Performance of Low-Cost Energy Dense Mixed Material MnO2-Cu2O Cathodes for Commercially Scalable Aqueous Zinc Batteries
Abstract
1. Introduction
2. Experimental Section
- Materials: Electrolytic manganese dioxide (EMD) was purchased from Borman (Las Vegas, NV, USA). Zinc (Zn) powders were purchased from Grillo-Werke AG (Duisburg, Germany). Potassium hydroxide (KOH) was purchased from Fisher Scientific (Waltham, MA, USA). Graphite (MX-25 and BNB-90) was purchased from Imerys Graphite & Carbon (Terrebonne, QC, Canada, previously known as TIMCAL). Carbon nanotubes (CNTs) were purchased from CNano (Reno, NV, USA). Bismuth oxide (Bi2O3), copper oxide (Cu2O) powder was purchased from Sigma-Aldrich (St. Louis, MO, USA). Nickel and copper mesh were purchased from PPG Engineered Materials (Wallingford, CT, USA, formerly known as Dexmet Corporation). Dispersion Teflon was purchased from DuPont (Wilmington, DE, USA). Sintered nickel electrodes were purchased from Jiangsu HighStar Battery Manufacturing (Qidong, China). Cellophane was purchased from Innovia Films (Wigton, UK). Pellon was purchased from Freudenberg (Weinheim, Germany). Crosslinked polyvinyl alcohol (PVA) separators were made in-house by casting a water solution of 5–10wt.% PVA [36].
- Electrode Fabrication: Electrodes were made by first mixing the raw materials with dispersion Teflon into a homogenous mixture and rolled into sheets using a rolling pin or a rolling machine. These sheets were dried at 60 °C overnight. The dried sheets were then pressed onto current collectors. Cathode sheets were pressed onto nickel mesh while anode sheets were pressed onto copper mesh.The cathodes were composed of 29 wt.