage 2024, 93, 112294. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, X.; Yu, G. Material and Structural Design of Novel Binder Systems for High-Energy, High-Power Lithium-Ion Batteries. Acc. Chem. Res. 2017, 50, 2642–2652. [Google Scholar] [CrossRef]
- Zou, F.; Manthiram, A. A Review of the Design of Advanced Binders for High-Performance Batteries. Adv. Energy Mater. 2020, 10, 2002508. [Google Scholar] [CrossRef]
- Wang, L.; He, X.; Li, J.; Gao, J.; Fang, M.; Tian, G.; Wang, J.; Fan, S. Graphene-Coated Plastic Film as Current Collector for Lithium/Sulfur Batteries. J. Power Sources 2013, 239, 623–627. [Google Scholar] [CrossRef]
- Wang, J.; Yao, Z.; Monroe, C.W.; Yang, J.; Nuli, Y. Carbonyl-β-Cyclodextrin as a Novel Binder for Sulfur Composite Cathodes in Rechargeable Lithium Batteries. Adv. Funct. Mater. 2013, 23, 1194–1201. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, Q.; Gentle, I.R.; Wang, D.-W. Carboxymethyl Cellulose Binders Enable High-Rate Capability of Sulfurized Polyacrylonitrile Cathodes for Li–S Batteries. J. Mater. Chem. A 2017, 5, 5460–5465. [Google Scholar] [CrossRef]
- Eng, A.Y.S.; Nguyen, D.-T.; Kumar, V.; Subramanian, G.S.; Ng, M.-F.; Seh, Z.W. Tailoring Binder–Cathode Interactions for Long-Life Room-Temperature Sodium–Sulfur Batteries. J. Mater. Chem. A 2020, 8, 22983–22997. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Kim, H.M.; Sun, Y.-K. High Performance Potassium–Sulfur Batteries Based on a Sulfurized Polyacrylonitrile Cathode and Polyacrylic Acid Binder. J. Mater. Chem. A 2018, 6, 14587–14593. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, Y.; NuLi, Y.; Wang, B.; Yang, J.; Wang, J. Sodium Polyacrylate as a Promising Aqueous Binder of S@pPAN Cathodes for Magnesium–Sulfur Batteries. J. Phys. Chem. C 2020, 124, 20712–20721. [Google Scholar] [CrossRef]
- Wong, A.J.Y.; Lieu, W.Y.; Chinnadurai, D.; Ng, M.-F.; Seh, Z.W. Uncovering the Binder Interactions with S-PAN and MXene for Room Temperature Na–S Batteries. Nano Lett. 2023, 23, 3592–3598. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.; Yang, H.; Lei, J.; Naveed, A.; Yang, J.; Nuli, Y.; Wang, J. Towards Practical Li–S Battery with Dense and Flexible Electrode Containing Lean Electrolyte. Energy Storage Mater. 2020, 27, 307–315. [Google Scholar] [CrossRef]
- Yang, H.; Chen, J.; Yang, J.; Nuli, Y.; Wang, J. Dense and High Loading Sulfurized Pyrolyzed Poly (Acrylonitrile)(S@pPAN) Cathode for Rechargeable Lithium Batteries. Energy Storage Mater. 2020, 31, 187–194. [Google Scholar] [CrossRef]
- Niesen, S.; Kappler, J.; Trück, J.; Veith, L.; Weil, T.; Soczka-Guth, T.; Buchmeiser, M.R. Influence of the Drying Temperature on the Performance and Binder Distribution of Sulfurized Poly(Acrylonitrile) Cathodes. J. Electrochem. Soc. 2021, 168, 050510. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, H.; Yu, S.; Liu, P. Communication—Binder Effects on Cycling Performance of High Areal Capacity SPAN Electrodes. J. Electrochem. Soc. 2021, 168, 110504. [Google Scholar] [CrossRef]
- Miao, Z.; Xiao, X.; Li, J.; Xu, X.; Chen, W.; Yuan, L.; Sun, Q.; Li, Z.; Huang, Y. Evaluating the Effect of Binder for Sulfurized Polyacrylonitrile Cathode via Optical Fiber Sensors. Adv. Funct. Mater. 2024, 34, 2301736. [Google Scholar] [CrossRef]
- Yuan, H.; Guo, C.; Chen, J.; Lu, H.; Yang, J.; Nuli, Y.; Wang, J. A Crosslinking Hydrogel Binder for High-Sulfur Content S@pPAN Cathode in Rechargeable Lithium Batteries. J. Energy Chem. 2021, 60, 360–367. [Google Scholar] [CrossRef]
- Lin, H.-C.; Hong, J.-L. Metalized Polyacrylates as Efficient Binder for a Sulfurized Polyacrylonitrile/Polydopamine Active Material in Sulfur Cathodes for Room Temperature Sodium–Sulfur Batteries. ACS Appl. Energy Mater. 2022, 5, 11304–11316. [Google Scholar] [CrossRef]
- Sun, T.; Wang, S.; Xu, M.; Qiao, N.; Zhu, Q.; Xu, B. High-Performance Sulfurized Polyacrylonitrile Cathode by Using MXene as a Conductive and Catalytic Binder for Room-Temperature Na/S Batteries. ACS Appl. Mater. Interfaces 2024, 16, 10093–10103. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Hwang, T.H.; Kim, B.G.; Min, J.; Choi, J.W. A Lithium-Sulfur Battery with a High Areal Energy Density. Adv. Funct. Mater. 2014, 24, 5359–5367. [Google Scholar] [CrossRef]
- Cho, G.; Jeong, J.; Chae, M.; Noh, J.; Cho, K.; Kim, J.; Ahn, H.; Nam, T.; Kim, K. Electrochemical Properties of Sulfurized Poly-Acrylonitrile (SPAN) Cathode Containing Carbon Fiber Current Collectors. Surf. Coat. Technol. 2017, 326, 443–449. [Google Scholar] [CrossRef]
- Hara, T.; Konarov, A.; Mentbayeva, A.; Kurmanbayeva, I.; Bakenov, Z. High Mass-Loading of Sulfur-Based Cathode Composites and Polysulfides Stabilization for Rechargeable Lithium/Sulfur Batteries. Front. Energy Res. 2015, 3, 22. [Google Scholar] [CrossRef]
- Baikalov, N.; Serik, N.; Kalybekkyzy, S.; Kurmanbayeva, I.; Bakenov, Z.; Mentbayeva, A. High Mass-Loading Sulfur-Composite Cathode for Lithium-Sulfur Batteries. Front. Energy Res. 2020, 8, 207. [Google Scholar] [CrossRef]
- Zhang, Y.; Lou, J.; Shuai, Y.; Chen, K.; He, X.; Wang, Y.; Li, N.; Zhang, Z.; Gan, F. A Novel Rechargeable Potassium–Sulfur Battery Based on Liquid Alloy Anode. Mater. Lett. 2019, 242, 5–8. [Google Scholar] [CrossRef]
- Kalybekkyzy, S.; Mentbayeva, A.; Yerkinbekova, Y.; Baikalov, N.; Kahraman, M.V.; Bakenov, Z. Electrospun 3D Structured Carbon Current Collector for Li/S Batteries. Nanomaterials 2020, 10, 745. [Google Scholar] [CrossRef]
- Liu, F.; Chiluwal, S.; Childress, A.S.; Etteh, C.; Miller, K.; Washington, M.; Rao, A.M.; Podila, R. Graphene Foam Current Collector for High-Areal-Capacity Lithium–Sulfur Batteries. ACS Appl. Nano Mater. 2021, 4, 53–60. [Google Scholar] [CrossRef]
- Sapkota, N.; Chiluwal, S.; Parajuli, P.; Rowland, A.; Podila, R. Insights into the Pseudocapacitive Behavior of Sulfurized Polymer Electrodes for Li–S Batteries. Adv. Sci. 2023, 10, 2206901. [Google Scholar] [CrossRef]
- Shuai, Y.; Hu, Y.; Lou, J.; Gong, X.; Li, M.; Xu, Z.; Deng, D.; Jiang, F.; Li, M. Fluorinated Aluminum Foam for Dendrite-Free Na Metal Anodes. J. Electrochem. Soc. 2023, 170, 030519. [Google Scholar] [CrossRef]
- Lou, J.; Zhou, J.; Ma, X.; Chen, K.; Chen, S. A Novel Sodium–Potassium Anode Supported by Fluorinated Aluminum Foam. Materials 2023, 16, 7269. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, J.; Lei, J.; Liu, D.; Xie, Z.; Qu, D.; Li, K.; Deng, T.; Tang, H. Advanced Separators for Lithium-Ion and Lithium–Sulfur Batteries: A Review of Recent Progress. ChemSusChem 2016, 9, 3023–3039. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, R.; Xiao, Y.; Cao, F.; Zhang, H. Recent Progress of Separators in Lithium-Sulfur Batteries. Energy Storage Mater. 2021, 40, 439–460. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, J.; Xu, Z.; Li, H.; Guo, Y.; Liang, C.; Wang, J. Suppressing Dendrite Growth of a Lithium Metal Anode by Modifying Conventional Polypropylene Separators with a Composite Layer. ACS Appl. Energy Mater. 2020, 3, 506–513. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, C.; Ding, F.; Li, H.; Zhang, S.; Liu, X.; Xu, Q. A Thermo-Stable Poly(Propylene Carbonate)-Based Composite Separator for Lithium-Sulfur Batteries under Elevated Temperatures. Int. J. Energy Res. 2020, 44, 10295–10306. [Google Scholar] [CrossRef]
- Zhang, T.; Li, X.; Miao, X.; Sun, R.; Li, J.; Zhang, Z.; Wang, R.; Wang, C.; Li, Z.; Yin, L. Achieve Stable Lithium Metal Anode by Sulfurized-Polyacrylonitrile Modified Separator for High-Performance Lithium Batteries. ACS Appl. Mater. Interfaces 2022, 14, 14264–14273. [Google Scholar] [CrossRef]