% EMD, 35 wt.% Cu2O, 23 wt.% graphite or carbon nanotubes 10 wt.% Bi2O3 and 3 wt.% Teflon, while the anodes were composed of 97 wt.% Zn and 3 wt.% Teflon. These compositions remained the same for prismatic or cylindrical form factor cells. Zn electrodes were only used in “full cells” when cathode performance vs. Zn anodes needed to be tested. In “half-cell” tests, we used a sintered nickel counter electrode and a mercury (Hg)|mercury oxide (HgO) reference electrode to test only the cathode performance.The fabricated electrodes size changed with the type of experiment performed. Usually, the prismatic electrode sizes were 2 in × 3 in and 3 in × 6 in while cylindrical electrode sizes ranged from 3 in × 12 in to 3 in × 17 in.
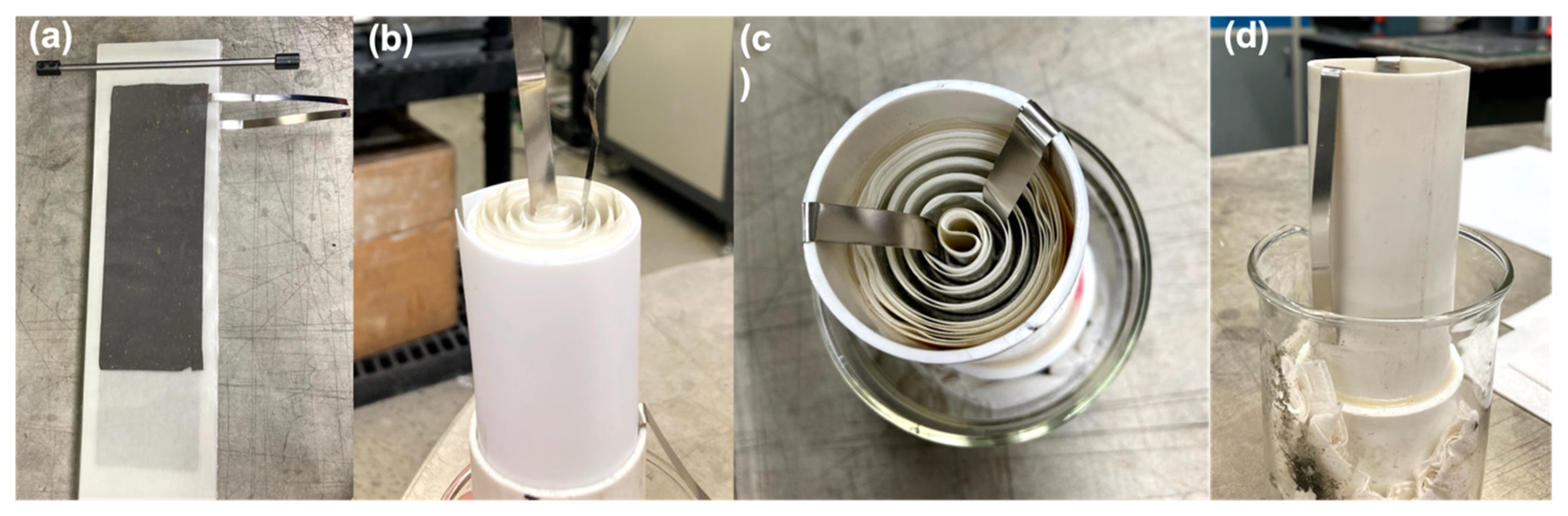
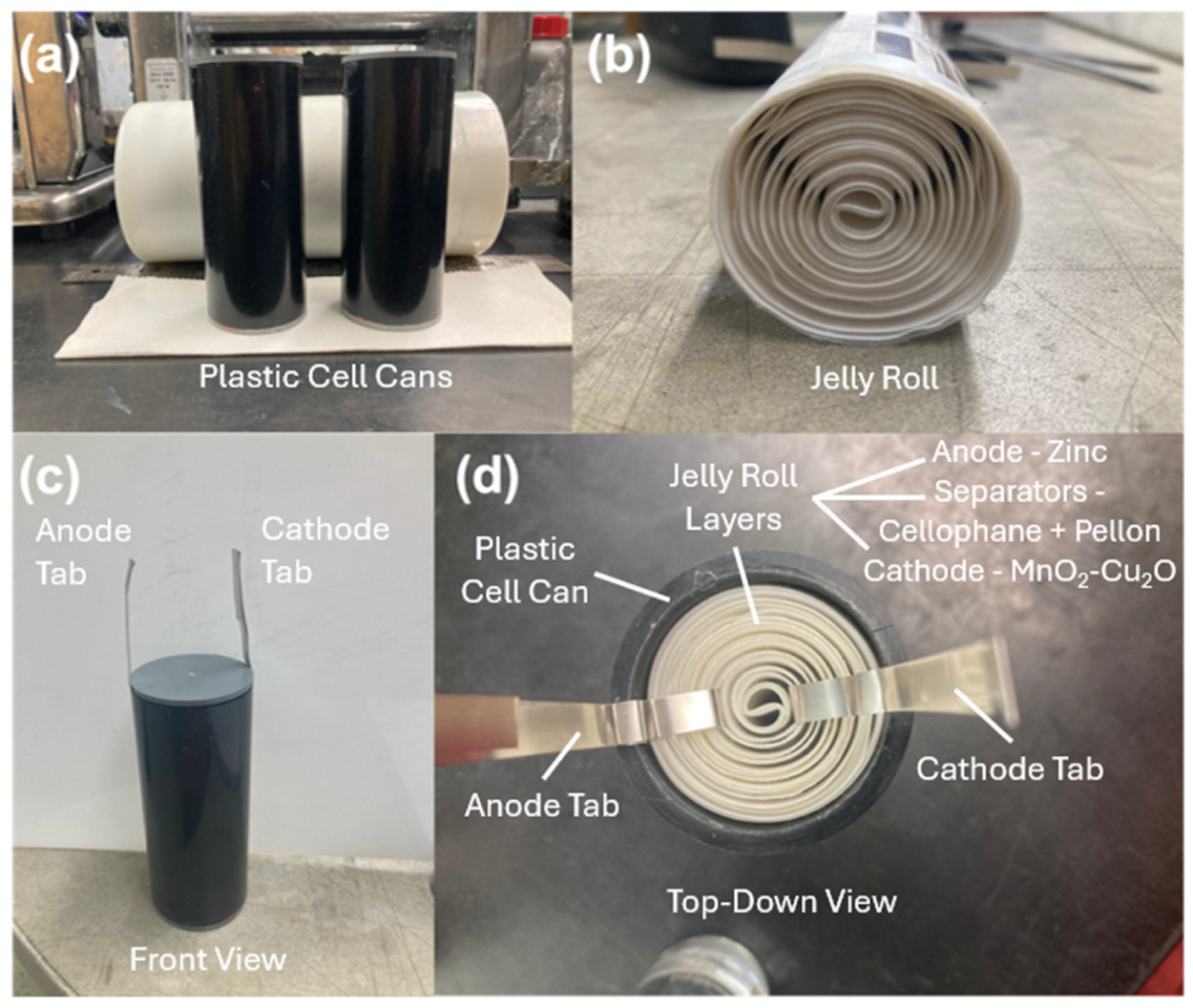
- Battery Fabrication: Prismatic and cylindrical form factor cells were tested. Prismatic cells were conducted in polysulfone boxes whose dimensions were 3.26 in × 2.23 in × 6.25 in (width × depth × height). Electrode packs consisting of anodes and cathodes were wrapped in one or two layers of cellophane (0.001 in each layer) or crosslinked polyvinyl alcohol (PVA) (0.002 ± 0.001 in) separator. We also used 3.44 in × 1.85 in × 8.13 in (width × depth × height) polypropylene boxes