- Anbunathan, A.; Karuppiah, C.; Chang, J.-K.; Jose, R.; Yang, C.-C. In Situ Grown ZIF67 Particles on a Glass Fiber Separator: The Performance Booster and Anode Defender for Lithium–Sulfurized Polyacrylonitrile (SPAN) Batteries. ACS Appl. Energy Mater. 2023, 6, 3549–3565. [Google Scholar] [CrossRef]
- Zhao, H.; Deng, N.; Yan, J.; Kang, W.; Ju, J.; Ruan, Y.; Wang, X.; Zhuang, X.; Li, Q.; Cheng, B. A Review on Anode for Lithium-Sulfur Batteries: Progress and Prospects. Chem. Eng. J. 2018, 347, 343–365. [Google Scholar] [CrossRef]
- Shuai, Y.; Zhang, B.; Ding, X.; Lou, J.; Wang, Y.; Chen, S.; Chen, K. Enhancing Fast Ion Transport at Interfaces of Lithium Metal Anode in Lithium-Sulfurized Polyacrylonitrile Batteries. Energy Technol. 2020, 8, 1901287. [Google Scholar] [CrossRef]
- Guo, C.; Yang, H.; Naveed, A.; Nuli, Y.; Yang, J.; Cao, Y.; Yang, H.; Wang, J. AlF3-Modified Carbon Nanofibers as a Multifunctional 3D Interlayer for Stable Lithium Metal Anodes. Chem. Commun. 2018, 54, 8347–8350. [Google Scholar] [CrossRef]
- Li, Y.; Mao, E.; Min, Z.; Cai, Z.; Chen, Z.; Fu, L.; Duan, X.; Wang, L.; Zhang, C.; Lu, Z.; et al. Hybrid Polymer-Alloy-Fluoride Interphase Enabling Fast Ion Transport Kinetics for Low-Temperature Lithium Metal Batteries. ACS Nano 2023, 17, 19459–19469. [Google Scholar] [CrossRef]
- Wei, S.; Choudhury, S.; Xu, J.; Nath, P.; Tu, Z.; Archer, L.A. Highly Stable Sodium Batteries Enabled by Functional Ionic Polymer Membranes. Adv. Mater. 2017, 29, 1605512. [Google Scholar] [CrossRef]
- Tian, K.; Wei, C.; Wang, Z.; Li, Y.; Xi, B.; Xiong, S.; Feng, J. Stable Sodium Metal Anode Enabled by Interfacial Room-Temperature Liquid Metal Engineering for High-Performance Sodium–Sulfur Batteries with Carbonate-Based Electrolyte. Interdiscip. Mater. 2024, 3, 425–436. [Google Scholar] [CrossRef]
- Zhang, T.; Hong, M.; Yang, J.; Xu, Z.; Wang, J.; Guo, Y.; Liang, C. A High Performance Lithium-Ion–Sulfur Battery with a Free-Standing Carbon Matrix Supported Li-Rich Alloy Anode. Chem. Sci. 2018, 9, 8829–8835. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zeng, Q.; Lv, R.; Lv, W.; Yang, Q.-H.; Amal, R.; Wang, D.-W. A Li-Ion Sulfur Full Cell with Ambient Resistant Al-Li Alloy Anode. Energy Storage Mater. 2018, 15, 209–217. [Google Scholar] [CrossRef]
- Zhang, T.; Lu, H.; Yang, J.; Xu, Z.; Wang, J.; Hirano, S.; Guo, Y.; Liang, C. Stable Lithium Metal Anode Enabled by a Lithiophilic and Electron/Ion Conductive Framework. ACS Nano 2020, 14, 5618–5627. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ke, X.; Cheng, Y.; Fan, M.; Wu, W.; Huang, X.; Liang, Y.; Zhong, Y.; Ao, Z.; Lai, Y.; et al. Boosting the Electrochemical Performance of 3D Composite Lithium Metal Anodes through Synergistic Structure and Interface Engineering. Energy Storage Mater. 2020, 26, 56–64. [Google Scholar] [CrossRef]
- Wang, S.; Chen, J.; Lu, H.; Zhang, Y.; Yang, J.; Nuli, Y.; Wang, J. Artificial Alloy/Li3N Double-Layer Enabling Stable High-Capacity Lithium Metal Anodes. ACS Appl. Energy Mater. 2021, 4, 13132–13139. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, C.; Li, Z.; Teng, Z.; Wang, Y.; Chen, K.; Zhu, C. In-Situ Construction of LiF/NaF-Rich Hybrid Solid Electrolyte Interphase for Dendrite-Free and Stable Li-Na Alloy Anodes. J. Electroanal. Chem. 2024, 968, 118489. [Google Scholar] [CrossRef]
- Duan, X.; Wang, L.; Li, G.; Liu, X.; Wan, M.; Du, J.; Zhan, R.; Wang, W.; Li, Y.; Tu, S.; et al. Revealing the Intrinsic Uneven Electrochemical Reactions of Li Metal Anode in Ah-Level Laminated Pouch Cells. Adv. Funct. Mater. 2023, 33, 2210669. [Google Scholar] [CrossRef]
- Du, J.; Li, G.; Tan, Y.; Duan, X.; Fan, T.; Du, C.; Zhou, A.; Wang, Y. Tuning the Li–Sn Alloy Dispersity to Improve the Lithiophilicity of Lithium Metal Anodes towards Stable Lithium Metal Batteries. Inorg. Chem. Front. 2025, 12, 3137–3146. [Google Scholar] [CrossRef]
- Sun, H.; He, X.; Ren, J.; Li, J.; Jiang, C.; Wan, C. Hard Carbon/Lithium Composite Anode Materials for Li-Ion Batteries. Electrochim. Acta 2007, 52, 4312–4316. [Google Scholar] [CrossRef]
- Uzakbaiuly, B.; Mentbayeva, A.; Konarov, A.; Kurmanbayeva, I.; Zhang, Y.; Bakenov, Z. Evaluating Sulfur-Composite Cathode Material with Lithiated Graphite Anode in Coin Cell and Pouch Cell Configuration. Front. Energy Res. 2020, 8, 595481. [Google Scholar] [CrossRef]
- Wang, H.; Ye, Y.; Liu, H.; Li, Q.; Wang, K.; Sun, S.; Wu, F.; Chen, R.; Li, L. Coassembly of Ultrathin Lithium with Dual Lithium-Free Electrodes for Long-Lasting Sulfurized Polyacrylonitrile Batteries. Nano Lett. 2025, 25, 2266–2274. [Google Scholar] [CrossRef] [PubMed]
- Mao, E.; Du, J.; Duan, X.; Wang, L.; Wang, X.; Li, G.; Fu, L.; Sun, Y. Preparation and Electrochemical Performance of Ultra-Thin Reduced Graphene Oxide/Lithium Metal Composite Foils. New Carbon Mater. 2023, 38, 754–763. [Google Scholar] [CrossRef]
- Dai, Z.; Wang, M.; Zhang, Y.; Wang, B.; Luo, H.; Zhang, X.; Wang, Q.; Zhang, Y.; Wu, H. Engineering Bifunctional Host Materials of Sulfur and Lithium-Metal Based on Nitrogen-Enriched Polyacrylonitrile for Li–S Batteries. Chem.—Eur. J. 2020, 26, 8784–8793. [Google Scholar] [CrossRef]
- Yu, S.; Wang, S.; Miao, Q.; Hui, Z.; Hyun, G.; Holoubek, J.; Yu, X.; Gao, J.; Liu, P. Composite Lithium Metal Structure to Mitigate Pulverization and Enable Long-Life Batteries. Adv. Energy Mater. 2023, 13, 2302400. [Google Scholar] [CrossRef]
- Shao, M.; Wu, N.; Chen, T.; Han, X.; Shen, Y.; Zhang, W.; Zheng, B.; Li, S.; Huo, F. Amorphous Hollow Carbon Film as a Flexible Host for Liquid Na-K Alloy Anode. Chin. Chem. Lett. 2023, 34, 107767. [Google Scholar] [CrossRef]
- Tang, X.; Zhou, D.; Li, P.; Guo, X.; Sun, B.; Liu, H.; Yan, K.; Gogotsi, Y.; Wang, G. MXene-Based Dendrite-Free Potassium Metal Batteries. Adv. Mater. 2020, 32, 1906739. [Google Scholar] [CrossRef]
- Shi, L.; Liu, Y.; Wang, W.; Wang, A.; Jin, Z.; Wu, F.; Yang, Y. High-Safety Lithium-Ion Sulfur Battery with Sulfurized Polyacrylonitrile Cathode, Prelithiated SiOx/C Anode and Carbonate-Based Electrolyte. J. Alloys Compd. 2017, 723, 974–982. [Google Scholar] [CrossRef]
- Huang, S.-S.; Tung, M.T.; Huynh, C.D.; Hwang, B.-J.; Bieker, P.M.; Fang, C.-C.; Wu, N.-L. Engineering Rice Husk into a High-Performance Electrode Material through an Ecofriendly Process and Assessing Its Application for Lithium-Ion Sulfur Batteries. ACS Sustain. Chem. Eng. 2019, 7, 7851–7861. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, Z.; Guo, Y.; Liang, C.; Wang, J.; Yang, J. Building High Performance Silicon–Oxygen and Silicon–Sulfur Battery by in-Situ Lithiation of Fibrous Si/C Anode. J. Alloys Compd. 2019, 806, 335–342. [Google Scholar] [CrossRef]
- Shuai, Y.; Lou, J.; Wang, Y.; Chen, K. An Air-Stable Prelithiation Technology for Lithium Ion-Sulfurized Polyacrylonitrile Battery. Funct. Mater. Lett. 2020, 13, 1950094. [Google Scholar] [CrossRef]
- Ye, Y.; Zhao, Y.; Zhao, T.; Xu, S.; Xu, Z.; Qian, J.; Wang, L.; Xing, Y.; Wei, L.; Li, Y.; et al. An Antipulverization and High-Continuity Lithium Metal Anode for High-Energy Lithium Batteries. Adv. Mater. 2021, 33, 2105029. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Wang, Y.; Zhang, Y.; Tan, L.; Qian, Y.; Tao, Y.; Xiong, S.; Feng, J. Flexible and Stable 3D Lithium Metal Anodes Based on Self-Standing MXene/COF Frameworks for High-Performance Lithium-Sulfur Batteries. Nano Res. 2021, 14, 3576–3584. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, J.; Hu, X.; Li, Y.; Du, H.; Wang, K.; Du, Z.; Gong, X.; Ai, W.; Huang, W. Lithiophilic Sites Dependency of Lithium Deposition in Li Metal Host Anodes. Nano Energy 2022, 94, 106883. [Google Scholar] [CrossRef]
- Trück, J.; Wang, P.; Buch, E.; Groos, J.; Niesen, S.; Buchmeiser, M.R. Communication—Lithium Titanate as Mg-Ion Insertion Anode for Mg-Ion Sulfur Batteries Based on Sulfurated Poly(Acrylonitrile) Composite. J. Electrochem. Soc. 2022, 169, 010505. [Google Scholar] [CrossRef]
- Takemoto, K.; Wakasugi, J.; Kubota, M.; Kanamura, K.; Abe, H. Dual Additive of Lithium Titanate and Sulfurized Pyrolyzed Polyacrylonitrile in Sulfur Cathode for High Rate Performance in Lithium–Sulfur Battery. Phys. Chem. Chem. Phys. 2022, 25, 351–358. [Google Scholar] [CrossRef]
- Berhe, G.B.; Su, W.-N.; Huang, C.-J.; Hagos, T.M.; Hagos, T.T.; Bezabh, H.K.; Weret, M.A.; Abrha, L.H.; Yang, Y.-W.; Hwang, B.-J. A New Class of Lithium-Ion Battery Using Sulfurized Carbon Anode from Polyacrylonitrile and Lithium Manganese Oxide Cathode. J. Power Sources 2019, 434, 126641. [Google Scholar] [CrossRef]
- Berhe, G.B.; Su, W.-N.; Abrha, L.H.; Bezabh, H.K.; Hagos, T.M.; Hagos, T.T.; Huang, C.-J.; Sahalie, N.A.; Jote, B.A.; Thirumalraj, B.; et al. Garnet–PVDF Composite Film Modified Lithium Manganese Oxide Cathode and Sulfurized Carbon Anode from Polyacrylonitrile for Lithium-Ion Batteries. J. Mater. Chem. A 2020, 8, 14043–14053. [Google Scholar] [CrossRef]
- Yamano, A.; Kubo, T.; Chujo, F.; Yamashita, N.; Morishita, M.; Yanagida, M.; Furusawa, S.; Kikuchi, N.; Sakai, T. Rubber-Derived Sulfur Composite as a High Capacity Anode for Li-Ion Battery Using 5 V-Class LiNi0.5Mn1.5O4 Cathode. Electrochemistry 2022, 90, 127004. [Google Scholar] [CrossRef]
- Berhe, G.B.; Su, W.-N.; Hagos, T.T.; Bezabh, H.K.; Hagos, T.M.; Hwang, B.J. Partially Fluorinated Electrolyte for High-Voltage Cathode with Sulfurized Carbon Anode from Polyacrylonitrile for Lithium-Ion Battery. J. Power Sources 2023, 558, 232567. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, W.; Ming, J.; Li, M.; Xie, L.; He, X.; Wang, J.; Liang, S.; Wu, Y. An Exploration of New Energy Storage System: High Energy Density, High Safety, and Fast Charging Lithium Ion Battery. Adv. Funct. Mater. 2019, 29, 1805978. [Google Scholar] [CrossRef]
- Shuai, Y.; He, X.; Zhang, Z.; Chen, K.; Lou, J.; Wang, Y.; Li, N. A Highly Reversible and Low-Cost Sulfur-Graphite Dual-Ion Battery. Energy Technol. 2019, 7, 1800729. [Google Scholar] [CrossRef]
- Luo, F.; Feng, X.; Zeng, L.; Lin, L.; Li, X.; Kang, B.; Xiao, L.; Chen, Q.; Wei, M.; Qian, Q. In Situ Simultaneous Encapsulation of Defective MoS2 Nanolayers and Sulfur Nanodots into SPAN Fibers for High Rate Sodium-Ion Batteries. Chem. Eng. J. 2021, 404, 126430. [Google Scholar] [CrossRef]
- Deng, L.; Hong, Y.; Yang, Y.; Zhang, J.; Niu, X.; Wang, J.; Zeng, L.; Hao, W.; Guo, L.; Zhu, Y. Sulfurized Polyacrylonitrile as a High-Performance and Low-Volume Change Anode for Robust Potassium Storage. ACS Nano 2021, 15, 18419–18428. [Google Scholar] [CrossRef]
- Kakiage, K.; Yano, T.; Uehara, H.; Kakiage, M. Ultra-Lightweight Rechargeable Battery with Enhanced Gravimetric Energy Densities >750 Wh Kg−1 in Lithium–Sulfur Pouch Cell. Commun. Eng. 2024, 3, 177. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, G. Developments in Nanostructured Cathode Materials for High-Performance Lithium-Ion Batteries. Adv. Mater. 2008, 20, 2251–2269. [Google Scholar] [CrossRef]
- Cai, C.; Wang, Y. Novel Nanocomposite Materials for Advanced Li-Ion Rechargeable Batteries. Materials 2009, 2, 1205–1238. [Google Scholar] [CrossRef]
- Yin, Y.-X.; Xin, S.; Guo, Y.-G.; Wan, L.-J. Lithium–Sulfur Batteries: Electrochemistry, Materials, and Prospects. Angew. Chem. Int. Ed. 2013, 52, 13186–13200. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Su, Y.-S. Challenges and Prospects of Lithium–Sulfur Batteries. Acc. Chem. Res. 2013, 46, 1125–1134. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, G.; Cui, Y. Nanostructured Sulfur Cathodes. Chem. Soc. Rev. 2013, 42, 3018–3032. [Google Scholar] [CrossRef]
- Wang, D.-W.; Zeng, Q.; Zhou, G.; Yin, L.; Li, F.; Cheng, H.-M.; Gentle, I.R.; Lu, G.Q.M. Carbon–Sulfur Composites for Li–S Batteries: Status and Prospects. J. Mater. Chem. A 2013, 1, 9382–9394. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.-G.; Yang, Z.; Lemmon, J.P.; Imhoff, C.; Graff, G.L.; Li, L.; Hu, J.; Wang, C.; Xiao, J.; et al. Materials Science and Materials Chemistry for Large Scale Electrochemical Energy Storage: From Transportation to Electrical Grid. Adv. Funct. Mater. 2013, 23, 929–946. [Google Scholar] [CrossRef]
- Bresser, D.; Passerini, S.; Scrosati, B. Recent Progress and Remaining Challenges in Sulfur-Based Lithium Secondary Batteries—A Review. Chem. Commun. 2013, 49, 10545–10562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S. Sulfurized Carbon: A Class of Cathode Materials for High Performance Lithium/Sulfur Batteries. Front. Energy Res. 2013, 1, 10. [Google Scholar] [CrossRef]
- Fedorková, A.; Oriňáková, R.; Čech, O.; Sedlaříková, M. New Composite Cathode Materials for Li/S Batteries: A Review. Int. J. Electrochem. Sci. 2013, 8, 10308–10319. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Chung, S.-H.; Zu, C.; Su, Y.-S. Rechargeable Lithium–Sulfur Batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef]
- Chen, L.; Shaw, L.L. Recent Advances in Lithium–Sulfur Batteries. J. Power Sources 2014, 267, 770–783. [Google Scholar] [CrossRef]
- Zhang, S.; Ueno, K.; Dokko, K.; Watanabe, M. Recent Advances in Electrolytes for Lithium–Sulfur Batteries. Adv. Energy Mater. 2015, 5, 1500117. [Google Scholar] [CrossRef]
- Adelhelm, P.; Hartmann, P.; Bender, C.L.; Busche, M.; Eufinger, C.; Janek, J. From Lithium to Sodium: Cell Chemistry of Room Temperature Sodium–Air and Sodium–Sulfur Batteries. Beilstein J. Nanotechnol. 2015, 6, 1016–1055. [Google Scholar] [CrossRef] [PubMed]
- Pope, M.A.; Aksay, I.A. Structural Design of Cathodes for Li-S Batteries. Adv. Energy Mater. 2015, 5, 1500124. [Google Scholar] [CrossRef]
- Li, Z.; Huang, Y.; Yuan, L.; Hao, Z.; Huang, Y. Status and Prospects in Sulfur–Carbon Composites as Cathode Materials for Rechargeable Lithium–Sulfur Batteries. Carbon 2015, 92, 41–63. [Google Scholar] [CrossRef]
- Lin, Z.; Liang, C. Lithium–Sulfur Batteries: From Liquid to Solid Cells. J. Mater. Chem. A 2014, 3, 936–958. [Google Scholar] [CrossRef]
- Fang, X.; Peng, H. A Revolution in Electrodes: Recent Progress in Rechargeable Lithium–Sulfur Batteries. Small 2015, 11, 1488–1511. [Google Scholar] [CrossRef]
- Pang, Q.; Liang, X.; Kwok, C.Y.; Nazar, L.F. Review—The Importance of Chemical Interactions between Sulfur Host Materials and Lithium Polysulfides for Advanced Lithium-Sulfur Batteries. J. Electrochem. Soc. 2015, 162, A2567. [Google Scholar] [CrossRef]
- Zhang, S.S. Heteroatom-Doped Carbons: Synthesis, Chemistry and Application in Lithium/Sulphur Batteries. Inorg. Chem. Front. 2015, 2, 1059–1069. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Bakenova, Z.; Bakenov, Z. Carbon/Sulfur Composite Cathodes for Flexible Lithium/Sulfur Batteries: Status and Prospects. Front. Energy Res. 2015, 3, 2. [Google Scholar] [CrossRef]
- Seh, Z.W.; Sun, Y.; Zhang, Q.; Cui, Y. Designing High-Energy Lithium–Sulfur Batteries. Chem. Soc. Rev. 2016, 45, 5605–5634. [Google Scholar] [CrossRef]
- Liang, J.; Sun, Z.-H.; Li, F.; Cheng, H.-M. Carbon Materials for Li–S Batteries: Functional Evolution and Performance Improvement. Energy Storage Mater. 2016, 2, 76–106. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, N.; Cui, Y. Promises and Challenges of Nanomaterials for Lithium-Based Rechargeable Batteries. Nat. Energy 2016, 1, 16071. [Google Scholar] [CrossRef]