for testing larger electrodes. These packs were compressed in prismatic cell boxes between polypropylene shims. The shims were placed on each side of the multiple electrode pack. Prismatic and cylindrical cells were filled with either 25 or 37 wt.% potassium hydroxide (KOH) electrolyte as noted.PVC pipes (purchased from McMaster Carr, Elmhurst, IL, USA) were used for cylindrical can designs. The pipe outer diameter was 2 in and the inner diameter was 1.6 in. The pipes were cut to different heights for different cell capacity testing. The cylindrical electrodes were rolled with the help of a rolling pin to obtain the right alignment. The electrodes were separated by cellophane and Pellon.
- Battery Testing and Equipment: A multi-channel ARBIN BT-2000 (College Station, TX, USA) and PEC tester (Leuven, Belgium) were used for cycling the cells. Cell capacities and C-rates were determined by the amount of active material (MnO2 and Cu2O) used in the cells. Bi2O3 has a known capacity (345.11 mAh/g) but was not counted here since only a small amount was used and has a negligible capacity contribution depending on the discharge voltage cutoff used. Voltaiq was used for battery data plotting and analysis. Batteries were cycled under capacity limits or voltage limits. Capacity limits were set to 280 mAh/g active material (MnO2 + Cu2O) while the voltage limits between 0.3 V and −1.0 V vs. Hg|HgO (equivalent to ~1.65–1.7 V and ~0.35 V vs. Zn) were utilized to access to the entire active material capacity available in the cathode. For cells utilizing graphite in place of carbon nanotubes, a modified capacity-limited cycling protocol was used.