- Griebel, J.J.; Glass, R.S.; Char, K.; Pyun, J. Polymerizations with Elemental Sulfur: A Novel Route to High Sulfur Content Polymers for Sustainability, Energy and Defense. Prog. Polym. Sci. 2016, 58, 90–125. [Google Scholar] [CrossRef]
- Xie, J.; Zhao, C.; Lin, Z.; Gu, P.; Zhang, Q. Nanostructured Conjugated Polymers for Energy-Related Applications beyond Solar Cells. Chem.—Asian J. 2016, 11, 1489–1511. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cheruvally, G.; Kim, C.; Cho, K.-K.; Ahn, H.-J.; Kim, K.-W.; Ahn, J.-H. Lithium/Sulfur Secondary Batteries: A Review. J. Electrochem. Sci. Technol. 2016, 7, 97–114. [Google Scholar] [CrossRef]
- Gu, X.; Zhang, S.; Hou, Y. Graphene-Based Sulfur Composites for Energy Storage and Conversion in Li-S Batteries. Chin. J. Chem. 2016, 34, 13–31. [Google Scholar] [CrossRef]
- Gu, X.; Hencz, L.; Zhang, S. Recent Development of Carbonaceous Materials for Lithium–Sulphur Batteries. Batteries 2016, 2, 33. [Google Scholar] [CrossRef]
- Peng, H.-J.; Huang, J.-Q.; Cheng, X.-B.; Zhang, Q. Review on High-Loading and High-Energy Lithium–Sulfur Batteries. Adv. Energy Mater. 2017, 7, 1700260. [Google Scholar] [CrossRef]
- Dirlam, P.T.; Glass, R.S.; Char, K.; Pyun, J. The Use of Polymers in Li-S Batteries: A Review. J. Polym. Sci. Part Polym. Chem. 2017, 55, 1635–1668. [Google Scholar] [CrossRef]
- Xin, S.; Chang, Z.; Zhang, X.; Guo, Y.-G. Progress of Rechargeable Lithium Metal Batteries Based on Conversion Reactions. Natl. Sci. Rev. 2017, 4, 54–70. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, H.; Kim, C.-S.; Zaghib, K.; Mauger, A.; Julien, C.M. Advances in Lithium—Sulfur Batteries. Mater. Sci. Eng. R Rep. 2017, 121, 1–29. [Google Scholar] [CrossRef]
- Zeng, L.-C.; Li, W.-H.; Jiang, Y.; Yu, Y. Recent Progress in Li–S and Li–Se Batteries. Rare Met. 2017, 36, 339–364. [Google Scholar] [CrossRef]
- Wang, T.; Kretschmer, K.; Choi, S.; Pang, H.; Xue, H.; Wang, G. Fabrication Methods of Porous Carbon Materials and Separator Membranes for Lithium–Sulfur Batteries: Development and Future Perspectives. Small Methods 2017, 1, 1700089. [Google Scholar] [CrossRef]
- Li, G.; Wang, S.; Zhang, Y.; Li, M.; Chen, Z.; Lu, J. Revisiting the Role of Polysulfides in Lithium–Sulfur Batteries. Adv. Mater. 2018, 30, 1705590. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Huang, J.-Q.; Peng, H.-J.; Titirici, M.-M.; Xiang, R.; Chen, R.; Liu, Q.; Zhang, Q. A Review of Functional Binders in Lithium–Sulfur Batteries. Adv. Energy Mater. 2018, 8, 1802107. [Google Scholar] [CrossRef]
- Yang, X.; Li, X.; Adair, K.; Zhang, H.; Sun, X. Structural Design of Lithium–Sulfur Batteries: From Fundamental Research to Practical Application. Electrochem. Energy Rev. 2018, 1, 239–293. [Google Scholar] [CrossRef]
- Kumar, R.; Liu, J.; Hwang, J.-Y.; Sun, Y.-K. Recent Research Trends in Li–S Batteries. J. Mater. Chem. A 2018, 6, 11582–11605. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, H.; Bae, J.; Chung, S.-H.; Zhang, W.; Manthiram, A.; Yu, G. Nanostructured Host Materials for Trapping Sulfur in Rechargeable Li–S Batteries: Structure Design and Interfacial Chemistry. Small Methods 2018, 2, 1700279. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, X.; Zhang, S.; Hou, Y. Sulfur Hosts against the Shuttle Effect. Small Methods 2018, 2, 1700345. [Google Scholar] [CrossRef]
- Ould Ely, T.; Kamzabek, D.; Chakraborty, D.; Doherty, M.F. Lithium–Sulfur Batteries: State of the Art and Future Directions. ACS Appl. Energy Mater. 2018, 1, 1783–1814. [Google Scholar] [CrossRef]
- Liu, J.; Wang, M.; Xu, N.; Qian, T.; Yan, C. Progress and Perspective of Organosulfur Polymers as Cathode Materials for Advanced Lithium-Sulfur Batteries. Energy Storage Mater. 2018, 15, 53–64. [Google Scholar] [CrossRef]
- Guo, W.; Fu, Y. A Perspective on Energy Densities of Rechargeable Li-S Batteries and Alternative Sulfur-Based Cathode Materials. Energy Environ. Mater. 2018, 1, 20–27. [Google Scholar] [CrossRef]
- Li, S.-Y.; Wang, W.-P.; Duan, H.; Guo, Y.-G. Recent Progress on Confinement of Polysulfides through Physical and Chemical Methods. J. Energy Chem. 2018, 27, 1555–1565. [Google Scholar] [CrossRef]
- Zhao, F.; Li, Y.; Feng, W. Recent Advances in Applying Vulcanization/Inverse Vulcanization Methods to Achieve High-Performance Sulfur-Containing Polymer Cathode Materials for Li–S Batteries. Small Methods 2018, 2, 1800156. [Google Scholar] [CrossRef]
- Zeng, Z.; Liu, X. Sulfur Immobilization by “Chemical Anchor” to Suppress the Diffusion of Polysulfides in Lithium–Sulfur Batteries. Adv. Mater. Interfaces 2018, 5, 1701274. [Google Scholar] [CrossRef]
- Conder, J.; Marino, C.; Novák, P.; Villevieille, C. Do Imaging Techniques Add Real Value to the Development of Better Post-Li-Ion Batteries? J. Mater. Chem. A 2018, 6, 3304–3327. [Google Scholar] [CrossRef]
- Arias, A.N.; Tesio, A.Y.; Flexer, V. Review—Non-Carbonaceous Materials as Cathodes for Lithium-Sulfur Batteries. J. Electrochem. Soc. 2018, 165, A6119. [Google Scholar] [CrossRef]
- Wang, D.-Y.; Guo, W.; Fu, Y. Organosulfides: An Emerging Class of Cathode Materials for Rechargeable Lithium Batteries. Acc. Chem. Res. 2019, 52, 2290–2300. [Google Scholar] [CrossRef]
- Lu, Y.; Rong, X.; Hu, Y.-S.; Chen, L.; Li, H. Research and Development of Advanced Battery Materials in China. Energy Storage Mater. 2019, 23, 144–153. [Google Scholar] [CrossRef]
- Hong, X.; Mei, J.; Wen, L.; Tong, Y.; Vasileff, A.J.; Wang, L.; Liang, J.; Sun, Z.; Dou, S.X. Nonlithium Metal–Sulfur Batteries: Steps Toward a Leap. Adv. Mater. 2019, 31, 1802822. [Google Scholar] [CrossRef]
- Kopeć, M.; Lamson, M.; Yuan, R.; Tang, C.; Kruk, M.; Zhong, M.; Matyjaszewski, K.; Kowalewski, T. Polyacrylonitrile-Derived Nanostructured Carbon Materials. Prog. Polym. Sci. 2019, 92, 89–134. [Google Scholar] [CrossRef]
- Huang, S.; Guan, R.; Wang, S.; Xiao, M.; Han, D.; Sun, L.; Meng, Y. Polymers for High Performance Li-S Batteries: Material Selection and Structure Design. Prog. Polym. Sci. 2019, 89, 19–60. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, X.-Q.; Huang, J.-Q.; Liu, Q.; Zhang, Q. Lithium-Anode Protection in Lithium–Sulfur Batteries. Trends Chem. 2019, 1, 693–704. [Google Scholar] [CrossRef]
- Gu, X.; Tang, T.; Liu, X.; Hou, Y. Rechargeable Metal Batteries Based on Selenium Cathodes: Progress, Challenges and Perspectives. J. Mater. Chem. A 2019, 7, 11566–11583. [Google Scholar] [CrossRef]
- Teng, Y.; Zhou, Q.; Gao, P. Applications and Challenges of Elemental Sulfur, Nanosulfur, Polymeric Sulfur, Sulfur Composites, and Plasmonic Nanostructures. Crit. Rev. Environ. Sci. Technol. 2019, 49, 2314–2358. [Google Scholar] [CrossRef]
- Liu, D.; Li, Z.; Li, X.; Cheng, Z.; Yuan, L.; Huang, Y. Recent Advances in Cathode Materials for Room-Temperature Sodium−Sulfur Batteries. ChemPhysChem 2019, 20, 3164–3176. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Sivaram, S. Separator Membranes for Lithium–Sulfur Batteries: Design Principles, Structure, and Performance. Energy Technol. 2019, 7, 1800819. [Google Scholar] [CrossRef]
- Pan, Y.; Li, S.; Yin, M.; Li, J. Electrolyte Evolution Propelling the Development of Nonlithium Metal–Sulfur Batteries. Energy Technol. 2019, 7, 1900164. [Google Scholar] [CrossRef]
- Poizot, P.; Gaubicher, J.; Renault, S.; Dubois, L.; Liang, Y.; Yao, Y. Opportunities and Challenges for Organic Electrodes in Electrochemical Energy Storage. Chem. Rev. 2020, 120, 6490–6557. [Google Scholar] [CrossRef]
- Zhao, M.; Li, B.-Q.; Peng, H.-J.; Yuan, H.; Wei, J.-Y.; Huang, J.-Q. Lithium–Sulfur Batteries under Lean Electrolyte Conditions: Challenges and Opportunities. Angew. Chem. Int. Ed. 2020, 59, 12636–12652. [Google Scholar] [CrossRef]
- Liu, T.; Hu, H.; Ding, X.; Yuan, H.; Jin, C.; Nai, J.; Liu, Y.; Wang, Y.; Wan, Y.; Tao, X. 12 Years Roadmap of the Sulfur Cathode for Lithium Sulfur Batteries (2009–2020). Energy Storage Mater. 2020, 30, 346–366. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, K.; Sun, Z.; Hu, G.; Xiao, R.; Cheng, H.-M.; Li, F. Structure-Related Electrochemical Performance of Organosulfur Compounds for Lithium–Sulfur Batteries. Energy Environ. Sci. 2020, 13, 1076–1095. [Google Scholar] [CrossRef]
- Fang, R.; Xu, J.; Wang, D.-W. Covalent Fixing of Sulfur in Metal–Sulfur Batteries. Energy Environ. Sci. 2020, 13, 432–471. [Google Scholar] [CrossRef]
- Chen, Y.; Zhuo, S.; Li, Z.; Wang, C. Redox Polymers for Rechargeable Metal-Ion Batteries. EnergyChem 2020, 2, 100030. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, H.; Fan, W.; Zhong, C.; Hu, W.; Mitlin, D. Review of Emerging Potassium–Sulfur Batteries. Adv. Mater. 2020, 32, 1908007. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Naveed, A.; Nuli, Y.; Yang, J.; Wang, J. Designing an Intrinsically Safe Organic Electrolyte for Rechargeable Batteries. Energy Storage Mater. 2020, 31, 382–400. [Google Scholar] [CrossRef]
- Yang, H.; Chen, J.; Yang, J.; Wang, J. Prospect of Sulfurized Pyrolyzed Poly(Acrylonitrile) (S@pPAN) Cathode Materials for Rechargeable Lithium Batteries. Angew. Chem. 2020, 132, 7374–7386. [Google Scholar] [CrossRef]
- Li, S.; Zhang, W.; Zeng, Z.; Cheng, S.; Xie, J. Selenium or Tellurium as Eutectic Accelerators for High-Performance Lithium/Sodium–Sulfur Batteries. Electrochem. Energy Rev. 2020, 3, 613–642. [Google Scholar] [CrossRef]
- Zhao, X.; Lu, Y.; Qian, Z.; Wang, R.; Guo, Z. Potassium-Sulfur Batteries: Status and Perspectives. EcoMat 2020, 2, e12038. [Google Scholar] [CrossRef]
- Mukkabla, R.; Buchmeiser, M.R. Cathode Materials for Lithium–Sulfur Batteries Based on Sulfur Covalently Bound to a Polymeric Backbone. J. Mater. Chem. A 2020, 8, 5379–5394. [Google Scholar] [CrossRef]
- Huang, X.; Luo, B.; Chen, P.; Searles, D.J.; Wang, D.; Wang, L. Sulfur-Based Redox Chemistry for Electrochemical Energy Storage. Coord. Chem. Rev. 2020, 422, 213445. [Google Scholar] [CrossRef]
- Siczek, K. The Toxicity of Secondary Lithium-Sulfur Batteries Components. Batteries 2020, 6, 45. [Google Scholar] [CrossRef]
- Dias, J.A.; Santagneli, S.H.; Messaddeq, Y. Methods for Lithium Ion NASICON Preparation: From Solid-State Synthesis to Highly Conductive Glass-Ceramics. J. Phys. Chem. C 2020, 124, 26518–26539. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, T.; Tian, H.; Su, D.; Zhang, Q.; Wang, G. Advances in Lithium–Sulfur Batteries: From Academic Research to Commercial Viability. Adv. Mater. 2021, 33, 2003666. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Liu, S.; Li, G.-R.; Gao, X.-P. Strategy of Enhancing the Volumetric Energy Density for Lithium–Sulfur Batteries. Adv. Mater. 2021, 33, 2003955. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.-P.; Zhang, X.-Q.; Li, B.-Q.; Zhang, Q. Challenges and Promises of Lithium Metal Anode by Soluble Polysulfides in Practical Lithium–Sulfur Batteries. Mater. Today 2021, 45, 62–76. [Google Scholar] [CrossRef]
- Li, Y.; Guo, S. Material Design and Structure Optimization for Rechargeable Lithium-Sulfur Batteries. Matter 2021, 4, 1142–1188. [Google Scholar] [CrossRef]
- Eng, A.Y.S.; Kumar, V.; Zhang, Y.; Luo, J.; Wang, W.; Sun, Y.; Li, W.; Seh, Z.W. Room-Temperature Sodium–Sulfur Batteries and Beyond: Realizing Practical High Energy Systems through Anode, Cathode, and Electrolyte Engineering. Adv. Energy Mater. 2021, 11, 2003493. [Google Scholar] [CrossRef]
- Sun, J.; Du, Z.; Liu, Y.; Ai, W.; Wang, K.; Wang, T.; Du, H.; Liu, L.; Huang, W. State-Of-The-Art and Future Challenges in High Energy Lithium–Selenium Batteries. Adv. Mater. 2021, 33, 2003845. [Google Scholar] [CrossRef]
- Shadike, Z.; Tan, S.; Wang, Q.-C.; Lin, R.; Hu, E.; Qu, D.; Yang, X.-Q. Review on Organosulfur Materials for Rechargeable Lithium Batteries. Mater. Horiz. 2021, 8, 471–500. [Google Scholar] [CrossRef]
- Liu, G.; Sun, Q.; Li, Q.; Zhang, J.; Ming, J. Electrolyte Issues in Lithium–Sulfur Batteries: Development, Prospect, and Challenges. Energy Fuels 2021, 35, 10405–10427. [Google Scholar] [CrossRef]
- Han, Z.; Li, S.; Wu, Y.; Yu, C.; Cheng, S.; Xie, J. Challenges and Key Parameters in Exploring the Cyclability Limitation of Practical Lithium–Sulfur Batteries. J. Mater. Chem. A 2021, 9, 24215–24240. [Google Scholar] [CrossRef]
- Lyu, H.; Sun, X.-G.; Dai, S. Organic Cathode Materials for Lithium-Ion Batteries: Past, Present, and Future. Adv. Energy Sustain. Res. 2021, 2, 2000044. [Google Scholar] [CrossRef]
- Tan, J.; Matz, J.; Dong, P.; Ye, M.; Shen, J. Appreciating the Role of Polysulfides in Lithium-Sulfur Batteries and Regulation Strategies by Electrolytes Engineering. Energy Storage Mater. 2021, 42, 645–678. [Google Scholar] [CrossRef]
- Chai, J.; Du, J.; Li, Q.; Han, N.; Zhang, W.; Tang, B. Recent Breakthroughs in the Bottleneck of Cathode Materials for Li–S Batteries. Energy Fuels 2021, 35, 15455–15471. [Google Scholar] [CrossRef]
- Li, S.; Lorandi, F.; Wang, H.; Liu, T.; Whitacre, J.F.; Matyjaszewski, K. Functional Polymers for Lithium Metal Batteries. Prog. Polym. Sci. 2021, 122, 101453. [Google Scholar] [CrossRef]
- Angulakshmi, N.; Dhanalakshmi, R.B.; Sathya, S.; Ahn, J.-H.; Stephan, A.M. Understanding the Electrolytes of Lithium−Sulfur Batteries. Batter. Supercaps 2021, 4, 1064–1095. [Google Scholar] [CrossRef]
- Jin, F.; Wang, B.; Wang, J.; Wang, Y.; Ning, Y.; Yang, J.; Zhang, Z.; Liu, P.; Zhou, Y.; Wang, D.; et al. Boosting Electrochemical Kinetics of S Cathodes for Room Temperature Na/S Batteries. Matter 2021, 4, 1768–1800. [Google Scholar] [CrossRef]
- Chiluwal, S.; Rao, A.M.; Podila, R. Strategies for Improving Rechargeable Lithium-Ion Batteries: From Active Materials to CO2 Emissions. Nanotechnol. Rev. 2021, 10, 1993–2026. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, L.; Zhang, C.; Liu, L.; Li, Y.; Qiao, Z.; Lin, J.; Wei, Q.; Wang, L.; Xie, Q.; et al. Recent Advances and Strategies toward Polysulfides Shuttle Inhibition for High-Performance Li–S Batteries. Adv. Sci. 2022, 9, 2106004. [Google Scholar] [CrossRef]
- Deng, R.; Wang, M.; Yu, H.; Luo, S.; Li, J.; Chu, F.; Liu, B.; Wu, F. Recent Advances and Applications Toward Emerging Lithium–Sulfur Batteries: Working Principles and Opportunities. Energy Environ. Mater. 2022, 5, 777–799. [Google Scholar] [CrossRef]
- Pan, Z.; Brett, D.J.L.; He, G.; Parkin, I.P. Progress and Perspectives of Organosulfur for Lithium–Sulfur Batteries. Adv. Energy Mater. 2022, 12, 2103483. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Zhang, L. Designing Principles of Advanced Sulfur Cathodes toward Practical Lithium-Sulfur Batteries. SusMat 2022, 2, 34–64. [Google Scholar] [CrossRef]
- Xing, C.; Chen, H.; Qian, S.; Wu, Z.; Nizami, A.; Li, X.; Zhang, S.; Lai, C. Regulating Liquid and Solid-State Electrolytes for Solid-Phase Conversion in Li–S Batteries. Chem 2022, 8, 1201–1230. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, Q.; Hao, S.-M.; Deng, S.; He, Q.; Lin, Z.; Yang, Y. Polymers in Lithium–Sulfur Batteries. Adv. Sci. 2022, 9, 2103798. [Google Scholar] [CrossRef]
- Zou, R.; Liu, W.; Ran, F. Sulfur-Containing Polymer Cathode Materials: From Energy Storage Mechanism to Energy Density. InfoMat 2022, 4, e12319. [Google Scholar] [CrossRef]
- Rafie, A.; Kim, J.W.; Sarode, K.K.; Kalra, V. A Review on the Use of Carbonate-Based Electrolytes in Li-S Batteries: A Comprehensive Approach Enabling Solid-Solid Direct Conversion Reaction. Energy Storage Mater. 2022, 50, 197–224. [Google Scholar] [CrossRef]