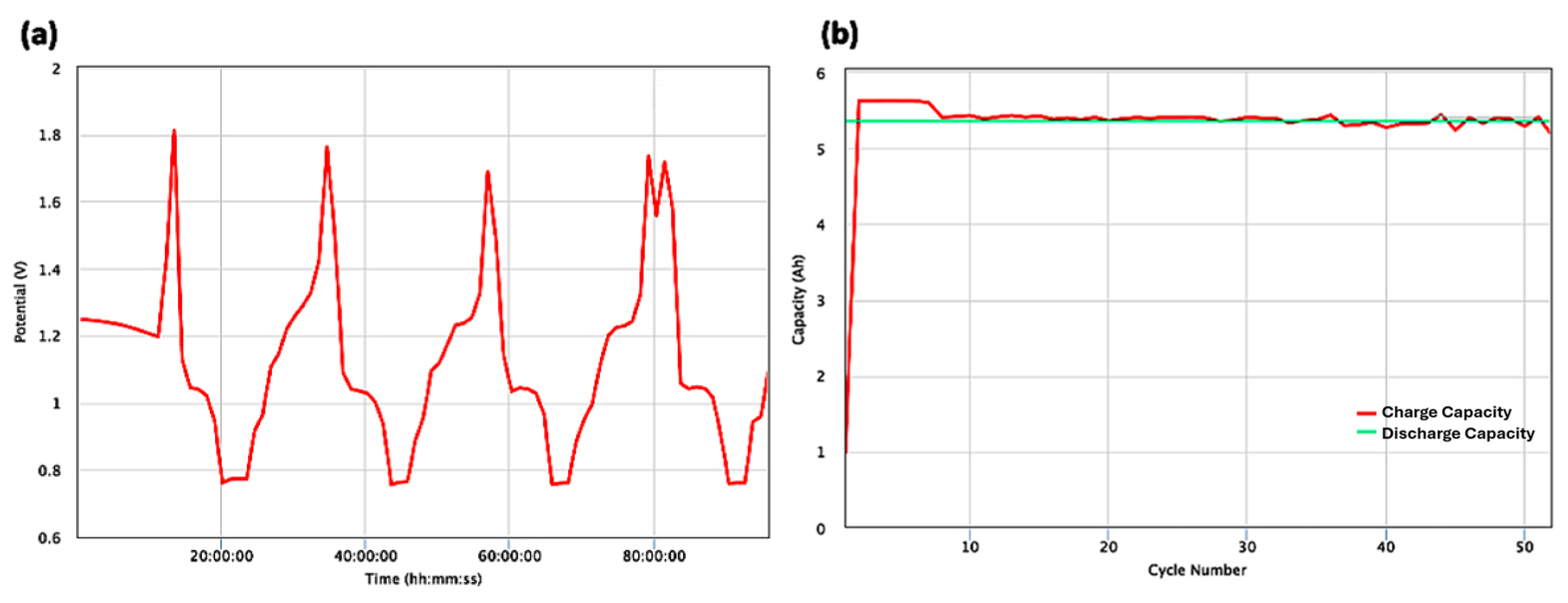
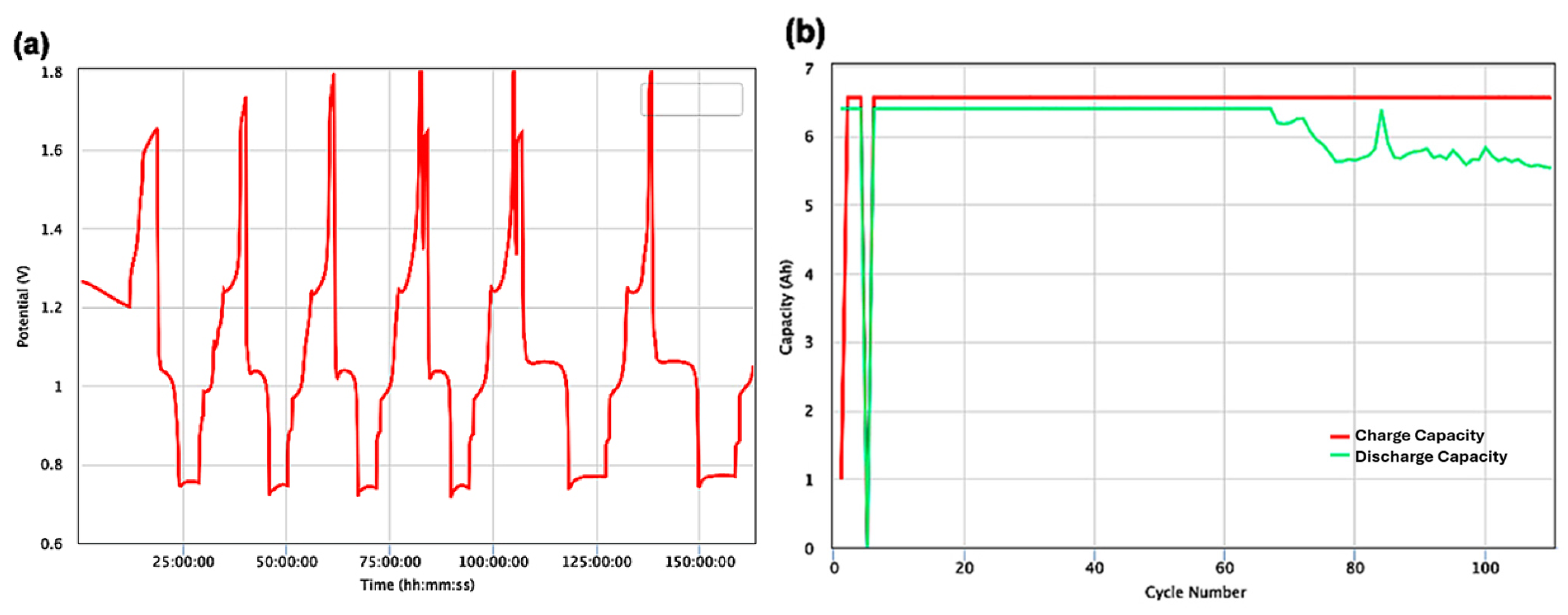
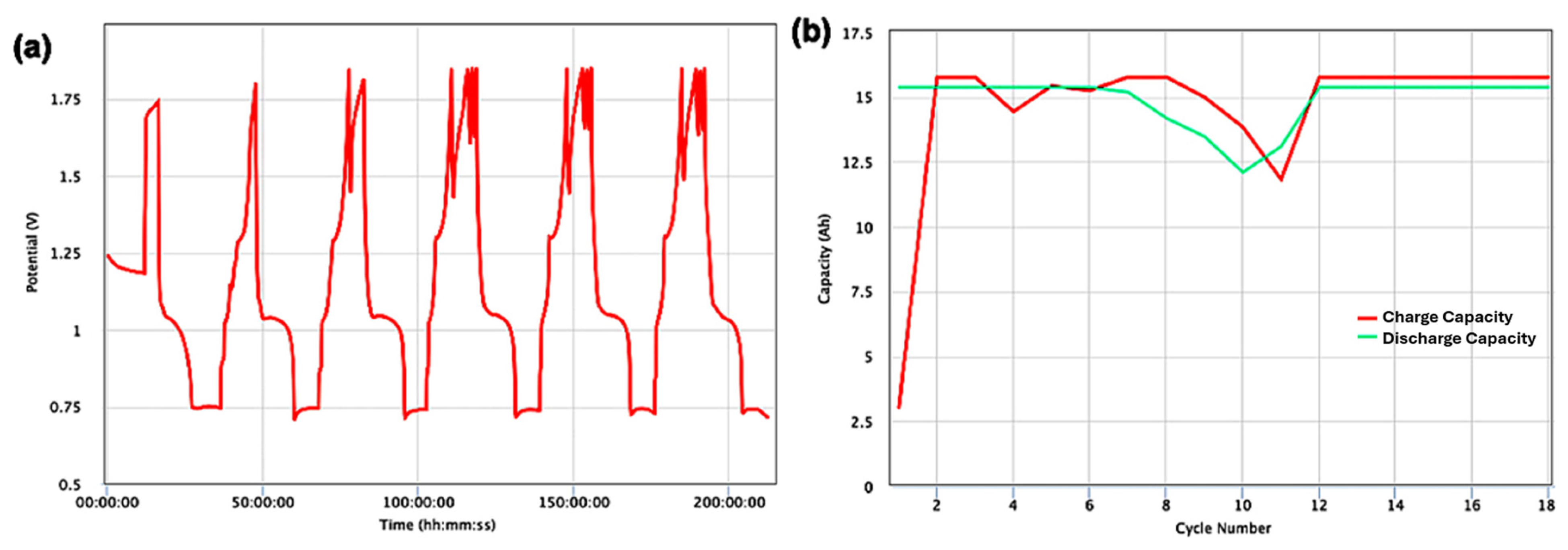
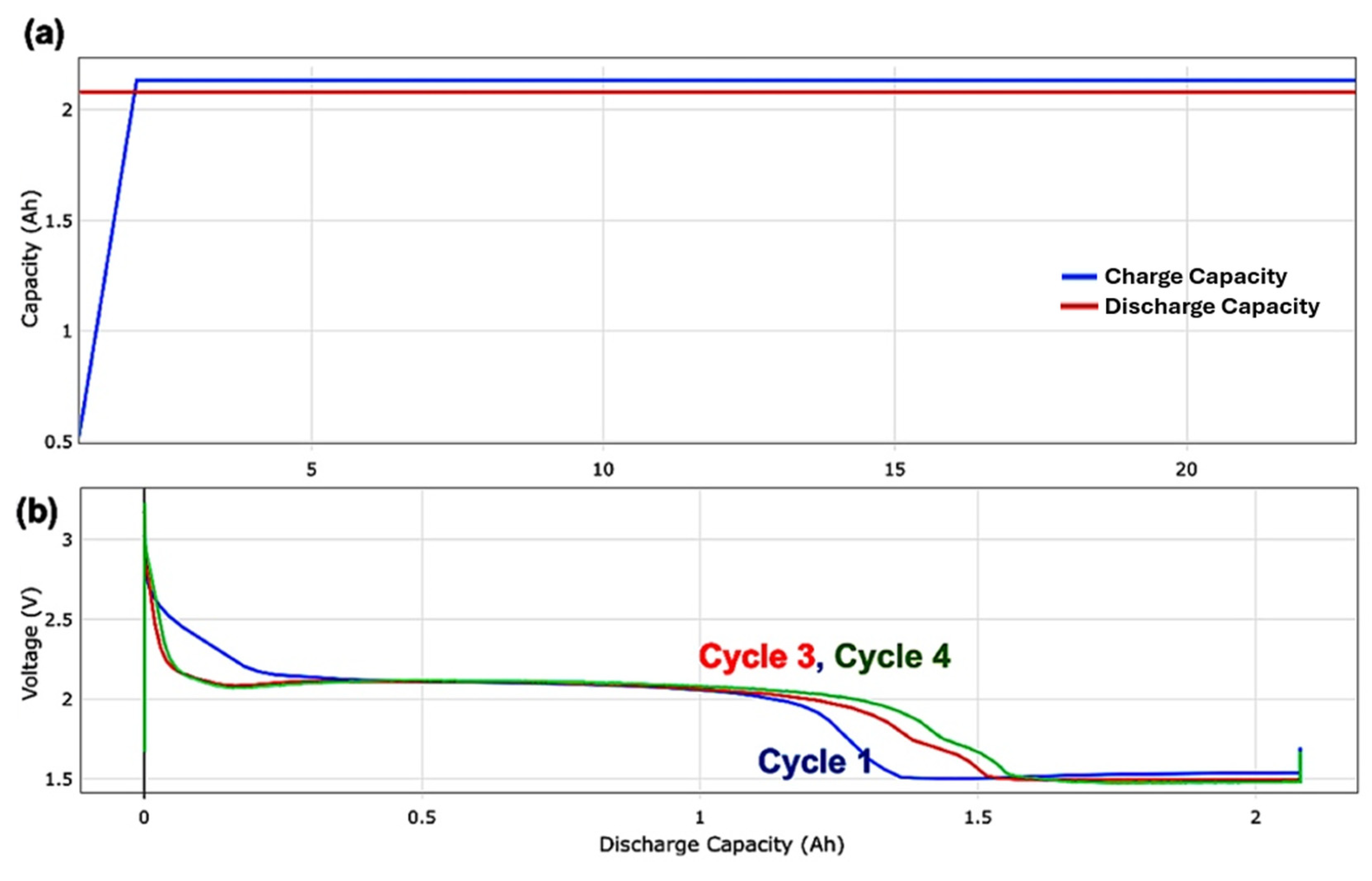
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cho, J.; Yadav, G.G.; Weiner, M.; Huang, J.; Upreti, A.; Wei, X.; Yakobov, R.; Hawkins, B.E.; Nyce, M.; Lambert, T.N.; et al. Hydroxyl Conducting Hydrogels Enable Low-Maintenance Commercially Sized Rechargeable Zn–MnO2 Batteries for Use in Solar Microgrids. Polymers 2022, 14, 417. [Google Scholar] [CrossRef]
- Lim, M.B.; Lambert, T.N.; Chalamala, B.R. Rechargeable Alkaline Zinc–Manganese Oxide Batteries for Grid Storage: Mechanisms, Challenges and Developments. Mater. Sci. Eng. R Rep. 2021, 143, 100593. [Google Scholar] [CrossRef]
- Yadav, G.G.; Cho, J.; Turney, D.; Hawkins, B.; Wei, X.; Huang, J.; Banerjee, S.; Nyce, M. Going beyond Intercalation Capacity of Aqueous Batteries by Exploiting Conversion Reactions of Mn and Zn electrodes for Energy-Dense Applications. Adv. Energy Mater. 2019, 9, 1902270. [Google Scholar] [CrossRef]
- Lim, M.; Lambert, T.N. Rechargeable Zinc Batteries for Grid Storag. In DOE Energy Storage Handbook; DOE Office of Electricity Energy Storage Program: Washington, DC, USA, 2021. Available online: https://www.sandia.gov/ess-ssl/eshb/ (accessed on 31 July 2025).
- Turney, D.E.; Gallaway, J.W.; Yadav, G.G.; Ramirez, R.; Nyce, M.; Banerjee, S.; Chen-Wiegart, Y.-C.K.; Wang, J.; D’aMbrose, M.J.; Kolhekar, S.; et al. Rechargeable Zinc Alkaline Anodes for Long-Cycle Energy Storage. Chem. Mater. 2017, 29, 4819–4832. [Google Scholar] [CrossRef]
- Debnath, S.; Maiti, A.; Naskar, P.; Banerjee, A. Rechargeable Manganese Dioxide−Zinc Batteries: A Review Focusing on Challenges and Optimization Strategies under Alkaline and Mild Acidic Electrolyte Media. ChemNanoMat 2022, 8, 2200261. [Google Scholar] [CrossRef]
- Zhong, C.; Liu, B.; Ding, J.; Liu, X.; Zhong, Y.; Li, Y.; Sun, C.; Han, X.; Deng, Y.; Zhao, N.; et al. Decoupling electrolytes towards stable and high-energy rechargeable aqueous zinc–manganese dioxide batteries. Nat. Energy 2020, 5, 440–449. [Google Scholar] [CrossRef]
- Durena, R.; Zukuls, A. A Short Review: Comparison of Zinc–Manganese Dioxide Batteries with Different pH Aqueous Electrolytes. Batteries 2023, 9, 311. [Google Scholar] [CrossRef]
- Huang, J.; Yadav, G.G.; Gallaway, J.W.; Wei, X.; Nyce, M.; Banerjee, S. A calcium hydroxide interlayer as a selective separator for rechargeable alkaline Zn/MnO2 batteries. Electrochem. Commun. 2017, 81, 136–140. [Google Scholar] [CrossRef]
- Cho, J.; Turney, D.E.; Yadav, G.G.; Nyce, M.; Wygant, B.R.; Lambert, T.N.; Banerjee, S. Use of Hydrogel Electrolyte in Zn-MnO2 Rechargeable Batteries: Characterization of Safety, Performance, and Cu2+ Ion Diffusion. Polymers 2024, 16, 658. [Google Scholar] [CrossRef]
- Poosapati, A.; Vadnala, S.; Negrete, K.; Lan, Y.; Hutchison, J.; Zupan, M.; Madan, D. Rechargeable Zinc-Electrolytic Manganese Dioxide (EMD) Battery with a Flexible Chitosan-Alkaline Electrolyte. ACS Appl. Energy Mater. 2021, 4, 4248–4258. [Google Scholar] [CrossRef]
- Kordesch, K.; Gsellmann, J.; Peri, M.; Tomantschger, K.; Chemelli, R. The rechargeability of manganese dioxide in alkaline electrolyte. Electrochimica Acta 1981, 26, 1495–1504. [Google Scholar] [CrossRef]
- Ingale, N.D.; Gallaway, J.W.; Nyce, M.; Couzis, A.; Banerjee, S. Rechargeability and economic aspects of alkaline zinc–manganese dioxide cells for electrical storage and load leveling. J. Power Sources 2015, 276, 7–18. [Google Scholar] [CrossRef]
- Yao, Y.F.; Gupta, N.; Wroblowa, H. Rechargeable manganese oxide electrodes: Part I. Chemically modified materials. J. Electroanal. Chem. Interfacial Electrochem. 1987, 223, 107–117. [Google Scholar] [CrossRef]
- Wroblowa, H.S.; Gupta, N. Rechargeable manganese oxide electrodes: Part II. physically modified materials. J. Electroanal. Chem. Interfacial Electrochem. 1987, 238, 93–102. [Google Scholar] [CrossRef]
- Dzieciuch, M.A.; Gupta, N.; Wroblowa, H.S. Rechargeable Cells with Modified MnO2 Cathodes. J. Electrochem. Soc. 1988, 135, 2415–2418. [Google Scholar] [CrossRef]