- Weret, M.A.; Su, W.-N.; Hwang, B.J. Strategies towards High Performance Lithium-Sulfur Batteries. Batter. Supercaps 2022, 5, e202200059. [Google Scholar] [CrossRef]
- Zhang, S.; Andreas, N.S.; Li, R.; Zhang, N.; Sun, C.; Lu, D.; Gao, T.; Chen, L.; Fan, X. Mitigating Irreversible Capacity Loss for Higher-Energy Lithium Batteries. Energy Storage Mater. 2022, 48, 44–73. [Google Scholar] [CrossRef]
- Li, X.; Yuan, L.; Liu, D.; Xiang, J.; Li, Z.; Huang, Y. Solid/Quasi-Solid Phase Conversion of Sulfur in Lithium–Sulfur Battery. Small 2022, 18, 2106970. [Google Scholar] [CrossRef]
- Xu, J.; Liu, K.; Khan, M.A.; Wang, H.; He, T.; Zhao, H.; Ye, D.; Tang, Y.; Zhang, J. Sub-Zero Temperature Electrolytes for Lithium-Sulfur Batteries: Functional Mechanisms, Challenges and Perspectives. Chem. Eng. J. 2022, 443, 136637. [Google Scholar] [CrossRef]
- Luo, J.; Guan, K.; Lei, W.; Zhang, S.; Jia, Q.; Zhang, H. One Dimensional Carbon-Based Composites as Cathodes for Lithium-Sulfur Battery. J. Mater. Sci. Technol. 2022, 122, 101–120. [Google Scholar] [CrossRef]
- Lim, C.Y.J.; Seh, Z.W. Quasi-Solid-State Conversion Cathode Materials for Room-Temperature Sodium–Sulfur Batteries. Battery Energy 2022, 1, 20220008. [Google Scholar] [CrossRef]
- Jiao, L.; Li, H.; Zhang, C.; Jiang, H.; Yang, S.; Shu, D.; Li, C.; Cheng, B.; Yang, Q.; Zhang, W. Molecular Engineering of Sulfur-Providing Materials for Optimized Sulfur Conversion in Li-S Chemistry. EcoMat 2022, 4, e12262. [Google Scholar] [CrossRef]
- Ren, S.; Sang, P.; Guo, W.; Fu, Y. Organosulfur Polymer-Based Cathode Materials for Rechargeable Batteries. Polym. Chem. 2022, 13, 5676–5690. [Google Scholar] [CrossRef]
- Yi, Y.; Hai, F.; Guo, J.; Tian, X.; Zheng, S.; Wu, Z.; Wang, T.; Li, M. Progress and Prospect of Practical Lithium-Sulfur Batteries Based on Solid-Phase Conversion. Batteries 2023, 9, 27. [Google Scholar] [CrossRef]
- Li, X.-Y.; Zhao, C.-X.; Li, B.-Q.; Huang, J.-Q.; Zhang, Q. Advances on Composite Cathodes for Lithium-Sulfur Batteries. J. Electrochem. 2022, 28, 28–40+4–5. [Google Scholar] [CrossRef]
- Sang, P.; Chen, Q.; Wang, D.-Y.; Guo, W.; Fu, Y. Organosulfur Materials for Rechargeable Batteries: Structure, Mechanism, and Application. Chem. Rev. 2023, 123, 1262–1326. [Google Scholar] [CrossRef] [PubMed]
- Raza, H.; Bai, S.; Cheng, J.; Majumder, S.; Zhu, H.; Liu, Q.; Zheng, G.; Li, X.; Chen, G. Li-S Batteries: Challenges, Achievements and Opportunities. Electrochem. Energy Rev. 2023, 6, 29. [Google Scholar] [CrossRef]
- Sun, J.; Liu, Y.; Liu, L.; Bi, J.; Wang, S.; Du, Z.; Du, H.; Wang, K.; Ai, W.; Huang, W. Interface Engineering Toward Expedited Li2S Deposition in Lithium–Sulfur Batteries: A Critical Review. Adv. Mater. 2023, 35, 2211168. [Google Scholar] [CrossRef]
- Bi, C.-X.; Hou, L.-P.; Li, Z.; Zhao, M.; Zhang, X.-Q.; Li, B.-Q.; Zhang, Q.; Huang, J.-Q. Protecting Lithium Metal Anodes in Lithium–Sulfur Batteries: A Review. Energy Mater. Adv. 2023, 4, 0010. [Google Scholar] [CrossRef]
- Liu, X.; Mariani, A.; Adenusi, H.; Passerini, S. Locally Concentrated Ionic Liquid Electrolytes for Lithium-Metal Batteries. Angew. Chem. Int. Ed. 2023, 62, e202219318. [Google Scholar] [CrossRef]
- Lv, Z.-C.; Wang, P.-F.; Wang, J.-C.; Tian, S.-H.; Yi, T.-F. Key Challenges, Recent Advances and Future Perspectives of Rechargeable Lithium-Sulfur Batteries. J. Ind. Eng. Chem. 2023, 124, 68–88. [Google Scholar] [CrossRef]
- Kim, J.T.; Hao, X.; Wang, C.; Sun, X. Cathode Materials for Single-Phase Solid-Solid Conversion Li-S Batteries. Matter 2023, 6, 316–343. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, Q.; Wang, R.; Liu, Z.; Zhai, Y.; Pang, Y.; Tang, Y.; Wang, Q.; Wu, K.; Wu, H.; et al. Recent Progress in Advanced Organosulfur Cathode Materials for Rechargeable Lithium Batteries. Mater. Today 2023, 65, 100–121. [Google Scholar] [CrossRef]
- Zhang, M.; Mao, H.; Liang, Y.; Yu, X. Recent Progress in Zeolitic Imidazolate Frameworks (ZIFs)-Derived Nanomaterials for Effective Lithium Polysulfide Management in Lithium–Sulfur Batteries. J. Mater. Chem. A 2023, 11, 17892–17919. [Google Scholar] [CrossRef]
- Yang, S.; Wang, B.; Lv, Q.; Zhang, N.; Zhang, Z.; Jing, Y.; Li, J.; Chen, R.; Wu, B.; Xu, P.; et al. Recent Advances in Cathodes for All-Solid-State Lithium-Sulfur Batteries. Chin. Chem. Lett. 2023, 34, 107783. [Google Scholar] [CrossRef]
- Pang, Z.; Zhang, H.; Wang, L.; Song, D.; Shi, X.; Ma, Y.; Kong, L.; Zhang, L. Towards Safe Lithium-Sulfur Batteries from Liquid-State Electrolyte to Solid-State Electrolyte. Front. Mater. Sci. 2023, 17, 230630. [Google Scholar] [CrossRef]
- Xin, S.; Zhang, X.; Wang, L.; Yu, H.; Chang, X.; Zhao, Y.-M.; Meng, Q.; Xu, P.; Zhao, C.-Z.; Chen, J.; et al. Roadmap for Rechargeable Batteries: Present and Beyond. Sci. China Chem. 2024, 67, 13–42. [Google Scholar] [CrossRef]
- Fei, Y.; Li, G. Unveiling the Pivotal Parameters for Advancing High Energy Density in Lithium-Sulfur Batteries: A Comprehensive Review. Adv. Funct. Mater. 2024, 34, 2312550. [Google Scholar] [CrossRef]
- Song, X.; Liang, X.; Eko, J.; Sun, H.H.; Kim, J.-M.; Kim, H.; Sun, Y.-K. Toward Practical Li–S Batteries: On the Road to a New Electrolyte. Adv. Energy Mater. 2024, 14, 2402506. [Google Scholar] [CrossRef]
- Liang, F.; Wang, S.; Liang, Q.; Zhong, A.; Yang, C.; Qian, J.; Song, H.; Chen, R. Insight into All-Solid-State Li–S Batteries: Challenges, Advances, and Engineering Design. Adv. Energy Mater. 2024, 14, 2401959. [Google Scholar] [CrossRef]
- Rao, X.-Y.; Xiang, S.-F.; Zhou, J.; Zhang, Z.; Xu, X.-Y.; Xu, Y.-Y.; Zhou, X.-C.; Pan, Z.-D.; Tan, S.-C.; Dong, S.-X.; et al. Recent Progress and Strategies of Cathodes toward Polysulfides Shuttle Restriction for Lithium-Sulfur Batteries. Rare Met. 2024, 43, 4132–4161. [Google Scholar] [CrossRef]
- Qian, W.; Guo, Y.; Zuo, W.; Wu, X.; Zhang, L. Toward Practical Lithium–Sulfur Batteries. Mater. Chem. Front. 2024, 8, 2556–2577. [Google Scholar] [CrossRef]
- Tan, J.; Ma, L.; Wang, Y.; Yi, P.; Ye, C.; Fang, Z.; Li, Z.; Ye, M.; Shen, J. Lithium Sulfur Batteries: Insights from Solvation Chemistry to Feasibility Designing Strategies for Practical Applications. Energy Environ. Mater. 2024, 7, e12688. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, T.; Yang, S.; Qu, Z.; Xiao, R.; Wang, G.; Sun, Z.; Li, F. Organosulfur Cathodes in Lithium Metal Batteries: Bridging the Gap between Fundamentals and Practical Applications. Adv. Funct. Mater. 2024, 34, 2405122. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Yang, J.; Nuli, Y.; Wang, J. Post Lithium-Sulfur Battery Era: Challenges and Opportunities towards Practical Application. Sci. China Chem. 2024, 67, 106–121. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Li, C.; Wang, Z.; Ma, Y.; Shi, X.; Zhang, H.; Song, D.; Zhang, L. Review on Polymer Electrolytes for Lithium-Sulfurized Polyacrylonitrile Batteries. EcoEnergy 2025, 3, 56–76. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, H.; Lu, Q. From Non-Carbon Host toward Carbon-Free Lithium-Sulfur Batteries. Nano Res. 2024, 17, 1337–1365. [Google Scholar] [CrossRef]
- Cai, D.; Zheng, F.; Li, Y.; Zhang, C.; Qin, Z.; Li, W.; Liu, Y.; Li, A.; Zhang, J. Design of Coatings for Sulfur-Based Cathode Materials in Lithium-Sulfur Batteries: A Review. Chem.—Asian J. 2024, 19, e202400099. [Google Scholar] [CrossRef]
- Huang, Z.; Cai, Y.; Zhang, S.; Wang, R.; Li, X.; Liu, Z. 3D Aligned Architectures for Lithium Batteries: Mechanism, Design, and Manufacture. Energy Storage Mater. 2025, 75, 103999. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, F.; Baek, J.; Lee, D.H.; Kim, M.; Song, H.-W.; Lee, S. From Non-Aqueous Liquid to Solid-State Li–S Batteries: Design Protocols, Challenges and Solutions. Mater. Adv. 2024, 5, 8772–8786. [Google Scholar] [CrossRef]