- Meng, L.; Zhu, Y.; Lu, Y.; Liang, T.; Zhou, L.; Fan, J.; Kuo, Y.; Guan, P.; Wan, T.; Hu, L.; et al. Rechargeable Zn−MnO2 Batteries: Progress, Challenges, Rational Design, and Perspectives. ChemElectroChem 2024, 11, e202300495. [Google Scholar] [CrossRef]
- Liu, X.; Yi, J.; Wu, K.; Jiang, Y.; Liu, Y.; Zhao, B.; Li, W.; Zhang, J. Rechargeable Zn–MnO2 batteries: Advances, challenges and perspectives. Nanotechnology 2020, 31, 122001. [Google Scholar] [CrossRef]
- Zimmerer, E.K.; Gallaway, J.W. Structural identification of disordered γ-MnOOH in the alkaline MnO2 discharge mechanism. MRS Commun. 2025, 1–8. [Google Scholar] [CrossRef]
- Darıcıoğlu, N.Ö.; Akbaş, Y.; Öztürk, T. Development of MnO2 Based Cathodes for Alkaline Batteries via Combinatorial Approach. J. Electrochem. Soc. 2022, 169, 120529. [Google Scholar] [CrossRef]
- Daniel-Ivad, J. Alkaline Manganese Dioxide Cell with Improved Open Circuit Voltage; CA2455548A1; Canadian Intellectual Property Office: Gatineau, QC, Canada, 2004.
- Yadav, G.G.; Gallaway, J.W.; Turney, D.E.; Nyce, M.; Huang, J.; Wei, X.; Banerjee, S. Regenerable Cu-intercalated MnO2 layered cathode for highly cyclable energy dense batteries. Nat. Commun. 2017, 8, 14424. [Google Scholar] [CrossRef]
- Yadav, G.G.; Wei, X.; Huang, J.; Gallaway, J.W.; Turney, D.E.; Nyce, M.; Secor, J.; Banerjee, S. A conversion-based highly energy dense Cu2+ intercalated Bi-birnessite/Zn alkaline battery. J. Mater. Chem. A 2017, 5, 15845–15854. [Google Scholar] [CrossRef]
- Yadav, G.G.; Wei, X.; Gallaway, J.W.; Chaudhry, Z.; Shin, A.; Huang, J.; Yakobov, R.; Nyce, M.; Vanderklaauw, N.; Banerjee, S. Rapid electrochemical synthesis of δ-MnO2 from γ-MnO2 and unleashing its performance as an energy dense electrode. Mater. Today Energy 2017, 6, 198–210. [Google Scholar] [CrossRef]
- Gallaway, J.W.; Yadav, G.G.; Turney, D.E.; Nyce, M.; Huang, J.; Chen-Wiegart, Y.-C.K.; Williams, G.; Thieme, J.; Okasinski, J.S.; Wei, X.; et al. An Operando Study of the Initial Discharge of Bi and Bi/Cu Modified MnO2. J. Electrochem. Soc. 2018, 165, A2935–A2947. [Google Scholar] [CrossRef]
- Wu, J.; Faiz, Y.; Hussain, S.; Faiz, F.; Zarshad, N.; Rahman, A.U.; Masood, M.A.; Deng, Y.; Pan, X.; Ahmad, M. Defects rich- Cu-doped MnO2 nanowires as an efficient and durable electrode for high performance aqueous supercapacitors. Electrochimica Acta 2023, 443, 141927. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, T.; Javed, M.S.; Zeng, W.; Hussain, S.; Zhang, Y.; Peng, X. Rational synthesis of Cu-doped porous δ-MnO2 microsphere for high performance supercapacitor applications. Electrochimica Acta 2016, 191, 716–723. [Google Scholar] [CrossRef]
- Yang, G.; Ma, K.; Wang, C. Unconventional Copper Electrochemistry in Aqueous Zn‖CuMn2O4 Batteries. Adv. Energy Mater. 2024, 14, 2303695. [Google Scholar] [CrossRef]
- Lan, R.; Gkanas, E.; Sahib, A.J.S.; Greszta, A.; Bhagat, R.; Roberts, A. The effect of copper doping in α-MnO2 as cathode material for aqueous Zinc-ion batteries. J. Alloy. Compd. 2024, 992, 174528. [Google Scholar] [CrossRef]
- Wu, T.-H.; Liang, W.-Y.; Lin, Y.-Q. Facile synthesis of Cu−intercalated MnO2 nanoflakes cathode for enhanced energy storage in zinc−ion batteries. J. Taiwan Inst. Chem. Eng. 2022, 131, 104172. [Google Scholar] [CrossRef]
- Spoerke, E.D.; Passell, H.; Cowles, G.; Lambert, T.N.; Yadav, G.G.; Huang, J.; Banerjee, S.; Chalamala, B. Driving Zn-MnO2 grid-scale batteries: A roadmap to cost-effective energy storage. MRS Energy Sustain. 2022, 9, 13–18. [Google Scholar] [CrossRef]
- Schorr, N.B.; Arnot, D.J.; Bruck, A.M.; Duay, J.; Kelly, M.; Habing, R.L.; Ricketts, L.S.; Vigil, J.A.; Gallaway, J.W.; Lambert, T.N. Rechargeable Alkaline Zinc/Copper Oxide Batteries. ACS Appl. Energy Mater. 2021, 4, 7073–7082. [Google Scholar] [CrossRef]
- Shin, J.; Seo, J.K.; Yaylian, R.; Huang, A.; Meng, Y.S. A review on mechanistic understanding of MnO2 in aqueous electrolyte for electrical energy storage systems. Int. Mater. Rev. 2019, 65, 356–387. [Google Scholar] [CrossRef]
- Shang, W.; Ma, Y.; Tan, P. A Multifaceted Competitive Zn–Cu Battery Unleashed by Optimizing Charge Protocols. Adv. Funct. Mater. 2024, 34, 2315782. [Google Scholar] [CrossRef]
- Hao, Q.; Zhou, D.; Huang, J.; Xia, J.; Qu, Z.; Li, Z.; Teng, C. Multicomponent hierarchical Cu-based advanced cathode for ultrahigh capacity rechargeable Cu-Zn battery. J. Alloy. Compd. 2024, 1006, 176289. [Google Scholar] [CrossRef]
- Huang, J.; Yadav, G.; Turney, D.E.; Cho, J.; Nyce, M.; Wygant, B.R.; Lambert, T.N.; Banerjee, S. Ion-Selective Graphene Oxide/Polyvinyl Alcohol Composite Membranes for Rechargeable Alkaline Zinc Manganese Dioxide Batteries. ACS Appl. Energy Mater. 2022, 5, 9952–9961. [Google Scholar] [CrossRef]
- Minakshi, M.; Mitchell, D.R. The influence of bismuth oxide doping on the rechargeability of aqueous cells using MnO2 cathode and LiOH electrolyte. Electrochimica Acta 2008, 53, 6323–6327. [Google Scholar] [CrossRef]
- Bruck, A.M.; Kim, M.A.; Ma, L.; Ehrlich, S.N.; Okasinski, J.S.; Gallaway, J.W. Bismuth Enables the Formation of Disordered Birnessite in Rechargeable Alkaline Batteries. J. Electrochem. Soc. 2020, 167, 110514. [Google Scholar] [CrossRef]
- Ramirez-Meyers, K.; Wu, X.; Whitacre, J.F. Exploring the Voltage Stability of Birnessitic Bi-MnO2 Cathodes Formed In Situ in NaOH and KOH-Based Electrolytes. J. Electrochem. Soc. 2024, 171, 030524. [Google Scholar] [CrossRef]
- Wu, X.; Whitacre, J.F. Reducing Manganese Dissolution in Electrolytic Manganese Dioxide Electrodes in NaOH Electrolyte. J. Electrochem. Soc. 2024, 171, 070542. [Google Scholar] [CrossRef]
- Zhu, C.; Schorr, N.B.; Qi, Z.; Wygant, B.R.; Turney, D.E.; Yadav, G.G.; Worsley, M.A.; Duoss, E.B.; Banerjee, S.; Spoerke, E.D.; et al. Direct Ink Writing of 3D Zn Structures as High-Capacity Anodes for Rechargeable Alkaline Batteries. Small Struct. 2022, 4, 2200323. [Google Scholar] [CrossRef]