- Feng, J.; Yu, S.; Shi, C.; Tang, X.; Zhao, X.; Chen, S.; Song, J. Advanced Cathode Designs for High-Energy Lithium/Sodium–Selenium Battery. Adv. Funct. Mater. 2025, 35, 2422013. [Google Scholar] [CrossRef]
- Liu, X.; Xu, C.; Adenusi, H.; Wu, Y.; Passerini, S. Development of PFAS-Free Locally Concentrated Ionic Liquid Electrolytes for High-Energy Lithium and Aluminum Metal Batteries. Acc. Chem. Res. 2025, 58, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Yue, H.; Xiao, Y.; Huang, Y.; Li, X.; Gao, X.; He, N.; Zhan, C.; Nan, D. A Review of Organic Sulfur Applications in Lithium-Sulfur Batteries. J. Power Sources 2025, 625, 235717. [Google Scholar] [CrossRef]
- Cao, Z.; Wei, B. A Perspective: Carbon Nanotube Macro-Films for Energy Storage. Energy Environ. Sci. 2013, 6, 3183–3201. [Google Scholar] [CrossRef]
- Holze, R.; Wu, Y.P. Intrinsically Conducting Polymers in Electrochemical Energy Technology: Trends and Progress. Electrochim. Acta 2014, 122, 93–107. [Google Scholar] [CrossRef]
- Huang, J.-Q.; Zhang, Q.; Wei, F. Multi-Functional Separator/Interlayer System for High-Stable Lithium-Sulfur Batteries: Progress and Prospects. Energy Storage Mater. 2015, 1, 127–145. [Google Scholar] [CrossRef]
- Liang, Y.; Yao, Y. Positioning Organic Electrode Materials in the Battery Landscape. Joule 2018, 2, 1690–1706. [Google Scholar] [CrossRef]
- Yuan, H.; Peng, H.-J.; Huang, J.-Q.; Zhang, Q. Sulfur Redox Reactions at Working Interfaces in Lithium–Sulfur Batteries: A Perspective. Adv. Mater. Interfaces 2019, 6, 1802046. [Google Scholar] [CrossRef]
- Yang, X.; Luo, J.; Sun, X. Towards High-Performance Solid-State Li–S Batteries: From Fundamental Understanding to Engineering Design. Chem. Soc. Rev. 2020, 49, 2140–2195. [Google Scholar] [CrossRef]
- Li, H.; Yang, H.; Ai, X. Routes to Electrochemically Stable Sulfur Cathodes for Practical Li–S Batteries. Adv. Mater. 2023, 2305038. [Google Scholar] [CrossRef]
- Wang, Y.; Chai, J.; Li, Y.; Li, Q.; Du, J.; Chen, Z.; Wang, L.; Tang, B. Strategies to Mitigate the Shuttle Effect in Room Temperature Sodium–Sulfur Batteries: Improving Cathode Materials. Dalton Trans. 2023, 52, 2548–2560. [Google Scholar] [CrossRef]
- Tan, S.-J.; Feng, X.-X.; Wang, Y.-H.; Guo, Y.-G.; Xin, S. Nonconventional Electrochemical Reactions in Rechargeable Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2024, 16, 67002–67009. [Google Scholar] [CrossRef]
- Manthiram, A.; Chung, S.-H.; Zu, C. Lithium–Sulfur Batteries: Progress and Prospects. Adv. Mater. 2015, 27, 1980–2006. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, Y.-S.; Yang, J. Sulfur-Based Composite Cathode Materials for High-Energy Rechargeable Lithium Batteries. Adv. Mater. 2015, 27, 569–575. [Google Scholar] [CrossRef]
- Ma, J.; Li, Y.; Grundish, N.S.; Goodenough, J.B.; Chen, Y.; Guo, L.; Peng, Z.; Qi, X.; Yang, F.; Qie, L.; et al. The 2021 Battery Technology Roadmap. J. Phys. Appl. Phys. 2021, 54, 183001. [Google Scholar] [CrossRef]
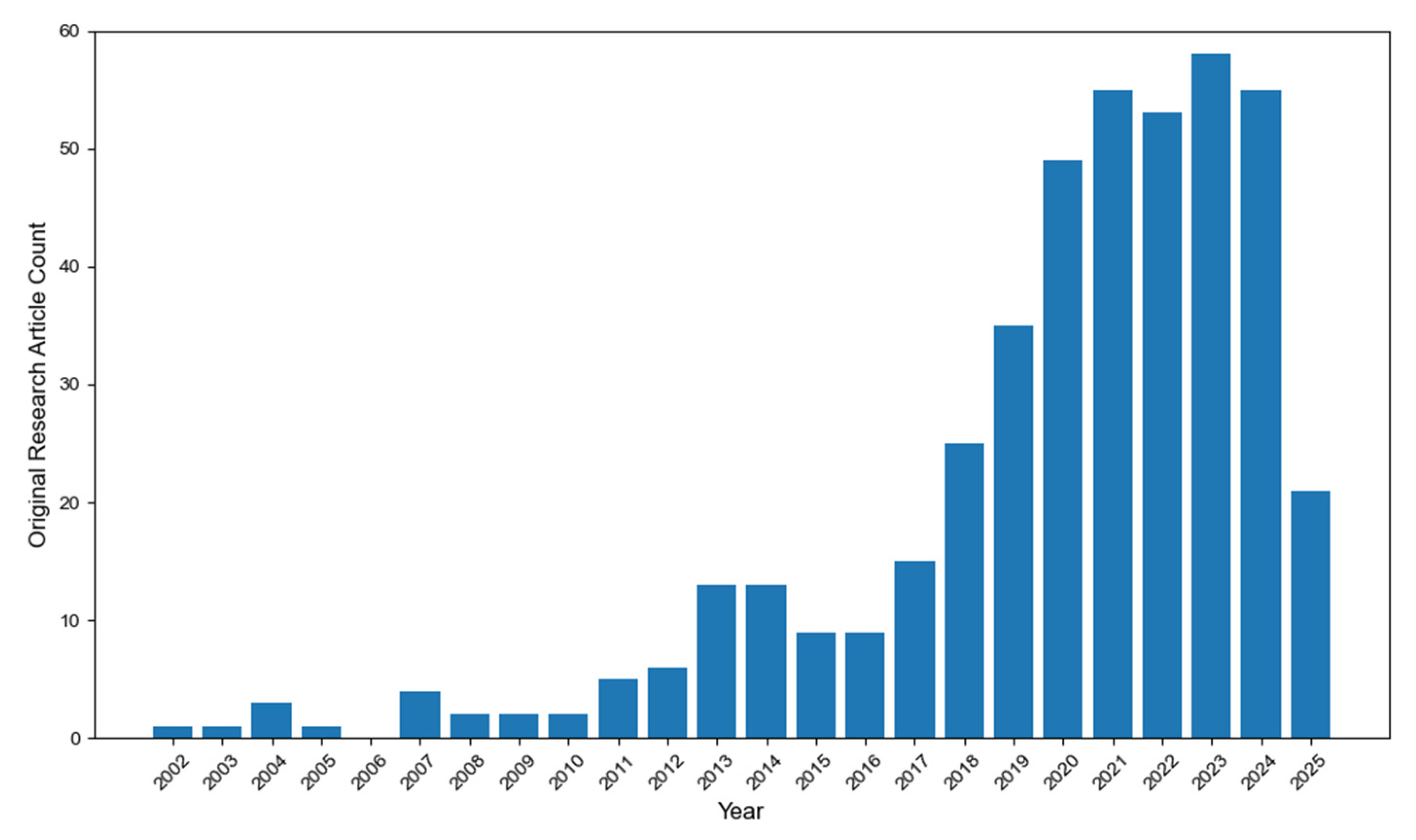
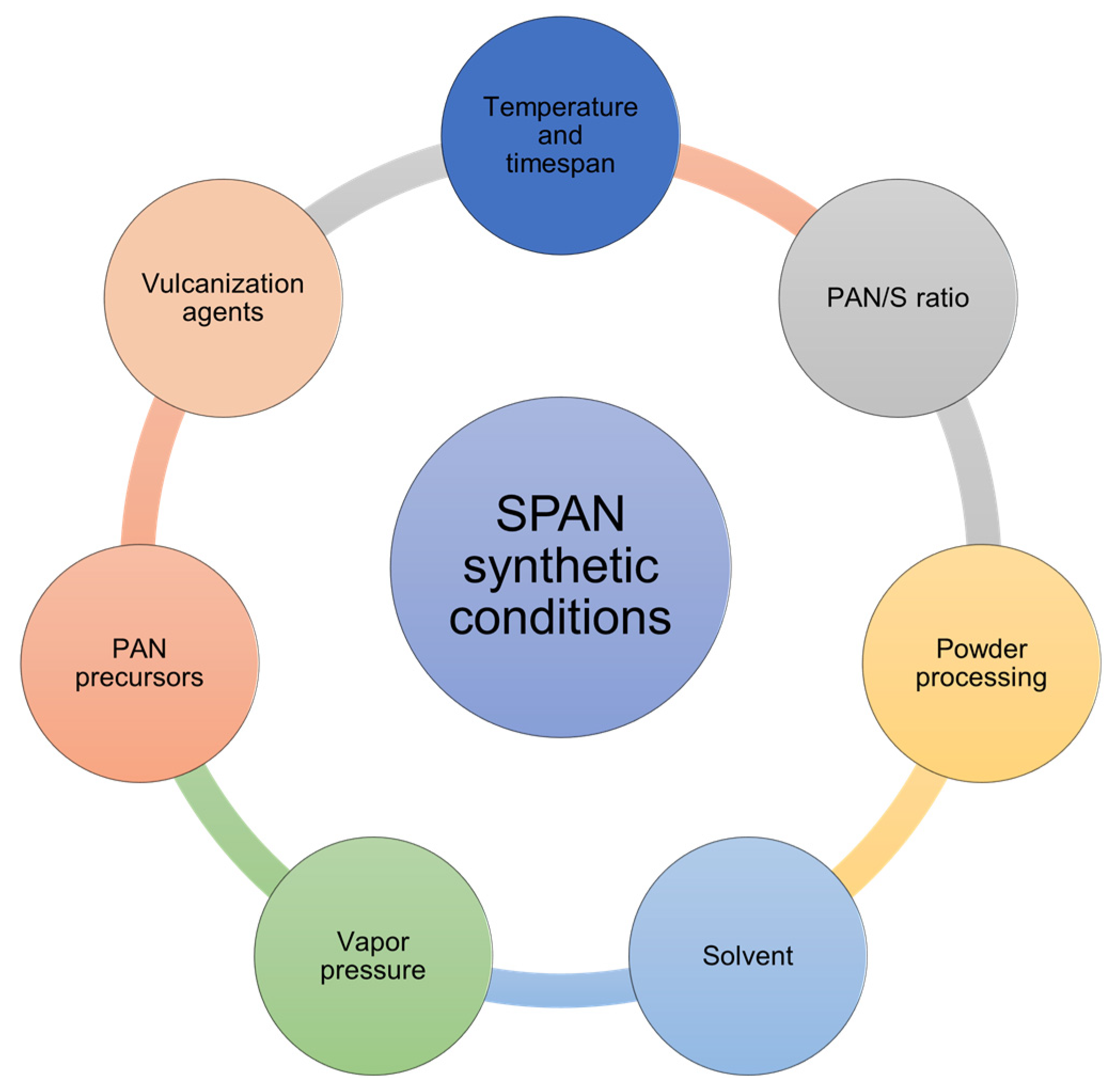

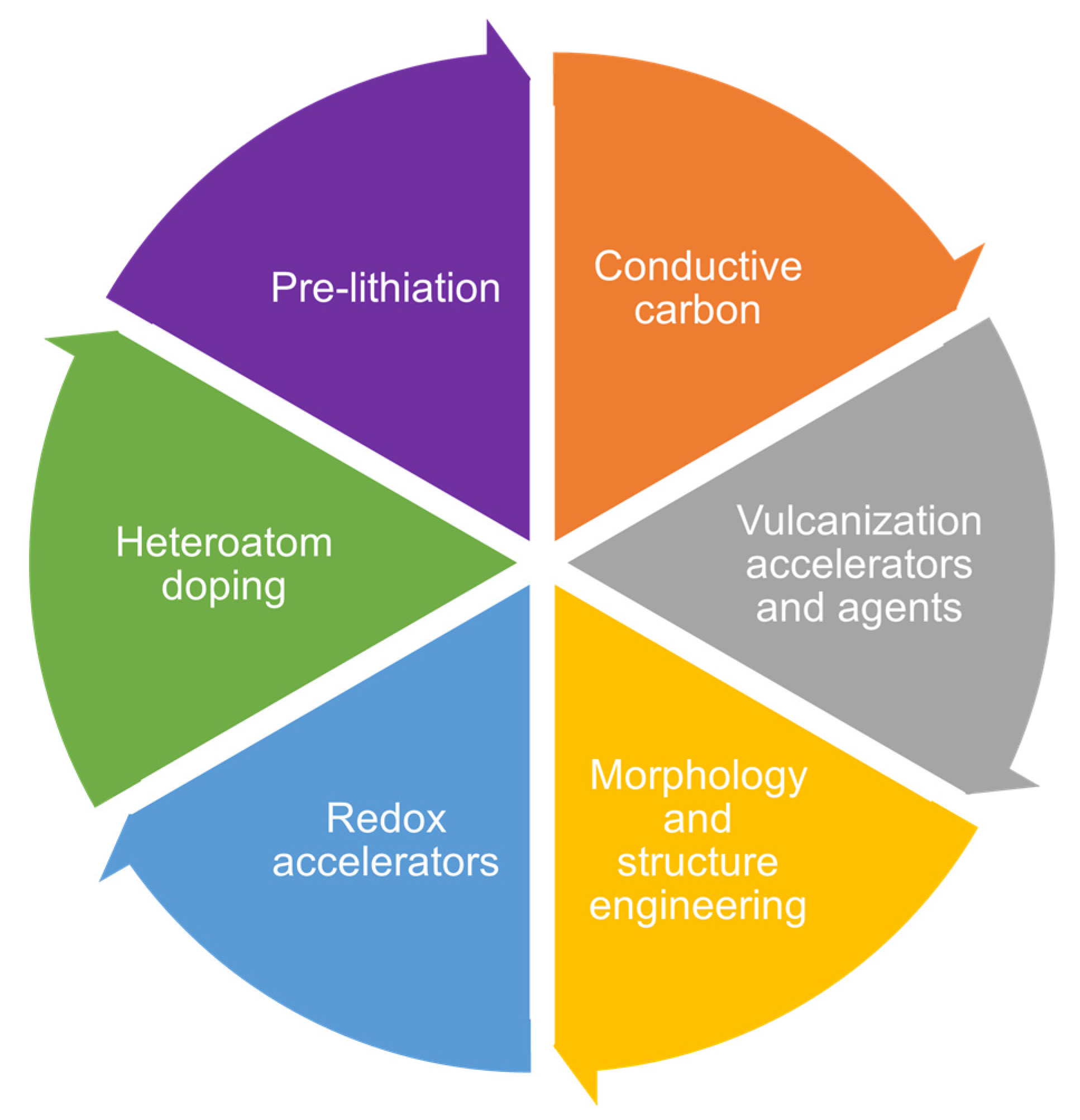
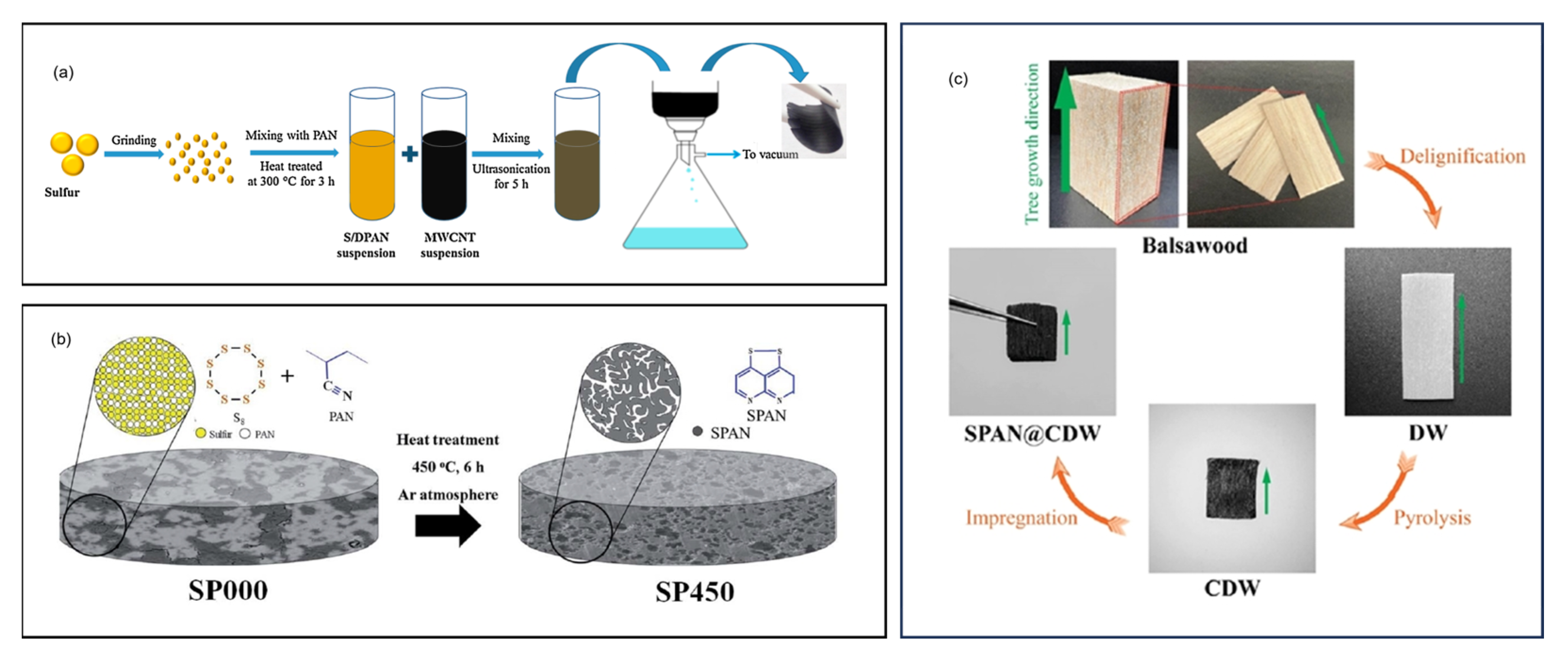
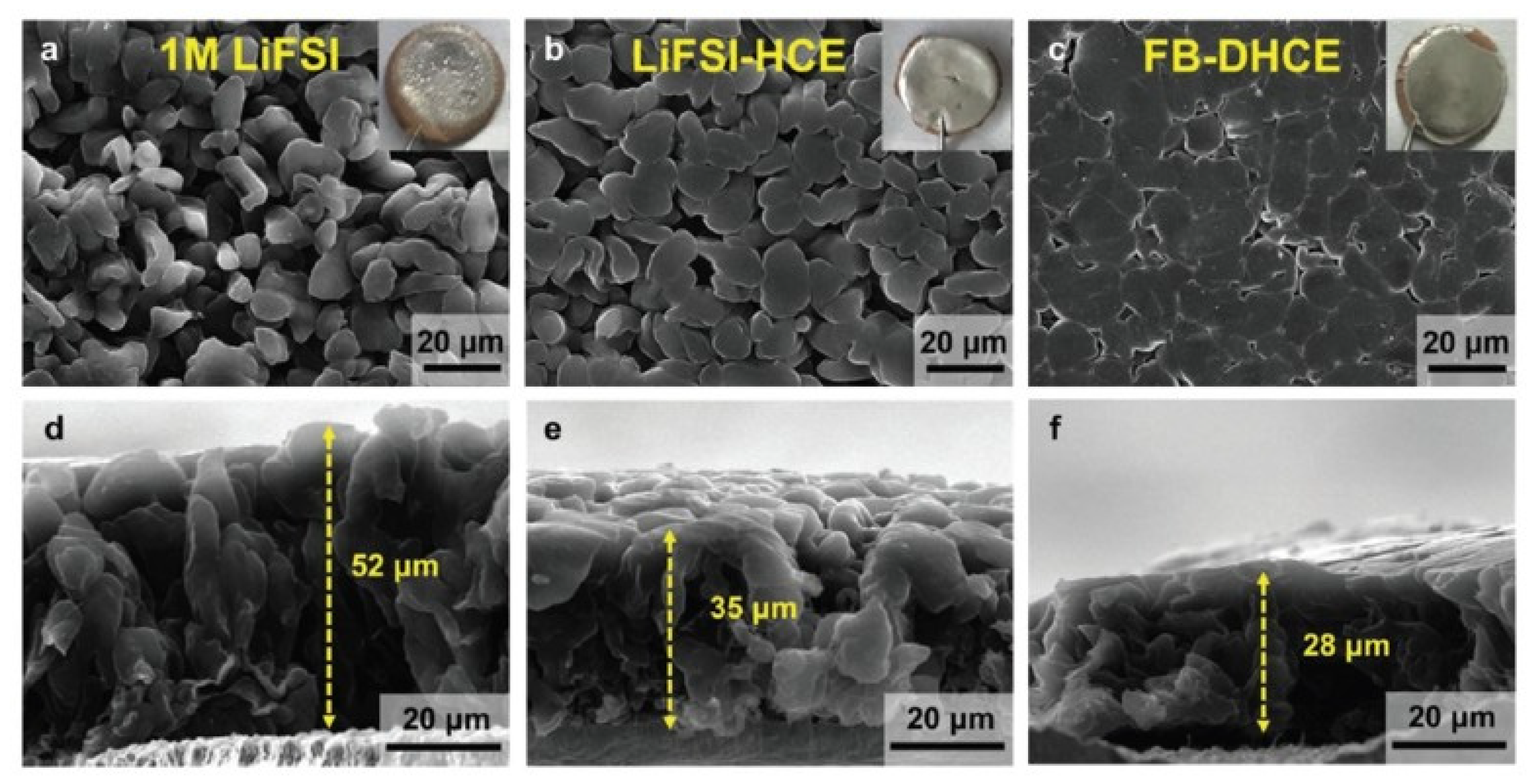
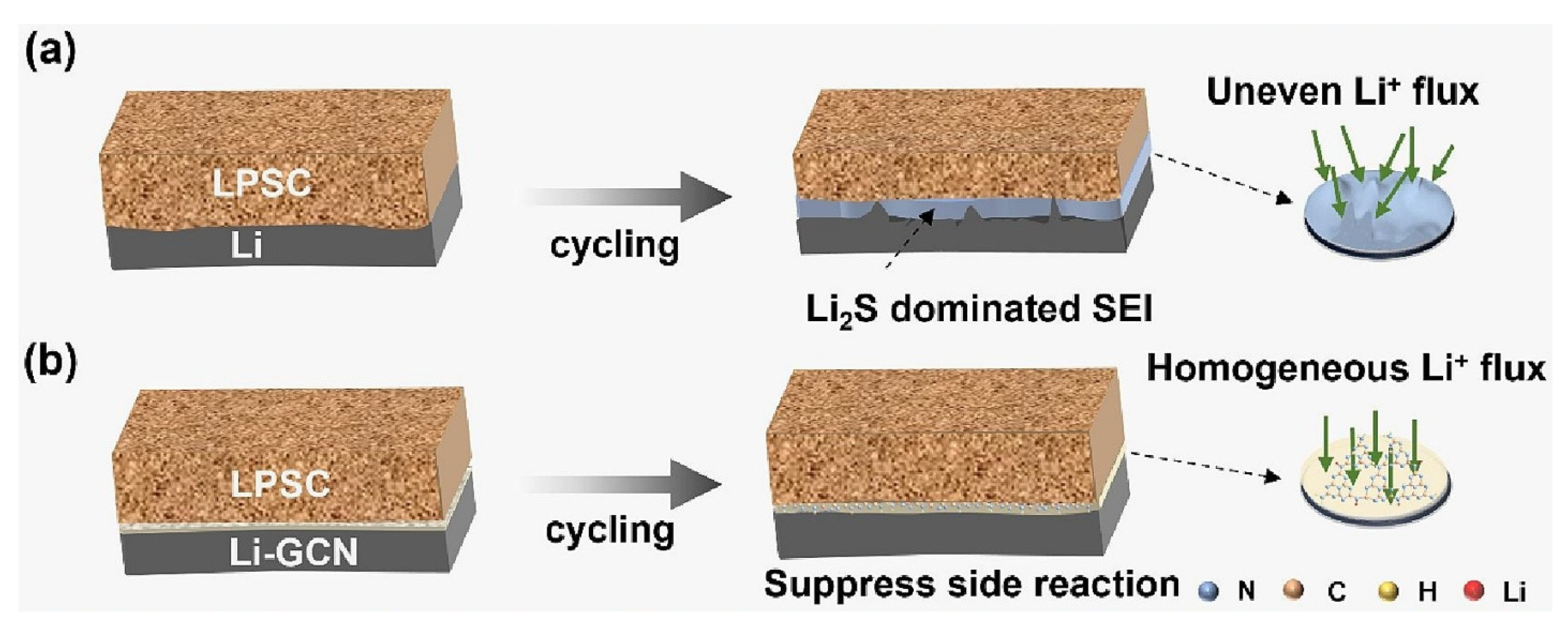

| Cathode | Synthetic Temperature (°C) | Cathode Loading | Initial Discharge Capacity | Cycles | Final Discharge Capacity | Capacity Retention | Rate/Current Density | Ref. |
|---|---|---|---|---|---|---|---|---|
| CSM-300 | 300 | 4.5 mgcomposite | 510 mAh g−1 (2nd) | 50 | 470 mAh g−1 | 92.2% | 0.2 mA cm−2 | [57] |
| CSM-350 | 350 | 4.5 mgcomposite | 570 mAh g−1 (2nd) | 50 | 490 mAh g−1 | 86.0% | 0.2 mA cm−2 | [57] |
| CSM-450 | 450 | 4.5 mgcomposite | 520 mAh g−1 (2nd) | 50/380 | 503/470 mAh g−1 | 96.7%/90.4% | 0.2 mA cm−2 | [57] |
| CSM-550 | 550 | 4.5 mgcomposite | 315 mAh g−1 (2nd) | 50 | 375 mAh g−1 | 119.0% | 0.2 mA cm−2 | [57] |
| CSM-650 | 650 | 4.5 mgcomposite | 245 mAh g−1 (2nd) | 50 | 290 mAh g−1 | 118.4% | 0.2 mA cm−2 | [57] |
| CSM-800 | 800 | 4.5 mgcomposite | 115 mAh g−1 (2nd) | 50 | 130 mAh g−1 | 113.0% | 0.2 mA cm−2 | [57] |
| SPAN-300 | 300 | N/A | 702.4 mAh g−1 (2nd) | 50 | 552.7 mAh g−1 | 78.7% | 100 mA g−1 | [58] |
| SPAN-350 | 350 | N/A | 801.5 mAh g−1 (2nd) | 50 | 795.4 mAh g−1 | 98.1% | 100 mA g−1 | [58] |
| SPAN-400 | 400 | N/A | 650.6 mAh g−1 (2nd) | 50 | 559.6 mAh g−1 | 86.1% | 100 mA g−1 | [58] |
| SPAN-300 | 300 | 2 mgcomposite cm−2 | 1195 mAh g−1 (3nd) | 100 | 239 mAh g−1 | 20.0% | C/10 | [59] |
| SPAN-350 | 350 | 2 mgcomposite cm−2 | 696 mAh g−1 (3nd) | 100 | 675 mAh g−1 | 97.0% | C/10 | [59] |
| PAN/S-250 | 250 | N/A | 751 mAh g−1 | 50 | 467 mAh g−1 | 62.2% | 100 mA g−1 | [60] |
| PAN/S-300 | 300 | N/A | 723 mAh g−1 | 50 | 505 mAh g−1 | 69.8% | 100 mA g−1 | [60] |
| PAN/S-350 | 350 | N/A | 725 mAh g−1 | 50 | 506 mAh g−1 | 69.8% | 100 mA g−1 | [60] |
| PAN/S-400 | 400 | N/A | 810 mAh g−1 | 50 | 579 mAh g−1 | 71.5% | 100 mA g−1 | [60] |
| PAN/S-450 | 450 | N/A | 586 mAh g−1 | 50 | 412 mAh g−1 | 70.3% | 100 mA g−1 | [60] |
| Cathode | Timespan (h) | Cathode Loading | Initial Discharge Capacity | Cycles | Final Discharge Capacity | Capacity Retention | Rate/Current Density | Ref. |
|---|---|---|---|---|---|---|---|---|
| PAN/S-6 | 6 | N/A | 789 mAh g−1 | 50 | 607 mAh g−1 | 76.9% | 100 mA g−1 | [60] |
| PAN/S-12 | 12 | N/A | 836 mAh g−1 | 50 | 648 mAh g−1 | 77.5% | 100 mA g−1 | [60] |
| PAN/S-18 | 18 | N/A | 840 mAh g−1 | 50 | 612 mAh g−1 | 72.9% | 100 mA g−1 | [60] |
| Cathode | PAN/S Ratio | Cathode Loading | Initial Discharge Capacity | Cycles | Final Discharge Capacity | Capacity Retention | Rate/Current Density | Ref. |
|---|---|---|---|---|---|---|---|---|
| SPAN_3:1 | 1:3 | 5 mgcomposite cm−2 | N/A | 40 | 429 mAh g−1composite | ~100% | C/10 | [65] |
| SPAN_5:1 | 1:5 | 5 mgcomposite cm−2 | N/A | 40 | 514 mAh g−1composite | ~100% | C/10 | [65] |
| SPAN_10:1 | 1:10 | 5 mgcomposite cm−2 | N/A | 40 | 557 mAh g−1composite | ~100% | C/10 | [65] |
| SPAN_15:1 | 1:15 | 5 mgcomposite cm−2 | N/A | 40 | 629 mAh g−1composite | ~100% | C/10 | [65] |
| SPAN_60:1 | 1:60 | 5 mgcomposite cm−2 | N/A | 40 | 614 mAh g−1composite | ~100% | C/10 | [65] |
| PAN/S-1:1.5 | 1:1.5 | N/A | 856 mAh g−1 | 50 | 678 mAh g−1 | 79.2% | 100 mA g−1 | [60] |
| PAN/S-1:1.8 | 1:1.8 | N/A | 843 mAh g−1 | 50 | 523 mAh g−1 | 62.0% | 100 mA g−1 | [60] |
| PAN/S-1:2 | 1:2 | N/A | 819 mAh g−1 | 50 | 525 mAh g−1 | 64.1% | 100 mA g−1 | [60] |
| Cathode | Vapor Pressure | Cathode Loading | Initial Discharge Capacity | Cycles | Final Discharge Capacity | Capacity Retention | Rate/Current Density | Ref. |
|---|---|---|---|---|---|---|---|---|
| S@pPAN-0 | open | 1.9 mgcomposite cm−2 | 1176 mAh g−1sulfur (2nd) | 100 | 721 mAh g−1sulfur | 61.3% | 200 mA g−1 | [64] |
| S@pPAN-2 | 2 MPa | 1.9 mgcomposite cm−2 | 1320 mAh g−1sulfur (2nd) | 100 | 1007 mAh g−1sulfur | 76.3% | 200 mA g−1 | [64] |
| S@pPAN-5 | 5 MPa | 1.9 mgcomposite cm−2 | 1542 mAh g−1sulfur (2nd) | 100 | 1357 mAh g−1sulfur | 88.0% | 200 mA g−1 | [64] |
| S@pPAN-8 | 8 MPa | 1.9 mgcomposite cm−2 | 1538 mAh g−1sulfur (2nd) | 100 | 1277 mAh g−1sulfur | 83.0% | 200 mA g−1 | [64] |
| SPAN | Open system | N/A | 1295 mAh g−1sulfur | 200 | 1253 mAh g−1sulfur | 96.8% | C/3 | [68] |
| SPAN | Closed system | N/A | 1301 mAh g−1sulfur | 200 | 1170 mAh g−1sulfur | 89.9% | C/3 | [68] |
| Cathode | Cathode Loading | Initial Discharge Capacity | Cycles | Final Discharge Capacity | Capacity Retention | Rate/Current Density | Ref. |