- Hopkins, B.J.; Sassin, M.B.; Chervin, C.N.; DeSario, P.A.; Parker, J.F.; Long, J.W.; Rolison, D.R. Fabricating Architected Zinc Electrodes with Unprecedented Volumetric Capacity in Rechargeable Alkaline Cells. Energy Storage Mater. 2020, 27, 370–376. [Google Scholar] [CrossRef]
- Arnot, D.J.; Lim, M.B.; Bell, N.S.; Schorr, N.B.; Hill, R.C.; Meyer, A.; Cheng, Y.; Lambert, T.N. High Depth-of-Discharge Zinc Rechargeability Enabled by a Self-Assembled Polymeric Coating. Adv. Energy Mater. 2021, 11, 2101594. [Google Scholar] [CrossRef]
- Yang, P.K.; Turney, D.E.; Nyce, M.; Wygant, B.R.; Lambert, T.N.; O’Brien, S.; Yadav, G.G.; Weiner, M.; Yang, S.; Hawkins, B.E.; et al. Performance and failure mechanisms of alkaline zinc anodes with addition of calcium zincate (Ca[Zn(OH)3]2·2H2O) under industrially relevant conditions. Energy Adv. 2024, 3, 1932–1947. [Google Scholar] [CrossRef]
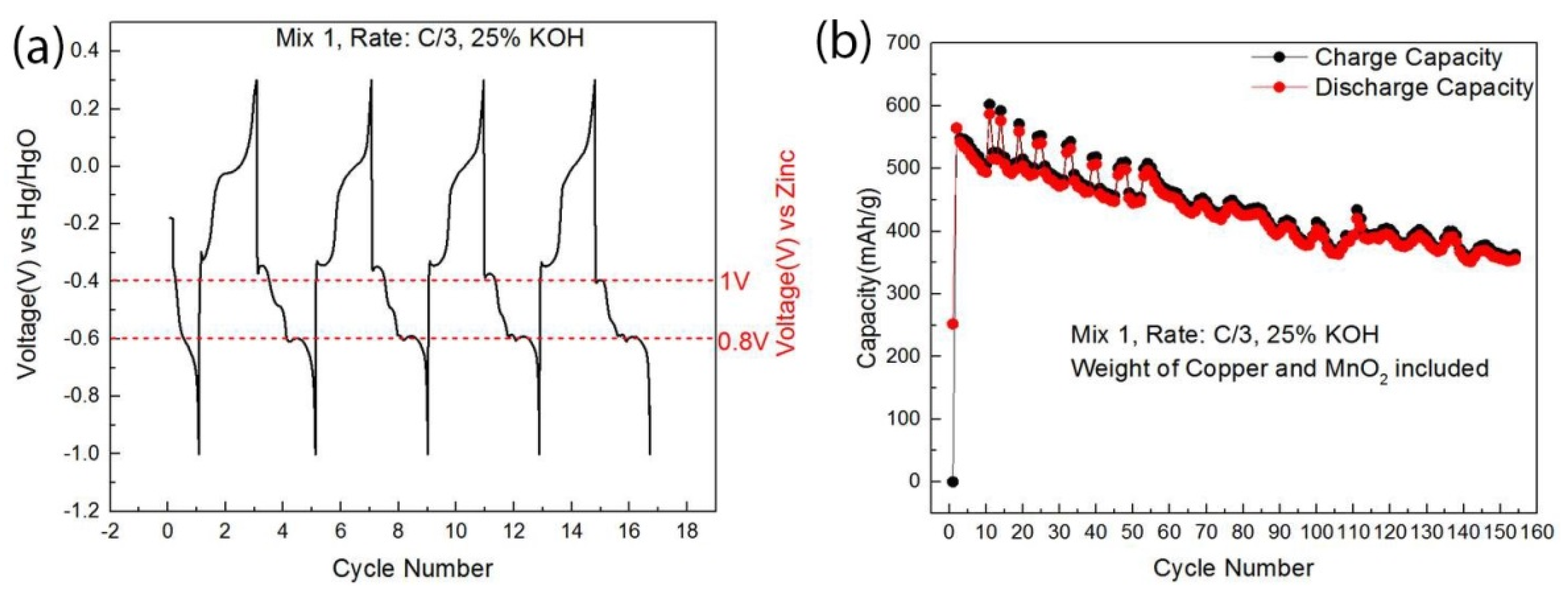
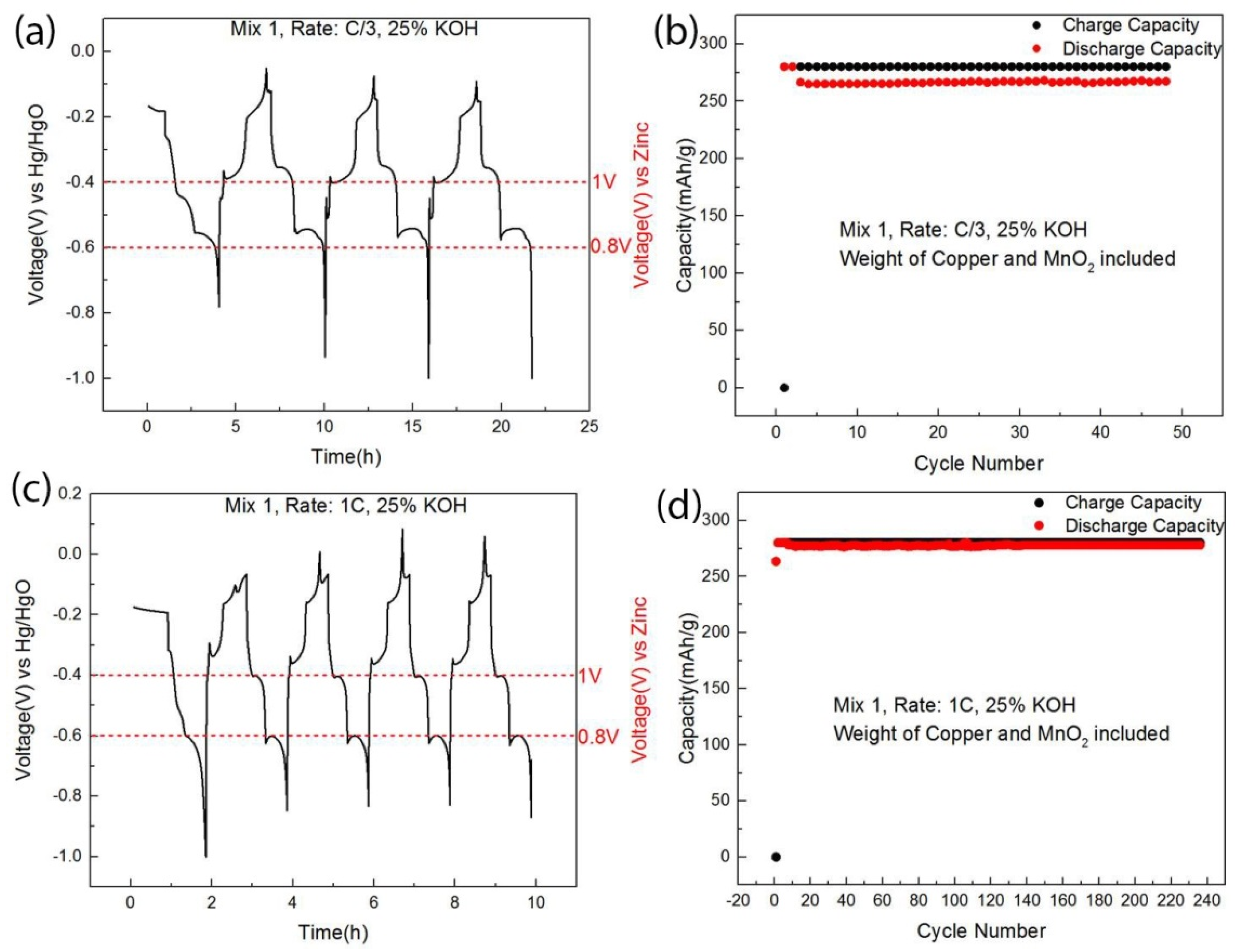
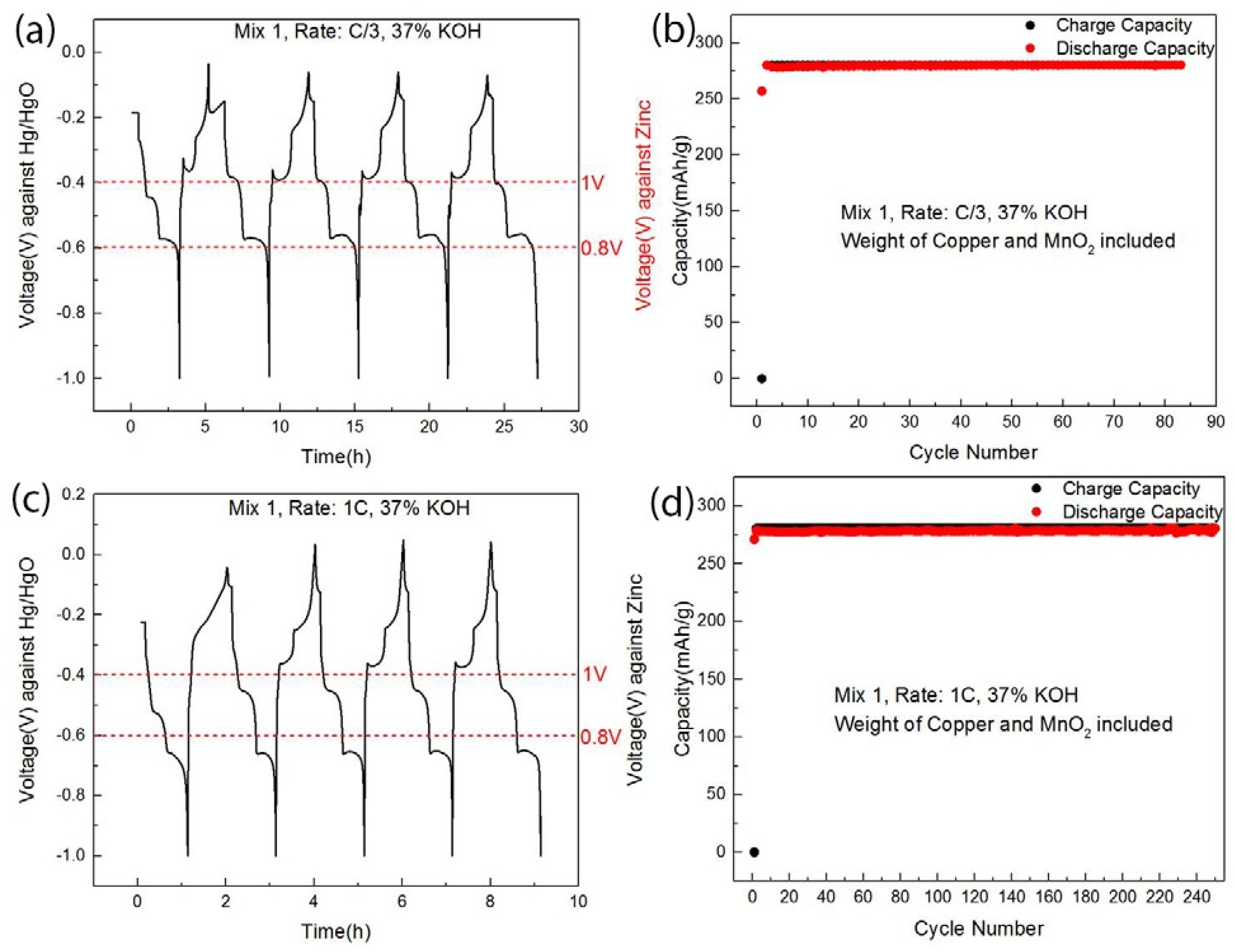
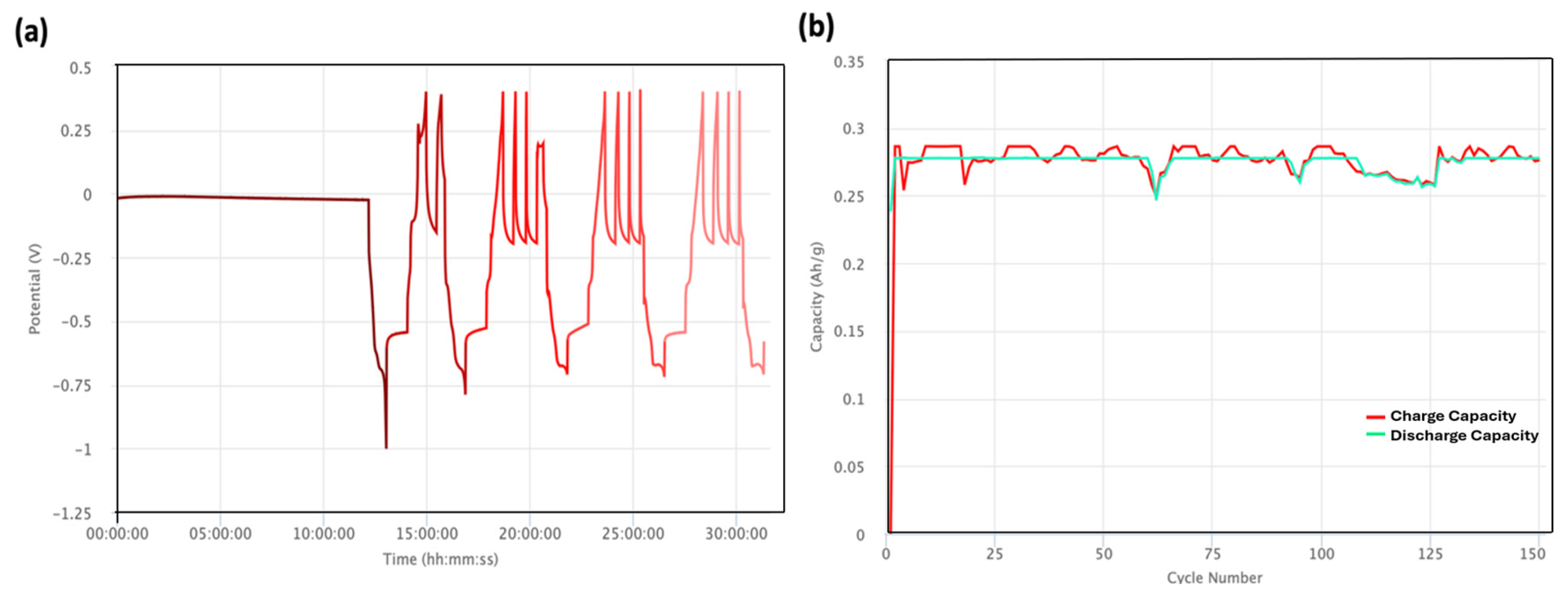
| Cathode Material | Raw Material Price ($/kg) | Specific Capacity 1 (Ah/kg) | Discharge Voltage 2 (V) | Specific Energy (Wh/kg) | Cathode Active Material Cost ($/kWh) |
|---|---|---|---|---|---|
| MnO2 | 1.5 | 308 | 1.3 | 400 | 3.7 |
| V2O5 | 17 | 295 | 0.8 | 236 | 72 |
| Ni(OH)2 | 25 | 288 | 1.6 | 460 | 54 |
| Bromine | 3 | 335 | 1.5 | 500 | 6 |
| CuO | 7.5 | 337 | 0.7 | 236 | 32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, G.G.; Sammy, M.; Cho, J.; Booth, M.N.; Nyce, M.; Huang, J.; Lambert, T.N.; Turney, D.E.; Wei, X.; Banerjee, S. Performance of Low-Cost Energy Dense Mixed Material MnO2-Cu2O Cathodes for Commercially Scalable Aqueous Zinc Batteries. Batteries 2025, 11, 291. https://doi.org/10.3390/batteries11080291
Yadav GG, Sammy M, Cho J, Booth MN, Nyce M, Huang J, Lambert TN, Turney DE, Wei X, Banerjee S. Performance of Low-Cost Energy Dense Mixed Material MnO2-Cu2O Cathodes for Commercially Scalable Aqueous Zinc Batteries. Batteries. 2025; 11(8):291. https://doi.org/10.3390/batteries11080291
Chicago/Turabian StyleYadav, Gautam G., Malesa Sammy, Jungsang Cho, Megan N. Booth, Michael Nyce, Jinchao Huang, Timothy N. Lambert, Damon E. Turney, Xia Wei, and Sanjoy Banerjee. 2025. "Performance of Low-Cost Energy Dense Mixed Material MnO2-Cu2O Cathodes for Commercially Scalable Aqueous Zinc Batteries" Batteries 11, no. 8: 291. https://doi.org/10.3390/batteries11080291
APA StyleYadav, G. G., Sammy, M., Cho, J., Booth, M. N., Nyce, M., Huang, J., Lambert, T. N., Turney, D. E., Wei, X., & Banerjee, S. (2025). Performance of Low-Cost Energy Dense Mixed Material MnO2-Cu2O Cathodes for Commercially Scalable Aqueous Zinc Batteries. Batteries, 11(8), 291. https://doi.org/10.3390/batteries11080291