|---|---|---|---|---|---|---|---|
| pPAN-S@MWCNT | N/A | 697 mAh g−1composite (2nd) | 50 | 592 mAh g−1composite | 85.0% | C/10 | [82] |
| SPAN/MWCNT | 4 mgcomposite cm−2 | N/A | 100 | N/A | 96.5% | C/2 | [78] |
| SPAN/C | N/A | 500 mAh g−1 | 100 | 400 mAh g−1 | 80.0% | 1 C | [83] |
| SPAN-CNT20 | 0.9–1.1 mgsulfur cm−2 | N/A | 500 | 1106 mAh g−1sulfur | N/A | 1 C | [84] |
| CoS2-SPAN-CNT | 2.4 mgsulfur cm−2 | 1799 mAh g−1 | 100 | 1240 mAh g−1 | 68.9% | 0.2 C | [85] |
| Co10-SPAN-CNT | 1 mgsulfur cm−2 | 1252 mAh g−1sulfur | 1500 | 1020 mAh g−1sulfur | 81.5% | 1 C | [86] |
| SPAN/CNT-12 | 2 mgsulfur cm−2 | N/A | 1000 | 1180 mAh gsulfur−1 | ~100% | 0.2 C | [87] |
| SPAN/CNT-500 | N/A | 1814 mAh g−1 | 200 | 1280 mAh g−1 | 70.6% | 400 mA g−1 | [88] |
| CNT1@SPAN | 5 mgsulfur cm−2 | N/A | 50 | 1079.1 mAh g−1 | N/A | 0.1 C | [89] |
| ICIHP-SPAN | N/A | 614.8 mAh g−1 | 500 | 500 mAh g−1 | 81.3% | 5 C | [90] |
| PDA@SPAN | 7.16 mgcomposite cm−2 | N/A | 160 | N/A | 87.18% | 0.1 C | [91] |
| SPAN/MWCNTs-NH2 | N/A | 400 | 594.8 mAh g−1 | 90.0% | 1 C | [92] |
| Cathode | Cathode Loading | Initial Discharge Capacity | Cycles | Final Discharge Capacity | Capacity Retention | Rate/Current Density | Ref. |
|---|---|---|---|---|---|---|---|
| pPAN-S/GNS 4% | N/A | 1500 mAh g−1sulfur | 100 | 1200 mAh g−1sulfur | 80% | C/10 | [94] |
| pPAN-S/mGO-S | 1–2 mgcomposite cm−2 | 900 mAh g−1composite | 50 | 650 mAh g−1composite | N/A | C/10 | [95] |
| S/PAN/Graphene | 3.5 mgcomposite cm−2 | 612 mAh g−1 (2nd cycle) | 100 | N/A | 77% | C/10 | [96] |
| pPAN-S@GNS | 2 mgcomposite cm−2 | 681.2 mAh g−1composite (2nd cycle) | 300 | N/A | 88.8% | 0.2 C | [97] |
| S/DPAN/rGO | N/A | 1490 mAh g−1sulfur (2nd cycle) | 100 | N/A | 92% | 0.2 C | [98] |
| PAN/S/GO | 2.4 mgsulfur cm−2 | 1402 mAh g−1sulfur | 50 | 1096 mAh g−1sulfur | N/A | 0.2 C | [99] |
| 3DHG/PS2 | 15.2 mgcomposite cm−2 | N/A | 1500 | 581.6 mAh g−1sulfur | 81.5% | 2 C | [100] |
| 2D-SPAN/G | 10 mgsulfur cm−2 | N/A | 300 | N/A | 79.0% | 0.25 C | [101] |
| SFPAN-g-rGO (2000:1) | 1.2–1.6 mgcomposite cm−2 | 1303 mAh g−1 (after activation) | 800 | 1129 mAh g−1 | N/A | 1 C | [73] |
| SPAN/RGO | 8.0 mgcomposite cm−2 | N/A | 60 | 670.2 mAh g−1 | N/A | 0.1 C | [102] |
| Cathode | Cathode Loading | Initial Discharge Capacity | Cycles | Final Discharge Capacity | Capacity Retention | Rate/Current Density | Ref. |
|---|---|---|---|---|---|---|---|
| PAN-S/C | N/A | N/A | 50 | 658.8 mAh g−1SPAN | 95% | C/10 | [105] |
| pPAN-KB/S | 4.4 mgsulfur cm−2 | N/A | 50 | 513 mAh g−1sulfur | N/A | C/2 | [106] |
| SPAN@D-KB | 2 mgcomposite cm−2 | N/A | 350 | 653 mAh g−1composite | N/A | 0.2 C | [107] |
| pPAN1@C/S | 0.6–0.8 mgsulfur cm−2 | 1269 mAh g−1sulfur | N/A | N/A | N/A | 0.5 C | [108] |
| S/MCPs-PAN-52 | 1 mgsulfur cm−2 | 789.7 mAh g−1composite | 200 | 666.2 mAh g−1composite | N/A | 160 mA g−1 | [109] |
| S/rSP@SPAN | 0.79–0.81 mgcomposite cm−2 | 1500 mAh g−1sulfur (2nd cycle) | 100 | 1251 mAh g−1sulfur | N/A | 0.1 C | [110] |
| PANC@BP/PAN/S | N/A | 700 mAh g−1composite (2nd cycle) | 400 | 612 mAh g−1composite | 87.6% | 100 mA g−1 | [111] |
| PBD622-400 | 2.5–6.7 mgcomposite cm−2 | 1431 mAh g−1sulfur | 150 | 806 mAh g−1sulfur | N/A | 1050 mA g−1 | [112] |
| Cathode | Cathode Loading | Initial Discharge Capacity | Cycles | Final Discharge Capacity | Capacity Retention | Rate/Current Density | Ref. |
|---|---|---|---|---|---|---|---|
| PAN-S-VA | N/A | 494 mAh g−1composite (2nd cycle) | 200 | 477 mAh g−1composite | 95% | 0.25 C | [119] |
| S@PAN-DG | 5 mgSPAN cm−2 | N/A | 100 | 815 mAh g−1composite | 91.5% | 0.25 C | [120] |
| SPAN-DG | 5 mgSPAN cm−2 | 730 mAh g−1composite (2nd cycle) | 500 | 620 mAh g−1composite | 84.93% | 1 C | [121] |
| SPAN-1 | 2 mgsulfur cm−2 | N/A | 2000 | 857 mAh g−1 | N/A | 5 A g−1 | [122] |
| Se0.05S0.95PAN-TI11 | 2 mg cm−2 | N/A | 1000 | N/A | 89.4% | 2 C | [123] |
| SPAN-1.5%HBO | N/A | 853 mAh g−1 | 100 | 577 mAh g−1 | N/A | 1 C | [124] |
| Cathode | Template | Cathode Loading | Initial Discharge Capacity | Cycles | Final Discharge Capacity | Capacity Retention | Rate/Current Density | Ref. |
|---|---|---|---|---|---|---|---|---|
| fibrous SPAN | PMMA | 0.672 mgsulfur cm−2 | 1672 mAh g−1sulfur | N/A | N/A | N/A | 0.5 C | [131] |
| MSPAN | SBA-15 | 2.45 mg cm−2 | N/A | 900 | 610 mAh g−1 | N/A | 2 C | [132] |
| Porous PAN/S | PMMA | N/A | 1620 mAh g−1sulfur | 500 | 794 mAh g−1sulfur | N/A | 2 C | [134] |
| pPAN-SeS2 | PS | 2 mgcomposite cm−2 | 871 mAh g−1 | 2000 | 633 mAh g−1 | N/A | 4 A g−1 | [135] |
| MSPAN-2 | PS | 1 mgcomposite cm−2 | 523 mAh g−1 | 100 | 437 mAh g−1 | N/A | 0.1 A g−1 | [136] |
| H-SPAN | PEO | 2.2 mgcomposite cm−2 | N/A | 300 | 1250 mAh g−1sulfur | N/A | 0.1 C | [137] |
| TPSPAN | Sodium bicarbonate | N/A | 962 mAh g−1 | 2000 | N/A | 94.6% | 2 A g−1 | [138] |
| Cathode | Redox Accelerator | Cathode Loading | Initial Discharge Capacity | Cycles | Final Discharge Capacity | Capacity Retention | Rate/Current Density | Ref. |
|---|---|---|---|---|---|---|---|---|
| NiS2-SPAN | NiS2 | 1.15 mgsulfur cm−2 | 1722 mAh g−1sulfur | 100 | 1533 mAh g−1sulfur | 89% | 200 mA g−1 | [162] |
| FeS@SPAN | FeS | 1–1.2 mgcomposite cm−2 | 844.5 mAh g−1composite | 500 | 688.6 mAh g−1composite | N/A | 1 A g−1 | [163] |
| SPAN-1 | Fe1−xS | 2 mgsulfur cm−2 | N/A | 2000 | 857 mAh g−1 | N/A | 5 A g−1 | [122] |
| CoS2-SPAN-CNT | CoS2 | 2.4 mgsulfur cm−2 | 1799 mAh g−1 | 100 | 1240 mAh g−1 | 68.9% | 0.2 C | [85] |
| CoS2-SFPAN-3 | CoS2 | 1–1.4 mgsulfur cm−2 | N/A | 500 | 631 mAh g−1 | N/A | 1 C | [164] |
| CoSe2-10@SPAN | CoSe2 | 1.67 mgcomposite cm−2 | ~800 mAh g−1 | 500 | 675 mAh g−1 | 71.1% | 1 A g−1 | [165] |
| Co10-SPAN-CNT | Co-N4S | 1 mgsulfur cm−2 | 1252 mAh g−1sulfur | 1500 | 1020 mAh g−1sulfur | 81.5% | 1 C | [86] |
| MoS2@SPAN | MoS2 | 2 mgcomposite cm−2 | N/A | 500 | 264 mAh g−1 | N/A | 2 A g−1 | [166] |
| FeMn@GN-SPAN | FexMn1-xS | 3 mgsulfur cm−2 | 967 mAh g−1 | 500 | 845 mAh g−1 | N/A | 0.3 C | [147] |
| WS2-SPAN | WS2 | N/A | N/A | 450 | 464 mAh g−1 | N/A | 0.5 A g−1 | [167] |
| WSSe-Se@PAN-2 | WSxSe2−x | N/A | N/A | 700 | 467 mAh g−1 | N/A | 2 A g−1 | [168] |
| BiSbSx@SPAN-450 | BiSbSx | N/A | N/A | 2000 | 472 mAh g−1composite | N/A | 1 A g−1 | [169] |
| SnS2@SPAN | SnS2 | 0.5–1 mg cm−2 | N/A | 500 | 117.5 mAh g−1 | ~100% | 4 A g−1 | [170] |
| SnS2-SPAN | SnS2 | N/A | N/A | 700 | 218 mAh g−1 | N/A | 0.5 A g−1 | [171] |
| S/PAN/Mg0.6Ni0.4O | Mg0.6Ni0.4O | 4 mg cm−2 | 1545 mAh g−1sulfur | 100 | 1223 mAh g−1sulfur | N/A | 0.1 C | [172] |
| S/PAN/SiO2 | SiO2 | 2.5 mg cm−2 | N/A | 100 | 1106 mAh g−1 | N/A | 0.2 C | [173] |
| SPAN/Ti | TiO2 | 1 mgsulfur cm−2 | 1885 mAh g−1sulfur | 200 | ~1600 mAh g−1sulfur | N/A | 0.5 C | [174] |
| SPAN/Ti-Y | TiO2/Y2O3 | 1 mg cm−2 | 1528 mAh g−1sulfur & oxides (5th cycle) | 850 | 907 mAh g−1sulfur & oxides | N/A | 3 C | [175] |
| FeNb@SPAN | FexNbyO | 1 mg cm−2 | N/A | 80 | 201 mAh g−1 | N/A | 50 mA g−1 | [176] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, M. Sulfurized Polyacrylonitrile for Rechargeable Batteries: A Comprehensive Review. Batteries 2025, 11, 290. https://doi.org/10.3390/batteries11080290
Wei M. Sulfurized Polyacrylonitrile for Rechargeable Batteries: A Comprehensive Review. Batteries. 2025; 11(8):290. https://doi.org/10.3390/batteries11080290
Chicago/Turabian StyleWei, Mufeng. 2025. "Sulfurized Polyacrylonitrile for Rechargeable Batteries: A Comprehensive Review" Batteries 11, no. 8: 290. https://doi.org/10.3390/batteries11080290
APA StyleWei, M. (2025). Sulfurized Polyacrylonitrile for Rechargeable Batteries: A Comprehensive Review. Batteries, 11(8), 290. https://doi.org/10.3390/batteries11080290